Abstract
Background
Clinical outcomes reported after treatment of infrapopliteal lesions with drug‐eluting stents (DESs) have been more favorable compared with percutaneous transluminal angioplasty with a bailout bare metal stent (PTA‐BMS) through midterm follow‐up in patients with critical limb ischemia. In the present study, long‐term results of treatment of infrapopliteal lesions with DESs are presented.
Methods and Results
Adults with critical limb ischemia (Rutherford category ≥4) and infrapopliteal lesions were randomized to receive PTA‐BMS or DESs with paclitaxel. Long‐term follow‐up consisted of annual assessments up to 5 years after treatment or until a clinical end point was reached. Clinical end points were major amputation (above ankle level), infrapopliteal surgical or endovascular reintervention, and death. Preserved primary patency (≤50% restenosis) of treated lesions was an additional morphological end point, assessed by duplex sonography. In total, 74 limbs (73 patients) were treated with DESs and 66 limbs (64 patients) were treated with PTA‐BMS. The estimated 5‐year major amputation rate was lower in the DES arm (19.3% versus 34.0% for PTA‐BMS; P=0.091). The 5‐year rates of amputation‐ and event‐free survival (survival free from major amputation or reintervention) were significantly higher in the DES arm compared with PTA‐BMS (31.8% versus 20.4%, P=0.043; and 26.2% versus 15.3%, P=0.041, respectively). Survival rates were comparable. The limited available morphological results showed higher preserved patency rates after DESs than after PTA‐BMS at 1, 3, and 4 years of follow‐up.
Conclusions
Both clinical and morphological long‐term results after treatment of infrapopliteal lesions in patients with critical limb ischemia are improved with DES compared with PTA‐BMS.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT00471289.
Keywords: critical limb ischemia, drug‐eluting stent, endovascular treatment, peripheral artery disease
Subject Categories: Peripheral Vascular Disease, Stent, Revascularization, Angiography, Ischemia
Introduction
Critical limb ischemia (CLI) manifests with chronic ischemic pain at rest, loss of tissue in the limb, or both. At present, the incidence of this final stage of peripheral arterial disease is estimated at 500 to 1000 cases per 1 million inhabitants every year in the Western world.1Major risk factors in the development of CLI are diabetes mellitus, smoking, and increasing age.1, 2 With an aging Western population and increasing prevalence of diabetes mellitus, the burden of CLI and its costs are likely to increase.3
The main goal of treatment for CLI is to prevent major amputation. This mutilating procedure is associated with high periprocedural morbidity and mortality, and functional outcome after amputation is often poor.4
Restoration of unobstructed pulsatile blood flow to the foot is imperative to relieve symptoms and to prevent amputation. Revascularization can be achieved by means of either endovascular or surgical techniques.1, 3, 5, 6 In case of CLI due to infrapopliteal lesions, percutaneous transluminal angioplasty (PTA) with bailout bare metal stent (BMS) is probably still the most frequently used endovascular technique. Drug‐eluting stents (DESs) in infrapopliteal lesions have demonstrated lower restenosis rates than either PTA or BMS in several randomized clinical trials, but only a few of these studies also reported improved clinical results such as lower amputation rates.7, 8, 9, 10, 11, 12 Reasons for this may include study design,7 small numbers of patients,8, 9 or inclusion of participants with intermittent claudication and thus not at risk of major amputation.8, 9, 10, 11 In addition, only 1 study so far has reported long‐term clinical results.13
The Percutaneous transluminal Angioplasty versus Drug eluting stents for Infrapopliteal lesions (PADI) trial was designed to compare the performance of paclitaxel‐eluting DESs and PTA‐BMS of infrapopliteal lesions in a population consisting solely of CLI patients.14 Short‐ and midterm results of this study have been published elsewhere and showed more favorable clinical outcomes after DESs compared with PTA‐BMS, with fewer major amputations after DESs and a trend toward significance at 2‐year follow‐up and significantly fewer minor amputations during the first 6 months of follow‐up.12 This paper presents the outcomes at long‐term follow‐up of this multicenter randomized controlled trial.
Methods
Study Design, Population, and Procedures
The purpose of the PADI trial was to assess the performance of paclitaxel‐eluting DESs compared with PTA‐BMS in infrapopliteal lesions causing CLI. Patients were enrolled between October 2007 and February 2013 at 3 major vascular centers in the Netherlands. The study protocol was approved by the medical ethics boards of the participating centers, and all enrolled patients gave written informed consent. Adults with a Rutherford category15 ≥4 due to infrapopliteal lesions, as assessed with pretreatment imaging, were randomly allocated to one of the 2 treatment arms. Randomization was per limb. When a patient was included for both limbs, each limb was randomized separately. A maximum of 3 lesions per limb could be included, with each of these lesions allocated to the same treatment arm. In the DES arm, target lesions were treated with paclitaxel‐eluting stainless steel coronary stents (TAXUS Liberté; Boston Scientific). Patients in the PTA‐BMS arm received PTA with optional bailout stenting using non–drug‐eluting BMS. All patients were treated with carbasalate calcium (100 mg daily, indefinitely) and clopidogrel (loading dose of 300 mg directly after the procedure followed by 75 mg daily for at least 6 months). Details of the study design and short‐ and midterm results have been reported previously.12, 14 The trial was registered at ClinicalTrials.gov (identifier NCT00471289).
Long‐Term Follow‐up and End Points
Long‐term follow‐up involved annual patient assessments for 5 years after inclusion or until a clinical end point was reached. Assessments consisted of medical history, physical examination, and duplex sonography of the treated limb. When patients were not willing or able to visit the hospital, passive follow‐up was obtained by contacting patients or their general practitioners by phone or by retrieving data from the hospital electronic medical records. Survival was recorded for patients who underwent a major amputation or infrapopliteal surgical or endovascular retreatment of the target limb. Causes of death were registered as CLI related or unrelated, if known.
Clinical end points of the long‐term follow‐up registry of the PADI trial were major amputation (above ankle level) of the treated limb, infrapopliteal surgical or endovascular reintervention attempted on the treated limb, or death, throughout the entire observation period. Preserved primary patency of treated lesions was an additional morphological end point of long‐term follow‐up. This end point was assessed by duplex sonography, defined as ≤50% restenosis (peak systolic velocity ratio ≤2.016). An ordinal score was used to grade the severity of treatment failure on a continuum from vessel restenosis (>50% stenosis, peak systolic velocity ratio >2.016) to vessel occlusion to clinical failure (treatment in interim, major amputation, or CLI‐related death).
Statistical Analysis
We compared categorical variables with the 2‐sided χ2 test, ordinal variables with the Mann–Whitney test, and continuous variables with the 2‐sided Student t test. The observed rates of major amputation per limb and survival per patient were estimated with the Kaplan–Meier method. In addition, estimated rates of amputation‐free survival (defined as survival free from major amputation of the index limb) and event‐free survival (defined as survival free from major amputation or reintervention of the index limb) were analyzed with this same method. Limbs and patients were censored at end of follow‐up.
We performed additional subgroup analyses in patients with and without diabetes mellitus; with normal and impaired renal function; with and without tissue loss; and with 1, 2, or 3 treated lesions. Unadjusted and adjusted hazard ratios were calculated for the risk of major amputation, major amputation or death, and an event (major amputation, reintervention, or death).
For the analyses regarding the end points per lesion, a weighted χ2 test was used, with weights equal to the inverse number of lesions per limb. In patients included for both limbs, limbs were considered independently for the analysis.
All primary and secondary end points were evaluated in the modified intention‐to‐treat analysis, excluding patients who did not fulfill all inclusion criteria or who were incorrectly included. End points per lesion were also evaluated in the modified intention‐to‐treat analysis, so they were included in the arm for which they were randomized.
A 2‐sided P≤0.05 was considered to indicate statistical significance. Analyses were performed in SPSS version 23 for Mac (IBM Corp).
Results
Baseline Characteristics and Short‐Term Outcomes
From October 2007 through February 2013, 75 limbs in 74 patients were randomly assigned to the DES arm and 69 limbs in 67 patients were assigned to the PTA‐BMS arm (Figure 1). One patient (1 limb) in the DES arm and 3 patients (3 limbs) in the PTA arm were excluded from the modified intention‐to‐treat analysis. Overall, 91 lesions were treated in the PTA‐BMS arm and 121 lesions were treated in the DES arm, an average of 1.4 and 1.6 lesions per limb, respectively.
Figure 1.
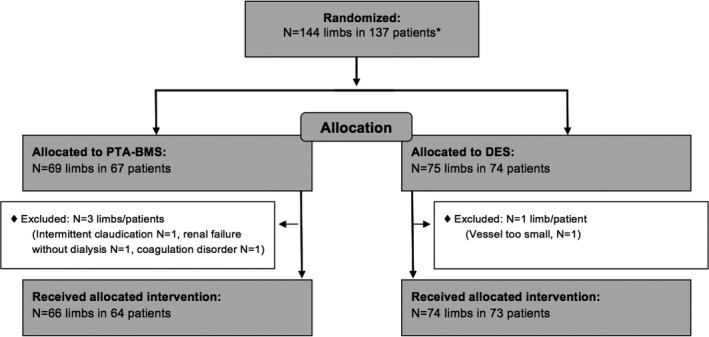
Flow diagram of inclusion. *Four patients included for 2 limbs, with 1 limb in each arm. BMS indicates bare metal stent; DES, drug‐eluting stent; PTA, percutaneous transluminal angioplasty.
The comparable baseline characteristics and short‐term outcomes have been published elsewhere.12
Long‐Term Clinical Outcomes
Patients were followed for a mean duration of 163.8 weeks (SD 107.1 weeks), equivalent to 430 patient‐years of observation.
Figure 2 shows the clinical outcome per patient at 1, 2, 3, 4, and 5 years after treatment (the exact numbers can be found in Tables S1–S5). At all observation points, the percentage of preserved limbs was higher in the DES arm than in the PTA‐BMS arm; however, these outcomes did not differ significantly.
Figure 2.
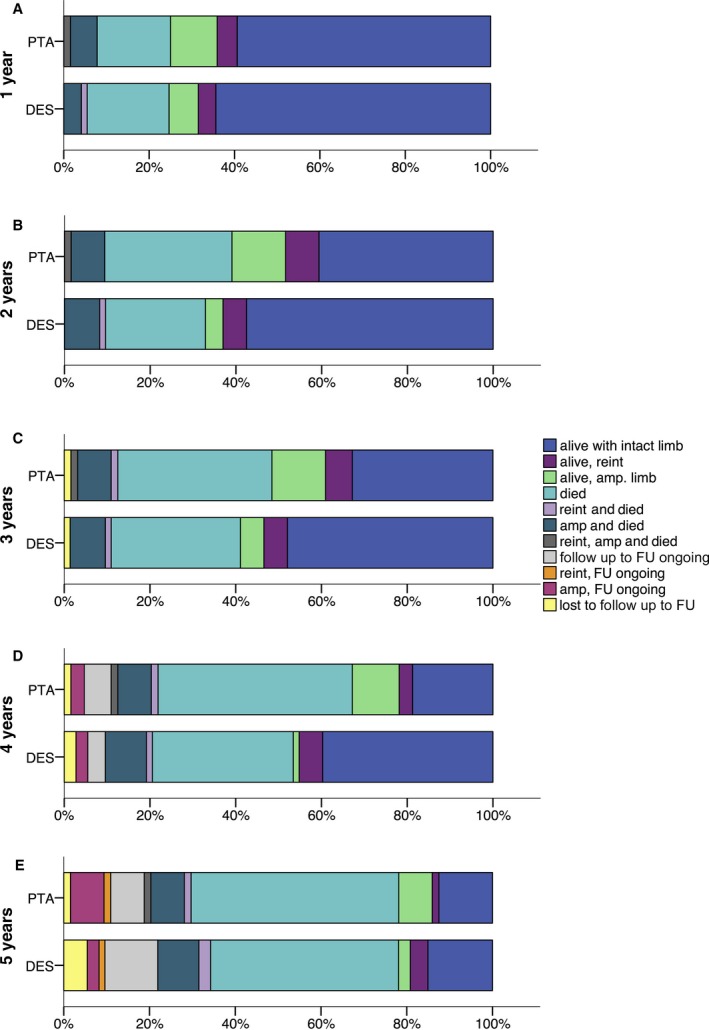
Clinical outcome per patient at 1 year (a), 2 years (b), 3 years (c), 4 years (d), and 5 years (e) of follow‐up. Amp indicates amputation; DES, drug‐eluting stent; FU, follow‐up; PTA, percutaneous transluminal angioplasty; reint, reintervention.
The estimated major amputation rate was lower in the DES arm than in the PTA‐BMS arm (19.3% versus 34.0% after 5 years, respectively), with a trend toward significance (P=0.091) (Table 1 and Figure 3A). The amputation‐ and event‐free survival rates were significantly higher in the DES arm than in the PTA‐BMS arm (31.8% versus 20.4%, P=0.043; and 26.2% versus 15.3%, P=0.041, respectively), as is shown in Table 1 and Figure 3B and 3C. Survival rates were comparable (Table 1, Figure 3D).
Table 1.
Estimated Major Amputation, Major Amputation or Death, Event, and Survival Rates
| PTA‐BMS (66 Limbs, 64 Patients) | DES (74 Limbs, 73 Patients) | Overall P Valuea | |||
|---|---|---|---|---|---|
| n | % (95% CI) | n | % (95% CI) | ||
| Major amputation rate per limb | |||||
| 0–6 months | 13 | 20.5 (10.5–30.5) | 7 | 9.8 (2.9–16.7) | |
| 0–1 year | 13 | 20.5 (10.5–30.5) | 8 | 11.4 (4.0–18.8) | |
| 0–2 years | 15 | 24.8 (13.6–36.0) | 9 | 13.2 (5.2–21.2) | |
| 0–3 years | 15 | 24.8 (13.6–36.0) | 10 | 15.1 (6.5–23.7) | |
| 0–4 years | 16 | 28.0 (15.8–40.2) | 10 | 15.1 (6.5–23.7) | |
| 0–5 years | 17 | 34.0 (18.1–49.9) | 11 | 19.3 (7.7–30.9) | 0.091 |
| Major amputation or death rate per patient | |||||
| 0–6 months | 18 | 28.1 (17.1–39.1) | 15 | 20.5 (11.3–29.7) | |
| 0–1 year | 23 | 35.9 (24.1–47.7) | 23 | 31.5 (20.9–42.1) | |
| 0–2 years | 33 | 51.6 (39.4–63.8) | 27 | 37.0 (25.8–48.2) | |
| 0–3 years | 39 | 60.9 (48.9–72.9) | 33 | 45.2 (33.8–56.6) | |
| 0–4 years | 46 | 73.5 (62.3–84.7) | 35 | 48.3 (36.7–59.9) | |
| 0–5 years | 49 | 79.6 (69.0–90.2) | 45 | 68.2 (56.0–80.4) | 0.043 |
| Event rate per patient | |||||
| 0–6 months | 20 | 31.2 (19.8–42.6) | 17 | 23.3 (13.7–32.9) | |
| 0–1 year | 26 | 40.6 (28.6–52.6) | 26 | 35.6 (24.6–46.6) | |
| 0–2 years | 38 | 59.4 (47.4–71.4) | 31 | 42.5 (31.1–53.9) | |
| 0–3 years | 43 | 67.2 (55.6–78.8) | 37 | 50.7 (39.1–62.3) | |
| 0–4 years | 50 | 79.0 (68.8–89.2) | 39 | 53.8 (42.2–65.4) | |
| 0–5 years | 53 | 84.7 (75.5–93.9) | 49 | 73.8 (62.2–85.4) | 0.041 |
| Survival rate per patient | |||||
| 0–6 months | 54 | 84.4 (75.6–93.2) | 63 | 86.3 (78.5–94.1) | |
| 0–1 year | 48 | 75.0 (64.4–85.6) | 55 | 75.3 (65.5–85.1) | |
| 0–2 years | 39 | 60.9 (48.9–72.9) | 49 | 67.1 (56.3–77.9) | |
| 0–3 years | 33 | 53.1 (40.9–65.3) | 43 | 60.3 (49.1–71.5) | |
| 0–4 years | 21 | 41.8 (29.3–54.3) | 34 | 55.5 (43.9–67.1) | |
| 0–5 years | 13 | 37.0 (24.3–49.7) | 17 | 37.7 (25.2–50.2) | 0.45 |
BMS indicates bare metal stent; DES, drug‐eluting stent; PTA, percutaneous transluminal angioplasty.
Overall log‐rank test.
Figure 3.
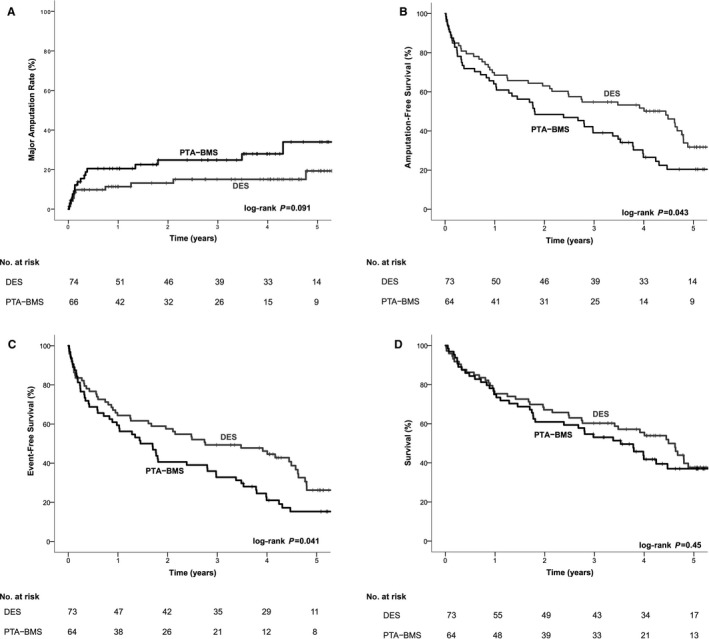
A, Kaplan–Meier curves representing estimated 5‐year cumulative incidence rates after PTA‐BMS and DES. A, Major amputation per limb. B, Amputation‐free survival per patient. C, Event‐free survival per patient. D, Survival per patient. BMS indicates bare metal stent; DES, drug‐eluting stent; PTA, percutaneous transluminal angioplasty.
Additional analyses of the major amputation and death rates and event rates were performed in subgroups of patients with and without diabetes mellitus; with normal and impaired renal function; with and without tissue loss; and with 1, 2, or 3 treated lesions (Table 2). In all subgroups, the percentage of events was lower in patients treated with DESs. This difference was statistically significant only in the subgroup of patients with impaired renal function.
Table 2.
Subgroup Analyses
| PTA‐BMS | DES | P Valuea | |||||
|---|---|---|---|---|---|---|---|
| Total (n) | Event (n) | % (95% CI) | Total (n) | Event (n) | % (95% CI) | ||
| 5‐year major amputation/death rate per patient | |||||||
| DM | 43 | 34 | 81.0 (68.8–93.2) | 44 | 26 | 68.3 (52.2–84.4) | 0.07 |
| No DM | 21 | 15 | 78.2 (58.0–98.4) | 29 | 19 | 68.9 (50.7–87.1) | 0.56 |
| Tissue loss | 56 | 45 | 83.0 (72.6–93.4) | 63 | 41 | 71.3 (58.8–83.8) | 0.05 |
| No tissue loss | 8 | 4 | 50.0 (15.3–84.7) | 10 | 4 | 47.5 (11.0–84.0) | 0.59 |
| Impaired RF | 10 | 9 | 90.0 (71.4–100) | 11 | 6 | 56.4 (26.0–86.8) | 0.017 |
| Normal RF | 54 | 40 | 77.9 (65.9–89.9) | 62 | 39 | 70.9 (57.8–84.0) | 0.18 |
| 1 lesion | 46 | 35 | 78.9 (66.4–91.4) | 40 | 26 | 74.9 (58.8–91.0) | 0.36 |
| 2 lesions | 9 | 8 | 88.9 (68.3–100) | 19 | 10 | 56.1 (31.6–80.6) | 0.09 |
| 3 lesions | 9 | 6 | 72.2 (40.4–100) | 14 | 9 | 64.3 (39.2–89.4) | 0.45 |
| 5‐year event rate per patient | |||||||
| DM | 43 | 36 | 84.7 (73.7–95.7) | 44 | 29 | 75.1 (60.2–90.0) | 0.15 |
| No DM | 21 | 17 | 86.4 (69.9–100) | 29 | 20 | 72.8 (55.2–90.4) | 0.30 |
| Tissue loss | 56 | 47 | 85.7 (76.1–95.3) | 63 | 45 | 77.7 (66.1–89.3) | 0.13 |
| No tissue loss | 8 | 6 | 75.0 (45.0–100) | 10 | 4 | 47.5 (11.0–84.0) | 0.09 |
| Impaired RF | 10 | 9 | 90.0 (71.4–100) | 11 | 6 | 56.4 (26.0–86.8) | 0.017 |
| Normal RF | 54 | 44 | 83.9 (73.5–94.3) | 62 | 43 | 77.6 (65.3–89.9) | 0.20 |
| 1 lesion | 46 | 38 | 83.9 (72.9–94.9) | 40 | 27 | 75.6 (59.9–91.3) | 0.25 |
| 2 lesions | 9 | 9 | 100 (100–100) | 19 | 12 | 68.4 (43.9–92.9) | 0.07 |
| 3 lesions | 9 | 6 | 72.2 (40.4–100) | 14 | 10 | 71.4 (47.7–95.1) | 0.66 |
BMS indicates bare metal stent; DES, drug‐eluting stent; DM, diabetes mellitus; N, number of patients; PTA, percutaneous transluminal angioplasty, RF, renal function.
Overall log‐rank test.
Univariate and multivariate regression analysis showed no significant association between DESs and the risk of major amputation (Table 3); however, DESs were associated with a significantly lower risk of the composite end points of major amputation or death and of major amputation, reintervention, or death (Tables 4 and 5, respectively). Factors associated with a higher risk of major amputation were diabetes mellitus and higher Rutherford category. Regarding the risk of major amputation or death, further factors with significant hazard ratios were age, current smoking, Rutherford category, and an ankle‐brachial index >1.4 or unmeasurable ankle‐brachial index. Age and high or unmeasurable ankle‐brachial index were also associated with a higher risk of major amputation, reintervention, or death.
Table 3.
Cox Regression Analysis of the Risk of Major Amputation
| Variables at Baseline | Hazard Ratio | 95% CI | P Value |
|---|---|---|---|
| Univariate analysis | |||
| Treatment with DES | 0.53 | 0.25–1.12 | 0.10 |
| Multivariate analysis | |||
| Treatment with DES | 0.61 | 0.27–1.36 | 0.22 |
| Age | 1.01 | 0.98–1.04 | 0.60 |
| Diabetes mellitus | 3.43 | 1.15–10.24 | 0.027 |
| Impaired renal functiona | 1.80 | 0.68–4.74 | 0.24 |
| Stroke | 0.88 | 0.32–2.46 | 0.81 |
| Coronary disease | 1.32 | 0.57–3.05 | 0.52 |
| Current smoker | 1.86 | 0.70–4.94 | 0.21 |
| Former smoker | 1.20 | 0.39–3.71 | 0.75 |
| Rutherford category | 2.12 | 1.06–4.24 | 0.035 |
| Low ABI (<0.7) | 1.30 | 0.53–3.19 | 0.57 |
| High ABI (>1.4/unmeasurable) | 2.04 | 0.67–6.23 | 0.21 |
| Number of included lesions | 0.75 | 0.43–1.30 | 0.31 |
ABI indicates ankle‐brachial index; DES, drug‐eluting stent.
Impaired renal function defined as estimated glomerular filtration rate <30 mL/min/1.73 m2.
Table 4.
Cox Regression Analysis of Risk of Major Amputation or Death
| Variables at Baseline | Hazard Ratio | 95% CI | P Value |
|---|---|---|---|
| Univariate analysis | |||
| Treatment with DES | 0.67 | 0.45–0.99 | 0.045 |
| Multivariate analysis | |||
| Treatment with DES | 0.59 | 0.39–0.89 | 0.012 |
| Age | 1.05 | 1.03–1.08 | <0.001 |
| Diabetes mellitus | 1.12 | 0.72–1.76 | 0.62 |
| Impaired renal functiona | 1.27 | 0.70–2.33 | 0.44 |
| Stroke | 1.36 | 0.79–2.33 | 0.26 |
| Coronary disease | 1.15 | 0.74–1.80 | 0.53 |
| Current smoker | 1.82 | 1.05–3.14 | 0.032 |
| Former smoker | 1.48 | 0.86–2.55 | 0.16 |
| Rutherford category | 1.51 | 1.05–2.18 | 0.026 |
| Number of treated lesions | 0.80 | 0.60–1.06 | 0.13 |
| Low ABI (<0.7) | 0.89 | 0.55–1.44 | 0.63 |
| High ABI (>1.4/unmeasurable) | 3.38 | 1.71–6.68 | <0.001 |
ABI indicates ankle‐brachial index; DES, drug‐eluting stent.
Impaired renal function defined as estimated glomerular filtration rate <30 mL/min/1.73 m2.
Table 5.
Cox Regression Analysis of Risk of Event
| Variables at Baseline | Hazard Ratio | 95% CI | P Value |
|---|---|---|---|
| Univariate analysis | |||
| Treatment with DES | 0.67 | 0.46–0.99 | 0.043 |
| Multivariate analysis | |||
| Treatment with DES | 0.62 | 0.42–0.92 | 0.019 |
| Age | 1.04 | 1.02–1.07 | <0.001 |
| Diabetes mellitus | 1.14 | 0.74–1.76 | 0.53 |
| Impaired renal functiona | 1.06 | 0.59–1.92 | 0.85 |
| Stroke | 1.53 | 0.90–2.58 | 0.12 |
| Coronary disease | 1.33 | 0.87–2.05 | 0.19 |
| Former smoker | 1.21 | 0.71–2.05 | 0.48 |
| Current smoker | 1.58 | 0.93–2.71 | 0.09 |
| Rutherford category | 1.39 | 0.97–1.99 | 0.08 |
| Number of treated lesions | 0.81 | 0.62–1.06 | 0.13 |
| Low ABI (<0.7) | 0.95 | 0.59–1.50 | 0.81 |
| High ABI (>1.4/unmeasurable) | 2.88 | 1.50–5.53 | 0.001 |
ABI indicates ankle‐brachial index; DES, drug‐eluting stent.
Impaired renal function defined as estimated glomerular filtration rate <30 mL/min/1.73 m2.
Long‐Term Patency Rates
Tables 6, 7, 8, 9 through 10 show the results of the modified intention‐to‐treat analysis of the patency rates of the treated lesions, as assessed on duplex sonography. These results of duplex sonography are available in a limited percentage of limbs. Many patients were unable to visit the hospital, and during follow‐up, a substantial percentage of patients died from unrelated or unknown causes. The number of limbs without imaging is comparable in both groups.
Table 6.
Patency Per Lesion at 1‐Year Follow‐up (Duplex)
| Modified Intention‐to‐Treat Analysis | PTA‐BMS | DES | P Valueb |
|---|---|---|---|
| n=71a | n=90a | ||
| Lesions with preserved patency | 30 (42.3) | 59 (65.6) | 0.007 |
| Ordinal score | 0.021 | ||
| ≤50% stenotic | 30 (42.3) | 59 (65.6) | |
| >50% stenotic | 12 (16.9) | 2 (2.2) | |
| Occluded | 6 (8.5) | 5 (5.6) | |
| Amputation/CLI‐related death/treatment in interim | 23 (32.4) | 24 (26.7) |
Values are n (%). BMS indicates bare metal stent; CLI, critical limb ischemia; DES, drug‐eluting stent; PTA, percutaneous transluminal angioplasty.
Number of limbs/lesions with available diagnostic imaging and those with treatment failure. Imaging unavailable for 9 limbs in the PTA‐BMS group and 8 limbs in the DES group. Limbs/lesions in patients deceased due to unrelated causes were censored (10 limbs in PTA‐BMS, 10 limbs in DES).
P value weighted by number of lesions per patient.
Table 7.
Patency Per Lesion at 2‐Year Follow‐up (Duplex)
| Modified Intention‐to‐Treat Analysis | PTA‐BMS | DES | P Valueb |
|---|---|---|---|
| n=45a | n=48a | ||
| Lesions with preserved patency | 11 (24.4) | 15 (31.3) | 0.25 |
| Ordinal score | 0.64 | ||
| ≤50% stenotic | 11 (24.4) | 15 (31.3) | |
| >50% stenotic | 4 (8.9) | 2 (4.2) | |
| Occluded | 2 (4.4) | 4 (8.3) | |
| Amputation/CLI‐related death/treatment in interim | 28 (62.2) | 27 (56.3) |
Values are n (%). BMS indicates bare metal stent; CLI, critical limb ischemia; DES, drug‐eluting stent; PTA, percutaneous transluminal angioplasty.
Number of limbs/lesions with available diagnostic imaging and those with treatment failure. Imaging unavailable for 19 limbs in the PTA‐BMS group and 29 limbs in the DES group. Limbs/lesions in patients deceased due to unrelated causes were censored (18 limbs in PTA‐BMS, 14 limbs in DES).
P value weighted by number of lesions per patient.
Table 8.
Patency Per Lesion at 3‐Year Follow‐up (Duplex)
| Modified Intention‐to‐Treat Analysis | PTA‐BMS | DES | P Valueb |
|---|---|---|---|
| n=39a | n=53a | ||
| Lesions with preserved patency | 8 (20.5) | 20 (37.7) | 0.036 |
| Ordinal score | 0.18 | ||
| ≤50% stenotic | 8 (20.5) | 20 (37.7) | |
| >50% stenotic | 2 (5.1) | 2 (3.8) | |
| Occluded | 1 (2.6) | 2 (3.8) | |
| Amputation/CLI‐related death/treatment in interim | 28 (71.8) | 29 (54.7) |
Values are n (%). BMS indicates bare metal stent; CLI, critical limb ischemia; DES, drug‐eluting stent; PTA, percutaneous transluminal angioplasty.
Number of limbs/lesions with available diagnostic imaging and those with treatment failure. Imaging unavailable for 19 limbs in the PTA‐BMS group and 22 limbs in the DES group. Limbs/lesions in patients deceased due to unrelated causes were censored (22 limbs in PTA‐BMS, 19 limbs in DES).
P value weighted by number of lesions per patient.
Table 9.
Patency Per Lesion at 4‐Year Follow‐up (Duplex)
| Modified Intention‐to‐Treat Analysis | PTA‐BMS | DES | P Valueb |
|---|---|---|---|
| n=37a | n=46a | ||
| Lesions with preserved patency | 5 (13.5) | 15 (32.6) | 0.031 |
| Ordinal score | 0.08 | ||
| ≤50% stenotic | 5 (13.5) | 15 (32.6) | |
| >50% stenotic | 2 (5.4) | 0 | |
| Occluded | 0 | 2 (4.3) | |
| Amputation/CLI‐related death/treatment in interim | 30 (81.1) | 29 (63.0) |
Values are n (%). BMS indicates bare metal stent; CLI, critical limb ischemia; DES, drug‐eluting stent; PTA, percutaneous transluminal angioplasty.
Number of limbs/lesions with available diagnostic imaging and those with treatment failure. Imaging unavailable for 11 limbs in the PTA‐BMS group and 21 limbs in the DES group. Limbs/lesions in patients deceased due to unrelated causes were censored (27 limbs in PTA‐BMS, 21 limbs in DES). Follow‐up still ongoing for 6 limbs in the PTA‐BMS group and 5 limbs in the DES group.
P value weighted by number of lesions per patient.
Table 10.
Patency Per Lesion at 5‐Year Follow‐up (Duplex)
| Modified Intention‐to‐Treat Analysis | PTA‐BMS | DES | P Valueb |
|---|---|---|---|
| n=35a | n=43a | ||
| Lesions with preserved patency | 3 (8.6) | 5 (11.6) | 0.67 |
| Ordinal score | 0.52 | ||
| ≤50% stenotic | 3 (8.6) | 5 (11.6) | |
| >50% stenotic | 1 (2.9) | 0 | |
| Occluded | 0 | 2 (4.7) | |
| Amputation/CLI‐related death/treatment in interim | 31 (88.6) | 36 (83.7) |
Values are n (%). BMS indicates bare metal stent; CLI, critical limb ischemia; DES, drug‐eluting stent; PTA, percutaneous transluminal angioplasty.
Number of limbs/lesions with available diagnostic imaging and those with treatment failure. Imaging unavailable for 7 limbs in the PTA‐BMS group and 12 limbs in the DES group. Limbs/lesions in patients deceased due to unrelated causes were censored (30 limbs in PTA‐BMS, 28 limbs in DES). Follow‐up still ongoing for 11 limbs in the PTA‐BMS group and 12 limbs in the DES group.
P value weighted by number of lesions per patient.
Despite the limited number of lesions still available for follow‐up, the percentages of lesions with preserved primary patency were significantly higher in the DES arm than in the PTA‐BMS arm at 1, 3, and 4 years of follow‐up. After 2 and 5 years, the percentages of lesions with preserved patency were also higher in the DES group, but these differences did not reach statistical significance. The ordinal scores showed significantly more favorable outcomes in the DES group at 1‐year follow‐up.
Discussion
Our long‐term results show that up to 5 years after endovascular treatment of infrapopliteal lesions in CLI patients, the amputation‐ and event‐free survival rates are significantly higher in patients treated with paclitaxel‐eluting DESs compared with PTA‐BMS. When considering the major amputation and survival rates separately, both are more favorable in the DES group, with a trend toward significance for major amputation. These positive results of DESs were confirmed by univariate and multivariate regression analysis.
Morphological follow‐up of the treated infrapopliteal lesions in a limited number of patients shows that at 1‐, 3‐, and 4‐year follow‐up, the percentage of lesions with preserved binary patency is significantly higher in the DES group than in the PTA‐BMS group.
Few studies thus far have assessed the long‐term (>1‐year) follow‐up of patients with CLI due to infrapopliteal lesions that were treated with DESs. The published meta‐analyses considering the endovascular treatment of lesions below the knee with DESs versus either PTA or BMS evaluated the available outcomes until 1 year after treatment only.17, 18, 19, 20, 21 Of the individual randomized controlled trials reporting on DESs for the treatment of infrapopliteal lesions,7, 8, 9, 10, 11 only the YUKON trial evaluated the long‐term results.13 The mean follow‐up period of this trial, in which nonpolymer sirolimus‐eluting stents (SESs) were compared with BMS, was 1016 days, a few months shorter than our follow‐up period (1285 days). Reported event‐free survival rates (defined as freedom from target limb amputation, target vessel revascularization, myocardial infarction, and death) were 65.8% and 44.6% (P=0.02) for the SES and BMS group, respectively, and amputation rates were 2.6% and 12.2% (P=0.06), respectively. Furthermore, the authors found significantly stronger improvement in Rutherford category in the SES group.13 A prospective nonrandomized registry investigated the 3‐year angiographic and clinical outcomes after infrapopliteal revascularization with angioplasty and bailout SES or BMS in patients with CLI. The SES group showed significantly better primary patency and reduced binary restenosis rates and better repeat intervention‐free survival (hazard ratio 2.56, P=0.006; 77.6% in the SES group and 70.3% in the BMS group, P=0.049).22
Our results show that the long‐term prognosis of CLI patients remains poor, with estimated amputation‐ and event‐free survival rates after 5 years in our study population of 20.4% and 15.3%, respectively, in the PTA‐BMS group and 31.8% and 26.2%, respectively, in the DES group, respectively. In the above‐mentioned studies regarding long‐term follow‐up after infrapopliteal DES placement, the reported event‐free survival (with a slightly different definition compared with our definition) and reintervention‐free survival rates are higher. This can be explained by the difference in patient characteristics; not even half of the patients included in the YUKON trial suffered from CLI,13 and a larger percentage of patients included in the nonrandomized registry suffered from pain at rest.22 Clinical event rates in patients with claudication are reported to be very low1, 17 and patients with tissue loss have a much higher amputation rate than those with pain at rest.23
The estimated survival rates of our cohort are very poor in both groups (5‐year survival 37.0% in the PTA‐BMS group and 37.7% in the DES group). Reported mortality rates in CLI patients are as high as 40% at 1 year and 40% to 70% at 5 years, pointing out the systemic character of atherosclerotic disease in these patients, the majority of whom also suffer from coronary and cerebrovascular disease.1, 24, 25, 26, 27 These mortality rates even exceed those of several malignancies.28 A more recent meta‐analysis evaluated 1‐year amputation‐free survival and mortality rates in CLI patients without revascularization options and reported significant improvement of these outcomes over the past 2 decades.29 The most recent trial included in this meta‐analysis reported a 1‐year mortality rate of 19.8% (95% CI 11.6–31.7%),30 which is in line with our 1‐year survival rates. Insufficient data are available in the literature to determine whether this improvement, which has been suggested to be the result of improved secondary prevention, also occurs in the long term.27, 29
Additional subgroup analysis showed lower events in the DES subgroup, but because the numbers in the different subgroups are small, no further conclusions should be drawn about which specific patients would potentially benefit more from treatment with DESs.
We found a more favorable major amputation rate in the DES group, with a trend toward significance. Survival rates, however, were comparable in both groups, whereas it is generally assumed that survival is negatively affected after major amputation.4 Our results do not confirm this assumption; this may be explained by the lower major amputation rate (up to 34% after 5 years) in comparison with the overall high death rate (>60% after 5 years) in patients with abundant comorbidity. The fact that the difference in amputation rate did not reach statistical significance but the difference in amputation‐ and event‐free survival did is probably due to the higher number of events in the latter analyses.
DESs used in this study were balloon‐expandable coronary DESs, with the most important disadvantage being limited length only suited to treat lesions up to 90 mm. Infrapopliteal disease in CLI patients, especially those with diabetes mellitus, is known to consist of long and diffuse lesions.31, 32 The fact that the concept of DESs seems to be effective below the knee to reduce restenosis warrants the development of long and flexible self‐expandable DESs, enabling the treatment of longer infrapopliteal lesions with DESs.
An alternative drug‐eluting technique is PTA using a drug‐eluting balloon. To date, performance of the drug‐eluting balloon has not proved to be superior for infrapopliteal lesions. Although some studies found more favorable patency rates and lower restenosis rates in comparison with PTA,33, 34 the most recently conducted randomized trial did not show a difference in the primary efficacy end point of late lumen loss.35 Furthermore, this latter trial found a trend of higher amputation rates in the drug‐eluting balloon group (8.8% versus 3.6% with PTA; P=0.08).35
The major strength of the present study is the long follow‐up period up to 5 years after treatment, with very few patients lost to follow‐up. Furthermore, we included only CLI patients, who are actually at risk of clinical events.
Unfortunately, during the 5 years of follow‐up, a substantial number of patients were physically unable to visit the hospital for evaluation by the vascular surgeon and duplex sonography of the treated limb. Consequently, we could present major clinical end points, such as major amputation, reinterventions, and survival, for all patients and the primary patency rates for a subgroup only.
Another limitation is that we did not test the cost‐effectiveness of DESs. In fact, this is important to evaluate because drug‐eluting devices are costly. In addition to the already existing evidence of the superior performance of DESs in infrapopliteal lesions, cost‐effectiveness should be derived in the future.
In conclusion, this randomized controlled trial showed that long‐term amputation‐ and event‐free survival in patients with CLI due to infrapopliteal lesions is more favorable after treatment with DESs compared with the conventional endovascular strategy of PTA‐BMS. The limited available morphological results also showed higher preserved patency rates after DESs than after PTA‐BMS. Given the feasibility of DESs for infrapopliteal lesions, proven not only at short and midterm but also long term, one should consider treatment with a DES in patients with CLI caused by lesions below the knee.
Sources of Funding
The PADI trial received an unrestricted research grant during the conduct of the study from The Netherlands Society for Interventional Radiology, who had no role in study design, data collection, analysis, interpretation, or writing of the report.
Disclosures
Spreen has received a modest honorarium (speaking fee) from Boston Scientific, Marlborough, MA, USA; and van Overhagen has received modest honoraria (speaking fees) and expert witness fees (consulting fees) from Cook Medical, Bloomington, IN, USA; Boston Scientific, Marlborough, MA, USA; and Cardinal Health, Dublin, OH, USA; unrelated to the submitted work. There are no further personal conflicts of interest.
Supporting information
Table S1. Outcome at 1‐Year Follow‐up, per Patient
Table S2. Outcome at 2‐Year Follow‐up, per Patient
Table S3. Outcome at 3‐Year Follow‐up, per Patient
Table S4. Outcome at 4‐Year Follow‐up, per Patient
Table S5. Outcome at 5‐Yeas Follow‐up, per Patient
Acknowledgments
The effort of the clinical research coordinators in the participating centers is greatly appreciated.
(J Am Heart Assoc. 2017;6:e004877 DOI: 10.1161/JAHA.116.004877.)28411244
References
- 1. Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG. Inter‐society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg. 2007;45(suppl S):S5–S67. [DOI] [PubMed] [Google Scholar]
- 2. Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WR, Olin JW, Puschett JB, Rosenfield KA, Sacks D, Stanley JC, Taylor LM Jr, White CJ, White J, White RA, Antman EM, Smith SC Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Associations for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (writing committee to develop guidelines for the management of patients with peripheral arterial disease)–summary of recommendations. J Vasc Interv Radiol. 2006;17:1383–1397; quiz 1398. [DOI] [PubMed] [Google Scholar]
- 3. Adam DJ, Beard JD, Cleveland T, Bell J, Bradbury AW, Forbes JF, Fowkes FG, Gillepsie I, Ruckley CV, Raab G, Storkey H. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet. 2005;366:1925–1934. [DOI] [PubMed] [Google Scholar]
- 4. Nehler MR, Coll JR, Hiatt WR, Regensteiner JG, Schnickel GT, Klenke WA, Strecker PK, Anderson MW, Jones DN, Whitehill TA, Moskowitz S, Krupski WC. Functional outcome in a contemporary series of major lower extremity amputations. J Vasc Surg. 2003;38:7–14. [DOI] [PubMed] [Google Scholar]
- 5. van Overhagen H, Spiliopoulos S, Tsetis D. Below‐the‐knee interventions. Cardiovasc Intervent Radiol. 2013;36:302–311. [DOI] [PubMed] [Google Scholar]
- 6. Peregrin JH, Koznar B, Kovac J, Lastovickova J, Novotny J, Vedlich D, Skibova J. PTA of infrapopliteal arteries: long‐term clinical follow‐up and analysis of factors influencing clinical outcome. Cardiovasc Intervent Radiol. 2010;33:720–725. [DOI] [PubMed] [Google Scholar]
- 7. Bosiers M, Scheinert D, Peeters P, Torsello G, Zeller T, Deloose K, Schmidt A, Tessarek J, Vinck E, Schwartz LB. Randomized comparison of everolimus‐eluting versus bare‐metal stents in patients with critical limb ischemia and infrapopliteal arterial occlusive disease. J Vasc Surg. 2012;55:390–398. [DOI] [PubMed] [Google Scholar]
- 8. Falkowski A, Poncyljusz W, Wilk G, Szczerbo‐Trojanowska M. The evaluation of primary stenting of sirolimus‐eluting versus bare‐metal stents in the treatment of atherosclerotic lesions of crural arteries. Eur Radiol. 2009;19:966–974. [DOI] [PubMed] [Google Scholar]
- 9. Siablis D, Kitrou PM, Spiliopoulos S, Katsanos K, Karnabatidis D. Paclitaxel‐coated balloon angioplasty versus drug‐eluting stenting for the treatment of infrapopliteal long‐segment arterial occlusive disease: the IDEAS randomized controlled trial. JACC Cardiovasc Interv. 2014;7:1048–1056. [DOI] [PubMed] [Google Scholar]
- 10. Rastan A, Tepe G, Krankenberg H, Zahorsky R, Beschorner U, Noory E, Sixt S, Schwarz T, Brechtel K, Bohme C, Neumann FJ, Zeller T. Sirolimus‐eluting stents vs. bare‐metal stents for treatment of focal lesions in infrapopliteal arteries: a double‐blind, multi‐centre, randomized clinical trial. Eur Heart J. 2011;32:2274–2281. [DOI] [PubMed] [Google Scholar]
- 11. Scheinert D, Katsanos K, Zeller T, Koppensteiner R, Commeau P, Bosiers M, Krankenberg H, Baumgartner I, Siablis D, Lammer J, Van Ransbeeck M, Qureshi AC, Stoll HP. A prospective randomized multicenter comparison of balloon angioplasty and infrapopliteal stenting with the sirolimus‐eluting stent in patients with ischemic peripheral arterial disease: 1‐year results from the ACHILLES trial. J Am Coll Cardiol. 2012;60:2290–2295. [DOI] [PubMed] [Google Scholar]
- 12. Spreen MI, Martens JM, Hansen BE, Knippenberg B, Verhey E, van Dijk LC, de Vries JP, Vos JA, de Borst GJ, Vonken EJ, Wever JJ, Statius van Eps RG, Mali WP, van Overhagen H. Percutaneous transluminal angioplasty and drug‐eluting stents for infrapopliteal lesions in critical limb ischemia (PADI) trial. Circ Cardiovasc Interv. 2016;9:e002376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rastan A, Brechtel K, Krankenberg H, Zahorsky R, Tepe G, Noory E, Schwarzwalder U, Macharzina R, Schwarz T, Burgelin K, Sixt S, Tubler T, Neumann FJ, Zeller T. Sirolimus‐eluting stents for treatment of infrapopliteal arteries reduce clinical event rate compared to bare‐metal stents: long‐term results from a randomized trial. J Am Coll Cardiol. 2012;60:587–591. [DOI] [PubMed] [Google Scholar]
- 14. Martens JM, Knippenberg B, Vos JA, de Vries JP, Hansen BE, van Overhagen H. Update on PADI trial: percutaneous transluminal angioplasty and drug‐eluting stents for infrapopliteal lesions in critical limb ischemia. J Vasc Surg. 2009;50:687–689. [DOI] [PubMed] [Google Scholar]
- 15. Rutherford RB, Baker JD, Ernst C, Johnston KW, Porter JM, Ahn S, Jones DN. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg. 1997;26:517–538. [DOI] [PubMed] [Google Scholar]
- 16. Soule B, Hingorani A, Ascher E, Kallakuri S, Yorkovich W, Markevich N, Costa T, Schutzer R. Comparison of magnetic resonance angiography (MRA) and duplex ultrasound arterial mapping (DUAM) prior to infrainguinal arterial reconstruction. Eur J Vasc Endovasc Surg. 2003;25:139–146. [DOI] [PubMed] [Google Scholar]
- 17. Jens S, Conijn AP, Koelemay MJ, Bipat S, Reekers JA. Randomized trials for endovascular treatment of infrainguinal arterial disease: systematic review and meta‐analysis (Part 2: below the knee). Eur J Vasc Endovasc Surg. 2014;47:536–544. [DOI] [PubMed] [Google Scholar]
- 18. Antoniou GA, Chalmers N, Kanesalingham K, Antoniou SA, Schiro A, Serracino‐Inglott F, Smyth JV, Murray D. Meta‐analysis of outcomes of endovascular treatment of infrapopliteal occlusive disease with drug‐eluting stents. J Endovasc Ther. 2013;20:131–144. [DOI] [PubMed] [Google Scholar]
- 19. Katsanos K, Spiliopoulos S, Diamantopoulos A, Karnabatidis D, Sabharwal T, Siablis D. Systematic review of infrapopliteal drug‐eluting stents: a meta‐analysis of randomized controlled trials. Cardiovasc Intervent Radiol. 2013;36:645–658. [DOI] [PubMed] [Google Scholar]
- 20. Baerlocher MO, Kennedy SA, Rajebi MR, Baerlocher FJ, Misra S, Liu D, Nikolic B. Meta‐analysis of drug‐eluting balloon angioplasty and drug‐eluting stent placement for infrainguinal peripheral arterial disease. J Vasc Interv Radiol. 2015;26:459–473.e4; quiz 474. [DOI] [PubMed] [Google Scholar]
- 21. Fusaro M, Cassese S, Ndrepepa G, Tepe G, King L, Ott I, Nerad M, Schunkert H, Kastrati A. Drug‐eluting stents for revascularization of infrapopliteal arteries: updated meta‐analysis of randomized trials. JACC Cardiovasc Interv. 2013;6:1284–1293. [DOI] [PubMed] [Google Scholar]
- 22. Siablis D, Karnabatidis D, Katsanos K, Diamantopoulos A, Spiliopoulos S, Kagadis GC, Tsolakis J. Infrapopliteal application of sirolimus‐eluting versus bare metal stents for critical limb ischemia: analysis of long‐term angiographic and clinical outcome. J Vasc Interv Radiol. 2009;20:1141–1150. [DOI] [PubMed] [Google Scholar]
- 23. Benoit E, O'Donnell TF Jr, Iafrati MD, Asher E, Bandyk DF, Hallett JW, Lumsden AB, Pearl GJ, Roddy SP, Vijayaraghavan K, Patel AN. The role of amputation as an outcome measure in cellular therapy for critical limb ischemia: implications for clinical trial design. J Transl Med. 2011;9:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Becker F, Robert‐Ebadi H, Ricco JB, Setacci C, Cao P, de Donato G, Eckstein HH, De Rango P, Diehm N, Schmidli J, Teraa M, Moll FL, Dick F, Davies AH, Lepäntalo M, Apelqvist J. Chapter I: definitions, epidemiology, clinical presentation and prognosis. Eur J Vasc Endovasc Surg. 2011;42:S4–S12. [DOI] [PubMed] [Google Scholar]
- 25. Varu VN, Hogg ME, Kibbe MR. Critical limb ischemia. J Vasc Surg. 2010;51:230–241. [DOI] [PubMed] [Google Scholar]
- 26. Dormandy J, Heeck L, Vig S. The fate of patients with critical leg ischemia. Semin Vasc Surg. 1999;12:142–147. [PubMed] [Google Scholar]
- 27. Teraa M, Conte MS, Moll FL, Verhaar MC. Critical limb ischemia: current trends and future directions. J Am Heart Assoc. 2016;5:e002938 DOI: 10.1161/JAHA.115.002938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Feiring AJ. Footnotes on critical limb ischemia. J Am Coll Cardiol. 2008;51:1975–1976. [DOI] [PubMed] [Google Scholar]
- 29. Benoit E, O'Donnell TF Jr, Kitsios GD, Iafrati MD. Improved amputation‐free survival in unreconstructable critical limb ischemia and its implications for clinical trial design and quality measurement. J Vasc Surg. 2012;55:781–789. [DOI] [PubMed] [Google Scholar]
- 30. Belch J, Hiatt WR, Baumgartner I, Driver IV, Nikol S, Norgren L, Van Belle E. Effect of fibroblast growth factor NV1FGF on amputation and death: a randomised placebo‐controlled trial of gene therapy in critical limb ischaemia. Lancet. 2011;377:1929–1937. [DOI] [PubMed] [Google Scholar]
- 31. Graziani L, Silvestro A, Bertone V, Manara E, Andreini R, Sigala A, Mingardi R, De Giglio R. Vascular involvement in diabetic subjects with ischemic foot ulcer: a new morphologic categorization of disease severity. Eur J Vasc Endovasc Surg. 2007;33:453–460. [DOI] [PubMed] [Google Scholar]
- 32. Rueda CA, Nehler MR, Perry DJ, McLafferty RB, Casserly IP, Hiatt WR, Peyton BD. Patterns of artery disease in 450 patients undergoing revascularization for critical limb ischemia: implications for clinical trial design. J Vasc Surg. 2008;47:995–999; discussion 999‐1000. [DOI] [PubMed] [Google Scholar]
- 33. Liistro F, Porto I, Angioli P, Grotti S, Ricci L, Ducci K, Falsini G, Ventoruzzo G, Turini F, Bellandi G, Bolognese L. Drug‐eluting balloon in peripheral intervention for below the knee angioplasty evaluation (DEBATE‐BTK): a randomized trial in diabetic patients with critical limb ischemia. Circulation. 2013;128:615–621. [DOI] [PubMed] [Google Scholar]
- 34. Fanelli F, Cannavale A, Boatta E, Corona M, Lucatelli P, Wlderk A, Cirelli C, Salvatori FM. Lower limb multilevel treatment with drug‐eluting balloons: 6‐month results from the DEBELLUM randomized trial. J Endovasc Ther. 2012;19:571–580. [DOI] [PubMed] [Google Scholar]
- 35. Zeller T, Baumgartner I, Scheinert D, Brodmann M, Bosiers M, Micari A, Peeters P, Vermassen F, Landini M, Snead DB, Kent KC, Rocha‐Singh KJ; Investigators IPDT . Drug‐eluting balloon versus standard balloon angioplasty for infrapopliteal arterial revascularization in critical limb ischemia: 12‐month results from the IN.PACT DEEP randomized trial. J Am Coll Cardiol. 2014;64:1568–1576. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Outcome at 1‐Year Follow‐up, per Patient
Table S2. Outcome at 2‐Year Follow‐up, per Patient
Table S3. Outcome at 3‐Year Follow‐up, per Patient
Table S4. Outcome at 4‐Year Follow‐up, per Patient
Table S5. Outcome at 5‐Yeas Follow‐up, per Patient


