Abstract
Background
Small artery pathophysiology is frequently invoked as a cause of obesity‐related diastolic heart failure. However, evidence to support this hypothesis is scant, particularly in humans.
Methods and Results
To address this, we studied human small artery structure and function in obesity and looked for correlations between vascular parameters and diastolic function. Seventeen obese patients with metabolic syndrome and 5 control participants underwent echocardiography and subcutaneous gluteal fat biopsy. Small arteries were isolated from the biopsy and pressure myography was used to study endothelial function and wall structure. In comparison with the control group, small arteries from obese participants exhibited significant endothelial dysfunction, assessed as the vasodilatory response to acetylcholine and also pathological growth of the wall. For the obese participants, multiple regression analysis revealed an association between left atrial volume and both the small artery wall thickness (β=0.718, P=0.02) and wall‐to‐lumen ratio (β=0.605, P=0.02). Furthermore, the E:E′ ratio was associated with wall‐to‐lumen ratio (β=0.596, P=0.02) and inversely associated with interleukin‐6 (β=−0.868, P=0.03). By contrast, endothelial function did not correlate with any of the echocardiographic parameters studied.
Conclusions
Although the small arteries studied were not cardiac in origin, our results support a role for small artery remodeling in the development of diastolic dysfunction in humans. Further direct examination of the structure and function of the myocardial resistance vasculature is now warranted, to elucidate the temporal association between metabolic risk factors, small artery injury, and diastolic impairment.
Keywords: diastolic dysfunction, endothelial dysfunction, heart failure, obesity, vascular remodeling
Subject Categories: Vascular Biology, Endothelium/Vascular Type/Nitric Oxide, Metabolic Syndrome, Hypertension, Heart Failure
Introduction
Heart failure describes a clinical spectrum with a number of different etiologies. Traditionally, although the clinical focus has been on heart failure associated with reduced left ventricular ejection fraction, a second category of heart failure is recognized, in which systolic function is preserved but symptoms and complications still occur. The natural history of heart failure with preserved ejection fraction (HFpEF) describes a preclinical phase, in which there is diastolic dysfunction in the absence of symptoms.1 Imaging studies with echocardiography at this early stage of disease (Grade I or Ia) reveal characteristic patterns of impaired diastolic relaxation, with or without mild evidence of increased filling pressures. The reduction in left ventricular (LV) compliance leading to diastolic dysfunction causes progressive change in atrial morphology and function that represent early surrogate markers for HFpEF. Although our understanding of the causes of HFpEF is some years behind heart failure with reduced ejection fraction, epidemiological studies have shown that there are disproportionately high rates of diabetes mellitus, hypertension, and obesity in these patients.2, 3 This has become an area of intense interest, and a number of conceptual models have been proposed to explain the connection between these risk factors and the development of symptomatic heart failure.4 One such model is that microvascular disease underpins changes to the myocardium seen in HFpEF, through inflammatory and redox pathways.5 When considering the profound effect that these same cardiovascular6 and metabolic risk7 factors have on small artery structure and function,8 and consequently the prognostic relevance of these changes to target organ damage,9 positioning resistance vessels at the center of any mechanistic framework in HFpEF seems justified. However, there are currently no studies that have explored the relationship between small resistance arteries and the heart in the setting of human obesity. In contrast to the paucity of small artery studies, large artery‐to‐heart studies have yielded many valuable insights into the vascular origins of myocardial dysfunction,10 correlating aortic pulse wave velocity with left atrial remodeling in hypertensive patients11 or demonstrating an association between reductions in arterial compliance and progressively abnormal diastolic function in hypertensive patients with exertional dyspnea.12 Pathological small artery remodeling frequently precedes larger artery dysfunction and target organ damage. Furthermore, metabolic disease, especially diabetes mellitus and obesity, influences growth of the small artery wall in response to elevated blood pressures.8, 13 Thus, we hypothesized that alterations in small artery wall‐to‐lumen ratio and arterial wall hypertrophy would correlate with left atrial remodeling and diastolic impairment in patients with metabolic syndrome. We investigated this by performing echocardiography in patients with obesity and metabolic syndrome and pairing this with small artery structural and functional profiling of subcutaneous small arteries.
Methods
Study Population
Seventeen participants with National Cholesterol Education Program Adult Treatment Panel III defined metabolic syndrome14 and 5 control participants were recruited from a general medicine clinic and a day case programmed investigation unit at Manchester Royal Infirmary. All participants gave full written informed consent for the study, which was approved by the Local Research Ethics Committee. On the day of study, fasting venous blood samples were taken to assess renal function, glucose, and lipid profile in addition to the inflammatory markers: high‐sensitivity C‐reactive protein,tumor necrosis factor‐α (TNF‐α), and interleukin‐6 (IL‐6). Blood pressure was measured with subjects sitting, after 15 minutes of rest, by a semiautomatic machine (OMRON 705 CP, White Medical) with a mean of 3 readings recorded. Anthropometric measurements were also taken. Apart from hypertension or abnormalities of lipid metabolism, patients were excluded if diagnosed with chronic disease (eg, diabetes mellitus, heart, renal, or inflammatory disease).
Definition of Metabolic Syndrome and Insulin Resistance
The 2001 National Cholesterol Education Program Adult Treatment Panel III was used to classify patients for this study. The metabolic syndrome was defined as 3 or more of the following characteristics: central obesity as measured by a waist circumference >102 cm in men or 88 cm in women, triglycerides >1.7 mmol/L, high‐density lipoprotein cholesterol (<1.03 mmol/L in men or <1.29 mmol/L in women), fasting blood glucose >6.1 mmol/L, and hypertension (systolic >130 mm Hg or diastolic >85 mm Hg, or current use of antihypertensive drugs).14, 15
Pressure Myography
A single subcutaneous gluteal fat biopsy was obtained from each subject by using 3 to 5 mL of 2% lignocaine, allowing tissue (2×1.5×1.5 cm) to be harvested and placed immediately in ice‐cold physiological saline solution (PSS). Small arteries 100 to 150 μm in diameter were dissected from the tissue and carefully cleaned under a dissecting microscope. Isolated vessels were then transferred to an arteriographic bath chamber (Living Systems Instrumentation) and cannulated as described previously.16
The chamber was placed on the stage of an inverted microscope and superfused with PSS, gassed with 5% CO2/95% air (pH 7.4–7.45) at 37°C, at a superfusion rate of 20 mL/min. PSS composition was (mmol/L) 139 NaCl, 4.7 KCl, 25 NaHCO3, 1.17 KH2PO4, 1.17 MgSO4, 0.026 EDTA, 1.6 CaCl2, and 5.5 glucose. Lumen diameter was recorded with the use of a Video Dimension Analyser (Living Systems Instrumentations) connected to a chart recorder. Vessels were connected to a pressure servo system (Living Systems Instrumentation) and pressurized to 60 mm Hg; any vessel with a leak was discarded. Vessels were allowed to equilibrate to 37°C for 1 hour and then challenged with 60 mmol/L KPSS until a steady vasoconstriction was attained.
Pressure Myography: Pharmacological Assessment
After viability assessment with KPSS, each vessel was stimulated as follows: (1) Cumulative addition of norepinephrine (Sigma‐Aldrich), 10−9, 3×10−9, 10−8, 3×10−8, 10−7, 3×10−7, 10−6, 3×10−6, 10−5 mol/L with 3 to 5 minutes incubation per concentration. (2) Endothelial function was assessed via the cumulative response to acetylcholine (Sigma‐Aldrich) achieved by adding serial concentrations (mol/L) 10−9, 3×10−9, 10−8, 3×10−8, 10−7, 3×10−7, 10−6, 3×10−6, 10−5 to a preconstricted vessel with 10−5 norepinephrine. After 1 hour of incubation with 5×10−5 mol/L N G‐monomethyl‐l‐arginine (Sigma), a NO synthase inhibitor, the responses to acetylcholine were repeated as in step 2 above.
Pressure Myography: Passive Structure Measurement
The vessel was superfused for 20 minutes with Ca‐free PSS containing 2 mmol/L ethylene glycol‐bis (‐amino ethyl ether)‐N,N,N 1 ,N 1‐tetraacetic acid to ensure the vessels were devoid of active tone. To determine the structural properties of the arteries, the intraluminal pressure was reduced to 3 mm Hg to determine the unstressed diameter and then increased in steps to 20, 40, 60, 80, 100, 120, 140, 160, and 180 mm Hg.
Calculations
The wall/lumen ratio was calculated as WT/D×100, where WT is wall thickness and D is lumen diameter.
Wall cross‐sectional area (CSA) was calculated as:
Echocardiography
Echocardiographic studies were performed by a single operator (ML) who was blinded to the patient status at the time of the scan. Standard parasternal and apical imaging planes were obtained with commercially available echocardiographic equipment (Sonos 5500; Philips Medical Systems, Andover, MA). Images were stored as digital loops to optical disc for later analysis. Cardiac chamber quantification was performed according to American Society of Echocardiography guidelines.17 Left atrial volume was calculated using the modified Simpsons method in the 4‐ and 2‐chamber apical views at end‐systole. Transmitral pulsed wave Doppler was used to obtain early (E wave) and late (A wave) diastolic filling velocities and E/A ratio as a marker of diastolic function. Myocardial performance index was calculated as has been described previously.18 LV mass was calculated using the formula:
Tissue Doppler parameters were measured using pulsed‐wave tissue Doppler imaging with the sampling cursor positioned at the septal and lateral mitral valve annulus. Early (E′) and late (A′) myocardial diastolic velocities were measured and E/E′ ratio was calculated as a marker of left atrial pressure.
Statistical Analysis
Data are expressed as mean±SD. Comparison of characteristics between groups were made using the Mann–Whitney U test. Data for all diastolic parameters (mean tissue Doppler imaging E:A, mean E:E′, mean E:A and 2‐chamber left atrial volume were transformed using log base 10 to normalize skewed distributions. The independent variables for this study were therefore CSA, wall thickness, wall:lumen ratio, endothelial function, and high‐sensitivity C‐reactive protein. Multivariable linear regression models were used to investigate the influence of the independent variables on the variance of diastolic parameters. Stepwise model building was used to estimate the relative contribution of each independent variable and the variability of the diastolic parameters. All analyses were adjusted for age and sex. Test collinearity diagnostics indicated that CSA, wall thickness, and wall:lumen ratio could be used in the same model. The unstandardized (B±SE) coefficients are the coefficients of the estimated regression model. The standardized coefficients (β) are an attempt to make the regression coefficients more comparable. The change in R 2 (the coefficient of variations) is the increased percentage of the variation explained when each variable was added to the model. Statistical analysis was performed using SPSS statistical software (version 21; SPSS, Chicago, IL).
Results
Patient Demographics
A comparison of the demographic data between the metabolic syndrome group and controls is given in Table 1. Those with metabolic syndrome had significantly higher mean body mass index and leptin. The mean level of high‐density lipoprotein cholesterol was significantly lower in the metabolic syndrome group, while there was no significant difference in the mean level of triglycerides between the 2 groups.
Table 1.
Demographic Details of Participants With Metabolic Syndrome and Controls
| Characteristics | MetS (n=17) | Controls (n=5) | P Value |
|---|---|---|---|
| Age, y | 54.4±11.2 | 49.6±12.3 | 0.45 |
| Body mass index, kg/m2 | 33.3±4.8 | 25.0±2.7 | 0.001 |
| Systolic blood pressure, mm Hg | 139.8±14.6 | 140.4±19.3 | 0.94 |
| Diastolic blood pressure, mm Hg | 83.7±9.6 | 85.8±6.0 | 0.76 |
| Total cholesterol, mg/dL | 169.4±38.1 | 249.6±11.4 | 0.002 |
| HDL cholesterol, mg/dL | 39.9±8.8 | 57.7±9.4 | 0.001 |
| Total/HDL cholesterol ratio | 4.4±1.1 | 4.4±0.6 | 1.00 |
| Triglycerides, mg/dL | 138.0±51.9 | 116.6±23.7 | 0.36 |
| Fasting glucose, mg/dL | 109.6±15.9 | 92.6±8.9 | 0.10 |
| Heart rate, bpm | 66.5±9.7 | 59.2±5.2 | 0.12 |
| hsCRP, mg/L | 5.14±5.26 | 1.44±0.66 | 0.10 |
| Leptin, μg/L | 29.58±15.78 | 8.78±7.67 | 0.002 |
Baseline characteristics and differences between control subjects (n=5) and patients with metabolic syndrome (n=17). Values are mean±SD. HDL indicates high‐density lipoprotein; hsCRP, high‐sensitivity C‐reactive protein).
Echocardiography, Small Artery Structure and Function in Metabolic Syndrome
Endothelial function (Figure 1A) was measured as average maximal relaxation to acetylcholine. Those with metabolic syndrome had significantly impaired endothelial function compared with control participants (86.74±11.60% versus 98.70±4.31%, P=0.004). This level of damage is consistent with that seen in other clinical studies of small artery function in obesity. Endothelial function ranged from normal (99.2% of resting diameter after maximal acetylcholine concentrations) to significantly impaired (59.6% of resting diameter) in the metabolic syndrome group. Data were also collected on acetylcholine relaxations after 1‐hour incubation with a NOS inhibitor (N G‐monomethyl‐l‐arginine) and presented in the dose–response curve in Figure 1B. Of the small artery structural indices, mean wall thickness was significantly greater in patients with metabolic syndrome compared with control participants, consistent with previous studies. There were no significant differences in cross‐sectional area and wall‐to‐lumen ratio of the small arteries between the 2 groups (Table 2). There were no significant differences in left ventricular ejection fraction between the control group and patients with metabolic syndrome; however, diastolic parameters were deranged: the mean E:A ratio was significantly lower in the metabolic syndrome group (metabolic syndrome: 0.93±0.23 versus control participants: 1.19±0.17, P=0.048), representing impaired relaxation and grade I diastolic dysfunction. There were no significant differences in the lateral E:E′ ratio (8.90±4.17 in metabolic syndrome versus 5.73±1.68 in control participants, P=0.16). A value less than 8 corresponds with normal ventricular relaxation. LV mass was similar in both groups.
Figure 1.
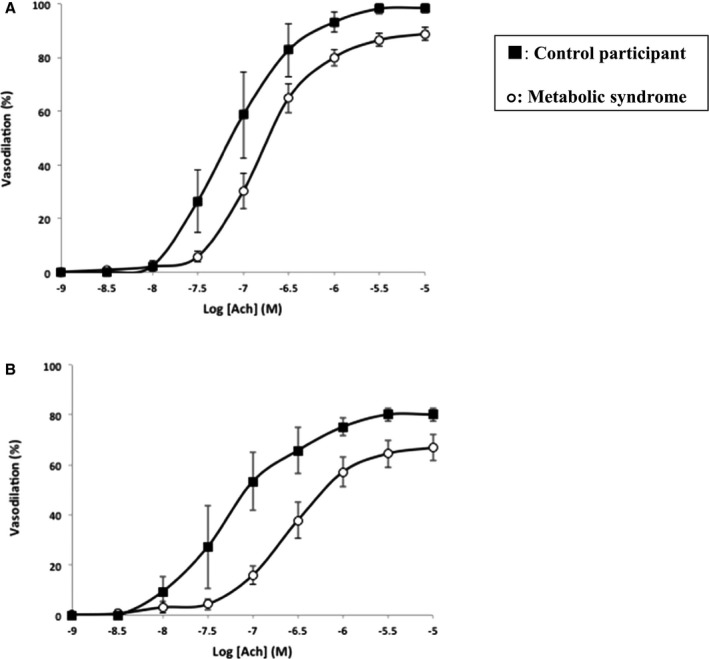
Assessment of endothelial function in patients with metabolic syndrome and controls. A, Effect of acetylcholine dilation on preconstricted small arteries from subcutaneous gluteal fat biopsy samples from control participants and patients with metabolic syndrome measured in a pressurized system. B, Acetylcholine relaxations after 1‐hour incubation with L‐NMMA in control participants and those with metabolic syndrome. l‐NMMA indicates N G‐monomethyl‐l‐arginine.
Table 2.
Small Artery Profile and Echocardiographic Examination of Patients With Metabolic Syndrome and Control Subjects
| Characteristics | Obese (n=17) | Controls (n=5) | P Value |
|---|---|---|---|
| Endothelial function | 86.74±11.60 | 98.70±4.31 | 0.08 |
| Endo l‐NMMA | 76.85±9.95 | 78.00±9.90 | 0.88 |
| CSA | 11 634.12±3454.51 | 9303.04±3122.23 | 0.54 |
| Wall:lumen ratio | 20.77±5.72 | 16.13±4.45 | 0.12 |
| Wall thickness | 24.79±5.06 | 19.90±4.79 | 0.07 |
| LV mass | 183.51±43.51 | 152.95±41.14 | 0.26 |
| LV mass/m2 | 88.42±22.26 | 83.51±15.22 | 0.75 |
| Mean LA volume | 52.81±16.77 | 52.84±11.27 | 0.94 |
| E:A ratio | 0.93±0.23 | 1.19±0.17 | 0.048 |
| Mean TDI E:A | 0.78±0.30 | 0.88±0.28 | 0.54 |
| Septal E:E′ | 11.16±3.72 | 9.63±2.67 | 0.49 |
| Lateral E:E′ | 8.90±4.17 | 5.73±1.68 | 0.16 |
Comparison of structural characteristics of small arteries from subcutaneous gluteal fat samples and echocardiographic assessment of diastolic parameters in patients with metabolic syndrome and healthy control participants. Values are mean±SD. CSA indicates cross‐sectional area; l‐NMMA, N G‐monomethyl‐l‐arginine; LA, left atrial; LV, left ventricle; TDI, tissue Dopper imaging.
Correlations Between Subcutaneous Small Artery Structure and Function and Cardiac Parameters in Patients With Metabolic Syndrome
As summarized in Table 3 and Figure 2, following adjustment for age and sex, a statistically significant association was found between 2‐chamber left atrial volume and both wall thickness (β=0.718, P=0.02) and wall‐to‐lumen ratio (β=0.605, P=0.02). In addition, wall‐to‐lumen ratio was also significantly associated with lateral E:E′ values (β=0.596, P=0.02). A significant inverse relationship was observed between lateral E:E′ and IL‐6 (β=−0.868, P=0.03). In contrast, no significant associations were observed between cardiac parameters and endothelial function, before or after incubation with N G‐monomethyl‐l‐arginine.
Table 3.
Multiple Regression Analysis Models for Explaining the Variance of Diastolic Parameters Among Individuals With Metabolic Syndrome
| Variable | β | B±SE | P Value | R 2 Change |
|---|---|---|---|---|
| 2C LA Vol | ||||
| CSA | 0.621 | 0.0002±0.000 | 0.11 | 0.196 |
| Wall thickness | 0.718 | 0.017±0.006 | 0.02 | 0.377 |
| Wall:lumen ratio | 0.605 | 0.014±0.005 | 0.02 | 0.347 |
| Endothelial function | 0.210 | 0.002±0.004 | 0.57 | 0.040 |
| Endo l‐NMMA | 0.001 | 0.0002±0.004 | 1.00 | 0.000 |
| hsCRP | 0.023 | 0.001±0.007 | 0.94 | 0.001 |
| TNF | 0.300 | 0.009±0.012 | 0.49 | 0.062 |
| IL‐6 | 0.127 | 0.003±0.010 | 0.789 | 0.009 |
| Lateral E:E′ | ||||
| CSA | 0.206 | 0.0001±0.000 | 0.60 | 0.023 |
| Wall thickness | 0.495 | 0.017±0.010 | 0.11 | 0.195 |
| Wall:lumen ratio | 0.596 | 0.019±0.007 | 0.02 | 0.352 |
| Endothelial function | 0.088 | 0.001±0.005 | 0.80 | 0.007 |
| Endo l‐NMMA | 0.143 | 0.003±0.005 | 0.59 | 0.017 |
| hsCRP | −0.026 | −0.001±0.010 | 0.93 | 0.001 |
| TNF | −0.527 | −0.020±0.012 | 0.12 | 0.239 |
| IL‐6 | −0.868 | −0.024±0.009 | 0.03 | 0.429 |
| E:A ratio | ||||
| CSA | 0.291 | −0.00001±0.000 | 0.14 | 0.046 |
| Wall thickness | 0.119 | 0.002±0.003 | 0.48 | 0.011 |
| Wall:lumen ratio | −0.045 | −0.001±0.003 | 0.77 | 0.002 |
| Endothelial function | 0.010 | 0.0001±0.002 | 0.96 | 0.000 |
| Endo l‐NMMA | −0.092 | −0.001±0.002 | 0.64 | 0.007 |
| hsCRP | 0.011 | 0.000±0.003 | 0.94 | 0.000 |
| TNF | −0.096 | −0.002±0.004 | 0.57 | 0.008 |
| IL‐6 | −0.018 | 0.000±0.004 | 0.93 | 0.000 |
Multiple regression analysis is shown using left atrial volume, E:A ratio, lateral E:E′ as outcome variables and cross‐sectional area, wall thickness, wall‐to‐lumen ratio, endothelial function, endothelial function in presence of l‐NMMA, highly sensitive CRP, TNF, and IL‐6 as explanatory variables. Correlations were adjusted for age and sex. B±SE represents unstandardized coefficients (coefficients of the estimated regression model). β represents standardized coefficients. R 2 represents coefficient of variations. Values are mean±SD. CSA indicates cross‐sectional area; IL‐6, interleukin 6; 2C LA Vol, 2‐chamber left atrial volume; hsCRP, highly sensitive C‐reactive protein; l‐NMMA, N G‐monomethyl‐l‐arginine; TNF, tumor necrosis factor.
Figure 2.
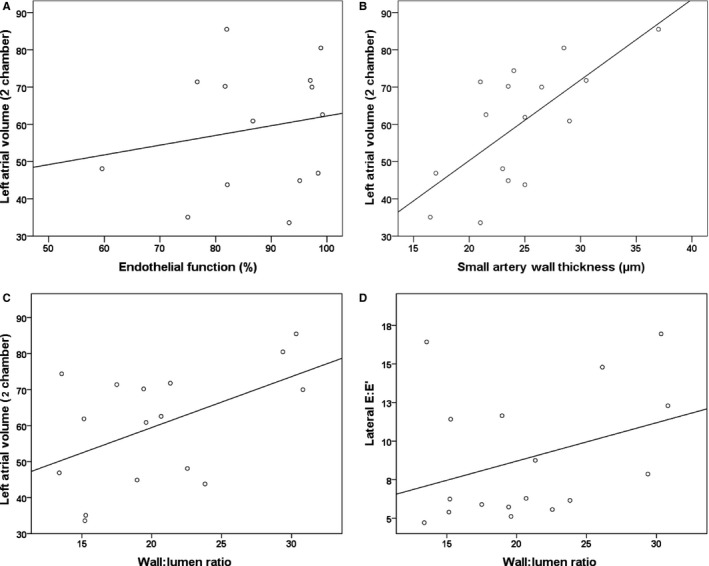
Correlation curves for small artery measurements against echocardiographic parameters. Correlation curves illustrating associations between (A) left atrial volume and endothelial function (r=0.210, P=0.57); (B) left atrial volume and small artery wall thickness (r=0.718, P=0.02); and (C) left atrial volume and small artery wall:lumen ratio (r=0.605, P=0.02); (D) lateral E:E′ and wall:lumen ratio (r=0.596, P=0.02).
Discussion
We herein report the first study to correlate small artery structural and functional indices with target organ diastolic impairment in patients with metabolic syndrome. Our most important observation was that in patients with metabolic syndrome, abnormal growth of the small artery wall was closely associated with the degree of diastolic dysfunction. By contrast, there was no correlation between the degree of endothelial damage and diastolic dysfunction, even though there was substantial impairment to the vasodilatory capacity of the vascular endothelium in obese patients. As such, our results lend weight to the increasingly held view that abnormal small artery remodeling is relevant to the pathogenesis of downstream target organ damage in obesity and type 2 diabetes mellitus.8, 19, 20
The microcirculation serves several critical functions, including autoregulation of hydrostatic pressures and control of local and systemic peripheral vascular resistance.21, 22 We examined small arteries of between 100 and 150 μm in lumen diameter, which form an important component of the resistance vasculature. The small artery profile, specifically the integrity of the myogenic response and structural changes to the arterial wall, are both robust prognostic parameters for subsequent target organ damage.9 Pathological alterations in subcutaneous small artery structure associate strongly with cardiovascular mortality,19, 23 particularly outward growth of the small artery wall.19 Obesity has a profound effect on both the structure and function of human subcutaneous small arteries: from a structural perspective, arteries exhibit an outward growth pattern with hypertrophy of the wall.24, 25 Inclusion of a small “control group” in our study served to indicate the small artery phenotype of the cohort with metabolic syndrome. This confirmed greater wall thickness in obese individuals. Other groups have described more pronounced hypertrophic changes in obesity26 and metabolic syndrome,25 with increased wall‐to‐lumen ratio and cross‐sectional areas when compared with lean individuals. Detailed assessment of structural changes in metabolic syndrome versus health was not an aim of the current study, but previous investigations from our group and others have demonstrated that a number of metabolic factors contribute to wall hypertrophy24 and that this is a dynamic process that is reversible with effective metabolic and blood pressure improvements.8, 24 Structural changes of the arterial wall therefore represent a spectrum of progressive change, which are influenced by both lifestyle and drug therapy. Given the heterogeneous nature of metabolic syndrome, there will naturally be pathophysiological variations between different cohorts, and we have therefore provided detailed characterization of our study group in the patient demographics.
Functionally, our ex vivo findings demonstrated reductions in acetylcholine‐induced vasodilation and thickening of the small artery wall in the obese patients. Echocardiography in obese participants also demonstrated lower E:A ratios compared with healthy participants, as described previously. Changes in the E:A ratio reflect elevated filling pressures, which in turn reduce the filling velocity, reflecting early diastolic disease. However, transmitral Doppler patterns such as the E:A ratio are sensitive to preload and therefore display a nonlinear progression in diastolic disease (“pseudonormalization”). By comparison, assessment with tissue Doppler imaging is less load dependent and as such is a more linear and reliable measure of progressive diastolic impairment. Here, E′ reflects the velocity of early myocardial relaxation as the mitral annulus ascends during early rapid LV filling. Thus, an E:E′ ratio (taking lateral annular values) <8 is normal, and values >10 correlate with elevated LV end‐diastolic pressures.27
The primary aim of the study was to test the hypothesis that increases in small artery wall thickness and wall‐to‐lumen ratio are associated with impaired diastolic indices in patients with metabolic syndrome. In this regard, the major observation from this study was that lateral E:E′ values significantly correlated with subcutaneous small artery wall‐to‐lumen ratio, even after adjustment for age and sex. The association of diastolic dysfunction with abnormal small artery remodeling was further strengthened by significant correlations between left atrial volume and both small artery wall thickness and the wall‐to‐lumen ratio. Left atrial dilatation is invariably present in heart failure, irrespective of ejection fraction,28 and it is established that left atrial volume increases proportionally with the severity of diastolic dysfunction, independent of comorbid risks.29 As such, left atrial volume is felt to be one of the most sensitive and specific measures to differentiate between HFpEF and hypertensive changes to the heart.28 In contrast, there were no significant relationships between the endothelial‐derived vasodilatory capacity of the artery and cardiac function. Our study is small and not powered to conclusively rule out relationships between small artery indices where no significance is seen. However, the lack of association between diastolic parameters and endothelial vasodilation is consistent with existing literature.30
As a driver for progressive cardiovascular dysfunction in obesity, inflammation is undoubtedly key.5 Specifically in regard to HFpEF, Paulus has recently proposed that obesity‐related inflammation and subsequent overproduction of reactive oxygen species limits local NO bioavailability within cardiomyocytes inducing dysfunction.5 Cytokines such as TNF‐α have been reported to disrupt NO bioavailability in obesity,31 which led us to assess systemic inflammation by measuring circulating TNF and IL‐6. Although no associations were identified between TNF‐α or high‐sensitivity C‐reactive protein and any of the echo parameters, there was a significant inverse relationship observed between lateral E:E′ and IL‐6 (β=−0.868, P=0.03). IL‐6 exhibits complex biology: In addition to inflammatory processes, IL‐6 is widely recognized for its the role in the regulation of metabolic, regenerative, and neural processes.32 Indeed, IL‐6 is released by skeletal muscle and has well‐described regenerative and anti‐inflammatory activities, working to inhibit cytokines such as TNF‐α.33 Furthermore, IL‐6−/− mice develop late‐onset obesity,34 glucose intolerance, and insulin resistance.35 Against this background, the inverse relationship between E:E′ and IL‐6 may provide insights into the functional pleiotropy of IL‐6 and its role in anti‐inflammatory processes in skeletal muscle.
Taken in totality, the associations observed between left atrial volume, the E:E′ ratio, and the abnormal wall growth of small arteries in patients with metabolic syndrome support an association between small artery dysfunction and diastolic heart failure. The interpretation of our results within the hypothesis outlined above rests on an implied assumption of small artery pathology; namely, that processes occurring in subcutaneous small arteries mirror those in the myocardium. As such the findings are associative, but previous studies have shown that structural and functional changes that occur in subcutaneous small arteries in response to hypertension and diabetes mellitus36 are mirrored in mesenteric,37 coronary,38 and cerebral39 arteries. Also, abnormal structure of small retinal arteries in patients with type 1 diabetes mellitus predicts not only retinopathy20 but also nephropathy,40 suggestive of a generalized microvascular burden. Although we have presented correlation analyses in graphical form, our adjusted logistic regression models validly describe the association between small vessel morphology with respect to CSA, wall thickness, wall‐to‐lumen ratio, and endothelial function, with corresponding echo parameters. In the absence of follow‐up on small artery morphology at baseline and incident changes in echo parameters over time, which is prohibitively challenging because of repeated invasive biopsies required, it would not be possible to imply causation or anything beyond an association. Although this might be considered a limitation in study design, direct interrogation of human arterial tissue samples provides mechanistic insights beyond that afforded by biomarker studies, where association is again an inherent limitation.
Patients with preclinical diastolic dysfunction and diabetes mellitus have more than double the rates of progression to symptomatic heart failure and mortality compared with those without diabetes mellitus.41 From a translational perspective, it is therefore important to examine the temporal link between development of risk factors, functional changes to small arteries, and myocardial dysfunction, to determine the sequence of events that ultimately lead to symptomatic heart failure and premature death in these patients. The data from this study suggest that manipulation of these molecular targets within small arteries may be relevant to the treatment of diastolic heart failure.
Sources of Funding
Khavandi acknowledges financial support from the Department of Health via the National Institute for Health Research (NIHR) comprehensive Biomedical Research Centre award to the Guy's and St Thomas' NHS Foundation Trust in partnership with King's College London and King's College Hospital NHS Foundation Trust, and is the recipient of a British Heart Foundation Clinical Research Training Fellowship award (FS/11/45/28859). He also receives funding from the NIHR through an Academic Clinical Fellowship award. Greenstein acknowledges funding support from the British Heart Foundation (FS/12/81/29882, FS/11/68/28821, IG/13/4/30317, FS/14/26/30767, PG/15/109/31931, FS/10/042/28372).
Disclosures
None.
Acknowledgments
We gratefully acknowledge the contribution of the nursing and administrative staff of the Manchester NIHR/Wellcome Trust Clinical Research Facility at Central Manchester Teaching Hospitals Foundation Trust who coordinated the study.
(J Am Heart Assoc. 2017;6:e004603 DOI: 10.1161/JAHA.116.004603.)28400366
References
- 1. Wan SH, Vogel MW, Chen HH. Pre‐clinical diastolic dysfunction. J Am Coll Cardiol. 2014;63:407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abhayaratna WP, Marwick TH, Smith WT, Becker NG. Characteristics of left ventricular diastolic dysfunction in the community: an echocardiographic survey. Heart. 2006;92:1259–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Redfield MM, Jacobsen SJ, Burnett JC Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. [DOI] [PubMed] [Google Scholar]
- 4. Komajda M, Lam CS. Heart failure with preserved ejection fraction: a clinical dilemma. Eur Heart J. 2014;35:1022–1032. [DOI] [PubMed] [Google Scholar]
- 5. Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. [DOI] [PubMed] [Google Scholar]
- 6. Heagerty AM, Aalkjaer C, Bund SJ, Korsgaard N, Mulvany MJ. Small artery structure in hypertension. Dual processes of remodeling and growth. Hypertension. 1993;21:391–397. [DOI] [PubMed] [Google Scholar]
- 7. Alexander CM, Landsman PB, Teutsch SM, Haffner SM. NCEP‐defined metabolic syndrome, diabetes, and prevalence of coronary heart disease among NHANES III participants age 50 years and older. Diabetes. 2003;52:1210–1214. [DOI] [PubMed] [Google Scholar]
- 8. Greenstein AS, Price A, Sonoyama K, Paisley A, Khavandi K, Withers S, Shaw L, Paniagua O, Malik RA, Heagerty AM. Eutrophic remodeling of small arteries in type 1 diabetes mellitus is enabled by metabolic control: a 10‐year follow‐up study. Hypertension. 2009;54:134–141. [DOI] [PubMed] [Google Scholar]
- 9. Rizzoni D, Porteri E, Boari GE, De Ciuceis C, Sleiman I, Muiesan ML, Castellano M, Miclini M, Agabiti‐Rosei E. Prognostic significance of small‐artery structure in hypertension. Circulation. 2003;108:2230–2235. [DOI] [PubMed] [Google Scholar]
- 10. Russo C, Jin Z, Palmieri V, Homma S, Rundek T, Elkind MS, Sacco RL, Di Tullio MR. Arterial stiffness and wave reflection: sex differences and relationship with left ventricular diastolic function. Hypertension. 2012;60:362–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lantelme P, Laurent S, Besnard C, Bricca G, Vincent M, Legedz L, Milon H. Arterial stiffness is associated with left atrial size in hypertensive patients. Arch Cardiovasc Dis. 2008;101:35–40. [DOI] [PubMed] [Google Scholar]
- 12. Mottram PM, Haluska BA, Leano R, Carlier S, Case C, Marwick TH. Relation of arterial stiffness to diastolic dysfunction in hypertensive heart disease. Heart. 2005;91:1551–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Endemann DH, Pu Q, De Ciuceis C, Savoia C, Virdis A, Neves MF, Touyz RM, Schiffrin EL. Persistent remodeling of resistance arteries in type 2 diabetic patients on antihypertensive treatment. Hypertension. 2004;43:399–404. [DOI] [PubMed] [Google Scholar]
- 14. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA. 2001;285:2486–2497. [DOI] [PubMed] [Google Scholar]
- 15. Yates AP, Laing I. Age‐related increase in haemoglobin A1c and fasting plasma glucose is accompanied by a decrease in beta cell function without change in insulin sensitivity: evidence from a cross‐sectional study of hospital personnel. Diabet Med. 2002;19:254–258. [DOI] [PubMed] [Google Scholar]
- 16. Halpern W, Osol G, Coy GS. Mechanical behavior of pressurized in vitro prearteriolar vessels determined with a video system. Ann Biomed Eng. 1984;12:463–479. [DOI] [PubMed] [Google Scholar]
- 17. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. [DOI] [PubMed] [Google Scholar]
- 18. Arnlov J, Ingelsson E, Riserus U, Andren B, Lind L. Myocardial performance index, a Doppler‐derived index of global left ventricular function, predicts congestive heart failure in elderly men. Eur Heart J. 2004;25:2220–2225. [DOI] [PubMed] [Google Scholar]
- 19. Izzard AS, Rizzoni D, Agabiti‐Rosei E, Heagerty AM. Small artery structure and hypertension: adaptive changes and target organ damage. J Hypertens. 2005;23:247–250. [DOI] [PubMed] [Google Scholar]
- 20. Wong TY, Islam FM, Klein R, Klein BE, Cotch MF, Castro C, Sharrett AR, Shahar E. Retinal vascular caliber, cardiovascular risk factors, and inflammation: the Multi‐Ethnic Study of Atherosclerosis (MESA). Invest Ophthalmol Vis Sci. 2006;47:2341–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Intengan HD, Schiffrin EL. Structure and mechanical properties of resistance arteries in hypertension: role of adhesion molecules and extracellular matrix determinants. Hypertension. 2000;36:312–318. [DOI] [PubMed] [Google Scholar]
- 22. Levy BI, Ambrosio G, Pries AR, Struijker‐Boudier HA. Microcirculation in hypertension: a new target for treatment? Circulation. 2001;104:735–740. [DOI] [PubMed] [Google Scholar]
- 23. De Ciuceis C, Porteri E, Rizzoni D, Rizzardi N, Paiardi S, Boari GE, Miclini M, Zani F, Muiesan ML, Donato F, Salvetti M, Castellano M, Tiberio GA, Giulini SM, Agabiti Rosei E. Structural alterations of subcutaneous small‐resistance arteries may predict major cardiovascular events in patients with hypertension. Am J Hypertens. 2007;20:846–852. [DOI] [PubMed] [Google Scholar]
- 24. De Ciuceis C, Porteri E, Rizzoni D, Corbellini C, La Boria E, Boari GE, Pilu A, Mittempergher F, Di Betta E, Casella C, Nascimbeni R, Rosei CA, Ruggeri G, Caimi L, Rosei EA. Effects of weight loss on structural and functional alterations of subcutaneous small arteries in obese patients. Hypertension. 2011;58:29–36. [DOI] [PubMed] [Google Scholar]
- 25. Grassi G, Seravalle G, Brambilla G, Facchetti R, Bolla G, Mozzi E, Mancia G. Impact of the metabolic syndrome on subcutaneous microcirculation in obese patients. J Hypertens. 2010;28:1708–1714. [DOI] [PubMed] [Google Scholar]
- 26. Grassi G, Seravalle G, Scopelliti F, Dell'Oro R, Fattori L, Quarti‐Trevano F, Brambilla G, Schiffrin EL, Mancia G. Structural and functional alterations of subcutaneous small resistance arteries in severe human obesity. Obesity (Silver Spring). 2010;18:92–98. [DOI] [PubMed] [Google Scholar]
- 27. Ho CY, Solomon SD. A clinician's guide to tissue Doppler imaging. Circulation. 2006;113:e396–e398. [DOI] [PubMed] [Google Scholar]
- 28. Gottdiener JS, Kitzman DW, Aurigemma GP, Arnold AM, Manolio TA. Left atrial volume, geometry, and function in systolic and diastolic heart failure of persons > or =65 years of age (the Cardiovascular Health Study). Am J Cardiol. 2006;97:83–89. [DOI] [PubMed] [Google Scholar]
- 29. Tsang TS, Barnes ME, Gersh BJ, Takemoto Y, Rosales AG, Bailey KR, Seward JB. Prediction of risk for first age‐related cardiovascular events in an elderly population: the incremental value of echocardiography. J Am Coll Cardiol. 2003;42:1199–1205. [DOI] [PubMed] [Google Scholar]
- 30. Brooks BA, Franjic B, Ban CR, Swaraj K, Yue DK, Celermajer DS, Twigg SM. Diastolic dysfunction and abnormalities of the microcirculation in type 2 diabetes. Diabetes Obes Metab. 2008;10:739–746. [DOI] [PubMed] [Google Scholar]
- 31. Virdis A, Santini F, Colucci R, Duranti E, Salvetti G, Rugani I, Segnani C, Anselmino M, Bernardini N, Blandizzi C, Salvetti A, Pinchera A, Taddei S. Vascular generation of tumor necrosis factor‐alpha reduces nitric oxide availability in small arteries from visceral fat of obese patients. J Am Coll Cardiol. 2011;58:238–247. [DOI] [PubMed] [Google Scholar]
- 32. Scheller J, Chalaris A, Schmidt‐Arras D, Rose‐John S. The pro‐and anti‐inflammatory properties of the cytokine interleukin‐6. Biochim Biophys Acta. 2011;1813:878–888. [DOI] [PubMed] [Google Scholar]
- 33. Pedersen BK, Steensberg A, Schjerling P. Muscle‐derived interleukin‐6: possible biological effects. J Physiol. 2001;536:329–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wallenius V, Wallenius K, Ahrén B, Rudling M, Carlsten H, Dickson SL, Ohlsson C, Jansson J‐O. Interleukin‐6‐deficient mice develop mature‐onset obesity. Nat Med. 2002;8:75–79. [DOI] [PubMed] [Google Scholar]
- 35. Matthews V, Allen T, Risis S, Chan M, Henstridge D, Watson N, Zaffino L, Babb J, Boon J, Meikle P. Interleukin‐6‐deficient mice develop hepatic inflammation and systemic insulin resistance. Diabetologia. 2010;53:2431–2441. [DOI] [PubMed] [Google Scholar]
- 36. Schofield I, Malik R, Izzard A, Austin C, Heagerty A. Vascular structural and functional changes in type 2 diabetes mellitus: evidence for the roles of abnormal myogenic responsiveness and dyslipidemia. Circulation. 2002;106:3037–3043. [DOI] [PubMed] [Google Scholar]
- 37. Tahvanainen A, Taurio J, Maki‐Jouppi J, Koobi P, Mustonen J, Kahonen M, Sand J, Nordback I, Porsti I. Increased wall tension in response to vasoconstrictors in isolated mesenteric arterial rings from patients with high blood pressure. Basic Clin Pharmacol Toxicol. 2006;99:440–449. [DOI] [PubMed] [Google Scholar]
- 38. Jenkins JT, Boyle JJ, McKay IC, Richens D, McPhaden AR, Lindop GB. Vascular remodelling in intramyocardial resistance vessels in hypertensive human cardiac transplant recipients. Heart. 1997;77:353–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rizzoni D, De Ciuceis C, Porteri E, Paiardi S, Boari GE, Mortini P, Cornali C, Cenzato M, Rodella LF, Borsani E, Rizzardi N, Platto C, Rezzani R, Rosei EA. Altered structure of small cerebral arteries in patients with essential hypertension. J Hypertens. 2009;27:838–845. [DOI] [PubMed] [Google Scholar]
- 40. Wong TY, Shankar A, Klein R, Klein BE. Retinal vessel diameters and the incidence of gross proteinuria and renal insufficiency in people with type 1 diabetes. Diabetes. 2004;53:179–184. [DOI] [PubMed] [Google Scholar]
- 41. From AM, Scott CG, Chen HH. The development of heart failure in patients with diabetes mellitus and pre‐clinical diastolic dysfunction a population‐based study. J Am Coll Cardiol. 2010;55:300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]


