Abstract
Nerve growth factor (NGF) delivery to the brain of patients appears to be an emerging potential therapeutic approach to neurodegenerative disease, such as Alzheimer's disease (AD). The intranasal route of administration could provide an alternative to intracere-broventricular infusion and gene therapy. We previously showed that intranasal administration of NGF determined an amelioration of cholinergic deficit and a decrease in the number of phosphotau-positive neurons and of β-amyloid accumulation in AD11 mice, which express transgenic antibodies neutralizing NGF action and exhibit a progressive Alzheimer-like neurodegeneration. In this study, we report that the Alzheimer-like neurodegeneration in AD11 mice is linked to progressive behavioral deficits in visual recognition memory and spatial memory starting from 4 months of age. To establish whether intranasal administration of NGF, started after the appearance of the first memory deficits, could revert the cognitive deficits in AD11 mice, we assessed the performance of NGF-treated or control AD11 mice in the object recognition test and in a test of memory for place and context. Deficits exhibited by untreated AD11 mice could be rescued by the intranasal administration of NGF. Thus, this route of administration provides a promising way to deliver NGF to the brain in a therapeutic perspective.
Keywords: Alzheimer's disease, behavior, mouse model
Nerve growth factor (NGF) (1, 2) is the most important target-derived trophic factor for basal forebrain cholinergic neurons (BFCNs). In rodents and nonhuman primates, NGF increases the synthesis of choline acetyltransferase and prevents BFCN atrophy caused by experimental injury or associated with physiological aging (3–8). Thus, NGF administration to the brain may counteract BFCN atrophy in physiological and pathological situations, such as aging and Alzheimer's disease (AD). This result, together with the fact that the progressive reduction of the BFCNs is responsible for the cognitive decline in AD patients, lays the groundwork to propose a therapeutic use of NGF in AD (9).
Despite the evidence of beneficial effects of NGF administration, therapeutic applications of NGF have several limitations. As for many other trophic factors, the blood–brain barrier represents a major problem in developing a NGF-based treatment for neurological diseases because it prevents this molecule from reaching the brain (10, 11). Until recently, the efficacy of NGF in rescuing BFCN atrophy was proved by using intracere-broventricular administrations to animal models (10, 12, 13). However, this route of administration is not practical in humans. A first clinical trial in AD patients, attempting to directly infuse NGF into human brain parenchyma, was suspended because of peripheral side effects of NGF (14). A second clinical trial, during which NGF was delivered by ex vivo gene therapy into the brain with stereological injections, ameliorated cognitive deficits of AD patients (15). However, this gene therapy approach requires the use of risky surgical procedures to implant modified cells in the patients' brain parenchyma.
The development of a less invasive delivery method for NGF therefore may significantly improve the prospects of NGF clinical uses. Frey and coworkers (16, 17) showed that NGF and other trophic factors, such as IGF-I, can be delivered to the brain via the olfactory and/or trigeminal pathways. NGF can be transported to the rat brain via an extraneuronal route into the brain via intercellular clefts in the olfactory epithelium (18).
The demonstration that the intranasal NGF delivery was effective in rescuing neurodegeneration was achieved by using the AD11 anti-NGF mouse model for AD. AD11 mice express recombinant antibodies neutralizing NGF biological activity (19). As a result of NGF deprivation, AD11 mice show a progressive neurodegeneration characterized not only by atrophy of BFCNs and nucleus of Meynert, but also by the intracellular accumulation of phosphorylated insoluble tau and the deposition of β-amyloid (19–22). By using the intranasal route of administration, we showed that NGF could rescue, in a well defined time window, all of the histological hallmarks characterizing the AD-like neurodegeneration in AD11 mice (23).
AD11 mice exhibit clear spatial and visual recognition memory deficits (24, 25). Whether these deficits build up progressively, as suggested by the progression of the neurodegeneration (24–27) and synaptic plasticity impairment in the cortex (ref. 28 and N. Origlia, unpublished data) and hippocampus (E. Cherubini, unpublished data), is still unknown. Also unknown is whether intranasal NGF administration, started after the first memory deficits are already apparent, is able to counteract them.
In this study, the behavioral analysis of AD11 mice was extended to show the progression of the memory deficits, by using the object recognition test (ORT) to investigate visual recognition memory and the Morris water maze (MWM) to test spatial memory. We found that the first memory deficits are revealed by the ORT, in good accordance with the precocious appearance of hyperphosphorylated tau in the enthorhinal cortex of AD11 mice. Under these experimental conditions, AD11 mice were tested to determine whether the intranasal route of administration of NGF could be used to revert recognition memory deficits. The results indicate that this hypothesis is indeed the case.
Materials and Methods
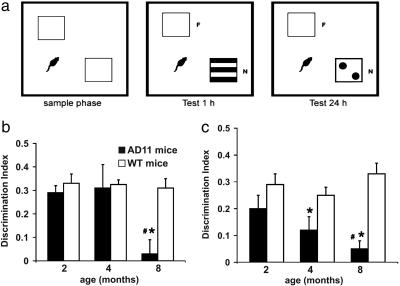
Visual ORT (vORT). The apparatus consisted of a square arena (60 × 60 × 30 cm) constructed in poly(vinyl chloride) with black walls and a white floor. The objects were cubes (12 cm wide) made of transparent Plexiglas that contained the visual patterns to be discriminated. The box and objects were cleaned between trials to stop the build-up of olfactory cues. Mice received three sessions of 10-min duration in the empty box to habituate them to the apparatus and test room. In the vORT, each mouse was first placed in the box and exposed to two identical sample stimuli (objects A1 and A2; e.g., two white cubes, 12 cm wide) for 5 min. This trial was called “sample phase” (Fig. 1a). The experimenter measured the total time the mouse spent exploring each of the two objects. Then the mouse was returned to its cage. During the 1- and 24-h retention interval, the experimenter removed both objects and replaced one of the two by its identical copy (A3) (to ensure that there was no carryover of olfactory cues) and the other object by a new one bearing a black-and- white pattern (object B). After a delay of 1 or 24 h, the mice were placed back in the box and exposed to the familiar object (A3, object identical to A1 and A2) and to a novel test object B for a further 5 min. The objects were placed in the same locations as the previous ones. The experimenter measured again the total time spent exploring each of the two objects (“test period”) (Fig. 1a). AD11 and age-matched wild-type (WT) mice were tested at 2 (n = 9 and 8, respectively), 4 (n = 9 for both), and 8 (n = 10 and 8, respectively) months of age.
Fig. 1.
Progression of vORT deficit in AD11 mice. (a) Schematic representation of sample and test conditions in vORT. (b and c) Performance in the vORT tested 1 h(b) and 24 h (c) after the end of the sample phase. The * denotes a significant (P < 0.01) difference between AD11 and WT mice at the given age (t test). The # denotes a significant difference with respect to the performance at 2 months of age for each genotype. Only AD11 mice show a significant decline of performance with age (P = 0.009 for the 1-h interval; P = 0.02 for the 24-h interval).
MWM. To establish whether aged AD11 mice show deficits in object location, the MWM test was used. A circular water tank, made from black polypropylene (diameter, 100 cm; height, 40 cm) was filled to a depth of 25 cm with water (23°C) and rendered opaque by the addition of a small amount of milk powder. Four positions around the edge of the tank were arbitrarily designated north (N), south (S), east (E), and west (W), which provided four alternative start positions and also defined the division of the tank into four quadrants: NE, SE, SW, and NW. A circular clear Perspex escape platform (diameter, 10 cm; height, 2 cm) was submerged 0.5 cm below the water surface and placed at the midpoint of one of the four quadrants. Mice were trained for four trials per day (with an intertrial interval of 30 min). The start position (N, S, E, or W) was pseudorandomized across trials. The hidden platform remained in the SW quadrant. Mice were allowed up to 60 sec to locate the escape platform, and their escape latency was recorded. On the last trial of the last training day, the mice received a single probe trial, during which the escape platform was removed from the tank, and the swimming path of each mouse was videorecorded over 60 sec while it searched for the missing platform. Mice at 4–5 (AD11 and WT mice, n = 9), 7 (AD11 and WT mice, n = 9), and 9 (AD11 mice, n = 5; WT mice, n = 9) months of age were tested in the MWM.
NGF Nasal Delivery. NGF administration was performed on anaesthetized mice as follows. First, 2,2,2-tribromethanol (Sigma–Aldrich) was dissolved in absolute ethanol at the concentration of 1 g/ml and stored at -20°C in the dark. After dilution in 0.9% NaCl at the final concentration of 2.5%, it was injected i.p. at the dosage of 10 μl/g of body weight to induce anesthesia, which followed within 5–10 min after injection. After anesthesia, mice were laid on their back, with the head in upright position, as described in refs. 16 and 23. A 10-μM solution of mouse NGF (Alomone Labs, Jerusalem) in PBS was administered intranasally to AD11 mice, 3 μl at a time, alternating the nostrils, with a lapse of 2 min between each administration, for a total of 14 times. The administration was repeated seven times at 2-day intervals. During these procedures, the nostrils were always kept open. As control, AD11 mice were treated with PBS.
Rescue of Behavioral Deficits by NGF. This study was divided in two parts. In the first part, 42 AD11 mice were used for a standard vORT. In the second set of experiments, 35 AD11 mice were tested in a block of three experimental conditions as follows: (i) object (shape) recognition test (ORT); (ii) object location test (OLT); and (iii) object context test (OCT).
For the vORT habituation phase, NGF-treated and untreated AD11 mice were placed in the empty arena to become familiar with the apparatus for 5 min. The sample phase started after 2 min. Two cubes with white visual patterns were presented in two opposite corners of the arena. The mice were left to explore the cubes in the arena for 5 min. The choice phase (5 min) was executed after 1 and 24 h.
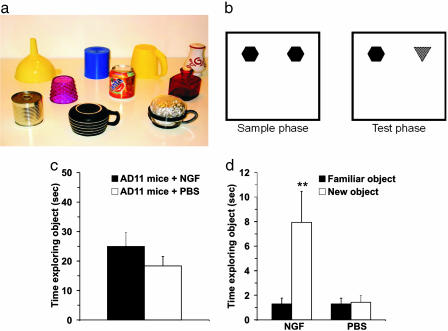
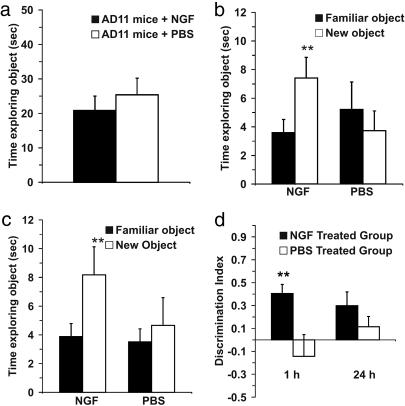
The ORT consisted of two phases, sample and test. The objects to be discriminated were made of plastic, metal, and glass and were too heavy to be displaced by the mice (Fig. 2a). The objects varied in size; the largest was ≈6 × 6 × 10 cm, and the smallest was ≈5 × 5 × 8 cm. (Fig. 2a). During the sample phase, NGF-treated and untreated mice were placed into arena with two identical sample objects, allowed to explore for 5 min, and then returned to their cage for 10 min of retention interval. In this phase, the objects were placed in two adjacent corners of the arena (Fig. 2b). In the test phase, the objects were replaced with two new objects: one was identical to that used in the sample phase, whereas the other was a novel object that the mice had never encountered before (Fig. 2b). Mice were left to explore the objects for 3 min.
Fig. 2.
NGF counteracts object-recognition deficits, as shown by the ORT. (a) Example of the objects used for the experiments. (b) Schematic representation of sample and test phase conditions in ORT. (c) Time spent in object exploration during the sample phase. There was no significant difference between the groups of treatment (P = 0.487). (d) Time spent exploring the familiar and the new objects in the test phase. The data show a significant difference within NGF-treated group (P = 0.006), whereas, for the PBS group, a significant difference was not found (P = 0.813). (Error bars represent SEM; **, P < 0.01.)
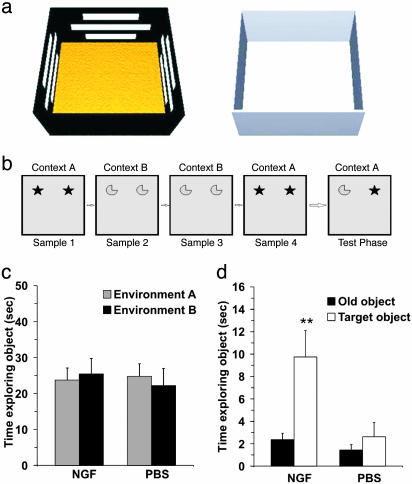
For OCT, two open field arenas (60 × 60 × 30 cm) made of poly(vinyl chloride) were used. Each arena constituted a different experimental condition (A and B). In condition A, horizontal white stripes were applied on the black walls of the arena. The floor was covered with rough Plexiglas (Fig. 3a). In condition B, the arena had gray walls, and the floor was made of Plexiglas (Fig. 3a). The particular object for a given test was randomly determined, but each object was used for only one experimental condition. Half of AD11 mice in each treatment group underwent the ORT in condition A, whereas the OLT was performed in condition B and vice versa. In this way, all of the mice were equally exposed to both environments before the OCT. The OCT was used to determine whether mice were sensitive to a change in context for a given object. Thus, previous familiarization with two environments was fundamental. The habituation phase started 2 days before the block of tests and consisted of four sessions. In each session, NGF-treated and untreated AD11 mice were exposed to both conditions (A and B). In the first and second sessions, mice were placed into the empty arena for 10 min. In the last two sessions, they were allowed to explore the arena for 3 min individually. The OCT was divided into four sample phases and a test phase, each lasting 3 min (Fig. 3b). The retention interval within the sample phases was 2 min. There was a 5-min interval between the last sample phase and the test phase. In the sample phase, two objects were placed in adjacent corners of the arena; phases 1 and 4 comprised objects A1 and A2 in environment A, and phases 2 and 3 comprised objects B1 and B2 in environment B (Fig. 3b). The test phase was in the same environment as sample phase 4, but one of the objects (A2) was replaced by B2. In this way, one object was in the same environment as in the sample phase, and the other object was in a different environment from the sample phase (Fig. 3b). To avoid the eventual preference for one of two environments, half of the mice began the sample phase in environment A with object A1 and A2 and finished with the same environment with object A1 and B2 and vice versa.
Fig. 3.
NGF counteracts object-recognition deficits, as shown by the OCT. (a) Three-dimensional representation of conditions A (Left)and B(Right) in OCT. (b) Schematic representation of sample/test conditions in OCT. Condition A is represented by the shaded box, and condition B is shown by the plain box. Objects are represented by the symbols. (c) Object exploration time in the sample phase. There was no difference in both groups (P = 0.600). (d) Mean during exploration for the old object and the target object within each group. A clear difference was evident in the NGF-treated group (P = 0.003). For the PBS group, no significant difference was found (P = 0.397). (Error bars represent SEM; **, P < 0.01.)
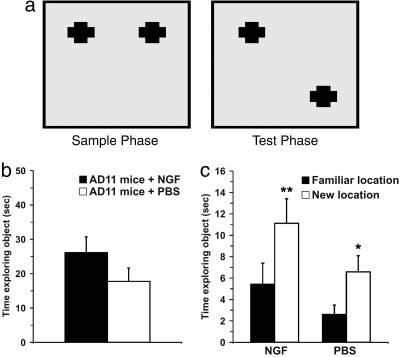
The sample phase of the OLT was exactly the same as the ORT. After a delay of 10 min, the test phase began. In this phase, the objects were replaced by their identical copies, one of which was placed in the same position, whereas the other one was moved to the other adjacent corner, so that the two objects were now in diagonally opposite corners (Fig. 4a). Thus, in the test phase both objects were equally familiar, but one had changed location. The mice were exposed to the objects for 3 min.
Fig. 4.
NGF counteracts object-recognition deficits, as shown by OLT. (a) Representation of sample and test phase conditions in OLT. (b) Mean time spent exploring the objects during the sample phase. There was no significant difference between the groups (P = 0.132). (c) Mean time spent exploring the familiar and the new locations in the test phase. The data show a significant difference in both groups (NGF group, P < 0.001; PBS group, P = 0.032). (Error bars represent SEM; *, P < 0.05; **, P < 0.01.)
Measurements and Statistics. The standard measure for the statistical analysis in the ORTs was the time spent exploring the two objects. The exploration of an object was defined as directing the nose to the object at a distance of <2 cm and touching it with the nose. Turning around, climbing over, or sitting on the object were not included. In the sample phase, if the exploration time was <3 sec, the mice were discarded from the sample. Mice also were excluded from the sample if they spent <1 sec exploring both new and familiar objects in the test phase. In the sample phase, the total time spent exploring each object was recorded and compared across different genotypes or treatments with the Student t test and across different ages or different conditions with one-way ANOVA. For OCT, two-way ANOVA was used. In the test phase, comparisons between time spent exploring the new and old objects were performed within groups (analysis performed by using paired t tests). A discrimination index (DI) was calculated as the difference between the time spent exploring new and old object divided by the total time spent exploring the objects [(n - f)/(f + n), where n represents new and f represents familiar]. DIs were compared across ages for the same genotype with one-way ANOVA, across the two time intervals for the same genotype and age with a paired t test, and across genotypes or treatments for the same age and time interval with a t test. For the MWM, performance in the learning phase was compared with two-way ANOVA, time versus genotype, for repeated measures. Performance in the probe test was compared with one-way ANOVA across quadrants for each genotype.
Results
Progression of Behavioral Deficits in AD11 Mice. The vORT revealed that no differences in visual recognition memory between 2-month-old AD11 mice and age-matched WT mice could be shown. At this age, both groups spent significantly more time exploring the new object both at the 1- and the 24-h interval between the sample and the test phase. The DIs at 1 and 24 h did not differ between AD11 and WT mice. At 4 months of age, AD11 mice showed a deficit, with respect to WT mice (significantly lower DI, t test, P < 0.05), at the 24-h interval between the sample and the test phase (Fig. 1b), whereas at 8 months of age, the deficits were observed both at 1- and 24-h interval (Fig. 1c, t test, P = 0.006 and P < 0.001, respectively). There was a clear decline of performance for AD11 mice with age for both 1- and 24-h intervals (one-way ANOVA, P < 0.05 and P < 0.01, respectively). On the contrary, the performance of WT mice did not significantly vary as a function of age (one-way ANOVA, P > 0.05).
We previously showed a progressive deficit in spatial memory tasks in an eight-arms radial maze paradigm (N.B., unpublished data). In the all-arms baited version of the radial maze, a deficit in the learning curves appeared starting from 4 months of age.
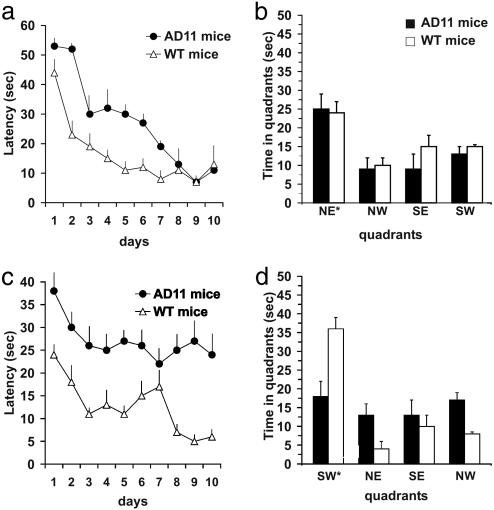
The MWM test showed a clear progression of spatial memory deficits. Five-month-old AD11 mice were able to learn the task as well as their age-matched WT controls, and the probe test was performed equally well (data not shown). At 7 months of age, AD11 mice showed significantly slower learning with respect to age-matched WT mice (two-way repeated-measures ANOVA: genotype, P < 0.001; days, P < 0.001, interaction, P = 0.007) (Fig. 5a). However, there was a significantly longer time spent in the target quadrant in the probe test for both genotypes (one-way ANOVA, P < 0.001) (Fig. 5b), indicating that AD11 mice remembered the location of the hidden platform at this age. At 9 months, AD11 mice showed a significantly worse performance in the learning curve (two-way ANOVA, genotype, P < 0.01) (Fig. 5c) and did not remember the location of the hidden platform in the probe test (Fig. 5d; P > 0.05).
Fig. 5.
Progression of MWM deficit in AD11 mice. (a) Learning curves for WT and AD11 mice at the age of 7 months. The difference between WT and AD11 mice is significant (genotype, P < 0.001; time, P < 0.001). (b) Results of the probe trial at 7 months of age. The platform (located in the NE quadrant during learning) was removed. Both WT and AD11 mice spent significantly more time in the NE quadrant (P < 0.001). (c) Learning curves for AD11 and WT mice ages 9–10 months. The difference between WT and AD11 is significant (genotype, P < 0.01; time, P < 0.01). (d) Results of the probe trial at 9–10 months of age. The platform (located in the SW quadrant during learning) was removed. WT mice spent significantly more time in the SW quadrant, whereas AD11 mice did not show any significant preference for the SW quadrant (P < 0.001 and P > 0.05, respectively).
Rescue of Behavioral Deficits by NGF. vORT. Fig. 6a shows the time spent exploring objects in the sample phase. There were no group differences in exploration time (Student's t test, P = 0.481). These data indicated that, if any significant differences between the groups in terms of discrimination on the test phase were observed, they did not result from differences in time spent exploring in the sample phase. In the test phase, NGF-treated AD11 mice spent significantly more time with the new object after the 1-h (Fig. 6b) and 24-h (Fig. 6c) delay (paired t test, P = 0.001 and P = 0.005, respectively). The placebo group did not show a significant difference in either 1- or 24-h delay (paired t test, P = 0.282 and P = 0.138, respectively). In the test phase (Fig. 6d), the DI data revealed that the NGF-treated group had an exploration index that was significantly higher than the PBS-treated group in 1-h delay (ANOVA, F = 8.896; P = 0.007), whereas in the 24-h delay there was not a significant difference between the two groups of animals (ANOVA, F = 1.132; P = 0.300).
Fig. 6.
NGF counteracts object recognition deficits, as shown by the vORT. (a) Time engaged in object exploration during the sample phases for vORT. There was not a significant difference between the groups. (Error bars represent SEM.) (b). Time engaged in exploring each object type (new and familiar) during the test phase performed 1 h after the end of the sample phase. (Error bars represent SEM; **, P < 0.01.) (c) Time engaged in exploring each object type (new and familiar) during the test phase performed 24 h after the end of the sample phase. (Error bars represent SEM; **, P < 0.01.) (d) DI in the vORT. Data revealed that in the 1-h delay there was significant difference between the groups (P = 0.007), whereas in the 24-h delay there was not a significant difference (P = 0.300). (Error bars represent SEM; **, P < 0.01.)
Object (shape) recognition test. During the sample phase, analysis of the total time spent in exploration revealed no significant differences between the NGF- and PBS-treated groups (Student's t test, P = 0.487) (Fig. 2c). The NGF-treated group of AD11 mice showed at the 10-min time interval a significantly greater exploration time dedicated to the novel object compared with the familiar object (paired t test, P = 0.006), whereas the PBS-treated group did not show a significant difference (paired t test, P = 0.813) (Fig. 2d). These data confirmed that AD11 mice treated with PBS failed to discriminate the novel object, whereas the NGF-treated AD11 mice clearly were able to discriminate between the two objects. One-way ANOVA revealed an effect of NGF treatment (F = 6.215; P = 0.025) indicated in the fact that the NGF-treated group of mice had a DI significantly higher than those of PBS group (Fig. 7).
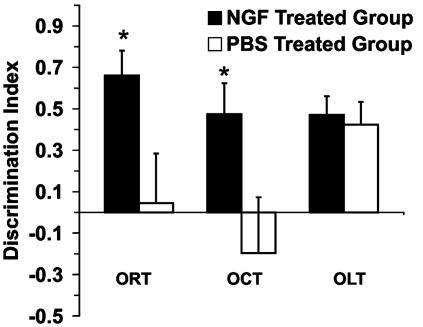
Fig. 7.
DI in each experimental condition. The graph shows that the performance of the NGF-treated group was greater than that of the PBS group in the ORT and OCT (P = 0.025 and P = 0.045, respectively). OLT revealed that there was not a significant difference between treated and untreated mice (P = 0.760). (Error bars represent SEM; *, P < 0.05.)
OCT. In the sample phase of context version, taking into account both environments, two-way ANOVA (treatment vs. environment) showed that there was not a significant difference for either factor (P = 0.783 for treatment factor; P = 0.914 for the environment factor). The effect of different treatments did not depend on what environment was present. There was no statistically significant interaction between both factors (P = 0.600). These data showed no significant preference between the two environments (Fig. 3c). In the test phase, one paired t test was used to verify the eventual difference in the exploration time between the object that was in the changed environment (target object) and the object that was in the same environment (old object). The results showed that the NGF-treated group was able to discriminate between the old and target objects (P = 0.003), whereas no significant difference was found in the PBS group (P = 0.397) (Fig. 3d). One-way ANOVA indicated that the NGF-treated group could discriminate between the two objects significantly better than the PBS group (F = 4.68 and P = 0.045) (Fig. 7).
OLT. The analysis in the sample test revealed that no significant differences were found in the total amount of exploration time between the two groups of mice (Student's t test, P = 0.132; Fig. 4b). In the test phase, exploration times for both groups of treatment demonstrated a clear preference of the object placed in a novel location compared with the object placed in a familiar location (paired t test: NGF-treated group, P = <0.001; PBS group, P = 0.032) (Fig. 4c). The PBS-treated AD11 mice showed they were able to discriminate the novel location from the familiar location. In fact, one-way ANOVA between the groups, considering the DI, revealed that there was no significant difference between groups of treatment (F = 0.096 and P = 0.760) (Fig. 7).
Discussion
The main findings of this study were that AD11 mice showed a progressive behavioral deficit and that NGF intranasal delivery increased the ability of AD11 mice in remembering a familiar object and in associating an object to a particular context.
We used tasks that exploited the rodents' spontaneous preference for novel objects, which were first introduced by Ennaceur and Delacour (29). The introduction of these tests allowed overcoming the disadvantages of lengthy training procedures. More importantly, the ORT strongly relies on visual memory. Visual recognition memory is currently under examination as a potential early diagnostic marker of AD, because neurofibrillary tangles initially develop in subregions of the parahippocampal gyrus known to be important for visual recognition memory (24, 25, 30, 31). In this context, it is very interesting that in AD11 mice, the first memory deficits become apparent during the vORT, not in the MWM, in line with the first appearance of hyperphosphorylated tau in the entorhinal cortex (26). ORTs have the advantage that, in addition to examining the exploration of a novel object, they can be used to examine other aspects of recognition, such as object location and context. Thus, both spatial and nonspatial working memory can be tested by using the same paradigm (22), allowing a more stringent comparison between deficits in spatial and nonspatial memory.
In this study, we show that AD11 mice display progressive behavioral deficits in visual recognition and spatial memory. Although impairment in object recognition starts at 4 months of age, deficits in spatial memory appear later (9 months of age), as assessed by using the MWM. In keeping with these latter findings, the OLT performed at 6 months of age did not reveal any impairment in AD11 mice. In addition, AD11 mice were found to be insensitive to the combination of object and environment. Thus, unlike normal rats and mice (32), but as in rats with hippocampal damage (27), AD11 mice cannot link the event of exploring and experiencing an object with the contextual cues surrounding that particular object, not being able to provide parallels with episodic memory. The deficits in object and contextual recognition both were counteracted by the intranasal administration of NGF.
In conclusion, although it was already shown that the intranasal administration of trophic factors, such as insulin, can improve memory and mood in healthy (33) and AD subjects (M. Roger and S. Craft, unpublished data), there was no evidence for such an effect after NGF intranasal delivery. Thus, to our knowledge, this study provides, for the first time, the evidence that the noninvasive intranasal administration of NGF determines not only the rescue of the main hallmarks of AD-like neurodegeneration, such as phosphotau and β-amyloid (23), but also counteracts functional cognitive deficits. In the context of the emerging role that NGF and its precursors could play in the onset and therapy of AD, these results highlight the possibility that the olfactory pathway can be a promising, noninvasive route of administration for the delivery of NGF agonists, allowing a long-term treatment of AD.
Acknowledgments
We thank Dr. Marco Stebel and the staff of the animal house at the University of Trieste (Trieste) for help. This work was supported in part by Telethon Grant GGP030416 and Ministero dell'Istruzione, dell'Università e della Ricerca Cofinanziamento.
Author contributions: R.D.R., A.A.G., and N.B. designed research; R.D.R., A.A.G., and C.B. performed research; L.M. contributed new reagents/analytic tools; R.D.R., A.A.G., S.C., N.B., and A.C. analyzed data; and R.D.R., A.A.G., S.C., N.B., and A.C. wrote the paper.
Abbreviations: NGF, nerve growth factor; BFCN, basal forebrain cholinergic neuron; AD, Alzheimer's disease; ORT, object recognition test; vORT, visual ORT; MWM, Morris water maze; OLT, object location test; OCT, object context test; DI, discrimination index.
References
- 1.Levi-Montalcini, R. (1952) Ann. N.Y. Acad. Sci. 55, 330-344. [DOI] [PubMed] [Google Scholar]
- 2.Levi-Montalcini, R. (1987) Science 237, 1154-1162. [DOI] [PubMed] [Google Scholar]
- 3.Hefti, F., Dravid, A. & Hartikka, J. (1984) Brain Res. 293, 305-311. [DOI] [PubMed] [Google Scholar]
- 4.Fischer, W., Wictorin, K., Bjorklund, A., Williams, L. R., Varon, S. & Gage, F. H. (1987) Nature 329, 65-68. [DOI] [PubMed] [Google Scholar]
- 5.Koliatsos, V. E., Price, D. L., Clatterbuck, R. E., Markowska, A. L., Olton, D. S. & Wilcox, B. J. (1993) Ann. NY Acad. Sci. 695, 292-299. [DOI] [PubMed] [Google Scholar]
- 6.Tuszynski, M. H., Sang, H., Yoshida, K. & Gage, F. H. (1991) Ann. Neurol. 30, 625-636. [DOI] [PubMed] [Google Scholar]
- 7.Markowska, A. L., Koliatsos, V. E., Breckler, S. J., Price, D. L. & Olton, D. S. (1994) J. Neurosci. 14, 4815-4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Markowska, A. L., Price, D. & Koliatsos, V. E. (1996) J. Neurosci. 16, 3541-3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Auld, D. S., Kornecook, T. J., Bastianetto, S. & Quirion, R. (2002) Prog. Neurobiol. 68, 209-245. [DOI] [PubMed] [Google Scholar]
- 10.Kordower, J. H., Winn, S. R., Liu, Y. T., Mufson, E. J., Sladek, J. R., Jr., Hammang, J. P., Baetge, E. E. & Emerich, D. F. (1994) Proc. Natl. Acad. Sci. USA 91, 10898-10902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thorne, R. G. & Frey, W. H., II (2001) Clin. Pharmacokinet. 40, 907-946. [DOI] [PubMed] [Google Scholar]
- 12.Tuszynski, M. H., Roberts, J., Senut, M. C., U, H. S. & Gage, F. H. (1996) Gene Ther. 3, 305-314. [PubMed] [Google Scholar]
- 13.Tuszynski, M. H., Smith, D. E., Roberts, J., McKay, H. & Mufson, E. (1998) Exp. Neurol. 154, 573-582. [DOI] [PubMed] [Google Scholar]
- 14.Eriksdotter Jonhagen, M., Nordberg, A., Amberla, K., Backman, L., Ebendal, T., Meyerson, B., Olson, L., Seiger, Shigeta, M., Theodorsson, E., et al. (1998) Dementia Geriatr. Cognit. Disorders 9, 246-257. [DOI] [PubMed] [Google Scholar]
- 15.Tuszynski, M. H. & Blesch, A. (2004) Prog. Brain Res. 146, 441-449. [DOI] [PubMed] [Google Scholar]
- 16.Frey, W. H., II, Liu, J., Chen, X., Thorne, R. G., Fawcett, J. R., Ala, T. A. & Rahman, Y. E. (1997) Drug Delivery 4, 87-92. [Google Scholar]
- 17.Thorne, R.G., Pronk, G., Padmanabhan, V. & Frey, W. H., II (2004) Neuroscience 127, 481-496. [DOI] [PubMed] [Google Scholar]
- 18.Chen, X. Q., Fawcett, J. R., Rahman, Y. E., Ala, T. A. & Frey, W. H., II (1998) J. Alzheimer's Dis. 1, 35-44. [DOI] [PubMed] [Google Scholar]
- 19.Ruberti, F., Capsoni, S., Comparini, A., Di Daniel, E., Franzot, J., Gonfloni, S., Rossi, G., Berardi, N. & Cattaneo, A. (2000) J. Neurosci. 20, 2589-2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Capsoni, S., Ugolini, G., Comparini, A., Ruberti, F., Berardi, N. & Cattaneo, A. (2000) Proc. Natl. Acad. Sci. USA 97, 6826-6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Capsoni, S., Giannotta, S. & Cattaneo, A. (2002) Mol. Cell. Neurosci. 21, 15-28. [DOI] [PubMed] [Google Scholar]
- 22.Capsoni, S., Giannotta, S. & Cattaneo, A. (2002) Brain Aging 2, 24-42. [Google Scholar]
- 23.Capsoni, S., Giannotta, S. & Cattaneo, A. (2002) Proc. Natl. Acad. Sci. USA 99, 12432-12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murray, E. A. & Richmond, B. J. (2001) Curr. Opin. Neurobiol. 11, 188-193. [DOI] [PubMed] [Google Scholar]
- 25.Van Hoesen, G. W., Hyman, B. T. & Damasio, A. R. (1991) Hippocampus 1, 1-8. [DOI] [PubMed] [Google Scholar]
- 26.Capsoni, S., Giannotta, S. & Cattaneo, A. (2002) Brain Aging 2, 24-43. [Google Scholar]
- 27.Mumby, D. G., Gaskin, S., Glenn, M. J., Schramek, T. E. & Lehmann, H. (2002) Learn. Mem. 9, 49-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pesavento, E., Capsoni, S., Domenici, L. & Cattaneo, A. (2002) Eur. J. Neurosci. 15, 1030-1036. [DOI] [PubMed] [Google Scholar]
- 29.Ennaceur, A. & Delacour, J. (1988) Behav. Brain Res. 31, 47-59. [DOI] [PubMed] [Google Scholar]
- 30.Barbeau, E., Didic, M., Tramoni, E., Felician, O., Joubert, S., Sontheimer, A., Ceccaldi, M. & Poncet, M. (2004) Neurology 62, 1317-1322. [DOI] [PubMed] [Google Scholar]
- 31.Winters, B. D., Forwood, S. E., Cowell, R. A., Saksida, L. M. & Bussey, T. J. (2004) J. Neurosci. 24, 5901-5908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dix, S. L. & Aggleton, J. P. (1999) Behav. Brain Res. 99, 191-200. [DOI] [PubMed] [Google Scholar]
- 33.Benedict, C., Hallschmid, M., Hatke, A., Schultes, B., Fehm, H. L., Born, J. & Kern, W. (2004) Psychoneuroendocrinology 29, 1326-1334. [DOI] [PubMed] [Google Scholar]