Abstract
Background
Medications that impact insulin sensitivity or cause weight gain may increase heart failure risk. Our aim was to compare heart failure and cardiovascular death outcomes among patients initiating sulfonylureas for diabetes mellitus treatment versus metformin.
Methods and Results
National Veterans Health Administration databases were linked to Medicare, Medicaid, and National Death Index data. Veterans aged ≥18 years who initiated metformin or sulfonylureas between 2001 and 2011 and whose creatinine was <1.4 (females) or 1.5 mg/dL (males) were included. Each metformin patient was propensity score‐matched to a sulfonylurea initiator. The outcome was hospitalization for acute decompensated heart failure as the primary reason for admission or a cardiovascular death. There were 126 867 and 79 192 new users of metformin and sulfonylurea, respectively. Propensity score matching yielded 65 986 per group. Median age was 66 years, and 97% of patients were male; hemoglobin A1c 6.9% (6.3, 7.7); body mass index 30.7 kg/m2 (27.4, 34.6); and 6% had heart failure history. There were 1236 events (1184 heart failure hospitalizations and 52 cardiovascular deaths) among sulfonylurea initiators and 1078 events (1043 heart failure hospitalizations and 35 cardiovascular deaths) among metformin initiators. There were 12.4 versus 8.9 events per 1000 person‐years of use (adjusted hazard ratio 1.32, 95%CI 1.21, 1.43). The rate difference was 4 heart failure hospitalizations or cardiovascular deaths per 1000 users of sulfonylureas versus metformin annually.
Conclusions
Predominantly male patients initiating treatment for diabetes mellitus with sulfonylurea had a higher risk of heart failure and cardiovascular death compared to similar patients initiating metformin.
Keywords: acute heart failure, comparative effectiveness, diabetes mellitus, pharmacoepidemiology
Subject Categories: Quality and Outcomes; Diabetes, Type 2; Heart Failure; Complications
Introduction
Patients with underlying heart disease and diabetes mellitus have metabolic disturbances including hyperinsulinemia and insulin resistance that can influence heart failure incidence and progression.1, 2, 3 It has been hypothesized that medications that improve insulin sensitivity and limit the potential for weight gain, such as metformin, could prevent heart failure,1, 4 whereas medications that increase endogenous hyperinsulinemia5 and facilitate weight gain may increase heart failure risk.1, 6, 7, 8
The theory that insulin sensitization may also improve cardiovascular outcomes compared to insulin provision prompted the Bypass Angioplasty Revascularization Investigation 2 Diabetes trial.9 That trial used a factorial design to randomize patients with diabetes mellitus and cardiovascular disease to either early revascularization or intensive medical therapy. Medical therapy was further randomized as insulin sensitization (metformin and/or a thiazolidinedione) or insulin provision (sulfonylurea and/or insulin). Heart failure was considered an adverse outcome and occurred in 22.6% of those randomized to insulin sensitization compared with 20.0% of those randomized to insulin provision (P=0.13). The effects of metformin and thiazolidinedione could not be separated, and by 3 years 75% of patients in the insulin‐sensitizing group were taking thiazolidinedione and more than 25% had also added insulin and/or a sulfonylurea. Although randomized trials are ideal for assessing efficacy, they often lack the ability to assess whether treatments work under real‐world conditions with a broader set of participants.10
A recent statement by the American Heart Association reported that metformin remains concerning for patients with established heart failure (level C evidence) because of the risk of lactic acidosis that was described with its predecessor, phenformin. Sulfonylureas were not considered in their report of drugs associated with heart failure, which focused on more frequently described associations including thiazolidinediones and saxagliptin.11 It remains uncertain if common initial diabetes mellitus medications such as sulfonylurea differ from metformin on heart failure outcomes because heart failure has been an infrequent primary outcome in clinical trials.12 Our aim was to test the hypothesis that heart failure outcomes would be higher among patients initiating sulfonylurea for diabetes mellitus treatment compared to metformin because of the potential for more weight gain.
Methods
Study Design and Data Sources
We assembled a retrospective cohort of Veterans Health Administration (VHA) patients.13 Pharmacy data included dispensed prescriptions, date filled, days supplied, and number of pills. Demographic, diagnostic, and procedure information identified inpatient and outpatient encounters. We collected laboratory results and vital signs data from clinical sources. For Medicare or Medicaid enrollees, we obtained enrollment, claims files, and prescription (Part D) data.14, 15, 16, 17 We obtained dates and cause of death from vital status and the National Death Index files.18 The institutional review board of Tennessee Valley Healthcare System approved this study with a waiver of informed consent.
Study Population
The population was made up of veterans aged ≥18 years who received regular VHA care at least once every 365 days for 2 or more years. New users of oral hypoglycemics were identified as patients who filled a first hypoglycemic prescription from October 2001 through December 2011 with ≥730 days of baseline data available and without any diabetic drug fill in the 180 days prior to that first fill (Figure S1). The date of first new use was termed the index date. We selected those who were adherent by including patients who refilled their incident medication at least once in the 180 days after the index date. This prevented the inclusion of those with early nonadherence and those who switched to alternate regimens. We excluded patients receiving hospice care. We also excluded patients with evidence of chronic kidney disease including females with creatinine >1.4 mg/dL and males with a creatinine >1.5 mg/dL on the index date because during this time in the United States metformin was not recommended for these patients.19
Exposures
The exposures were metformin and a sulfonylurea (glyburide, glipizide, or glimepiride). Follow‐up began at 180 days after the incident prescription and continued through an outcome (described below) or censoring event. Patients were censored on the 181st day without medical contact (inpatient, outpatient, or pharmacy use) or nonpersistence, defined as the 91st day without the hypoglycemic therapy or addition of a second hypoglycemic drug, reaching the previously described creatinine threshold, death, or study end (December 31, 2011). Seventy percent of our population received 90‐day prescriptions, and 93% and 94% of metformin and sulfonylurea users, respectively, refilled their prescriptions within 90 days.20
Outcome
The primary outcome was a composite of either hospitalization for a diagnosis of heart failure or cardiovascular death. The secondary outcomes evaluated each component separately, and the composite primary outcome also included emergency department visits for heart failure that did not result in hospitalization.
We defined heart failure hospitalization by adapting the validated definitions used in the Mini‐Sentinel to identify heart failure outcomes.21 Events were defined as a primary discharge diagnosis of heart failure (ICD9‐CM: 425.X; 428.X; 404.01, 404.03, 404.11, 404.13, 398.91, 402.01, 402.11, 402.91, 404.91, 404.93) or a diagnosis‐related group (DRG) code for heart failure (DRG 127 before fiscal year 2008; and 291‐293 after fiscal year 2008). Cardiovascular deaths were identified based on death certificates with an ICD‐10 coded underlying cause of death including I00‐I78 (cardiovascular deaths) or R98, R99, R960, R961 (unattended deaths), excluding I30.X (diseases of the pericardium). This definition was derived from the Centers for Disease Control and Prevention and validated strategies for identification of sudden cardiac deaths.22
Emergency department visits for heart failure were included if there was a coded visit (CPT code 99281, 99282, 99283, 99284, 99285) and a primary heart failure diagnosis (listed above) on the same day. Any emergency department visit that resulted in hospitalization within a 48‐hour time frame was considered a single hospitalization event.
Covariates
Study covariates were measured during the 730 days before the index date and included age, sex, race (white, black, other), fiscal year, healthcare utilization (hospitalization, nursing home, number of outpatient visits or medications, Medicare or Medicaid use in past year), and physiologic variables (body mass index [BMI], blood pressure, hemoglobin [Hb]A1c, low‐density lipoprotein levels, presence of proteinuria, and creatinine), which were used to calculate estimated glomerular filtration rate using the Chronic Kidney Disease Epidemiology Collaboration equation,23 selected medications, smoking, and comorbidities (Table S1). Missing covariates were handled with multiple imputations using predictive mean matching with bootstrapping.24 All covariates from the primary analysis as well as an indicator for each Veterans Integrated Service Networks were included in 20 imputation models to compute final estimates.
Statistical Analyses
The primary analysis compared the hazard of heart failure hospitalization or cardiovascular death between exposure groups in a propensity score–matched cohort. The propensity score modeled the probability of sulfonylurea given covariates and VHA medical center. The 1:1 matching was performed on the log odds of the propensity scores using an 8:1 digit matching algorithm (Table S2, Figure S2).25, 26 Cox proportional hazards models were used to compare outcomes for sulfonylurea versus metformin (referent) in the matched cohort adjusted for covariates. The proportional hazards assumptions were verified through examination of log‐log plots.
Evaluation of a Positive Control: Sensitivity and Subgroup Analyses
We conducted planned sensitivity analyses. First, we used the initial regimen that defined exposure and ignored subsequent regimen changes or the 90‐day refill requirement (persistent exposure not required). This analysis is akin to intention‐to‐treat analysis in clinical trials; however, although it increases follow‐up time and events, it allows for exposure time misclassification due to patient non‐adherence. Second, because the main analysis included a matched subset of the population, we conducted an inverse probability of treatment weighted analysis to include all patients. For these analyses, we used the previously described propensity score and weighted the sulfonylurea users to resemble the metformin population and approximate a balanced cohort. For this analysis we also included new users of thiazolidinedione (a small, select group27) as a positive control group because of the well‐described association of thiazolidinediones with heart failure outcomes.28, 29 Third, in the cohort construction we were interested in long‐term outcomes; therefore, follow‐up began 180 days post–treatment initiation to minimize the inclusion of those with early nonadherence and regimen switching. To evaluate early outcome differences between groups, we performed an alternate weighted analysis with a new‐user design in which follow‐up began at the index date and continued through the first 180 days.30, 31 For this analysis we also included thiazolidinedione users. We conducted subgroup analyses, stratifying by history of heart failure diagnosis (yes, no), age (≥65, <65 years), and race (black, white). Finally, we explored the sensitivity of our main analysis to potential unmeasured confounding.32 For this we assessed the strength of the association of an unmeasured binary confounder and its hypothetical distribution between exposure groups that would be required to explain our findings. Analyses were conducted using R (http://www.r-project.org) and SAS for Windows 9.2. (SAS Institute Cary, North Carolina).
Results
Study Cohort and Patient Characteristics
There were 407 145 patients who started an antidiabetic medication (no hypoglycemics filled in the previous 180 days). Of these, 102 457 initiated regimens other than metformin or a sulfonylurea; 142 were excluded for data errors; 21 474 were excluded for elevated creatinine or hospice care; 23 207 died or were censored during the 6‐month lag time, and 53 806 were not persistent on their initial regimen at the start of follow‐up (early stoppers N=33 363; early intensifiers N=20 443). Thus, there were 126 867 metformin initiators and 79 192 sulfonylurea initiators (46.2% glipizide, 53.1% glyburide, and 0.7% glimepiride; Figure 1). After 1:1 propensity score matching, our study included 65 986 patients in each group, and baseline characteristics were similar (Table 1, Figure S3). Characteristics of patients excluded from the PS match are shown in Table S3 Characteristics of patients included in the weighted analysis (including 6945 thiazolidinedione new users as a positive control group) are listed in Table S4
Figure 1.
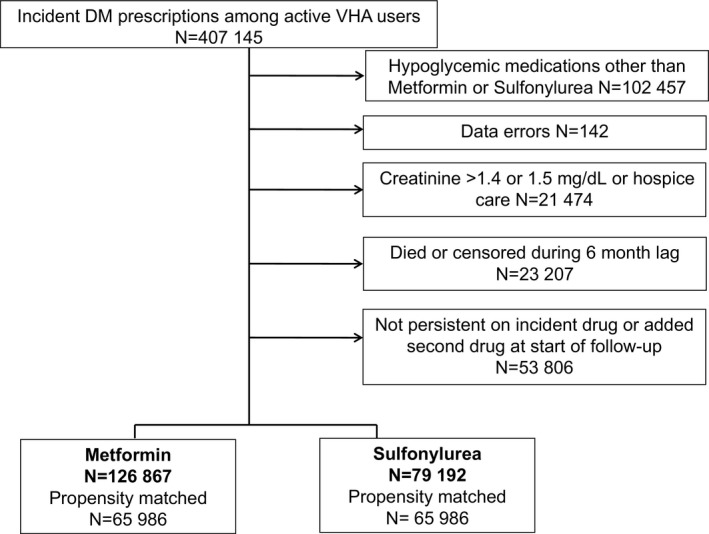
Flow of eligible patients included.
Table 1.
Characteristics of Patients in the Unmatched and Matched Cohorts
| Characteristics | Full Cohort | Propensity‐Matched Cohort | |||
|---|---|---|---|---|---|
| Sulfonylurea (N=79 192) | Metformin (N=126 867) | Sulfonylurea (N=65 986) | Metformin (N=65 986) | Standardized Differences for Matched Cohorta | |
| Age, median (IQR) | 68 (58, 77) | 62 (56, 72) | 66 (57, 75) | 66 (58, 75) | 0.013 |
| Male, % | 97 | 95 | 97 | 97 | 0.006 |
| Race, % | |||||
| White | 77 | 76 | 77 | 77 | 0.002 |
| Black | 14 | 13 | 13 | 14 | 0.003 |
| Hispanic/other | 5 | 4 | 5 | 5 | 0.004 |
| Missing | 4 | 7 | 5 | 4 | 0.008 |
| HbA1c, % median (IQR) | 6.9 (6.3, 7.8) | 6.8 (6.3, 7.5) | 6.9 (6.3, 7.7) | 6.9 (6.3, 7.6) | 0.024 |
| Missing measurement, % | 22 | 19 | 21 | 21 | 0 |
| Low‐density lipoprotein, mg/dL, median (IQR) | 98 (77, 122) | 99 (79, 123) | 98 (78, 123) | 98 (78, 122) | 0.007 |
| Missing measurement, % | 31 | 25 | 29 | 29 | 0.002 |
| Creatinine mg/dL, median (IQR) | 1.1 (0.9, 1.2) | 1.0 (0.9, 1.1) | 1.0 (0.9, 1.2) | 1.0 (0.9, 1.2) | 0.029 |
| Glomerular filtration rate mL/min, median (IQR) | 77 (64, 94) | 84 (71, 99) | 80 (67, 97) | 79 (67, 96) | 0.019 |
| Missing measurement, % | 18 | 14 | 17 | 17 | 0 |
| Proteinuria, (%) negative | 47 | 51 | 48 | 48 | 0.001 |
| Urine protein trace or 1+ | 11 | 10 | 11 | 11 | 0.001 |
| Proteinuria present at 2+ | 2 | 1 | 2 | 2 | 0 |
| Proteinuria present at 3+ | 0.41 | 0.27 | 0 | 0 | 0 |
| Proteinuria present at 4+ | 0.05 | 0.02 | 0 | 0 | 0 |
| Missing measurement, % | 40 | 39 | 40 | 39 | 0.002 |
| Systolic blood pressure, mm Hg, median (IQR) | 136 (124, 148) | 135 (124, 146) | 136 (124, 148) | 136 (124, 148) | 0.001 |
| Diastolic blood pressure, mm Hg, median (IQR) | 76 (68, 83) | 77 (70, 84) | 76 (68, 84) | 76 (68, 84) | 0.006 |
| Missing measurement, % | 2.6 | 1.9 | 2 | 2 | 0.001 |
| Body mass index, kg/m2, median (IQR) | 30.2 (26.9, 34.1) | 31.8 (28.4, 36.0) | 30.7 (27.4, 34.6) | 30.7 (27.4, 34.6) | 0.003 |
| Missing measurement, % | 4.3 | 2.9 | 4 | 4 | 0.002 |
| Baseline comorbidities, %b | |||||
| Malignancy | 7 | 5 | 6 | 6 | 0.002 |
| Liver/respiratory failure | 2 | 1 | 1 | 1 | 0.005 |
| HIV | 0.6 | 0.4 | 1 | 0 | 0.004 |
| Congestive heart failure | 10 | 4 | 6 | 6 | 0.003 |
| Cardiovascular disease | 28 | 22 | 27 | 27 | 0.001 |
| Serious mental illness | 16 | 17 | 17 | 17 | 0 |
| Smoking | 11 | 12 | 11 | 11 | 0.001 |
| Chronic obstructive pulmonary disease | 15 | 12 | 13 | 13 | 0 |
| Cardiac valve disease | 2 | 1 | 2 | 2 | 0.001 |
| Arrhythmia | 11 | 7 | 9 | 9 | 0.002 |
| Parkinson | 0.8 | 0.5 | 1 | 1 | 0.002 |
| Use of medications, % | |||||
| Angiotensin‐converting enzyme inhibitors | 53 | 53 | 53 | 53 | 0.003 |
| Angiotensin II receptor blockers | 7 | 8 | 7 | 7 | 0.003 |
| β‐Blockers | 44 | 40 | 42 | 42 | 0.005 |
| Calcium channel blockers | 26 | 24 | 26 | 26 | 0.002 |
| Thiazide and potassium‐sparing diuretics | 31 | 33 | 31 | 31 | 0.006 |
| Nonselective α blockers | 16 | 14 | 15 | 16 | 0.009 |
| Loop diuretics | 18 | 10 | 14 | 14 | 0.003 |
| Other antihypertensive medications | 26 | 24 | 25 | 25 | 0.002 |
| Statin lipid‐lowering drugs | 58 | 64 | 60 | 60 | 0.002 |
| Nonstatin lipid‐lowering drugs | 15 | 18 | 16 | 16 | 0.001 |
| Antiarrhythmics, digoxin, and inotropes | 2 | 2 | 2 | 2 | 0.005 |
| Anticoagulants, platelet inhibitors | 8 | 5 | 7 | 7 | 0.001 |
| Nitrates | 15 | 11 | 14 | 14 | 0.004 |
| Aspirin | 18 | 17 | 18 | 18 | 0 |
| Antipsychotics | 7 | 8 | 8 | 8 | 0.002 |
| Oral glucocorticoids | 12 | 11 | 12 | 12 | 0.001 |
| Indicators of health care utilization, % | |||||
| Hospitalized in last year (Veterans Health) | 9 | 6 | 8 | 8 | 0.007 |
| Hospitalized in last year (Medicare/Medicaid) | 11 | 6 | 8 | 8 | 0 |
| Hospitalized within 30 days (Veterans Health) | 4 | 3 | 3 | 3 | 0.003 |
| Hospitalized within 30 days (Medicare/Medicaid) | 3 | 1 | 2 | 2 | 0.004 |
| Days from prior heart failure hospitalization to incident diabetes mellitus drug, median (IQR) | 218 (65, 427) | 266 (97, 456) | 268 (81, 476) | 257 (95, 440) | 0.032 |
| Nursing home encounter in last year | 0.07 | 0.05 | 0 | 0 | 0.001 |
| Number medications | 10 (7, 14) | 9 (6, 14) | 10 (6, 14) | 10 (6, 14) | 0.003 |
| Outpatient visits in past year | 5 (3, 9) | 5 (3, 9) | 5 (3, 9) | 5 (3, 9)] | 0.003 |
| Medicare use in last year | 34 | 26 | 32 | 32 | 0.002 |
| Medicaid use in last year | 15 | 9 | 12 | 12 | 0.001 |
IQR indicates interquartile range.
Standardized mean differences are the absolute difference in means or percentage divided by an evenly weighted pooled standard deviation, or the difference between groups in number of standard deviations. In the matched cohort all standardized differences were not statistically significant (see Figure S3 for the plot of the mean standardized differences of the prematched and matched cohort).
Definitions of comorbidities included in Table S1.
In the primary analysis, the median (interquartile range [IQR]) follow‐up prior to censoring or reaching an outcome was 1.1 (0.4, 2.7) years among metformin users and 0.9 (0.4, 2.1) year among sulfonylurea users. Reasons for censoring were nonpersistence (49% metformin versus 46% sulfonylurea); additional therapy (24% versus 28%); no healthcare contact (5% versus 5%); reaching creatinine threshold (10% versus 11%); study end (9% versus 5%); or death (2% versus 2%). In the sensitivity analysis in which regimen persistence was not required, the median follow‐up time was 5.1 (3.6, 6.6) versus 5.0 (3.0, 6.5) years among metformin and sulfonylurea users, respectively.
Time to Heart Failure Events or Cardiovascular Death
There were 1236 events (1184 heart failure hospitalizations and 52 cardiovascular deaths) among sulfonylurea initiators and 1078 events (1043 heart failure hospitalizations and 35 cardiovascular deaths) among metformin initiators, yielding 12.4 versus 8.9 events per 1000 person‐years of use, respectively (adjusted hazard ratio [aHR] 1.32, 95%CI [1.21, 1.43]) (Table 2, Figure 2). Event rates for heart failure hospitalization alone comprised the majority of the outcomes and were 11.9 and 8.6 per 1000 person‐years among sulfonylurea and metformin users (aHR 1.30 [1.20, 1.42]). Event rates for cardiovascular death alone were 5.2 and 2.9 per 10 000 person years among sulfonylurea and metformin users (aHR 1.76 [1.14, 2.71]). The secondary outcome that added emergency room visits yielded event rates of 15.1 versus 11.0 per 1000 person‐years among sulfonylurea and metformin users (aHR 1.30 [1.20, 1.40]).
Table 2.
Rates and Adjusted Hazard Ratios for Risk of Congestive Heart Failure Events or Cardiovascular Deaths Among Those Who Initiate Metformin vs Sulfonylurea Among Propensity Score–Matched and Weighted Cohort
| Metformin | Sulfonylurea | |
|---|---|---|
| Persistent exposure requireda | ||
| N at risk | 65 986 | 65 986 |
| Composite heart failure hospitalization or cardiovascular death | 1078 | 1236 |
| Person‐years | 121 406 | 99 872 |
| Unadjusted rate/1000 person‐years | 8.9 (8.4, 9.4) | 12.4 (11.7, 13.1) |
| Adjusted hazard ratiob (95%CI) | Reference | 1.32 (1.21, 1.43) |
| Heart failure hospitalization alone | 1043 | 1184 |
| Unadjusted rate/1000 person‐years | 8.6 (8.1, 9.1) | 11.9 (11.2, 12.5) |
| Adjusted hazard ratiob (95%CI) | Reference | 1.30 (1.20, 1.42) |
| Cardiovascular death alone | 35 | 52 |
| Unadjusted rate/10 000 person‐years | 2.9 (2.1, 4.0) | 5.2 (3.9, 6.8) |
| Adjusted hazard ratiob (95%CI) | Reference | 1.76 (1.14, 2.71) |
| Composite heart failure emergency department visit, hospitalization, or cardiovascular death | 1334 | 1449 |
| Person‐years | 121 147 | 99 600 |
| Unadjusted rate/1000 person‐years | 11.0 (10.4, 11.6) | 15.1 (14.3, 15.8) |
| Adjusted hazard ratiob (95%CI) | Reference | 1.30 (1.20, 1.40) |
| Persistent exposure not requiredc | ||
| N at risk | 65 986 | 65 986 |
| Composite heart failure hospitalization or cardiovascular death | 4007 | 4573 |
| Person‐years | 323 268 | 311 040 |
| Unadjusted rate/1000 person‐years | 12.4 (12.0, 12.8) | 14.7 (14.3, 15.1) |
| Adjusted hazard ratiob (95%CI) | Reference | 1.21 (1.16, 1.27) |
| Weighted analysis of full cohort | ||
| N at risk (weighted) | 126 867 | 125 362 |
| Composite heart failure hospitalization or cardiovascular death | 1499 | 1699 |
| Person‐years | 240 948 | 190 773 |
| Unadjusted rate/1000 person‐years | 6.2 (5.9, 6.5) | 8.9 (8.5, 9.3) |
| Adjusted hazard ratiob (95%CI) | Reference | 1.43 (1.32, 1.55) |
Primary analysis considers patients persistent on incident regimen until they do not have oral antidiabetic medications for 90 days.
Cox proportional hazards model for time to event. Adjusted for age, sex, race, fiscal year of cohort entry, number of medications, number of outpatient visits, baseline HbA1c, body mass index, estimated glomerular filtration rate, low‐density lipoprotein cholesterol, blood pressure, use of medications and health care utilization (see Table S1), smoking‐related illness, cardiovascular disease, serious liver/respiratory disease, cancer, Parkinson disease, mental illness, arrhythmia, cardiac valve disease, asthma/obstructive pulmonary disease, procedures for carotid/peripheral artery revascularization or bypass or lower extremity amputation. All continuous variables were modeled as restricted cubic splines.
Persistent exposure not required analysis in which patients remain in their exposure group, regardless of persistence on drug therapy, until outcome or end of the study.
Figure 2.
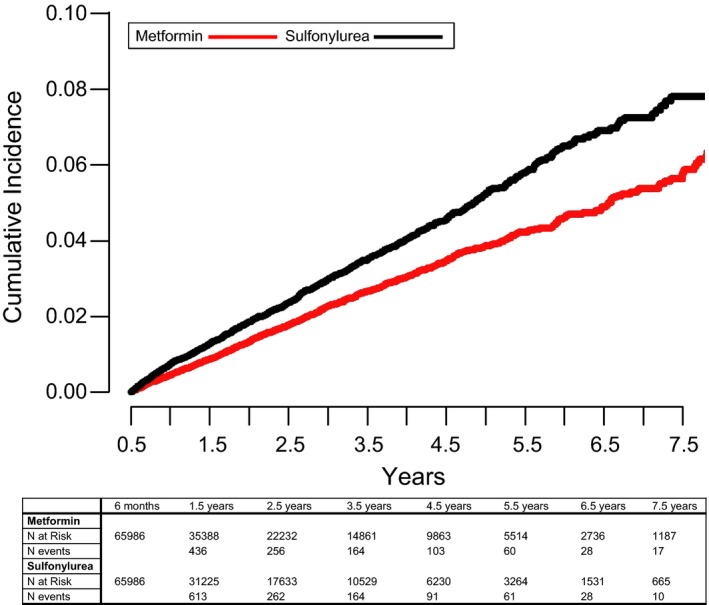
Cumulative incidence of heart failure hospitalization or cardiovascular death over time.
We assessed median [interquartile range] HbA1c and BMI on the index date and over time for the matched cohort. Baseline HbA1c was 6.9% (51.9 mmol/mol) in both groups, and declined to 6.4% [6.0, 6.9] (46.4 mmol/mol [42.1, 51.9]) and 6.5% [6.0, 7.1] (47.5 mmol/mol [42.1, 54.1]) in metformin and sulfonylurea initiators, respectively, by 1.5 years after drug initiation. The HbA1c difference of 0.1% (1.1 mmol/mol) was maintained between groups over follow‐up. Median BMI declined rapidly in metformin initiators, yielding a maximum difference of 0.9 BMI units between groups by 1.5 years. This difference narrowed to 0.5 BMI unit difference at 7.5 years (Figure 3).
Figure 3.
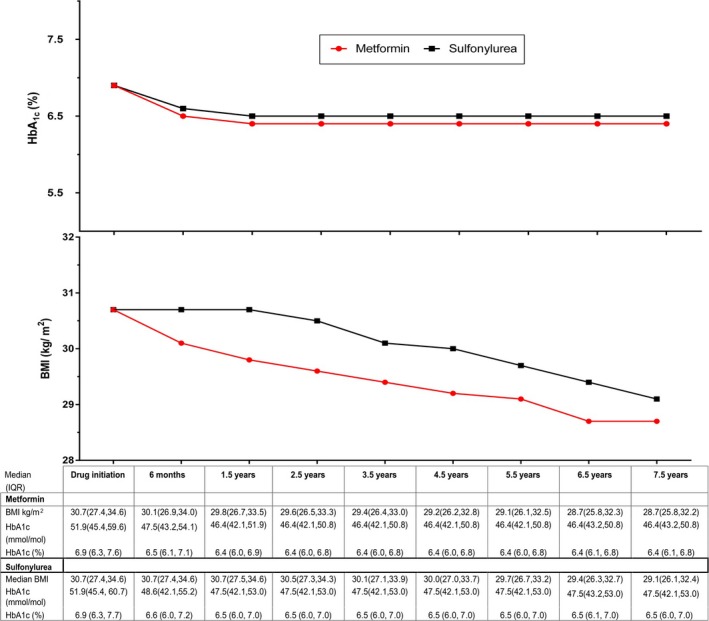
Median (interquartile range) glycated hemoglobin (HbA1c) and body mass index (BMI) of at‐risk patients over time.
Sensitivity and Subgroup Analyses
In sensitivity analyses in which patients remained in their original exposure group, (persistent exposure not required), there were 4573 events (4366 heart failure hospitalizations, 207 cardiovascular deaths) among sulfonylurea initiators and 4007 events (3830 heart failure hospitalizations, 177 cardiovascular deaths) among metformin initiators, yielding 14.7 and 12.4 events per 1000 person‐years (aHR 1.21 [1.16, 1.27]) (Table 2).
In analyses in which sulfonylurea users were weighted to resemble metformin users, there were 1699 and 1499 events among sulfonylurea and metformin users, yielding 8.9 and 6.2 events per 1000 person‐years (aHR 1.43, [1.32, 1.55]) (Table 2). As a positive control, thiazolidinedione users were compared to weighted metformin users; there were 141 and 154 events, yielding 16.5 (14.0, 19.4) and 10.5 (8.9, 12.2) events per 1000 person‐years (aHR 1.44, [1.20, 1.74]).
In the primary analyses, follow‐up began 6 months after the index date to minimize early nonadherence and regimen switching. The alternate weighted analysis of new users evaluating this first 6‐month period found sulfonylurea (N=163 995) versus metformin (N=166 397) had 11.7 (11.0, 12.5) versus 7.8 (7.2, 8.4) events per 1000 person‐years (aHR 1.50 [1.35, 1.66]). Those who initiated thiazolidinedione (N=10 164) versus metformin (N=10 200) had 25.7 (21.6, 30.6) versus 14.9 (11.9, 18.7) events per 1000 person‐years (aHR 1.72 [1.36, 2.18]) (Table S5). Subgroup analyses demonstrated consistent results including patients both with and without a prior history of heart failure. There was no evidence of effect modification (Figure 4).
Figure 4.
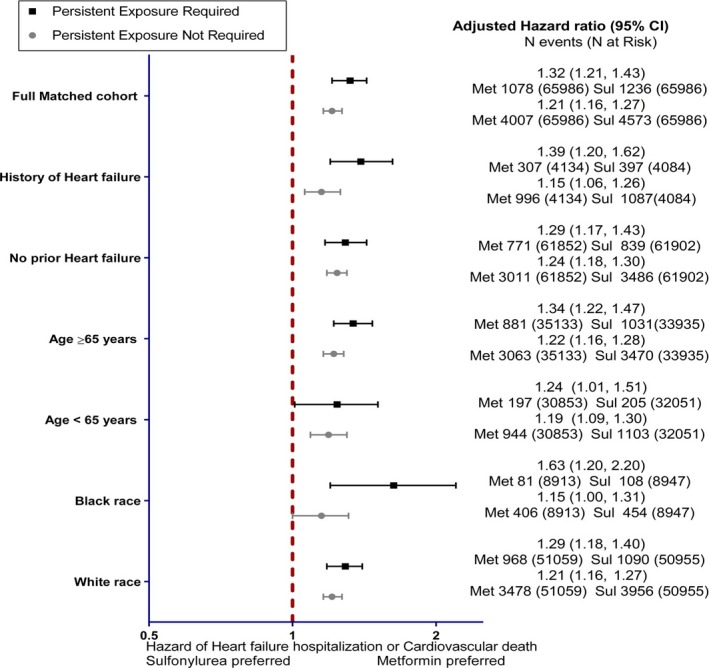
Adjusted hazard ratio and 95%CIs of subgroups. Two medication adherence requirements tested: persistence to medication required with 90‐day gaps or persistence not required. Sul indicates sulfonylurea; Met, metformin.
Our finding of increased hazard for the composite outcome among sulfonylurea users could in theory have resulted from an unmeasured covariate that is associated with heart failure and was more prevalent among sulfonylurea than metformin users. For example, we observed heart failure history to have a HR of 2.3 for our outcome. An unmeasured confounder of this strength would need to be at least 17% more prevalent among sulfonylurea users. For comparison in the unmatched cohort, baseline heart failure history was only 5% more prevalent. Thus, if an unmeasured confounder comparable to heart failure history existed, it would not change this paper's main conclusions (Table S6).
Discussion
Type 2 diabetes mellitus is linked to obesity and is an independent risk factor for cardiomyopathy.33, 34 Patients with type 2 diabetes mellitus have abnormalities in carbohydrate metabolism (insulin resistance) and elevated insulin levels (hyperinsulinemia), which contribute to the development of heart failure.4, 35, 36, 37 Medications that improve insulin sensitivity and limit weight gain, such as metformin, may be more beneficial than medications that increase endogenous insulin and result in weight gain, such as sulfonylureas. Many hypoglycemics have not been rigorously evaluated for the risk of heart failure.12, 38 Clinical trials of diabetes mellitus medications, including the United Kingdom Prospective Diabetes Study, excluded patients with heart failure. The associations reported between heart failure and thiazolidinediones or saxagliptin have been the subject of much debate, in part because these associations were identified as adverse event reports, not as prespecified outcomes in clinical trials that had other surrogate or cardiovascular events as outcomes.7, 8, 39
In this national cohort of veterans who initiated either metformin or a sulfonylurea for first‐line diabetes mellitus treatment, we found that sulfonylurea initiation was associated with an increased risk of heart failure hospitalization and cardiovascular death compared with metformin initiation. Our comparison groups were carefully matched on important covariates including BMI and HbA1c at therapy initiation. Interestingly, among patients who remained at risk by 1.5 years after initiation, metformin users had on average almost 1 BMI unit lower weight than patients prescribed a sulfonylurea (≈2.9 kg for an average 5 foot 10 inch male) and a 0.1% lower HbA1c. The weight differences largely persisted for the study duration. These weight differences are also consistent with findings from the systematic review and a recent network meta‐ analysis.12, 40 Bennett reported a pooled 2.5‐kg relative difference in weight for monotherapy between metformin and a sulfonylurea with a high strength of evidence. The network meta‐analysis by Palmer et al12 reported a standardized mean difference of 0.19‐kg higher weight for sulfonylurea monotherapy users compared with those taking metformin. Nevertheless, it remains unclear to what extent the degree of glycemic control or changes in weight affected the heart failure risk in our study.
Several lines of evidence suggest that weight changes during diabetes mellitus management are associated with heart failure. A meta‐analysis and metaregression41 combined information from multiple trials to investigate whether glucose‐lowering drugs (predominantly thiazolidinedione or dipeptidyl peptidase‐4 inhibitors) or management strategies (standard versus intensive control) were associated with heart failure. There were 95 502 patients included from 14 trials. The meta‐analysis demonstrated that, compared with standard glucose control, heart failure risk increased with intensive control (risk ratio 1.14 [1.01, 1.30]) and was also associated with weight gain (P=0.02 for meta‐regression). Each 1.0 kg increase in weight was associated with a 7.1% (95%CI 1.0‐13.6) relative increase in heart failure risk. Conversely, weight loss was associated with a decreased heart failure risk [risk ratio 0.80 (0.62, 1.04)]. Another recent observational cohort followed more than 10 000 patients in a United Kingdom diabetes registry for more than 10 years.42 The risk of incident heart failure was 2 times higher among obese patients (BMI ≥30 kg/m2) in all age tertiles compared with patients whose BMI was between 18.5 and 24.9 kg/m2.
Possible explanations for our findings include differential medication effects on BMI, as evident in our cohort and others,42, 43 and/or differential effects on insulin levels or insulin resistance.36, 37 Our study is not mechanistic and cannot establish a causal relationship or distinguish among these hypotheses. However, the BMI difference observed between metformin and sulfonylurea users is consistent with a differential risk of heart failure. We also verified the expected increased association of heart failure with thiazolidinediones versus metformin. This finding and consistent results using different methodologic approaches lend credence to the increased risk observed with sulfonylureas versus metformin. Our results are consistent with the United Kingdom Prospective Diabetes Study and a large cohort within the Clinical Practice Research Database, which found beneficial effects of metformin on heart failure but no benefit from sulfonylurea.44, 45 The study by Tzoulaki in the Clinical Practice Research Database found that sulfonylurea was associated with a higher risk of heart failure than metformin, but confidence intervals were wide in fully adjusted models, most likely due to a smaller number of outcome events than in our current study. We estimated sulfonylurea users to have an average of 4 excess heart failure hospitalizations or cardiovascular deaths per 1000 users annually compared to the metformin users.
Our study does have limitations. First, although we utilized multiple strategies to address confounding by indication and disease severity including exclusions, propensity score matching, and covariate adjustment, residual confounding from unmeasured factors, such as patient frailty, remains possible. Our findings were robust when we assessed sensitivity to unmeasured confounders. A hypothetical unmeasured confounder resembling the baseline heart failure history prevalence in prematching imbalance and with a similar strength of association with the outcome would not explain the statistically significant results from our primary analysis (Table S6). Second, veterans may not receive all their care or medications at veteran facilities,15, 16 resulting in missing outcomes or medications, which we partially addressed through supplementation with Medicare/Medicaid information. Third, we did not account for time‐varying nonadherence to other medications, such as diuretics, which may lead to heart failure exacerbations. Our groups were matched on baseline characteristics, including medications and comorbidities associated with heart failure risk, and consistent associations were also observed among patients without a history of heart failure. Fourth, to reduce exposure misclassification, follow‐up started 180 days after initiation, and because we required persistence on drug for our primary analysis, the median follow‐up time was short, ≈1 year. Although this approach excluded the initial exposure period, separate evaluations examined the first 6 months and also allowed for nonpersistence and increased follow‐up to an average of 5 years. Both sensitivity analyses produced consistent results. Finally, our population reflects a typical veteran population, predominantly male; therefore, caution is warranted when extrapolating to other settings and to females.
At age 40, the lifetime risk of developing heart failure is 1 in 5. It remains the primary reason for hospital admission among both VHA and Medicare beneficiaries and a major contributor to the $37.2 billion in heart failure costs in the United States. We found that using sulfonylurea as an initial therapy for diabetes mellitus was associated with more heart failure outcomes than initiation of metformin. Metformin is already the preferred first‐line medical therapy for diabetes mellitus and now can be used safely in another insulin‐resistant state, mild to moderate kidney disease.46 Despite the recommendation to use metformin, sulfonylurea remains an initial choice for diabetes mellitus treatment in 20%47 to 30%27 of the insured and VHA populations, respectively, because of physician preference, relative ease of initiation and titration, and lack of gastrointestinal side effects. Given the clinically important increase in heart failure and other cardiovascular risk associated with sulfonylureas compared with metformin,13, 44 it is urgent to determine whether other drugs should be preferred over sulfonylureas for those intolerant to metformin.
Author Contributions
Drs Roumie, Greevy, Grijalva, Hung, Elasy, and Griffin designed the study. Drs Roumie, Min, Presley, Greevy, and Griffin conducted the study and collected data. Analysis was done by Drs D'Agostino McGowan, Hackstadt, and Greevy. The manuscript was drafted by Dr Roumie, and critical revision of the manuscript was performed by Drs Roumie, Min, Presley, Hung, D'Agostino McGowan, Hackstadt, Greevy, Grijalva, Elasy, and Griffin. Drs Roumie and Greevy had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Sources of Funding
This project was funded by the VA Clinical Science Research and Development Investigator initiated grant I01CX000570 (Roumie) and supported in part by the Agency for Healthcare Research and Quality as part of the Developing Evidence to Inform Decisions about Effectiveness (DEcIDE) program (Griffin). Drs Roumie and Elasy were supported in part by Center for Diabetes Translation Research P30DK092986. Dr Min was supported by the Clinical and Translational Science Award (CTSA) No. TL1TR000447‐09 from the National Center for Advancing Translational Sciences. Dr Hung (2‐031‐09S) was supported by a VA Career Development Award. Dr Presley was supported by the Office of Academic Affiliations VA Quality Scholars Program. Support for Veterans Affairs/Centers for Medicare & Medicaid Services data was provided by the Department of Veterans Affairs, Veterans Affairs Health Services Research and Development Service, and the Veterans Affairs Information Resource Center (project numbers SDR 02‐237 and 98‐004).
Disclosures
None.
Supporting information
Table S1. Definitions of Comorbid Conditions and Medications on the Basis of Codes and Prescriptions in 730 Days Before Treatment Intensification
Table S2. Details for the Construction of the Propensity Score Model
Table S3. Characteristics of Patients Who Did Not Match in 1 to 1 Propensity Score Matching
Table S4. Description and Characteristics of the Weighted Analysis Cohort
Table S5. Sensitivity Analyses Evaluating the Hazard of Heart Failure in First 180 Days of Use of Sulfonylurea vs Metformin and Thiazolidinedione vs Metformin Using New‐User Design and Inverse Probability Treatment Weighted Analysis
Table S6. Analysis of Sensitivity to Unmeasured Confounding
Figure S1. Study design schematic.
Figure S2. Distribution of propensity scores by drug.
Figure S3. Mean standardized difference plot comparing metformin vs sulfonylurea.
(J Am Heart Assoc. 2017;6:e005379 DOI: 10.1161/JAHA.116.005379.)28424149
References
- 1. DeFronzo RA, Ferrannini E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991;14:173–194. [DOI] [PubMed] [Google Scholar]
- 2. Inzucchi SE, Masoudi FA, McGuire DK. Metformin therapy in patients with type 2 diabetes complicated by heart failure. Am Heart J. 2007;154:e45. [DOI] [PubMed] [Google Scholar]
- 3. Inzucchi SE, Masoudi FA, Wang Y, Kosiborod M, Foody JM, Setaro JF, Havranek EP, Krumholz HM. Insulin‐sensitizing antihyperglycemic drugs and mortality after acute myocardial infarction: insights from the National Heart Care Project. Diabetes Care. 2005;28:1680–1689. [DOI] [PubMed] [Google Scholar]
- 4. Nichols GA, Gullion CM, Koro CE, Ephross SA, Brown JB. The incidence of congestive heart failure in type 2 diabetes: an update. Diabetes Care. 2004;27:1879–1884. [DOI] [PubMed] [Google Scholar]
- 5. Ekeruo IA, Solhpour A, Taegtmeyer H. Metformin in diabetic patients with heart failure: safe and effective? Curr Cardiovasc Risk Rep. 2013;7:417–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Crowley MJ, Diamantidis CJ, McDuffie JR, Cameron CB, Stanifer JW, Mock CK, Wang X, Tang S, Nagi A, Kosinski AS, Williams JW Jr. Clinical outcomes of metformin use in populations with chronic kidney disease, congestive heart failure, or chronic liver disease: a systematic review. Ann Intern Med. 2017;166:191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, Ohman P, Frederich R, Wiviott SD, Hoffman EB, Cavender MA, Udell JA, Desai NR, Mosenzon O, McGuire DK, Ray KK, Leiter LA, Raz I. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317–1326. [DOI] [PubMed] [Google Scholar]
- 8. Udell JA, Bhatt DL, Braunwald E, Cavender MA, Mosenzon O, Steg PG, Davidson JA, Nicolau JC, Corbalan R, Hirshberg B, Frederich R, Im K, Umez‐Eronini AA, He P, McGuire DK, Leiter LA, Raz I, Scirica BM. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes and moderate or severe renal impairment: observations from the SAVOR‐TIMI 53 Trial. Diabetes Care. 2015;38:696–705. [DOI] [PubMed] [Google Scholar]
- 9. Frye RL, August P, Brooks MM, Hardison RM, Kelsey SF, MacGregor JM, Orchard TJ, Chaitman BR, Genuth SM, Goldberg SH, Hlatky MA, Jones TL, Molitch ME, Nesto RW, Sako EY, Sobel BE. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009;360:2503–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Frangakis C. The calibration of treatment effects from clinical trials to target populations. Clin Trials. 2009;6:136–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Page RL II, O'Bryant CL, Cheng D, Dow TJ, Ky B, Stein CM, Spencer AP, Trupp RJ, Lindenfeld J. Drugs that may cause or exacerbate heart failure: a scientific statement from the American Heart Association. Circulation. 2016;134:e32–e69. [DOI] [PubMed] [Google Scholar]
- 12. Palmer SC, Mavridis D, Nicolucci A, Johnson DW, Tonelli M, Craig JC, Maggo J, Gray V, De Berardis G, Ruospo M, Natale P, Saglimbene V, Badve SV, Cho Y, Nadeau‐Fredette AC, Burke M, Faruque L, Lloyd A, Ahmad N, Liu Y, Tiv S, Wiebe N, Strippoli GF. Comparison of clinical outcomes and adverse events associated with glucose‐lowering drugs in patients with type 2 diabetes: a meta‐analysis. JAMA. 2016;316:313–324. [DOI] [PubMed] [Google Scholar]
- 13. Roumie CL, Hung AM, Greevy RA, Grijalva CG, Liu X, Murff HJ, Elasy TA, Griffin MR. Comparative effectiveness of sulfonylurea and metformin monotherapy on cardiovascular events in type 2 diabetes mellitus: a cohort study. Ann Intern Med. 2012;157:601–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. VHA: Information Exchange Agreement (IEA) between Department of Health and Human Services, the Centers for Medicare & Medicaid Services and Department of Veterans Affairs, Veterans Health Administration. 2009. Available at: https://www.va.gov/ORO/Docs/Agreements/VHA_CMS_InformationExchangeAgreement100109.pdf. Accessed April 8, 2017.
- 15. Humensky J, Carretta H, de Groot K, Brown MM, Tarlov E, Hynes D. Service utilization of veterans dually eligible for VA and Medicare fee‐for‐service: 1999–2004. Medicare Medicaid Res Rev. 2012;2:E1–E21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hynes DM, Koelling K, Stroupe K, Arnold N, Mallin K, Sohn MW, Weaver FM, Manheim L, Kok L. Veterans' access to and use of Medicare and Veterans Affairs health care. Med Care. 2007;45:214–223. [DOI] [PubMed] [Google Scholar]
- 17. Department of VHA . Access to Centers for Medicare and Medicaid Services (CMS) data for VHA users within the VA Information technology (IT) systems. 2010. Available at: http://www1.va.gov/vhapublications/ViewPublication.asp?pub_ID=2228. Accessed April 8, 2017.
- 18. McCarthy JF, Valenstein M, Kim HM, Ilgen M, Zivin K, Blow FC. Suicide mortality among patients receiving care in the Veterans Health Administration health system. Am J Epidemiol. 2009;169:1033–1038. [DOI] [PubMed] [Google Scholar]
- 19. US Food and Drug Administration . Glucophage final printed labeling. 2001. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2000/20357S019_Glucophage_prntlbl.pdf. Accessed April 8, 2017.
- 20. Greevy RA Jr, Huizinga MM, Roumie CL, Grijalva CG, Murff H, Liu X, Griffin MR. Comparisons of persistence and durability among three oral antidiabetic therapies using electronic prescription‐fill data: the impact of adherence requirements and stockpiling. Clin Pharmacol Ther. 2011;90:813–819. [DOI] [PubMed] [Google Scholar]
- 21. Saczynski JS, Andrade SE, Harrold LR, Tjia J, Cutrona SL, Dodd KS, Goldberg RJ, Gurwitz JH. A systematic review of validated methods for identifying heart failure using administrative data. Pharmacoepidemiol Drug Saf. 2010;21(suppl 1):129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chung CP, Murray KT, Stein CM, Hall K, Ray WA. A computer case definition for sudden cardiac death. Pharmacoepidemiol Drug Saf. 2010;19:563–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Coresh J, Turin TC, Matsushita K, Sang Y, Ballew SH, Appel LJ, Arima H, Chadban SJ, Cirillo M, Djurdjev O, Green JA, Heine GH, Inker LA, Irie F, Ishani A, Ix JH, Kovesdy CP, Marks A, Ohkubo T, Shalev V, Shankar A, Wen CP, de Jong PE, Iseki K, Stengel B, Gansevoort RT, Levey AS. Decline in estimated glomerular filtration rate and subsequent risk of end‐stage renal disease and mortality. JAMA. 2014;311:2518–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. vanBuuren S. Flexible Imputation of Missing Data. Boca Raton, FL: CRC Press; Taylor and Francis Group; 2012. [Google Scholar]
- 25. Parsons L. Performing a 1:N case‐control match on propensity score. In: SAS User's Group International Conference Proceedings; 2004.
- 26. D'Agostino R, Rubin D. Estimating and using propensity scores with partially missing data. J Am Stat Assoc. 2000;95:749–759. [Google Scholar]
- 27. Roumie CL, Greevy RA, Grijalva CG, Hung AM, Liu X, Griffin MR. Diabetes treatment intensification and associated changes in HbA1c and body mass index: a cohort study. BMC Endocr Disord. 2016;16:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Erdmann E, Charbonnel B, Wilcox RG, Skene AM, Massi‐Benedetti M, Yates J, Tan M, Spanheimer R, Standl E, Dormandy JA. Pioglitazone use and heart failure in patients with type 2 diabetes and preexisting cardiovascular disease: data from the PROactive study (PROactive 08). Diabetes Care. 2007;30:2773–2778. [DOI] [PubMed] [Google Scholar]
- 29. Varas‐Lorenzo C, Margulis AV, Pladevall M, Riera‐Guardia N, Calingaert B, Hazell L, Romio S, Perez‐Gutthann S. The risk of heart failure associated with the use of noninsulin blood glucose‐lowering drugs: systematic review and meta‐analysis of published observational studies. BMC Cardiovasc Disord. 2014;14:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moride Y, Abenhaim L. Evidence of the depletion of susceptibles effect in non‐experimental pharmacoepidemiologic research. J Clin Epidemiol. 1994;47:731–737. [DOI] [PubMed] [Google Scholar]
- 31. Ray WA. Evaluating medication effects outside of clinical trials: new‐user designs. Am J Epidemiol. 2003;158:915–920. [DOI] [PubMed] [Google Scholar]
- 32. Schneeweiss S, Glynn RJ, Tsai EH, Avorn J, Solomon DH. Adjusting for unmeasured confounders in pharmacoepidemiologic claims data using external information: the example of COX2 inhibitors and myocardial infarction. Epidemiology. 2005;16:17–24. [DOI] [PubMed] [Google Scholar]
- 33. Grundy SM. Cardiovascular and metabolic risk factors: how can we improve outcomes in the high‐risk patient? Am J Med. 2007;120:S3–S8; discussion S9. [DOI] [PubMed] [Google Scholar]
- 34. Tofler GH, Muller JE, Stone PH, Willich SN, Davis VG, Poole WK, Braunwald E. Factors leading to shorter survival after acute myocardial infarction in patients ages 65 to 75 years compared with younger patients. Am J Cardiol. 1988;62:860–867. [DOI] [PubMed] [Google Scholar]
- 35. Nichols GA, Koro CE, Gullion CM, Ephross SA, Brown JB. The incidence of congestive heart failure associated with antidiabetic therapies. Diabetes Metab Res Rev. 2005;21:51–57. [DOI] [PubMed] [Google Scholar]
- 36. Tenenbaum A, Motro M, Fisman EZ, Leor J, Freimark D, Boyko V, Mandelzweig L, Adler Y, Sherer Y, Behar S. Functional class in patients with heart failure is associated with the development of diabetes. Am J Med. 2003;114:271–275. [DOI] [PubMed] [Google Scholar]
- 37. Ingelsson E, Sundstrom J, Arnlov J, Zethelius B, Lind L. Insulin resistance and risk of congestive heart failure. JAMA. 2005;294:334–341. [DOI] [PubMed] [Google Scholar]
- 38. Khan SS, Butler J, Gheorghiade M. Management of comorbid diabetes mellitus and worsening heart failure. JAMA. 2014;311:2379–2380. [DOI] [PubMed] [Google Scholar]
- 39. Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, Kravitz BG, Lachin JM, O'Neill MC, Zinman B, Viberti G. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427–2443. [DOI] [PubMed] [Google Scholar]
- 40. Bennett WL, Maruthur NM, Singh S, Segal JB, Wilson LM, Chatterjee R, Marinopoulos SS, Puhan MA, Ranasinghe P, Block L, Nicholson WK, Hutfless S, Bass EB, Bolen S. Comparative effectiveness and safety of medications for type 2 diabetes: an update including new drugs and 2‐drug combinations. Ann Intern Med. 2011;154:602–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Udell JA, Cavender MA, Bhatt DL, Chatterjee S, Farkouh ME, Scirica BM. Glucose‐lowering drugs or strategies and cardiovascular outcomes in patients with or at risk for type 2 diabetes: a meta‐analysis of randomised controlled trials. Lancet Diabetes Endocrinol. 2015;3:356–366. [DOI] [PubMed] [Google Scholar]
- 42. Costanzo P, Cleland JG, Pellicori P, Clark AL, Hepburn D, Kilpatrick ES, Perrone‐Filardi P, Zhang J, Atkin SL. The obesity paradox in type 2 diabetes mellitus: relationship of body mass index to prognosis: a cohort study. Ann Intern Med. 2015;162:610–618. [DOI] [PubMed] [Google Scholar]
- 43. Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, Kannel WB, Vasan RS. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–313. [DOI] [PubMed] [Google Scholar]
- 44. Tzoulaki I, Molokhia M, Curcin V, Little MP, Millett CJ, Ng A, Hughes RI, Khunti K, Wilkins MR, Majeed A, Elliott P. Risk of cardiovascular disease and all cause mortality among patients with type 2 diabetes prescribed oral antidiabetes drugs: retrospective cohort study using UK general practice research database. BMJ. 2009;339:b4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Effect of intensive blood‐glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:854–865. [PubMed] [Google Scholar]
- 46. FDA Drug Safety Communication: FDA revises warnings regarding use of the diabetes medicine metformin in certain patients with reduced kidney function. 2016. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm493244.htm. Accessed April 8, 2017.
- 47. Desai NR, Shrank WH, Fischer MA, Avorn J, Liberman JN, Schneeweiss S, Pakes J, Brennan TA, Choudhry NK. Patterns of medication initiation in newly diagnosed diabetes mellitus: quality and cost implications. Am J Med. 2012;125:302.e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Definitions of Comorbid Conditions and Medications on the Basis of Codes and Prescriptions in 730 Days Before Treatment Intensification
Table S2. Details for the Construction of the Propensity Score Model
Table S3. Characteristics of Patients Who Did Not Match in 1 to 1 Propensity Score Matching
Table S4. Description and Characteristics of the Weighted Analysis Cohort
Table S5. Sensitivity Analyses Evaluating the Hazard of Heart Failure in First 180 Days of Use of Sulfonylurea vs Metformin and Thiazolidinedione vs Metformin Using New‐User Design and Inverse Probability Treatment Weighted Analysis
Table S6. Analysis of Sensitivity to Unmeasured Confounding
Figure S1. Study design schematic.
Figure S2. Distribution of propensity scores by drug.
Figure S3. Mean standardized difference plot comparing metformin vs sulfonylurea.


