Abstract
Background
Renal dysfunction, commonly associated with cardiac dysfunction, has predictive value for adverse long‐term outcomes in heart failure (HF). We previously identified a novel renal biomarker, interleukin‐34 (IL‐34), elevated in HF patients and associated with kidney dysfunction and coronary artery disease during HF. However, the prognostic value of IL‐34 in HF remains unclear, so that the present study aimed to determine it.
Methods and Results
This prospective, observational study included 510 consecutive HF patients with their serum IL‐34 as well as other variables measured at baseline, and they were followed up for 2 years. The primary end point was a composite of cardiovascular death or a first HF hospitalization, with cardiovascular death, HF hospitalization, and all‐cause mortality as secondary outcomes. There was a significant and gradual increase in risk as IL‐34 increased, determined by log‐rank tests with Kaplan–Meier curves. Serum IL‐34 was also a significant prognostic predictor of the primary end point (1.301 [1.115–1.518]; P=0.001), cardiovascular death (1.347 [1.096–1.655]; P=0.005), HF hospitalization (1.234 [1.018–1.494]; P=0.032), and all‐cause mortality (1.343 [1.115–1.618]; P=0.002) in HF as per SD increase in the log IL‐34 level after adjusting for age, sex, traditional risk factors, and N‐terminal pro‐brain natriuretic peptide. Especially, IL‐34 had a more‐significant prognostic value in HF patients with kidney impairment than those without.
Conclusions
IL‐34 is a significant predictor of cardiovascular death, HF hospitalization, and all‐cause mortality in chronic HF, especially when concomitant with renal dysfunction. Serum IL‐34 measurement may provide new insights linking kidney impairment to poor HF outcomes beyond other renal markers.
Keywords: heart failure, interleukin‐34, prognosis, renal insufficiency
Subject Categories: Heart Failure, Biomarkers, Prognosis
Introduction
Renal dysfunction is common in patients with heart failure (HF) and is associated with high morbidity and mortality.1 The interaction between cardiac and renal dysfunction is a critical factor that determines the progression and prognosis of HF,2 and biomarkers for impaired renal function were recently identified as strong risk factors for developing and worsening HF and as having predictive value for adverse long‐term outcomes.3 Moreover, renal dysfunction during HF is regulated by a number of factors, including the inflammation process and innate immunity.1
Interleukin‐34 (IL‐34) is a newly identified cytokine that regulates the differentiation, proliferation, and survival of mononuclear phagocytes, including monocytes, macrophages, and osteoclasts.4, 5 IL‐34 has been found to play a role in rheumatoid arthritis, inflammatory bowel disease, Sjogren's syndrome, coronary artery disease (CAD), and other diseases, inducing proinflammatory cytokines and chemokines such as interleukin‐6 (IL‐6), interleukin‐8 (IL‐8), and monocyte chemoattractant protein‐1 and regulating innate immunity.5 Recent studies have also demonstrated that IL‐34 mediates acute kidney injury and worsens subsequent chronic kidney disease (CKD), mainly through regulating myeloid cells influx into the ischemic kidney,6 which is similar to the hemodynamic abnormalities caused by reduced cardiac output during HF.7
In our recent study, we found that the proinflammatory cytokine, IL‐34, is elevated in patients with HF compared with those without. It could be used as an independent risk factor for HF in multivariable logistic regression models. We also found that IL‐34 is significantly correlated with the presence and severity of kidney dysfunction and CAD in patients with HF. However, the prognostic value of IL‐34 in chronic HF patients, especially in those with renal dysfunction, is not well understood. Therefore, the present study aimed to examine the relationship between IL‐34 and cardiovascular death, HF hospitalization, and all‐cause mortality in patients with HF, especially in those with kidney impairment.
Methods
Patient Population and Study Design
A total of 510 consecutive patients with stable HF for ≥3 months of diuretics use and hospitalized for HF at least once within a year before enrollment were included in the study (NCT02776384). Moreover, to be enrolled, N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) level had to be over 300 pg/mL for those with a sinus rhythm and 900 pg/mL for those with atrial fibrillation at baseline. All subjects enrolled were women and men aged 18 years or older. However, patients with acute coronary syndrome during the previous 4 weeks or significant concomitant diseases, such as infection or an autoimmune disease, were excluded. Furthermore, patients who suffered from myocardial infarction, stroke, or underwent open‐heart surgery within 4 weeks before enrollment and those who underwent mechanical ventilation, renal replacement therapy, or postcardiac transplantation were also excluded. All study patients underwent an echocardiographic evaluation of their cardiac structure and function at the time of blood sample collection at baseline. A medical history was obtained to document etiology, symptoms according New York Heart Association functional classes (NYHA), and coexisting diseases. Concomitant cardiac medications and routine laboratory test results were also recorded at enrollment. Subjects were followed prospectively by direct contact as well as telephone follow‐up and chart review to record HF hospitalizations, mortality, and other adverse events.
Measurements
Renal function
Estimated glomerular filtration rate (eGFR) was calculated by using a modified Modification of Diet in Renal Disease (MDRD) equation as follows: eGFRMDRD=30 849×serum creatinine (μmol/L)−1.154−age−0.203 [×0.742 if women]. Preserved and impaired renal function were defined as eGFRMDRD ≥60 mL/min per 1.73 m2 and eGFRMDRD <60 mL/min per 1.73 m2, respectively.
Measurement of IL‐34 in serum
Serum IL‐34 was measured in the fasting venous blood samples by using an ELISA (Human IL‐34 Quantikine ELISA Kit, D3400; R&D Systems, Minneapolis, MN), according to the manufacturer guidelines.
Outcomes
According to the prospective observational study design, patients were followed until death or to the last visit. The primary end point was a composite of cardiovascular death or a first HF hospitalization, with cardiovascular death, a first HF hospitalization, and all‐cause mortality as secondary outcomes. Death and hospitalization were documented during planned clinic assessments every 6 months.
Clinical outcomes were prospectively determined during the ensuing 2 years for all subjects after enrollment, adjudicated, and verified by source documentation. Both primary and secondary end points were analyzed as the time to the first event for this cohort of patients over the duration of the 2‐year program. The date was recorded, and information regarding the cause was obtained. In brief, deaths were identified mainly by review of medical records and direct contact with the patients' family. All deaths were classified as cardiovascular deaths unless an unequivocal noncardiovascular cause was established. In total, serum IL‐34 levels were measured and follow‐up data were available for 94.9% of included patients. Institutional review board approval was obtained accordingly. The study was approved by the institutional review committee of the Rui Jin Hospital affiliated to Shanghai Jiao Tong University School of Medicine (Shanghai, China) and was in accord with the principle of the Helsinki Declaration II. A previous signed informed consent was obtained from each patient before screening and data collection.
Statistical Analysis
Continuous variables are expressed as the mean and SD if normally distributed; median with interquartile ranges (IQRs) are provided when data were not normally distributed. Categorical data were summarized as proportions and frequencies. Log transformations were performed to normalize non‐normally distributed variables, such as IL‐34, NT‐proBNP, high‐sensitivity C‐reactive protein (hsCRP), cystatin C (CysC), and other factors. Means were compared using independent Student t or 1‐way ANOVA tests, as appropriate. Chi‐squared tests were used to compare categorical variables.
Kaplan–Meier (KM) event curves were constructed to predict the 2‐year outcome, including each end point (primary end point, cardiovascular death, HF hospitalization, and all‐cause mortality). The number of events or survival situation among groups was compared using the log‐rank test. Cox proportional hazard analysis was used to estimate hazard ratios (HRs) with 95% CIs and assess whether IL‐34 is a predictor of prognosis in HF using continuous standardized increments of IL‐34 levels, natural log‐transformed IL‐34 levels, as well as quartiles of IL‐34 both as an ordinal variable and as categorical data. The assumption of proportional hazards was examined using log cumulative hazard functions as well as Schoenfeld's residuals, which was found to be statistically nonsignificant. Uni‐ and multivariable Cox proportional hazard models were used to evaluate separately the association of IL‐34 as well as other influential variables of cardiac function and renal function with all‐cause mortality, cardiovascular death, HF hospitalization, and the composite primary outcome. The results were first adjusted for age and sex, and then for the full model, including age, sex, BMI, current smoking status, history of hypertension or diabetes mellitus (DM), serum or plasma NT‐proBNP, hsCRP, hemoglobin, albumin, CysC, and NYHA functional class. Moreover, each of the analyses was further performed separately in HF patients with and without renal insufficiency. Area under the receiver operating characteristics (AUC‐ROC) curves of multivariable analyses (c‐statistic analyses), including several conventional risk factors with and without IL‐34 as the predictors of the primary end point, were also performed to evaluate the prognostic value of IL‐34 in risk stratification, among all HF patients, as well as HF patients with and without renal dysfunction respectively.
Cut‐off finder analysis using R software (version 2.15.0; R Foundation for Statistical Computing, Vienna, Austria) was used to estimate the cut‐off value of IL‐34 (110.4 pg/mL) that best predicted the primary end point in patients with HF. The event rates of each end point were compared between patients below and above the cut‐off value. Cox proportional hazard analysis was used to estimate corresponding HRs using the IL‐34 level as a binary variable. All statistical analyses were performed using SPSS software (version 19.0; SPSS, Inc, Chicago, IL). Statistical significance was set at 2‐tailed, and P<0.05 was considered statistically significant. All authors had full access to all the data in the study and took responsibility for the integrity of data and accuracy of data analysis.
Results
Baseline Characteristics of Heart Failure Patients According to Quartile of IL‐34 Levels
In our previous study, we have demonstrated that serum levels of IL‐34 were significantly increased in patients with HF compared with those without. In particular, it was significantly associated with renal insufficiency in patients with HF at baseline. In the present study, we further divided all HF patients into 4 groups according to quartile of IL‐34 levels: first quartile (<54.56 pg/mL); second quartile (54.56–75.57 pg/mL); third quartile (75.57–114.79 pg/mL); and fourth quartile (≥114.79 pg/mL). Mean IL‐34 was 116.24±144.46 pg/mL for all patients with HF. Clinical and laboratory characteristics of the 510 patients at baseline are presented in Table 1.
Table 1.
Baseline Characteristics According to Quartile of IL‐34 Levels in HF Patients
| IL‐34 <54.56 pg/mL (n=127) | 54.56 pg/mL ≤ IL‐34 <75.57 pg/mL (n=128) | 75.57 pg/mL ≤ IL‐34 <114.79 pg/mL (n=127) | IL‐34 ≥114.79 pg/mL (n=128) | P Value | |
|---|---|---|---|---|---|
| Age, y | 61.86±11.86 | 65.91±12.23 | 64.87±11.92 | 66.96±11.53 | 0.005 |
| Male, sex | 102 (80.3) | 101 (78.9) | 96 (75.6) | 106 (82.8) | 0.547 |
| Smoking | 64 (49.6) | 52 (40.6) | 56 (44.1) | 57 (44.5) | 0.549 |
| Alcohol use | 32 (25.2) | 27 (21.1) | 27 (21.3) | 30 (23.4) | 0.843 |
| BMI, kg/m2 | 24.99±3.41 | 24.75±3.68 | 24.33±3.83 | 23.95±3.16 | 0.096 |
| Systolic blood pressure, mm Hg | 126.46±24.93 | 129.01±21.84 | 125.17±20.10 | 129.59±22.33 | 0.342 |
| Diastolic blood pressure, mm Hg | 75.37±15.58 | 74.16±14.30 | 75.72±13.45 | 74.88±12.12 | 0.825 |
| Heart rate, beats/min | 79.66±14.09 | 79.70±17.41 | 79.71±12.16 | 78.74±13.80 | 0.938 |
| Medical history | |||||
| Atrial fibrillation | 19 (15.0) | 23 (18.0) | 21 (16.5) | 20 (15.6) | 0.924 |
| CAD | 88 (69.3) | 102 (79.7) | 105 (82.7) | 112 (87.5) | 0.003 |
| CKD | 26 (20.5) | 35 (27.3) | 35 (27.6) | 51 (39.8) | 0.007 |
| DM | 46 (36.2) | 47 (36.7) | 46 (36.2) | 47 (36.7) | 1.000 |
| Hypertension | 75 (59.1) | 86 (67.2) | 88 (69.3) | 85 (66.4) | 0.341 |
| Dyslipidemia | 41 (32.3) | 48 (37.5) | 40 (31.5) | 28 (21.9) | 0.054 |
| Echocardiography | |||||
| LVEF, % | 43.84±11.78 | 45.24±10.15 | 43.15±11.38 | 45.81±11.23 | 0.211 |
| LVEDd, mm | 59.64±9.58 | 58.64±7.83 | 60.01±8.36 | 59.24±7.53 | 0.619 |
| LVESd, mm | 46.72±10.75 | 45.17±8.99 | 46.81±9.56 | 45.26±8.85 | 0.357 |
| LAd, mm | 43.86±6.57 | 43.57±5.95 | 43.79±5.71 | 44.29±5.34 | 0.810 |
| Lab. examination | |||||
| WBC, ×109/L | 7.98±3.39 | 7.33±2.29 | 7.03±2.25 | 7.00±2.00 | 0.007 |
| Hemoglobin, g/L | 134.09±16.84 | 132.94±17.69 | 130.37±16.70 | 125.92±19.78 | 0.001 |
| Platelet, ×109/L | 197.38±65.36 | 179.81±67.39 | 178.84±59.72 | 175.12±61.80 | 0.026 |
| Fasting glucose, mmol/L | 6.32±3.48 | 5.90±1.61 | 5.94±2.23 | 5.71±1.97 | 0.237 |
| HbA1c, % | 6.51±1.23 | 6.53±1.25 | 6.59±1.28 | 6.41±1.30 | 0.738 |
| ALT, IU/L | 46.16±94.24 | 39.75±63.65 | 29.03±17.53 | 29.25±19.40 | 0.049 |
| Albumin, g/L | 35.75±4.55 | 35.91±4.40 | 35.86±5.29 | 34.57±5.26 | 0.090 |
| BUN, mmol/L | 6.69±3.14 | 6.78±3.27 | 6.66±2.81 | 7.72±4.41 | 0.042 |
| Creatinine, μmol/L | 90.89±34.03 | 91.27±26.23 | 91.43±31.07 | 124.88±132.86 | <0.001 |
| Uric acid, μmol/L | 364.80±122.94 | 379.93±136.53 | 377.50±123.11 | 380.79±140.74 | 0.748 |
| eGFRMDRD, mL/min per 1.73 m2 | 77.16±21.21 | 73.99±20.98 | 74.94±24.72 | 65.21±23.76 | <0.001 |
| CysC, mg/L | 1.16±0.43 | 1.25±0.40 | 1.29±0.41 | 1.60±1.25 | <0.001 |
| Total cholesterol, mmol/L | 4.08±1.05 | 3.98±1.09 | 3.99±1.05 | 3.82±0.97 | 0.265 |
| Triglyceride, mmol/L | 1.56±0.83 | 1.67±1.26 | 1.45±0.65 | 1.36±0.71 | 0.036 |
| LDL‐C, mmol/L | 2.54±0.85 | 2.34±0.83 | 2.44±0.83 | 2.23±0.79 | 0.027 |
| HDL‐C, mmol/L | 0.99±0.29 | 0.97±0.25 | 0.99±0.26 | 1.00±0.27 | 0.856 |
| hsCRP, mg/L | 14.89±35.54 | 22.90±52.17 | 13.48±28.23 | 23.88±50.01 | 0.145 |
| NT‐proBNP, pg/mL | 2569.50±3382.66 | 2612.11±4267.53 | 3394.43±5419.57 | 4136.48±5375.58 | 0.029 |
| CAG Gensini score | 33.37±40.77 | 42.18±42.09 | 44.82±44.77 | 54.11±44.89 | 0.002 |
| Medications | |||||
| ACEI/ARB | 89 (70.1) | 102 (79.7) | 104 (81.9) | 91 (71.1) | 0.060 |
| β‐blocker | 109 (85.8) | 114 (89.1) | 108 (85.0) | 103 (80.5) | 0.284 |
| Digoxin | 21 (16.5) | 15 (11.7) | 28 (22.0) | 17 (13.3) | 0.113 |
| Antiplatelet drugs | 98 (77.2) | 108 (84.4) | 107 (84.3) | 107 (83.6) | 0.367 |
| Nitrates | 45 (35.4) | 46 (35.9) | 52 (40.9) | 74 (57.8) | 0.001 |
| Statin | 90 (70.9) | 99 (77.3) | 108 (85.0) | 99 (77.3) | 0.061 |
| CCB | 16 (12.6) | 20 (15.6) | 23 (18.1) | 20 (15.6) | 0.687 |
| Hypoglycemic drugs | 20 (15.7) | 23 (18.0) | 30 (23.6) | 28 (21.9) | 0.377 |
Data are presented as the mean±SD or n (%). ACEI indicates angiotensin‐converting enzyme inhibitor; ALT, alanine aminotransferase; ARB, angiotensin receptor blocker; BUN, blood urea nitrogen; CAD, coronary artery disease; CAG, coronary angiogram; CCB, calcium‐channel blocker; CKD, chronic kidney disease; CysC, cystatin C; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; HDL‐C, high‐density lipoprotein cholesterol; HF, heart failure; hsCRP, high‐sensitivity C‐reactive protein; IL, interleukin; LAd, left atrium diameter; LDL‐C, low‐density lipoprotein cholesterol; LVEDd, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; LVESd, left ventricular end‐systolic diameter; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; WBC, white blood cell.
As expected, patients with higher IL‐34 level tended to have lower eGFR (65.21±23.76 mL/min per 1.73 m2 in the fourth quartile versus 77.16±21.21 mL/min per 1.73 m2 in the first quartile; P<0.001), higher creatinine (124.88±132.86 μmol/L in the fourth quartile versus 90.89±34.03 μmol/L in the first quartile; P<0.001) and CysC level (1.60±1.25 mg/L in the fourth quartile versus 1.16±0.43 mg/L in the first quartile; P<0.001), all indicating poorer renal function. Also, serum IL‐34 levels were significantly higher in HF patients with CKD than in those without (145.87±178.01 versus 104.24±126.74 pg/mL; P=0.001). Regarding general conditions, patients in the higher IL‐34 group tended to be older (P=0.005); however, there was shown to be no significant difference between male and female patients. Medical history, including DM, hypertension, or atrial fibrillation, was comparable among all groups except for the fact that patients in the upper quartile of IL‐34 were more likely to combine CKD and CAD. Although there was no remarkable difference in left ventricular ejection fraction (LVEF) among these 4 groups, patients in the upper IL‐34 quartile showed markedly higher NT‐proBNP levels (P=0.029). Moreover, patients with higher IL‐34 levels had lower white blood count (WBC; P=0.007), hemoglobin (P=0.001), and platelet levels (P=0.026) and also tended to have lower serum lipid levels. Medications were also comparable among these groups.
Heart Failure Patients With Higher Serum IL‐34 Levels Tended to Have Worse Prognosis
This prospective observational study had a median follow‐up time until end point or last visit of 706 days (IQR, 690–719), and 94% of included patients completed the entire observation period, whereas 6% were lost to follow‐up. During the follow‐up period, 132 patients (25.7%) reached the combined primary end point of cardiovascular death or first HF hospitalization, with a mean time to the primary end point of 300 days (IQR, 174–403). Incidences of cardiovascular death, first HF hospitalization, and all‐cause mortality at any time during the whole follow‐up were 74 (14.3%), 90 (17.6%), and 92 (18.4%), respectively.
All patients were divided into quartiles according to their serum IL‐34 levels as described before. Among the HF patients, 21 patients (16.5%) with serum IL‐34 levels in the first quartile, 27 (21.1%) with levels in the second, 35 (27.6%) with levels in the third, and 49 (38.3%) with levels in the fourth met the primary end point. Figure 1 shows the KM curves for occurrence of the primary end point (Figure 1A), cardiovascular death (Figure 1B), HF hospitalization (Figure 1C), and all‐cause mortality (Figure 1D), according to the 4 quartiles of serum IL‐34 levels. As shown, there was a significant and gradual increase in risk for those events given that IL‐34 level increased in the log‐rank test.
Figure 1.
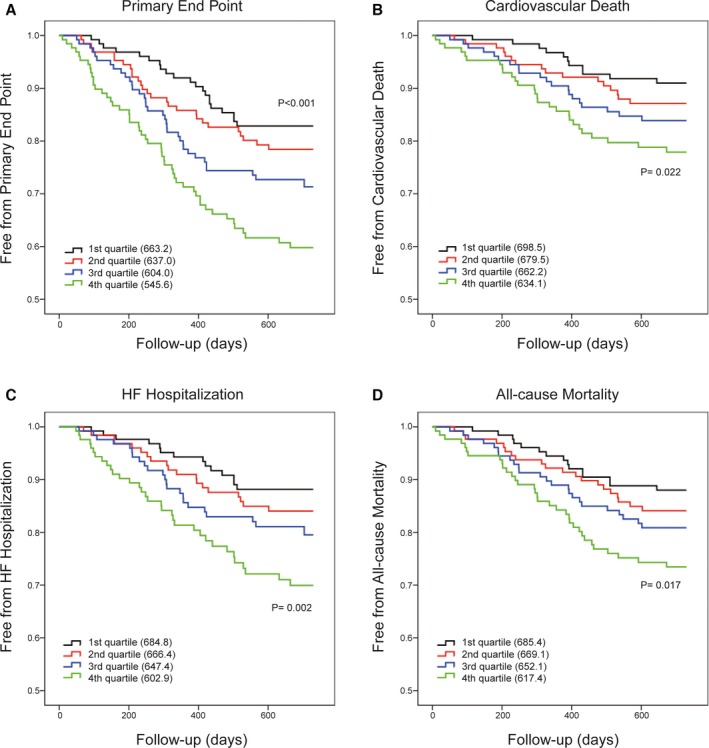
Serum IL‐34 level can predict poor outcomes in heart failure. Kaplan–Meier curves for the primary end point (A) and the three secondary end points of cardiovascular death (B), HF hospitalization (C), and all‐cause mortality (D) according to the quartiles of serum IL‐34 levels. Differences among groups were evaluated with the log‐rank test. HF indicates heart failure; IL, interleukin.
In both uni‐ and multivariable analyses, IL‐34 significantly predicted the primary end point and 3 secondary end points (cardiovascular death, HF hospitalization, and all‐cause mortality) separately, as did NT‐proBNP, eGFR, and CysC, both as a continuous variable and when divided into quartiles. In the step‐wise adjusted models, unadjusted, adjusted for age and sex (model 1), and adjusted for the full model with various influencing factors (model 2), higher IL‐34 levels were consistently significantly associated with an increased risk of both the primary and secondary end points (Table 2). To be specific, for the primary end point in the fully adjusted model, the HR was 1.301 (1.115–1.518) per SD increase in the log IL‐34 level (P=0.001), whereas the HR was 1.155 (1.023–1.303; P=0.020) per SD increase in the IL‐34 level without log adjustment. Moreover, when divided into 4 quartiles according to the IL‐34 level, the HR was 1.314 (1.104–1.564; P=0.002) as an ordinal variable and the HR was 2.205 (1.248–3.896; P=0.006) in the highest quartile using the lowest quartile as a reference value. IL‐34 had a similar significant predictive value for each of the secondary end point. The corresponding HRs for cardiovascular death, HF hospitalization, and all‐cause mortality were 1.347 (1.096–1.655; P=0.005), 1.234 (1.018–1.494; P=0.032), and 1.343 (1.115–1.618; P=0.002), respectively, per SD increase in the log IL‐34 level. Moreover, there is no significant effect measure modification between IL‐34 and renal dysfunction in predicting each of these end points.
Table 2.
Uni‐ and Multivariable Cox Proportional Hazard Models for Serum IL‐34 Level as a Predictor of End Points in HF
| Unadjusted | Adjusted for Model 1 | Adjusted for Model 2 | ||||
|---|---|---|---|---|---|---|
| HR (CI) | P Value | HR (CI) | P Value | HR (CI) | P Value | |
| Primary end point | ||||||
| IL‐34 per SD | 1.249 (1.119–1.395) | <0.001 | 1.237 (1.104–1.387) | <0.001 | 1.155 (1.023–1.303) | 0.020 |
| Log IL‐34 per SD | 1.442 (1.248–1.667) | <0.001 | 1.401 (1.209–1.622) | <0.001 | 1.301 (1.115–1.518) | 0.001 |
| IL‐34 quartiles | 1.433 (1.221–1.681) | <0.001 | 1.398 (1.189–1.642) | <0.001 | 1.314 (1.104–1.564) | 0.002 |
| 1st quartile | 1 | 1 | 1 | |||
| 2nd quartile | 1.307 (0.739–2.312) | 0.357 | 1.157 (0.652–2.053) | 0.618 | 1.246 (0.671–2.312) | 0.486 |
| 3rd quartile | 1.795 (1.045–3.083) | 0.034 | 1.663 (0.967–2.861) | 0.066 | 1.785 (0.988–3.226) | 0.055 |
| 4th quartile | 2.868 (1.720–4.783) | <0.001 | 2.554 (1.527–4.273) | <0.001 | 2.205 (1.248–3.896) | 0.006 |
| Cardiovascular death | ||||||
| IL‐34 per SD | 1.332 (1.160–1.507) | <0.001 | 1.306 (1.139–1.498) | <0.001 | 1.278 (1.102–1.481) | 0.001 |
| Log IL‐34 per SD | 1.513 (1.251–1.830) | <0.001 | 1.450 (1.195–1.759) | <0.001 | 1.347 (1.096–1.655) | 0.005 |
| IL‐34 quartiles | 1.394 (1.127–1.725) | 0.002 | 1.342 (1.083–1.664) | 0.007 | 1.167 (0.917–1.486) | 0.210 |
| 1st quartile | 1 | 1 | 1 | |||
| 2nd quartile | 1.458 (0.677–3.141) | 0.336 | 1.244 (0.575–2.693) | 0.579 | 1.707 (0.712–4.093) | 0.231 |
| 3rd quartile | 1.886 (0.904–3.937) | 0.091 | 1.702 (0.814–3.558) | 0.157 | 2.021 (0.868–4.702) | 0.103 |
| 4th quartile | 2.771 (1.374–5.586) | 0.004 | 2.359 (1.164–4.778) | 0.017 | 1.739 (0.758–3.991) | 0.192 |
| HF hospitalization | ||||||
| IL‐34 per SD | 1.210 (1.045–1.401) | 0.011 | 1.200 (1.033–1.394) | 0.017 | 1.114 (0.927–1.338) | 0.251 |
| Log IL‐34 per SD | 1.408 (1.176–1.686) | <0.001 | 1.383 (1.153–1.658) | <0.001 | 1.234 (1.018–1.494) | 0.032 |
| IL‐34 quartiles | 1.432 (1.179–1.740) | <0.001 | 1.411 (1.160–1.716) | 0.001 | 1.274 (1.036–1.568) | 0.022 |
| 1st quartile | 1 | 1 | 1 | |||
| 2nd quartile | 1.381 (0.692–2.755) | 0.359 | 1.288 (0.643–2.580) | 0.474 | 1.525 (0.698–3.330) | 0.290 |
| 3rd quartile | 1.778 (0.915–3.455) | 0.090 | 1.702 (0.874–3.313) | 0.118 | 2.048 (0.966–4.339) | 0.061 |
| 4th quartile | 2.934 (1.570–5.484) | 0.001 | 2.744 (1.462–5.148) | 0.002 | 2.566 (1.241–5.305) | 0.011 |
| All‐cause mortality | ||||||
| IL‐34 per SD | 1.307 (1.158–1.475) | <0.001 | 1.291 (1.139–1.465) | <0.001 | 1.239 (1.088–1.410) | 0.001 |
| Log IL‐34 per SD | 1.477 (1.242–1.756) | <0.001 | 1.422 (1.193–1.695) | <0.001 | 1.343 (1.115–1.618) | 0.002 |
| IL‐34 quartiles | 1.351 (1.118–1.634) | 0.002 | 1.307 (1.079–1.582) | 0.006 | 1.166 (0.943–1.443) | 0.157 |
| 1st quartile | 1 | 1 | 1 | |||
| 2nd quartile | 1.338 (0.685–2.613) | 0.394 | 1.167 (0.595–2.289) | 0.652 | 1.392 (0.658–2.945) | 0.388 |
| 3rd quartile | 1.661 (0.872–3.167) | 0.123 | 1.519 (0.795–2.900) | 0.205 | 1.597 (0.782–3.262) | 0.199 |
| 4th quartile | 2.486 (1.350–4.578) | 0.003 | 2.153 (1.164–3.981) | 0.014 | 1.650 (0.817–3.330) | 0.163 |
IL‐34 levels were analyzed as a continuous variable, a log‐transformed continuous variable, an ordinal variable divided according to quartiles of IL‐34 levels, and a categorical variable using the lowest quartile as a reference. Primary end point indicates a composite end point of cardiovascular death or first‐time HF hospitalization. Model 1: adjusted for age and sex. Model 2: adjusted for age, sex, body mass index, smoking status, history of DM, history of hypertension, hsCRP, NT‐proBNP, cystatin C, hemoglobin, albumin, and NYHA class. Hazard ratios (HRs) are per 1 SD. DM indicates diabetes mellitus; HF, heart failure; hsCRP, high sensitivity C reactive protein; IL, interleukin; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; NYHA, New York Heart Association.
The Prognostic Value of the Serum IL‐34 Level in Heart Failure Patients With Chronic Kidney Disease
CKD and non‐CKD groups were further divided into higher IL‐34 and lower IL‐34 groups using the median IL‐34 level (75.57 pg/mL) as a cut‐off value, and the groups were compared using the KM curves and log‐rank tests. In HF patients with CKD, those with higher IL‐34 levels were significantly more likely to meet the primary end point (log‐rank test, P=0.004); however, the difference was not significant among those without CKD (P=0.088; Figure 2A). Similarly, when using the median CysC level (1.155 mg/L) as the cut‐off value for kidney function, serum IL‐34 level could only predict the primary end point for patients with CysC levels above the median (P<0.001 versus P=0.651; Figure 2B).
Figure 2.
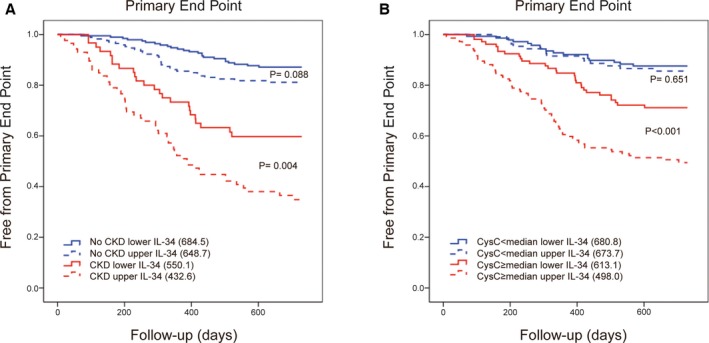
Serum IL‐34 level predicted poor outcomes in heart failure, especially in those with renal dysfunction. A, Kaplan–Meier curves for the primary end point of 4 groups divided using the median IL‐34 level and an eGFR=60 mL/min per 1.73 m2 as the cut‐off values: non‐CKD with IL‐34 below the median, non‐CKD with IL‐34 above the median, CKD with IL‐34 below the median, and CKD with IL‐34 above the median. Log‐rank tests were used to compare the impact of IL‐34 on patients with or without CKD. B, Kaplan–Meier curves for the primary end point according to 4 groups divided using the median IL‐34 and median cystatin C level as the cut‐off values, similar to that illustrated in (A). Log‐rank tests were also performed. CKD indicates chronic kidney disease; CysC, cystatin C; eGFR, estimated glomerular filtration rate; IL, interleukin.
Cox regression models were also analyzed to further confirm the predictive value of IL‐34. Among HF patients with renal dysfunction, high levels of IL‐34 indicated a significantly increased risk of cardiovascular death, HF hospitalization, all‐cause mortality, and the primary end point in the unadjusted Cox models. The significantly predictive value for each end point remained in the Cox regression models after adjusting for several confounding factors (age, sex, BMI, smoke, history of DM, history of hypertension, hsCRP, NT‐proBNP, CysC, hemoglobin, albumin, and NYHA class; Table 3). Nevertheless, in the non‐CKD group, high levels of IL‐34 were not capable enough to predict the risk of cardiovascular death or HF hospitalization in either the unadjusted Cox regression model or the fully adjusted model. Serum IL‐34 levels seemed to be predictive of the primary end point and all‐cause mortality in the univariate analysis; however, when further adjusted for covariate variables, this was no longer significant (Table 3). The predictive value of IL‐34 was also quite more significant in HF patients with renal impairment using ROC curves (Table 4).
Table 3.
Uni‐ and Multivariate Cox Proportional Hazard Models for Serum IL‐34 as a Predictor of End Points in HF Patients With or Without CKD
| Unadjusted | Adjusted for Model | |||
|---|---|---|---|---|
| HR (CI) | P Value | HR (CI) | P Value | |
| Primary end point | ||||
| Non‐CKD | 1.365 (1.044–1.786) | 0.023 | 1.275 (0.973–1.670) | 0.078 |
| CKD | 1.595 (1.274–1.997) | <0.001 | 1.345 (1.117–1.619) | 0.002 |
| Cardiovascular death | ||||
| Non‐CKD | 1.389 (0.970–1.989) | 0.073 | 1.332 (0.929–1.910) | 1.332 |
| CKD | 1.345 (1.067–1.696) | 0.012 | 1.347 (1.051–1.727) | 0.019 |
| HF hospitalization | ||||
| Non‐CKD | 1.270 (0.937–1.722) | 0.123 | 1.163 (0.815–1.659) | 0.407 |
| CKD | 1.305 (1.039–1.638) | 0.022 | 1.273 (1.014–1.600) | 0.038 |
| All‐cause mortality | ||||
| Non‐CKD | 1.376 (1.014–1.869) | 0.041 | 1.330 (0.976–1.814) | 0.071 |
| CKD | 1.391 (1.062–1.637) | 0.012 | 1.306 (1.056–1.615) | 0.014 |
The primary end point indicates a composite end point of cardiovascular death or first‐time HF hospitalization. IL‐34 level was analyzed as a log‐transformed continuous variable. Model: adjusted for age, sex, body mass index, smoking status, history of DM, history of hypertension, hsCRP, NT‐proBNP, cystatin C, hemoglobin, albumin, and NYHA class. Hazard ratios (HRs) are per 1 SD. DM indicates diabetes mellitus; HF, heart failure; hsCRP, high‐sensitivity C reactive protein; IL, interleukin; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; NYHA, New York Heart Association.
Table 4.
C‐Statistic Analyses for Multivariable Analyses Including Different Risk Factors With and Without IL‐34 as Predictors of the Primary End Point in HF Patients
| AUC‐ROC | |||
|---|---|---|---|
| Total Cohort (n=510) | Non‐CKD (n=363) | CKD (n=147) | |
| IL‐34 |
0.627 (0.572–0.682) (P<0.001) |
0.574 (0.491–0.657) (P=0.082) |
0.630 (0.540–0.720) (P=0.007) |
| Without IL‐34 |
0.827 (0.784–0.869) (P<0.001) |
0.781 (0.707–0.855) (P<0.001) |
0.724 (0.641–0.808) (P<0.001) |
| With IL‐34 |
0.837 (0.795–0.878) (P<0.001) |
0.786 (0.716–0.857) (P<0.001) |
0.768 (0.687–0.848) (P<0.001) |
| P value | 0.364 | 0.784 | 0.033 |
Multivariable analysis with or without IL‐34 consists of several risk factors for the primary end point, including age, sex, body mass index, smoking status, history of DM, history of hypertension, hsCRP, NT‐proBNP, cystatin C, hemoglobin, albumin, and NYHA class. AUC‐ROC indicates area under the curve‐receiver operating characteristics; DM, diabetes mellitus; HF, heart failure; hsCRP, high‐sensitivity C reactive protein; IL, interleukin; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; NYHA, New York Heart Association.
The Additive Value of IL‐34 in Risk Prediction
In order to further evaluate the prognostic value of IL‐34 in improving risk stratification, increment in C‐statistic with IL‐34 was calculated as a measure of model discrimination in all HF patients, as well as in HF patients with and without renal dysfunction, respectively (Table 4). Using AUC‐ROC curves of multivariable analyses including several conventional risk factors with and without IL‐34 as the predictors of the primary end point, we found that the prognostic incremental value of IL‐34 was significant especially in HF patients with renal dysfunction (P=0.033).
Moreover, the cut‐off value for the IL‐34 level that best predicted the primary end point in patients with HF was calculated as 110.4 pg/mL. During the follow‐up period, 20.8% of patients with IL‐34 levels below the cut‐off value met the primary end point, whereas the event rate was 39.6% among patients with IL‐34 levels above the cut‐off value (P<0.001). The IL‐34 cut‐off value was also able to predict the risks of cardiovascular death, HF hospitalization, and all‐cause mortality in patients with HF (Figure 3A), as indicated by a 2‐fold increased risk for the primary end point (HR, 2.295 [1.623–3.245]; P<0.001) and the 3 secondary end points (cardiovascular death: HR, 2.077 [1.305–3.304]; P=0.002; HF hospitalization: HR, 2.209 [1.446–3.374]; P<0.001; all‐cause mortality: HR, 2.020 [1.332–3.066]; P=0.001) in the Cox regression analyses. Moreover, the log‐rank test demonstrated a significant difference between the 2 groups divided by the IL‐34 cut‐off level (Figure 3B).
Figure 3.
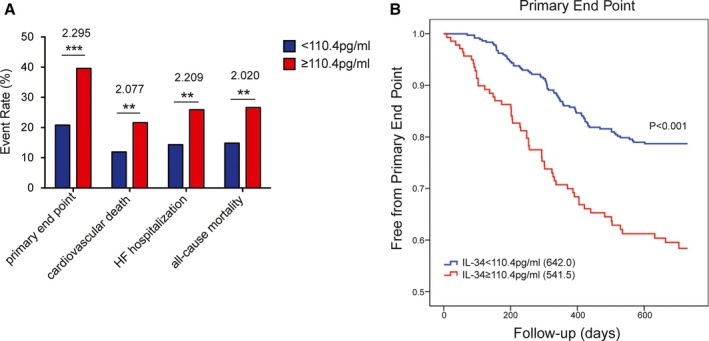
Event rates and Kaplan–Meier curve analysis according to the estimated IL‐34 cut‐off value. A, Event rates of the primary end point, cardiovascular death, HF hospitalization, and all‐cause mortality for HF patients below and above the IL‐34 cut‐off value (110.4 pg/mL). The numbers above each bar indicate the corresponding hazard ratio using the IL‐34 level as a binary variable divided by the cut‐off value in Cox regression analyses. B, Kaplan–Meier curve for the primary end point dichotomized according to a clinical cut‐off value of 110.4 pg/mL for the serum IL‐34 level. ** P<0.01; *** P<0.001. HF indicates heart failure; IL, interleukin.
Finally, combining IL‐34 with NT‐proBNP further refined the risk stratification better than with either marker alone. After dividing IL‐34 and NT‐proBNP levels into tertiles, over half (57.4%) of the patients with the highest levels for both IL‐34 and NT‐proBNP reached the primary end point compared with 5.6% of patients with the lowest levels for both IL‐34 and NT‐proBNP (P<0.0001; Figure 4).
Figure 4.
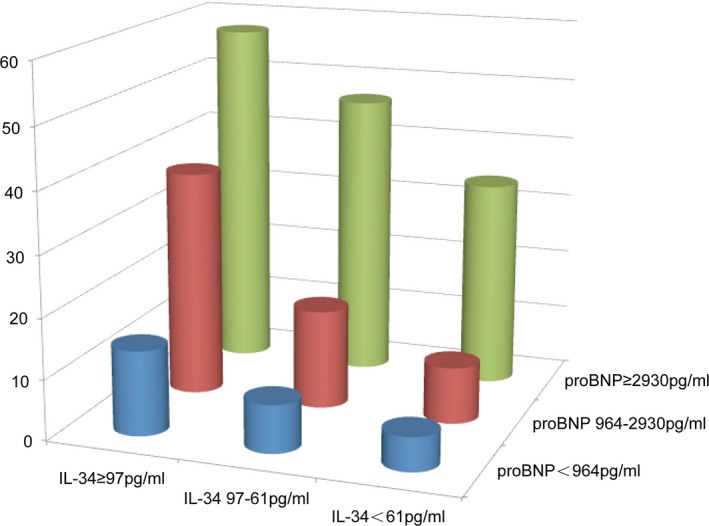
Risk stratification in patients with heart failure according to tertiles of serum IL‐34 and NT‐proBNP levels. The risk of the primary end point (cardiovascular death or HF hospitalization) significantly increases in patients with both biomarkers in the highest tertile compared with those with both biomarkers in the lowest tertile (P<0.001). HF indicates heart failure; IL, interleukin; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide.
Discussion
IL‐34 has been identified as a novel biomarker and a potential risk factor for HF in our previous study. In the present prospective observational analysis with 2 years of follow‐up, serum IL‐34 levels were significantly predictive of the composite primary end point including cardiovascular death or HF hospitalization, as well as the 3 secondary end points, cardiovascular death, HF hospitalization, and all‐cause mortality, among patients with HF, especially when accompanied by kidney dysfunction.
IL‐34 Impacts the Prognosis of Heart Failure by Affecting Renal Dysfunction Among Heart Failure Patients
It is possible that the pathophysiological role of IL‐34 in progressive renal impairment may be more prominent than its direct impact on cardiac pathology, and this relation with renal dysfunction may, in part, determine the prognostic value of IL‐34 in HF.1, 3 Renal insufficiency is relatively common in patients with congestive HF (CHF). Accumulating evidence suggests that renal dysfunction could also independently contribute to an increased cardiovascular morbidity and mortality risk in HF patients, regardless of the LVEF value.8 Even minor kidney dysfunction is associated with altered homeostasis during HF, including increased inflammatory cytokine levels and endothelial dysfunction, which predicts cardiovascular mortality. Therefore, as previously reported,9 kidney dysfunction may be a confounding variable that indicates the severity of poorer cardiac function and prognosis attributed to a shared etiology, for example, atherosclerotic disease burden or HF.
Serum IL‐34 levels are increased in HF patients, especially those with renal insufficiency, partly because of ischemic injury to the kidneys during HF,10 which causes tubular epithelial cells to upregulate IL‐34 production and release it into the circulation. IL‐34 stimulates bone marrow myeloid cell proliferation and increases the recruitment of circulating myeloid cells to the kidney and simultaneously increases renal interstitial macrophages and neutrophils, thus further aggravating the inflammation injury and renal dysfunction.6 Impaired renal dysfunction could, in turn, worsen the process of HF,1 leading to poor clinical outcomes.
Many renal biomarkers have been reported to be powerful predictors of mortality and HF severity in recent years.11 eGFR has long been viewed as the most powerful predictor of mortality, and it had a greater significance than the LVEF and NYHA class in a landmark analysis of 1906 patients.12 CysC was also identified as a prognostic biomarker in HF independent of body mass, protein intake, or catabolism; it might be a better biomarker than creatinine and has been shown to be an accurate index of the GFR, with great sensitivity for the early detection of kidney dysfunction.11, 13 The correlation between IL‐34 and several renal markers, such as eGFR, CysC, and creatinine, in addition to its predictive value for CKD, suggests that IL‐34 does mediate poor outcomes in HF, mainly attributed to progressively impaired renal function.
In addition to the markers analyzed in this study, markers of tubular damage, such as neutrophil gelatinase‐associated lipocalin (NGAL),14, 15 kidney injury molecule (KIM‐1),16 and albuminuria,11 are potentially more useful for the early detection of renal dysfunction during HF because tubular cells may be injured at an earlier stage than the glomerulus.17, 18 Indeed, expression and secretion of IL‐34 have been linked to renal tubular destruction and the development of renal fibrosis in animal models.16 Specifically, serum levels of NGAL and albuminuria are higher in wild type mice compared with IL34−/− mice, and renal fibrosis was more severe in wild type mice, suggesting that IL‐34 mediates inflammation and tubule injury during both acute kidney injury and CKD, together with increased KIM‐1, NGAL, and aggravated renal fibrosis.6 KIM‐1 and NGAL have been shown to be independent prognostic markers in patients with HF, and an increase in urinary KIM‐1 or either serum or urinary NGAL can predict an increased risk of mortality and HF hospitalization independent of eGFR because of their associations with tubular damage,14, 15, 16, 17, 18 which are stronger than those of eGFR and CysC.15 Thus, considering all of these findings, IL‐34, together with changes in KIM‐1 or NGAL levels, may predict mortality and HF hospitalization in HF patients, mainly because of its role in renal tubule damage or renal fibrosis, earlier than reductions in the eGFR.
IL‐34 Affects the Prognosis of Heart Failure by Further Aggravating Coronary Artery Disease in Heart Failure Patients
Our previous study illustrated that among HF patients, serum IL‐34 levels were significantly higher in those with CAD than in those without, and IL‐34 was a significant predictor of CAD using a logistic regression model adjusted for conventional risk factors, which is in accord with another cross‐sectional study of a small group of CAD patients and a control group.19
Several studies have shown that HF patients with CAD exhibited greater deterioration of cardiac function and increased mortality compared with patients without CAD.20, 21 It is possible that IL‐34 may be involved in the pathogenesis of CAD as a proinflammatory cytokine, promoting the release of other proinflammatory cytokines, including interleukin‐1β (IL‐1β), IL‐6, and tumor necrosis factor‐α,5 further aggravating the process of CAD and, ultimately, worsening HF.
Study Limitations
Our present study did have some limitations. First, we were not able to analyze the renal function and cardiac function of any patient during follow‐up. Thus, it is still necessary to explore the effect of IL‐34 on long‐term changes in renal function.
Second, because of the limited scale of the study, the relatively small number of patients limited the statistical power of the findings. In addition, the subjects included in the study were all Asian, and no conclusions can be drawn for ethnic groups. A prospective confirmation of the present findings and an analysis of a larger, more‐diverse population are necessary.
Last, we did not compare the prognostic value of IL‐34 with other markers for tubule injury, NGAL and KIM‐1, in CHF. In addition, future studies are needed to examine other inflammatory markers and compare with IL‐34 for prognostic value in HF.
Conclusions
The present study demonstrated the incremental prognostic value of serum IL‐34 in patients with HF, especially in those with kidney impairment. Thus, measurement of IL‐34 may provide new insights into kidney function beyond eGFR and CysC and into the elusive mechanisms linking kidney insufficiency to poor HF outcomes. It is likely that investigation of IL‐34 will lead to new avenues of therapeutic intervention for several cardiovascular diseases, especially the vicious circle between HF and kidney dysfunction.
Sources of Funding
This study was supported by the National Natural Science Foundation of China (81370256, 81670352 to Tao; 81400362, 81670457 to Yan; 81570316 to Ruiyan Zhang), Shanghai Municipal Education Commission‐Gaofeng Clinical Medicine Grant Support (20152205 to Tao), ‘973’ grant from National Science and Technology of China (2015CBS553604 to Lu), and a Shanghai Rising‐Star Program grant to Yan (17QA1402300). There are no relationships with industry.
Disclosures
None.
Acknowledgments
We acknowledge the contribution of all doctors from the Department of Cardiology at Ruijin Hospital to the study. They also gave written permission for the publication of data and conclusions.
(J Am Heart Assoc. 2017;6:e004911 DOI: 10.1161/JAHA.116.004911.)28365566
Contributor Information
Rong Tao, Email: rongtao@hotmail.com.
Xiaoxiang Yan, Email: cardexyanxx@hotmail.com.
References
- 1. Metra M, Cotter G, Gheorghiade M, Dei Cas L, Voors AA. The role of the kidney in heart failure. Eur Heart J. 2012;33:2135–2142. [DOI] [PubMed] [Google Scholar]
- 2. Ather S, Chan W, Bozkurt B, Aguilar D, Ramasubbu K, Zachariah AA, Wehrens XH, Deswal A. Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. J Am Coll Cardiol. 2012;59:998–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dries DL, Exner DV, Domanski MJ, Greenberg B, Stevenson LW. The prognostic implications of renal insufficiency in asymptomatic and symptomatic patients with left ventricular systolic dysfunction. J Am Coll Cardiol. 2000;35:681–689. [DOI] [PubMed] [Google Scholar]
- 4. Lin H, Lee E, Hestir K, Leo C, Huang M, Bosch E, Halenbeck R, Wu G, Zhou A, Behrens D, Hollenbaugh D, Linnemann T, Qin M, Wong J, Chu K, Doberstein SK, Williams LT. Discovery of a cytokine and its receptor by functional screening of the extracellular proteome. Science. 2008;320:807–811. [DOI] [PubMed] [Google Scholar]
- 5. Masteller EL, Wong BR. Targeting IL‐34 in chronic inflammation. Drug Discov Today. 2014;19:1212–1216. [DOI] [PubMed] [Google Scholar]
- 6. Baek JH, Zeng R, Weinmann‐Menke J, Valerius MT, Wada Y, Ajay AK, Colonna M, Kelley VR. IL‐34 mediates acute kidney injury and worsens subsequent chronic kidney disease. J Clin Invest. 2015;125:3198–3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Damman K, Navis G, Smilde TD, Voors AA, van der Bij W, van Veldhuisen DJ, Hillege HL. Decreased cardiac output, venous congestion and the association with renal impairment in patients with cardiac dysfunction. Eur J Heart Fail. 2007;9:872–878. [DOI] [PubMed] [Google Scholar]
- 8. Hillege HL, Nitsch D, Pfeffer MA, Swedberg K, McMurray JJ, Yusuf S, Granger CB, Michelson EL, Ostergren J, Cornel JH, de Zeeuw D, Pocock S, van Veldhuisen DJ, Candesartan in Heart Failure: Assessment of Reduction in M, Morbidity I . Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation. 2006;113:671–678. [DOI] [PubMed] [Google Scholar]
- 9. Palmer SC, Yandle TG, Frampton CM, Troughton RW, Nicholls MG, Richards AM. Renal and cardiac function for long‐term (10 year) risk stratification after myocardial infarction. Eur Heart J. 2009;30:1486–1494. [DOI] [PubMed] [Google Scholar]
- 10. Damman K, van Deursen VM, Navis G, Voors AA, van Veldhuisen DJ, Hillege HL. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol. 2009;53:582–588. [DOI] [PubMed] [Google Scholar]
- 11. van Veldhuisen DJ, Ruilope LM, Maisel AS, Damman K. Biomarkers of renal injury and function: diagnostic, prognostic and therapeutic implications in heart failure. Eur Heart J. 2016;37:2577–2585. [DOI] [PubMed] [Google Scholar]
- 12. Hillege HL, Girbes AR, de Kam PJ, Boomsma F, de Zeeuw D, Charlesworth A, Hampton JR, van Veldhuisen DJ. Renal function, neurohormonal activation, and survival in patients with chronic heart failure. Circulation. 2000;102:203–210. [DOI] [PubMed] [Google Scholar]
- 13. Damman K, van der Harst P, Smilde TD, Voors AA, Navis G, van Veldhuisen DJ, Hillege HL. Use of cystatin c levels in estimating renal function and prognosis in patients with chronic systolic heart failure. Heart. 2012;98:319–324. [DOI] [PubMed] [Google Scholar]
- 14. Damman K, van Veldhuisen DJ, Navis G, Voors AA, Hillege HL. Urinary neutrophil gelatinase associated lipocalin (NGAL), a marker of tubular damage, is increased in patients with chronic heart failure. Eur J Heart Fail. 2008;10:997–1000. [DOI] [PubMed] [Google Scholar]
- 15. van Deursen VM, Damman K, Voors AA, van der Wal MH, Jaarsma T, van Veldhuisen DJ, Hillege HL. Prognostic value of plasma neutrophil gelatinase‐associated lipocalin for mortality in patients with heart failure. Circ Heart Fail. 2014;7:35–42. [DOI] [PubMed] [Google Scholar]
- 16. Jungbauer CG, Birner C, Jung B, Buchner S, Lubnow M, von Bary C, Endemann D, Banas B, Mack M, Boger CA, Riegger G, Luchner A. Kidney injury molecule‐1 and N‐acetyl‐beta‐D‐glucosaminidase in chronic heart failure: possible biomarkers of cardiorenal syndrome. Eur J Heart Fail. 2011;13:1104–1110. [DOI] [PubMed] [Google Scholar]
- 17. Damman K, Masson S, Hillege HL, Maggioni AP, Voors AA, Opasich C, van Veldhuisen DJ, Montagna L, Cosmi F, Tognoni G, Tavazzi L, Latini R. Clinical outcome of renal tubular damage in chronic heart failure. Eur Heart J. 2011;32:2705–2712. [DOI] [PubMed] [Google Scholar]
- 18. Damman K, Van Veldhuisen DJ, Navis G, Vaidya VS, Smilde TD, Westenbrink BD, Bonventre JV, Voors AA, Hillege HL. Tubular damage in chronic systolic heart failure is associated with reduced survival independent of glomerular filtration rate. Heart. 2010;96:1297–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li Z, Jin D, Wu Y, Zhang K, Hu P, Cao X, Chen Z. Increased serum interleukin‐34 in patients with coronary artery disease. J Int Med Res. 2012;40:1866–1870. [DOI] [PubMed] [Google Scholar]
- 20. Hwang SJ, Melenovsky V, Borlaug BA. Implications of coronary artery disease in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2014;63:2817–2827. [DOI] [PubMed] [Google Scholar]
- 21. Rusinaru D, Houpe D, Szymanski C, Levy F, Marechaux S, Tribouilloy C. Coronary artery disease and 10‐year outcome after hospital admission for heart failure with preserved and with reduced ejection fraction. Eur J Heart Fail. 2014;16:967–976. [DOI] [PubMed] [Google Scholar]


