Abstract
Background
Patients with recent non–ST‐segment elevation myocardial infarction commonly have heterogeneous characteristics that may be challenging to assess clinically.
Methods and Results
We prospectively studied the diagnostic accuracy of 2 novel (T1, T2 mapping) and 1 established (T2‐weighted short tau inversion recovery [T2W‐STIR]) magnetic resonance imaging methods for imaging the ischemic area at risk and myocardial salvage in 73 patients with non–ST‐segment elevation myocardial infarction (mean age 57±10 years, 78% male) at 3.0‐T magnetic resonance imaging within 6.5±3.5 days of invasive management. The infarct‐related territory was identified independently using a combination of angiographic, ECG, and clinical findings. The presence and extent of infarction was assessed with late gadolinium enhancement imaging (gadobutrol, 0.1 mmol/kg). The extent of acutely injured myocardium was independently assessed with native T1, T2, and T2W‐STIR methods. The mean infarct size was 5.9±8.0% of left ventricular mass. The infarct zone T1 and T2 times were 1323±68 and 57±5 ms, respectively. The diagnostic accuracies of T1 and T2 mapping for identification of the infarct‐related artery were similar (P=0.125), and both were superior to T2W‐STIR (P<0.001). The extent of myocardial injury (percentage of left ventricular volume) estimated with T1 (15.8±10.6%) and T2 maps (16.0±11.8%) was similar (P=0.838) and moderately well correlated (r=0.82, P<0.001). Mean extent of acute injury estimated with T2W‐STIR (7.8±11.6%) was lower than that estimated with T1 (P<0.001) or T2 maps (P<0.001).
Conclusions
In patients with non–ST‐segment elevation myocardial infarction, T1 and T2 magnetic resonance imaging mapping have higher diagnostic performance than T2W‐STIR for identifying the infarct‐related artery. Compared with conventional STIR, T1 and T2 maps have superior value to inform diagnosis and revascularization planning in non–ST‐segment elevation myocardial infarction.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT02073422.
Keywords: acute coronary syndrome, area at risk, edema, mapping, noninvasive imaging, non–ST‐segment elevation acute coronary syndrome
Subject Categories: Magnetic Resonance Imaging (MRI)
Introduction
Following acute myocardial infarction (MI), MRI techniques have been shown to retrospectively estimate the ischemic area at risk (AAR), that is, the myocardial perfusion bed directly affected by ischemia because of acute occlusion of a coronary artery.1 Furthermore, as a result of even transient ischemia producing differences in the myocardial longitudinal (T1) and transverse (T2) relaxation times, newer techniques have shown improved sensitivity in identifying myocardial injury.2, 3 Dark‐blood fat‐suppressed (short tau inversion recovery [STIR]) T2‐weighted MRI methods (T2W‐STIR) are widely used for clinical and research purposes.4, 5, 6, 7 T2W‐STIR, however, is prone to artifacts from motion, blood–tissue borders, and coil sensitivity issues that cause signal loss with depth of field8, 9 and may lead to diagnostic uncertainty. Novel fast T1 and T2 parametric methods for mapping myocardial injury have been described in patients with acute coronary syndrome—predominantly in patients with ST‐segment elevation MI (STEMI). In these cohorts, both T1 and T2 mapping techniques demonstrated superior accuracy compared with T2W‐STIR.3, 10 Given that edema may persist for days or weeks after acute ischemic injury, STEMI patients have generally been a focus for research with MRI following revascularization. Non‐STEMI (NSTEMI) is a condition typified by smaller degrees of myocardial injury and nonocclusive culprit lesions. Compared with STEMI, the clinical utility of identifying the territory of acute injury in NSTEMI patients may be enhanced because NSTEMI is a more heterogeneous condition associated with diagnostic uncertainty,11 and MRI can be performed before a decision to proceed to an invasive management strategy. Nevertheless, data allowing direct comparison of T1 and T2 mapping sequences and T2W‐STIR in NSTEMI, particularly at 3.0 T, are lacking and may not necessarily be congruent with findings in the STEMI cohorts.
We performed a prospective study of medically stabilized NSTEMI patients referred for coronary angiography to compare the diagnostic accuracy of T1 and T2 mapping compared with T2‐weighted dark‐blood edema imaging (T2W‐STIR) for detection of the infarct‐related artery (IRA). We also compared these MRI methods for estimation of the angiographic ischemic AAR.
Methods
Patient Population
Patients with a diagnosis of type 1 NSTEMI12 with at least 1 cardiac risk factor scheduled for early coronary angiography were prospectively recruited from 2 cardiac institutions. Exclusion criteria represented standard contraindications to MRI, including metallic devices and severe kidney disease (an estimated glomerular filtration rate <30 mL/min per 1.73 m2). The participants in this study were enrolled in the Fractional Flow Reserve Versus Angiographically Guided Management to Optimise Outcomes in Unstable Coronary Syndromes (FAMOUS NSTEMI) clinical trial, a multicenter study examining the utility of fractional flow reserve use in this population (ClinicalTrials.gov identifier NCT02073422). Only patients enrolled in centers with access to cardiac MRI (CMR) were approached to participate. All patients provided written informed consent. The local ethics board approved the study. Pharmacological management of patients reflected contemporary guidelines including treatment with dual‐antiplatelet therapy, an HMG‐CoA enzyme inhibitor, and a beta blocker.13 Troponin checks were routinely performed before and 12 hours after the procedure to assess for procedure‐related (type 4a) MI.
MRI Acquisition
MRI was performed on a Siemens MAGNETOM Verio 3.0‐T scanner with an 8‐element phased‐array cardiac surface coil. The MRI protocol included steady‐state free procession cine MRI, T1 and T2 parametric maps10, and T2W‐STIR before gadolinium contrast administration. Cine steady‐state free procession images with 2‐fold accelerated parallel imaging (GRAPPA [generalized autocalibrating partially parallel acquisition]) were acquired in a stack of short‐axis views of the left ventricle. Shimming and a radio frequency localizer were used at the outset of the scan to optimize data quality. Imaging parameters were repetition time 3.4 ms, echo time 1.51 ms, flip angle 50°, typical field of view 340×286 mm2, matrix 256×216, slice thickness 7 mm, slice gap 3 mm, receiver bandwidth 977 Hz/pixel, 25 cardiac phases. Late gadolinium enhancement (LGE) CMR was performed with a T1‐weighted, segmented, gradient echo, phase‐sensitive inversion recovery sequence, with following parameters: echo time 760 ms, repetition time 1.56 ms, flip angle 20°. The inversion time was adjusted for optimal suppression of signal from normal myocardium (inversion time ≈340 ms). Typical field of view was 350×262 mm2, matrix 256×192, slice thickness 7 mm, slice gap 2.8 mm, and bandwidth 465 Hz/pixel. Images were collected 10 to 15 minutes after the administration of 0.1 mmol/kg of contrast agent (Gadovist; Bayer).
The ECG‐gated single‐shot modified Look‐Locker inversion recovery (MOLLI) method (3[3]3[3]5 protocol) was used to measure myocardial longitudinal relaxation times (T1).14, 15 The MOLLI parameters included a T1 start of 100 ms, an increment of 80 ms, and a trigger delay of 160 ms. The protocol included a GRAPPA acceleration factor of 2. Following pixel‐wise T1 fitting, T1 (ms) was displayed on a quantitative, color, scaled map.
Myocardial transverse (T2) relaxation times were estimated using a T2 mapping technique that involved a T2 prepared True Fast Imaging with Steady state Precession (TrueFISP) pulse sequence to produce single‐shot T2 prepared images, each with different T2 preparation times.16 The T2 prepared TrueFISP images were acquired at intervals of at least 2 R‐R intervals to allow for sufficient magnetization recovery in between acquisitions. Motion correction was enabled to prevent misregistration between images. T2 was estimated by pixel‐wise fitting assuming monoexponential signal decay, and a color, scaled, motion‐corrected myocardial T2 map was then generated. For both T1 and T2 maps, an algorithm for motion correction based on previous work was applied before curve fitting.17
The breath‐hold black‐blood triple inversion recovery sequence (T2W‐STIR) involves a pair of selective and nonselective 180° inversion pulses to null the blood pool signal followed by a third inversion pulse (STIR) to null the fat signal.4 Surface coil intensity correction was routinely performed with prescan normalization and slice‐related shimming, as appropriate. Typical imaging parameters were echo time of 2 R‐R intervals, repetition time of 47 ms, and flip angle of 180°, with 310 Hz/pixel bandwidth and turbo factor 15. In‐plane spatial resolution was 2.2×1.4 mm (with acquisition matrix of 151×256, GRAPPA=2), and slice thickness was 6 mm. Short‐axis left ventricular (LV) views were sequentially acquired with T1/T2 parametric maps and T2W‐STIR matched to the same slice position. We obtained 3 slices: basal, mid, and apical. Sample images are shown in Figure 1.
Figure 1.
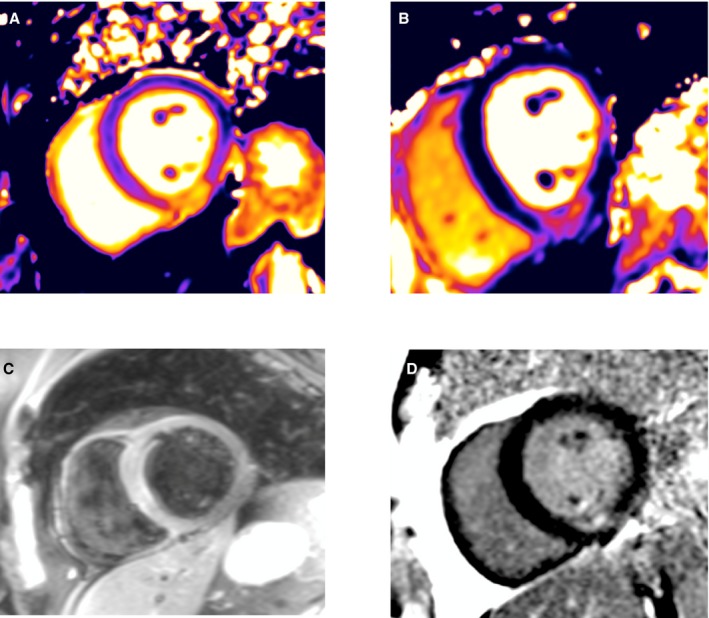
Magnetic resonance imaging findings in a 62‐year‐old male patient 4 days after hospitalization because of an acute non–ST‐segment elevation myocardial infarction: (A) T2 map, (B) T2‐weighted short tau inversion recovery (T2W‐STIR), (C) T1 map, (D) late gadolinium enhancement (LGE). Inferior subendocardial infarction, as revealed by LGE, corresponds with the transmural extent of myocardial injury revealed with T1 and T2 maps, but this is not seen with T2W‐STIR. Conversely, the anterior wall is hyperintense with T2W‐STIR.
MRI Analysis
Anonymized images were analyzed in random order on a Siemens workstation by 2 MRI‐trained cardiologists with >3 years experience with imaging myocardial edema. An image analyst coordinated data management to ensure blinded analysis. A third MRI‐trained cardiologist and an MRI‐trained technician performed quantitative assessments of infarct area and AAR as determined by both T1 and T2 maps.
Two MRI‐trained cardiologists who were blind to the patients' clinical presentation reviewed hyperintense zones on T1‐ and T2‐weighted MRI images independently. Specific edema imaging sequences were analyzed randomly and independently from each other to avoid observer bias. Each observer assessed for the presence or absence of edema in each short‐axis slice and ascribed it to a segment in accordance with the 16‐segment model of the American Heart Association. A third observer resolved discordance between cardiologists. Each observer was also asked to assign a culprit coronary arterial territory for each patient based on each edema imaging modality. In addition, each observer was asked to categorize overall image quality (nondiagnostic, poor, adequate, good, or excellent) and to comment on the presence or absence of artifacts for each edema imaging sequence. An artifact was recorded only if there was concordance between 2 of the 3 observers.
The jeopardized myocardial territory of acute injury was defined as the percentage of LV area delineated by the hyperintense zone on T1 and T2 images. The signal intensity threshold indicating injured myocardium was set at 2 SD above the mean intensity of reference (region of interest) placed in remote unaffected myocardium (180° from the affected zone with no visible evidence of infarction, edema, or wall motion abnormalities).18, 19 Myocardial salvage was calculated by subtraction of the percentage of infarct size from the percentage of the area of acute injury.18 The extent of infarct scar was delineated as an area of myocardial enhancement (cm2) mapped on LGE images, using a signal intensity threshold of >5 SD above remote region and expressed as a percentage of total LV mass.20, 21 Microvascular obstruction (MVO) was defined as a dark zone present within an area of gadolinium enhancement. Infarct regions with evidence of MVO were included within the infarct area, and MVO area was also recorded individually as a percentage of total LV mass.
Angiographic Analysis
The attending interventional cardiologist used a combination of clinical history, ECG, and coronary angiographic findings to identify the culprit coronary artery. Culprit artery assignment was independently analyzed and verified by an accredited interventional cardiologist (M.M.C.). The Alberta Provincial Project for outcome Assessment in Coronary Heart Disease (APPROACH) lesion score was used to provide estimates of the AAR.22 In addition, all angiograms were independently analyzed to assess plaque characteristics by a core lab (J.C., V.T.).
Statistical Analyses
Statistical analyses were carried out using IBM SPSS Statistics software, version 21.0. Normality was tested with the Shapiro–Wilk test. All results are given as mean±SD unless otherwise stated. Correlations between AAR were quantified with T1/T2 maps and T2W‐STIR. APPROACH lesion scores were tested by Pearson or Spearman methods, as appropriate. Comparisons of normally distributed continuous data between T1/T2 maps and T2W‐STIR were undertaken using a Student t test. Between‐group comparisons of nonnormally distributed data were performed with a Mann–Whitney test. The level of agreement between the myocardial territory of acute injury was quantified with T1/T2 maps, and T2W‐STIR was assessed using Bland–Altman plots and 95% limits of agreement. The 95% limits of agreement were calculated using the mean difference between areas of acute injury quantified by the 2 imaging modalities ±2 SD of these differences and contained ≈95% of all such differences. The McNemar exact test was used to compare accuracy of the T1, T2, and T2W‐STIR sequences in identifying the IRA compared with the current gold standard method of coronary angiography. In addition, binomial tests (with 95% CIs calculated using the likelihood ratio) were also used to determine how well the novel mapping techniques correctly identified the IRA compared with other methods such as ECG, wall motion abnormalities on MRI, and LGE. To compare the degree of overlap between remote and injured zones for each mapping technique, effect sizes (in the form of Cohen's d) were calculated, and these values were converted to overlapping coefficients using a formula described previously.23
An interobserver agreement reliability analysis, using the κ statistic, was performed to determine the consistency of IRA diagnosis among observers. To assess reproducibility, quantitative assessment of the area of acute injury on T1 and T2 maps was performed by a second observer in a subset of 18 patients. The level of agreement between the 2 observers assessing the AAR on T1 and T2 maps was assessed using Bland–Altman plots and 95% limits of agreement. P<0.05 was considered statistically significant.
The study is publicly registered (ClinicalTrials.gov identifier NCT02073422).
Results
Patient Characteristics
Between February 2012 and May 2013, we recruited 73 NSTEMI patients (mean age 57±10 years, 78% male) who underwent CMR within 6.5±3.5 days of invasive management. All patients fulfilled the diagnostic criteria for acute NSTEMI, and the infarct‐related coronary arteries were identified by clinical criteria including ECG and coronary angiography. Patient demographics and clinical characteristics are provided in Table 1. The mean Global Registry of Acute Coronary Events score at hospital was 156±37. Overall, 27.4% of patients were imaged before and 72.6% were imaged after coronary angiography. Angiographic characteristics, in particular, the IRA characteristics and assignments, are provided in Table 1. Overall, 63% of the study population underwent percutaneous coronary intervention. All patients had evidence of coronary artery disease at angiography.
Table 1.
Patient Characteristics
| Characteristic | Value |
|---|---|
| Age, y | 57±10 |
| Male, n (%) | 57 (78) |
| Body mass index, kg/m2 | 28.7±4.8 |
| Smoker, n (%) | 52 (72.2) |
| Hypertension, n (%) | 25 (34.7) |
| Hyperlipidemia, n (%) | 23 (32.4) |
| Diabetes mellitus, n (%) | 9 (12.3) |
| Previous PCI, n (%) | 6 (8.2) |
| GRACE score | 156±37.25 |
| Troponin, mg/dL | 0.92 (0.2, 5.3) |
| Infarct‐related artery | |
| Left anterior descending, n (%) | 33 (45) |
| Circumflex, n (%) | 23 (32) |
| Right coronary, n (%) | 17 (23) |
| Angiographic characteristics | |
| Thrombus | 17.6% |
| Ulcerated | 58.8% |
| Irregular | 64.7% |
| Calcified | 17.6% |
| TIMI flow <3 | 19.6% |
| Stenosis, % | 84.79±15.7% |
| Multivessel disease, n (%) | 36 (49) |
| Treatment strategies | |
| Medical therapy only, n (%) | 21 (28.7) |
| Coronary artery bypass grafting, n (%) | 6 (8.2) |
| PCI, n (%) | 46 (63) |
| APPROACH lesion score, %LV mass | 23.45±12.6 |
GRACE indicates Global Registry of Acute Coronary Events; LV, left ventricular; PCI, percutaneous coronary intervention; TIMI, Thrombolysis in Myocardial Infarction.
Cardiac MRI Findings
The CMR findings are summarized in Tables 2 and 3. Of 26 patients (35.6%) without evidence of LGE, 16 (61.5%) had evidence of acute myocardial injury revealed by T1 and T2 mapping, whereas fewer participants (7, 27%) had evidence of myocardial injury using T2W‐STIR. The mean infarct size as a percentage of LV volume was 5.9±8.0%. There was no evidence of acute myocardial injury using 2 of the 3 imaging methods in 10 patients (13.6%).
Table 2.
MRI findings
| MRI Parameter | Value |
|---|---|
| LV dimensions and function, mean±SD | |
| LV ejection fraction, % | 57±12 |
| End‐diastolic volume index, mL/m2 | 93±17 |
| End‐systolic volume index, mL/m2 | 41±16 |
| LV mass index, g/m2 | 71±10 |
| LGE | |
| Patients with evidence of LGE, n (%) | 48 (66) |
| Acute infarct size, %LV mass, mean±SD | 5.9±8.0 |
| Microvascular obstruction, n (%) | 10 (14) |
Mean±SD heart rate in patients at the time of the MRI scan was 67±22. LGE indicates late gadolinium enhancement; LV, left ventricular.
Table 3.
Comparisons of the presence and extent of myocardial injury and salvage as revealed by T1 and T2 mapping and T2W‐STIR
| T1 | T2 | T2W‐STIR | P Value | |
|---|---|---|---|---|
| Patients with evidence of myocardial injury | 64 (88) | 63 (86) | 42 (58) | <0.001 |
| Area of myocardial injury (%LV volume) | 15.8±10.6 | 16.0±11.8 | 7.8±11.6 | <0.001 |
| Myocardial salvage (%LV volume) | 10.1±8.9 | 10.5±9.5 | 4.7±9.7 | <0.001 |
LV indicates left ventricular.
Microvascular obstruction was detected in 10 patients (14%). The troponin concentration in patients with MVO was numerically higher (7.43±−9.9) than in patients without MVO (4.37±8.01; P=0.57). The mean LV ejection fraction was 57±12%.
On average, 3 short‐axis slices were available for native T1 and T2 maps and T2W‐STIR images. Overall, 83.3% of patients had either excellent or good image quality. No patient was excluded because of poor image quality. There were significantly more artifacts with T2W‐STIR (57.1% of images) compared with T1 maps (12.78%) and T2 maps (3.7%; P<0.0001. A detailed breakdown of artifacts is provided in Table 4. When comparing the 2 parametric mapping techniques directly, there were significantly more artifacts with T1 maps compared with T2 maps (P<0.001).
Table 4.
Detailed Breakdown of Artifacts
| T1 (219) | T2 (219) | T2W‐STIR (219) | |
|---|---|---|---|
| MOCO | 18 | 12 | |
| M | 5 | 4 | 79 |
| RF | 28 | ||
| SB | 10 | ||
| G | 4 | ||
| FE | 1 | 2 | |
| W | 4 | 2 | 1 |
| SSFP | 1 | 1 |
F indicates flow; FE, field effects; G, gating; M, motion (eg, breathing); MOCO, motion correction; RF, radiofrequency field inhomogeneity; S, susceptibility; SB, stagnant blood; SSFP, steady‐state free precession off‐resonance bands; T2W‐STIR, T2‐weighted short tau inversion recovery; W, Wrap.
Detection of Acute Myocardial Injury by T1 and T2 Maps and T2‐Weighted Short Tau Inversion Recovery
There was no significant difference between T1 and T2 maps for classification of acute myocardial injury (T1 versus T2 maps: 64 [88%] versus 63 [86%] patients, respectively); however, there were significant differences when comparing T2W‐STIR (42, 58%) with T1 maps (P=0.019) and T2 maps (P=0.012).
Regarding patient characteristics among those with any myocardial injury (any) compared with those without, patients with injury were significantly more likely to have LGE (44 of 66 versus 1 of 7, χ2=7.34, P=0.007) and higher troponin (mean 5.26±8.54 versus 0.24±0.31, P<0.001). There were no significant differences in TIMI (Thrombolysis in Myocardial Infarction) flow (2.71±0.7 versus 2.86±0.38, P=0.59), fractional flow reserve values (0.78±0.19 versus 0.82±0.17, P=0.57), ECG evidence of ischemia (37 of 66 versus 2 of 7, P=0.16), or MVO (10 of 66 versus 0 of 7, P=0.26).
Diagnostic Accuracy of T1 and T2 Maps and Dark‐Blood T2‐Weighted Short Tau Inversion Recovery
The diagnostic accuracy of each imaging method for identification of the IRA, as defined by clinical data including ECG and coronary angiogram, are shown in Table 5. The IRA was correctly identified more often with T2 maps compared with other imaging methods (77% T2 maps, 71% T1 maps, 44% T2W‐STIR); however, when assessing diagnostic accuracy, there was no difference when comparing T1 and T2 maps (P=0.125). In contrast, differences in diagnostic accuracy were shown between T1 maps and T2W‐STIR (P<0.001) and between T2 maps and T2W‐STIR (P<0.001). For IRA identification, a high level of interobserver agreement was found with T1 maps (κ=0.790, P<0.001) and T2 maps (κ=0.794, P<0.001), whereas the level of agreement was moderate (κ=0.555, P<0.001) with T2W‐STIR. Moreover, there was a lower level of discordance between observers for individual patients using T1 maps (discordant findings in 13.69%) and T2 maps (15.06%) compared with dark‐blood T2W‐STIR (30.14% cases, P<0.001). We also compared each technique with other methods of identifying the IRA, such as ECG, presence of LGE, and regional wall motion abnormalities. The proportions of correctly identified infarct‐related arteries for T1 (0.84, 95% CI 0.74–0.91, P<0.001), T2 (0.86, 95% CI 0.77–0.93, P<0.001), and regional wall motion abnormalities (0.64, 95% CI 0.53–0.75, P=0.019) were significantly higher than chance alone. For several other techniques, however, the proportions of accurate results did not significantly differ from 0.50: ECG 0.53 (95% CI 0.42–0.65, P=0.640), LGE 0.62 (95% CI 0.50–0.72, P=0.061), T2W‐STIR 0.51 (95% CI 0.39–0.62, P=0.999).
Table 5.
Diagnostic Accuracies of Each Imaging Method for Identification of the IRA
| IRA Territory Identified | T2 Map | All | ||
|---|---|---|---|---|
| Correct (n=56) | Incorrect (n=17) | |||
| T1 map | Correct (n=52) | 52 | 0 | 52 (71%) |
| Incorrect (n=21) | 4 | 17 | 21 (29%) | |
| 56 (77%) | 17 (23%) | P=0.13 | ||
| IRA Territory Identified | T2W‐STIR Map | |||
|---|---|---|---|---|
| Correct (n=32) | Incorrect (n=41) | |||
| T1 map | Correct (n=52) | 31 | 21 | 52 (71%) |
| Incorrect (n=21) | 1 | 20 | 21 (29%) | |
| 32 (44%) | 41 (56%) | P<0.001 | ||
| IRA Correctly Identified | T2W‐STIR Map | |||
|---|---|---|---|---|
| Correct (n=32) | Incorrect (n=41) | |||
| T2 map | Correct (n=56) | 31 | 25 | 56 (77%) |
| Incorrect (n=17) | 1 | 16 | 17 (23%) | |
| 32 (44%) | 41 (56%) | P<0.01 | ||
IRA indicates infarct‐related artery; T2W‐STIR, T2‐weighted short tau inversion recovery.
When assessing the accuracy of these techniques in specific subgroups, there were no differences in the numbers of patients with correctly identified infarct‐related arteries, depending on whether they had multivessel disease, using T1 maps (29 of 36 versus 32 of 37, χ2=0.47, P=0.494), T2 maps (31 of 36 versus 31 of 36, χ2=0.00, P=1.00), and T2W‐STIR (18 of 36 versus 19 of 37, χ2=0.01, P=0.908).
T1 and T2 Values in Areas of Acute Myocardial Injury in Non‐ST‐Segment Elevation Myocardial Infarction
The mean signal intensity of remote and injured myocardium is shown in Table 3 for each imaging method. The measurements from acutely ischemic regions were significantly different compared with remote, unaffected myocardium for each method (injured versus remote: T1 maps, 1323±68 ms versus 1152±43 ms, P<0.001; T2 map, 57±5 ms versus 45±3 ms, P<0.001; T2W‐STIR, 146±55 versus 90±25, P<0.001); however, there was some overlap between distributions. For T1 maps, there was a large effect size (d=3.1, P<0.001) for the difference between infarct zone and remote scores with a small amount of overlap (12.5%). For T2 maps, there was an equally large effect (d=2.9) for the difference between infarct zone and remote scores with a small amount of overlap (14.9%). For T2W‐STIR, there was a somewhat smaller effect size (d=1.3) for the difference between infarct zone and remote scores with much greater overlap (51.0%) (Figure 2).
Figure 2.
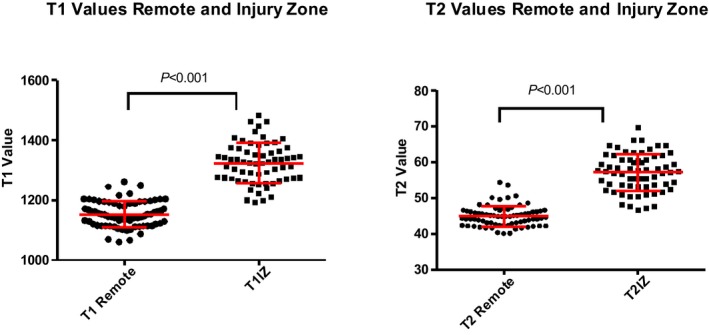
T1 and T2 values (m/s) in remote territory and in the injury zone (IZ).
For the extent of acute myocardial injury (percentage of LV volume) estimated by 2 independent observers using T1 mapping, there was good correlation between observers 1 and 2 (r 2=99.7%). The 95% limits of agreement were −1.76 and 1.63 (percentage of LV volume), respectively, and there was no evidence of bias (0.25). For T2 mapping between these observers, there was good correlation (r 2=98.9%), with 95% limits of agreement of −2.98 and 3.36 (percentage of LV volume) and no evidence of bias (0.19).
Variable Quantification of the Extent of Acute Myocardial Injury by T1 and T2 Maps and T2‐Weighted Short Tau Inversion Recovery
There was good correlation between T1 and T2 maps for estimation of myocardial injury (r=0.82, P<0.001; T1 maps versus T2 maps: 15.8±10.6% versus 16.0±11.8% of LV volume, P=0.838). The 95% limits of agreement for mean myocardial injury estimated with T1 versus T2 maps were −13% and 13%, respectively, of LV volume with a minimal bias (0.2±6.8%) (Figures 3 and 4).
Figure 3.
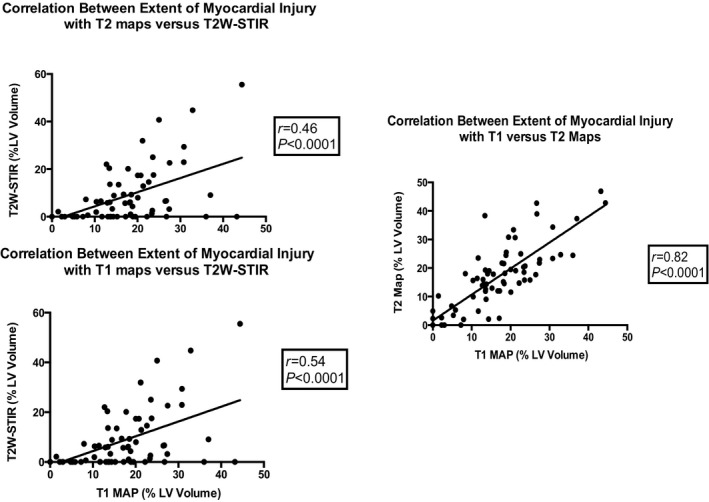
Correlation between the extent of myocardial injury estimated with T1 and T2 mapping and dark‐blood T2W‐STIR imaging. LV indicates left ventricular; T2W‐STIR, T2‐weighted short tau inversion recovery.
Figure 4.
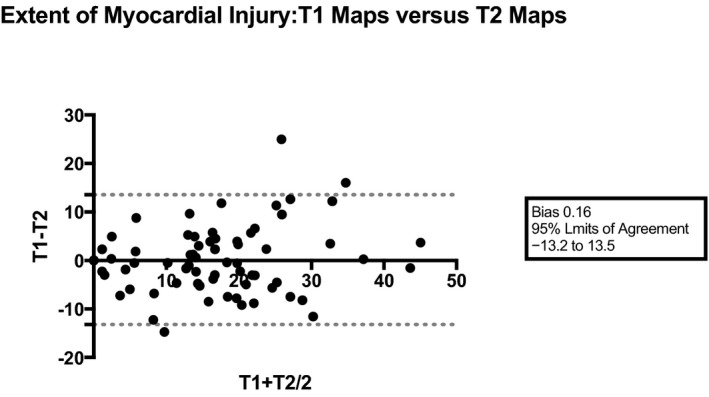
Bland–Altman plot comparing the extent of myocardial injury using T1 vs T2 mapping sequences.
There were moderate correlations between areas of acute injury estimated with T1 maps versus T2W‐STIR (r=0.54, P<0.001) and injury estimated with T2 maps versus T2W‐STIR (r=0.46, P<0.001). Mean area of acute injury estimated with T2W‐STIR (7.8±11.6% of LV volume) was smaller than that estimated with T1 (P<0.001) or T2 (P<0.001). The 95% limits of agreement for mean area of acute injury estimated with T1 maps versus T2W‐STIR and T2 maps versus T2W‐STIR were wider with a higher level of bias compared with the comparison for T1 versus T2 maps (T1 versus T2W‐STIR: −28% and 12% of LV volume with a bias of −8.0±10.6%; T2 versus T2W‐STIR: −32% and 16% with a bias of −8.1±12.1%).
The average amount of myocardial salvage (percentage of LV myocardial volume) estimated with T1 maps (10.1±8.9%) compared with T2 maps (10.5±9.5%, P=0.616) was similar. However, the mean myocardial salvage estimated with T2W‐STIR (4.7±9.7% of LV volume) was significantly lower than estimates with T1 maps (P=0.027) or T2 maps (P=0.009).
Correlation of the Extent of Acute Myocardial Injury Estimated by T1‐ and T2‐Weighted MRI Versus APPROACH Lesion Score
The mean APPROACH lesion score was 23.4±12.6 of the LV mass. CMR estimates of extent of acute myocardial injury did not correlate with the APPROACH lesion score. Myocardial injury estimates were compared for T1 maps versus APPROACH (r=0.13, P=0.29), T2 maps versus APPROACH (r=0.11, P=0.37), and T2W‐STIR versus APPROACH (r=0.08, P=0.49).
Discussion
The main results of our study are as follows. First, using 3.0‐T MRI, native T1 and T2 maps have higher diagnostic accuracy than dark‐blood T2W‐STIR and other techniques, including ECG, in detecting the IRA in patients with recent NSTEMI. Second, T1 and T2 maps produced highly congruent results when estimating the extent of myocardial injury in contrast to dark‐blood T2W‐STIR imaging that tended to underestimate the extent of injury compared with these methods. Third, reflecting the heterogeneity of NSTEMI patients (including interindividual variations in natural history, presence of multivessel disease, and typical preservation of culprit artery blood flow), T2‐weighted CMR does not reliably quantify the ischemic area of acute injury. Fourth, there was a high level of interobserver agreement for identification of the IRA with T1 and T2 maps compared with dark‐blood T2W‐STIR. Finally, artifacts were significantly more prevalent with dark‐blood imaging compared with T1 and T2 maps.
Unlike patients with STEMI, identification of the IRA in patients with the IRA in patients with NSTEMI for several reasons including a lack of ECG changes and the presence of multivessel disease. Furthermore, a significant proportion of patients who present with NSTEMI have normal coronary arteries at the time of coronary angiography.24 This number could be expected to increase with the increasing use of high‐sensitivity troponin. Our data demonstrate that novel T1 and T2 maps have higher accuracy for predicting the IRA in patients with NSTEMI compared with standard diagnostic techniques such as ECG and location of regional wall motion abnormalities. Consequently, the use of CMR in the diagnostic workup of patients with NSTEMI may enable selection of appropriate patients for invasive treatment and direct treatment to the appropriate coronary territory.
Imaging Acute Myocardial Injury
The detection of acute myocardial injury using a dark‐blood turbo spin‐echo technique has been shown to facilitate prompt diagnosis of acute coronary syndromes and to allow identification of both the AAR and the amount of myocardial salvage after reperfusion.25, 26, 27 Yet, despite great theoretical promise, the clinical reality of using dark‐blood T2W‐STIR imaging has been far from optimal because of methodological problems inherent in the technique.28, 29 Recently, a noncontrast quantitative T2 mapping sequence using T2 steady‐state free procession has been proposed and shown to be more accurate than conventional T2W‐STIR for predicting myocardial injury in animal and human models.10, 30
Previous experimental studies have also shown that T1 values increase with increasing myocardial water content and thus with ischemia and infarction.30 By directly quantifying T1 values for each voxel in the myocardium, a parametric map can be generated representing the T1 relaxation times of any region of the heart. The most widely adopted T1 mapping sequence is based on the MOLLI technique.14, 31 The use of T1 maps has also been shown to be more accurate at predicting edematous myocardium than dark‐blood T2W‐STIR.32 However, most of this work concerning the superior precision of T2 and T1 maps has been performed in the STEMI population; typically, this syndrome is associated with large degrees of myocardial injury.
Dark‐Blood Imaging
Following its development by Simonetti and colleagues, dark‐blood T2W‐STIR has been widely used to assess for the presence of myocardial edema.4 Several investigators have shown prognostic information using STIR imaging. In a prospective study involving 88 patients with NSTEMI, for example, Raman and colleagues highlighted the potential for T2W‐STIR imaging in identifying acute myocardial injury and distinguishing patients who required coronary revascularization from those who did not.5 Moreover, the presence of an increase in regional signal intensity was associated with a higher hazard of a cardiovascular event or death within 6 months after a non–ST‐segment elevation acute coronary syndrome.5
Recent studies have shown improved accuracy in the detection of acute myocardial injury with both T1 mapping32 and T2 mapping techniques compared with dark‐blood imaging at 1.5 T.10 These findings were echoed by McAlindon and colleagues, who compared the accuracy of these techniques in 40 patients with reperfused STEMI.33 These investigators demonstrated that T2 mapping had less variability and improved detection of the IRA compared with dark‐blood T2‐weighted imaging.
NSTEMI is the most common form of acute coronary syndrome, yet to date, no study has directly compared T1 and T2 maps with dark‐blood T2W‐STIR in NSTEMI. Our study showed—for the first time in a large NSTEMI population—that at 3.0 T, the diagnostic performance of dark‐blood imaging is inferior to newer novel mapping sequences, with underestimation of the area of acute injury as well as myocardial salvage. The identification of acute myocardial injury and the diagnostic accuracy of dark‐blood imaging were also limited, which is important from a clinical perspective. Previous studies comparing dark‐blood imaging with newer T2 or T1 methods have included only small numbers of patients with NSTEMI. Overall, the mean infarct size in these subpopulations have been moderate—16.7% of LV mass reported by Payne et al34—and in the study by Verhaert and colleagues, the mean peak troponin concentration was 50 mg/dL.10 In our NSTEMI population, the mean infarct size was 5.9% and the troponin value was 0.92 mg/dL—a much lower infarct burden. The poor performance of STIR in this population calls into question the use of dark‐blood edema MRI for imaging NSTEMI patients, either for clinical or research purposes. This point becomes all the more relevant because the sensitivity of the biochemical detection of NSTEMI increases with the adoption of high‐sensitivity troponin assays. The higher sensitivity of T1 and T2 mapping for detecting subtle alterations in signal intensity that can occur in NSTEMI patients suggests that these methods may be more useful in clinical practice.
T1 and T2 Maps in Non‐ST‐Segment Elevation Myocardial Infarction
Dall'Armellina and colleagues assessed T1 with T2‐weighted bright‐blood imaging in 41 patients presenting with MI at 3.0 T.3 Overall, 73% of the study cohort presented with STEMI. T1‐ and T2‐weighted imaging had similar diagnostic performance for detecting edematous injured myocardium. In a subgroup analysis performed in only 9 patients with NSTEMI, the authors demonstrated superior diagnostic performance of T1 maps over T2 imaging and less variability in T1 maps. In a much larger cohort of NSTEMI patients and using a different T1 mapping method, our results indicate that T1 and T2 maps had similar accuracy for estimating the myocardial AAR in patients with NSTEMI. In contrast to the findings of Dall'Armellina and colleagues, although there was no advantage in terms of accuracy, there were significantly fewer artifacts with T2 maps—suggesting an advantage for this mapping sequence—compared with T1 maps. Importantly, and despite this lower degree of injury, the utility of T1 and T2 mapping was preserved.
Angiographic Area at Risk
Several studies have clearly demonstrated the accuracy of both T1 and T2 maps in the assessment of the angiographic AAR in patients with acute coronary syndromes; however, the patient populations have largely consisted of STEMI patients. The APPROACH score has been shown to correlate with the myocardium at risk derived through CMR,18 and the magnitude of the correlation increased with the transmural extent of infarction.22 Typically, NSTEMI patients have smaller, subendocardial infarcts; therefore, it is not surprising to find that the AAR, as measured with edema imaging sequences, in our population did not correlate with the APPROACH score.
Clinical Application
T1 and T2 maps may prove useful to help identify the artery that should be treated before an invasive percutaneous coronary intervention is performed. CMR could potentially provide useful information to optimize the percutaneous coronary intervention plan by targeting the intervention to the culprit artery and by providing clinically relevant information on regional LV systolic function and myocardial viability. Our data support the potential role of CMR imaging as an adjunctive test to facilitate the invasive management of NSTEMI patients. Further research is warranted.
Retrospective imaging of the ischemic AAR is clinically relevant because the amount of salvageable myocardium is a predictor of prognosis and potential response to therapy.30 This study demonstrates the greater accuracy of T1 and T2 maps in identifying ischemically jeopardized myocardium compared with T2W‐STIR imaging in patients with NSTEMI. Patients who experience an NSTEMI tend to be older and have more concomitant health problems and multivessel coronary disease. Using a more sensitive marker of myocardial injury than dark‐blood T2W‐STIR in this heterogeneous population may allow for improved risk stratification for patients who would benefit from early invasive assessment and may accurately identify the culprit territory when faced with a patient who has multivessel coronary disease.
Study Limitations
The timing of MRI after MI was influenced by the clinical course of the patients (eg, recurrent ischemia, the timing of invasive angiography). Although this variation in timing may have introduced some measurement heterogeneity, we think the MRI data became more representative of real‐life clinical practice, reflecting the timing of when MRI might actually be performed in relation to acute presentation and/or invasive management. The participants were also enrolled in a clinical trial, but the intervention in this trial was not relevant to the MRI study, and the characteristics of our patients were similar to those of the trial participants and registry patients.
Despite higher accuracy for predicting the IRA, novel mapping sequences still failed to identify the IRA in ≈20% of cases. This may limit the clinical usefulness of the technique in a nonselected NSTEMI population.
MOLLI is sensitive to heart rate and this method may lead to modest inaccuracies in T1 (ms) in patients with a rapid heart rate, an irregular heart rate, or frequent ectopic beats. T2 mapping is also heart rate dependent; however, the majority of patients were on beta blockers before the MRI scan, with a controlled heart rate to minimize this effect.
We obtained only 3 slices to cover the left ventricle, and thus a theoretical concern exists that small NSTEMIs could have been missed using the MRI mapping technique.
Using the angiogram as the gold standard to identify the IRA is not without its flaws and could lead to a situation in which the mapping technique is correct but the IRA is incorrectly identified.
We used 6‐mm slice thickness for STIR imaging in the study, and this may have enhanced imaging artifacts; however, this is within the range implemented by other researchers.
From an anatomical perspective, interindividual variation in coronary artery distribution may confound IRA assignment. In NSTEMI, multivessel coronary artery disease is common, and there may be >1 culprit lesion. Therefore, it could be that an IRA that was missed on angiography was correctly identified with the MRI maps. Because patients underwent CMR after invasive management with percutaneous coronary intervention, it is possible that the myocardial injury detected with T1 maps, T2 maps, and T2W‐STIR actually originated from the percutaneous coronary intervention treatment rather than the initial MI. However, according to the current Universal Definition of Myocardial Infarction,12 none of the participants in this study experienced periprocedural MI.
Conclusion
T1 and T2 maps are novel quantitative imaging methods that have high diagnostic accuracy in detecting the IRA in NSTEMI. They produce congruent results when estimating AAR, whereas T2W‐STIR imaging yielded less accurate results with more artifacts. Findings suggest that T1 and T2 mapping represents a clinically valuable novel imaging method, with particular utility for the assessment of reversible myocardial injury and salvaged myocardium. Based on our findings, the utility of T2W‐STIR imaging at 3.0‐T for the assessment of edema in patients with NSTEMI is limited.
Sources of Funding
A project grant from the British Heart Foundation (PG/11/55/28999) and the Chief Scientist Office of the Scottish Government supported the trial. The funders had no role in the design or conduct of the study or in the analysis, interpretation or presentation of the results. The authors disclose a research agreement with Siemens Healthcare. There are no other disclosures relevant to this manuscript. The sponsor is the National Waiting Times Centre, NHS Scotland. The authors are solely responsible for the design and conduct of the study, all analyses, the drafting and editing of the paper and its final contents.
Disclosures
None.
Acknowledgments
We thank the patients who participated in this study. We also thank all of the university and NHS staff that supported the project. We thank Peter Weale and Patrick Revell from Siemens Healthcare UK for providing the quantitative mapping sequences.
(J Am Heart Assoc. 2017;6:e004759 DOI: 10.1161/JAHA.116.004759.)28364045
References
- 1. Friedrich MG, Kim HW, Kim RJ. T2‐weighted imaging to assess post‐infarct myocardium at risk. JACC Cardiovasc Imaging. 2011;4:1014–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Payne AR, Casey M, McClure J, McGeoch R, Murphy A, Woodward R, Saul A, Bi X, Zuehlsdorff S, Oldroyd KG, Tzemos N, Berry C. Bright‐blood T2‐weighted MRI has higher diagnostic accuracy than dark‐blood short tau inversion recovery MRI for detection of acute myocardial infarction and for assessment of the ischemic area at risk and myocardial salvage. Circ Cardiovasc Imaging. 2011;4:210–219. [DOI] [PubMed] [Google Scholar]
- 3. Dall'Armellina E, Piechnik SK, Ferreira VM, Si QL, Robson MD, Francis JM, Cuculi F, Kharbanda RK, Banning AP, Choudhury RP, Karamitsos TD, Neubauer S. Cardiovascular magnetic resonance by non contrast T1‐mapping allows assessment of severity of injury in acute myocardial infarction. J Cardiovasc Magn Reson. 2012;14:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Simonetti OP, Finn JP, White RD, Laub G, Henry DA. “Black blood” T2‐weighted inversion‐recovery MR imaging of the heart. Radiology. 1996;199:49–57. [DOI] [PubMed] [Google Scholar]
- 5. Raman SV, Simonetti OP, Winner MW III, Dickerson JA, He X, Mazzaferri EL Jr, Ambrosio G. Cardiac magnetic resonance with edema imaging identifies myocardium at risk and predicts worse outcome in patients with non‐ST‐segment elevation acute coronary syndrome. J Am Coll Cardiol. 2010;55:2480–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eitel I, Desch S, Fuernau G, Hildebrand L, Gutberlet M, Schuler G, Thiele H. Prognostic significance and determinants of myocardial salvage assessed by cardiovascular magnetic resonance in acute reperfused myocardial infarction. J Am Coll Cardiol. 2010;55:2470–2479. [DOI] [PubMed] [Google Scholar]
- 7. Thiele H, Hildebrand L, Schirdewahn C, Eitel I, Adams V, Fuernau G, Erbs S, Linke A, Diederich KW, Nowak M, Desch S, Gutberlet M, Schuler G. Impact of high‐dose N‐acetylcysteine versus placebo on contrast‐induced nephropathy and myocardial reperfusion injury in unselected patients with ST‐segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. The LIPSIA‐N‐ACC (Prospective, Single‐Blind, Placebo‐Controlled, Randomized Leipzig Immediate PercutaneouS Coronary Intervention Acute Myocardial Infarction N‐ACC) Trial. J Am Coll Cardiol. 2010;55:2201–2209. [DOI] [PubMed] [Google Scholar]
- 8. Keegan J, Gatehouse PD, Prasad SK, Firmin DN. Improved turbo spin‐echo imaging of the heart with motion‐tracking. J Magn Reson Imaging. 2006;24:563–570. [DOI] [PubMed] [Google Scholar]
- 9. Kellman P, Aletras AH, Mancini C, McVeigh ER, Arai AE. T2‐prepared SSFP improves diagnostic confidence in edema imaging in acute myocardial infarction compared to turbo spin echo. Magn Reson Med. 2007;57:891–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Verhaert D, Thavendiranathan P, Giri S, Mihai G, Rajagopalan S, Simonetti OP, Raman SV. Direct T2 quantification of myocardial edema in acute ischemic injury. JACC Cardiovasc Imaging. 2011;4:269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Carrick D, Behan M, Foo F, Christie J, Hillis WS, Norrie J, Oldroyd KG, Berry C. Usefulness of fractional flow reserve to improve diagnostic efficiency in patients with non‐ST elevation myocardial infarction. Am J Cardiol. 2013;111:45–50. [DOI] [PubMed] [Google Scholar]
- 12. Thygesen K, Alpert JS, White HD; Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction , Jaffe AS, Apple FS, Galvani M, Katus HA, Newby LK, Ravkilde J, Chaitman B, Clemmensen PM, Dellborg M, Hod H, Porela P, Underwood R, Bax JJ, Beller GA, Bonow R, Van der Wall EE, Bassand JP, Wijns W, Ferguson TB, Steg PG, Uretsky BF, Williams DO, Armstrong PW, Antman EM, Fox KA, Hamm CW, Ohman EM, Simoons ML, Poole‐Wilson PA, Gurfinkel EP, Lopez‐Sendon JL, Pais P, Mendis S, Zhu JR, Wallentin LC, Fernández‐Avilés F, Fox KM, Parkhomenko AN, Priori SG, Tendera M, Voipio‐Pulkki LM, Vahanian A, Camm AJ, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck‐Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Morais J, Brener S, Harrington R, Morrow D, Lim M, Martinez‐Rios MA, Steinhubl S, Levine GN, Gibler WB, Goff D, Tubaro M, Dudek D, Al‐Attar N. Universal definition of myocardial infarction. Circulation. 2007;116:2634–2653. [DOI] [PubMed] [Google Scholar]
- 13. Hamm CW, Bassand JP, Agewall S, Bax J, Boersma E, Bueno H, Caso P, Dudek D, Gielen S, Huber K, Ohman M, Petrie MC, Sonntag F, Uva MS, Storey RF, Wijns W, Zahger D; ESC Committee for Practice Guidelines . ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation: The Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST‐segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2011;32:2999–3054. [DOI] [PubMed] [Google Scholar]
- 14. Messroghli DR, Radjenovic A, Kozerke S, Higgins DM, Sivananthan MU, Ridgway JP. Modified Look‐Locker inversion recovery (MOLLI) for high‐resolution T1 mapping of the heart. Magn Reson Med. 2004;52:141–146. [DOI] [PubMed] [Google Scholar]
- 15. Messroghli DR, Walters K, Plein S, Sparrow P, Friedrich MG, Ridgway JP, Sivananthan MU. Myocardial T1 mapping: application to patients with acute and chronic myocardial infarction. Magn Reson Med. 2007;58:34–40. [DOI] [PubMed] [Google Scholar]
- 16. Giri S, Chung YC, Merchant A, Mihai G, Rajagopalan S, Raman SV, Simonetti OP. T2 quantification for improved detection of myocardial edema. J Cardiovasc Magn Reson. 2009;11:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hermosillo G, Chefd'Hotel C, Faugeras O. International Journal of Computer Vision 2002;50:329. [Google Scholar]
- 18. Berry C, Kellman P, Mancini C, Chen MY, Bandettini WP, Lowrey T, Hsu LY, Aletras AH, Arai AE. Magnetic resonance imaging delineates the ischemic area at risk and myocardial salvage in patients with acute myocardial infarction. Circ Cardiovasc Imaging. 2010;3:527–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim RJ, Fieno DS, Parrish TB, Harris K, Chen EL, Simonetti O, Bundy J, Finn JP, Klocke FJ, Judd RM. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation. 1999;100:1992–2002. [DOI] [PubMed] [Google Scholar]
- 20. Carrick D, Oldroyd KG, McEntegart M, Haig C, Petrie MC, Eteiba H, Hood S, Owens C, Watkins S, Layland J, Lindsay M, Peat E, Rae A, Behan M, Sood A, Hillis WS, Mordi I, Mahrous A, Ahmed N, Wilson R, Lasalle L, Genereux P, Ford I, Berry C. A randomized trial of deferred stenting versus immediate stenting to prevent no‐or slow reflow in acute ST‐elevation myocardial infarction (DEFER‐STEMI). J Am Coll Cardiol. 2014;63:2088–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moon JC, Messroghli DR, Kellman P, Piechnik SK, Robson MD, Ugander M, Gatehouse PD, Arai AE, Friedrich MG, Neubauer S, Schulz‐Menger J, Schelbert EB. Myocardial T1 mapping and extracellular volume quantification: a Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement. J Cardiovasc Magn Reson. 2013;15:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ortiz‐Perez JT, Meyers SN, Lee DC, Kansal P, Klocke FJ, Holly TA, Davidson CJ, Bonow RO, Wu E. Angiographic estimates of myocardium at risk during acute myocardial infarction: validation study using cardiac magnetic resonance imaging. Eur Heart J. 2007;28:1750–1758. [DOI] [PubMed] [Google Scholar]
- 23. Reiser B, Faraggi D. Confidence Intervals for the Overlapping Coefficient: the Normal Equal Variance Case. J R Stat Soc Ser. 1999;48:413–418. [Google Scholar]
- 24. Cortell A, Sanchis J, Bodi V, Nunez J, Mainar L, Pellicer M, Minana G, Santas E, Dominguez E, Palau P, Llacer A. Non‐ST‐elevation acute myocardial infarction with normal coronary arteries: predictors and prognosis. Rev Esp Cardiol. 2009;62:1260–1266. [DOI] [PubMed] [Google Scholar]
- 25. Abdel‐Aty H, Cocker M, Meek C, Tyberg JV, Friedrich MG. Edema as a very early marker for acute myocardial ischemia: a cardiovascular magnetic resonance study. J Am Coll Cardiol. 2009;53:1194–1201. [DOI] [PubMed] [Google Scholar]
- 26. Aletras AH, Tilak GS, Natanzon A, Hsu LY, Gonzalez FM, Hoyt RF Jr, Arai AE. Retrospective determination of the area at risk for reperfused acute myocardial infarction with T2‐weighted cardiac magnetic resonance imaging: histopathological and displacement encoding with stimulated echoes (DENSE) functional validations. Circulation. 2006;113:1865–1870. [DOI] [PubMed] [Google Scholar]
- 27. Cury RC, Shash K, Nagurney JT, Rosito G, Shapiro MD, Nomura CH, Abbara S, Bamberg F, Ferencik M, Schmidt EJ, Brown DF, Hoffmann U, Brady TJ. Cardiac magnetic resonance with T2‐weighted imaging improves detection of patients with acute coronary syndrome in the emergency department. Circulation. 2008;118:837–844. [DOI] [PubMed] [Google Scholar]
- 28. Arai AE. Using magnetic resonance imaging to characterize recent myocardial injury: utility in acute coronary syndrome and other clinical scenarios. Circulation. 2008;118:795–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pennell D. Myocardial salvage: retrospection, resolution, and radio waves. Circulation. 2006;113:1821–1823. [DOI] [PubMed] [Google Scholar]
- 30. Ugander M, Bagi PS, Oki AJ, Chen B, Hsu LY, Aletras AH, Shah S, Greiser A, Kellman P, Arai AE. Myocardial edema as detected by pre‐contrast T1 and T2 CMR delineates area at risk associated with acute myocardial infarction. JACC Cardiovasc Imaging. 2012;5:596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Messroghli DR, Greiser A, Frohlich M, Dietz R, Schulz‐Menger J. Optimization and validation of a fully‐integrated pulse sequence for modified look‐locker inversion‐recovery (MOLLI) T1 mapping of the heart. J Magn Reson Imaging. 2007;26:1081–1086. [DOI] [PubMed] [Google Scholar]
- 32. Ferreira VM, Piechnik SK, Dall'Armellina E, Karamitsos TD, Francis JM, Choudhury RP, Friedrich MG, Robson MD, Neubauer S. Non‐contrast T1‐mapping detects acute myocardial edema with high diagnostic accuracy: a comparison to T2‐weighted cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2012;14:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McAlindon E, Pufulete M, Lawton C, Angelini GD, Bucciarelli‐Ducci C. Quantification of infarct size and myocardium at risk: evaluation of different techniques and its implications. Eur Heart J Cardiovasc Imaging. 2015;16:738–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Payne AR, Berry C, Kellman P, Anderson R, Hsu LY, Chen MY, McPhaden AR, Watkins S, Schenke W, Wright V, Lederman RJ, Aletras AH, Arai AE. Bright‐blood T(2)‐weighted MRI has high diagnostic accuracy for myocardial hemorrhage in myocardial infarction: a preclinical validation study in swine. Circ Cardiovasc Imaging. 2011;4:738–745. [DOI] [PMC free article] [PubMed] [Google Scholar]


