Abstract
Background and Aim:
Monitoring carbon dioxide (CO2) is of utmost importance in neurosurgical patients. It is measured by partial pressure of arterial CO2 (PaCO2) and end-tidal CO2 (ETCO2). We aimed to study the correlation between PaCO2 and ETCO2 in neurosurgical patients in the intraoperative and postoperative period on mechanical ventilation in Postanesthesia Care Unit (PACU).
Methodology:
This was prospective observational study done at tertiary care teaching public hospital over a period of 1 year. We studied 30 patients undergoing elective craniotomy intraoperatively and in the postoperative period on mechanical ventilation for 24 h. Serial measurement of ETCO2 and PaCO2 at baseline, hourly intraoperatively and every 6 hourly in the PACU were studied. Data analysis was done using SPSS software version 20.
Results:
The mean PaCO2–ETCO2 gradient intraoperatively over 4 h is 3.331 ± 2.856 and postoperatively over 24 h is 2.779 ± 2.932 and lies in 95% confidence interval. There was statistically significant correlation between PaCO2 and ETCO2 intraoperatively baseline, 1 h, 2 h, 3 h, and 4 h with Pearson's correlation coefficients of 0.799, 0.522, 0582, 0.439, and 0.547, respectively (P < 0.05). In PACU at baseline, 6 h, 12 h, 18 h, and 24 h Pearson's correlation coefficients were. 534, −0.032, 0.522, 0.242, 0.592, and 0.547, respectively, which are highly significant at three instances (P < 0.01).
Conclusion:
ETCO2 correlates PaCO2 with acceptable accuracy in neurosurgical patients in the intraoperative and postoperative period on mechanical ventilation in Intensive Care Unit. Thus, continuous and noninvasive ETCO2 can be used as a reliable guide to estimate arterial PCO2 during neurosurgical procedures and in PACU.
Keywords: End-tidal carbondioxide, neurosurgery, partial pressure of arterial carbon dioxide, postoperative care unit
Introduction
In neurosurgeries monitoring of arterial partial pressure of carbon dioxide (PaCO2) is most vital as it affects intracranial pressure (ICP), cerebral blood flow, volume and cerebral perfusion pressure (CPP). Increase in PaCO2 increases ICP thereby decreasing CPP. Raised ICP can be reduced through therapeutic hyperventilation; however, excessive hyperventilation (<20 mmHg) could result in regional cerebral hypoxia.[1,2] Hence, continuous monitoring of CO2 is of utmost importance. End-tidal CO2 (ETCO2) is another method to estimate CO2 continuously and noninvasively. A good alveolar ventilation-perfusion matching results in an ETCO2 that closely correlates with PaCO2; hence in patients without significant cardiopulmonary disorders, PaCO2 may be estimated by using actual ETCO2 measurements. The difference between PaCO2 and ETCO2 (P(a-ET) CO2 gradient) is reported to be 3.6–4.6 mmHg in healthy awake patients. However, in literature various studies mention substantial variability in patients undergoing craniotomy in different positions and mechanically ventilated neurosurgical Intensive Care Unit (ICU) patients.[3,4,5,6] In addition, in diseased lungs, impaired cardiac function, increased dead space ventilation, ventilation-perfusion (V/Q) mismatch, sampling line error, and critical illness may widen the above gradient.[7,8] Nevertheless, monitoring ETCO2 has many advantages such as it reduces the need for invasive arterial blood gas (ABG) sampling, allowing safe, comfortable, and continuous monitoring. A sudden change in ETCO2 can prompt the clinician to measure PaCO2 via an ABG sample thus before the patient is compromised, early intervention is guaranteed.[9] This also has important implication in Postanesthesia Care Unit (PACU) for more cautious postoperative care. There is lot of contradiction in the recent literature regarding ETCO2 and PaCO2 correlation in neurosurgical patients.[1,3,4,5,6,7,10,11] Hence, we decided to do the present study in Indian population.
The aim was to study the correlation between PaCO2 and ETCO2 in patients undergoing neurosurgery in the intraoperative as well as in the postoperative period on mechanical ventilation in PACU.
Methodology
This was prospective observational study done at a tertiary care teaching public hospital in neurosurgery operation theater and PACU after approval from the Institutional Ethics Committee and written informed valid consent. The study was conducted over a period of 1 year from June 2014 to June 2015. We studied 30 patients aged between 18 and 60 years, belonging to the American Society of Anesthesiologists (ASA) Grade 1/2 undergoing elective craniotomy (surgical duration of 4–5 h) in the supine position. In the postoperative period, we included only those patients who required mechanical ventilation for minimum 24 h period. We excluded patients with lung disease and hemodynamically unstable patients.
After adequate preoxygenation and premedication, induction was done with intravenous (IV) fentanyl 2 μg/kg and thiopentone 5 mg/kg. Vecuronium 0.08 mg/kg was used to facilitate tracheal intubation. After intubation with an appropriate-sized cuffed endotracheal tube, intermittent positive pressure ventilation was given using a volume-controlled mode with a tidal volume of 7–10 ml/kg and a respiratory rate of 10–12 breaths per minute and continous ETCO2 was monitored using a side-stream capnometer (Patient Monitor 9000 Express side-stream CO2, Penlon Limited, Abington, Oxon). Postinduction radial artery cannulated and baseline ABG were collected. Anesthesia was maintained with oxygen (40–50%), air and desflurane (minimum alveolar concentration 0.8–1.0). The first postinduction measurement of ETCO2 and PaCO2 was taken as a baseline and then repeated for every 1 h until the end of surgery. In the postoperative period in PACU, patients were maintained on volume-synchronized intermittent mandatory ventilation (volume SIMV) with inspired oxygen fraction (FiO2) 40–50% and adequate sedation and analgesia with titrated doses of IV midazolam and fentanyl. Continuous ETCO2 was recorded by using a sidestream capnometer which was connected by angle piece connector in between the endotracheal tube and breathing circuit. After stabilizing the patient in PACU, a baseline measurement of ETCO2 and PaCO2 was recorded and thereafter every 6 hourly. Simultaneous measurement of blood pressure, heart rate, respiratory rate, central venous pressure, tidal volume, and FiO2, peak inspiratory pressure were recorded at each sampling time. Standard calibration of sidestream CO2 of patient monitor 9000 express was done with the same gas mixture before induction of each case as per the specifications of manufacturer.[12]
Statistical analysis
We calculated sample size with reference to Husaini and Choy, 2008, by taking into consideration Pearson's correlation between PaCO2 and ETCO2, with Type I error of 0.05 and Type II error of 0.20, with power equal to 0.80, which came to be 21.[1]
We decided to go ahead with a sample size of 30, which was appropriate for the study design and institutional settings. Quantitative data are presented with the help of mean, median, standard deviation (SD), interquartile range (IQR), minimum and maximum values. Qualitative data are presented with the help of frequency and percentage table. Data were initially analyzed using Pearson's correlation to assess the relationship between PaCO2 and ETCO2 at different stages of the operation. P < 0.05 was considered statistically significant with 95% confidence interval (CI).
Data analysis is done with the help of IBM Corp. Released 2011. IBM SPSS Statistics for windows, Version 20.0. Armonk, NY:IBM Corp.
Results
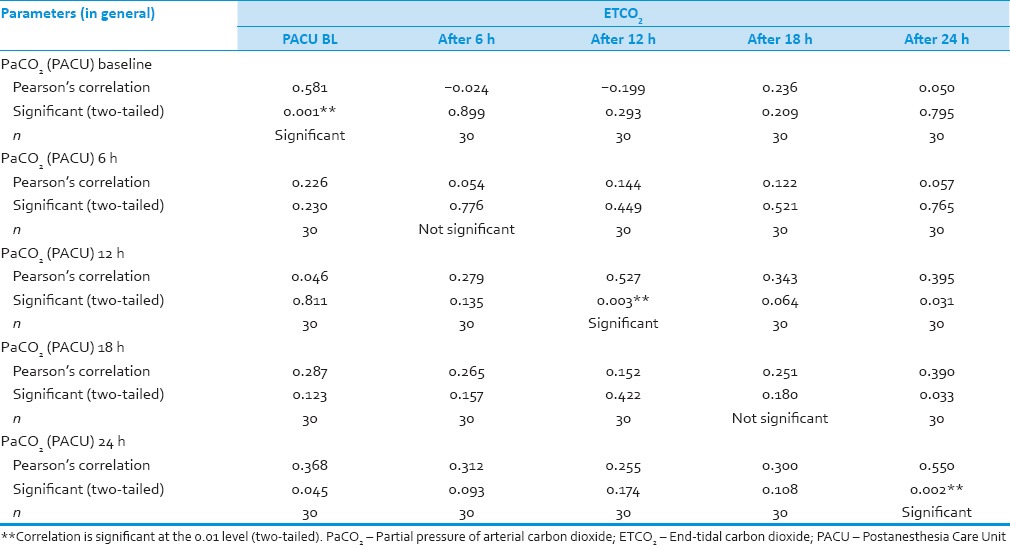
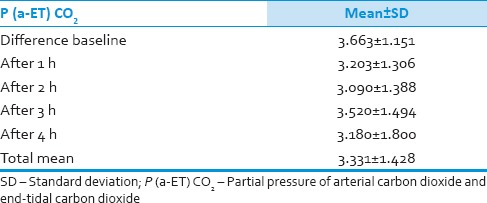
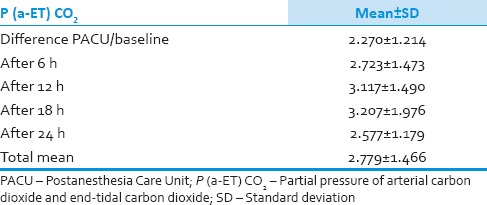
We analyzed 30 patients in the age group of 18–60 years with youngest being 23 years old and oldest 59 years and 11 of them belonged to the age group of 31–40. Among these 60% (18) were male and rest 40% (12) female with ASA Grade 1 as 40% (12) and ASA Grade 2 60% (18). There was no significant correlation of PaCO2 and ETCO2 values and demographic data, ASA grades. The various neurosurgeries included in the study with percentage distribution are depicted in Figure 1 with no significant correlation between diagnosis and correlation between PaCO2 and ETCO2. The parameters ETCO2 and PaCO2 andP(a-ET) CO2 gradient at regular intervals were recorded with mean, SD, median, IQR, minimum and maximum values as depicted in Table 1. The meanP(a-ET) CO2 gradient at each time interval in both intraoperative and the postoperative period is represented in Table 1, Figures 2 and 3. The mean ofP(a-ET) CO2 gradient intraoperatively over 4 h is found to be 3.331 ± 2.856 and postoperatively over 24 h 2.779 ± 2.932 and lies in 95% CI. Correlations between PaCO2 and ETCO2 at different intervals during intraoperative period and in PACU are depicted in Tables 2 and 3. Data are analyzed by using Pearson's correlation to study the relationship between PaCO2 and ETCO2 at regular intervals. Table 2 shows correlation between PaCO2 and ETCO2 intraoperatively with statistically significant Pearson's correlation coefficients. Table 3 shows the correlation between PaCO2 and ETCO2 postoperatively in PACU, displaying highly significant correlation at three occasions however not significant at two occasions.
Figure 1.
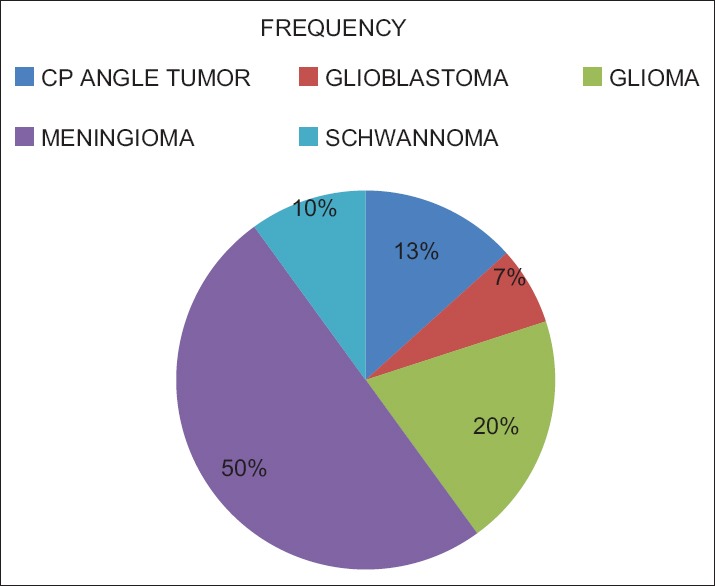
Distribution of study group as per diagnosis
Table 1.
Arterial and end-tidal carbon dioxide values in intraoperative and postoperative period
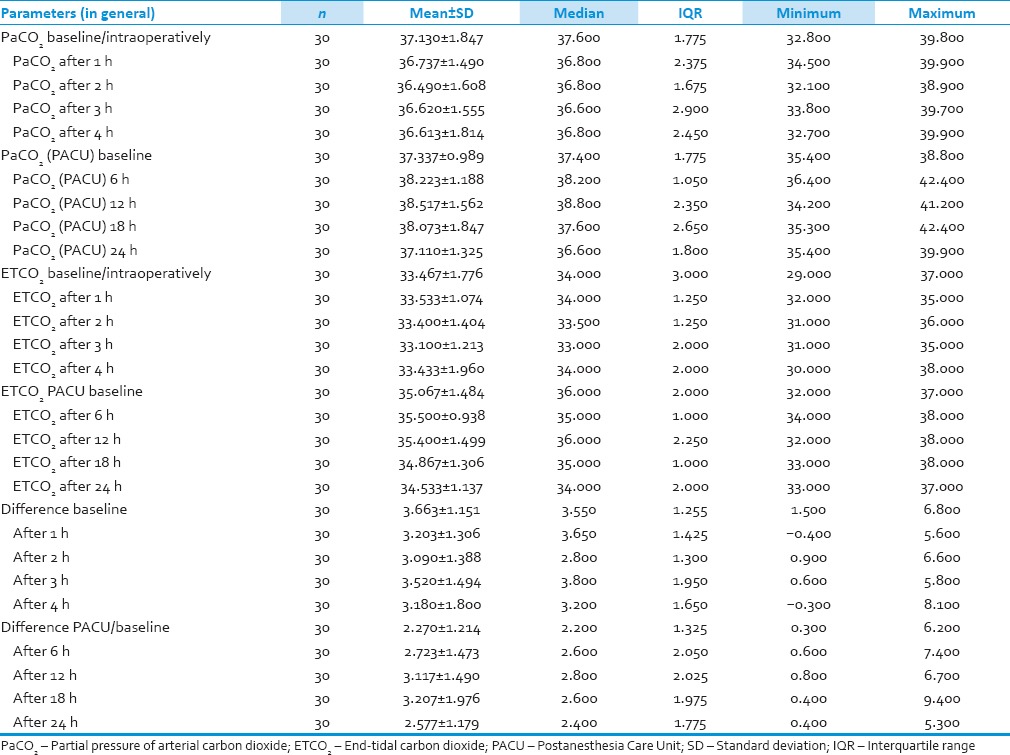
Figure 2.
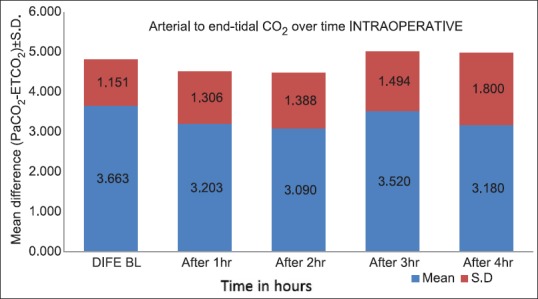
The arterial to end-tidal carbon dioxide differences over time for intraoperative period (mean ± standard deviation)
Figure 3.
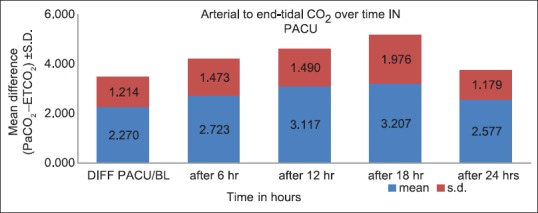
The arterial to end-tidal carbon dioxide differences over time for postoperative period (mean ± standard deviation)
Table 2.
Correlation between partial pressures of arterial and end-tidal carbon dioxide during craniotomy
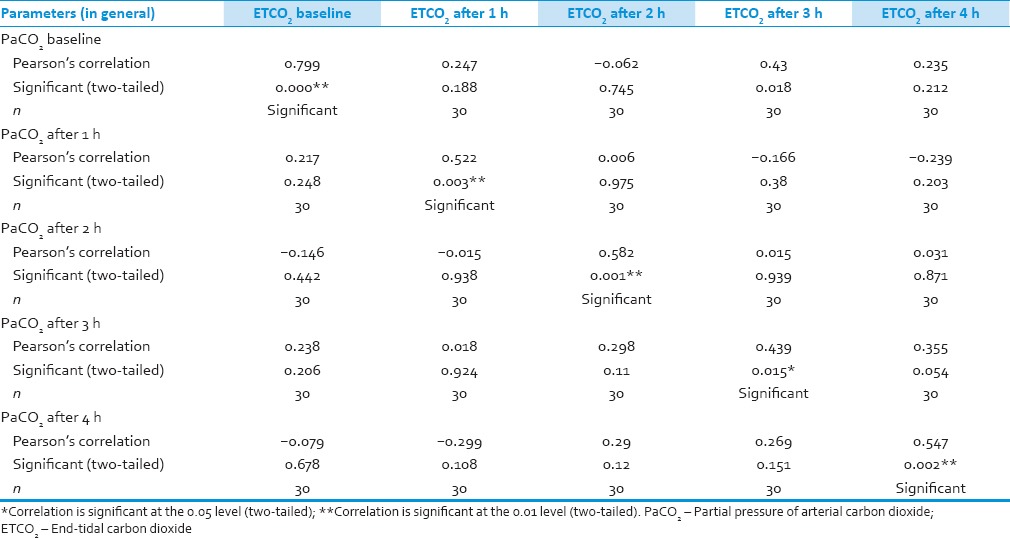
Table 3.
Correlation between partial pressures of arterial and end-tidal carbon dioxide in Postanesthesia Care Unit
Figures 4–13 show correlation between two methods of CO2 measurement at given point of time by plotting a scatter diagram with R as the correlation coefficient between each set of values.
Figure 4.
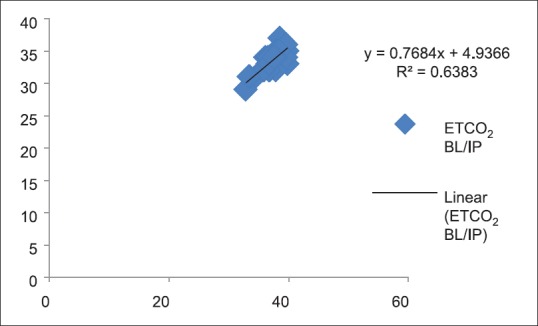
Correlation between partial pressure of arterial carbon dioxide and end-tidal carbon dioxide (P < 0.05)
Figure 13.
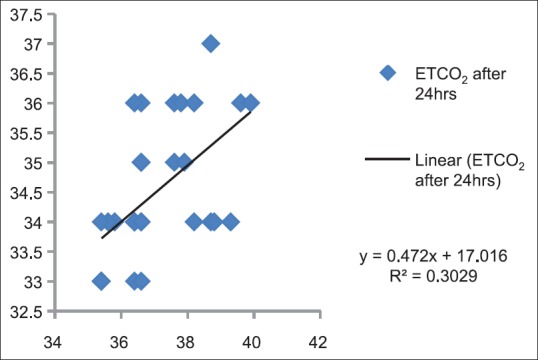
Correlation between partial pressure of arterial carbon dioxide and end-tidal carbon dioxide after 24 h in Postanesthesia Care Unit (P < 0.05)
Figure 5.
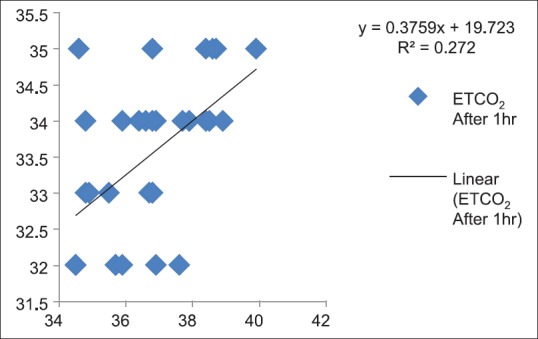
Correlation between partial pressure of arterial carbon dioxide and end-tidal carbon dioxide after 1 h (P < 0.05)
Figure 6.
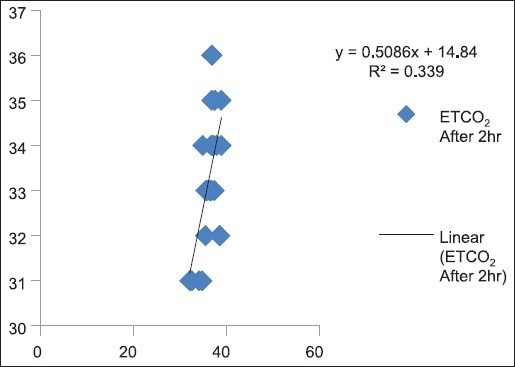
Correlation between partial pressure of arterial carbon dioxide and end-tidal carbon dioxide after 2 h (P < 0.05)
Figure 7.
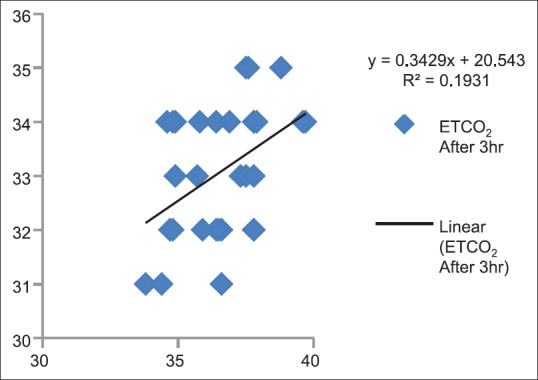
Correlation between partial pressure of arterial carbon dioxide and end-tidal carbon dioxide after 3 h (P < 0.05)
Figure 8.
Correlation between partial pressure of arterial carbon dioxide and end-tidal carbon dioxide after 4 h (P < 0.05)
Figure 9.
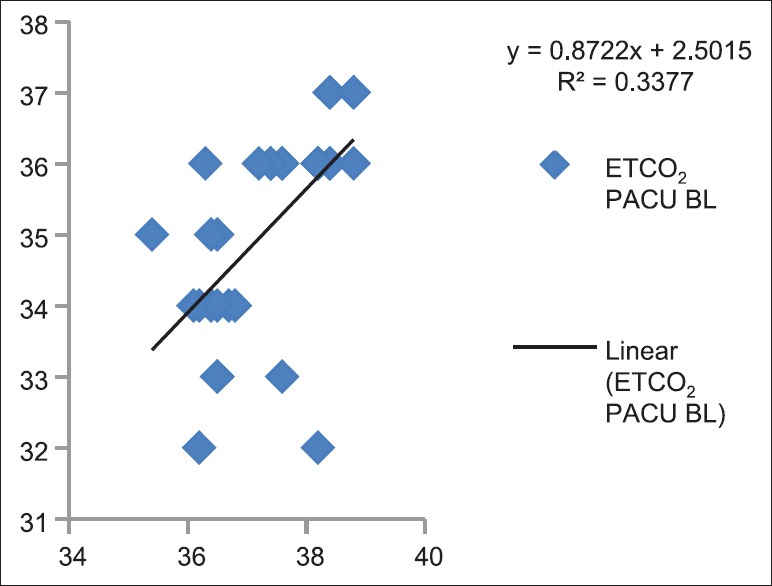
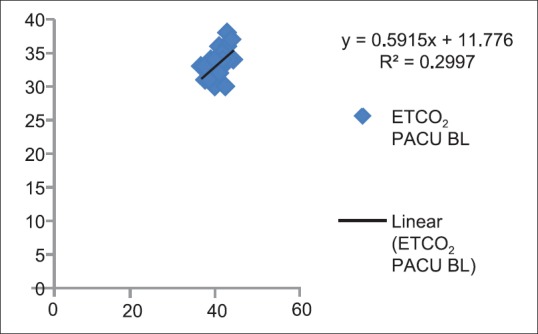
Correlation between partial pressure of arterial carbon dioxide and end-tidal carbon dioxide baseline in Postanesthesia Care Unit (P < 0.05)
Figure 10.
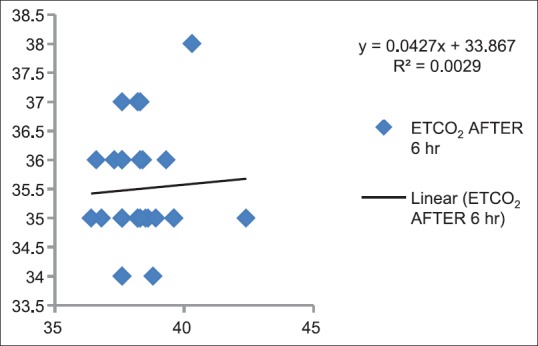
Correlation between partial pressure of arterial carbon dioxide and end-tidal carbon dioxide after 6 h in Postanesthesia Care Unit (P > 0.05)
Figure 11.
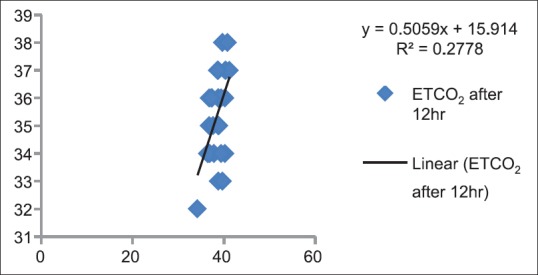
Correlation between partial pressure of arterial carbon dioxide and end-tidal carbon dioxide after 12 h in Postanesthesia Care Unit (P < 0.05)
Figure 12.
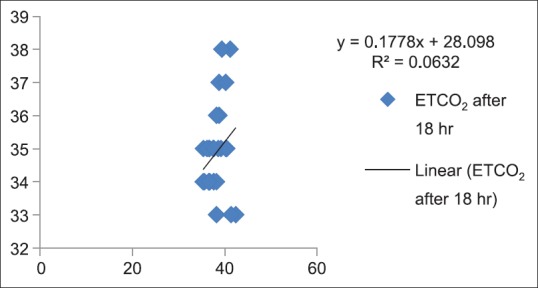
Correlation between partial pressure of arterial carbon dioxide and end-tidal carbon dioxide after 18 h in Postanesthesia Care Unit (P > 0.05)
Discussion
ETCO2 monitoring is considered the standard of care during general anesthesia and ICU care. The monitoring of PaCO2 and control in a narrow range is necessary during neurosurgical procedures as this affects and ICP dynamics and CPP.
ABG measurement of PaCO2 is considered gold standard for monitoring changes in CO2, which is invasive, expensive, and provides only intermittent measures of PaCO2. ETCO2 which is continuous respiratory measure of CO2 can also reflect an indirect quantity of PaCO2.[1] The ETCO2 may be used as a surrogate marker for monitoring PaCO2 in neurosurgical and ICU patients and thus reducing repetitive invasive ABG sampling. However, various studies have shown inconsistent results regarding this correlation during intraoperative period and in ICU patients. Hence, we conducted this study to evaluate the correlations in patients undergoing neurosurgeries who are also requiring postoperative ventilatory support for at least 24 h. Hence, this is the first initiative to assess the correlation of CO2 level through invasive and noninvasive methods in the intraoperative period as well as in the postoperative period in the same set of patients.
Thirty patients aged between 18 and 60 years, undergoing elective craniotomy and those who required postoperative mechanical ventilation for minimum 24 h period, were studied. We found no significant correlation between PaCO2 and ETCO2 with respect to demographic data, ASA grading, and diagnosis. Kenichi Satoh 2015 concluded that partial pressure gradient of PaCO2 to ETCO2 increases with increasing age in patients undergoining surgeries in general anesthesia in supine position however we did not observe similar finding.[8]
During intraoperative period mean difference between PaCO2 and ETCO2 was found to be 3.31 ± 2.856 with 95% CI [Table 4 and Figure 2]. In our study, PaCO2 values always exceeded ETCO2 and at any point of time it never went in opposite directions. P(a-ET) CO2 gradient lies between 3.6 and 4.6 mmHg in healthy awake patients. This gradient mainly depends on the degree of alveolar dead space.[12] Under stable physiologic conditions, with completely accurate monitoring, P(a-ET) CO2 gradient should be close to zero, thus PaCO2 values can be implicated accurately and constantly from ETCO2 values. The various causes of widened gradient are V/Q mismatch and poor sampling of gas at patients end, impaired cardiac function, and critical illness.[3] Khan et al. 2007, Sharma et al. 1995, Hemmati et al. 2012 showed similar mean values which remained satisfactorily persistent with ETCO2 for predicting PaCO2 under anesthesia.[3,6,11] However, above positive gradient was not consistently found in all the patients in the study of Russell and Graybeal 1995.[4]
Table 4.
Mean of gradient of partial pressures of arterial carbon dioxide and end- tidal carbon dioxide during intraoperative period
During the postoperative period, the mean of P(a-ET) CO2 gradient was found to be 2.78 ± 2.932 with 95% CI [Table 5 and Figure 3]. In these values also, there was a positive gradient in all values with PaCO2 exceeding ETCO2 values. According to Razi et al. 2012, in healthy subjects there are close correlation between PaCO2 and ETCO2, and it is commonly accepted that PaCO2 measurements vary approximately 2–5 mmHg above ETCO2 values.[7] Russell and Graybeal et al. 1992 found a significant correlation between the gradients in total study population but not in individual patients. The direction of PaCO2 change was also inaccurately predicted by ETCO2 changes. ETCO2 does not provide a stable reflection of PaCO2 in all neuro-intensive care patients.[5]
Table 5.
Mean of gradient of partial pressure of arterial carbon dioxide and end-tidal carbon dioxide in Postanesthesia Care Unit
We also calculated the correlation between PaCO2 and ETCO2 at given time and found out the positive correlation with each value, which was statistically significant (P < 0.05). During intraoperative period monitoring of each hourly value of PaCO2 and ETCO2, a positive significant correlation was found [Table 2]. These results are consistent with Khan et al. 2007, Husaini and Choy 2008, Hemmati et al. 2012, which showed a positive correlation at each time interval,[1,3,11] whereas Russell and Graybeal 1995 did not show positive correlation in all patients undergoing craniotomy.[4]
In the same patients in the postoperative period in ICU on volume SIMV mode for 24 h, we obtained a highly significant positive correlation (P < 0.01) at three occasions, however not significant at two [Table 3]. Razi et al. 2012 assessed in neurological patients admitted in intensive care in various modes of ventilation at a given point of time. They have found a positive correlation in each mode of ventilation (volume SIMV, continuous positive airway pressure, T-piece).[7] Kerr et al. 1996 studied the relationship between PaCO2 and ETCO2 in mechanically ventilated adults with severe head trauma and also observed ETCO2 monitoring correlating well with PaCO2 in patients without respiratory complications or without spontaneous breathing.[10] Russell and Graybeal 1992 did not obtain positive correlation in all ICU patients.[5] The reasons for this variability in the above gradient in our study and other studies as well are explained by various factors such as dead space fraction, ventilation-perfusion mismatch, the site of sampling, and nonuniform alveoli CO2 emptying patterns.[7,9] Cheifetz and Myers et al. 2007 have emphasized capnography as the standard of care in all respects right from operation theater to ICUs during mechanical ventilation.[9]
The present study was not without limitations as we did not study patients with major hemodynamic changes, severe lung disease, or positions other than supine. In healthy lungs and hemodynamically stable patients due to good alveolar ventilation and perfusion matching, ETCO2 closely correlates with PaCO2 but in above set of patients it may not correlate well, hence it is practical to verify with ABG analysis. Hence, future research should be directed in including all these patients. In addition, we used sidestream capnography intraoperatively as well as postoperatively, as mainstream was unavailable. In the PACU, we did not compare different modes of ventilation with respect to ETCO2 and PaCO2 correlation. Hence, above factors must be considered when generalizing the results.
Conclusions
From this study, we conclude that ETCO2 reflects PaCO2 with acceptable accuracy. In addition, ETCO2 correlates PaCO2 in patients undergoing neurosurgery in the intraoperative as well as in the postoperative period on mechanical ventilation (SIMV mode) in PACU. The above correlation is perfect in patients who are hemodynamically stable and with healthy lungs. Thus, simple, continuous, and noninvasive ETCO2 can be used as a reliable guide to estimate PaCO2 during neurosurgical procedures and in PACU.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to acknowledge the support from the Department of Neurosurgery, Topiwala National Medical College and BYL Nair Charitable Hospital, Mumbai Central, Mumbai - 400 008, Maharashtra, India.
References
- 1.Husaini J, Choy YC. End-tidal to arterial carbon dioxide partial pressure difference during craniotomy in anaesthetised patients. Med J Malaysia. 2008;63:384–7. [PubMed] [Google Scholar]
- 2.Smith M. Anesthesia and neurosurgery. Br J Anaesth. 2002;89:189. [Google Scholar]
- 3.Khan FA, Khan M, Abbasi S. Arterial to end-tidal carbon dioxide difference in neurosurgical patients undergoing craniotomy: A review of practice. J Pak Med Assoc. 2007;57:446–8. [PubMed] [Google Scholar]
- 4.Russell GB, Graybeal JM. The arterial to end-tidal carbon dioxide difference in neurosurgical patients during craniotomy. Anesth Analg. 1995;81:806–10. doi: 10.1097/00000539-199510000-00025. [DOI] [PubMed] [Google Scholar]
- 5.Russell GB, Graybeal JM. End-tidal carbon dioxide as an indicator of arterial carbon dioxide in neurointensive care patients. J Neurosurg Anesthesiol. 1992;4:245–9. doi: 10.1097/00008506-199210000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Sharma SK, McGuire GP, Cruise CJ. Stability of the arterial to end-tidal carbon dioxide difference during anaesthesia for prolonged neurosurgical procedures. Can J Anaesth. 1995;42:498–503. doi: 10.1007/BF03011688. [DOI] [PubMed] [Google Scholar]
- 7.Razi E, Moosavi GA, Omidi K, Khakpour Saebi A, Razi A. Correlation of end-tidal carbon dioxide with arterial carbon dioxide in mechanically ventilated patients. Arch Trauma Res. 2012;1:58–62. doi: 10.5812/atr.6444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Satoh K, Ohashi A, Kumagai M, Sato M, Kuji A, Joh S. Evaluation of differences between PaCO2 and ETCO2 by Age as measured during general anesthesia with patients in a supine position. J Anesthesiol 2015. 2015:5. [Google Scholar]
- 9.Cheifetz IM, Myers TR. Respiratory therapies in the critical care setting. Should every mechanically ventilated patient be monitored with capnography from intubation to extubation? Respir Care. 2007;52:423–38. [PubMed] [Google Scholar]
- 10.Kerr ME, Zempsky J, Sereika S, Orndoff P, Rudy EB. Relationship between arterial carbon dioxide and end-tidal carbon dioxide in mechanically ventilated adults with severe head trauma. Crit Care Med. 1996;24:785–90. doi: 10.1097/00003246-199605000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Hemmati N, Hamid A, Karbasforooshan A. Correlation between end-tidal and arterial carbon dioxide partial pressure in patients undergoing craniotomy. Congr Iran Neurosurgeons. 2012;4:2012. [Google Scholar]
- 12.Al-Shaikh B, Stacey S. Essentials of Anaesthetic Equipment E-Book. 4th ed. Edinburgh: Churchill Livingstone Elsevier; 2013. Non invasive monitoring; pp. 147–57. [Google Scholar]


