Abstract
Background
Smoking cessation and weight management are recommended after acute coronary syndrome (ACS); however, little is known about the effects of smoking cessation on weight change after ACS. We aimed to assess the effect of smoking cessation after ACS on weight over a 12‐month follow‐up period.
Methods and Results
Data were prospectively collected from the EVITA (Evaluation of Varenicline in Smoking Cessation for Patients Post‐Acute Coronary Syndrome) trial. Weight change was compared among 3 groups of patients: those who were completely abstinent (n=70), those who smoked intermittently (n=68), and those who smoked persistently (n=34). Patients' mean baseline weight was 83.9 kg (SD 17.7) with a mean body mass index of 28.5 (SD 5.4). Patients smoked a mean of 37.7 years (SD 17.7) and a mean of 21.0 cigarettes (SD 9.0) per day prior to their ACS. Weight change varied across groups, with abstainers gaining a mean of 4.8 kg (SD 8.6), intermittent smokers gaining a mean of 2.0 kg (SD 8.9) and persistent smokers losing a mean of 0.7 kg (SD 7.4). At 52 weeks, abstainers were more likely to gain weight than persistent smokers (difference in means 5.5 kg; 95% CI 2.3–8.8). This weight gain was not associated with an increase in the use of antihypertensive or antidiabetic medications.
Conclusions
Following an ACS, significant weight is gained by patients who quit smoking. Weight‐management interventions among smokers who quit after ACS should be a focus of investigation in future research so that the cardiovascular benefits achieved by smoking cessation are not offset by weight gain in this high‐risk population.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT00794573.
Keywords: acute coronary syndrome, secondary prevention, smoking, weight gain
Subject Categories: Cardiovascular Disease, Lifestyle, Secondary Prevention, Clinical Studies
Introduction
The benefits of smoking cessation and weight loss after myocardial infarction in preventing recurrent events are well documented.1, 2 The American Heart Association/American College of Cardiology Foundation guidelines advocate complete cessation of smoking after an acute coronary syndrome (ACS; class I recommendation with level of evidence A), weight reduction to achieve a body mass index between 18.5 and 24.9 (class I, level of evidence B), and an initial goal of reducing body weight by ≈5% to 10% (class I, level of evidence C).3 Of all the modifiable risk factors in patients with established cardiovascular disease, smoking cessation is most consistently associated with a reduction in both morbidity4 and mortality.5, 6, 7
Smoking cessation, however, is known to cause weight gain and obesity, which markedly worsens cardiovascular risk factors including hypertension, diabetes mellitus (DM), and hyperlipidemia. To what extent the resultant elevated blood pressure (BP) and impaired glucose metabolism nullifies benefits of smoking cessation remains unknown.8, 9, 10 An analysis of the effect of smoking status on weight change demonstrated significant weight gain in those who stopped smoking 12 months after an ACS.10 This increase in weight was associated with an increase in prevalence of hypertension and DM. Therefore, a planned secondary analysis was designed to answer this question prospectively in the EVITA (Evaluation of Varenicline in Smoking Cessation for Patients Post‐Acute Coronary Syndrome) trial.11 We sought to compare changes in weight between smokers who return to smoking and those who remain abstinent after myocardial infarction. We also examined the effect of weight change on BP, the need for BP‐lowering medications, and the need for antidiabetic medications.
Methods
We used data from the multicenter, double‐blind, placebo‐controlled EVITA trial, in which smokers hospitalized with an ACS were randomized to varenicline or placebo for 12 weeks.11 Institutional review committee approval was obtained for all centers, and participants provided informed consent prior to enrollment. Enrollment for the EVITA trial began in November 2009, and follow‐up continued through December 2015. All 302 patients received low‐intensity counseling from a research nurse at baseline and follow‐up visits. Patients enrolled in the trial had to have smoked ≥10 cigarettes per day in the past year, have had an ACS (either enzymatically positive myocardial infarction or unstable angina with significant coronary artery disease), and be motivated to quit smoking. Seven‐day point prevalence abstinence was defined as 0 reported cigarettes smoked in the previous 7 days with an expired carbon monoxide level ≤10 parts per million.
We restricted analyses of this study to patients who completed all follow‐up clinic visits (n=172). Participants' heights and weights were measured at baseline. At each clinic visit, a research nurse also measured weight and assessed participants' smoking status. We calculated body mass index (BMI) using the standard formula (weight in kilograms divided by height in square meters) and categorized it into 4 groups: underweight <18.5, normal weight 18.5 to 24.9, overweight 25.0 to 29.9, and obese ≥30.0.
The research nurse also measured BP at each clinic visit and collected data regarding the use of antihypertensive and antidiabetic medications. We defined change in weight as the difference in weight between baseline and the 12‐month follow‐up visit. We compared weight change among patients who were smoking persistently, smoking intermittently, and completely abstinent during the 12‐month follow‐up. We classified patients who were abstinent at all follow‐up visits as abstainers, those who reported smoking at all follow‐ups as persistent smokers, and those who were both abstinent and smoking at some follow‐ups during the 12 months as intermittent smokers. We selected variables believed to influence weight change a priori and assessed them prospectively. The severity of dependence on nicotine was one such variable and was assessed by administering the Fagerström Test for Nicotine Dependence12 to each patient. With a score ranging from 0 to 10, scores >4 and ≥7 indicate moderate and severe dependence on nicotine, respectively. Severity of depressive symptoms was another variable and was measured using the Beck Depression Inventory.13 Other variables included use of antidepression medications, number of years smoked, baseline weight and BMI, hypertension, hyperlipidemia, DM, prior use of nicotine replacement therapies, prior use of varenicline, and previous quit attempts.
Data Analysis
We presented baseline demographic, smoking, and clinical characteristics as mean (SD), median (interquartile range [IQR]), and proportion, as applicable. We compared participants included in our analyses with those excluded with respect to a number of baseline characteristics by estimating mean differences or percentage differences, as applicable, with corresponding 95% CIs.
The smoking patterns (abstainers versus intermittent smokers versus persistent smokers) of the participants included in our analyses were presented as proportions, and differences in means were estimated to compare the number of cigarettes smoked per day at 12 months for intermittent and persistent smokers.
We assessed differences in weight change between groups of smokers by estimating differences in the means of weight change within each group from baseline to 12 months, with corresponding 95% CIs. To adjust for potential confounders, we conducted a multivariable linear regression analysis that controlled for age, sex, baseline weight, number of years smoked, hypertension, DM, hyperlipidemia, treatment group, Beck Depression Inventory scores, and medications taken for depression. We also reported the proportions of participants in each smoking group who belonged to the different BMI categories at baseline and at 12 months.
In addition, we estimated differences in the means of change in BP between those who gained, lost, and maintained their baseline weight. Weight change for the purpose of this analysis was defined as a >5% change from baseline weight. This threshold was selected based on expert opinion that has previously considered a >5% change in weight to be clinically significant.14 We also used this definition to assess changes in use of antihypertensive and antidiabetic medication among those who gained, lost, or maintained their baseline weight.
We used multiple linear regression to identify independent baseline predictors of weight change at 12 months. Potential predictors in our model included age, sex, baseline weight, number of years smoked, hypertension, DM, hyperlipidemia, treatment group, Beck Depression Inventory scores, and medications taken for depression. Potential confounders were assessed by checking the degree to which regression coefficients changed when potential confounders were removed from the models.
Throughout all analyses, our sample sizes were sufficiently large such that 95% CIs could be based on standard normal theory. This is especially true because many of our comparisons were derived from within‐subject differences, which remove much of the concern about the normality of underlying distributions.
Statistical analyses were conducted using SAS statistical software (version 9.3; SAS Institute Inc) and R (CRAN, version 3.0.1; R Core Team).
Results
Patient Characteristics
The majority of the 172 participants included in our analyses were male (78.0%) with a mean age of 57.0 years (SD 9.1). At baseline, the mean weight was 83.9 kg (SD 17.7), the mean BMI was 28.5 (SD 5.4), and 73.8% of patients were obese or overweight. The prevalence of DM and hypertension was 15.7% and 43.6%, respectively. Patients had been smoking a mean of 37.7 years (SD 17.7) and close to 1 pack of cigarettes (mean of 21.0 cigarettes, SD 9.0) per day prior to their ACS (Table). The mean Fagerström test score was 5.2 (SD 2.1), indicating at least moderate dependence on nicotine.
Table 1.
Patient Characteristics by Smoking Category
| Characteristics | Persistent Smokers (n=34) | Intermittent Smokers (n=68) | Abstainers (n=70) |
|---|---|---|---|
| Demographic and smoking | |||
| Age, mean (SD) | 57.3 (10.1) | 58.2 (9.1) | 55.7 (8.6) |
| Male, % | 73.5 | 79.4 | 78.6 |
| Weight, mean (SD) | 84.7 (16.0) | 83.3 (18.1) | 84.3 (11.2) |
| BMI, mean (SD) | 28.6 (5.3) | 28.2 (5.8) | 28.8 (5.0) |
| No. years smoked, mean (SD) | 19.7 (12.3) | 21.8 (11.5) | 20.8 (8.6) |
| No. cigarettes/day (past year), mean (SD) | 28.6 (5.3) | 28.2 (10.2) | 28.8 (8.5) |
| Other smokers at home, % | 50 | 39.7 | 38.6 |
| Prior quit attempts, % | 10.1 | 85.3 | 87.1 |
| Fagerström Test for Nicotine Dependence, mean (SD) | 8.5 (7.1) | 5.2 (2.3) | 5.1 (1.9) |
| Beck Depression score, mean (SD) | 8.5 (7.1) | 4.9 (5.1) | 5.3 (5.8) |
| Any previous serious quit attempts % | 88.3 | 85.3 | 87.1 |
| 0 | 11.8 | 14.7 | 12.9 |
| 1 | 38.2 | 23.5 | 30.0 |
| 2 | 11.8 | 25.0 | 21.4 |
| ≥3 | 38.2 | 36.8 | 35.7 |
| Prior use of abstinence aids, % | 55.9 | 55.9 | 52.9 |
| Nicotine replacement therapya | 38.2 | 45.6 | 47.1 |
| Bupropion | 20.6 | 13.2 | 17.1 |
| Varenicline | 0 | 4.6 | 4.6 |
| Counseling | 7.7 | 16.3 | 0 |
| Clinical characteristics | |||
| Medical history, % | |||
| Hyperlipidemia | 64.7 | 69.1 | 54.3 |
| Hypertension | 47.1 | 44.1 | 41.4 |
| Diabetes mellitus | 20.6 | 20.6 | 8.6 |
| Prior MI | 26.5 | 22.1 | 10.0 |
| Prior PCI | 23.5 | 16.2 | 8.6 |
| Prior CABG | 2.9 | 2.9 | 0 |
| STEMI, % | 55.9 | 55.9 | 70.0 |
| Medications, % | |||
| ACE inhibitors | 53.0 | 67.7 | 68.6 |
| Antilipid agent | 100.0 | 98.5 | 97.1 |
| ARBs | 14.7 | 8.8 | 1.4 |
| Beta blockers | 91.2 | 83.8 | 80.0 |
| Insulin | 5.8 | 5.9 | 2.9 |
| Oral hypoglycemic agent | 14.7 | 14.7 | 8.6 |
ACE indicates angiotensin‐converting enzyme; ARB, angiotensin II receptor blocker; BMI, body mass index; CABG, coronary artery bypass grafting; MI, myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST‐segment elevation myocardial infarction.
Includes nicotine patch, gum, and inhaler.
Compared with the participants excluded from our analyses (n=130), those included were older, had a lower mean weight and BMI at baseline, smoked longer, and had higher prevalence of hypertension and hyperlipidemia (Table S1). In addition, a larger proportion of those included had previously attempted to quit smoking and reported prior use of abstinence aids.
Smoking Patterns
Of the 172 patients who attended all clinic visits during the 12 months of follow‐up, 34 remained persistent smokers, 68 became intermittent smokers, and 70 became abstainers. In other words, close to 60% continued to smoke during the first year after ACS. At the 12‐month follow‐up, intermittent and persistent smokers reported smoking a median of 3.6 (IQR 0.3–13) and 13.4 (IQR 5.7–20.0) cigarettes daily, respectively. Patients in all 3 groups reported a substantial decrease in their median number of cigarettes smoked per day from baseline: abstainers, −20.0 (IQR −25.0 to −15.0); intermittent smokers, −12.2 (IQR −20.0 to −8.4), and persistent smokers, −5.8 (IQR −9.3 to 0). There was a significant difference in the number of reported cigarettes smoked per day at 12 months between intermittent and persistent smokers (difference in means −6.7; 95% CI −10.8 to −2.7).
Twelve‐Month Weight Change and Body Mass Index
Mean weight change between baseline and the 12‐month follow‐up in the entire cohort was 2.6 kg (SD 8.7), with 43.0% of patients gaining >5% of their baseline body weight and 16.9% losing >5% of their baseline body weight. At 12 months, when stratified by smoking status, 60.0% of abstainers gained >5% of their baseline body weight compared with 23.5% of persistent smokers. In contrast, 12.9% of abstainers lost >5% of their body weight compared with 29.4% of persistent smokers (Figure 1). When intermittent and persistent smokers were grouped together in follow‐up, roughly 3 in 10 (32 of 102) gained >5% of their baseline body weight compared with 6 in 10 (42 of 70) abstainers. Conversely, 1 in 5 (20 of 102) of the cohort composed of intermittent and persistent smokers lost >5% of baseline body weight compared with 1 in 10 (9 of 70) abstainers.
Figure 1.
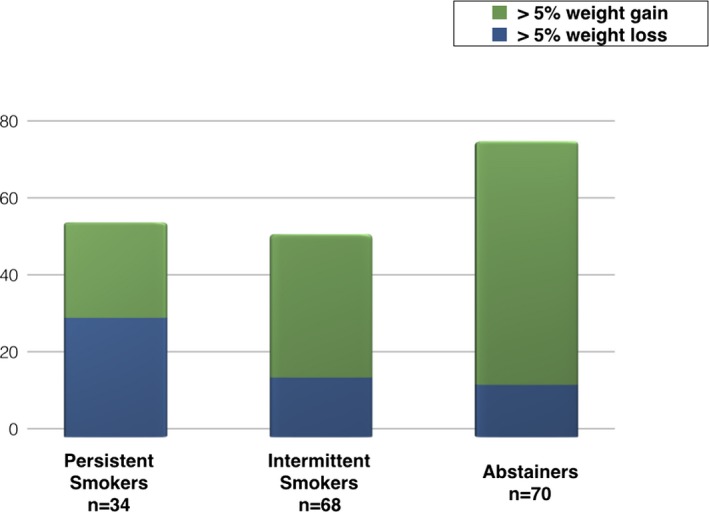
Proportion of patients with >5% body weight gain or loss according to pattern of smoking at 52 weeks. Compared with persistent smokers, many more abstainers gained >5% of their body weight over 12 months of follow‐up.
Weight change between baseline and 12 months varied across groups. Abstainers gained a mean of 4.8 kg (SD 8.6), intermittent smokers gained a mean of 2.0 kg (SD 8.9), and persistent smokers lost a mean of 0.7 kg (SD 7.4). At 12 months, patients who had been abstinent were more likely to have gained weight than those who had smoked persistently (difference in means, 5.5 kg; 95% CI 2.3–8.8). Similarly, abstainers had gained more weight than intermittent smokers; however, the difference did not reach statistical significance (difference in means 2.8 kg; 95% CI −0.1 to 5.7) (Figure 2). Results of our multivariable linear regression analysis are consistent with our crude estimates and indicate that, compared with persistent smokers, abstainers experienced a significant weight increase of 4.7 kg (95% CI 1.07–8.25), and intermittent smokers experienced a weight increase of 2.4 kg (95% CI −1.28 to 6.07).
Figure 2.
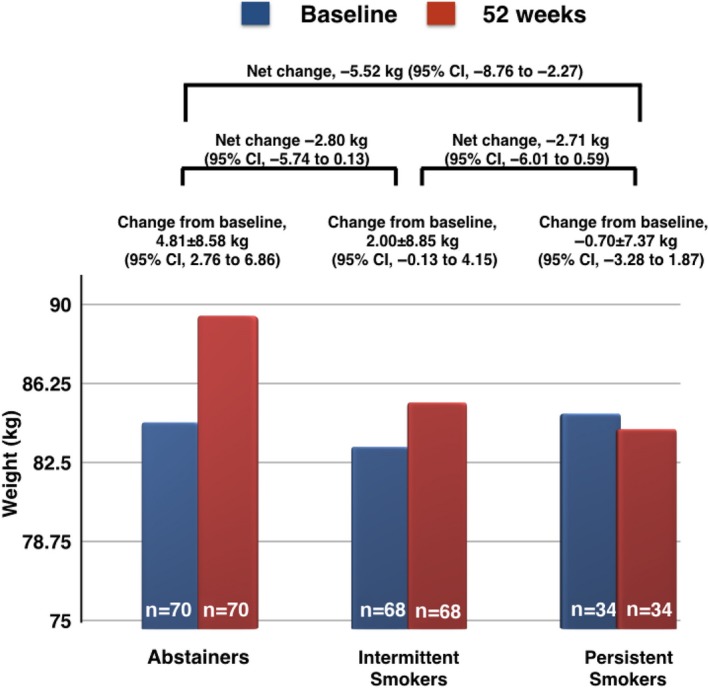
Weight changes within and between different categories of smokers. A significant difference in change of weight from baseline to 52 weeks was observed between smokers who became abstainers and smokers who continued to smoke persistently.
At 12 months, 18.6% of patients had increased by a BMI category and 8.1% had decreased by a BMI category. The most notable change in BMI category occurred in abstainers, in whom the proportion of patients with BMI ≥30 increased from roughly a third to half of the entire cohort (Figure 3).
Figure 3.
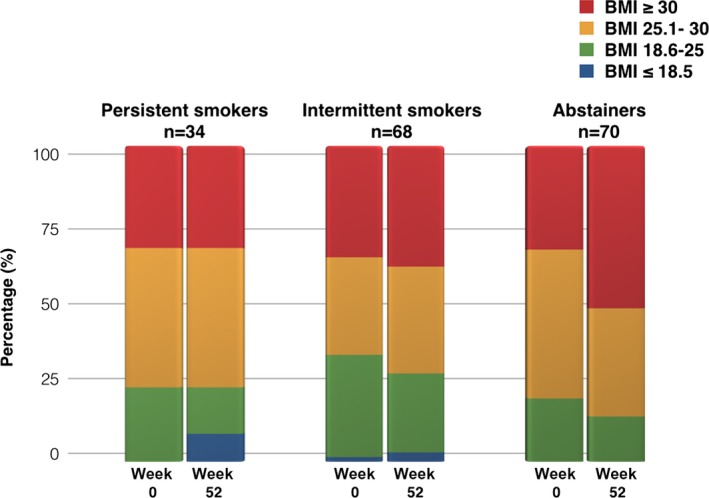
Changes in BMI according to pattern of smoking at week 52. The most notable change in BMI over the course of follow‐up was observed in the abstainers. The patients classified as obese (BMI ≥30.0) changed from 33% to 51%. BMI indicates body mass index.
Predictors of Weight Change at 12 Months
We conducted multivariable regression analyses to identify predictors of weight gain at 12 months after ACS. Our results revealed 2 potential predictors of weight change: smoking abstinence (difference of 5.5 kg compared with persistent smoking; 95% CI 2.0–8.9) and number of years smoked (difference of −0.2 kg per year smoked; 95% CI −0.3 to −0.04). No other variables appeared to be important, including sex, age, treatment (varenicline), baseline weight and BMI, prior use of nicotine replacement therapies or varenicline, and prior quit attempts. Nevertheless, because of wide CIs, the results of our analysis are inconclusive.
Weight Change and Blood Pressure
Patients who gained >5% of their body weight between baseline and 12 months experienced an increase in mean systolic BP of 12.3 mm Hg (SD 19.6) and an increase in mean diastolic BP of 6.5 mm Hg (SD 14.3). However, there appeared to be no significant difference in change of diastolic BP (difference in means −0.56 mm Hg; 95% CI −7.68 to 6.55) or systolic BP (difference in means 3.81 mm Hg; 95% CI −6.3 to 13.98) between those who had gained and those who had lost >5% of their baseline weight (Figure 4). Of the patients who moved up ≥1 BMI class (n=32), systolic BP increased a mean of 8.8 mm Hg (SD 23.8) and diastolic BP increased a mean of 4.4 mm Hg (SD 15.2). This increase in BP, however, did not translate into a higher net use of antihypertensive medications because there was an equal number of patients among those whose BMI increased who decreased antihypertensive medication use as those who increased its use (n=4, 12.5%).
Figure 4.
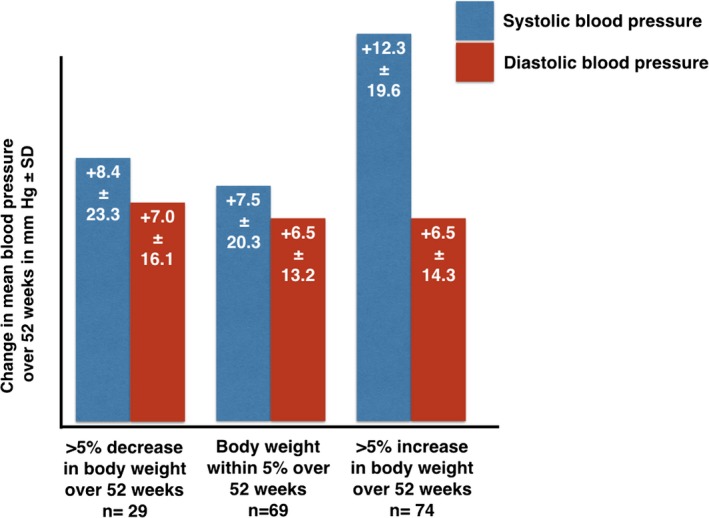
Changes in blood pressure according to percentage body weight gain. The entire cohort's blood pressure increased over the course of follow‐up with a numerically higher increase in systolic blood pressure in patients who gained >5%.
Weight Gain and Use of Antidiabetic Medications
In patients who gained >5% of their baseline weight (n=74), there was no change in the use of antidiabetic medications between baseline and the 12‐month visit (12.2% and 10.9%, respectively, of patients using antidiabetic medication). Similarly, in those who lost >5% of their baseline weight (n=29), there was no change in the number of patients using antidiabetic medications between baseline and 12 months (6.9% and 10.3%, respectively). The majority of patients (86.6%) reported no use of antidiabetic medications at baseline, and that remained unchanged at the end of follow‐up.
Discussion
Our study was designed to prospectively assess changes in weight in patients attempting to stop smoking following an ACS. Our results indicate that although patients smoked fewer cigarettes than they had before their index ACS, 3 in 5 patients were still smoking at 1 year after ACS. In addition, even though 73% of the entire cohort was overweight or obese at baseline, the weight of the entire cohort changed only by a mean of 2.6 kg between baseline and 12 months. Moreover, those who successfully stopped smoking gained an average of 4.8 kg, resulting in half of that cohort having a BMI ≥30. These findings suggest that it is important to target multiple lifestyle changes, including a comprehensive weight management strategy, such that the cardiovascular benefits achieved by smoking cessation are not counterbalanced by the untoward effects of weight gain in high‐risk patients after ACS.
The 1‐year relapse rate of returning to smoking in post‐ACS patients has been reported to be very high, between 73% to 78%.15 Despite their motivation to quit and the presence of a “teachable moment” while recovering from their myocardial infarction in the hospital, the relapse rate in our patient population was only slightly lower at 59%. This is likely due to the proven efficacy of varenicline.11 Patients in our trial had at least moderate nicotine dependence, as reflected by their Fagerström scores. The reduced consumption of cigarettes is encouraging because cutting the number of cigarettes reduces the future risk of reinfarction in a dose‐dependent fashion.16
Contrary to guideline recommendations, most patients have negligible weight loss in the 1 year following an ACS. In the PREMIER (Prospective Registry Evaluating Myocardial Infarction: Event and Recovery) cohort, 1253 patients were followed for 1 year, and the mean weight change was 0.2% (SD 7%).17 In a follow‐up analysis of weight change in 2408 patients after myocardial infarction in the ENRICHD (Enhancing Recovery in Coronary Heart Disease) program, average BMI was 28.95 (SD 6.02) at baseline and 28.41 (SD 5.72) at follow up.18 In fact, 18% experienced >5% weight gain at 2‐year follow‐up. In the most contemporary report of 179 smokers after ACS, there was an increase in median weight of 4.0 kg (IQR 0–7.0) between baseline and 12 months.10 Despite the elevated baseline mean BMI of 28.5 (SD 5.4), our own analysis showed a trend toward median weight gain of 3 kg (IQR −1.7 to 7.4). ACS patients' adherence to guideline‐directed therapies after discharge declines over the first year,19 with worse performance in the high‐risk patients compared with the low‐ and intermediate‐risk groups. Factors that could contribute to low adherence rates include a lack of continued emphasis by physicians during follow‐up on the importance of guideline‐directed medical therapies, inadequate patient education, and polypharmacy.
Weight gain in smokers who successfully stop smoking is well documented, with mean weight gain varying between 3 and 6 kg in North America.20 In a recent analysis of smokers in the post‐ACS population, abstainers gained a median of 4.8 kg (IQR 1.0–8.6) at 12 months, which was significantly more than the gain in patients who continued to smoke intermittently and/or persistently.10 Our trial is the only study that extends these findings in a similar cohort of patients hospitalized for an ACS. Although weight gain after smoking cessation is well documented, its implications after ACS have not been adequately studied. Despite a convincing link between smoking cessation and weight gain, the purported mechanisms to explain this association are less convincing. These mechanisms include decreased resting metabolic rate, decreased physical activity, and increased lipoprotein lipase activity.20 In addition, nicotine has a depressive effect on appetite; therefore, its withdrawal could lead to increased caloric intake.21 The above mechanistic studies, however, have not been performed in the post‐ACS patient—the subject of our analysis. There may be multiple reasons specific to our patient population that may lead to changes in weight. The acuity of presentation of the ACS (eg, ST‐segment elevation myocardial infarction), choice of pharmacotherapy to assist with smoking cessation (nicotine replacement therapy versus varenicline versus bupropion), or baseline demographics such as how obese the patient was at baseline could explain changes in body weight 12 months after ACS. Interestingly, other than abstaining from smoking at 12 months and number of years smoked, none of the factors mentioned seemed predictive of weight changes in our multivariable regression analyses. Nevertheless, the results of this analysis are inconclusive. The dose‐response curve observed in our study and that of Grandi et al,10 with the degree to which one stops smoking correlating with the amount of weight one gains, has implications about which populations should be targeted most aggressively for counseling regarding potential weight gain.
The secondary question that we attempted to answer in our analysis was whether this increase in weight leads to changes in measurable outcomes such as BP, BP‐lowering medications, or antidiabetic medications. In a community‐based cohort, 4‐year weight gain that occurred following smoking cessation did not modify its beneficial effect of reducing the number of cardiovascular events.22 Although our study was not powered for clinical end points, we found a similar trend in our analysis of post‐ACS patients. There was no significant association between weight gain and an increase in BP or the use of BP‐lowering medications and antidiabetic medications. Possible explanations include the fact that glucose tolerance and insulin sensitivity are impaired in smokers compared with nonsmokers,23 and by removing this metabolically offending agent, the negative consequences of weight gain are nullified. There is also evidence that abstainers experience improvements in their lipid profiles, independent of potential weight gain, such as a decrease in low‐density lipoprotein cholesterol and triglyceride levels and an increase in high‐density lipoprotein cholesterol levels.24, 25 Consequently, despite weight gain and its known deleterious effect on patients' metabolic profiles, the overall benefit still favors smoking cessation.
This study has several potential limitations. First, the participants included in our study differed significantly from those excluded regarding a number of baseline characteristics including age, weight, BMI, number of years smoked, prevalence of hypertension and hyperlipidemia, previous quit attempts, and prior use of abstinence aids. These discrepancies may affect the generalizability of our results. Second, only weight and BMI were recorded without any determination of body composition, waist‐to‐hip ratio, or cardiorespiratory fitness. However, although BMI does not always reflect true body fatness26, 27 or fitness,28 many of the cohort studies that assess the relationship between obesity and survival have repeatedly used BMI. Third, there is an inherent bias in smoking cessation trials in that patients who manage to quit smoking—independent of requiring pharmacological assistance—tend to be more health conscious and to adopt a healthier lifestyle than those continuing to smoke.29 This is reflected in a larger cohort of persistent smokers who are lost to follow‐up compared with abstainers.11, 15 Fourth, details such as medication dosages, metabolic profile such as hemoglobin A1c, and lipids were not routinely measured in follow‐up; therefore, inferences about prescribed medical therapy correlating to poor BP and DM control may be problematic. Finally, data on factors such as participants' physical activity levels, which may explain the apparent differences in weight change between smoking status groups, were not collected. Our results would have been more informed had physical activity levels been measured and compared between groups.
Conclusion
Our study was designed to evaluate the effect of smoking cessation on weight change at 12 months after ACS. Our study showed that >50% of patients return to smoking, albeit smoking fewer cigarettes, within 12 months. In addition, most of the patients gained weight, with abstainers gaining a mean of 4.8 kg. This weight gain was not associated with increased use of antihypertensive and antidiabetic medications. Weight management interventions among smokers who quit after ACS should be a focus of investigation in future research so that the cardiovascular benefits achieved by smoking cessation are not offset by weight gain in this high‐risk population.
Sources of Funding
The EVITA trial was an investigator‐initiated trial with funding and study drug/placebo provided by Pfizer Inc. Pfizer Inc had no role in the design, conduct, analysis, interpretation of data, or reporting of the EVITA trial. For this analysis, no additional funding was provided.
Disclosures
Drs Eisenberg and Dehghani received honoraria from Pfizer Inc. for providing continuing medical education on smoking cessation. The remaining authors have no disclosures to report.
Supporting information
Appendix S1. EVITA Trial Investigators and Committee Members.
Table S1. Comparison of Baseline Characteristics Between Those Included and Excluded From the Study
Acknowledgments
The authors would like to thank Patrick Belisle for his work on the statistical data analyses. A complete list of the EVITA Trial Investigators and Committee Members can be found in Appendix S1.
(J Am Heart Assoc. 2017;6:e004785 DOI: 10.1161/JAHA.116.004785.)28420644
References
- 1. Rea TD, Heckbert SR, Kaplan RC, Psaty BM, Smith NL, Lemaitre RN, Lin D. Body mass index and the risk of recurrent coronary events following acute myocardial infarction. Am J Cardiol. 2001;88:467–472. [DOI] [PubMed] [Google Scholar]
- 2. Critchley J, Capewell S. Smoking cessation for the secondary prevention of coronary heart disease. Cochrane Database Syst Rev. 2004;1:CD003041. [DOI] [PubMed] [Google Scholar]
- 3. Smith SC Jr, Benjamin EJ, Bonow RO, Braun LT, Creager MA, Franklin BA, Gibbons RJ, Grundy SM, Hiratzka LF, Jones DW, Lloyd‐Jones DM, Minissian M, Mosca L, Peterson ED, Sacco RL, Spertus J, Stein JH, Taubert KA; World Heart F, the Preventive Cardiovascular Nurses A . AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation. 2011;124:2458–2473. [DOI] [PubMed] [Google Scholar]
- 4. Zhang YJ, Iqbal J, van Klaveren D, Campos CM, Holmes DR, Kappetein AP, Morice MC, Banning AP, Grech ED, Bourantas CV, Onuma Y, Garcia‐Garcia HM, Mack MJ, Colombo A, Mohr FW, Steyerberg EW, Serruys PW. Smoking is associated with adverse clinical outcomes in patients undergoing revascularization with PCI or CABG: the SYNTAX trial at 5‐year follow‐up. J Am Coll Cardiol. 2015;65:1107–1115. [DOI] [PubMed] [Google Scholar]
- 5. Wilson K, Gibson N, Willan A, Cook D. Effect of smoking cessation on mortality after myocardial infarction: meta‐analysis of cohort studies. Arch Intern Med. 2000;160:939–944. [DOI] [PubMed] [Google Scholar]
- 6. Critchley JA, Capewell S. Mortality risk reduction associated with smoking cessation in patients with coronary heart disease: a systematic review. JAMA. 2003;290:86–97. [DOI] [PubMed] [Google Scholar]
- 7. Gerber Y, Rosen LJ, Goldbourt U, Benyamini Y, Drory Y; Israel Study Group on First Acute Myocardial I . Smoking status and long‐term survival after first acute myocardial infarction a population‐based cohort study. J Am Coll Cardiol. 2009;54:2382–2387. [DOI] [PubMed] [Google Scholar]
- 8. Janzon E, Hedblad B, Berglund G, Engstrom G. Changes in blood pressure and body weight following smoking cessation in women. J Intern Med. 2004;255:266–272. [DOI] [PubMed] [Google Scholar]
- 9. Nilsson P, Lundgren H, Soderstrom M, Fagerstrom KO, Nilsson‐Ehle P. Effects of smoking cessation on insulin and cardiovascular risk factors—a controlled study of 4 months' duration. J Intern Med. 1996;240:189–194. [DOI] [PubMed] [Google Scholar]
- 10. Grandi SM, Filion KB, Gervais A, Joseph L, O'Loughlin J, Paradis G, Rinfret S, Pilote L, Grondin FR, Lutchmedial S, Eisenberg MJ. Weight change in patients attempting to quit smoking post‐myocardial infarction. Am J Med. 2014;127:641–649.e641. [DOI] [PubMed] [Google Scholar]
- 11. Eisenberg MJ, Windle SB, Roy N, Old W, Grondin FR, Bata I, Iskander A, Lauzon C, Srivastava N, Clarke A, Cassavar D, Dion D, Haught H, Mehta SR, Baril JF, Lambert C, Madan M, Abramson BL, Dehghani P; Investigators E . Varenicline for smoking cessation in hospitalized patients with acute coronary syndrome. Circulation. 2016;133:21–30. [DOI] [PubMed] [Google Scholar]
- 12. Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. [DOI] [PubMed] [Google Scholar]
- 13. Beck AT, Steer RA, Ball R, Ciervo CA, Kabat M. Use of the Beck Anxiety and Depression Inventories for primary care with medical outpatients. Assessment. 1997;4:211–219. [DOI] [PubMed] [Google Scholar]
- 14. Stevens J, Truesdale KP, McClain JE, Cai J. The definition of weight maintenance. Int J Obes. 2005;30:391–399. [DOI] [PubMed] [Google Scholar]
- 15. Eisenberg MJ, Grandi SM, Gervais A, O'Loughlin J, Paradis G, Rinfret S, Sarrafzadegan N, Sharma S, Lauzon C, Yadav R, Pilote L; Investigators Z . Bupropion for smoking cessation in patients hospitalized with acute myocardial infarction: a randomized, placebo‐controlled trial. J Am Coll Cardiol. 2013;61:524–532. [DOI] [PubMed] [Google Scholar]
- 16. Teo KK, Ounpuu S, Hawken S, Pandey MR, Valentin V, Hunt D, Diaz R, Rashed W, Freeman R, Jiang L, Zhang X, Yusuf S; Investigators IS . Tobacco use and risk of myocardial infarction in 52 countries in the INTERHEART study: a case‐control study. Lancet. 2006;368:647–658. [DOI] [PubMed] [Google Scholar]
- 17. Fadl YY, Krumholz HM, Kosiborod M, Masoudi FA, Peterson PN, Reid KJ, Weintraub WS, Buchanan DM, Spertus JA. Predictors of weight change in overweight patients with myocardial infarction. Am Heart J. 2007;154:711–717. [DOI] [PubMed] [Google Scholar]
- 18. Lopez‐Jimenez F, Wu CO, Tian X, O'Connor C, Rich MW, Burg MM, Sheps D, Raczynski J, Somers VK, Jaffe AS. Weight change after myocardial infarction—the Enhancing Recovery in Coronary Heart Disease patients (ENRICHD) experience. Am Heart J. 2008;155:478–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shore S, Jones PG, Maddox TM, Bradley SM, Stolker JM, Arnold SV, Parashar S, Peterson P, Bhatt DL, Spertus J, Ho PM. Longitudinal persistence with secondary prevention therapies relative to patient risk after myocardial infarction. Heart. 2015;101:800–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Filozof C, Fernandez Pinilla MC, Fernandez‐Cruz A. Smoking cessation and weight gain. Obes Rev. 2004;5:95–103. [DOI] [PubMed] [Google Scholar]
- 21. Koopmann A, Dinter C, Grosshans M, von der Goltz C, Hentschel R, Dahmen N, Gallinat J, Wagner M, Grunder G, Thurauf N, Wienker T, Brinkmeyer J, Mobascher A, Spreckelmeyer KN, Clepce M, de Millas W, Wiedemann K, Winterer G, Kiefer F. Psychological and hormonal features of smokers at risk to gain weight after smoking cessation—results of a multicenter study. Horm Behav. 2011;60:58–64. [DOI] [PubMed] [Google Scholar]
- 22. Clair C, Rigotti NA, Porneala B, Fox CS, D'Agostino RB, Pencina MJ, Meigs JB. Association of smoking cessation and weight change with cardiovascular disease among adults with and without diabetes. JAMA. 2013;309:1014–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Facchini FS, Hollenbeck CB, Jeppesen J, Chen YD, Reaven GM. Insulin resistance and cigarette smoking. Lancet. 1992;339:1128–1130. [DOI] [PubMed] [Google Scholar]
- 24. Gepner AD, Piper ME, Johnson HM, Fiore MC, Baker TB, Stein JH. Effects of smoking and smoking cessation on lipids and lipoproteins: outcomes from a randomized clinical trial. Am Heart J. 2011;161:145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Eliasson B, Attvall S, Taskinen MR, Smith U. Smoking cessation improves insulin sensitivity in healthy middle‐aged men. Eur J Clin Invest. 1997;27:450–456. [DOI] [PubMed] [Google Scholar]
- 26. Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009;53:1925–1932. [DOI] [PubMed] [Google Scholar]
- 27. Lavie CJ, De Schutter A, Patel D, Artham SM, Milani RV. Body composition and coronary heart disease mortality—an obesity or a lean paradox? Mayo Clin Proc. 2011;86:857–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lavie CJ, De Schutter A, Patel DA, Milani RV. Body composition and fitness in the obesity paradox—body mass index alone does not tell the whole story. Prev Med. 2013;57:1–2. [DOI] [PubMed] [Google Scholar]
- 29. Senechal M, Swift DL, Johannsen NM, Blair SN, Earnest CP, Lavie CJ, Church TS. Changes in body fat distribution and fitness are associated with changes in hemoglobin A1c after 9 months of exercise training: results from the HART‐D study. Diabetes Care. 2013;36:2843–2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. EVITA Trial Investigators and Committee Members.
Table S1. Comparison of Baseline Characteristics Between Those Included and Excluded From the Study


