Abstract
Background
Patients with chronic kidney disease (CKD) are at increased risk for bleeding, transfusion, and dialysis after cardiac catheterization. Whether rates of these complications are increased in this high‐risk population undergoing transradial access compared with transfemoral access is unknown.
Methods and Results
From the Veterans Affairs (VA) Clinical Assessment Reporting and Tracking program, we identified 229 108 patients undergoing cardiac catheterization between 2007 and 2014, of which 48 155 (21.0%) had baseline glomerular filtration rate (GFR) between 15 and 59 mL/min. We used multivariable Cox modeling to determine the independent association between transradial access and postprocedure transfusion as well as progression to new dialysis by degree of renal dysfunction. Overall, 35 979 (15.7%) of patients underwent Transradial access. Transradial patients tended to be slightly younger, but, overall, had similar rates of CKD compared to transfemoral patients (24.3% vs 27.1%). Transradial patients had longer fluoroscopy times (7.2 vs 6.0 minutes; P<0.001), but lower contrast use (85.0 vs 100.0 mL; P<0.001). The estimated rate of blood transfusion within 48 hours was lower among transradial patients (0.85% vs 1.01%) as were rates of new dialysis at 1 year (0.58% vs 0.71%). After multivariable adjustment, transradial access was associated with lower rates of progression to dialysis at 1 year overall (hazard ratio [HR], 0.83; 95% CI, 0.70–0.98), with no trend of increased risk for dialysis by degree of CKD compared with transfemoral access. Transradial access was associated with greater reduction in transfusion rates with increasing degree of CKD (P value for trend=0.04: non‐CKD: HR, 0.99; 95% CI, 0.73–1.34; GFR 45–59 mL/min: HR, 0.93; 95% CI, 0.70–1.23; GFR 30–44 mL/min: HR, 0.73; 95% CI, 0.51–1.03; GFR 15–29 mL/min: HR, 0.43; 95% CI, 0.20–0.90).
Conclusions
Among patients undergoing cardiac catheterization in the VA health system, transradial access was associated with lower risk for postprocedure transfusion within 48 hours among patients with more‐severe CKD, and with lower risk of progression to end‐stage renal disease at 1 year compared with transfemoral access. These data provide additional evidence that transradial access may provide significant benefit in this high‐risk population.
Keywords: blood transfusion, chronic kidney disease, dialysis, radial artery catheter
Subject Categories: Quality and Outcomes, Percutaneous Coronary Intervention, Coronary Artery Disease
Introduction
Chronic kidney disease (CKD) is common among patients with coronary artery disease (CAD) and is independently associated with increased risk for adverse cardiovascular and renal outcomes.1, 2, 3 Clinical trials often exclude patients with CKD because of their likelihood of suffering bleeding complications with antithrombotic therapy and progression to dialysis after cardiac catheterization.4 As such, evidence‐based approaches to patients with coexisting CKD and CAD are lacking.
The risk for dialysis after cardiac catheterization can be attributed to atheroemboli from aortic atherosclerosis,5, 6, 7 direct renal injury from iodinated contrast,8 or a combination of these factors. The only accepted strategies to reduce the incidence of renal injury are volume expansion and minimization of contrast load.9 Similarly, the increased risk for bleeding in patients with CKD may be attributed to reduced clearance of antithrombotic agents, increased vascular calcification and stiffness leading to vascular complications, or both.10 In this context, the use of radial artery access for cardiac catheterization and percutaneous coronary intervention (PCI) is an attractive strategy to reduce both the risk for bleeding and dialysis, because the catheter avoids the abdominal aorta and the radial artery is superficial and lends itself more easily to hemostasis. On the other hand, it may be prudent to avoid radial arterial access because the attendant damage to the artery can increase the risk for radial artery occlusion and complicate the placement of permanent dialysis access. Thus, the role of a radial approach in patients with CKD remains unclear.
Accordingly, we used data from the Veterans Affairs (VA) Clinical Assessment Reporting and Tracking (CART) program to compare procedural characteristics, rates of blood transfusion within 48 hours of cardiac catheterization, and progression to new dialysis within 1 year in patients undergoing cardiac catheterization by the transradial and transfemoral approach, stratified by degree of renal dysfunction. We hypothesized that patients undergoing transradial access would have lower rates of transfusion within 48 hours, but similar rates of progression to new dialysis by 1 year, with greatest potential benefit observed in patients with more‐severe renal disease.
Methods
Data Sources
The Veterans Affairs (VA) Clinical Assessment Reporting and Tracking (CART) program is a national clinical quality program for all VA cardiac catheterization laboratories. It collects data about catheterization procedures performed in all 78 VA cardiac catheterization laboratories using a software application that is embedded within the VA electronic medical record, which then allows for data linkage in order to assess short‐ and long‐term longitudinal outcomes. Institutional review board and VA research and development approvals were obtained for the creation of the data set and for this particular study. This study was approved by the Colorado Multiple Institutional Review Board (COMIRB) with waiver of informed consent, given the retrospective nature of the study.
The data elements included within the CART program are standardized by the American College of Cardiology's National Cardiovascular Data Registry11 and include information on procedural indications, demographic and clinical characteristics, presentation details, procedures performed, access site, periprocedural complications, and preprocedure and intraprocedure medications. Continuous monitoring, maintenance, and updating of the CART application are performed by a dedicated staff, and the quality and integrity of the data are maintained through the use of standardized data definitions, uniform data transmission protocols, and routine data quality checks and audits. Additional details of the design and conduct of this registry have been previously described.12, 13, 14, 15
Study Population and Data Definitions
We studied all veterans undergoing cardiac catheterization for any indication in the VA Health System between October 1, 2007 and September 30, 2014. Among the 261 274 patients in the initial sample, we excluded patients with no information about access site (N=2863), access site crossover (N=2134), no administrative data for follow‐up (N=41), past cardiac transplant (N=1552), treated at sites with no transradial access patients (N=1102), with estimated glomerular filtration rate (eGFR) <15 mL/min (N=7477), those on dialysis at the time of the catheterization (N=2877), and those with eGFR >59 mL/min but a documented history of CKD (N=14 120). eGFR was calculated using the abbreviated Modification of Diet in Renal Disease equation,16 based on the most recent measured creatinine before catheterization, which was ≤30 days before the date of catheterization. We identified patients undergoing cardiac catheterization by a transradial or transfemoral route. Patients were stratified by degree of CKD into 4 categories based on the National Kidney Foundation Kidney Disease Outcomes Quality Initiative: ≥59 mL/min (no CKD), 45 to 59 mL/min (stage 3A), 30 to 44 mL/min (stage 3B), and 15 to 29 mL/min (stage 4).
Outcomes of Interest
The primary clinical events of interest were the occurrence of blood transfusion within 48 hours and progression to dialysis within 1 year of the index catheterization. Blood transfusion was used as a surrogate for bleeding complications given that bleeding events in CART are not adjudicated. Progression to new dialysis was assessed by dialysis procedures recorded in VA administrative and fee‐based data sources after the date of the catheterization through September 2014. Because outcomes were censored for some patients, we used survival methods that account for censoring in the analysis, as described below.
Statistical Analysis
We compared baseline demographic, clinical, and presentation characteristics between patients undergoing cardiac catheterization by a transradial versus a transfemoral approach. Continuous variables are expressed as median values with 25th and 75th percentiles whereas categorical values are presented as percentages. We used Pearson chi‐squared tests for categorical variables and Wilcoxon rank‐sum tests for continuous variables.
We compared the unadjusted rates of each outcome of interest using cumulative incidence plots, estimated event rates, and Gray's test, accounting for both censoring and death as a competing risk. We then used Cox proportional hazards modeling with a robust covariance estimator to account for correlation by hospital in order to determine the independent association between transradial access and postprocedure transfusion as well as progression to new dialysis, using transfemoral as the reference. We modeled this relationship between access site and the outcomes with and without an interaction term between access site and degree of CKD and evaluated the trend across degree of CKD using type 3 tests in the SAS Phreg procedure. The outcomes were adjusted for the following variables: demographics (age, sex, race, Hispanic ethnicity, and obesity); medical history (hypertension, hyperlipidemia, diabetes mellitus, tobacco use, chronic obstructive pulmonary disease [COPD], peripheral artery disease [PAD], past myocardial infarction [MI], past cardiac transplant, past coronary artery bypass surgery [CABG], congestive heart failure [CHF], cerebrovascular disease, post‐traumatic stress disorder [PTSD], depression, obstructive sleep apnea); anemia; and presence of cardiogenic shock or heart failure on admission.
We performed a sensitivity analysis in which we repeated the Cox models comparing the risk of the outcomes for transradial access relative to transfemoral access in the subset of patients with both ad‐hoc PCI and nonmissing contrast volume (N=47 412). We performed another sensitivity analysis restricting the study population to sites performing transradial access in at least 5% of patients. Additionally, we sought to explore whether the association between access site and progression to dialysis was mediated by the decrease in transfusion among patients undergoing transradial access.17 We performed the 4‐step mediation analysis developed by Baron and Kenney using similar Cox proportional hazards models to those described above.18
A P value of 0.05 was considered statistically significant. All statistical analyses were performed by the CART Coordinating Center at the Denver VA Medical Center using SAS (version 9.4; SAS Institute Inc., Cary, NC) and R software (version 3.2.2; R Foundation for Statistical Computing, Vienna, Austria). The study was approved by the COMIRB.
Results
Between 2007 and 2014, 261 274 patients underwent cardiac catheterization. After applying exclusions, the final study sample consisted of 229 108 patients at 78 cardiac catheterization facilities across the VA Health System, of which 35 979 (15.7%) underwent catheterization by transradial access (Figure 1). Baseline characteristics for the 2 groups are listed in Table 1. Transradial access patients were younger, more likely African American, and more likely obese, but had lower rates of past cardiac events and procedures compared to transfemoral access patients; most differences were statistically significant but clinically modest. Patients with past cardiac procedures were less likely to undergo catheterization by a transradial route. Transradial patients had longer fluoroscopy times (7.2 vs 6.0 minutes; P<0.001), but lower contrast use (85.0 vs 100.0 mL; P<0.001). When stratified by degree of CKD, transradial access patients were less likely to have stage 3B or 4 CKD than transfemoral patients: no CKD: 80.5% versus 78.7%; GFR 45 to 59 mL/min: 14.8% versus 15.5%; GFR 30 to 44 mL/min: 4.1% versus 4.9%; GFR 15 to 29 mL/min: 0.6% versus 0.9% (P<0.001); however, overall differences were modest. Rates of transradial versus transfemoral access are described in Table S1.
Figure 1.
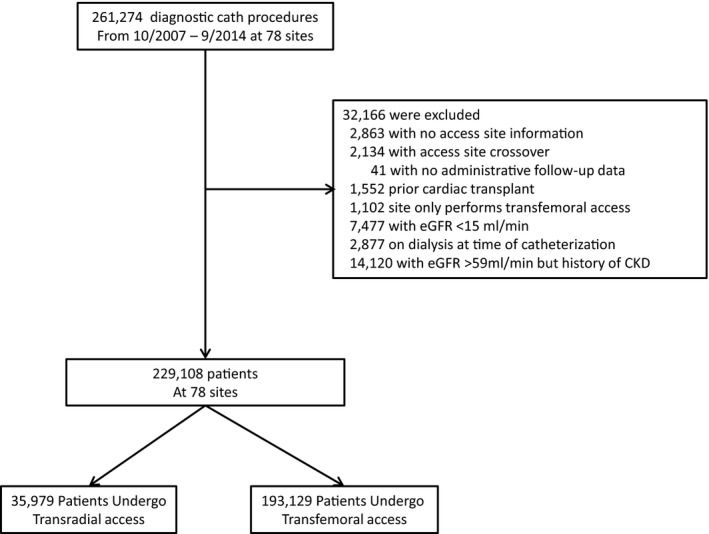
Study population characteristics. This figure displays the study population characteristics, including exclusions. CKD indicates chronic kidney disease; eGFR, estimated glomerular filtration rate.
Table 1.
Baseline Characteristicsa
| Overall (N=229 108) | Transradial Access (n=35 979) | Transfemoral Access (n=193 129) | |
|---|---|---|---|
| Demographics | |||
| Age in y, median (IQR) | 64 (59–70) | 64 (59–68) | 64 (60–70) |
| Male | 97.0 | 96.8 | 97.0 |
| Race | |||
| White | 79.2 | 74.8 | 80.0 |
| Black | 13.9 | 18.1 | 13.1 |
| Other | 6.9 | 7.1 | 6.9 |
| Hispanic | 4.7 | 3.6 | 4.9 |
| BMI, median (IQR) | 29.9 (26.4–34.0) | 30.3 (26.7–35.1) | 29.8 (26.4–33.9) |
| Past medical history | |||
| Past MI | 32.7 | 30.2 | 33.1 |
| Past CHF | 26.4 | 25.0 | 26.6 |
| Past CVA | 16.1 | 15.6 | 16.2 |
| Past PCI | 32.4 | 30.0 | 32.8 |
| Past CABG | 21.8 | 11.7 | 23.7 |
| Hypertension | 88.2 | 89.4 | 88.0 |
| Diabetes mellitus | 45.2 | 46.3 | 45.0 |
| Dyslipidemia | 86.5 | 86.7 | 86.5 |
| PAD | 18.4 | 20.4 | 18.1 |
| COPD | 22.2 | 21.4 | 22.4 |
| PTSD | 17.4 | 20.8 | 16.7 |
| Depression | 31.5 | 33.2 | 31.1 |
| Sleep apnea | 20.8 | 25.1 | 20.0 |
| Presenting features | |||
| Acute coronary syndrome | 20.3 | 18.6 | 20.6 |
| Heart failure | 6.0 | 5.6 | 6.1 |
| Cardiogenic shock within 24 hours | |||
| Systolic BP on presentation | 132 (123–141) | 133 (124–142) | 132 (123–141) |
| Heart rate on presentation | 69 (61–80) | 70 (62–80) | 69 (61–79) |
| Hemoglobin, g/dL | 13.7 (12.5–14.7) | 13.7 (12.5–14.7) | 13.8 (12.6–14.8) |
| Creatinine | 1.0 (0.9–1.2) | 1.0 (0.9–1.2) | 1.0 (0.9–1.2) |
| Procedural features | |||
| Fluoroscopy time | 6.1 (3.5–11.2) | 7.2 (4.4–12.3) | 6.0 (3.3–11.0) |
| Contrast volume, mL | 100 (70–136) | 85.0 (60.0–125.0) | 100 (70–140) |
| LV ventriculography | 38.7 | 33.8 | 39.6 |
BMI indicates body mass index; BP, blood pressure; CABG, coronary artery bypass graft surgery; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; IQR, interquartile range; LV, left ventricular; MI, myocardial infarction; PAD, peripheral artery disease; PCI, percutaneous coronary intervention; PTSD, post‐traumatic stress disorder.
Data are expressed as percentage of patients for categorical variables, median (interquartile range) for continuous variables.
Rates of Blood Transfusion Within 48 Hours and Progression to New Dialysis During Follow‐up
The cumulative incidence curve for the rate of blood transfusion within 48 hours is shown in Figure 2. The estimated rate of blood transfusion within 48 hours of cardiac catheterization was 0.99% (95% CI, 0.94–1.02); it was 0.85% (95% CI, 0.75–0.94) among those who underwent catheterization by transradial access and 1.01% (95% CI, 0.96–1.05) by transfemoral access (P=0.004). Within each access category, transfusion rates were higher among patients with more‐advanced CKD (Table 2).
Figure 2.
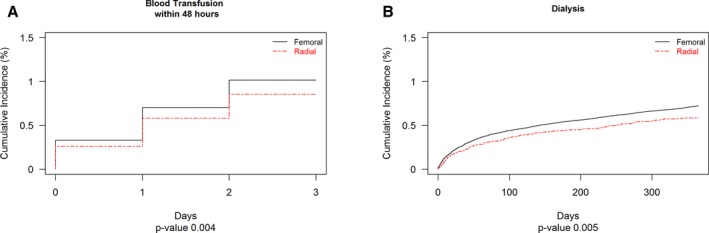
Cumulative incidence of reported outcomes. This figure displays the cumulative incidence of (A) blood transfusion within 48 hours and (B) progression to dialysis at 1 year.
Table 2.
Estimated Cumulative Incidence of Outcomes (%; 95% CI)
| Total (N=229 108) | Femoral | Radial | |||||||
|---|---|---|---|---|---|---|---|---|---|
| GFR >59, mL/min | GFR: 45 to 59, mL/min | GFR: 30 to 44, mL/min | GFR: 15 to 29, mL/min | GFR >59, mL/min | GFR: 45 to 59, mL/min | GFR: 30 to 44, mL/min | GFR: 15 to 29, mL/min | ||
| N=152 006 (66.3%) | N=29 892 (13.0%) | N=9491 (4.1%) | N=1740 (0.8%) | N=28 947 (12.6%) | N=5315 (2.3%) | N=1485 (0.6%) | N=232 (0.1%) | ||
| New dialysis | 0.69 (0.65, 0.72) | 0.31 (0.28, 0.34) | 0.95 (0.84, 1.06) | 3.21 (2.81, 3.51) | 19.26 (16.49, 20.13) | 0.28 (0.22, 0.34) | 1.08 (0.8, 1.35) | 2.59 (1.75, 3.36) | 14.36 (8.87, 17.63) |
| Transfusion within 48 hours | 0.99 (0.94, 1.02) | 0.79 (0.74, 0.83) | 1.3 (1.16, 1.42) | 2.72 (2.37, 3.02) | 6.41 (5.12, 7.42) | 0.72 (0.62, 0.82) | 1.13 (0.84, 1.41) | 1.9 (1.19, 2.58) | 3.05 (0.79, 5.2) |
GFR indicates glomerular filtration rate.
The cumulative incidence curve for the progression to dialysis at 1 year is shown in Figure 2. An estimated 0.69% (95% CI, 0.65–0.72) of patients progressed to new dialysis during the follow‐up period. Unadjusted rates of progression to dialysis were slightly lower among transradial patients (0.58%; 95% CI, 0.50–0.65 vs 0.71%; 95% CI, 0.67–0.75; P=0.005) and increased with increasing severity of CKD for both groups.
Multivariable Analyses
We performed Cox proportional hazards modeling to compare risks of blood transfusion and progression to new dialysis between patients undergoing catheterization by each access strategy. For each outcome, we tested the interaction between transradial access and degree of CKD, and the trend in association of transradial with outcomes by degree of CKD. After multivariable adjustment, transradial access was associated with decreasing rates of blood transfusion within 48 hours with increasing severity of CKD, with significant association only for patients with most‐severe CKD (P value for trend=0.04; no CKD: hazard ratio [HR]=0.99; 95% CI, 0.73–1.34; GFR 45–59 mL/min: HR=0.93; 95% CI, 0.70–1.23; GFR 30–44 mL/min: HR=0.73; 95% CI, 0.51–1.03; GFR 15–29 mL/min: HR=0.43; 95% CI, 0.20–0.90; Figure 3). Combining all patients, risk of transfusion did not differ significantly by access site (HR, 0.91; 95% CI, 0.72–1.15; P=0.44), but among CKD patients only, risk for transfusion was lower among transradial patients (HR, 0.79; 95% CI, 0.64–0.98; P=0.03).
Figure 3.
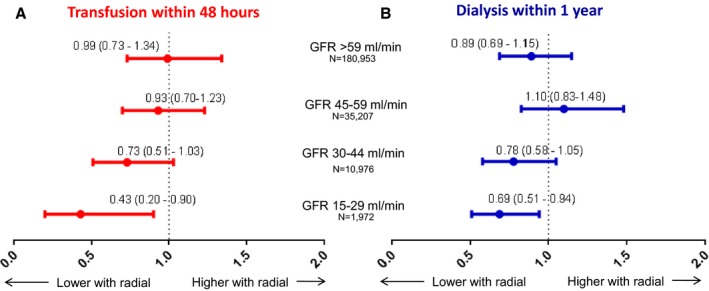
Hazard ratios for reported outcomes, stratified by degree of chronic kidney disease. This figure displays the adjusted hazard ratio for (A) blood transfusion within 48 hours and (B) progression to dialysis at 1 year, stratified by degree of chronic kidney disease. GFR indicates glomerular filtration rate.
Among all patients undergoing cardiac catheterization, risk of progression to end‐stage renal disease (ESRD) within 1 year was lower among patients undergoing transradial access (HR, 0.83; 95% CI, 0.70–0.98; P=0.03) and was similar when restricted only to patients with CKD (HR, 0.80; 95% CI, 0.68–0.94; P=0.008). The benefit of transradial access did not vary significantly with degree of CKD, nor was there a significant trend by degree of CKD (P value for interaction=0.15; P value for trend=0.08), although the HRs were lowest among those with the most‐severe CKD (no CKD: HR=0.89; 95% CI, 0.69–1.15; GFR 45–59 mL/min: HR=1.1; 95% CI, 0.83–1.48; GFR 30–44 mL/min: HR=0.78; 95% CI, 0.58–1.05; GFR 15–29 mL/min: HR=0.69; 95% CI, 0.51–0.94; Figure 3).
In sensitivity analysis, after adjusting for contrast volume and excluding patients who did not undergo ad‐hoc PCI, similar trends were observed with respect to blood transfusion and progression to dialysis (Table S2). After repeating the analysis excluding 28 sites performing transradial access in <5% of patients, results similar to those in the primary analysis were observed (Table S3). We also excluded patients with a history of past CABG (Table S4) and demonstrated results similar to those in the primary analysis. To explore the mediating effect of lower transfusion rates among transradial access patients in progression to dialysis, we adjusted for transfusions as a potential intermediary mechanism in the model of progression to dialysis. After adjustment, the HR for access site (transradial vs transfemoral) was 0.81 (95% CI, 0.67–0.96; P=0.01), with the occurrence of transfusion within 48 hours being independently associated with dialysis (HR, 2.97; 95% CI, 2.26–3.92; P<0.001), suggesting that although bleeding was associated with higher risk for dialysis, the reduction in bleeding with transradial access did not seem to be the mediator of the decreased risk for dialysis with transradial access.
Discussion
In this large, national sample of veterans undergoing cardiac catheterization, we observed that transradial access was associated with: (1) slightly increased fluoroscopy times yet no increased used in contrast; (2) significantly lower rates of blood transfusion within 48 hours among patients with CKD, with increasing observed benefit with increasing severity of renal dysfunction; and (3) lower rates of progression to dialysis at 1 year, particularly among patients with severe CKD, compared with transfemoral access; this effect was not mediated by the decreased bleeding with transradial access. Therefore, the results of this study suggest that transradial access is associated with lower risk of periprocedural bleeding in CKD patients with greatest benefit in patients with severe CKD. Additionally, rates of progression to ESRD appear to be lower among transradial patients. These data suggest that transradial may be safer in patients with CKD; however, prospective, randomized trials in this population are needed to confirm our observational findings.
Acute kidney injury (AKI) attributed to contrast‐induced nephropathy is a known complication of cardiac catheterization, and the risk of AKI is directly related to the degree of CKD before catheterization, amount of contrast used during the procedure, and past burden of atheroemboli in the aorta. We found slightly lower overall usage of contrast in patients undergoing catheterization by transradial access. Whereas the overall difference in contrast usage between the transradial and transfemoral patients is modest and clinically similar, previous studies have reported varying levels of contrast use during transradial procedures.19, 20 Importantly, patients undergoing transradial access were less likely to have past CABG (and thus require coronary bypass angiography) and also less likely to undergo left ventriculography. Nevertheless, our data provide some reassurance that transradial catheterization may not worsen CKD attributed to a higher volume of contrast during the procedure.
Clinically significant bleeding events are among the most common complications post‐PCI and have significant downstream consequences, including increased rates of stroke, nonfatal MI, and short‐ and long‐term mortality. Although estimates of major bleeding complications vary widely in the literature, depending on the specific population and the precise definition,21, 22, 23, 24 more‐recent estimates have placed the rate of major bleeding events at less than 5%, a decrease that has been attributed to better periprocedural anticoagulation and transradial access. CKD has consistently been shown to be a major risk factor for bleeding across clinical trials and registries.25, 26 Indeed, rates of bleeding among patients with CKD undergoing PCI have been reported to be even higher compared to patients without CKD. Saltzman et al noted an almost 3‐fold higher rate of major bleeding (19.3% vs 6.7%; P<0.001) among patients presenting with ST‐elevation myocardial infarction (STEMI) undergoing PCI in the HORIZONS‐AMI (heparin plus a glycoprotein IIb/IIIa inhibitor versus bivalirudin monotherapy and paclitaxel‐eluting stents versus bare‐metal stents in acute myocardial infarction) trial.27 In the non‐ST‐elevation myocardial infarction population, an analysis using the ACTION Registry—Get With the Guidelines noted that patients with stage 3 to 5 CKD had 2‐ to 6‐fold increased unadjusted risk of major bleeding compared to patients with an eGFR ≥60 mL/min per 1.73 m2, a difference that was attenuated, but persisted, after multivariable adjustment.27 Randomized trial data19, 28 as well as large‐scale registry data29 have described the significant reduction in major bleeding with transradial access compared with transfemoral access. Our study confirms and extends those findings in a number of ways. We report significantly lower bleeding rates among patients with CKD. Additionally, we demonstrate lower bleeding with a directional trend toward increased benefit in patients with the most‐severe CKD, a population that is at highest baseline risk of significant bleeding. These results suggest that the benefit of transradial access may be greatest in patients at highest risk of bleeding, which is a novel finding. Nevertheless, the benefits in major bleeding with transradial access need to be weighed against the overall risk of progression to ESRD, a situation in which preservation of potential access sites for dialysis is of primary importance. We report that an estimated 0.69% of patients progressed to new dialysis within 1 year, with lower rates among transradial versus transfemoral patients (0.58% vs 0.71%; P=0.005). There was a clear relationship between degree of renal insufficiency and progression to new dialysis, with 19.6% of patients with stage 4 CKD (GFR 15–29 mL/min) progressing to new dialysis, but only 1.0% of patients with stage 3A CKD (GFR 45–59 mL/min). There is concern that transradial access may damage the radial artery, making it potentially unusable for subsequent arteriovenous fistula (AVF) creation. Previous studies have noted increased inflammation by the introducer sheath30 and thrombus formation, and optical coherence tomography of accessed vessels have demonstrated intimal tears, medial dissections, and the formation of microthrombi.31 Although our data do not describe rates of access‐site complications that may complicate radiocephalic AVF creation, previous studies have underscored the risk of radial artery occlusion (RAO). The risk for RAO can be minimized with low‐profile equipment, adequate anticoagulation, prevention of radial artery spasm, and use of nonocclusive hemostasis after transradial procedures. These strategies have been shown to reduce RAO rates to 0.1% to 1.5%.32 Additionally, difficulty with radiocephalic AVF creation typically is attributed to complications with the cephalic vein, not the radial artery.33 These data, as well as our findings, should be taken into account when selecting an access site in CKD patients undergoing cardiac catheterization or PCI.
Our study must be considered in the context of several important limitations. First, our data only captured rates of transfusion within 48 hours after cardiac catheterization and do not capture actual rates of major or minor bleeding. It is possible that there is heterogeneity in individual providers' threshold for administering a transfusion.34 Nevertheless, large studies have described worse outcomes post‐PCI in patients that have required transfusion.35 Next, our data only describe progression to new dialysis at 1 year; it is likely that risk of developing worsening renal disease increases over time. Nevertheless, given the safety profile of transradial access with respect to bleeding, it would seem reasonable to consider a strategy that minimizes a known complication given the theoretical risk of challenging AVF creation further into the future. Third, we do not have systematic data on access site complications with either transfemoral or transradial access. We also do not have data regarding possible increased difficulty with creating a radiocephalic AVF after transradial access. Fourth, we did not capture right radial versus left radial access, which may have affected the total amount of fluoroscopy time and contrast usage among transradial patients. We also did not collect operator experience, which also may have affected our findings. Additionally, access‐site selection was not random, and there is the possibility that the observed benefit associated with transradial access was attributed to patient selection factors and not access route. Specifically, we do not have information on whether the patient present with STEMI, the patient population for which strong evidence exists regarding the superiority of transradial access. Because patients undergoing transradial access were less likely to have a past history of CABG and also less likely to undergo left ventriculography, lower use of contrast during the procedure may be affected by these factors more than the actual access site. Our sensitivity analyses adjusted for contrast use and did not demonstrate a significant change in the overall results. Finally, because this is an observational analysis, we cannot draw causal inferences from these results, and we cannot exclude the possibility of unmeasured confounding.
Conclusions
Among patients undergoing cardiac catheterization in the VA health system, transradial access was associated with greater reduction in postprocedure transfusion within 48 hours with increasing severity of CKD and less progression to ESRD at 1 year. These data provide additional evidence that transradial access may provide significant benefit in this high‐risk population and may be considered when selecting an access site for CKD patients undergoing PCI; however, prospective, randomized trials in this population are needed to confirm our observational findings.
Sources of Funding
The CART program is an operational program of the Department of Veterans Affairs Office of Information and Analytics. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the US government.
Disclosures
Dr Vora was funded by NIH T‐32 training grants T32 HL069749 and L30 HL124592. However, no relationships exist related to the analysis presented. Dr Nallamothu is supported by the following grants: United States Renal Data System Coordinating Center HHSN2762014 00001C (NIH) and End Stage Renal Disease (ESRD) Quality Measure Development, Maintenance, and Support HHSM‐500‐2013‐13017I (CMS). Dr Gurm reports research funding from the National Institutes of Health and consulting for Osprey Medical. Dr Rao reports research funding from Bellerophon; consulting for Terumo Medical, The Medicines Company, and ZOLL. The remaining authors have no disclosures to report.
Supporting information
Table S1. Rates of Transradial Versus Transfemoral Access by Degree of Renal Dysfunction
Table S2. After Adjustment for Contrast Use and Excluding Patients Undergoing Same‐Day PCI
Table S3. After Excluding Sites (n=28) With <5% Transradial Use
Table S4. Excluding Patients With Past CABG
(J Am Heart Assoc. 2017;6:e004819 DOI: 10.1161/JAHA.116.004819.)28420645
References
- 1. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. [DOI] [PubMed] [Google Scholar]
- 2. Menon V, Sarnak MJ. The epidemiology of chronic kidney disease stages 1 to 4 and cardiovascular disease: a high‐risk combination. Am J Kidney Dis. 2005;45:223–232. [DOI] [PubMed] [Google Scholar]
- 3. Chonchol M, Whittle J, Desbien A, Orner MB, Petersen LA, Kressin NR. Chronic kidney disease is associated with angiographic coronary artery disease. Am J Nephrol. 2008;28:354–360. [DOI] [PubMed] [Google Scholar]
- 4. Charytan D, Kuntz RE. The exclusion of patients with chronic kidney disease from clinical trials in coronary artery disease. Kidney Int. 2006;70:2021–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fukumoto Y, Tsutsui H, Tsuchihashi M, Masumoto A, Takeshita A. The incidence and risk factors of cholesterol embolization syndrome, a complication of cardiac catheterization: a prospective study. J Am Coll Cardiol. 2003;42:211–216. [DOI] [PubMed] [Google Scholar]
- 6. Johnson LW, Esente P, Giambartolomei A, Grant WD, Loin M, Reger MJ, Shaw C, Walford GD. Peripheral vascular complications of coronary angioplasty by the femoral and brachial techniques. Cathet Cardiovasc Diagn. 1994;31:165–172. [DOI] [PubMed] [Google Scholar]
- 7. Saklayen MG, Gupta S, Suryaprasad A, Azmeh W. Incidence of atheroembolic renal failure after coronary angiography. A prospective study. Angiology. 1997;48:609–613. [DOI] [PubMed] [Google Scholar]
- 8. Schweiger MJ, Chambers CE, Davidson CJ, Blankenship J, Bhalla NP, Block PC, Dervan JP, Gasperetti C, Gerber L, Kleiman NS, Krone RJ, Phillips WJ, Siegel RM, Uretsky BF, Laskey WK. Prevention of contrast induced nephropathy: recommendations for the high risk patient undergoing cardiovascular procedures. Catheter Cardiovasc Interv. 2007;69:135–140. [DOI] [PubMed] [Google Scholar]
- 9. Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM, Khot UN, Lange RA, Mauri L, Mehran R, Moussa ID, Mukherjee D, Nallamothu BK, Ting HH. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol. 2011;58:e44–e122. [DOI] [PubMed] [Google Scholar]
- 10. Lutz J, Menke J, Sollinger D, Schinzel H, Thurmel K. Haemostasis in chronic kidney disease. Nephrol Dial Transplant. 2014;29:29–40. [DOI] [PubMed] [Google Scholar]
- 11. Brindis RG, Fitzgerald S, Anderson HV, Shaw RE, Weintraub WS, Williams JF. The American College of Cardiology‐National Cardiovascular Data Registry (ACC‐NCDR): building a national clinical data repository. J Am Coll Cardiol. 2001;37:2240–2245. [DOI] [PubMed] [Google Scholar]
- 12. Byrd JB, Vigen R, Plomondon ME, Rumsfeld JS, Box TL, Fihn SD, Maddox TM. Data quality of an electronic health record tool to support VA cardiac catheterization laboratory quality improvement: the VA Clinical Assessment, Reporting, and Tracking System for Cath Labs (CART) program. Am Heart J. 2013;165:434–440. [DOI] [PubMed] [Google Scholar]
- 13. Box TL, McDonell M, Helfrich CD, Jesse RL, Fihn SD, Rumsfeld JS. Strategies from a nationwide health information technology implementation: the VA CART story. J Gen Intern Med. 2010;25(suppl 1):72–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tsai TT, Box TL, Gethoffer H, Noonan G, Varosy PD, Maddox TM, Fihn SD, Gross TP, Jesse RL, Rumsfeld JS. Feasibility of proactive medical device surveillance: the VA Clinical Assessment Reporting and Tracking (CART) program. Med Care. 2013;51:S57–S61. [DOI] [PubMed] [Google Scholar]
- 15. Maddox TM, Plomondon ME, Petrich M, Tsai TT, Gethoffer H, Noonan G, Gillespie B, Box T, Fihn SD, Jesse RL, Rumsfeld JS. A national clinical quality program for Veterans Affairs catheterization laboratories (from the Veterans Affairs clinical assessment, reporting, and tracking program). Am J Cardiol. 2014;114:1750–1757. [DOI] [PubMed] [Google Scholar]
- 16. Levey AS GT, Kusek JW, Beck GJ. A simplified equation to predict glomerular filtration rate from serum creatinine [Abstract]. J Am Soc Nephrol. 2000;11. [Google Scholar]
- 17. Kooiman J, Seth M, Dixon S, Wohns D, LaLonde T, Rao SV, Gurm HS. Risk of acute kidney injury after percutaneous coronary interventions using radial versus femoral vascular access: insights from the Blue Cross Blue Shield of Michigan Cardiovascular Consortium. Circ Cardiovasc Interv. 2014;7:190–198. [DOI] [PubMed] [Google Scholar]
- 18. Baron RM, Kenny DA. The moderator‐mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. [DOI] [PubMed] [Google Scholar]
- 19. Jolly SS, Yusuf S, Cairns J, Niemela K, Xavier D, Widimsky P, Budaj A, Niemela M, Valentin V, Lewis BS, Avezum A, Steg PG, Rao SV, Gao P, Afzal R, Joyner CD, Chrolavicius S, Mehta SR. Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes (RIVAL): a randomised, parallel group, multicentre trial. Lancet. 2011;377:1409–1420. [DOI] [PubMed] [Google Scholar]
- 20. Feldman DN, Swaminathan RV, Kaltenbach LA, Baklanov DV, Kim LK, Wong SC, Minutello RM, Messenger JC, Moussa I, Garratt KN, Piana RN, Hillegass WB, Cohen MG, Gilchrist IC, Rao SV. Adoption of radial access and comparison of outcomes to femoral access in percutaneous coronary intervention: an updated report from the National Cardiovascular Data Registry (2007–2012). Circulation. 2013;127:2295–2306. [DOI] [PubMed] [Google Scholar]
- 21. Batchelor WB, Anstrom KJ, Muhlbaier LH, Grosswald R, Weintraub WS, O'Neill WW, Peterson ED. Contemporary outcome trends in the elderly undergoing percutaneous coronary interventions: results in 7,472 octogenarians. National Cardiovascular Network Collaboration. J Am Coll Cardiol. 2000;36:723–730. [DOI] [PubMed] [Google Scholar]
- 22. Kinnaird TD, Stabile E, Mintz GS, Lee CW, Canos DA, Gevorkian N, Pinnow EE, Kent KM, Pichard AD, Satler LF, Weissman NJ, Lindsay J, Fuchs S. Incidence, predictors, and prognostic implications of bleeding and blood transfusion following percutaneous coronary interventions. Am J Cardiol. 2003;92:930–935. [DOI] [PubMed] [Google Scholar]
- 23. Lauer MA, Karweit JA, Cascade EF, Lin ND, Topol EJ. Practice patterns and outcomes of percutaneous coronary interventions in the United States: 1995 to 1997. Am J Cardiol. 2002;89:924–929. [DOI] [PubMed] [Google Scholar]
- 24. Popma JJ, Satler LF, Pichard AD, Kent KM, Campbell A, Chuang YC, Clark C, Merritt AJ, Bucher TA, Leon MB. Vascular complications after balloon and new device angioplasty. Circulation. 1993;88:1569–1578. [DOI] [PubMed] [Google Scholar]
- 25. Mehran R, Pocock SJ, Nikolsky E, Clayton T, Dangas GD, Kirtane AJ, Parise H, Fahy M, Manoukian SV, Feit F, Ohman ME, Witzenbichler B, Guagliumi G, Lansky AJ, Stone GW. A risk score to predict bleeding in patients with acute coronary syndromes. J Am Coll Cardiol. 2010;55:2556–2566. [DOI] [PubMed] [Google Scholar]
- 26. Subherwal S, Bach RG, Chen AY, Gage BF, Rao SV, Newby LK, Wang TY, Gibler WB, Ohman EM, Roe MT, Pollack CV Jr, Peterson ED, Alexander KP. Baseline risk of major bleeding in non‐ST‐segment‐elevation myocardial infarction: the CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA Guidelines) Bleeding Score. Circulation. 2009;119:1873–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Saltzman AJ, Stone GW, Claessen BE, Narula A, Leon‐Reyes S, Weisz G, Brodie B, Witzenbichler B, Guagliumi G, Kornowski R, Dudek D, Metzger DC, Lansky AJ, Nikolsky E, Dangas GD, Mehran R. Long‐term impact of chronic kidney disease in patients with ST‐segment elevation myocardial infarction treated with primary percutaneous coronary intervention: the HORIZONS‐AMI (Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction) trial. JACC Cardiovasc Interv. 2011;4:1011–1019. [DOI] [PubMed] [Google Scholar]
- 28. Romagnoli E, Biondi‐Zoccai G, Sciahbasi A, Politi L, Rigattieri S, Pendenza G, Summaria F, Patrizi R, Borghi A, Di Russo C, Moretti C, Agostoni P, Loschiavo P, Lioy E, Sheiban I, Sangiorgi G. Radial versus femoral randomized investigation in ST‐segment elevation acute coronary syndrome: the RIFLE‐STEACS (Radial Versus Femoral Randomized Investigation in ST‐Elevation Acute Coronary Syndrome) study. J Am Coll Cardiol. 2012;60:2481–2489. [DOI] [PubMed] [Google Scholar]
- 29. Hanna EB, Chen AY, Roe MT, Wiviott SD, Fox CS, Saucedo JF. Characteristics and in‐hospital outcomes of patients with non‐ST‐segment elevation myocardial infarction and chronic kidney disease undergoing percutaneous coronary intervention. JACC Cardiovasc Interv. 2011;4:1002–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Staniloae CS, Mody KP, Sanghvi K, Mindrescu C, Coppola JT, Antonescu CR, Shah S, Patel T. Histopathologic changes of the radial artery wall secondary to transradial catheterization. Vasc Health Risk Manag. 2009;5:527–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yonetsu T, Kakuta T, Lee T, Takayama K, Kakita K, Iwamoto T, Kawaguchi N, Takahashi K, Yamamoto G, Iesaka Y, Fujiwara H, Isobe M. Assessment of acute injuries and chronic intimal thickening of the radial artery after transradial coronary intervention by optical coherence tomography. Eur Heart J. 2010;31:1608–1615. [DOI] [PubMed] [Google Scholar]
- 32. Rao SV, Tremmel JA, Gilchrist IC, Shah PB, Gulati R, Shroff AR, Crisco V, Woody W, Zoghbi G, Duffy PL, Sanghvi K, Krucoff MW, Pyne CT, Skelding KA, Patel T, Pancholy SB. Best practices for transradial angiography and intervention: a consensus statement from the Society for Cardiovascular Angiography and Intervention's Transradial Working Group. Catheter Cardiovasc Interv. 2014;83:228–236. [DOI] [PubMed] [Google Scholar]
- 33. Jindal K, Chan CT, Deziel C, Hirsch D, Soroka SD, Tonelli M, Culleton BF. CHAPTER 4: vascular access. J Am Soc Nephrol. 2006;17:S16–S23. [DOI] [PubMed] [Google Scholar]
- 34. Sherwood MW, Wang Y, Curtis JP, Peterson ED, Rao SV. Patterns and outcomes of red blood cell transfusion in patients undergoing percutaneous coronary intervention. JAMA. 2014;311:836–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rao SV, Jollis JG, Harrington RA, Granger CB, Newby LK, Armstrong PW, Moliterno DJ, Lindblad L, Pieper K, Topol EJ, Stamler JS, Califf RM. Relationship of blood transfusion and clinical outcomes in patients with acute coronary syndromes. JAMA. 2004;292:1555–1562. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Rates of Transradial Versus Transfemoral Access by Degree of Renal Dysfunction
Table S2. After Adjustment for Contrast Use and Excluding Patients Undergoing Same‐Day PCI
Table S3. After Excluding Sites (n=28) With <5% Transradial Use
Table S4. Excluding Patients With Past CABG


