Abstract
Background
Elderly patients undergoing transcatheter aortic valve replacement (TAVR) are at risk of hospital readmission postprocedure. It is not known whether the index hospital length of stay and, specifically, early discharge post‐TAVR is associated with an increased risk of readmission. We hypothesized a nonlinear relationship whereby both short and long lengths of stay were associated with increased readmission risk.
Methods and Results
We performed a retrospective multicenter cohort analysis of patients undergoing elective transfemoral TAVR and surviving to discharge between January 2007 and March 2014. The exposure variable was hospital length of stay measured from the procedure date to the date of discharge and modeled as a continuous variable in a multivariable cause‐specific Cox regression. Main outcome measures were 30‐day and 1‐year all‐cause readmissions. The study population consisted of 709 patients with a median length of stay of 6 days (interquartile range, 4–8). At 30‐days and 1‐year, 13.5% and 44.0% of patients were readmitted, respectively. Although post‐TAVR length of stay was not associated with 30‐day all‐cause readmissions (P=0.925), there existed a significant association with 1‐year readmission (P=0.010) after adjustment for baseline clinical variables. The association between post‐TAVR length of stay and 1‐year readmission was linear (P=0.549 for nonlinearity) with no evidence supporting an increased readmission risk for shorter length of stays.
Conclusions
Among elderly survivors of elective transfemoral TAVR, a short postprocedural length of stay was not associated with an increased risk readmission within 30 days or 1 year. However, the risk of 1‐year readmission increased with longer post‐TAVR lengths of stay.
Keywords: hospitalization, length of stay, readmission, transcutaneous aortic valve implantation
Subject Categories: Valvular Heart Disease, Quality and Outcomes
Introduction
Readmission to hospital within 30 days after discharge is a proposed marker of hospital quality of care.1 Early readmission is both costly to hospital systems and detrimental to patients given the notable increased mortality after readmission across a wide spectrum of acute medical and surgical illnesses.2 Understanding the drivers of unplanned readmission are of paramount importance in order to develop strategies to mitigate this risk.
Hospital length of stay is a potentially modifiable factor for unplanned hospital readmission given that a longer length of stay may increase the risk of in‐hospital complications, such as acquired nosocomial infections3 and deconditioning,4 particularly in elderly patients with multiple comorbidities. Length of stay has gained increasing attention as healthcare systems move from global fixed budgets to bundled fixed payments for acute medical illnesses.5 However, this transition may promote financial constraints that inadvertently incentivize shorter length of stay so as to minimize costs.6 Recent reports in elderly patients with acute heart failure7 and ST segment elevation myocardial infarction8 demonstrate that both short and long length of stay are associated with increased early readmission risk and adverse outcomes, potentially attributed to inappropriate transitional care or residual or untreated medical illness at the time of early discharge. Thus, further understanding of the association between hospital length of stay and readmission is warranted, especially for high‐risk populations.
The incidence of severe and symptomatic aortic stenosis is rising as the population ages.9 Transcatheter aortic valve replacement (TAVR) is currently the preferred treatment over surgical aortic valve replacement in inoperable and high‐risk patients.10 Readmission post‐TAVR is common, costly, and associated with increased mortality risk.11 There are a paucity of data on the relationship between length of stay post‐TAVR and readmission. Shortening length of stay is attractive to healthcare systems given that it may potentially reduce adverse outcomes and TAVR‐related costs given that a substantial burden of the cost of TAVR is incurred after the index procedure.12 Accordingly, we conducted a retrospective multicenter cohort study of elective patients undergoing transfemoral TAVR in Ontario, Canada, in order to determine the relationship between length of stay and readmission risk. Specifically, we sought to determine whether the relationship was nonlinear and hypothesized that it was U‐shaped with an increased readmission risk with both short and long lengths of stay.
Methods
Research ethics board approval was obtained from Sunnybrook Health Sciences Centre (Toronto, Ontario, Canada). Given the data sources used for the analyses as outlined below, the need for patient consent was waived under Ontario's Personal Health Information Protection Act.
Study Population
We conducted a retrospective multicenter cohort study in Ontario, Canada. Ontario is Canada's largest province, with a population of ≈13.6 million, all of whom are provided universal medical coverage, which is publicly funded through a single third‐party payer, the Ministry of Health and Long Term Care (MOHLTC). All adult patients who underwent TAVR between January 1, 2007 and March 31, 2014 were identified using the Cardiac Care Network (CCN) of Ontario Cardiac Registry, which captures all TAVR referrals to any of the 10 tertiary cardiac centers within the province. The registry contains data on patient demographics and comorbidities, as well as periprocedural and intraoperative details. Over this time period, all TAVI procedures conducted in Ontario were under general anesthesia. We excluded patients who underwent nonelective TAVR, defined as having had an acute decompensation necessitating an urgent in‐patient procedure. Additional exclusions were patients who underwent nontransfemoral TAVR, died in hospital, were transferred to or from another acute care facility, and those under the age of 65 years, for whom information on drug coverage was not available (see below). Finally, we also excluded patients with outlier length of stays beyond the 99th percentile, which, in our study, corresponded to a length of stay longer than 28 days.
Data Sources
Clinical data from CCN Cardiac Registry were linked to administrative databases using unique encoded identifiers and analyzed at the Institute for Clinical Evaluative Sciences (ICES) to protect patient confidentiality. These databases included the Canadian Institute for Health Information Discharge Abstract Database (CIHI‐DAD),13 which contains information on all hospitalizations including information on discharges, transfers, and deaths. Using the CCN Cardiac Registry, CIHI‐DAD, the Ontario Diabetes Database14 (which contains information on incident and prevalent cases of diabetes mellitus), and Ontario Hypertension Database15 (which contains information on incident and prevalent cases of hypertension), we identified patient demographics as well as comorbidities within 3 years pre‐TAVR using the cardiac and noncardiac diagnosis codes presented in Tables S1 and S2. Frailty was ascertained by the presence of 1 of 12 clusters of frailty‐defining diagnoses defined by the Johns Hopkins Adjusted Clinical Groups predictive model.16 Further linkages were performed to the CIHI Same Day Surgery Database (CIHI‐SDS), and the Ontario Health Insurance Plan (OHIP) physician claims database, which, along with the CIHI‐DAD, were utilized to identify diagnostic, interventional, and surgical cardiac procedures occurring within the 10 years pre‐TAVR (Table S3).17, 18 Socioeconomic status was determined by using the median neighborhood income of the patient's place of residence at the time of referral in accord with their postal code. Preadmission and discharge prescription medication use within 90 days of hospitalization was ascertained by linkage to the Ontario Drug Benefit Database, which contains drug data on all patients above the age of 65 years, for whom full coverage is provided. In‐hospital complications post‐TAVR included acute kidney injury,19 bleeding classified as life‐threatening, disabling or major bleeding versus minor bleeding17 in accord with the Valve Academic Research Consortium‐2 (VARC‐2)20 criteria, and stroke of any subtype; these events were identified using the CIHI‐DAD21 (coded as an in‐hospital complication during the index TAVR admission), CIHI‐SDS, and OHIP databases (Table S4). Death was ascertained from linkage to the Registered Persons Database.
Primary Exposure
Our primary exposure variable was the length of stay during the index admission for elective transfemoral TAVR. We defined length of stay as the days elapsed between the date of TAVR and the date of discharge encoded within the CCN TAVR Registry. Patients undergoing elective TAVR are all admitted on either the day of the procedure or the day before, based on institutional practice. Because such practice was not clinically driven, we did not incorporate the preprocedural period into the length of stay metric given that our study focused on elective patients.
Outcomes
Primary outcomes of interest were all‐cause hospital readmissions occurring within 30 days and 1 year ascertained by linkage to the CIHI‐DAD. In sensitivity analysis (see below), readmissions were classified as cardiovascular and noncardiovascular based on the most responsible diagnosis code (Table S5).21 All outcomes were measured from the date of discharge.
Statistical Analysis
We modeled the effect of length of stay on the hazard of hospital readmission, treating mortality as a competing event.22, 23 To do so, we used marginal cause‐specific Cox proportional hazard models, with a robust (sandwich‐type) variance estimator in order to account for the clustering of patients within each of the 10 TAVR centers across the province. Length of stay was initially modeled as a continuous variable with restricted cubic splines with 3 knots at the 10th, 50th and 90th percentile (3, 6, and 13 days, respectively). A Wald test was utilized to test the null hypothesis that the relationship between length of stay and the hazard of readmission was linear. In the absence of evidence of nonlinearity, length of stay was modeled as having a linear relationship with the log‐hazard of the outcome.
Multivariable models were adjusted for candidate baseline and procedural variables, which were chosen based on clinical relevance. In order to avoid colinearity with our primary exposure variable, variance inflation factors were calculated, and candidate predictor variables were excluded from the multivariable model if the variance inflation factor was greater than 5. Models for the hazard of readmission within 30 days were adjusted for age, sex, frailty, left ventricular ejection fraction, peripheral vascular disease, cerebral vascular disease, chronic obstructive pulmonary disease, serum creatinine, recent heart failure hospitalization within 90 days, and calendar year of TAVR. Models for the hazard of readmission within 1 year were adjusted for these baseline variables as well as postdischarge warfarin use within 90 days. We conducted several sensitivity analyses. First, readmissions were classified as cardiovascular and noncardiovascular (Table S5),21 and each was modeled separately within 30 days and 1 year. Second, given the technological and clinical advances in TAVR over the period of our study, we built an additional model, incorporating an interaction term between period of TAVR implant and length of stay. We chose 2012 as the cutoff between the early and contemporary periods, because this was the year where TAVR received regulatory approval in Canada. Finally, we excluded patients who experienced a TAVR‐related complication (transfusion requirement, any bleeding, stroke, transient ischemic attack, need for permanent pacemaker, or acute kidney injury requiring dialysis) from the regression model. A 2‐sided P<0.05 was considered statistically significant. All analyses were performed using SAS software (version 9.3; SAS Institute, Inc, Cary, NC).
Results
Baseline Characteristics
We identified 709 patients over the age of 65 who underwent elective transfemoral TAVR and who survived their index hospitalization and were discharged home. Exclusions are shown in Figure 1. Baseline and procedural characteristics are presented in Table 1. Median age of our cohort was 84 years (interquartile range [IQR], 79–87) and 42% were female. A history of heart failure was the most prevalent cardiac comorbidity (89%). Within the cohort, 26% had a reduced left ventricular ejection fraction and 69% were moderately to severely symptomatic (New York Heart Association [NYHA], III–IV). A history of coronary artery bypass grafting was present in 34% of patients, 12% of patients had a preexisting permanent pacemaker, and 19.5% were classified as frail. The CoreValve was the most frequently implanted prosthesis (52%), followed by the Sapien or Sapien XT, which was implanted in 44% of patients. A comparison of baseline characteristics between survivors of elective transfemoral TAVR hospitalization with those who died in‐hospital postprocedure are available in Table S6. Baseline and procedural characteristics were similar across these 2 groups.
Figure 1.
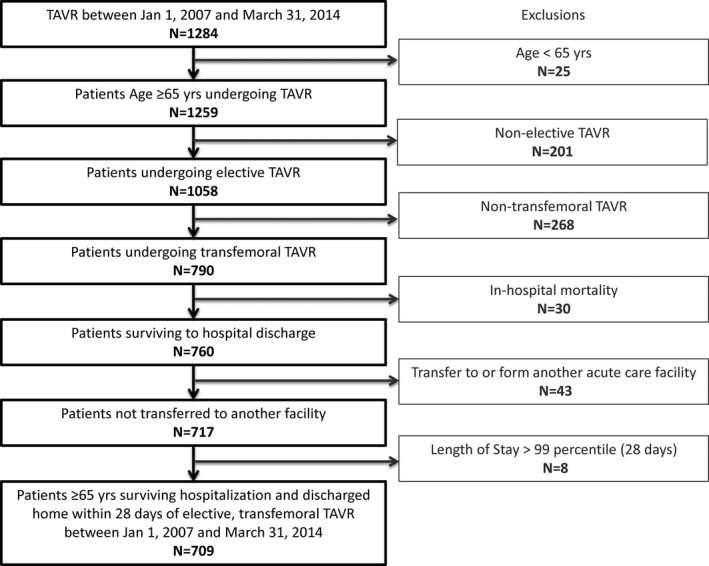
Flow diagram. TAVR indicates transcatheter aortic valve replacement.
Table 1.
Baseline and Procedural Characteristics
| Baseline and Procedural Characteristics | All Patients (N=709) |
|---|---|
| Demographic characteristics | |
| Age, median, y (IQR) | 84 (79–87) |
| Female, n (%) | 300 (42.3) |
| Socioeconomic status (%) | |
| 1st Quintile (lowest) | 124 (17.5) |
| 2nd Quintile | 135 (19.0) |
| 3rd Quintile | 138 (19.5) |
| 4th Quintile | 149 (21.0) |
| 5th Quintile (highest) | 159 (22.4) |
| Missing | SC |
| Cardiac history (%) | |
| Past myocardial infarction | 224 (31.6) |
| Ischemic heart disease | 514 (72.5) |
| History of heart failure | 632 (89.1) |
| Heart failure hospitalization within 90 days | 127 (17.9) |
| New York Heart Association Class | |
| I and II | 107 (15.1) |
| III and IV | 474 (66.8) |
| Missing | 128 (18.1) |
| Left ventricular ejection fraction | |
| ≤50% | 183 (25.8) |
| >50% | 514 (72.5) |
| Missing | 12 (1.7) |
| Past cardiac surgery, n (%) | |
| Coronary artery bypass grafting | 244 (34.4) |
| Aortic valve replacement | 65 (9.2) |
| Mitral valve replacement or repair | 16 (2.3) |
| Tricuspid valve replacement or repair | SC |
| History of percutaneous coronary intervention | 247 (34.8) |
| History of implantable cardiac defibrillator | 12 (1.7) |
| History of permanent pacemaker | 87 (12.3) |
| Atrial fibrillation/flutter | 228 (32.2) |
| Comorbid noncardiac conditions (%) | |
| Diabetes mellitus | 326 (46.0) |
| Hypertension | 678 (95.6) |
| Hyperlipidemia | 510 (71.9) |
| Peripheral vascular disease | 90 (12.7) |
| Cerebrovascular disease | 126 (17.8) |
| Chronic obstructive pulmonary disease | 104 (14.7) |
| History of cancer | 55 (7.8) |
| Cognitive impairment/dementia | 12 (1.7) |
| Dialysis | 23 (3.2) |
| Frailty | 138 (19.5) |
| Preprocedural blood work (%) | |
| Serum creatinine | |
| <120 μmol/L | 486 (68.5) |
| 120 to 200 μmol/L | 143 (20.2) |
| ≥200 μmol/L | 31 (4.4) |
| Missing | 49 (6.9) |
| Hemoglobin status | |
| Anemiaa | 448 (63.2) |
| Missing | 68 (9.6) |
| Preprocedural echocardiographic parameters | |
| Mean transvalvular gradient, mean (SD), mm Hgb | 46 (15) |
| Preprocedural risk score | |
| Society of Thoracic Surgeons score, mean (SD), %c | 13 (12) |
| Procedural characteristics (%) | |
| Year of transfemoral aortic valve replacement | |
| 2007 | 9 (1.3) |
| 2008 | 11 (1.6) |
| 2009 | 39 (5.5) |
| 2010 | 72 (10.2) |
| 2011 | 132 (18.6) |
| 2012 | 147 (20.7) |
| 2013 | 228 (32.2) |
| 2014 | 71 (10.0) |
| Prosthesis type | |
| Edwards Sapien | 312 (44.0) |
| Corevalve | 368 (51.9) |
| Missing | 24 (3.4) |
| Other | SC |
| Valve‐in‐valve | 33 (4.7) |
SC indicates small cell, in which patient numbers ≤5 and cannot be released because of privacy regulations; IQR, interquartile range.
Men <140 g/L and female <120 g/L.
n=90 (12.5%) missing.
n=458 (64.6%) missing.
Distribution of Post‐Transcatheter Aortic Valve Replacement Length of Stay and In‐Hospital Complications
The distribution of length of stay from date of TAVR to date of discharge is presented in Figure 2. Post‐TAVR length of stay was positively/right‐skewed. Median post‐TAVR length of stay was 6 days (IQR, 4–8) and the mean was 7.1 days (SD, 4.6). Five percent of patients were discharged by day 2 post‐TAVR whereas 18% were discharged by day 3. In contrast, 5% of patients had a length of stay greater than 17 days and 10% had a length of stay greater than 13 days.
Figure 2.
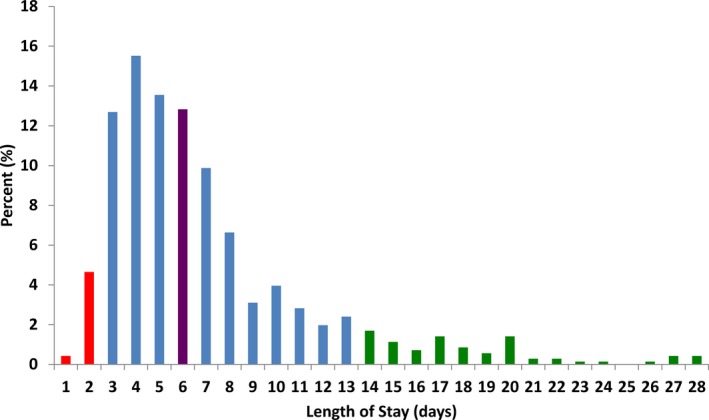
Distribution of length of stay post‐TAVR. The proportion of patients for each length of stay post‐TAVR is depicted. The lower 10th percentile is depicted in red and the upper 90th percentile in green. Median length of stay is depicted in purple. TAVR indicates transcatheter aortic valve replacement.
Twenty‐four percent of patients received a blood transfusion while in‐hospital, with 6% having a life‐threatening, disabling, or major bleeding and 5% having minor bleeding. Permanent pacemakers were implanted in 14% of patients during the postoperative period. The proportion of patients with a stroke or transient ischemic attack (1.4%) and acute kidney injury requiring dialysis (<1%) was low.
Readmission Outcomes and Length of Stay
The proportion of patients that were readmitted for any cause within 30‐days was 13.5%. Mortality within 30 days in this cohort of elective transfemoral TAVR patients who survived to hospital discharge was low, at <1%. Median 30‐day readmission length of stay was 5 days (IQR, 3–11), and the mean was 9.6 days (SD, 15.1). There was no statistically significant difference between mean (P=0.273) and median (P=0.166) post‐TAVR length of stay when comparing patients who were readmitted within 30 days to those who were not (Table 2). When patients were stratified above and below the median post‐TAVR hospital length of stay, the proportion of patients readmitted at 30 days was significantly higher (P=0.049) after a longer length of stay (≥6 days) when compared with a short post‐TAVR length of stay (<6 days). Although the mean and median readmission length of stay was longer in patients with a longer post‐TAVR length of stay, this did not reach statistical significance (P=0.151 for mean and P=0.239 for median; Table 3).
Table 2.
Post‐TAVR Length of Stay Stratified by Readmission Status
| Post‐TAVR Length of Stay (Days) | 30‐Day All‐Cause Readmission | 1‐Year All‐Cause Readmission | ||||
|---|---|---|---|---|---|---|
| Not Readmitted | Readmitted | P Value | Not Readmitted | Readmitted | P Value | |
| Mean (SD) | 7.0 (4.6) | 7.5 (4.8) | 0.273 | 6.5 (4.1) | 7.8 (5.2) | <0.001 |
| Median (IQR) | 6 (4–8) | 6 (4–10) | 0.166 | 5 (4–8) | 6 (4–10) | <0.001 |
IQR indicates interquartile range; TAVR, transcatheter aortic valve replacement.
Table 3.
Readmission Length of Stay Stratified by Short and Long Post‐TAVR Length of Stay
| Index TAVR Hospitalization | P Value | |||
|---|---|---|---|---|
| All Patients (N=709) | Length of Stay <6 Days (N=332) | Length of Stay ≥6 Days (N=377) | ||
| 30‐day all‐cause readmission | ||||
| Proportion readmitted, n (%) | 96 (13.5) | 36 (10.8) | 60 (15.9) | 0.049 |
| Readmission length of stay, days, median (IQR) | 5 (3–11) | 4 (3–8) | 6 (3–13) | 0.239 |
| Readmission length of stay, days, mean (SD) | 9.6 (15.1) | 6.8 (6.1) | 11.4 (18.4) | 0.151 |
| 1‐year all‐cause readmission | ||||
| Proportion readmitted, n (%) | 397 (44.0) | 126 (38.0) | 186 (49.3) | 0.002 |
| Readmission length of stay, days, median (IQR) | 5 (3–9) | 4 (3–7) | 6 (3–12) | <0.001 |
| Readmission length of stay, days, mean (SD) | 8.8 (13.9) | 5.6 (5.5) | 11.0 (17.1) | <0.001 |
IQR indicates interquartile range; TAVR, transcatheter aortic valve replacement.
The proportion of patients that were readmitted within 1 year was 44.0%, whereas 1‐year mortality was 10.6%. Median 1‐year readmission length of stay was 5 days (IQR, 3–9), and the mean was 8.8 days (SD, 13.9). Both mean and median post‐TAVR length of stay of patients who were readmitted at 1 year was significantly longer than mean and median post‐TAVR length of stay of patients that were not readmitted over the same time frame (both P<0.001; Table 2). When compared with a short post‐TAVR length of stay, more patients were readmitted by 1 year with significantly longer readmission mean and median length of stays after a longer initial post‐TAVR length of stay (P<0.001 for both; Table 3).
Association Between Post‐Transcatheter Aortic Valve Replacement Length of Stay and Readmissions
The association between post‐TAVR length of stay and the hazard of all‐cause readmissions is presented in Figure 3 (for 30‐day readmission) and Figure 4 (for 1‐year readmission). In each figure, we describe the relative increase in the hazard of readmission for a given length of stay compared with a patient with the median post‐TAVR length of stay of 6 days. In the unadjusted analysis, there was no significant association between post‐TAVR length of stay and the hazard of readmission within 30 days (P=0.125). However, there was a significant association between post‐TAVR length of stay and the hazard of readmission within 1 year (P<0.001), which was linear (P=0.110 for nonlinearity). Patients with the shortest post‐TAVR length of stay had the lowest readmission risk, and those with the longest post‐TAVR length of stay had the highest readmission risk. After adjustment for baseline covariates (Table S7), there remained no significant association between post‐TAVR length of stay and the hazard of readmission within 30 days (P=0.925). Furthermore, after multivariable adjustment, post‐TAVR length of stay demonstrated a significant association with the hazard of readmission within 1 year (P=0.010) that remained linear (P=0.549 for nonlinearity). There was a 3% increase in the adjusted hazard of readmission within 1 year (hazard ratio=1.03; 95% CI, 1.01–1.05; P=0.005) for every additional day spent in‐hospital post‐TAVR.
Figure 3.
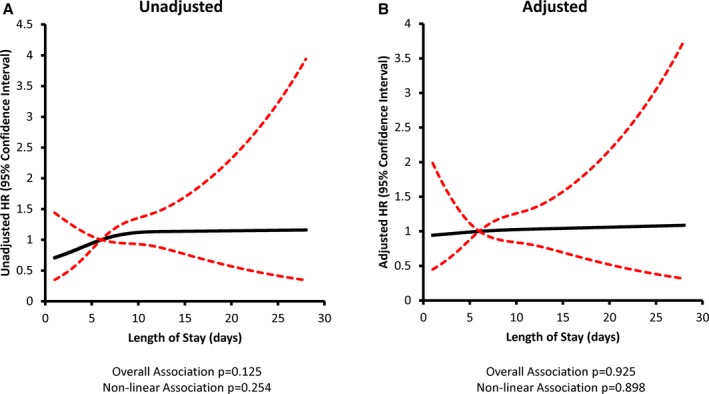
Post‐TAVR length of stay and 30‐day all‐cause readmission risk. (A) Unadjusted and (B) adjusted models are depicted with length of stay modeled as a restricted cubic spline with knots at 3, 6, and 13 days. All models accounted for correlation within hospitals. Risk adjustment included age, sex, frailty, left ventricular ejection fraction, peripheral vascular disease, cerebral vascular disease, chronic obstructive pulmonary disease, serum creatinine, recent heart failure hospitalization within 90 days, and year of TAVR. HR indicates hazard ratio; TAVR, transcatheter aortic valve replacement.
Figure 4.
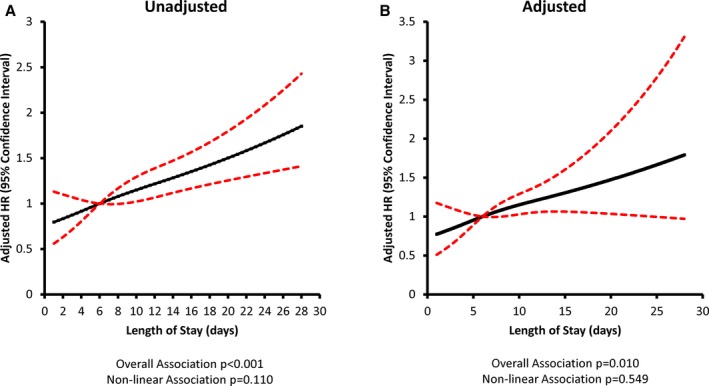
Post‐TAVR length of stay and 1‐year all‐cause readmission risk. (A) Unadjusted and (B) adjusted models are depicted with length of stay modeled as a restricted cubic spline with knots at 3, 6, and 13 days. All models accounted for correlation within hospitals. Risk adjustment included age, sex, frailty, left ventricular ejection fraction, peripheral vascular disease, cerebral vascular disease, chronic obstructive pulmonary disease, serum creatinine, recent heart failure hospitalization within 90 days, year of TAVR, and warfarin use within 90 days of discharge. HR indicates hazard ratio; TAVR, transcatheter aortic valve replacement.
Sensitivity Analysis
Within 30 days, 3.6% (n=26) of patients had a cardiovascular readmission and 10.5% (n=75) had a noncardiovascular readmission. Consistent with the primary outcome, there was no significant association between post‐TAVR length of stay and either cardiovascular (P=0.463 unadjusted and P=0.540 adjusted) or noncardiovascular readmission (P=0.204 unadjusted and P=0.766 adjusted) within 30 days. At 1 year, 16% (n=111) of patients had a cardiovascular readmission and 38% (n=273) had a noncardiovascular readmission. After multivariable adjustment, there was a linear association between post‐TAVR length of stay and the hazards of cardiovascular (P=0.036 for overall association and P=0.579 for nonlinearity) and noncardiovascular readmissions (P=0.009 for overall association and P=0.477 for nonlinearity) at 1 year. There was no evidence of a U‐shaped association for either outcome. As post‐TAVR length of stay increased, risk of cardiovascular and noncardiovascular readmission increased, whereas those with the shortest post‐TAVR length of stay had the lowest readmission risk. Thirty‐seven percent of the cohort underwent TAVR before 2012. There was no statistically significant interaction between period of TAVR (ie, pre‐ or post‐2012) and post‐TAVR length of stay for 30‐day (P=0.776 for interaction) and 1‐year (P=0.884 for interaction) readmission risk models. When 277 patients experiencing in‐hospital complications were excluded from the model, results remained similar. After adjustment, there was no association between post‐TAVR length of stay and 30‐day readmission risk (n=49/432 [11.3%] readmitted; P=0.829 for overall association). However, there was an association between post‐TAVR length of stay and 1‐year readmissions (n=179/432 [41.4%] readmitted; P=0.005 for overall association), which was linear (P=0.948 for nonlinearity).
Discussion
In this multicenter cohort study, we initially hypothesized that both a short and long hospital length of stay after elective transfemoral TAVR would be associated with an increased readmission risk. We found both 30‐day (13.5%) and 1‐year (44.0%) readmission rates were high. However, we did not detect a significant increase in 30‐day or 1‐year readmission risk for shorter post‐TAVR lengths of stay. In fact, post‐TAVR length of stay was not associated with 30‐day readmission even after multivariable adjustment and when cardiovascular and noncardiovascular readmissions were considered separately. In contrast, we found a linear association between post‐TAVR length of stay and 1‐year readmission risk. The 1‐year readmission risk was highest in patients with the longest post‐TAVR length of stay, and for each additional day in hospital after elective transfemoral TAVR, risk of all‐cause readmission increased by 3%. This association was consistent across both cardiovascular and noncardiovascular readmissions at 1 year.
In our cohort, post‐TAVR length of stay was not a marker for heightened 30‐day readmission risk, which is in contrast to other cardiac diseases, such as acute heart failure7 and myocardial infarction.8 This may be explained by our exclusion of nonelective patients. It is plausible that the preoperative clinical stability of the cohort selected for a lower‐risk group of patients who were not subjected to a high‐risk procedure amidst a systemic inflammatory state, and these patients had not accrued a significant number of days in hospital preoperatively. As a result, they may have been less likely to have (1) already incurred significant deconditioning preoperatively, (2) succumb to significant deconditioning postoperatively, or (3) been exposed to procedures and interventions that place them at risk of nosocomial infections. Alternatively, given the extensive and often prolonged preprocedural work‐up period, it maybe that there is greater attention to planning and establishing appropriate transitional care, such as homecare or rehabilitation in anticipation of the TAVR procedure. Indeed, such transition of care measures have been found to be effective methods of mitigating readmission risk.24, 25, 26 Further study of the impact of transitional care on readmission risk is warranted. In contrast, the association with increasing length of stay and heightened readmission risk at 1 year may have been a marker of the underlying complex cardiac disease and comorbidities common to high‐risk and inoperable patients with severe aortic stenosis, rather than a proxy for the index procedure and care delivered during the index hospitalization. Therefore, our data are in support of strategies directed toward shortening post‐TAVR length of stay.
Our findings are in keeping with 3 recently published single‐center studies. The first reported on a quality improvement initiative for 393 patients surviving hospitalization for transfemoral TAVR between 2012 and 2014 in Canada, of whom 150 were enrolled in a clinical pathway targeting discharge within 48 hours.27 This study found that the proportion of 30‐day readmission was not significantly different between the strategy targeting discharge within 48 hours and the standard discharge strategy. The second reported on 424 patients surviving transfemoral TAVR with the SAPIEN‐XT prosthesis between 2009 and 2013 in the United States.28 When compared to patients discharged greater than 72 hours post‐TAVR, early discharge within 72 hours was not associated with a significantly higher proportion of patients readmitted or dying within 30 days. Finally, a study from Italy reported on 465 patients surviving hospitalization for transfemoral TAVR between 2007 and 2014.29 After 2:1 propensity matching of patients discharged after 72 hours with patients discharge within 72 hours, the final cohort of 267 patients had a low 30‐day readmission rate (1.1%) without a significant difference in readmission or mortality between the 2 groups. Our multicenter, population‐level study complements the current evidence suggesting that early discharge may be safe, without an increase in readmission risk. Moreover, rather than predefining a specific cutoff for early discharge, we modeled the length of stay in its entirety. Regardless of whether 48 or 72 hours is considered, and after multivariable adjustment, we found that shorter length of stay is not associated with an increased readmission risk.
Our results have several important implications. There exists no consensus on the optimal length of stay in patients undergoing elective transfemoral TAVR, and this issue is not addressed in guideline statements.10 TAVR is a complex intervention targeted toward elderly patients with aortic stenosis, who, in addition to their inoperability or high‐risk surgical status, have multiple interacting medical comorbidities that may pose barriers to early discharge from hospital and predispose them to early readmission. Our results, however, reenforce that a shorter length of stay after elective TAVR may not be associated with harm and support initiatives directed toward shortening length of stay. Furthermore, TAVR remains costly, and despite the growing number of suitable candidates for intervention, it remains restricted to specialized tertiary care centers. Given that a substantial portion of the costs associated with TAVR are incurred during the time spent in‐hospital after the index procedure,12 shortening post‐TAVR length of stay holds promise in reducing costs and improving the efficiency of care without compromising quality of care, namely early readmission.1 Lastly, the high observed 30‐day (13.5%) and 1‐year (44.0%) readmission rates noted in elective cases highlights the need to develop strategies aimed at reducing readmission risk such as postdischarge transitional care and early physician follow‐up.25, 30
Our study should be interpreted in the context of several limitations that merit discussion. Since 2014, advances in technology, including reductions in catheter size, improvement in delivery systems, and increasing use of conscious sedation rather than general anesthesia, may limit the generalizability of our findings. However, the lack of association between a short length of stay post‐TAVR and readmission is still reassuring given that such advances are likely to promote earlier discharge, which appears safe and feasible. This study was not a randomized trial and therefore cannot inform on the optimal length of stay for patients undergoing elective transfemoral TAVR. It is possible that our study was underpowered to detect an association between length of stay and 30‐day readmissions. We excluded patients with an extremely prolonged length of stay greater than 28 days (ie, <99th percentile of the cohort); however, it is likely that these extreme outliers are noninformative to the general cohort of elective TAVR patients. Finally, despite adjustment for multiple covariates, we cannot rule out residual confounding that may have biased our results.
In conclusion, readmission rates after elective transfemoral TAVR remains high. However, there does not appear to be an increased risk of readmissions in patients with a short length of stay after elective transfemoral TAVR. Longer length of stay is associated with increased late readmission risk. These results support the safety of early discharge after elective transfemoral TAVR and highlight the need for prospective studies evaluating the optimal timing of discharge.
Sources of Funding
This study is funded by a Grant‐in‐Aid from the Heart and Stroke Foundation of Canada. Dr Wijeysundera had full access to all of the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis. This study was supported by the Institute for Clinical Evaluative Science (ICES), which is funded by an annual grant from the Ontario Ministry of Health and Long‐Term Care (MOHLTC). The opinions, results and conclusions reported in this article are those of the authors and are independent from the funding sources. No endorsement by ICES or the Ontario MOHLTC is intended or should be inferred. Parts of this material are based on data and information compiled and provided by the Canadian Institute for Health Information. However, the analyses, conclusions, opinions, and statements expressed herein are those of the authors, and not necessarily those of the Canadian Institute for Health Information. Dr Wijeysundera is supported by a Distinguished Clinical Scientist Award from the Heart and Stroke Foundation of Canada. Drs Ko and Lee are supported by a mid‐career personnel award from the Heart and Stroke Foundation, Ontario Provincial Office. Dr Lee is the Ted Rogers Chair in Heart Function Outcomes, a joint Hospital‐University Chair of the University Health Network and the University of Toronto. Dr Austin is supported, in part, by a Heart and Stroke Foundation (Ontario office) Career Investigator Award. Dr Czarnecki is supported by a Canadian Institute of Health Research Fellowship Award.
Disclosures
Dr Wijeysundera and Dr Wood received research funding from Edwards Lifesciences. Dr Wijeysundera received research funding from Medtronic Inc.
Supporting information
Table S1. Cardiac Disease Diagnosis Codes
Table S2. Noncardiac Disease Diagnosis Codes1
Table S3. List of Codes for Procedures2,3
Table S4. In Hospital Complications1,3,4
Table S5. Cause‐Specific Readmissions1
Table S6. Comparison Between Patients Surviving to Discharge and Dying In‐Hospital
Table S7. Effect Estimates of Baseline Predictor Variables Used in Multivariable Models
Acknowledgments
The authors acknowledge that the clinical registry data used in this publication are from CCN and its participating hospitals. CCN serves as an advisory body to the MOHLTC and is dedicated to improving the quality, efficiency, access, and equity of adult cardiovascular services in Ontario, Canada. CCN is funded by the MOHLTC.
(J Am Heart Assoc. 2017;6:e005460 DOI: 10.1161/JAHA.116.005460.)28438738
References
- 1. Ashton CM, Del Junco DJ, Souchek J, Wray NP, Mansyur CL. The association between the quality of inpatient care and early readmission: a meta‐analysis of the evidence. Med Care. 1997;35:1044–1059. [DOI] [PubMed] [Google Scholar]
- 2. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360:1418–1428. [DOI] [PubMed] [Google Scholar]
- 3. Hauck K, Zhao X. How dangerous is a day in hospital? A model of adverse events and length of stay for medical inpatients. Med Care. 2011;49:1068–1075. [DOI] [PubMed] [Google Scholar]
- 4. Wu HY, Sahadevan S, Ding YY. Factors associated with functional decline of hospitalised older persons following discharge from an acute geriatric unit. Ann Acad Med Singapore. 2006;35:17–23. [PubMed] [Google Scholar]
- 5. Mechanic R, Tompkins C. Lessons learned preparing for Medicare bundled payments. N Engl J Med. 2012;367:1873–1875. [DOI] [PubMed] [Google Scholar]
- 6. Ackerly DC, Grabowski DC. Post‐acute care reform—beyond the ACA. N Engl J Med. 2014;370:689–691. [DOI] [PubMed] [Google Scholar]
- 7. Sud M, Yu B, Austin PC, Wijeysundera HC, Ko DT, Cram P, Domanski P, Lee DS. Association between short and long length of stay with 30‐day readmission and mortality in hospitalized patients with heart failure. Eur Heart J. 2016;37(suppl):194–195. [DOI] [PubMed] [Google Scholar]
- 8. Swaminathan RV, Rao SV, McCoy LA, Kim LK, Minutello RM, Wong SC, Yang DC, Saha‐Chaudhuri P, Singh HS, Bergman G, Feldman DN. Hospital length of stay and clinical outcomes in older STEMI patients after primary PCI: a report from the National Cardiovascular Data Registry. J Am Coll Cardiol. 2015;65:1161–1171. [DOI] [PubMed] [Google Scholar]
- 9. Martinsson A, Li X, Andersson C, Nilsson J, Smith JG, Sundquist K. Temporal trends in the incidence and prognosis of aortic stenosis: a nationwide study of the Swedish population. Circulation. 2015;131:988–994. [DOI] [PubMed] [Google Scholar]
- 10. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP III, Guyton RA, O'Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM III, Thomas JD; Members AATF . 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:e521–e643. [DOI] [PubMed] [Google Scholar]
- 11. Nombela‐Franco L, del Trigo M, Morrison‐Polo G, Veiga G, Jimenez‐Quevedo P, Abdul‐Jawad Altisent O, Campelo‐Parada F, Biagioni C, Puri R, DeLarochelliere R, Dumont E, Doyle D, Paradis JM, Quiros A, Almeria C, Gonzalo N, Nunez‐Gil I, Salinas P, Mohammadi S, Escaned J, Fernandez‐Ortiz A, Macaya C, Rodes‐Cabau J. Incidence, causes, and predictors of early (</=30 days) and late unplanned hospital readmissions after transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2015;8:1748–1757. [DOI] [PubMed] [Google Scholar]
- 12. Wijeysundera HC, Li L, Braga V, Pazhaniappan N, Pardhan AM, Lian D, Leeksma A, Peterson B, Cohen EA, Forsey A, Kingsbury KJ. Drivers of healthcare costs associated with the episode of care for surgical aortic valve replacement versus transcatheter aortic valve implantation. Open Heart. 2016;3:e000468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Juurlink D, Preyra C, Croxford R, Chong A, Austin PC, Tu JV, Laupacis A. Canadian Institute for Health Information Discharge Abstract Database: A Validation Study. Toronto, Ontario: Institute for Clinical Evaluative Sciences; 2006. [Google Scholar]
- 14. Hux JE, Ivis F, Flintoft V, Bica A. Diabetes in Ontario: determination of prevalence and incidence using a validated administrative data algorithm. Diabetes Care. 2002;25:512–516. [DOI] [PubMed] [Google Scholar]
- 15. Tu K, Campbell NR, Chen ZL, Cauch‐Dudek KJ, McAlister FA. Accuracy of administrative databases in identifying patients with hypertension. Open Med. 2007;1:e18–e26. [PMC free article] [PubMed] [Google Scholar]
- 16. University JH . The Johns Hopkins Adjusted Clinical Groups technical reference guide, version 9. 2009.
- 17. Lee DS, Stukel TA, Austin PC, Alter DA, Schull MJ, You JJ, Chong A, Henry D, Tu JV. Improved outcomes with early collaborative care of ambulatory heart failure patients discharged from the emergency department. Circulation. 2010;122:1806–1814. [DOI] [PubMed] [Google Scholar]
- 18. Lee DS, Stitt A, Wang X, Yu JS, Gurevich Y, Kingsbury KJ, Austin PC, Tu JV. Administrative hospitalization database validation of cardiac procedure codes. Med Care. 2013;51:e22–e26. [DOI] [PubMed] [Google Scholar]
- 19. Wald R, Quinn RR, Luo J, Li P, Scales DC, Mamdani MM, Ray JG; University of Toronto Acute Kidney Injury Research G . Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA. 2009;302:1179–1185. [DOI] [PubMed] [Google Scholar]
- 20. Kappetein AP, Head SJ, Genereux P, Piazza N, van Mieghem NM, Blackstone EH, Brott TG, Cohen DJ, Cutlip DE, van Es GA, Hahn RT, Kirtane AJ, Krucoff MW, Kodali S, Mack MJ, Mehran R, Rodes‐Cabau J, Vranckx P, Webb JG, Windecker S, Serruys PW, Leon MB. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium‐2 consensus document. Eur Heart J. 2012;33:2403–2418. [DOI] [PubMed] [Google Scholar]
- 21. Tu JV, Chu A, Donovan LR, Ko DT, Booth GL, Tu K, Maclagan LC, Guo H, Austin PC, Hogg W, Kapral MK, Wijeysundera HC, Atzema CL, Gershon AS, Alter DA, Lee DS, Jackevicius CA, Bhatia RS, Udell JA, Rezai MR, Stukel TA. The cardiovascular health in ambulatory care research team (CANHEART): using big data to measure and improve cardiovascular health and healthcare services. Circ Cardiovasc Qual Outcomes. 2015;8:204–212. [DOI] [PubMed] [Google Scholar]
- 22. Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;133:601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee W, Wei LJ, Amato D. Cox‐Type Regression Analysis for Large Numbers of Small Groups of Correlated Failure Time Observations. Netherlands: Kluwer Academic; 1992. [Google Scholar]
- 24. Barnason S, Zimmerman L, Nieveen J, Schulz P, Young L. Patient recovery and transitions after hospitalization for acute cardiac events: an integrative review. J Cardiovasc Nurs. 2012;27:175–191. [DOI] [PubMed] [Google Scholar]
- 25. Frankenstein L, Frohlich H, Cleland JG. Multidisciplinary approach for patients hospitalized with heart failure. Rev Esp Cardiol (Engl Ed). 2015;68:885–891. [DOI] [PubMed] [Google Scholar]
- 26. Hall MH, Esposito RA, Pekmezaris R, Lesser M, Moravick D, Jahn L, Blenderman R, Akerman M, Nouryan CN, Hartman AR. Cardiac surgery nurse practitioner home visits prevent coronary artery bypass graft readmissions. Ann Thorac Surg. 2014;97:1488–1493; discussion 1493‐1485 [DOI] [PubMed] [Google Scholar]
- 27. Lauck SB, Wood DA, Baumbusch J, Kwon JY, Stub D, Achtem L, Blanke P, Boone RH, Cheung A, Dvir D, Gibson JA, Lee B, Leipsic J, Moss R, Perlman G, Polderman J, Ramanathan K, Ye J, Webb JG. Vancouver transcatheter aortic valve replacement clinical pathway: minimalist approach, standardized care, and discharge criteria to reduce length of stay. Circ Cardiovasc Qual Outcomes. 2016;9:312–321. [DOI] [PubMed] [Google Scholar]
- 28. Durand E, Eltchaninoff H, Canville A, Bouhzam N, Godin M, Tron C, Rodriguez C, Litzler PY, Bauer F, Cribier A. Feasibility and safety of early discharge after transfemoral transcatheter aortic valve implantation with the Edwards SAPIEN‐XT prosthesis. Am J Cardiol. 2015;115:1116–1122. [DOI] [PubMed] [Google Scholar]
- 29. Barbanti M, Capranzano P, Ohno Y, Attizzani GF, Gulino S, Imme S, Cannata S, Aruta P, Bottari V, Patane M, Tamburino C, Di Stefano D, Deste W, Giannazzo D, Gargiulo G, Caruso G, Sgroi C, Todaro D, di Simone E, Capodanno D, Tamburino C. Early discharge after transfemoral transcatheter aortic valve implantation. Heart. 2015;101:1485–1490. [DOI] [PubMed] [Google Scholar]
- 30. Scott IA. Preventing the rebound: improving care transition in hospital discharge processes. Aust Health Rev. 2010;34:445–451. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Cardiac Disease Diagnosis Codes
Table S2. Noncardiac Disease Diagnosis Codes1
Table S3. List of Codes for Procedures2,3
Table S4. In Hospital Complications1,3,4
Table S5. Cause‐Specific Readmissions1
Table S6. Comparison Between Patients Surviving to Discharge and Dying In‐Hospital
Table S7. Effect Estimates of Baseline Predictor Variables Used in Multivariable Models


