Abstract
Background
When transplanted into failing heart, autologous somatic tissue–derived cells yield functional recovery via paracrine effects that enhance native regeneration. However, the therapeutic effects are modest. We developed a method in which scaffold‐free cell sheets are attached to the epicardial surface to maximize paracrine effects. This Phase I clinical trial tested whether transplanting autologous cell–sheets derived from skeletal muscle is feasible, safe, and effective for treating severe congestive heart failure.
Methods and Results
Fifteen ischemic cardiomyopathy patients and 12 patients with dilated cardiomyopathy, who were in New York Heart Association functional class II or III and had been treated with the maximum medical and/or interventional therapies available, were enrolled. Scaffold‐free cell sheets of 3 to 9×108 cells derived from autologous muscle were transplanted over the LV free wall via left thoracotomy, without additional interventional treatments. There were no procedure‐related major complications during follow‐up. The majority of the ischemic cardiomyopathy patients showed marked symptomatic improvement in New York Heart Association classification (pre: 2.9±0.5 versus 6 months: 2.1±0.4, P<0.01; 1 year: 1.9±0.3, P<0.01) and the Six‐Minute Walk Test with significant reduction of serum brain natriuretic peptide level (pre: 308±72 pg/mL versus 6 months: 191±56 versus 1 year: 182±46, P<0.05), pulmonary artery pressure, pulmonary capillary wedge pressure, pulmonary vein resistance, and left ventricular wall stress after transplantation instead of limited efficacy in dilated cardiomyopathy patients.
Conclusions
Cell‐sheet transplantation as a sole therapy was feasible for treating cardiomyopathy. Promising results in the safety and functional recovery warrant further clinical follow‐up and larger studies to confirm this treatment's efficacy for severe congestive heart failure.
Clinical Trial Registration
URL: http://www.umin.ac.jp/english/. Unique identifier: UMIN000003273.
Keywords: autologous stem cell‐sheet, cellular transplantation, ejection fraction, growth factors and cytokines, ischemic cardiomyopathy, myocardial regeneration
Subject Categories: Cardiovascular Surgery, Cardiomyopathy
Introduction
Heart failure, caused primarily by ischemic cardiomyopathy (ICM) or dilated cardiomyopathy (DCM), is life‐threatening even with excellent treatment. Dedicated researchers have sought to combat these serious diseases with innovative drugs and such therapies as the left ventricular assist device (LVAD) and heart transplantation. However, these therapies have limited longevity, and heart transplantation is limited by a shortage of donors. This situation has led clinicians to consider alternative methods for treating heart failure.1
We developed a cell‐sheet implantation method that can heal severely damaged myocardium through cytokine paracrine effects, as evidenced by several experiments using infarction or DCM models in both large2 and small animals.3 Cell‐sheet implants are reported to offer better functional recovery than needle‐injection methods, mainly by cytokine paracrine effects4 despite poor cell survival.5, 6 Based on these findings from preclinical work, we previously conducted a First‐in‐Man Clinical Trial using cell‐sheet implants in a DCM patient with LVAD, which demonstrated the feasibility of the treatment with LVAD support.7
In the present study, we introduced cell‐sheet implants to treat cardiomyopathy patients without LVAD in a Phase I clinical trial to determine the safety, feasibility, and potential effectiveness of cell‐sheet implants as a sole therapy.
Methods
This study was approved by the institutional review committee of the Osaka University Graduate School of Medicine, Osaka, Japan, and approved by the institutional review board. All of the subjects provided prior informed consent to participate in the study.
Clinical Trial Registration Information
This study was conducted according to the Guidelines on Clinical Research Using Human Stem Cells from the Japanese Ministry of Health, Labour, and Welfare (UMIN ID; UMIN000003273; https://upload.umin.ac.jp/cgi-open-bin/ctr/ctr.cgi?function=brows&action=brows&recptno=R000003959&type=summary&language=J, UMIN000015892; https://upload.umin.ac.jp/cgi-open-bin/ctr/ctr.cgi?function=brows&action=brows&recptno=R000015089&type=summary&language=J).
Subjects
Twenty‐seven patients were enrolled from January 2010 to February 2015. All patients gave informed, written consent before being enrolled in the study. Patients with cardiomyopathy of ischemic (n=15) and nonischemic (n=12) etiology were considered eligible if they were 20 to 75 years of age, had an ejection fraction under 35% and a New York Heart Association (NYHA) classification of II or more, and had already received the maximum medical treatment—maximum β‐blocker or angiotensin‐converting enzyme inhibitor, and angiotensin receptor blocker. The patients with malignancy, end‐stage renal failure requiring hemodialysis, and infectious disease were excluded. NYHA classification of patients who receive myoblast sheets was over 2 (average 2.9) and the majority were stable NYHA class 3.
Eight patients had undergone cardiac resynchronized therapy and 7 received an implantable cardiac defibrillator.
In the patients with ischemic etiology, 10 patients received percutaneous coronary intervention and 5 patients underwent coronary artery bypass grafting more than 4 months before the cell‐sheet treatment. Two patients were receiving continuous catecholamine injections and had been in the hospital more than 2 months at the time of the cell‐sheet implantation surgery (Table 1). We have not changed medications or reset the condition of the resynchronization device after myoblast sheet implantation.
Table 1.
Characteristics of Study Patients
| Demographics | All | Ischemic | Nonischemic |
|---|---|---|---|
| No. of patients | 27 | 15 (55.6%) | 12 (44.4%) |
| Age, y (mean±SD) | 52.9±15.6 | 52.6±15.4 | 53.3±16.4 |
| >65 years | 8 (29.6%) | 4 (26.7%) | 4 (33.3%) |
| Male | 25 (92.6%) | 13 (86.7%) | 12 (100%) |
| Risk factor | |||
| Hypertension | 11 (40.7%) | 10 (66.7%) | 1 (8.3%) |
| Hyperlipidemia | 12 (44.4%) | 11 (73.3%) | 1 (8.3%) |
| Diabetes mellitus | 5 (18.5%) | 5 (33.3%) | 0 (0.0%) |
| Oral medication | 4 (14.8%) | 4 (26.7%) | 0 (0.0%) |
| Insulin | 1 (3.7%) | 1 (6.7%) | 0 (0.0%) |
| Cardiac history | |||
| ICD implantation | 7 (25.9%) | 3 (20.0%) | 4 (33.3%) |
| CRT‐D implantation | 8 (29.6%) | 2 (13.3%) | 6 (50.0%) |
| Myocardial infarction | 15 (55.6%) | 15 (100%) | 0 (0.0%) |
| History of coronary revascularization | |||
| PCI | 10 (37.0%) | 10 (66.7%) | 0 (0.0%) |
| CABG | 6 (22.2%) | 6 (40.0%) | 0 (0.0%) |
| History of valve surgery | |||
| Mitral valve surgery | 10 (37.0%) | 2 (13.3%) | 8 (66.7%) |
| Aortic valve surgery | 1 (3.7%) | 1 (6.7%) | 0 (0.0%) |
| IABP | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| LVAD | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Medication | |||
| ACE‐I | 18 (66.7%) | 10 (66.7%) | 8 (66.7%) |
| ARB | 9 (33.3%) | 7 (46.7%) | 2 (16.7%) |
| β‐blocker | 27 (100%) | 15 (100%) | 12 (100%) |
| Diuretics | 27 (100%) | 15 (100%) | 12 (100%) |
| Antiplatelet | 14 (51.9%) | 12 (80.0%) | 2 (16.7%) |
| Warfarin | 20 (74.1%) | 9 (60.0%) | 11 (91.7%) |
| Amiodarone | 9 (33.3%) | 2 (13.3%) | 7 (58.3%) |
| Statins | 13 (48.1%) | 12 (80.0%) | 1 (8.3%) |
| Continuous catecholamine infusion | 2 (7.4%) | 1 (6.7%) | 1 (8.3%) |
ACE‐I indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CABG, coronary artery bypass grafting; CRT‐D, cardiac resynchronization‐defibrillator therapy; IABP, intra‐aortic balloon pump; ICD, implantable cardioverter defibrillator; LVAD, left ventricular assist device; PCI, percutaneous coronary intervention.
Culture and Fabrication of Cell Sheets From Autologous Skeletal Muscle
Muscle specimens (≈10 g) were collected from the vastus medialis. Muscle fibers were collected by removing connective tissues with collagenase and TrypLE Select (Invitrogen), and were suspended in skBM medium supplemented with 20% bovine serum. The cell suspensions were placed in temperature‐responsive cell‐culture dishes (UpCell, CellSeed), the surface of which contained temperature‐responsive polymer (poly‐N‐isopropylacrylamide). After the temperature is reduced to 32°C, the surface rapidly becomes hydrated, prompting the complete detachment of adherent cells as a cell sheet.8 Cytokines in the supernatant from the cell sheets were measured by enzyme‐linked immunosorbent assay.
Implantation of Cell Sheets Onto Heart Tissues
To implant cell sheets, the left fifth intercostal space was opened with patients under anesthesia, and the pericardium was opened parallel to the phrenic nerve. While the LV lateral wall was dissected out, 3‐ or 4‐layer cell sheets were produced on a clean bench and transferred to the operating table. The cell sheets were placed and fixed with 7–0 Prolene onto the LV anterior and lateral wall as widely as possible. Fibrin was applied to the whole area of the top cell sheet after the sheets were piled up, so there was no fibrin between the cell sheets; no fibrin was found on the bottom of the cell‐sheet pile. Also, we added some glue on the top cell sheet after placement of cells sheets by a stitch to fix the epicardium of myocardium.
Cine‐Multidetector Computed Tomography
Cine‐multidetector computed tomography was performed with a 64‐channel multidetector row scanner before surgery, and again 6 months after implanting cell sheets. Regional end‐systolic wall stress was calculated using LV end‐systolic images with Janz's equation; end‐systolic wall stress=P×∆Ac/∆Aw, where P is LV end‐systolic pressure, and ∆Ac and ∆Aw are local cross‐sectional area of the LV cavity and local cross‐sectional area of the LV wall at end‐systole, respectively. In this study, LV end‐systolic pressure was estimated by the following equation: P=mean blood pressure ±7 mm Hg.9
Follow‐Up Examinations
Safety and feasibility
Safety and feasibility were determined based on the occurrence of a major cardiac adverse event up to 6 months after implanting the cell sheets. A major cardiac adverse event was defined as a composite of cardiovascular death, myocardial infarction, congestive heart failure (freedom from readmission for heart failure), hospitalization (any cause), infection, hemothorax, pneumothorax, cardiac tamponade, cardiovascular event, resuscitated sudden death, and arrhythmia, as assessed by Holter ECG findings. Arrhythmia included the following: (1) cardiac arrest, including ventricular fibrillation and asystole; (2) sustained monomorphic or polymorphic ventricular tachycardia (VT); (3) nonsustained monomorphic VT (≥10 beats, ˂30 s) and nonsustained polymorphic VT (≥10 beats, ˂30 s), including torsade de pointes (≥10 beats of changing morphology during a run with a long QTc interval); (4) atrial fibrillation (˃120 beats/minute) with rapid ventricular response, or new‐onset atrial fibrillation; (5) nonsustained supraventricular tachycardia (˃120 beats/minute) or new‐onset sustained supraventricular tachycardia (˃60 s); (6) third‐degree atrioventricular block; (7) nonsustained VT (≥3, <10 beats); or (8) premature ventricular complex. We evaluated arrhythmias as major cardiac adverse event by criteria described elsewhere.10
Heart failure event requiring in‐hospital treatment
Heart failure event requiring in‐hospital treatment was defined by the Framingham heart failure diagnostic criteria11 and preoperative admission for heart failure was retrospectively evaluated by their medical record. The rate of preoperative heart failure event was calculated by the number of events and duration from initial heart failure event.
Cardiac death
Cardiac death was defined as event as follows: (1) death caused by cardiac failure, (2) lethal arrhythmic event, (3) sudden death by unknown reason; or (4) deterioration of cardiac function requiring LVAD or depending on continuous catecholamine injection.
Cardiac echocardiography
An echocardiographic study was performed in all patients before treatment and 6 months and 1 year after treatment. We evaluated left ventricular ejection fraction (LVEF), left ventricular end‐diastolic dimension (LVEDD), and left ventricular end‐systolic dimension. LVEF was calculated by the modified Simpson method.
Catheterization
We evaluated pulmonary pressure and pulmonary vascular pressure by pressure studies using a Swan‐Ganz catheter via the right internal vein. We calculated cardiac output by thermodilution, and calculated pulmonary vein pressure as follows: Pulmonary vascular resistance (PVR)=(mean PAP‐mean LAP)/CO×79.92 (dyne·s·cm−5), where PAP is pulmonary artery pressure and LAP is left arterial pressure.
Evaluation of symptoms and exercise capacity
Patients were evaluated for exercise capacity before treatment and 6 months after implanting the cell sheets, using the Six‐Minute Walk Test.12 Changes in patients' symptoms were evaluated using the NYHA functional classification13 before treatment and again at 6 months, 1 year after implanting the cell sheets.
Statistical Analysis
All values are described as the mean±SD unless otherwise noted and for repeatedly measured data; missing values were imputed via last observation carried forward methods. The serial values were evaluated by Wilcoxon signed‐rank test and the P‐values were not adjusted for multiple testing, because this study was conducted to assess safety and feasibility. For repeatedly measured data, Friedman's test was performed separately for the patients with ischemic or nonischemic etiology to examine the treatment effects across 3 measurement times (pretreatment, 6 months, and 1 year). Complete‐case analyses were also performed as the sensitivity analysis for the repeatedly measured data. A value of P<0.05 was considered significant. The difference scores were also described in all continuous variabilities. Actuarial estimate of all‐cause mortality, cardiac death, major cardiac adverse event, and heart failure event requiring in‐hospital treatment were calculated using the Kaplan–Meier methods.
Results
Characterization of Implanted Autologous Skeletal Stem‐Cell Sheets
Cell sheets were round, with a thickness of about 100 μm and an approximate diameter of about 4 cm (Figure 1A). Hematoxylin and eosin staining and desmin staining showed abundant cells of skeletal origin (Figure 1B and 1C). Figure 1D shows expression of fibronectin indicating construction and preservation of extracellular matrix. Cell sheets were implanted to LV free wall through left thoracotomy (Figure 1E). Hepatocyte growth factor, vascular endothelial growth factor, and other cytokines were detected in the supernatant from the skeletal cells (Figure 1F).
Figure 1.
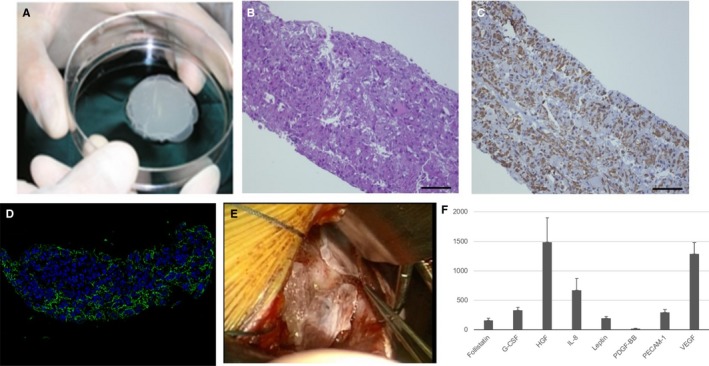
Characterization of implanted autologous skeletal stem‐cell sheets. A, The appearance of cell sheets. B, Hematoxylin and eosin staining. C, Desmin staining. D, Fibronectin staining. E, Cell sheets were implanted to LV free wall via left thoracotomy. F, In vitro study; various cytokines were detected in supernatant of cell sheet. G‐CSF indicates granulocyte colony‐stimulating factor; HGF, hepatocyte growth factor; IL‐8, interleukin‐8; PDGF‐BB, platelet‐derived growth factor‐BB; PECAM‐1, platelet endothelial cell adhesion molecule; VEGF, vascular endothelial growth factor.
Safety and Feasibility Analysis
All patients showed no procedural‐related complication and were discharged home at 49.6±57.6 days on average. An unusual long duration of stay was because of the need to monitor the patients for the occurrence of arrhythmias and had nothing to do with a complicated postoperative course. However, 2 patients who received continuous catecholamine injections preoperatively required long‐term hospitalization of 148 and 265 days, respectively. Lethal arrhythmias such as sustained VT and ventricular fibrillation were not observed in all patients by Holter ECG. We could not detect nonsustained VT or sustained VT for 1 month after operation in any patients by 24‐hour ECG monitoring. We have not observed any implantable cardioverter defibrillator operations to avoid lethal arrhythmias in any patients for 6 months after the operation. Only 2 patients with nonischemic etiology developed congestive heart failure within 6 months after the treatment.
Functional Changes After Cell‐Sheet Implantation
In the ischemic etiology, LVEDD decreased from 66.5±15.4% to 64.9±15.7% (P<0.05) and global LVEF increased from 26.74±8.0% to 30.7±10.0% (P<0.01) 1 year after the treatment, as compared with measurements taken before surgery, and 13 of 15 patients (86.7%) with ischemic etiology showed improvement of cardiac function (Figure 2A and 2B). However, in nonischemic etiology, LVEDD and LVEF were not statistically different after the treatment and less than one third of the patients showed improvement of these parameters (Figure 2C and 2D). The analyses for the repeated measures of LVEDD and LVEF also showed that there was significant improvement in both LVEDD and LVEF in the ischemic etiology during 1 year (Friedman's χ2=8.6 and 10.8, degrees of freedom [df]=2 and 2, P=0.014 and 0.005, respectively). In contrast, the changes of LVEDD and LVEF in the nonischemic etiology were not significant (Friedman's χ2=0.6 and 0.2, df=2 and 2, P=0.739 and 0.913, respectively). The results of the P‐values in the complete case analyses for LVEDD and LVEF in the ischemic etiology (n=15) and the nonischemic (n=8) supported the results (P=0.014 and 0.005 in the ischemic etiology and P=0.206 and 0.882 in the nonischemic, respectively).
Figure 2.
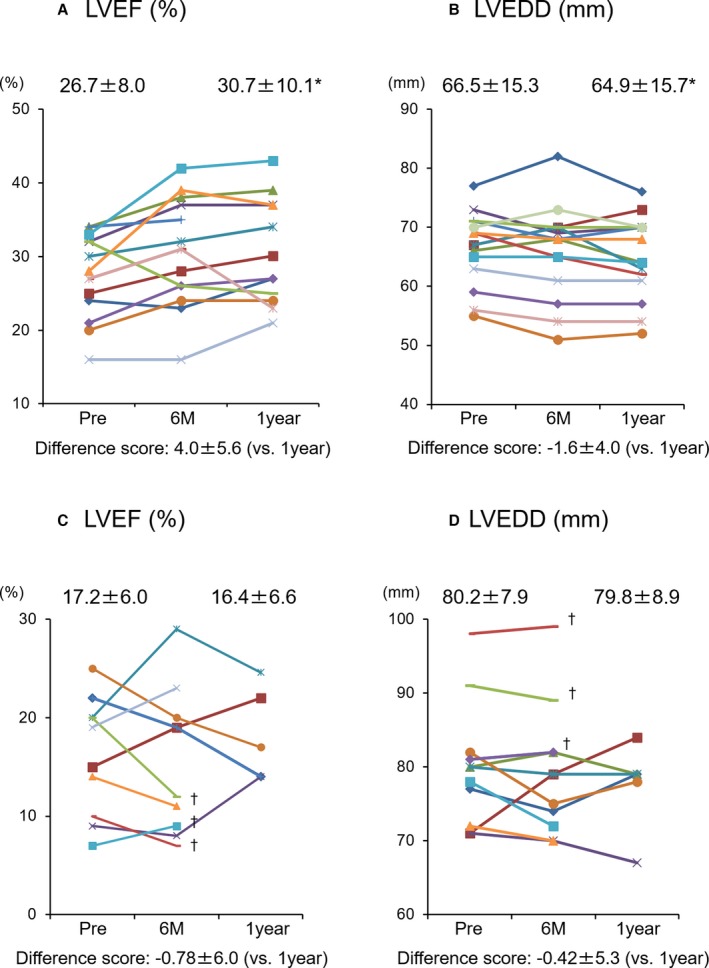
Echocardiographic analysis of cardiac function. A and B, Change of LVEF and LVEDD in the patients with ischemic etiology. C and D, Change of LVEF and LVEDD in the patients with nonischemic etiology. Each color bar corresponds to an individual patient. *vs pre P<0.05 (Friedman's test). †Examination was not performed because deterioration of cardiac function resulted in LVAD implantation or dependence on continuous catecholamine infusion within 1 year after treatment. LVAD indicates left ventricular assist device; LVEDD, left ventricular end‐diastolic dimension; LVEF, left ventricular ejection fraction.
Pressure Study
The parameters evaluated by Swan‐Ganz catheter were significantly decreased in the ischemic etiology as follows: mean pulmonary artery pressure, 26.9±12.2 mm Hg (pretreatment) versus 20.9±10.2 mm Hg, P<0.05 (6 months after treatment); pulmonary capillary wedge pressure (PCWP), 17.0±7.1 mm Hg versus 13.0±7.6 mm Hg, P<0.05; PVR, 205±131 dynes/sec±cm5 versus 158±50 dynes/sec±cm5, P<0.05 (Figure 3A through 3C). However, in the nonischemic etiology, they were not achieved after the treatment as follows: mean pulmonary artery pressure, 20.9±8.4 mm Hg (pretreatment) versus 22.2±10.1 mm Hg, P=0.45 (6 months after treatment); PCWP, 14.0±7.0 mm Hg versus 15.5±9.4 mm Hg, P=0.46; PVR, 141±64 dynes/sec±cm5 versus 166±77 dynes/sec±cm5, P=0.20 (Figure 3D through 3F).
Figure 3.
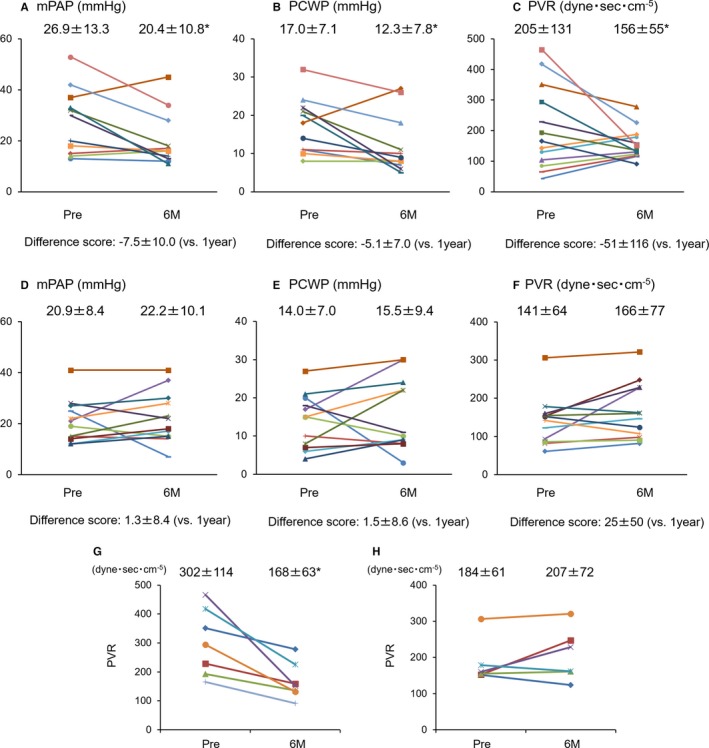
Pressure study evaluated by Swan‐Ganz catheter. Each color bar corresponds to an individual patient. A through C, Pressure study in the patients with ischemic etiology. D through F, Pressure study in the patients with nonischemic etiology. G, Change of PVR in the patients with ischemic etiology and preoperative pulmonary hypertension. H, Change of PVR in the patients with nonischemic etiology and preoperative pulmonary hypertension. mPAP indicates mean pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance. *vs pre P<0.05 (Wilcoxon signed‐rank test).
Prior to treatment, pressure studies showed pulmonary hypertension (PVR more than 150 dyne) in 7 of the patients with ischemic etiology and in 6 of the patients with nonischemic etiology. Compared with the pretreatment values, these parameters had improved even though the patients with pulmonary hypertension 6 months after treatment in the patients with ischemic etiology (Figure 3G). In contrast, in the patients with nonischemic etiology, such patients whose PVR was more than 150 did not show the same result (Figure 3H).
Evaluation of Symptoms and Exercise Capacity
In Six‐Minute Walk Tests conducted 6 months and 1 year after the cell‐sheet implantation, the walking distances in the patients with ischemic etiology had improved over the presurgery distances and these improvements were preserved for a long period (pre: 416±119 m versus 6 months: 475±125 m, P<0.05 and versus 1 year: 484±152 m, P<0.05) and those in the patients with nonischemic etiology showed no change after the treatment (pre: 413±129 m versus 6 months: 434±156 m and versus 1 year: 441±164 m, P=0.76) (Figure 4A and 4B). The analyses for the repeated measures of the walking distance also showed the significance in the ischemic etiology (Friedman's χ2=9.0, df=2, P=0.011) and nonsignificance in the nonischemic etiology (Friedman's χ2=0.5, df=2, P=0.761). Sensitivity analyses with complete cases supported these results.
Figure 4.
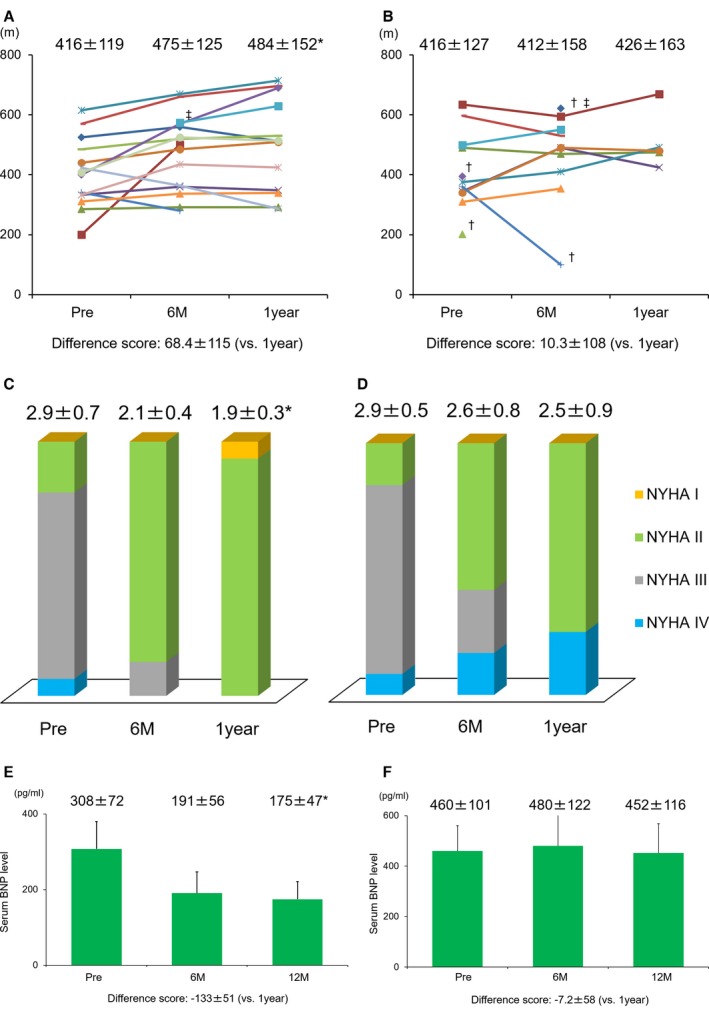
Exercise capacity and symptoms. A, The changes of exercise capacity evaluated by 6‐Minute Walk Test in the ischemic etiology. B, The changes of exercise capacity evaluated by 6‐Minute Walk Test in the nonischemic etiology. Each color bar in A and B corresponds to an individual patient. C, The changes of symptoms evaluated by NYHA functional classification in the ischemic etiology. D, The changes of symptoms evaluated by NYHA functional classification in the nonischemic etiology. E, The time course changes in serum level of BNP in the ischemic etiology (mean±SE). F, The time course changes in serum level of BNP in the nonischemic etiology (mean±SE). *vs pre P<0.05 (Friedman's test). †No examination was performed, because of deterioration of cardiac function after the treatment. ‡No examination was performed preoperatively because of depending on continuous catecholamine infusion. BNP indicates brain natriuretic peptide; NYHA, New York Heart Association.
In the ischemic etiology, the NYHA functional class had improved in all patients (pre: 2.9±0.7 versus 6 month: 2.1±0.4 P<0.01, 1 year: 1.9±0.3 P<0.01). On the other hand, in the nonischemic etiology, 3 patients developed heart failure within 1 year and they were considered as NYHA functional class IV. However, the NYHA functional class had also decreased on average, which was not statistically significant (pre: 2.9±0.5 versus 6 months: 2.6±0.8 P=0.05, 1 year: 2.5±0.9 P=0.20) (Figure 4C and 4D). The repeated‐measurement analyses for NYHA functional class also showed significance in the ischemic etiology (Friedman's χ2=20.5, df=2, P<0.001) and nonsignificance in the nonischemic etiology (Friedman's χ2=3.2, df=2, P=0.202).
Brain Natriuretic Peptide Blood Sampling
Brain natriuretic peptide serum levels gradually decreased in the ICM patients after surgery, eventually reaching about half of the pretreatment levels; the average level before treatment was 308±72 pg/mL, while the levels after treatment were 191±56 at 6 months, and 182±46 at 1 year (P<0.05) (the mean±SE). In the nonischemic etiology, brain natriuretic peptide serum level was not changed after the treatment; the average level before treatment was 460±101 pg/mL, while the level after the treatment was 480±122, and 452±116 pg/mL (P=0.80) (the mean±SE) (Figure 4E and 4F). The repeated‐measurement analyses for brain natriuretic peptide serum level also showed significance in the ischemic etiology (Friedman's χ2=6.5, df=2, P=0.038) and nonsignificance in the nonischemic etiology (Friedman's χ2=0.4, df=2, P=0.803).
End‐Systolic Wall Stress Evaluated by Cine‐Multidetector Computed Tomography
Cine‐multidetector computed tomography was avoided in 5 patients of 12 patients with nonischemic etiology because of the following reasons: renal failure in 2 patients and deterioration of cardiac function in 3 patients. Thus, the statistical analysis was not performed in nonischemic etiology. In the ischemic etiology, global end‐systolic wall stress, which was calculated by average of regional end‐systolic wall stress, was significantly decreased at 6 months after the treatment (380±70→333±50 kdyn/cm2 P<0.05) (Figure 5).
Figure 5.
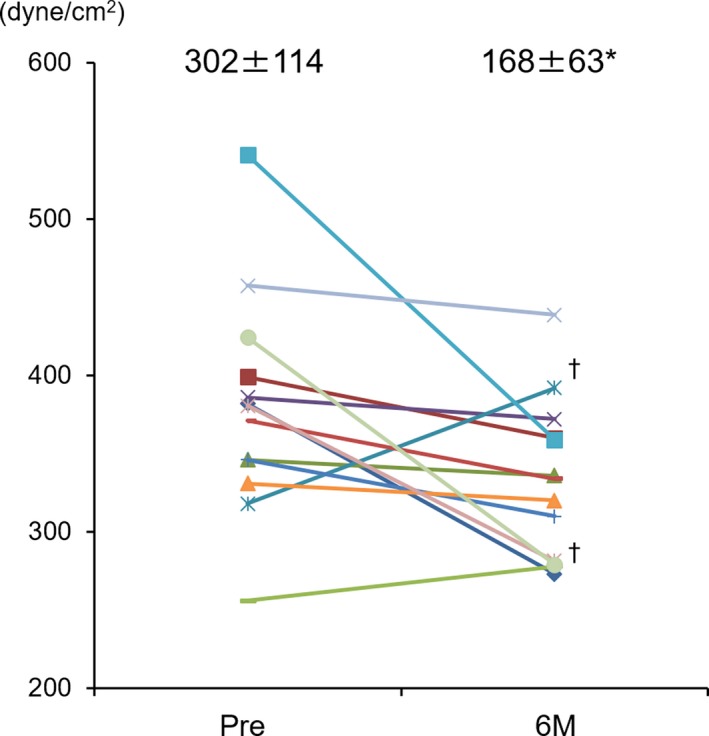
End‐systolic wall stress evaluated by MDCT. Each color bar corresponds to an individual patient. The changes of end‐systolic wall stress in the ischemic etiology: MDCT evaluation was not performed in 2 patients because of renal dysfunction. †Increasing of ESS was observed only in 2 patients in the ischemic etiology. *vs pre P<0.05 (Wilcoxon signed‐rank test). ESS indicates end‐systolic wall stress; MDCT, cine‐multidetector computed tomography.
Survival Rate
We encountered 3 late deaths attributable to gastrointestinal bleeding, arrhythmia, and heart failure. Survival rate was 96.0% at 1 year and 84.3% at 3 years, and there was no significant different between their etiologies (ischemic: 100% at 1 year and 90.9% at 3 years, nonischemic: 90.0% at 1 year and 75.0% at 3 years, P=0.31 Log rank) (Figure 6A).
Figure 6.
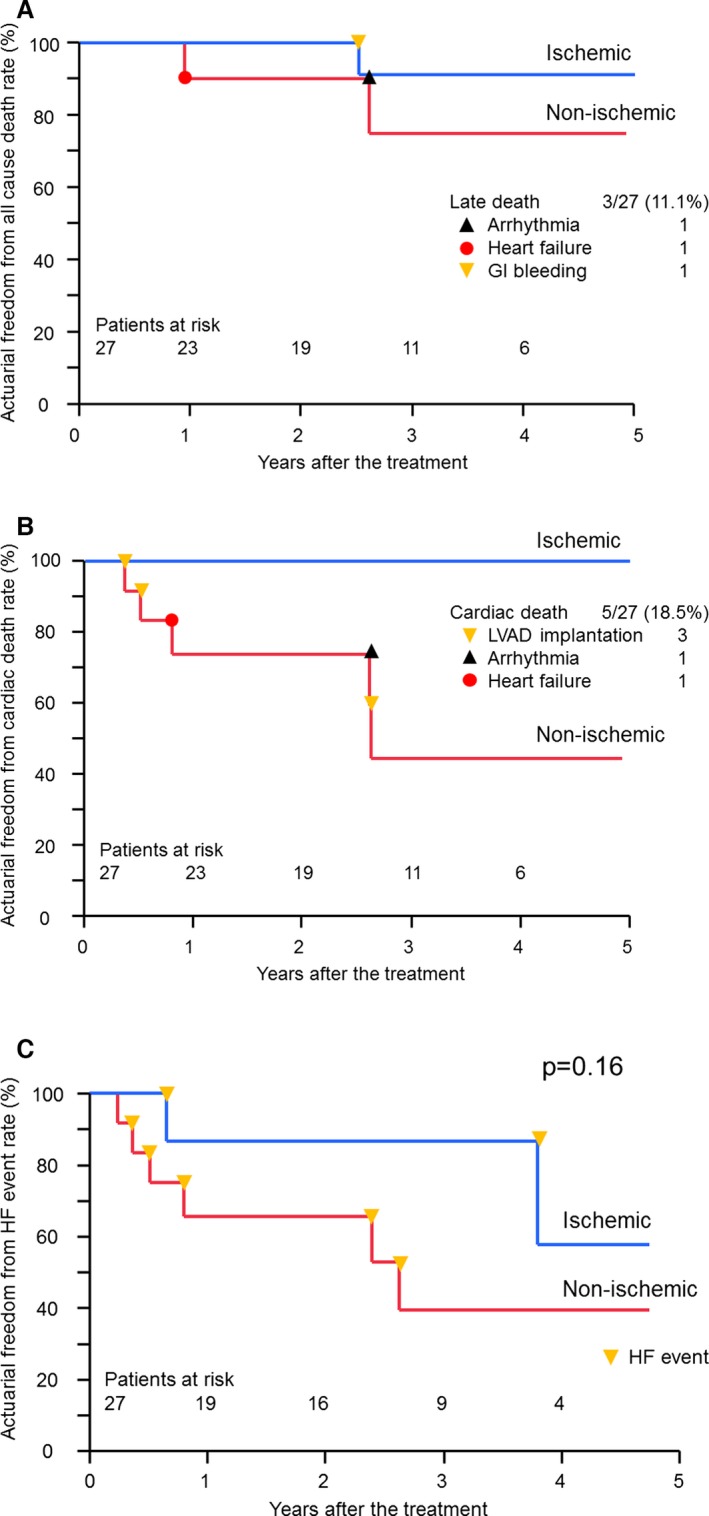
Clinical outcome of late periods. A, Freedom from all‐cause death in both etiologies. B, Freedom from cardiac death in both etiologies. C, Freedom from heart failure event requiring in‐hospital treatment. GI bleeding indicates gastrointestinal bleeding; HF event, heart failure event requiring in‐hospital treatment.
Cardiac Death‐Free Rate
In the ischemic etiology, there was no cardiac death event; however, 5 late cardiac deaths occurred in the nonischemic etiology. Three patients developed cardiac failure and underwent LVAD implantation (Table 2). Cardiac death‐free rate was 74.1% at 1 year and 74.1% at 2 years in the nonischemic etiology, which was statistically significant compared with the ischemic etiology (P<0.05 Log rank test) (Figure 6B).
Table 2.
Detail of All MACE Events
| Case | Etiology | Age | Events | Duration From Transplantation | LVAD | Result |
|---|---|---|---|---|---|---|
| 1 | DCM | 36 | Arrhythmia | 31 months | No | Deceased |
| 2 | DCM | 67 | Cerebral infarction | 29 months | No | Alive |
| 3 | DCM | 58 | Heart failure | 31 months | Yes | Alive |
| 4 | DCM | 62 | Heart failure | 10 months | Yes | Alive |
| 5 | DCM | 68 | Heart failure | 4 months | No | Deceased |
| 6 | DCM | 53 | Heart failure | 6 months | Yes | Alive |
| 7 | ICM | 68 | GI bleeding | 30 months | No | Deceased |
DCM indicates dilated cardiomyopathy; GI, gastrointestinal; ICM, ischemic cardiomyopathy; LVAD, left ventricular assist device; MACE, major adverse cardiac event.
Incidence and Rates of Freedom From Heart Failure Event Requiring In‐Hospital Treatment
Incidents of heart failure event was 0.91 event/patients‐years preoperatively and it was significantly decreased to 0.38 event/patients‐years in the postoperative period (P<0.01).
The rate of freedom from heart failure event was 86.7% for the first year and 86.7% for the third year in the ischemic etiology and 65.7% for the first year and 39.4% for the third year in the nonischemic etiology (Figure 6C), which was not statistically significant between the etiologies (P=0.16 Log rank test).
Discussion
We performed this Phase I clinical trial of our autologous skeletal stem‐cell sheet to evaluate its safety and feasibility as a sole therapy for patients with severe heart failure who had already received the maximum medical treatment available. This Phase I clinical trial demonstrated that the implantation of autologous skeletal stem‐cell sheets is feasible for heart‐failure patients. Almost all of the ICM patients improved in exercise capacity, as determined by the Six‐Minute Walk Test, and symptoms evaluated by NYHA. Reductions in PAP, PVR, PCWP, and LV wall stress were noted after cell‐sheet implantation in ICM patients. While systolic function did not dramatically improve after cell‐sheet implantation in some patients, the patients' symptoms and exercise capacity were notably recovered compared with their values before surgery. All the patients with pulmonary hypertension experienced marked reductions in PAP, PVR, and PCWP, which may have led to the remarkable recoveries of symptoms and exercise capacity. In addition to functional and symptomatic improvements, cardiac survival rate and lower freedom from heart failure events requiring in‐hospital treatment after sheet therapy, which lead to lower medical costs for heart failure, were satisfactory. Although some DCM patients showed marked functional and symptomatic recovery, other patients demonstrated preservation in cardiac function and symptoms in spite of gradual progress in heart failure. However, this study without controls and including some patients who may show functional recovery following coronary artery bypass graft or percutaneous coronary intervention could not exactly reveal safety and efficacy of the autologous skeletal stem‐cell sheet therapy, and further study may be needed to elucidate them.
It is interesting that cell‐sheet implantation reduced the PVR and pulmonary pressure. In severe heart failure, PVR may progress, by compensating for reduced blood inflow into the LV to avoid elevation of PCWP. Considering these mechanisms, PCWP may decrease as LV diastolic function improves after cell‐sheet implantation, reducing the compensation and leading to a reduction in PVR. We speculate that cell‐sheets continuously secrete very small amounts of cytokines, including hepatocyte growth factor, vascular endothelial growth factor, and Stromal cell‐derived factor‐1, which can reduce pulmonary hypertension over long periods of time. These cytokines may repair the diseased pulmonary vasculature as they circulate, reducing the PVR or pulmonary hypertension. We have already reported that implants of skeletal stem‐cell sheets in a right‐ventricle heart‐failure model in rats recovered the right‐ventricle function through cytokine paracrine effects.14 Further study is necessary to confirm the mechanisms that reduce pulmonary hypertension after cell‐sheet implantation. Furthermore, this study revealed that LV wall stress was remarkably declined after implantation, which is supposed to attenuate progressive LV fibrosis.
It is important to identify an appropriate first end point when evaluating therapies for heart failure. Unquestionably, improved quality of life, better survival rates, or lower medical costs are excellent surrogate markers of cell‐sheet efficacy. Although many cell‐therapy studies have set the LVEF as a first end point to evaluate the therapy's effectiveness,15 LVEF may not be the best indicator, because the LVEF is known to be easily influenced by LV pre‐ and afterloads, and it may change dramatically depending on volume load. Although previous clinical cell‐therapy studies have shown a 5% functional recovery in EF,15 we could not find an appropriate tool to reliably measure a 5% improvement in LVEF.16, 17
Recent studies have shown that a survival rate can be estimated based on several parameters; with this model, the efficacy of cell therapy could be estimated by comparing the real and estimated survival rates in a single‐arm study.18 However, it should be verified that this survival rate model mimics the real survival rates for the patients enrolled in a study. If verified and authorized, this model would be a powerful tool for evaluating the effectiveness of cell therapies for patients with severe heart failure and no other available treatment options. Thus, comprehensive surrogate parameters such as estimated survival rate, quality of life, exercise tolerance, and lower medical costs for heart failure may be the best way to evaluate cell‐therapy efficacy. In this respect, here we observed functional recovery, reverse LV remodeling, probably prolonged survival, improved quality of life, and increased exercise tolerance in ICM patients included in the study. Thus, registering a large number of candidates and using comprehensive surrogate markers may be the best approach for determining the effectiveness of cell‐sheet therapy in a single‐arm study.
In this study, we applied skeletal stem‐cell sheet therapy to idiopathic dilated cardiomyopathy. We previously reported that in DCM‐model hamsters, cell‐sheet implants preserved functional performance and attenuated dilation of the LV chamber compared with the untreated control group. Hamsters treated with cell‐sheet implants showed antifibrotic potential in the extracellular matrix, which increased survival.19 Considering these preclinical results, cell sheets may prolong the survival rate in human DCM patients by attenuating the LV dilation and deterioration in systolic function, which are common in DCM patients. However, further study may be needed to prove these hypotheses in clinical study. This clinical study clearly suggested that cell‐sheet implantation might have more functional impacts on ICM patients compared with DCM patients. It has been reported that regeneration mechanisms in cell sheet mainly depend on angiogenesis induced by secreted cytokines, so indication of cell‐sheet implantation may be more suitable to ICM patients compared with DCM patients, considering pathophysiological aspects. To improve effectiveness, the optimal dose of cells or extent of the ventricle that should be covered may be examined experimentally.
A preclinical study reported that the cell‐sheet method is less likely than needle‐injection delivery methods to evoke lethal ventricular arrhythmia.20 Myoblast injection triggered lethal ventricular arrhythmia in 1 clinical trial.10 Although we detected symptomless nonsustained VT in 3 patients after cell‐sheet implantation 3 or 6 months after operation, the arrhythmias were not life‐threatening and had been observed in these patients before surgery. Some studies have reported that implanted cell sheets cannot survive more than 6 months after implantation, and that arrhythmias are observed mainly in early phases after implantation.2 In our present study, all the patients were monitored by 24‐hour ECG for 1 month after cell‐sheet implantation, and we did not detect any lethal arrhythmias after implantation. Some studies have reported that 30% of patients with severe heart failure show sustained VT or nonsustained VT21; the probability of these arrhythmias may be lower in our present study. We can confirm that cell‐sheet implantation is safe, in terms of not inducing lethal arrhythmia. These arrhythmogenic differences might depend on the method of cell delivery, and as evidenced by preclinical studies20; the cell‐sheet method is less likely than needle‐injection methods to damage the myocardium.
This Phase I study found cell‐sheet transplantation as a sole therapy to be a feasible treatment for cardiomyopathy. The promising results in the safety and functional recovery seen in this study warrant further clinical follow‐up and larger studies to confirm the therapeutic efficacy of autologous skeletal stem‐cell sheets for severe congestive heart failure.
Sources of Funding
This work was supported by a Research on Regenerative Medicine for Clinical Application of Health and Labour Sciences Research and Japan Agency for Medical Research and Development (AMED) Grants in Japan.
Disclosures
Our laboratory received funding for cooperative research in cell sheet from the TERUMO Company.
Acknowledgments
We wish to thank Tomomi Shimamoto for her excellent assistance.
(J Am Heart Assoc. 2017;6:e003918 DOI: 10.1161/JAHA.116.003918.)28381469
References
- 1. Miyagawa S, Roth M, Saito A, Sawa Y, Kostin S. Tissue‐engineered cardiac constructs for cardiac repair. Ann Thorac Surg. 2011;91:320–329. [DOI] [PubMed] [Google Scholar]
- 2. Miyagawa S, Saito A, Sakaguchi T, Yoshikawa Y, Yamauchi T, Imanishi Y. Impaired myocardium regeneration with skeletal cell sheets–a preclinical trial for tissue‐engineered regeneration therapy. Transplantation. 2010;90:364–372. [DOI] [PubMed] [Google Scholar]
- 3. Memon IA, Sawa Y, Fukushima N, Matsumiya G, Miyagawa S, Taketani S, Sakakida S, Kondoh H, Aleshin AN, Shimizu T, Okano T, Matsuda H. Repair of impaired myocardium by means of implantation of engineered autologous myoblast sheets. J Thorac Cardiovasc Surg. 2005;130:1333–1341. [DOI] [PubMed] [Google Scholar]
- 4. Menasché P, Vanneaux V, Hagège A, Bel A, Cholley B, Cacciapuoti I, Parouchev A, Benhamouda N, Tachdjian G, Tosca L, Trouvin JH, Fabreguettes JR, Bellamy V, Guillemain R, Suberbielle Boissel C, Tartour E, Desnos M, Larghero J. Human embryonic stem cell‐derived cardiac progenitors for severe heart failure treatment: first clinical case report. Eur Heart J. 2015;36:2011–2017. [DOI] [PubMed] [Google Scholar]
- 5. Narita T, Shintani Y, Ikebe C, Kaneko M, Campbell NG, Coppen SR, Uppal R, Sawa Y, Yashiro K, Suzuki K. The use of scaffold‐free cell sheet technique to refine mesenchymal stromal cell‐based therapy for heart failure. Mol Ther. 2013;21:860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Menasche P. The future of stem cells: should we keep the “stem” and skip the “cells”? J Thorac Cardiovasc Surg. 2016;152:345–349. [DOI] [PubMed] [Google Scholar]
- 7. Sawa Y, Miyagawa S, Sakaguchi T, Fujita T, Matsuyama A, Saito A, Shimizu T, Okano T. Tissue engineered myoblast sheets improved cardiac function sufficiently to discontinue LVAS in a patient with DCM: report of a case. Surg Today. 2012;42:181–184. [DOI] [PubMed] [Google Scholar]
- 8. Shimizu T, Yamato M, Isoi Y, Akutsu T, Setomaru T, Abe K, Kikuchi A, Umezu M, Okano T. Fabrication of pulsatile cardiac tissue grafts using a novel 3‐dimensional cell sheet manipulation technique and temperature‐responsive cell culture surfaces. Circ Res. 2002;90:e40. [DOI] [PubMed] [Google Scholar]
- 9. Shudo Y, Matsumiya G, Sakaguchi T, Miyagawa S, Yoshikawa Y, Yamauchi T, Takeda K, Saito S, Nakatani S, Taniguchi K, Izutani H, Sawa Y. Assessment of changes in mitral valve configuration with multidetector computed tomography: impact of papillary muscle imbrication and ring annuloplasty. Circulation. 2010;122:S29–S36. [DOI] [PubMed] [Google Scholar]
- 10. Menasche P, Alfieri O, Janssens S, McKenna W, Reichenspurner H, Trinquart L, Vilquin JT, Marolleau JP, Seymour B, Larghero J, Lake S, Chatellier G, Solomon S, Desnos M, Hagège AA. The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial: first randomized placebo‐controlled study of myoblast transplantation. Circulation. 2008;117:1189–1200. [DOI] [PubMed] [Google Scholar]
- 11. McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285:1441–1446. [DOI] [PubMed] [Google Scholar]
- 12. ATS statement: guidelines for the six‐minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. [DOI] [PubMed] [Google Scholar]
- 13. Effects of pimobendan on adverse cardiac events and physical activities in patients with mild to moderate chronic heart failure: the effects of pimobendan on chronic heart failure study (EPOCH study). Circ J. 2002;66:149–157. [DOI] [PubMed] [Google Scholar]
- 14. Hoashi T, Matsumiya G, Miyagawa S, Ichikawa H, Ueno T, Ono M, Saito A, Shimizu T, Okano T, Kawaguchi N, Matsuura N, Sawa Y. Skeletal myoblast sheet transplantation improves the diastolic function of a pressure‐overloaded right heart. J Thorac Cardiovasc Surg. 2009;138:460–467. [DOI] [PubMed] [Google Scholar]
- 15. Abdel‐Latif A, Bolli R, Tleyjeh IM, Montori VM, Perin EC, Hornung CA, Zuba‐Surma EK, Al‐Mallah M, Dawn B. Adult bone marrow‐derived cells for cardiac repair: a systematic review and meta‐analysis. Arch Intern Med. 2007;167:989–997. [DOI] [PubMed] [Google Scholar]
- 16. Thavendiranathan P, Grant AD, Negishi T, Plana JC, Popović ZB, Marwick TH. Reproducibility of echocardiographic techniques for sequential assessment of left ventricular ejection fraction and volumes: application to patients undergoing cancer chemotherapy. J Am Coll Cardiol. 2013;61:77–84. [DOI] [PubMed] [Google Scholar]
- 17. Schepis T, Gaemperli O, Koepfli P, Valenta I, Strobel K, Brunner A, Leschka S, Desbiolles L, Husmann L, Alkadhi H, Kaufmann PA. Comparison of 64‐slice CT with gated SPECT for evaluation of left ventricular function. J Nucl Med. 2006;47:1288–1294. [PubMed] [Google Scholar]
- 18. Anand I, McMurray J, Cohn JN, Konstam MA, Notter T, Quitzau K, Ruschitzka F, Lüscher TF; EARTH investigators . Long‐term effects of darusentan on left‐ventricular remodelling and clinical outcomes in the EndothelinA Receptor Antagonist Trial in Heart Failure (EARTH): randomised, double‐blind, placebo‐controlled trial. Lancet. 2004;364:347–354. [DOI] [PubMed] [Google Scholar]
- 19. Kondoh H, Sawa Y, Miyagawa S, Sakakida‐Kitagawa S, Memon IA, Kawaguchi N, Matsuura N, Shimizu T, Okano T, Matsuda H. Longer preservation of cardiac performance by sheet‐shaped myoblast implantation in dilated cardiomyopathic hamsters. Cardiovasc Res. 2006;69:466–475. [DOI] [PubMed] [Google Scholar]
- 20. Narita T, Shintani Y, Ikebe C, Kaneko M, Harada N, Tshuma N, Takahashi K, Campbell NG, Coppen SR, Yashiro K, Sawa Y, Suzuki K. The use of cell‐sheet technique eliminates arrhythmogenicity of skeletal myoblast‐based therapy to the heart with enhanced therapeutic effects. Int J Cardiol. 2013;168:261–269. [DOI] [PubMed] [Google Scholar]
- 21. de Sousa MR, Morillo CA, Rabelo FT, Nogueira Filho AM, Ribeiro AL. Non‐sustained ventricular tachycardia as a predictor of sudden cardiac death in patients with left ventricular dysfunction: a meta‐analysis. Eur J Heart Fail. 2008;10:1007–1014. [DOI] [PubMed] [Google Scholar]


