Abstract
Background
Compromised lipophagy with unknown mechanisms may be critically involved in the intracellular accumulation of lipids, contributing to elevated atherosclerosis and liver steatosis. We hypothesize that toll‐interacting protein (Tollip), a key innate immune molecule involved in the fusion of autolysosome, may play a significant role in lipophagy and modulate lipid accumulation during the pathogenesis of atherosclerosis and liver steatosis.
Methods and Results
By comparing mice fed with either a Western high‐fat diet or a regular chow diet, we observed that both atherosclerosis and liver steatosis were aggravated in apolipoprotein E–deficient (ApoE−/−)/Tollip−/− mice as compared with ApoE−/− mice. Through electron microscopy analyses, we observed compromised fusion of lipid droplets with lysosomes within aortic macrophages as well as liver hepatocytes from ApoE−/−/Tollip−/− mice as compared with ApoE−/− mice. As a molecular indicator for disrupted lysosome fusion, the levels of p62 were significantly elevated in aortic and liver tissues from ApoE−/−/Tollip−/− mice. Molecules involved in facilitating lipophagy completion such as Ras‐related protein 7 and gamma‐aminobutyric acid receptor‐associated protein were reduced in ApoE−/−/Tollip−/− mice as compared with ApoE−/− mice. Intriguingly, ApoE−/−/Tollip−/− mice had reduced circulating levels of inflammatory cytokines such as tumor necrosis factor‐α and increased levels of transforming growth factor‐β. The reduced inflammation due to Tollip deficiency is consistent with a stable atherosclerotic plaque phenotype with increased levels of plaque collagen and smooth muscle cells in ApoE−/−/Tollip−/− mice.
Conclusions
Tollip deficiency selectively leads to enlarged yet stable atherosclerotic plaques, increased circulating lipids, liver steatosis, and reduced inflammation. Compromised lipophagy and reduced expression of inflammatory mediators due to Tollip deficiency may be the underlying causes. Our data suggest that lipid accumulation and inflammation may be intertwined yet independent processes during the progression of atherosclerosis and steatosis.
Keywords: animal model cardiovascular disease, atherosclerosis, hyperlipidemia, inflammation, lipophagy, Tollip
Subject Categories: Inflammation, Mechanisms
Introduction
Atherosclerosis and hepatic steatosis are closely associated chronic diseases with significant health concerns worldwide.1 Recent studies suggest that defective clearance of cellular lipids within tissues due to a compromised lipophagy pathway may play a highly important role for the development of liver steatosis and atherosclerosis.2 Through effective lipophagy, triglyceride (TG) and cholesterol are taken up by autophagosomes and delivered to lysosomes for degradation.2, 3 Free fatty acids generated by lipophagy from TG degradation fuel cellular respiration through mitochondrial β‐oxidation. Lipophagy therefore functions to modulate intracellular lipid storage, cellular levels of free lipids, and energy homeostasis. Intracellular lipids themselves may regulate autophagy by unclear mechanisms. Impaired lipophagy may lead to excessive tissue lipid accumulation such as hepatic steatosis and atherosclerosis.2, 4 However, cellular and molecular mechanisms responsible for the modulation of lipophagy are not well understood.
Toll‐interacting protein (Tollip) is a unique signaling protein involved in the modulation of innate immunity and inflammatory processes.5 Tollip contains a protein kinase C conserved region 2 domain in the central region that serves to anchor Tollip to endosomes and lysosomes.6, 7 Tollip also contains a coupling of ubiquitin to endoplasmic reticulum degradation domain in the C terminus and functions as an interaction motif for ubiquitinated proteins.8 The N terminus of Tollip contains a Tom1‐binding domain involved in the interaction with Tom1,9 through which Tollip may recruit clathrin onto the sorting endosomes. Potentially through its protein kinase C conserved region 2 domain, Tollip is required for the proper fusion of lysosome with autophagosome.7, 10 The involvements of Tollip during inflammatory processes are complex and highly context dependent. Under acute and strong inflammatory stress, Tollip may impair nuclear factor κB (NFκB) and serves to dampen excessive inflammatory reactions.11, 12 On the other hand, upon chronic low‐grade inflammatory challenges where NFκB may not be potently induced, Tollip can serve as a positive regulator of low‐grade inflammation through the induction of mitochondria reactive oxygen species.13 However, the potential role of Tollip in the process of lipophagy has not been explored.
Based on these studies, we hereby tested the hypothesis that Tollip may be critically involved in the process of lipophagy, and the lack of Tollip may compromise the proper clearance of intracellular lipids, thus contributing to the formation of lipid‐laden cells in vital tissues and the development of steatosis and atherosclerosis. Employing the animal models of apolipoprotein E–deficient (ApoE−/−) and ApoE−/−/Tollip−/− mice, we examined the pathologies of liver steatosis and atherosclerosis as well as the underlying mechanisms.
Materials and Methods
Mice
ApoE−/− and ApoE−/−/Tollip−/− mice were bred and maintained in the animal facility at Virginia Tech with the approved Institutional Animal Care and Use Committee protocol. Male mice were 8 to 9 weeks of age when the experiments were initiated. Mice were fed with either a normal diet (chow) or a Western high‐fat diet (HFD; Harlan Teklad 88137) for 8 weeks. Mice were properly anesthetized and euthanized in accordance with the approved animal protocol before tissue harvest and analyses.
Regents
The HFD was purchased from Harlan Laboratories, Inc (Dublin, VA). Oil‐Red‐O staining kit was from Newcomer Supply (Middleton, WI). Trichrome Stain (Masson) Kit and Cholesterol Quantitation Kit were from Sigma‐Aldrich (Louis, MO). Triglyceride Quantification Colorimetric/Fluorometric Kit and Alanine Aminotransferase (ALT or SGPT) Activity Colorimetric/Fluorometric Assay Kit were from BioVision Incorporated (Milpitas, CA). Mouse LIPA/Lysosomal Acid Lipase Sandwich ELISA Kit was purchased from LSBio Lifespan BioSciences, Inc (Seattle, WA). Mouse tumor necrosis factor‐α (TNF‐α) ELISA Kit, interleukin (IL)–6 ELISA, IL‐1 beta ELISA Kit, MCPT‐1 (mMCP‐1) ELISA Kit, and human/mouse transforming growth factor‐β (TGF‐β) 1 ELISA Kit were purchased from eBioscience (San Diego, CA). Anti‐mouse alpha smooth muscle actin (MSC), anti‐mouse F4/80 and anti‐SQSTM1/62 antibodies were from Abcam (Cambridge, MA). The anti‐mouse Ly6G, anti‐mouse Ly6C, anti‐mouse CD11b, anti‐mouse C‐X‐C motif chemokine receptor 2, and anti‐mouse TNF‐α were from BioLegend (San Diego, CA). Anti‐mouse IL12p40 and anti‐mouse Gr‐1 antibody was from eBioscience (San Diego, CA).
Histology
Parts of liver tissues were fixed in neutrally buffered formalin (10%) and embedded in paraffin. Paraffin‐embedded tissues were sectioned (5 μm) and stained with hematoxylin and eosin. Parts of liver tissues and aorta roots were performed on freshly frozen, optimal cutting temperature compound–embedded tissues of liver and aorta and used for generating frozen sections (10 μm). Slides were fixed in 4% neutral buffered formalin for 5 minutes. For immunohistology, frozen, optimal cutting temperature compound–embedded proximal aortic sections were fixed in 4% neutrally buffered formalin for 5 minutes and stained with Masson staining according to the manufacture's instruction. For smooth muscle cell staining, the sections were stained with an anti‐SMC Ab followed by a biotinylated anti‐Ig secondary Ab and streptavidin‐HRP/DAB with hematoxylin counter staining.
Immunofluorescence
Immunofluorescence analyses were performed on freshly frozen, optimal cutting temperature compound–embedded tissues. Slides were fixed in 4% neutrally buffered formalin for 5 minutes, and subsequently stained with anti‐mouse primary antibodies (anti mouse Gr‐1, F4/80, P62 antibodies) followed by a biotinylated anti‐Ig secondary antibody and Streptavidin‐PE or fluorescein isothiocyanate. DAPI was used to stain the nucleus.
Transmission Electron Microscopy
Tissues (1 mm3) were fixed in 2.5% glutaraldehyde in 100 mmol/L sodium cacodylate, pH7.4, and postfixed in 1% osmium tetroxide followed by 1% uranyl acetate. After ethanol dehydration, the tissues were embedded in LX112 resin (LADD Research Industries). Ultrathin sections were stained with uranyl acetate followed by lead citrate. All grids were viewed on a JEOL 100CX II transmission electron microscope at 80 kV.
ELISA of Cytokines and Chemokines
The levels of plasma TNF‐α, IL‐6, IL‐1β, TGF‐β, and C‐C motif chemokine ligand 2 were determined with ELISA Kit according to the manufacturer's instruction.
Flow Cytometry
Splenic cells were separated as previously described.14 Briefly, single‐cell suspensions were prepared from spleens. Prior to intracellular staining for cytokines, cells were cultured in vitro for 4 hours in RPMI‐completed medium with GolgiStop (BD Biosciences, San Jose, CA). Then, cells were fixed after surface marker staining, permeabilized, and stained with anti–TNF‐α and anti–IL‐12, according to the manufacturer's instructions (BD Biosciences). Samples were analyzed with a FACSCanto II (BD Biosciences). Fluorescence‐activated cell sorter plots shown were analyzed with FlowJo (Ashland, OR).
Real‐Time Polymerase Chain Reaction
RNA was extracted using RNeasy Mini Kit (QIAGEN), and 1.5 μg of RNA was reverse‐transcribed using a High‐Capacity cDNA Reverse Transcription Kit (ThermoFisher Scientific 4368813). Real‐time polymerase chain reaction was performed on a Bio‐Rad CFX96 machine using SsoAdvanced Universal SYBR Green Supermix (Bio‐Rad 1725271). Primers for mouse GAPDH (F 5′‐AACTTTGGCATTGTGGAAGGGCTC, R 5′‐TGGAAGAGTGGGAGTTGCTGTTGA), autophagocytosis‐associated protein 3 (F 5′‐TGTCTCTCCCTACTCCATTC, R 5′‐TGGCATACAGTTAGCACTTC), gamma‐aminobutyric acid receptor‐associated protein (F 5′‐GAAAGCGTCTATGGTCTGTG, R 5′‐TGAAGGAGGAACTGGATGT), autophagy‐related 9a (F 5′‐GGCTCTCTTATCACCATCCT, R 5′‐GAAGGAGTGGATCTCCCAATA), autophagy‐related protein 12 (F 5′‐GAGAGATTGACGTTAGAGTGTG, R 5′‐GAGACCTGCAATCCACATAC), and Ras‐related protein 7 (F 5′‐GGACTCTGGTGTTGGAAAG, R 5′‐CAGAAAGTCCGCTCCTATTG) were purchased from Integrated DNA Technologies, Inc (Coralville, IA). Readouts were analyzed by the ΔΔCQ method.
Immunoblotting
Tissues (≈100 mg) were harvested, immediately soaked in liquid nitrogen, and smashed into powder. Tissue powder was dissolved with sodium dodecyl sulfate lysis buffer containing the protease inhibitor mixture, and subjected to SDS‐PAGE. The protein bands were transferred onto an Immuno‐Blot PVDF membrane (Bio‐Rad). Western blot analyses were performed with the specified antibodies as previously described.7
Statistical Analysis
All experiments were performed at least 3 times. Representative and reproducible results were shown. Statistical analysis was performed with Prism software (GraphPad Software, La Jolla, CA). Values were expressed as means±SEM. Student t test was used for parametric analyses between the 2 groups. For nonparametric analyses between the 2 groups, the significance of the differences was assessed by Mann–Whitney U test. P<0.05 was considered statistically significant.
Results
Tollip Deficiency Promotes the Development of Atherosclerosis
We used the well‐established ApoE−/− mouse model for the systems analyses of atherosclerosis. Following 2 months of feeding with an HFD, compared with ApoE−/− mice, ApoE−/−/Tollip−/− mice developed aggravated atherosclerosis as measured by hematoxylin and eosin staining of the aorta area (Figure 1A and 1B). In addition, we observed increased lipid deposition within the atherosclerotic lesion areas of ApoE−/−/Tollip−/− mice as compared with ApoE−/− mice, as measured by the Oil‐Red‐O staining within the cross sections of the aorta (Figure 1C through 1E). We further examined the composition of aortic plaques through staining for smooth muscle cells and collagen content. Through immunohistochemical staining analyses of smooth muscle actin, we observed elevated levels of smooth muscle cells within the plaques in the aorta of ApoE−/−/Tollip−/− mice as compared with ApoE−/−/mice (Figure 1F). We also noted an increase in the collagen content as measured through Masson staining within the aorta of ApoE−/−/Tollip−/− mice as compared with ApoE−/−/mice (Figure 1F). Our results suggest that although Tollip deficiency may give rise to enlarged aortic plaques with increased lipid deposition, the formed plaques are more stable with increased collagen and smooth muscle cells. Enlarged atherosclerotic plaques were similarly observed in ApoE−/−/Tollip−/− mice as compared with ApoE−/− mice fed with regular chow (Figure S1).
Figure 1.

Toll‐interacting protein (Tollip) deficiency promotes the development of stable atherosclerotic plaques. A and B, Apolipoprotein E–deficient (ApoE−/−) and ApoE−/−/Tollip−/− mice (male, 8 weeks old) were fed with a high‐fat diet for an additional 8 weeks. A, Representative hematoxylin and eosin (H&E) staining images within the aortic roots of ApoE−/− and ApoE−/−/Tollip−/− mice. B, Representative Oil‐Red‐O staining images within the aortic roots. C, Quantification of Oil‐Red‐O staining–positive area (mm2) of aortic roots. D, Relative ratios of Oil‐Red‐O–positive areas over total plaque areas. E, Representative smooth muscle cell (SMC) staining of aorta areas from ApoE−/− and ApoE−/−/Tollip−/− mice. F, Representative Masson staining of aorta areas from ApoE−/− and ApoE−/−/Tollip−/− mice. Error bars represent SEM. *P<0.05; **P<0.01; ***P<0.001. Mann–Whitney U test.
Tollip Deficiency Results in the Disruption of Lipophagy in the Aorta
Mechanistically, studies from our laboratory and others reveal that Tollip is critically involved in the completion of lysosome fusion with autophagosome.7, 10 Since the proper fusion of lysosome with autophagosome also plays a key role during the lipophagy process, we tested the hypothesis that increased lipid deposition due to Tollip deficiency may be due to defective lipophagy.
To test this, we performed transmission electron microscopy analyses of aorta tissues from ApoE−/− mice and ApoE−/−/Tollip−/− mice fed with an HFD. Consistent with the conclusion drawn through the Oil‐Red‐O staining, we observed higher numbers of lipid droplets within aortic cells from ApoE−/−/Tollip−/− mice as compared with ApoE−/− mice (Figure 2A). The process of lysosome fusion with lipid droplet could be readily seen in aorta tissues from ApoE−/− mice but was absent within the aorta cells from ApoE−/−/Tollip−/− mice (Figure 2B).
Figure 2.

Toll‐interacting protein (Tollip) deficiency results in the disruption of lipophagy in aorta. Apolipoprotein E–deficient (ApoE−/−) and ApoE−/−/Tollip−/− mice (male, 8 weeks old) were fed with a high‐fat diet for an additional 8 weeks. A, Representative transmission electron microscopy (TEM) images of aortic sections from Apo−/− mice and ApoE−/−/Tollip−/− mice. B, Representative TEM images that demonstrate the lack of lysosome fusion with lipid droplets within aortic sections from ApoE−/−/Tollip−/− mice. LD indicates lipid droplet; Ly, lysosome.
At the molecular level, the disruption of lysosome fusion with autophagosomes and/or lipid droplets was associated with the elevated levels of p62.15 Thus, we subsequently stained for p62 levels within the aorta from ApoE−/− and ApoE−/−/Tollip−/− mice. We observed significantly elevated levels of p62 within plaque macrophages from ApoE−/−/Tollip−/− mice as compared with ApoE−/− mice (Figure 3A and 3B), further supporting the notion that lysosome fusion was disrupted in the aorta macrophages due to Tollip deficiency. The overall numbers of aortic macrophages within the plaque areas of ApoE−/−/Tollip−/− mice were not significantly elevated as compared with ApoE−/− mice (Figure S2). Together, our data reveal that the disruption of Tollip contributes to reduced levels of lysosome fusion, elevated accumulation of p62, and enhanced lipid deposition within aorta macrophages.
Figure 3.

Toll‐interacting protein (Tollip) deficiency results in compromised lysosome fusion within aorta. Apolipoprotein E–deficient (ApoE−/−) and ApoE−/−/Tollip−/− mice (male, 8 weeks old) were fed with a high‐fat diet for an additional 8 weeks. A, Representative images of p62 staining within plaque macrophages. B, Quantification of p62‐positive macrophages within aorta sections. Error bars represent SEM. *P<0.05. Mann–Whitney U test.
Tollip Deficiency Exacerbates Liver Steatosis
Next, we examined the liver pathology of ApoE−/−/Tollip−/− mice. Oil‐Red‐O staining revealed a significant elevation of lipid droplets within hepatocytes from Tollip−/−/ApoE−/− mice as compared with ApoE−/− mice (Figure 4A). Hematoxylin and eosin staining analyses also revealed fatty degeneration of hepatocytes, without apparent fibrosis (Figure 4B). The plasma lipid levels were also significantly elevated in ApoE−/−/Tollip−/− mice as compared with ApoE−/− mice (Figure S3).
Figure 4.

Toll‐interacting protein (Tollip) deficiency promotes liver steatosis. Apolipoprotein E–deficient (ApoE−/−) and ApoE−/−/Tollip−/− mice (male, 8 weeks old) were fed with a high‐fat diet for an additional 8 weeks. A, Representative Oil‐Red‐O staining images of liver sections. B, Representative hematoxylin and eosin staining images of liver sections. C, Representative transmission electron microscopy (TEM) images. D, Western blot analyses of liver lipoprotein lipase (LPL) protein levels. E, Immunohistochemical staining of neutrophils. Error bars represent SEM. ***P<0.001. Mann–Whitney U test.
Through the transmission electron microscopy analyses, we further confirmed that the numbers of lipid droplets within individual liver hepatocytes of ApoE−/−/Tollip−/− mice were higher than that of ApoE−/− mice (Figure 4C). Our data reveal that Tollip deficiency also contributes to excessive lipid accumulation in liver tissues. Lipoprotein lipase (LPL) is the key enzyme responsible for the modulation of circulating lipid levels, and a decrease in LPL activity was shown to be responsible for many cases of hyperlipidemia.16 We then tested the levels of LPL by Western blot analyses, and observed a significant increase of LPL protein levels in the liver tissues of ApoE−/−/Tollip−/− as compared with ApoE−/− mice fed with an HFD (Figure 4D).
Since Tollip deficiency caused an elevation of plasma lipid as well as liver lipid deposition, we next tested whether Tollip deficiency may also lead to elevated infiltration of inflammatory cells within liver tissues. Intriguingly, we observed that the number of infiltrated neutrophils were significantly lower in the liver tissues of ApoE−/−/Tollip−/− mice fed with an HFD as compared with ApoE−/− mice (Figure 4E). These data suggest that Tollip deficiency selectively affects lipid accumulation while decreasing the infiltration of inflammatory cells.
Tollip Deficiency Results in the Disruption of Lipophagy in Liver
To test whether Tollip deficiency may similarly contribute to the disruption of lipophagy completion in the liver, we performed transmission electron microscopy analyses of liver hepatocytes from ApoE−/− mice and ApoE−/−/Tollip−/− mice. We observed effective fusion between lysosome and lipid droplets in hepatocytes from ApoE−/− mice (Figure 5). In sharp contrast, despite close proximity of lysosomes with lipid droplets in hepatocytes from ApoE−/−/Tollip−/− mice, there was clear separation of membrane structures of lysosomes and lipid droplets, without any noticeable fusion (Figure 5). Intriguingly, there were significantly elevated numbers of lysosomes within hepatocytes from ApoE−/−/Tollip−/− mice as compared with ApoE−/− mice, perhaps due to the lack of lysosome fusion and/or a compensatory mechanism for elevated lysosome biogenesis (Figure 5B and 5C). Our results corroborate with the phenotypic data that Tollip is required for the process of lipophagy, and Tollip deficiency may cause a systemic compromise in the intracellular clearance of lipids.
Figure 5.

Toll‐interacting protein (Tollip) deficiency compromises liver lipophagy. Apolipoprotein E–deficient (ApoE−/−) and ApoE−/−/Tollip−/− mice (male, 8 weeks old) were fed with a high‐fat diet for an additional 8 weeks. A, Representative transmission electron microscopy (TEM) images of lipid droplets within liver hepatocytes. B, Representative TEM images of liver hepatocytes. C, The numbers of lysosomes within liver hepatocytes are quantified and shown in the graph. Error bars represent SEM. **P<0.01. Student t test. LD indicates lipid droplet; Ly, lysosome.
Next, we examined critical genes responsible for the fusion of autophagosome with lysosome from the liver tissues of ApoE−/− and ApoE−/−/Tollip−/− mice. As measured by real‐time reverse transcription polymerase chain reaction analyses, the expression levels of autophagocytosis‐associated protein 3, gamma‐aminobutyric acid receptor‐associated protein, autophagy‐related 9a, autophagy‐related protein 12, and Ras‐related protein 7 were significantly reduced in the liver tissues of ApoE−/−/Tollip−/− mice as compared with ApoE−/− mice (Figure 6A through 6E).
Figure 6.

Toll‐interacting protein (Tollip) deficiency reduces the expression of key genes involved in lysosome fusion. Apolipoprotein E–deficient (ApoE−/−)and ApoE−/−/Tollip−/− mice (male, 8 weeks old) were fed with a high‐fat diet for additional 8 weeks. A through E, Real‐time reverse transcription polymerase chain reaction data are shown for liver tissue expression of (A) autophagocytosis‐associated protein 3 (ATG3), (B) gamma‐aminobutyric acid receptor‐associated protein (GABARAP), (C) autophagy‐related 9a (ATG9a), (D) autophagy‐related protein 12 (ATG12), and (E) Ras‐related protein 7 (RAB7). Error bars represent SEM. *P<0.05. Student t test.
Tollip Deficiency Elicits Reduced Plasma Levels of Inflammatory Mediators
Tollip is involved not only in autophagy, but also in the modulation of inflammatory gene expressions.12, 13 However, previous studies on the exact function of Tollip during the expression of inflammatory mediators were inconclusive. Our previous data with in vitro cultured macrophages suggest that Tollip is involved in the generation of mitochondria reactive oxygen species and the expression of selected proinflammatory mediators under low‐grade inflammatory conditions.13 Based on these findings, we tested the expression levels of key inflammatory mediators in ApoE−/−/Tollip−/− mice. Consistent with our previous in vitro observation, we observed that the levels of plasma TNF‐α and IL‐6 were significantly lower in ApoE−/−/Tollip−/− mice as compared with ApoE−/− mice fed with an HFD (Figure 7). On the other hand, we observed elevated plasma levels of TGF‐β in ApoE−/−/Tollip−/− mice as compared with ApoE−/− mice fed with an HFD (Figure 7). The TGF‐β levels within the atherosclerotic plaque areas were also significantly higher in ApoE−/−/Tollip−/− mice as compared with ApoE−/− mice (Figure S4).
Figure 7.
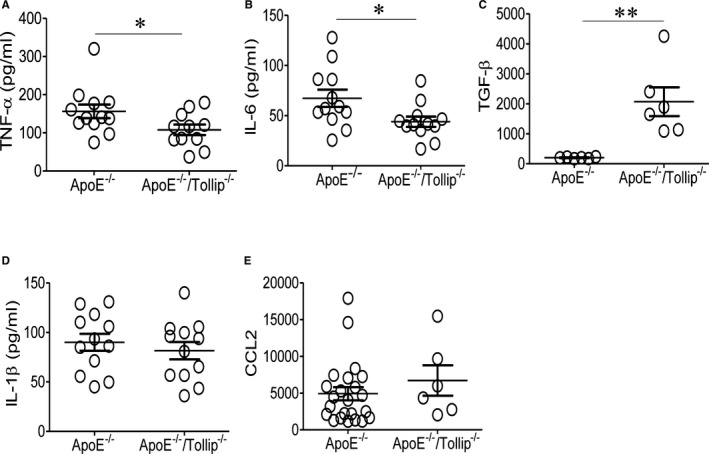
Toll‐interacting protein (Tollip) deficiency reduces the plasma levels of selected inflammatory cytokines. A through E, Apolipoprotein E–deficient (ApoE−/−) and ApoE−/−/Tollip−/− mice (male, 8 weeks old) were fed with a high‐fat diet for an additional 8 weeks. Plasma levels of (A) tumor necrosis factor‐α (TNF‐α), (B) interleukin (IL)–6, (C) transforming growth factor‐β (TGF‐β), (D) IL‐1β, and (E) C‐C motif chemokine ligand 2 (CCL2) were measured. Error bars represent SEM. *P<0.05; **P<0.01. Mann–Whitney U test.
To test the potential sources of TNF‐α expression, we examined the cellular levels of TNF‐α in splenic monocytes and neutrophils harvested from ApoE−/− and ApoE−/−/Tollip−/− mice through flow cytometry. As shown in Figure 8A and 8B, we observed significantly reduced intracellular levels of TNF‐α and IL‐12 in both splenic monocytes and neutrophils from ApoE−/−/Tollip−/− mice. In addition, the surface levels of C‐X‐C motif chemokine receptor 2 on splenic neutrophils as well as bone marrow neutrophils were significantly lower in ApoE−/−/Tollip−/− mice (Figure 8C and 8D). These data suggest that Tollip deficiency selectively increases intracellular lipid accumulation while reducing systemic proinflammatory response.
Figure 8.

Toll‐interacting protein (Tollip) deficiency reduces the expression of inflammatory mediators from monocytes and neutrophils. Apolipoprotein E–deficient (ApoE−/−) and ApoE−/−/Tollip−/− mice (male, 8 weeks old) were fed with a high‐fat diet for an additional 8 weeks. Flow cytometry analyses of intracellular tumor necrosis factor‐α (TNF‐α) and interleukin (IL)–12 in splenic neutrophils (A) and monocytes (B). Flow analyses of C‐X‐C motif chemokine receptor 2 (CXCR2) levels on splenic (C) and bone marrow (D) neutrophils. Error bars represent SEM. N=4, *P<0.05, **P<0.01. Student t test.
Discussion
Our study reveals dual roles of Tollip in facilitating lipophagy and systemic inflammation that reconcile the enlarged yet stable atherosclerosis phenotype associated with Tollip‐deficient mice. First, our data extend previous in vitro studies on the role of Tollip during the process of lysosome fusion.7, 10 Reports from others and our laboratory indicate that Tollip is critically involved in the fusion of lysosome with autophagosome in cultured cells. Our current study confirms these previous studies in an animal model in vivo, in the specific context of lipophagy. We observed a failure of lysosome fusion with lipid droplets within both aorta cells and hepatocytes of ApoE−/−/Tollip−/− mice through electron microscopy. Gene expression analyses revealed reduced expression of key genes involved in lysosome fusion as well as the lipoprotein lipase in ApoE−/−/Tollip−/− mice. The defective lipophagy and reduced LPL expression due to Tollip deficiency we observed in this study helps to explain elevated lipid deposition in atherosclerotic plaques as well as liver in ApoE−/−/Tollip−/− mice as compared with ApoE−/− mice.
Second, despite enlarged atherosclerotic plaques with elevated lipid deposition, the systemic levels of proinflammatory cytokines such as TNF‐α and IL‐6 were reduced in ApoE−/−/Tollip−/− mice as compared with ApoE−/− mice. Our study clarifies a novel contribution of Tollip in the context of low‐grade chronic inflammation in vivo. Previous studies suggest opposing roles of Tollip as capable of serving as both a positive and negative regulator of inflammation in cultured cells.12, 13, 17 Under acute and strong inflammatory signals, Tollip may serve as a negative regulator of NFκB signaling and suppress the expression of proinflammatory mediators.12 The induction of Tollip by higher‐dose inflammatory signals such as LPS lead to endotoxin tolerance.12, 18 In contrast, under lower inflammatory signals where NFκB signaling may not be the primary component for the low‐grade inflammatory state, Tollip serves as a positive regulator for the low‐level expression of proinflammatory mediators through facilitating the generation of mitochondria reactive oxygen species.13 Among multiple inducers of chronic inflammation, subclinical endotoxemia with super low levels of circulating LPS have been increasingly recognized in chronic diseases such as diabetes mellitus and atherosclerosis.19, 20 Super low–dose LPS induces nonresolving low‐grade inflammation, enhancing the proinflammatory function of Tollip through translocating Tollip from lysosome to mitochondria.7 On the other hand, dislocation of Tollip away from lysosome may compromise lipophagy and contribute to cellular lipid accumulation. Translocated Tollip may be responsible for altering the balance of cytokine expressions, favoring the expression of proinflammatory mediators such as TNF‐α and IL‐6 and reducing the expression of TGF‐β as shown in this study. Consistent with these mechanistic observations, we recently reported that injection of super low–dose LPS in ApoE single‐knockout mice induces an unstable atherosclerosis phenotype with increased plaque lipid deposition, increased systemic proinflammatory mediators, reduced TGF‐β, and reduced plaque collagen content.19 Our current observations in ApoE−/−/Tollip−/− mice complement our previous findings by demonstrating that complete deletion of Tollip compromises both lipophagy and proinflammatory cytokine expression in vivo. Reduced lipophagy explains elevated lipid deposition within enlarged atherosclerotic plaques in ApoE−/−/Tollip−/− mice. On the other hand, reduced systemic inflammation as reflected in reduced plasma levels of TNF‐α/IL‐6 and increased levels of TGF‐β due to Tollip deletion renders a stable atherosclerosis phenotype with increased plaque collagen and smooth muscle cells.
Our data not only address the dual roles of Tollip in lipophagy and inflammation but also help to clarify the relative contribution of rising plasma lipids and inflammation during the pathogenesis of atherosclerosis. Out data are consistent with previous findings that hyperlipidemia is a key driver for early development of atherosclerosis, and that high levels of circulatory inflammatory cytokines are not capable of stimulating the development of atherosclerosis in the absence of hyperlipidemia.21 Our data also suggest that hyperlipidemia without excessive inflammation may prevent the progression of unstable advanced atherosclerosis, as the atherosclerotic plaques manifested in ApoE−/−/Tollip−/− mice tend to have increased collagen contents and elevated smooth muscle cells sprouting over the plaque areas, characteristics of stable atherosclerotic plaques.
Likewise, despite elevated lipid deposition in liver tissues, we did not observe apparent fibrosis within liver tissues of ApoE−/−/Tollip−/− mice, which is distinct from conventional models of nonalcoholic fatty liver disease with an inflammatory component, where liver fibrosis is a common phenomenon and often leads to cirrhosis.22, 23 Animals with nonalcoholic fatty liver disease tend to have elevated liver inflammation as reflected by increased infiltration of liver neutrophils.24 In contrast, we observed less neutrophil infiltration in liver tissues from ApoE−/−/Tollip−/− mice as compared with ApoE−/− mice. Collectively, our data suggest that defective lipophagy may partially contribute to the pathogenesis of steatosis and that the lack of an inflammatory component may prevent the full‐blown development of nonalcoholic fatty liver disease with fibrosis and cirrhosis.
Conclusions
At the translational levels, our data suggest that restoring the proper function of Tollip instead of completely blocking Tollip may present the optimum approach to treat atherosclerosis and steatosis. Our data lend caution to related translational studies that aim to treat atherosclerosis by completely blocking target molecules through inhibitor approaches. Instead, balanced approaches to restore the proper functions of target molecules and pathways may hold better therapeutic promise in the effective treatment of chronic atherosclerosis.
Sources of Funding
This study was supported by grants from the National Institutes of Health R01 HL115835 and R56AI108264 to Dr Li. Dr Geng was supported by a Postdoctoral Fellowship Award from the American Heart Association.
Disclosures
None.
Supporting information
Figure S1. Toll‐interacting protein (Tollip) deficiency promotes the development of stable atherosclerotic plaques. A and B, Apolipoprotein E–deficient (ApoE−/−) and ApoE−/−/Tollip−/− mice (male, 8 weeks old) were fed with regular chow for an additional 8 weeks. A, Representative Oil Red O staining images within the aortic roots of ApoE−/− and ApoE−/−/Tollip−/− mice. B, Quantification of Oil Red O staining–positive area (mm2) of aortic roots. *P<0.05. Mann–Whitney U test.
Figure S2. Toll‐interacting protein (Tollip) deficiency does not lead to significant increase in aortic macrophages. Apolipoprotein E–deficient (ApoE−/−) and ApoE−/−/Tollip−/− mice (male, 8 weeks old) were fed with a high‐fat diet for an additional 8 weeks. Aortic sections were stained with monocyte/macrophage marker antibody (green) and DAPI (blue) to visualize aortic macrophages.
Figure S3. Toll‐interacting protein (Tollip) deficiency increases plasma levels of lipids. Apolipoprotein E–deficient (ApoE−/−) and ApoE−/−/Tollip−/− mice (male, 8 weeks old) were fed with a high‐fat diet for an additional 8 weeks. Plasma triglyceride, total cholesterol, and free cholesterol were measured. Error bars represent SEM. *P<0.05; **P<0.01; ***P<0.001. Mann–Whitney U test.
Figure S4. Toll‐interacting protein (Tollip) deficiency leads to elevated plaque levels of transforming growth factor‐β (TGF‐β). Apolipoprotein E–deficient (ApoE−/−) and ApoE−/−/Tollip−/− mice (male, 8 weeks old) were fed with a high‐fat diet for an additional 8 weeks. Aortic sections were stained with monocyte/macrophage marker antibody (green) and anti–transforming growth factor‐β. Error bars represent SEM. **P<0.01. Student t test.
Acknowledgement
The authors thank Kathy Lowe for her assistance in transmission electron microscopy.
(J Am Heart Assoc. 2017;6:e004078 DOI: 10.1161/JAHA.116.004078.)28396568
References
- 1. Braunwald E. Shattuck lecture–cardiovascular medicine at the turn of the millennium: triumphs, concerns, and opportunities. N Engl J Med. 1997;337:1360–1369. [DOI] [PubMed] [Google Scholar]
- 2. Liu K, Czaja MJ. Regulation of lipid stores and metabolism by lipophagy. Cell Death Differ. 2013;20:3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Martinez‐Lopez N, Singh R. Autophagy and lipid droplets in the liver. Annu Rev Nutr. 2015;35:215–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ouimet M, Franklin V, Mak E, Liao X, Tabas I, Marcel YL. Autophagy regulates cholesterol efflux from macrophage foam cells via lysosomal acid lipase. Cell Metab. 2011;13:655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Burns K, Clatworthy J, Martin L, Martinon F, Plumpton C, Maschera B, Lewis A, Ray K, Tschopp J, Volpe F. Tollip, a new component of the IL‐1RI pathway, links IRAK to the IL‐1 receptor. Nat Cell Biol. 2000;2:346–351. [DOI] [PubMed] [Google Scholar]
- 6. Katoh Y, Shiba Y, Mitsuhashi H, Yanagida Y, Takatsu H, Nakayama K. Tollip and Tom1 form a complex and recruit ubiquitin‐conjugated proteins onto early endosomes. J Biol Chem. 2004;279:24435–24443. [DOI] [PubMed] [Google Scholar]
- 7. Baker B, Geng S, Chen K, Diao N, Yuan R, Xu X, Dougherty S, Stephenson C, Xiong H, Chu HW, Li L. Alteration of lysosome fusion and low‐grade inflammation mediated by super‐low‐dose endotoxin. J Biol Chem. 2015;290:6670–6678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shih SC, Prag G, Francis SA, Sutanto MA, Hurley JH, Hicke L. A ubiquitin‐binding motif required for intramolecular monoubiquitylation, the CUE domain. EMBO J. 2003;22:1273–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yamakami M, Yoshimori T, Yokosawa H. Tom1, a VHS domain‐containing protein, interacts with tollip, ubiquitin, and clathrin. J Biol Chem. 2003;278:52865–52872. [DOI] [PubMed] [Google Scholar]
- 10. Lu K, Psakhye I, Jentsch S. Autophagic clearance of polyQ proteins mediated by ubiquitin‐Atg8 adaptors of the conserved CUET protein family. Cell. 2014;158:549–563. [DOI] [PubMed] [Google Scholar]
- 11. Zhu L, Wang L, Luo X, Zhang Y, Ding Q, Jiang X, Wang X, Pan Y, Chen Y. Tollip, an intracellular trafficking protein, is a novel modulator of the transforming growth factor‐beta signaling pathway. J Biol Chem. 2012;287:39653–39663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Piao W, Song C, Chen H, Diaz MA, Wahl LM, Fitzgerald KA, Li L, Medvedev AE. Endotoxin tolerance dysregulates MyD88‐ and Toll/IL‐1R domain‐containing adapter inducing IFN‐beta‐dependent pathways and increases expression of negative regulators of TLR signaling. J Leukoc Biol. 2009;86:863–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Maitra U, Deng H, Glaros T, Baker B, Capelluto DG, Li Z, Li L. Molecular mechanisms responsible for the selective and low‐grade induction of proinflammatory mediators in murine macrophages by lipopolysaccharide. J Immunol. 2012;189:1014–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Maitra U, Davis S, Reilly CM, Li L. Differential regulation of Foxp3 and IL‐17 expression in CD4 T helper cells by IRAK‐1. J Immunol. 2009;182:5763–5769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Puri R, Suzuki T, Yamakawa K, Ganesh S. Dysfunctions in endosomal‐lysosomal and autophagy pathways underlie neuropathology in a mouse model for Lafora disease. Hum Mol Genet. 2012;21:175–184. [DOI] [PubMed] [Google Scholar]
- 16. Suzuki T, Sawada S, Ishigaki Y, Tsukita S, Kodama S, Sugisawa T, Imai J, Yamada T, Yamaguchi T, Murano T, Katagiri H. Lipoprotein lipase deficiency (R243H) in a type 2 diabetes patient with multiple arterial aneurysms. Intern Med. 2016;55:1131–1136. [DOI] [PubMed] [Google Scholar]
- 17. Didierlaurent A, Brissoni B, Velin D, Aebi N, Tardivel A, Kaslin E, Sirard JC, Angelov G, Tschopp J, Burns K. Tollip regulates proinflammatory responses to interleukin‐1 and lipopolysaccharide. Mol Cell Biol. 2006;26:735–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li T, Hu J, Li L. Characterization of Tollip protein upon lipopolysaccharide challenge. Mol Immunol. 2004;41:85–92. [DOI] [PubMed] [Google Scholar]
- 19. Geng S, Chen K, Yuan R, Peng L, Maitra U, Diao N, Chen C, Zhang Y, Hu Y, Qi CF, Pierce S, Ling W, Xiong H, Li L. The persistence of low‐grade inflammatory monocytes contributes to aggravated atherosclerosis. Nat Commun. 2016;7:13436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mehta NN, McGillicuddy FC, Anderson PD, Hinkle CC, Shah R, Pruscino L, Tabita‐Martinez J, Sellers KF, Rickels MR, Reilly MP. Experimental endotoxemia induces adipose inflammation and insulin resistance in humans. Diabetes. 2010;59:172–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim EJ, Kim BH, Seo HS, Lee YJ, Kim HH, Son HH, Choi MH. Cholesterol‐induced non‐alcoholic fatty liver disease and atherosclerosis aggravated by systemic inflammation. PLoS One. 2014;9:e97841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313:2263–2273. [DOI] [PubMed] [Google Scholar]
- 23. Hubscher SG. Histological assessment of non‐alcoholic fatty liver disease. Histopathology. 2006;49:450–465. [DOI] [PubMed] [Google Scholar]
- 24. Rensen SS, Bieghs V, Xanthoulea S, Arfianti E, Bakker JA, Shiri‐Sverdlov R, Hofker MH, Greve JW, Buurman WA. Neutrophil‐derived myeloperoxidase aggravates non‐alcoholic steatohepatitis in low‐density lipoprotein receptor‐deficient mice. PLoS One. 2012;7:e52411. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Toll‐interacting protein (Tollip) deficiency promotes the development of stable atherosclerotic plaques. A and B, Apolipoprotein E–deficient (ApoE−/−) and ApoE−/−/Tollip−/− mice (male, 8 weeks old) were fed with regular chow for an additional 8 weeks. A, Representative Oil Red O staining images within the aortic roots of ApoE−/− and ApoE−/−/Tollip−/− mice. B, Quantification of Oil Red O staining–positive area (mm2) of aortic roots. *P<0.05. Mann–Whitney U test.
Figure S2. Toll‐interacting protein (Tollip) deficiency does not lead to significant increase in aortic macrophages. Apolipoprotein E–deficient (ApoE−/−) and ApoE−/−/Tollip−/− mice (male, 8 weeks old) were fed with a high‐fat diet for an additional 8 weeks. Aortic sections were stained with monocyte/macrophage marker antibody (green) and DAPI (blue) to visualize aortic macrophages.
Figure S3. Toll‐interacting protein (Tollip) deficiency increases plasma levels of lipids. Apolipoprotein E–deficient (ApoE−/−) and ApoE−/−/Tollip−/− mice (male, 8 weeks old) were fed with a high‐fat diet for an additional 8 weeks. Plasma triglyceride, total cholesterol, and free cholesterol were measured. Error bars represent SEM. *P<0.05; **P<0.01; ***P<0.001. Mann–Whitney U test.
Figure S4. Toll‐interacting protein (Tollip) deficiency leads to elevated plaque levels of transforming growth factor‐β (TGF‐β). Apolipoprotein E–deficient (ApoE−/−) and ApoE−/−/Tollip−/− mice (male, 8 weeks old) were fed with a high‐fat diet for an additional 8 weeks. Aortic sections were stained with monocyte/macrophage marker antibody (green) and anti–transforming growth factor‐β. Error bars represent SEM. **P<0.01. Student t test.


