Abstract
Background
Adenosine‐assisted transthoracic Doppler‐derived coronary flow reserve (TDE‐CFR) reflects coronary vascular function. The prognostic and incremental value of left anterior descending coronary artery TDE‐CFR above myocardial perfusion scintigraphy in patients with suspected myocardial ischemia has not yet been studied.
Methods and Results
Three hundred seventy‐one patients (mean age, 62.3±8.7 years; 46.8% males) referred to myocardial perfusion scintigraphy attributed to suspected myocardial ischemia were included in the study. The TDE‐CFR result was blinded to the referring physician. Patients were followed up regarding major cardiovascular events, defined as cardiovascular death, myocardial infarction, or acute revascularization during a median follow‐up time of 4.5 years. A TDE‐CFR value of ≤2.0 was considered reduced. Major cardiovascular events occurred during follow‐up in 60 patients (16.2%). A reduced TDE‐CFR was detected in 76 patients (20.5%). Patients with reduced TDE‐CFR had an event rate of 36.8% compared to 10.8% in patients with normal TDE‐CFR (unadjusted hazard ratio, 4.63; 95% CI, 2.78–7.69; P<0.001). In a multivariate model, TDE‐CFR remained a significant independent predictor of major cardiovascular events. The major cardiovascular events rate was 7.5% in patients without myocardial perfusion scintigraphy‐detected myocardial ischemia and normal TDE‐CFR (n=200), 24.2% in patients without ischemia but with reduced TDE‐CFR (n=33), and 46.5% in patients with both myocardial perfusion scintigraphy–detected myocardial ischemia and a reduced TDE‐CFR (n=43; P<0.001).
Conclusions
Coronary microvascular dysfunction, as determined by TDE‐CFR, is a strong independent predictor of cardiovascular events and adds incremental prognostic value compared with myocardial perfusion scintigraphy. The current study supports routine assessment of CFR in patients with suspected ischemic heart disease.
Keywords: adenosine, coronary blood flow reserve, echocardiography, myocardial perfusion imaging, prognosis
Subject Categories: Coronary Circulation, Ischemia, Prognosis, Ultrasound
Introduction
Myocardial perfusion scintigraphy (MPS) is a well‐established method to diagnose the presence and extent of myocardial ischemia featuring, in particular, a useful high negative predictive value.1 In fact, MPS‐verified myocardial ischemia is strongly related to the extent and severity of coronary artery disease (CAD) as verified by coronary angiogram.2, 3 Coronary flow velocity reserve (CFR), assessed by transthoracic echocardiography Doppler (TDE‐CFR), is an emerging diagnostic tool for CAD and has been used as a predictor of cardiovascular prognosis in different patient categories.4, 5, 6, 7, 8, 9 TDE‐CFR is known to reflect presence of macrovascular as well as microvascular disease in the coronary circulation with the advantages of being noninvasive, highly feasible, safe for patient and physician, and not associated with any radiation. Most frequently, the technique is used with a dipyridamole protocol in conjunction with a stress echocardiography examination.10
TDE‐CFR is known to provide an independent prognostic value for prediction of cardiovascular events and provide incremental prognostic value on top of stress echocardiographic wall motion criteria in different clinical settings.6 However, MPS is based on relative perfusion measurement and reflects most likely only the degree of the lumen obstructive CAD status, which may cause heterogeneous flow distribution during exercise or pharmacological challenge. In cases of balanced 3‐vessel disease or global microvascular dysfunction, MPS will have lower diagnostic and prognostic value. Moreover, we have previously reported a lack of correlation between the invasive assessment of CFR and noninvasive functional tests in patients without obstructive CAD.11 Thus, the quantitative measurement of TDE‐CFR may reflect more aspects of coronary vascular status and thereby provide an incremental value to MPS.
In the present study, we aim to evaluate the feasibility of adenosine‐induced TDE‐CFR in a patient population with suspected myocardial ischemia referred to MPS and hypothesize that TDE‐CFR may convey additional prognostic values above MPS.
Methods
The CEVENT‐Study
The CEVENT‐study (Coronary flow reserve and cardiovascular EVENTs) was conducted at the Department of Clinical Physiology, Sahlgrenska University Hospital (Gothenburg Sweden) from February 2006 until November 2008. We consecutively enrolled 371 patients presenting with clinical chest pain referred for MPS examination attributed to suspected myocardial ischemia. All patients underwent both MPS and TDE‐CFR examination and gave their informed and written consent to study participation. Inclusion criteria for TDE‐CFR were (1) eligibility to adenosine stress echocardiography and (2) ability to understand informed consent. Exclusion criterion was unwillingness to participate. Measurement of TDE‐CFR was performed within a week from MPS. In this clinical setting, the physician referring to the MPS examination was blinded to the result of the TDE‐CFR, which, consequently, did not affect or guide the clinical decision process in this experimental study. Patients were later followed up annually regarding cardiovascular events by semistructured telephone interviews performed by a nurse, reviewing of medical records by a physician, and also by data collection from the Swedish National Board of Health Registry. The study was approved by the Reginal Ethics Committee in Gothenburg, and all patients gave their written consent to study participation.
Echocardiography and Assessment of TDE‐CFR
A basic transthoracic echocardiography protocol was performed according to recommendations.12 The Sequoia C256 (Acuson Siemens, Mountain View, CA) ultrasound system was used with a 4‐MHz probe. Our protocol has been described previously.13 Briefly, the mid to distal part of the left anterior descending coronary artery was identified using 3.5‐MHz color Doppler in the interventricular sulcus in a modified 2‐chamber view. Pulsed Doppler was used to sample flow velocity signals at rest and during adenosine infusion (140 μg/min/kg) over 5 minutes. During the whole procedure, blood pressure and ECG were monitored. All studies were digitally stored for offline reviewing and measurements. Coronary flow velocity data were analyzed offline using the ultrasound software Image Arena (Tomtec, Unterschlissheim, Germany). Mean diastolic flow velocity at baseline and during peak hyperemia was measured by manual tracing of the diastolic Doppler flow signals. CFR was calculated as the ratio between the hyperemic and baseline flow velocity values. A CFR ratio of ≤2 was considered reduced.14
Myocardial Perfusion Scintigraphy Examinations
Myocardial perfusion scintigram was performed by a 2‐day stress/rest examination protocol according to a standard clinical protocol at the Department of Clinical Physiology, Sahlgrenska University Hospital. Our protocol has previously been described in detail.15 Briefly, stress provocation was performed by exercise test or pharmacological provocation. Radionuclide technetium (99mTc) sestamibi was administered and detected using single‐photon emission computed tomography. Images were obtained using dual‐head cameras (Infinia or Hawkeye; General Electric, Waukesha, WI) displaying perfusion and function of the left ventricle. Presence and extent of myocardial ischemia was determined by an experienced physician. Automatically generated variables from the software ECT toolbox (Syntermed, Atlanta, GA), such as reversibility stress score, was used as aid for clinical assessment. Severity of reversible ischemia was scored as no ischemia (0), mild (score 1), moderate (2), or severe (3), and extent of ischemia was scored as none (0), small (<10%, score 1), medium (10–19%, score 2), and large (>19%, score 3). No myocardial perfusion defects was defined as both severity and extent score=0.
Laboratory Analysis
Routine biochemical analyses were at the accredited coagulation laboratory at Sahlgrenska University Hospital. The laboratory participates in the ECAT foundation external quality assessment programme (www.ecat.nl). Triglycerides and cholesterol in serum were measured using reagent systems from Roche (Triglycerides/GB kit No. 12146029216; Cholesterol kit No. 2016630; Roche Diagnostics GMBH, Mannheim, Germany). Apolipoprotein A1 and B concentrations were measured with the turbidimetric technique, by use of polyclonal rabbit anti‐human antibodies (Q 0496 and Q 0497; Dako Cytomation, Glostrup, Denmark).
Follow‐up Data
Patients were followed up at least 4 years past study date by telephone interviews and medical records. The results of the TDE‐CFR measurement was blinded to the MPS referring physicians and consequently did not affect further clinical investigations or decision making. Major cardiovascular events (MACE) were defined as a composite of cardiovascular death, acute myocardial infarction, and need for urgent coronary revascularisation with percutaneous coronary intervention or coronary artery bypass grafting. Cause of death was established by data from the Swedish National Board of Health registries. Acute myocardial infarction was defined as presence of typical symptoms, ST changes in ECG, and impact on cardiac enzymes.16 Revascularization was considered to be urgent when a patient was admitted to the hospital with persistent chest pain (with or without ST‐segment or T‐wave changes or elevated biomarker levels), and the revascularization procedure was performed during the same hospitalization.
Statistical Analysis
Continuous variables are presented as mean±SD and categorical variables as frequencies. Normal distribution of variables was assessed by the Shapiro–Wilk test. Differences between groups in continuous normally distributed variables were tested with the Student t test. Differences in categorical variables were tested by chi‐square test or Fisher's exact test. Independent predictors of MACE were identified using the Cox proportional hazards model. The following variables were included in the model: sex, age, diabetes mellitus, smoking, hypertension, hypercholesterolemia, ejection fraction, CFR, MPS ischemia area, and anti‐ischemic treatment. Kaplan–Meier survival curves are shown for event‐free survival. Hazard ratios with the corresponding 95% CI were calculated by Cox regression analysis. Two‐sided P<0.05 was considered to be statistically significant. All data analysis were performed in the statistical software, SPSS (version 23.0; SPSS, Inc, Chicago, IL).
Results
TDE‐CFR and Myocardial Perfusion Scintigraphy‐Data
All examinations were performed successfully, and no major complications occurred. Baseline data are shown in Table 1. Of the 371 patients, 76 (20.5%) had reduced CFR (<2.0). MPS‐detected myocardial ischemia was present in 138 cases (37.2%). Patients with presence of MPS‐detected ischemia showed lower CFR values (2.50±0.95 versus 2.80±0.91; P=0.003). No difference in CFR was found between hypertensives and normotensives (2.56±1.01 versus 2.74±0.93; P=0.251), patients with and without hypercholesterolemia (2.58±0.96 versus 2.70±0.93; P=0.112), and patients with and without a family history of cardiovascular diseases (2.65±0.93 versus 2.66±0.90; P=0.911). However, patients with a smoking habit showed decreased CFR (2.53±0.85 versus 2.83±0.98; P=0.002) as well as those with diagnosis of diabetes mellitus (2.40±0.83 versus 2.73±0.95; P=0.015). Patients with reduced CFR showed increased MPS ischemia score and area compared with patients with normal CFR (1.0±1.1 versus 0.4±0.8; P<0.001 and 0.9±0.9 versus 0.4±0.7; P<0.001, respectively). Also, patients in the reduced CFR group showed greater reversibility stress score (70.1±124.0 versus 21.6±60.6; P=0.001). Furthermore, patients with reduced CFR showed increased infarct score and area (0.24±0.41 versus 0.11±0.30; P=0.015 and 0.50±0.98 versus 0.21±0.62; P=0.016, respectively).
Table 1.
Clinical Characteristics in Patients With Reduced and Normal CFR (Mean±SD or Number [%])
| All Patients (n=371) | CFR ≤2.0 (n=76) | CFR >2.0 (n=295) | P Value | |
|---|---|---|---|---|
| Age, y | 62.2±8.7 | 64.5±10.0 | 61.7±8.3 | 0.027 |
| Sex (male), n | 174 (47) | 46 (61) | 128 (43) | 0.008 |
| BMI, m2/kg | 26.2±3.8 | 26.1±3.9 | 26.2±3.8 | 0.87 |
| Diabetes mellitus, n | 46 (12%) | 13 (17%) | 33 (11%) | 0.17 |
| Hypertension, n | 47 (12%) | 10 (13%) | 37 (13%) | 0.89 |
| Smoking, n | 181 (49%) | 45 (59%) | 136 (46%) | 0.040 |
| Hypercholesterolemia, n | 184 (50%) | 42 (55%) | 142 (48%) | 0.24 |
| Ejection fraction, % | 63±12 | 59±13 | 64±11 | 0.001 |
| Laboratory data | ||||
| ApoB/ApoA1 ratio | 0.7±0.2 | 0.7±0.2 | 0.7±0.2 | 0.80 |
| Triglycerides, mmol/L | 1.4±0.9 | 1.6±1.2 | 1.4±0.8 | 0.084 |
| Total cholesterol, mmol/L | 5.3±1.3 | 5.2±1.3 | 5.3±1.3 | 0.51 |
| High‐density lipoprotein, mmol/L | 1.5±0.4 | 1.4±0.4 | 1.5±0.4 | 0.52 |
| Medication | ||||
| β‐blockers, n | 191 (52%) | 48 (63%) | 143 (48%) | 0.022 |
| Statins, n | 165 (45%) | 39 (51%) | 126 (43%) | 0.18 |
| ACE inhibitors, n | 91 (25%) | 26 (34%) | 65 (22%) | 0.028 |
| Aspirin, n | 195 (53%) | 48 (63%) | 150 (51%) | 0.055 |
| Clopidogrel, n | 24 (7%) | 5 (7%) | 19 (6%) | 0.97 |
ACE indicates angiotensin‐converting enzyme; Apo, apolipoprotein; BMI, body mass index; CFR, coronary flow reserve.
Follow‐up Data
There was no loss to follow‐up. During a median follow‐up time of 4.5 years, 28 MACE events (cardiovascular death, myocardial infarction, and acute revascularization) occurred in the 76 patients with CFR ≤2.0 (37%) and 32 events occurred in the 295 patients with CFR >2.0 (11%; P<0.001). Event‐free survival for the 2 groups is shown in Figure 1. Other important cardiovascular and neurological outcome variables in the 2 groups are presented in Table 2. Univariate and multivariate predictors of MACE are presented in Table 3. TDE‐CFR predicted spontaneous cardiovascular events (hazard ratio, 4.1; CI, 2.67–6.33; P<0.001). Even after adjustment for all significant outcome predictors in the univariate analysis, TDE‐CFR remained a significant independent predictor. Coronary flow at baseline and during hyperemia as well as TDE‐CFR data are shown in Table 4 depending on presence or not of MACE.
Figure 1.
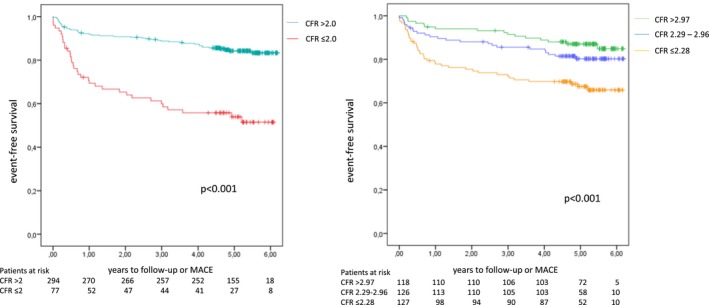
Left part of the image shows Kaplan–Meier event‐free survival in patients with preserved and reduced coronary flow reserve (CFR). To the right, Kaplan–Meier event‐free survival is shown by CFR tertiles. MACE indicates major cardiovascular events.
Table 2.
Cardiovascular and Cerebral Events in Patients With CFR ≤2.0 or CFR >2.0
| CFR ≤2.0 (n=76) | CFR >2.0 (n=295) | P Value (Chi‐Square) | |
|---|---|---|---|
| MACE | 28 (37%) | 32 (11%) | <0.001 |
| All‐cause death | 10 (13%) | 10 (3.4%) | <0.001 |
| Cardiovascular death | 8 (11%) | 2 (0.7%) | <0.001 |
| Myocardial infarction | 6 (7.9%) | 11 (3.7%) | 0.121 |
| Neurological events | 6 (7.9%) | 14 (4.7%) | 0.28 |
| Stroke | 4 (5.3%) | 4 (1.4%) | 0.036 |
| TIA | 2 (2.6%) | 10 (3.4%) | 0.74 |
| Revascularization | 28 (37%) | 42 (14%) | <0.001 |
| PCI | 19 (25%) | 36 (12%) | 0.005 |
| Acute | 7 (9.2%) | 20 (6.8%) | 0.47 |
| Elective | 12 (16%) | 16 (5.4%) | 0.002 |
| CABG | 9 (12%) | 6 (2.0%) | <0.001 |
| Acute | 0 | 2 (0.7%) | 0.47 |
| Elective | 9 (12%) | 4 (1.4%) | <0.001 |
| Episodes of unstable angina | 7 (9.2%) | 18 (6.1%) | 0.34 |
Numbers and %. CABG indicates coronary artery bypass grafting; CFR, coronary flow reserve; MACE, major adverse cardiovascular events (cardiovascular death, myocardial infarction, or acute revascularization); PCI, percutaneous coronary intervention; TIA, transient ischemic events.
Table 3.
Univariate and Multivariate Predictors for MACE
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Male sex | 3.47 (1.89–6.36) | <0.001 | 3.14 (1.46–6.75) | 0.003 |
| Age | 1.06 (1.02–1.10) | 0.001 | 1.04 (1.00–1.08) | 0.03 |
| Diabetes mellitus | 3.92 (1.99–7.75) | <0.001 | 3.21 (1.45–7.10) | 0.004 |
| Smoking | 2.08 (1.16–3.70) | 0.01 | 1.34 (0.68–2.65) | 0.403 |
| Hypertension | 1.27 (0.58–2.78) | 0.554 | ||
| Hypercholesterolemia | 2.19 (1.21–3.97) | 0.005 | 1.36 (0.68–2.70) | 0.385 |
| Ejection fraction | 0.96 (0.94–0.98) | <0.001 | 1.00 (0.96–1.02) | 0.519 |
| TDE‐CFR ≤2.0 | 4.79 (2.65–8.67) | <0.001 | 3.02 (1.51–6.04) | 0.002 |
| Scintigraphy ischemia area | 1.99 (1.45–2.72) | <0.001 | 1.38 (1.01–1.89) | 0.045 |
HR indicates hazard ratio; MACE, major adverse cardiovascular event; TDE‐CFR, transthoracic Doppler‐derived coronary flow reserve.
Table 4.
Coronary Flow Data in Patients With and Without MACE
| MACE (n=60) | No MACE (n=311) | P Value | |
|---|---|---|---|
| Baseline, cm/s | 0.34±0.18 | 0.30±0.10 | 0.23 |
| Hyperaemia, cm/s | 0.70±0.28 | 0.79±0.24 | 0.01 |
| Coronary flow reserve | 2.36±1.14 | 2.75±0.88 | 0.003 |
MACE indicates major adverse cardiovascular events.
In Figure 2, cumulative event‐free survival in patients with reduced or preserved TDE‐CFR and presence of ischemia on scinigraphy are depicted. Incidence of MACE was significantly higher in patients with reduced TDE‐CFR both in patients without scintigraphy‐verified ischemia (24% versus 8%; P=0.003) and in those with ischemia (47% versus 18%; P<0.001). In patients with negative scintigraphy, there were also significant differences between reduced and preserved TDE‐CFR in the incidence of all‐cause death, cardiovascular death, stroke, total revascularizations, and elective coronary artery bypass grafting (Table 5. In patients with scintigraphy‐verified ischemia, there was, besides MACE, also significant differences between reduced and preserved TDE‐CFR in the incidence of total revascularizations and elective coronary artery bypass grafting (Table 5.
Figure 2.
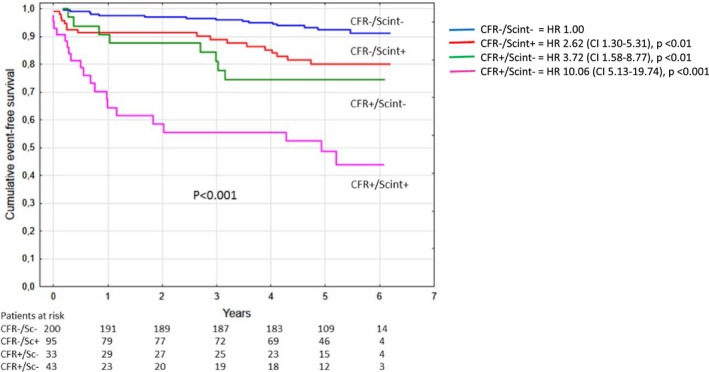
Kaplan–Meier event‐free survival in patients with reduced or preserved coronary flow reserve (CFR) in patients with or without ischemia on scintigraphy. There were significant differences between the CFR <2.0 and scintigraphy‐negative group, and all other groups and between the CFR ≤2.0 and scintigraphy‐positive group. In contrast, there was no significant difference between the CFR ≤2.0 and scintigraphy‐negative group and the CFR >2.0 and scintigraphy‐positive group. HR indicates hazard ratio.
Table 5.
Cardiovascular and Cerebral Events in Different Groups of Patients With CFR ≤2.0 or CFR >2.0 and Scintigraphy Indicating Ischemia or Not
| CFR ≤2.0 Scint Pos (n=43) | CFR ≤2.0 Scint Neg (n=33) | CFR >2.0 Scint Pos (n=95) | CFR >2.0 Scint Neg (n=200) | P Value (Chi‐Square) | |
|---|---|---|---|---|---|
| MACE | 20 (47%) | 8 (24%) † | 17 (18%) | 15 (7.5%) | <0.001 |
| All‐cause death | 5 (12%) | 5 (15%) ‡ | 6 (6.3%) | 4 (2.0%) | <0.001 |
| Cardiovascular death | 4 (9.3%) | 4 (12%) ‡ | 2 (2.1%) | 0 | 0.006 |
| Myocardial infarction | 5 (12%) | 1 (3.0%) | 5 (5.2%) | 6 (3.0%) | 0.097 |
| Neurological events | 2 (4.7%) | 4 (12%) | 5 (5.2%) | 9 (4.5%) | 0.35 |
| Stroke | 1 (2.3%) | 3 (9.1%) † | 1 (1.1%) | 3 (1.5%) | 0.037 |
| TIA | 1 (2.3%) | 1 (3.0%) | 4 (4.2%) | 6 (3.0%) | 0.93 |
| Revascularization | 21 (49%) § | 7 (21%)* | 26 (27%) | 16 (8.0%) | <0.001 |
| PCI | 16 (37%) | 3 (9.1%) | 22 (23%) | 14 (7.0%) | <0.001 |
| Acute | 6 (14%) | 1 (3.0%) | 12 (13%) | 8 (4.0%) | 0.012 |
| Elective | 10 (23%) § | 2 (6.1%) | 10 (11%) | 6 (3.0%) | <0.001 |
| CABG | 5 (12%) | 4 (12%) ‡ | 4 (4.2%) | 2 (1.0%) | <0.001 |
| Acute | 0 | 0 | 2 (2.1%) | 0 | 0.12 |
| Elective | 5 (12%) ∥ | 4 (12%) ‡ | 0 | 2 (1.0%) | <0.001 |
| Unstable angina | 7 (16%) | 0 | 9 (9.5%) | 9 (4.5%) | 0.01 |
Numbers and %. CABG indicates coronary artery bypass grafting; CFR, coronary flow reserve; MACE, major adverse cardiovascular events (cardiovascular death, myocardial infarction, or acute revascularization); PCI, percutaneous coronary intervention; TIA, transient ischemic events.
*P<0.05; † P<0.01; ‡ P<0.001 for CFR ≤2.0 and scint neg vs CFR >2.0 and scint neg; § P<0.05; ∥ P<0.001 for CFR ≤2.0 and scint pos vs CFR >2.0 and scint pos.
Discussion
In this study, we examined TDE‐CFR in patients undergoing MPS investigation attributed to suspected myocardial ischemia. Reduced TDE‐CFR was a significant predictor of MACE in this patient group. Moreover, after adjustment for significant cardiovascular variables in the univariate analysis, TDE‐CFR remained a significant independent predictor of MACE. More important, TDE‐CFR showed an incremental prognostic value to myocardial ischemia determined by MPS. The current study further supports the role and need for assessment of the coronary microcirculation for risk stratification in patients with suspected CAD.
The prognostic value of TDE‐CFR has previously been demonstrated with dipyridamole‐induced hyperemia in conjunction with a stress echocardiography examination. A reduced TDE‐CFR is known to predict an impaired cardiovascular prognosis in several patient groups, including patients with diabetes mellitus, hypertension, ischemic cardiomyopathy, normal coronary arteries, and CAD.4, 6, 7, 8, 9, 14, 17 Furthermore, TDE‐CFR has also been suggested as an additive prognostic marker to stress‐induced wall motion defects, which also has been demonstrated in multicenter studies.4, 6 Recently, TDE‐CFR, assessed by adenosine protocol, has been shown to predict cardiovascular prognosis in a post–acute coronary syndrome population.18 To our knowledge, this study is the first to evaluate prognostic value of the adenosine TDE‐CFR protocol in an experimental setting independent of the routine diagnostic route. Attributed to rapid onset and short half‐life of adenosine, the protocol has the advantages of being easily manageable, patient safe, and no antidote is required.
To date, MPS is the standard method for diagnosis of myocardial ischemia and to predict cardiovascular outcome.19 The standard interpretation of MPS is based on assessment of relative myocardial perfusion defects and refers to the region with the best perfusion as normal. Consequently, diagnostic problems arise in the case of multivessel disease or diffuse microvascular disease.20, 21 The results of the present study indicate that TDE‐CFR, as an integrated measure, might be comparable and independent in its prognostic value for future cardiovascular events, most likely attributed to its quantitative nature, as well as reflection of microvascular function. In this clinical setting, we demonstrate TDE‐CFR to provide incremental value to MPS detected ischemia.
It is now evident that CFR comprises a composite measure of coronary macrovascular and microvascular status.22 Besides lumen‐narrowing epicardial stenosis, intact endothelial function by nitric oxide pathway is required for maximal CFR response.23 In addition to vasodilation, healthy endothelium is also crucial for the atheroprotective and thromboprotective properties of the vascular wall. Thus, impaired coronary vasodilation in response to ischemic stress, in combination with dysfunctional protective mechanisms in the vascular wall, may be responsible for the poor outcome observed in patients with impaired CFR. CFR is known to relate to angiographic findings, and our group has previously shown CFR to be an independent predictor of the extent and severity of CAD in all 3 major coronary arteries as determined from coronary angiogram.24 In our study, the relatively high event rate during the first year of follow‐up may be explained by diffuse CAD or balanced 3‐vessel disease not detected by MPS. However, patients with CFR >2 still had an event rate of 10.8% over ≈5 years of follow‐up. Other important factors for cardiovascular events, such as blood viscosity and hypercoagulability, may contribute in acute situations concerning coronary plaque instability/rupture and thrombus formation, which is not is foresight by CFR per se.
CFR can be assessed by many imaging modalities. Concerning noninvasive techniques, positron emission tomography is considered golden standard.25 Positron emission tomography has the ability to study both ischemia extent, coronary blood flow, and CFR. Robidium/positron emission tomography–assessed CFR has shown similar incremental predictive values as TDE‐CFR above myocardial ischemia and other cardiac indexes.26 Positron emission tomography can also be performed, together with high‐resolution computed tomography, to provide further structural information.27 Furthermore, contrast computed tomography may be a new modality for myocardial perfusion imaging.28 However, the above‐mentioned techniques provide radiation, are more expensive, and have less clinical availability compared to a simple echocardiographic examination. Magnetic resonance imaging is a nonradioactive method, which also can assess CFR, but is relatively costly and time‐consuming. TDE‐CFR stands as attractive with the advantages of being safe for patient and physician, inexpensive, available, and feasible. Furthermore, its ability to predict cardiovascular prognosis appears to be similar to the more‐complex mentioned techniques.
Study Limitations
Attributed to the limited size of our patient population, a combined end point (MACE) with cardiovascular death, myocardial infarction, and acute revascularization was chosen as the outcome measure. Larger study populations are needed to analyze the role of TDE‐CFR for prediction of the individual MACE components. Also, the number of patients in the subgroups in Figure 2 are quite few. However, our study demonstrates the potential clinical role of TDE‐CFR alone in an independent unbiased setting on top of MPS‐derived data. In the current study, the CFR assessment was restricted to the left anterior descending distribution; it may be speculated that the assessment of TDE‐CFR in all 3 vessels will add diagnostic accuracy.
Conclusions
Our study demonstrates that coronary microvascular dysfunction, as was assessed by TDE‐CFR, is an independent and strong predictor of spontaneous cardiovascular events in patients with suspected myocardial ischemia. More important, in this clinical study, TDE‐CFR provides incremental prognostic value to MPS‐detected ischemia. The current study underscored the significant of the assessment and potential to develop future therapeutic intervention for microvascular dysfunction.
Sources of Funding
This study was funded by Swedish Research Grants ALF/LUA.
Disclosures
Dr Gan is employed by AstraZeneca. All other authors have nothing to disclose.
(J Am Heart Assoc. 2017;6:e004875 DOI: 10.1161/JAHA.116.004875).28420647
References
- 1. Hachamovitch R, Berman DS, Kiat H, Cohen I, Friedman JD, Shaw LJ. Value of stress myocardial perfusion single photon emission computed tomography in patients with normal resting electrocardiograms: an evaluation of incremental prognostic value and cost‐effectiveness. Circulation. 2002;105:823–829. [DOI] [PubMed] [Google Scholar]
- 2. Solot G, Hermans J, Merlo P, Chaudron JM, Luwaert R, Cheron P, Bodart F, Beauduin M. Correlation of 99Tcm‐sestamibi SPECT with coronary angiography in general hospital practice. Nucl Med Commun. 1993;14:23–29. [DOI] [PubMed] [Google Scholar]
- 3. Van Train KF, Garcia EV, Maddahi J, Areeda J, Cooke CD, Kiat H, Silagan G, Folks R, Friedman J, Matzer L. Multicenter trial validation for quantitative analysis of same‐day rest‐stress technetium‐99m‐sestamibi myocardial tomograms. J Nucl Med. 1994;35:609–618. [PubMed] [Google Scholar]
- 4. Cortigiani L, Rigo F, Galderisi M, Gherardi S, Bovenzi F, Picano E, Sicari R. Diagnostic and prognostic value of Doppler echocardiographic coronary flow reserve in the left anterior descending artery in hypertensive and normotensive patients. Heart. 2011;97:1758–1765. [DOI] [PubMed] [Google Scholar]
- 5. Cortigiani L, Rigo F, Gherardi S, Galderisi M, Sicari R, Picano E. Prognostic implications of coronary flow reserve on left anterior descending coronary artery in hypertrophic cardiomyopathy. Am J Cardiol. 2008;102:1718–1723. [DOI] [PubMed] [Google Scholar]
- 6. Cortigiani L, Rigo F, Gherardi S, Sicari R, Galderisi M, Bovenzi F, Picano E. Additional prognostic value of coronary flow reserve in diabetic and nondiabetic patients with negative dipyridamole stress echocardiography by wall motion criteria. J Am Coll Cardiol. 2007;50:1354–1361. [DOI] [PubMed] [Google Scholar]
- 7. Rigo F, Ciampi Q, Ossena G, Grolla E, Picano E, Sicari R. Prognostic value of left and right coronary flow reserve assessment in nonischemic dilated cardiomyopathy by transthoracic Doppler echocardiography. J Card Fail. 2011;17:39–46. [DOI] [PubMed] [Google Scholar]
- 8. Rigo F, Gherardi S, Galderisi M, Pratali L, Cortigiani L, Sicari R, Picano E. The prognostic impact of coronary flow‐reserve assessed by Doppler echocardiography in non‐ischaemic dilated cardiomyopathy. Eur Heart J. 2006;27:1319–1323. [DOI] [PubMed] [Google Scholar]
- 9. Rigo F, Gherardi S, Galderisi M, Sicari R, Picano E. The independent prognostic value of contractile and coronary flow reserve determined by dipyridamole stress echocardiography in patients with idiopathic dilated cardiomyopathy. Am J Cardiol. 2007;99:1154–1158. [DOI] [PubMed] [Google Scholar]
- 10. Sicari R, Nihoyannopoulos P, Evangelista A, Kasprzak J, Lancellotti P, Poldermans D, Voigt JU, Zamorano JL. Stress echocardiography expert consensus statement: European Association of Echocardiography (EAE) (a registered branch of the ESC). Eur J Echocardiogr. 2008;9:415–437. [DOI] [PubMed] [Google Scholar]
- 11. Cassar A, Chareonthaitawee P, Rihal CS, Prasad A, Lennon RJ, Lerman LO, Lerman A. Lack of correlation between noninvasive stress tests and invasive coronary vasomotor dysfunction in patients with nonobstructive coronary artery disease. Circ Cardiovasc Interv. 2009;2:237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Evangelista A, Flachskampf F, Lancellotti P, Badano L, Aguilar R, Monaghan M, Zamorano J, Nihoyannopoulos P. European Association of Echocardiography recommendations for standardization of performance, digital storage and reporting of echocardiographic studies. Eur J Echocardiogr. 2008;9:438–448. [DOI] [PubMed] [Google Scholar]
- 13. Wittfeldt A, Emanuelsson H, Brandrup‐Wognsen G, van Giezen JJ, Jonasson J, Nylander S, Gan LM. Ticagrelor enhances adenosine‐induced coronary vasodilatory responses in humans. J Am Coll Cardiol. 2013;61:723–727. [DOI] [PubMed] [Google Scholar]
- 14. Sicari R, Rigo F, Cortigiani L, Gherardi S, Galderisi M, Picano E. Additive prognostic value of coronary flow reserve in patients with chest pain syndrome and normal or near‐normal coronary arteries. Am J Cardiol. 2009;103:626–631. [DOI] [PubMed] [Google Scholar]
- 15. Svedlund S, Eklund C, Robertsson P, Lomsky M, Gan LM. Carotid artery longitudinal displacement predicts 1‐year cardiovascular outcome in patients with suspected coronary artery disease. Arterioscler Thromb Vasc Biol. 2011;31:1668–1674. [DOI] [PubMed] [Google Scholar]
- 16. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD; Writing Group on the Joint ESCAAHAWHFTFftUDoMI , Thygesen K, Alpert JS, White HD, Jaffe AS, Katus HA, Apple FS, Lindahl B, Morrow DA, Chaitman BA, Clemmensen PM, Johanson P, Hod H, Underwood R, Bax JJ, Bonow RO, Pinto F, Gibbons RJ, Fox KA, Atar D, Newby LK, Galvani M, Hamm CW, Uretsky BF, Steg PG, Wijns W, Bassand JP, Menasche P, Ravkilde J, Ohman EM, Antman EM, Wallentin LC, Armstrong PW, Simoons ML, Januzzi JL, Nieminen MS, Gheorghiade M, Filippatos G, Luepker RV, Fortmann SP, Rosamond WD, Levy D, Wood D, Smith SC, Hu D, Lopez‐Sendon JL, Robertson RM, Weaver D, Tendera M, Bove AA, Parkhomenko AN, Vasilieva EJ, Mendis S; Guidelines ESCCfP . Third universal definition of myocardial infarction. Eur Heart J. 2012;33:2551–2567. [DOI] [PubMed] [Google Scholar]
- 17. Cortigiani L, Rigo F, Gherardi S, Galderisi M, Bovenzi F, Picano E, Sicari R. Prognostic effect of coronary flow reserve in women versus men with chest pain syndrome and normal dipyridamole stress echocardiography. Am J Cardiol. 2010;106:1703–1708. [DOI] [PubMed] [Google Scholar]
- 18. Puddu PE, Mariano E, Voci P, Pizzuto F. Prediction of long‐term ischemic events by noninvasively assessed coronary flow reserve. J Cardiovasc Med (Hagerstown). 2012;13:483–490. [DOI] [PubMed] [Google Scholar]
- 19. Hachamovitch R, Berman DS, Shaw LJ, Kiat H, Cohen I, Cabico JA, Friedman J, Diamond GA. Incremental prognostic value of myocardial perfusion single photon emission computed tomography for the prediction of cardiac death: differential stratification for risk of cardiac death and myocardial infarction. Circulation. 1998;97:535–543. [DOI] [PubMed] [Google Scholar]
- 20. Bengel FM. Leaving relativity behind: quantitative clinical perfusion imaging. J Am Coll Cardiol. 2011;58:749–751. [DOI] [PubMed] [Google Scholar]
- 21. Knuuti J, Kajander S, Maki M, Ukkonen H. Quantification of myocardial blood flow will reform the detection of cad. J Nucl Cardiol. 2009;16:497–506. [DOI] [PubMed] [Google Scholar]
- 22. Gan LM, Wikstrom J, Fritsche‐Danielson R. Coronary flow reserve from mouse to man—from mechanistic understanding to future interventions. J Cardiovasc Transl Res. 2013;6:715–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Buus NH, Bottcher M, Hermansen F, Sander M, Nielsen TT, Mulvany MJ. Influence of nitric oxide synthase and adrenergic inhibition on adenosine‐induced myocardial hyperemia. Circulation. 2001;104:2305–2310. [DOI] [PubMed] [Google Scholar]
- 24. Haraldsson I, Gan LM, Svedlund S, Wittfeldt A, Ramunddal T, Angeras O, Albertsson P, Matejka G, Omerovic E. Non‐invasive evaluation of coronary flow reserve with transthoracic Doppler echocardiography predicts the presence of significant stenosis in coronary arteries. Int J Cardiol. 2014;176:294–297. [DOI] [PubMed] [Google Scholar]
- 25. Bergmann SR, Herrero P, Markham J, Weinheimer CJ, Walsh MN. Noninvasive quantitation of myocardial blood flow in human subjects with oxygen‐15‐labeled water and positron emission tomography. J Am Coll Cardiol. 1989;14:639–652. [DOI] [PubMed] [Google Scholar]
- 26. Murthy VL, Naya M, Foster CR, Hainer J, Gaber M, Di Carli G, Blankstein R, Dorbala S, Sitek A, Pencina MJ, Di Carli MF. Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation. 2011;124:2215–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kajander S, Joutsiniemi E, Saraste M, Pietila M, Ukkonen H, Saraste A, Sipila HT, Teras M, Maki M, Airaksinen J, Hartiala J, Knuuti J. Cardiac positron emission tomography/computed tomography imaging accurately detects anatomically and functionally significant coronary artery disease. Circulation. 2010;122:603–613. [DOI] [PubMed] [Google Scholar]
- 28. Choo KS, Hwangbo L, Kim JH, Park YH, Kim JS, Kim J, Chun KJ, Jeong DW, Lim SJ. Adenosine‐stress low‐dose single‐scan CT myocardial perfusion imaging using a 128‐slice dual‐source CT: a comparison with fractional flow reserve. Acta Radiol. 2013;54:389–395. [DOI] [PubMed] [Google Scholar]


