Abstract
Background
In peripartum cardiomyopathy, the prevalence of focal myocardial damage detected by late gadolinium enhancement (LGE) cardiovascular magnetic resonance is important to elucidate mechanisms of myocardial injury and cardiac dysfunction. LGE equates irreversible myocardial injury, but LGE prevalence in peripartum cardiomyopathy is uncertain.
Methods and Results
Among 100 women enrolled within the Investigations of Pregnancy Associated Cardiomyopathy cohort, we recruited 40 women at 13 centers to undergo LGE cardiovascular magnetic resonance, enrolled within the first 13 weeks postpartum. Follow‐up scans occurred at 6 months postpartum, and death/transplant rates at 12 months. Baseline characteristics did not differ significantly in the parent cohort according to cardiovascular magnetic resonance enrollment except for mechanical circulatory support. LGE was noted only in 2 women (5%) at baseline. While left ventricular dysfunction with enlargement was prevalent at baseline cardiovascular magnetic resonance scans (eg, ejection fraction 38% [Q1–Q3 31–50%], end diastolic volume index=108 mL/m2 [Q1–Q3 83–134 mL/m2]), most women demonstrated significant improvements at 6 months, consistent with a low prevalence of LGE. LGE was not related to baseline clinical variables, ejection fraction, New York Heart Association heart failure class, or mortality. Neither of the 2 women who died exhibited LGE. LGE was inversely associated with persistent left ventricular ejection fraction at 6 months (P=0.006).
Conclusions
Factors other than focal myocardial damage detectable by LGE explain the initial transient depressions in baseline left ventricular ejection fraction, yet focal myocardial damage may contribute to persistent myocardial dysfunction and hinder recovery in a small minority. Most women exhibit favorable changes in ventricular function over 6 months.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT01085955.
Keywords: cardiovascular magnetic resonance, heart failure, myocardial fibrosis, peripartum cardiomyopathy, pregnancy and postpartum
Subject Categories: Cardiomyopathy, Heart Failure
Introduction
In peripartum cardiomyopathy (PPCM), the prevalence of focal irreversible myocardial damage detected by late gadolinium enhancement (LGE) imaging cardiac magnetic resonance (CMR) is unclear. This knowledge is important to inform mechanisms of disease in PPCM, a clinical condition that manifests a broad spectrum of severity.1 The overall favorable prognosis of PPCM where most women recover systolic function and fare well2 suggests a low prevalence of LGE. Yet, a recent study by Haghikia and colleagues reported an unexpectedly high prevalence of LGE (71%)3 in PPCM, which greatly exceeds the ≈30% prevalence of LGE reported in large case series of dilated cardiomyopathy patients.4 The reported high prevalence of LGE in PPCM appears at odds with the clinical course of PPCM as well as prior reports from smaller PPCM case series.5, 6, 7 Indeed, despite the high prevalence of LGE, 59% of the women (16 of 27) in the Haghikia study still had full recovery of systolic function. LGE is clinically important.
While LGE can occur even when ejection fraction is preserved,8, 9, 10 LGE is generally associated with persistent myocardial dysfunction despite medical therapy,11, 12 arrhythmia,4, 13, 14 and in turn, hospitalization for heart failure, arrhythmia, and mortality.4, 8, 10, 14, 15 Regardless of etiology, LGE is prognostically adverse across the spectrum of left ventricular ejection fraction (LVEF), even when preserved.4, 8, 9, 10 LGE detects foci of irreversible myocardial damage manifest by enhancement, regardless of the mechanism or the timing of injury. The spatial distribution of LGE, its relation to myocardial mass, and the clinical context inform disease mechanisms. The volume of distribution for extracellular gadolinium contrast agents (Gd) increases in necrotic myocardium (where cell membranes lose integrity to exclude extracellular molecules), fibrotic myocardium (where collagen fibrils accumulate in the extracellular space), or even infiltrated myocardium (eg, extracellular amyloid protein). Gd accumulates in foci of irreversibly damaged myocardium not only during the initial insult characterized by necrosis (or apoptosis), but also throughout the transition to fibrosis.16, 17 Fibrosis may also occur from primary fibroblast activation and not solely from “replacement” of necrotic or apoptotic myocytes.18
To understand the prevalence of focal myocardial damage in PPCM, we conducted a prospective multicenter study within the Peripartum Cardiomyopathy Network. We enrolled 40 women with PPCM and acquired CMR examinations with LGE to characterize their myocardium.
Methods
Patients
The Investigations of Pregnancy Associated Cardiomyopathy (IPAC) cohort has been described previously.1 Briefly, among 100 women from 30 centers enrolled within the IPAC study, we recruited a subset of 40 women who provided written and oral informed consent after institutional review board approval to undergo CMR scans with Gd contrast to permit LGE imaging. This subset of women was enrolled within the first 13 weeks postpartum at 13 centers (Table S1) between December 2009 and September 2012. Inclusion criteria were as follows: age ≥18 years of age, lack of prior cardiac disease, estimated ejection fraction <45% by echocardiography at enrollment, and clinical evaluation consistent with idiopathic nonischemic cardiomyopathy. Exclusion criteria were as follows: significant valvular disease, coronary artery disease (>50% stenosis of a major epicardial vessel or a positive noninvasive study), evidence of ongoing bacterial septicemia (eg, positive blood cultures), ongoing substance abuse, history of chemotherapy or chest radiation with 5 years of enrollment, or a history of a previous cardiomyopathy. Demographic information (including self‐designated race), comorbidity, previous clinical evaluations, and current medical therapy were recorded at the time of enrollment.
CMR Scans
CMR scans with Gd were performed within 2 weeks of study entry. A sample protocol with parameters was provided to centers (Table S1). A repeat scan was performed at 6 months postpartum. Scans included cine images for volumetric assessment and LGE to identify focal myocardial damage.
After localizer images, breath held retrospectively ECG‐gated steady‐state free precession cine images were acquired over the entire left ventricle to permit direct measurement of left ventricular mass, volumes, and ejection fraction (sample parameters: 30 phases per cardiac cycle, field of view 360×320 cm, 256×144 matrix, bandwidth 700 Hz/pixel, slice thickness 6 mm, gap 4 mm, and TR/TE 4/1.8 ms). Additional 2‐, 3‐, and 4‐chamber views provided assessment of wall motion, systolic function, and valvular function. End‐systolic and end‐diastolic phases were identified and endocardial and epicardial borders were traced to compute volumes, ejection fraction, and left ventricular mass without geometric assumptions in the standard fashion. Volumes and mass were indexed to body surface area using the Mosteller formula. Previously published age‐dependent reference values defined left ventricular enlargement.19 LVEF <55% was considered abnormal. Right ventricular size and function were assessed qualitatively with “eyeball” estimation of normal, mild, moderate, or severe dysfunction/enlargement given values of 1, 2, 3, and 4, respectively. The tricuspid annular plane systolic excursion was quantified from the 4‐chamber cine. Myocardial edema on T2‐weighted imaging acquired before contrast administration was interpreted qualitatively with the intent of prioritizing specificity over sensitivity. Thresholding approaches were not pursued, given the lack of surface intensity correction and variability of scan parameters across sites.
To identify focal myocardial damage, LGE was performed 10 minutes after an intravenous dose of standard extracellular Gd contrast (0.2 mmol/kg) with agents possessing similar relaxivity of gadoteridol (Gd HP‐DO3A), ≈4 to 5 L/mmol per second. Use of plasma protein bound contrast agents (eg, gadobenate dimeglumine) was avoided because of their lower partition coefficient that diminishes dispersion into the interstitial space, a property that could obscure myocardial disease.20, 21 When available, use of phase‐sensitive inversion recovery was preferred since signal intensity reflects the extent of T1 recovery, varies minimally as a function of distance from the coils, and has greater signal‐to‐noise ratios compared to conventional inversion recovery magnitude images without phase‐sensitive reconstruction (which are not truly T1‐weighted and lack surface coil intensity correction).22 The inversion time was set to null normal myocardium (for magnitude LGE images) and manually increased to adjust for the clearance of Gd contrast; segmented k‐space lines were acquired every other heartbeat with a parallel imaging acceleration factor of 2. If available, free breathing single‐shot LGE images with steady‐state free precession readout were also acquired.
CMR scans were interpreted qualitatively in the core lab at the University of Pittsburgh/UPMC cardiovascular Magnetic Resonance Center blinded to all clinical information (by author EBS with over a decade of clinical CMR experience and research that involves LGE validation work,23 LGE population and cohort studies,8, 24 and emerging novel parametric mapping techniques25, 26), and 6‐month scans were interpreted in no particular order blinded to the baseline scan. Identification of foci of LGE required confirmation in orthogonal imaging planes. Formal assessment of interobserver and intraobserver variability was not pursued because of (1) the variation in pulse sequences and parameters across centers, which would limit generalizability; and (2) the low prevalences of LGE and T2‐weighted abnormalities.
Statistical Analysis
Continuous variables were summarized by their medians and interquartile ranges, where unpaired data were compared with the Wilcoxon‐Mann–Whitney test, and paired data were compared with the Wilcoxon signed‐rank sum test. Since the sample size was small, we made no assumptions about normal distributions. Categorical variables were summarized by frequencies and compared with the χ2 test or Fisher's test as appropriate. Differences in LGE prevalence at baseline and at 6 months were assessed using McNemar's test. Continuous variables (eg, LVEF) were modeled with linear regression, and dichotomous variables (eg, LGE) were modeled with logistic regression. Analyses were performed using SAS 9.3 (Cary, NC). There were few deaths, so the relationship between LGE and death was assessed qualitatively without formal hypothesis testing, focusing on the direction of the association (whether LGE was found in survivors or those who died) rather than the magnitude.
Results
Enrollment
Among participants in the parent study, baseline characteristics among those who enrolled to receive CMR scans (n=40) did not differ significantly compared to those who did not (n=60), with the exception of the need for mechanical support (Table 1). Patient characteristics did not vary according to receipt of any CMR scanning. For example, women who received the baseline CMR scan, follow‐up CMR scan, or both CMR scans did not different significantly from the parent cohort (data not shown). Also, among women who received at least 1 CMR scan, those who received the baseline scan or follow‐up scan did not differ from those who did not (data not shown). In the non‐CMR group, 2 women had intra‐aortic balloon pumps, and 1 woman had a ventricular assist device, whereas no patient had mechanical support in the CMR group. One individual could not complete the baseline CMR scan and withdrew from further CMR scanning, leaving a total of 39 patients who received CMR scans. Thirty‐four individuals received baseline scans, which occurred 31±24 days postpartum and 25 individuals received 6‐month scans 188±30 days postpartum, 3 of whom did not have a baseline scan. Thus, 22 women had paired baseline and 6‐month scans.
Table 1.
Patient Characteristics According to Whether Cardiovascular Magnetic Resonance (CMR) Scans Were Performed
| Variable | CMR Performed (N=40) | No CMR Performed (N=60) | P Value |
|---|---|---|---|
| Demographics | |||
| Age, y, median (Q1–Q3) | 31 (27–33) | 30 (24–35) | 0.92 |
| Hispanic, n (%) | 8 (20%) | 7 (12%) | 0.27 |
| Black, n (%) | 9 (23%) | 21 (35%) | 0.27 |
| Days elapsed to enroll after delivery, median (Q1–Q3) | 22 (10–44) | 24 (12–52) | 0.40 |
| Comorbidity and past medical history | |||
| Diabetes mellitus, n (%) | 3 (8%) | 8 (13%) | 0.51 |
| Hypertension, n (%) | 18 (45%) | 27 (45%) | 1.00 |
| Smoking, n (%) | 13 (33%) | 22 (37%) | 0.44 |
| Substance abuse, n (%) | 3 (8%) | 7 (12%) | 0.74 |
| Autoimmune disease, n (%) | 2 (5%) | 3 (5%) | 1.00 |
| Family history of dilated cardiomyopathy, n (%) | 2 (5%) | 8 (13%) | 0.31 |
| Gravida, n (%) | 2 (1–4%) | 2 (1–4%) | 0.92 |
| Para, n (%) | 2 (1–3) | 2 (1–3) | 0.89 |
| Cesarean delivery, n (%) | 18 (45%) | 32 (53%) | 0.41 |
| Multiple birth, n (%) | 7 (18%) | 12 (20%) | 0.75 |
| NYHA class distribution (I, II, III, IV), n (%) | 7, 18, 9, 6 (18%, 45%, 23%, 15%) | 5, 28, 16, 11 (8%, 47%, 27%, 18%) | 0.57 |
| Biometrics | |||
| BMI, median (Q1–Q3) | 29 (24–35) | 27 (24–33) | 0.41 |
| Height, in, median (Q1–Q3) | 65 (64–66) | 63 (61–66) | 0.05 |
| Weight, lbs, median (Q1–Q3) | 177 (132–216) | 149 (130–191) | 0.29 |
| Systolic blood pressure, mm Hg, median (Q1–Q3) | 109 (98–118) | 110 (100–128) | 0.38 |
| Diastolic blood pressure, mm Hg, median (Q1–Q3) | 71 (59–80) | 68 (62–79) | 0.77 |
| Heart rate, bpm, median (Q1–Q3) | 86 (75–100) | 86 (72–99) | 0.40 |
| Medications | |||
| Inotropes, n (%) | 4 (10%) | 11 (18%) | 0.25 |
| ACE inhibitors, n (%) | 32 (80%) | 40 (80%) | 1.00 |
| Angiotensin receptor blocker, n (%) | 1 (3%) | 0 (0%) | 0.40 |
| β‐Blocker, n (%) | 34 (85%) | 54 (90%) | 0.54 |
| Diuretic, n (%) | 27 (68%) | 43 (72%) | 0.66 |
| Digoxin, n (%) | 3 (8%) | 5 (8%) | 1.00 |
| Laboratories | |||
| Sodium, median (Q1–Q3) | 139 (137–141) | 139 (137–141) | 0.34 |
| BUN, median (Q1–Q3) | 13 (9–16) | 14 (10–20) | 0.23 |
| Cr, median (Q1–Q3) | 0.85 (0.70–1.00) | 0.80 (0.70–1.00) | 0.74 |
| Hematocrit, %, median (Q1–Q3) | 35 (31–38) | 35 (32–39) | 0.78 |
| White blood cells, median (Q1–Q3) | 7.8 (5.6–9.8) | 7.2 (6.0–9.0) | 0.65 |
ACE indicates angiotensin‐converting enzyme; BMI, body mass index; bpm, beats per minute; BUN, blood urea nitrogen; Cr, creatinine; NYHA, New York Heart Association.
Left Ventricular Parameters
Left ventricular dysfunction with low LVEF and enlargement of both end‐systolic and end‐diastolic volumes was commonly observed at baseline CMR scans, but most women demonstrated significant improvements at 6 months (Table 2 and Figure 1). At the time of the baseline scan, 9 of 34 women (26%) already had improvement in LV function with LVEF >50%. These women with preserved LVEF had their baseline scans on average 51±20 days postpartum. Twenty‐six of 34 women (76%) had LVEF <50% at baseline, whereas 5 of 25 women (20%) had ventricular ejection fraction <50% at 6 months. Measures of left ventricular mass, end‐diastolic volume, and end‐systolic volume also decreased over time (Figure 1, Table 2). The 2 women with the worst LVEF at 6 months exhibited alternate pathologies potentially distinct from other women with PPCM (Figure 1).
Table 2.
Cardiovascular Magnetic Resonance (CMR) Data for Those With Paired Studies (n=22)
| CMR Parameter | Baseline CMR Scan | 6‐Month CMR Scan | Difference | P Value |
|---|---|---|---|---|
| LV ejection fraction (%), median (Q1–Q3) | 41 (31–53) | 57 (51–60) | 11 (3–24) | <0.001 |
| LV end diastolic volume, mL, median (Q1–Q3) | 171 (137–259) | 145 (124–167) | −22 (−55 to 0) | <0.001 |
| LV end diastolic volume index, mL/m2, median (Q1–Q3) | 101 (81–129) | 84 (70–96) | −12 (−32 to 0) | <0.001 |
| LV end systolic volume, mL, median (Q1–Q3) | 101 (63–171) | 60 (52–74) | −25 (−74 to −7) | <0.001 |
| LV end systolic volume index, mL/m2, median (Q1–Q3) | 64 (36–92) | 34 (30–42) | −13 (−38 to −5) | <0.001 |
| LV mass, g (Q1–Q3) | 94 (80–112) | 79 (58–104) | −22 (−32 to −2) | 0.009 |
| LV mass index, g/m2 (Q1–Q3) | 56 (44–62) | 43 (34–55) | −12 (−18 to −1) | 0.009 |
| Prevalence of LGE, N (%) | 2 (9%) | 3 (14%) | 1 (5%) | 0.31a |
LGE indicates late gadolinium enhancement; LV, left ventricular.
For the 20 individuals with paired data; P value reflects McNemar's test.
Figure 1.
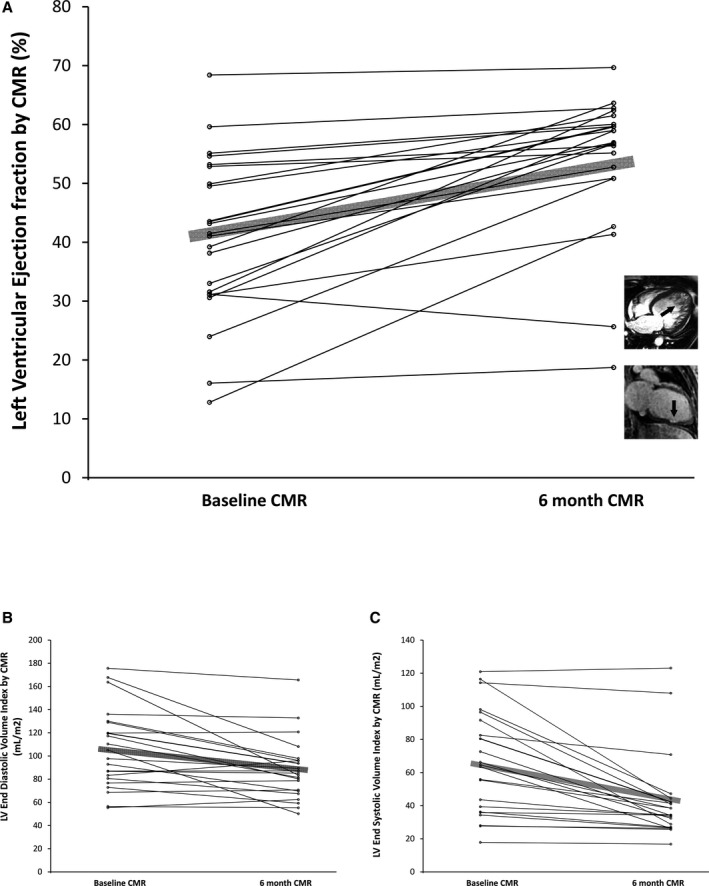
Left ventricular ejection fraction improved over time in most participants (A), resulting from decreased end diastolic volumes (B) and end systolic volumes (C) at 6 months compared to baseline. Since cardiovascular magnetic resonance (CMR) occurred 31±24 days postpartum, some women exhibited significant recovery by the time they were scanned. The 2 women with the worst ejection fraction at 6 months exhibited evidence of pathology distinct from the others (A, adjacent thumbnail insets). One woman exhibited marked trabeculations on cine images suggesting left ventricular noncompaction cardiomyopathy (arrow), and the other woman exhibited focal myocardial scar on late gadolinium enhancement (LGE) images indicating irreversible myocardial injury (arrow) also shown in Figure 2C. Alternate pathologies may exist given the nonspecific diagnostic criteria of peripartum cardiomyopathy.
Right Ventricular Parameters
Right ventricular systolic dysfunction was not prevalent, noted in 21% (7 of 34) at baseline and 8% (2 of 25) at 6 months when assessed qualitatively. Six women at baseline had tricuspid annular peak systolic excursion ≤1.5 cm at baseline, but only 3 women had tricuspid annular plane systolic excursion ≤1.5 cm at 6 months. Right ventricular ejection fraction related to left ventricular parameters. For example, qualitative “eyeball” estimation of right ventricular size correlated inversely with LVEF (P<0.01). Qualitative ratings of right ventricular function as well as tricuspid annular plane systolic excursion correlated positively with LVEF (P<0.01 and P=0.035, respectively).
Late Gadolinium Enhancement
Consistent with recovery of left ventricular systolic function observed in the volumetric data, irreversible myocardial damage detectable by LGE was not prevalent, being present on only 2 women at baseline and 3 women at 6 months (Figure 2) on blinded interpretations. One individual exhibited a pattern suggestive of myocarditis with midwall and epicardial LGE (Figure 2A). Another participant with suboptimal CMR data quality was equivocally read as negative for LGE at baseline because of uncertainty about LGE confirmation on orthogonal views, but subsequent retrospective review of all images indicated the presence of bona fide enhancement (Figure 2C). The prevalence of LGE was not significantly different across time for the 22 participants with paired images (P=0.31). The 6‐month LVEFs were 53%, 41%, and 19% for the participants with LGE in Figure 2A through 2C, respectively.
Figure 2.
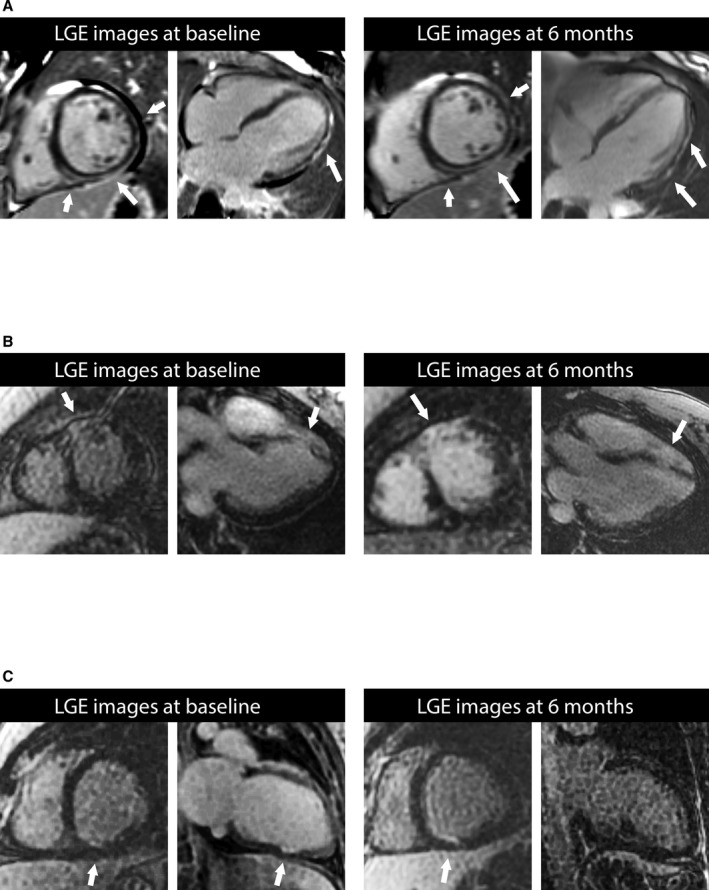
Examples of focal myocardial damage detected by late gadolinium enhancement (LGE, arrows) in 3 participants. In (C), the 2‐chamber orientation is rotated, rendering the LGE not clearly visible. None of these participants exhibited extensive myocardial damage, and none experienced adverse events. The LGE pattern in patient in (A) resembles the injury pattern in myocarditis. The 6‐month left ventricular ejection fractions were 53%, 41%, and 19% for the participants in (A through C), respectively. Coronary artery disease in the patients in (B and C) could not be excluded definitively. Given: (1) the low pretest probability of coronary disease in younger premenopausal women, and (2) the limited involvement in the long‐axis direction (ie, base to apex) that is unusual for coronary disease, we believed the probability of coronary artery disease was low. Still, we could not exclude vasospasm, embolization, cocaine, or recanalized myocardial infarction as potential etiologies.
LGE was not related to mortality or baseline clinical variables. No LGE was detected in the 2 individuals who subsequently died (Figure 3). No clinical variables were associated with LGE at baseline or 6 months. In logistic models, age, gravida, para, diabetes mellitus, hypertension, active smoking, history of illicit substance abuse, history of autoimmune disease, family history of cardiomyopathy, multiple births, and delivery method were not significantly related to LGE (P>0.25 for all).
Figure 3.
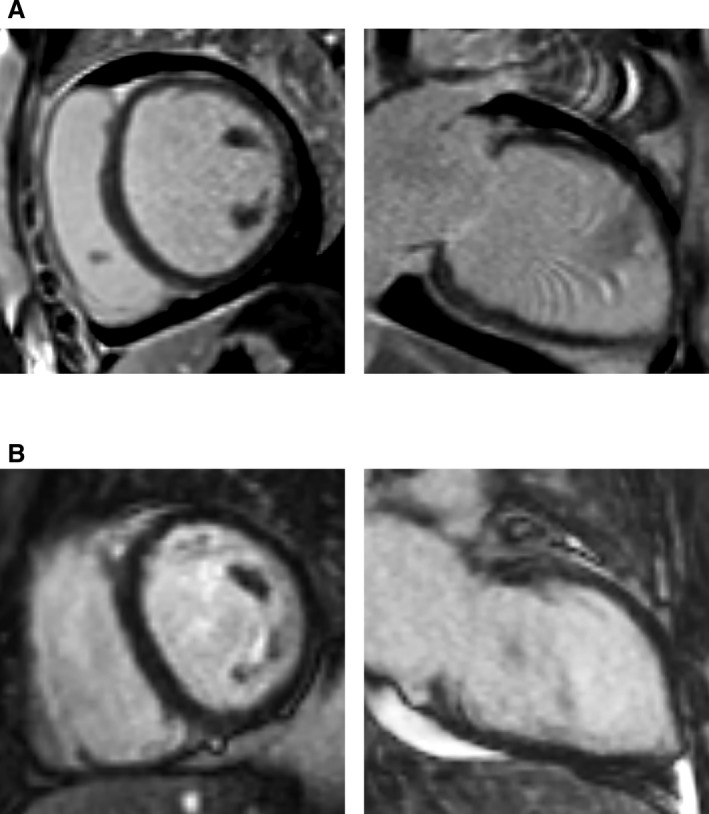
No late gadolinium enhancement (LGE) was detected for the 2 individuals who died (A and B). The woman in (A) had a measured left ventricular ejection fraction of 15%, and died after left ventricular assist device implantation and eventual orthotopic heart transplantation. The woman in (B) had a measured left ventricular ejection fraction of 17% and died prior to surgical interventions.
LGE was not related to baseline function or New York Heart Association heart failure class. Baseline LV ejection fraction was not associated with baseline LGE in univariable linear regression models (P=0.83). Yet, LGE did relate to a lack of functional recovery at 6 months. The presence of LGE at 6 months was inversely associated with LV ejection fraction at 6 months (P=0.006) in a linear regression model.
Other CMR Data
No atrial or ventricular thrombi were observed by LGE at any time point. No foci of regional myocardial edema were observed on T2‐weighted spin echo sequences for any of the CMR scans.
Discussion
In this multicenter prospective study employing CMR with LGE to characterize PPCM, we observed a low prevalence of LGE. Consistent with the absence of focal myocardial damage on LGE imaging, most patients exhibited significant improvements in left ventricular systolic function with favorable changes in left ventricular mass and volumes. Baseline ejection fraction was not associated with LGE, whereas 6‐month ejection fraction was associated with LGE in the minority who experienced persistent systolic dysfunction. Regardless of function, LGE did not appear to be related to baseline clinical variables in the few women who exhibited LGE. The 2 women who died did not exhibit LGE. Right ventricular systolic dysfunction was uncommon and less prevalent over time. No cardiac thrombi were observed by LGE imaging. No foci of myocardial edema were observed on T2‐weighted imaging.
These observations suggest that factors other than focal myocardial irreversible damage detectable by LGE explain the initial transient depressions in baseline LVEF seen in PPCM. Indeed, neither of the 2 women who died exhibited LGE in their baseline CMR scan, which further supports the concept that focal myocardial damage is not a major contributor to PPCM pathophysiology. In the small minority of women in whom LGE was encountered, focal myocardial damage may have contributed to persistent myocardial dysfunction and hindered recovery. We could not exclude that the few women with baseline LGE may have had other non‐PPCM concurrent disease processes (eg, idiopathic or familial dilated cardiomyopathy) that was simply exacerbated by pregnancy. We also could not exclude that some may have developed myocarditis in the peripartum period. Since the diagnostic criteria for PPCM are not entirely specific, this issue of diagnostic criteria for PPCM requires further study. Indeed, the 2 women with the worst LVEF at 6 months exhibited pathology potentially distinct from PPCM, suggesting that alternate pathology may exist given the nonspecific diagnostic criteria of PPCM, especially when follow‐up LVEF remains poor. As our understanding of PPCM advances with identification of causal factors, more specific criteria might emerge.
CMR has emerged as an important tool to identify the etiology of nonischemic cardiomyopathies,27 and predict risk of adverse outcomes based on the extent of LGE.4, 8, 10, 14, 15, 27 LGE can detect foci of myocardial damage as small as 0.7 g of myocardial tissue.28 In the absence of coronary artery disease associated with ischemic cardiomyopathy,29 LGE can likely detect myocardial damage associated with vasospasm, embolization, cocaine, or recanalized myocardial infarction.30
PPCM data remain significantly limited, and our results contrast with another multicenter PPCM study by Haghikia and colleagues.3 The origins of the discrepancy in LGE prevalence between our study and the study by Haghikia and colleagues remain uncertain. Yet, the low prevalence of LGE reported in our work is concordant with the observed improvement in systolic function shown by echocardiographic results from the parent study.1 Since LGE indicates irreversible focal myocardial damage, even when ejection fraction is preserved,8, 9, 10 the overall low prevalence of LGE in this study represents an optimistic finding. Indeed, the CMR literature consistently indicates that LGE is associated with persistent myocardial dysfunction despite medical therapy,11, 12 arrhythmia,4, 13, 14 and in turn, adverse clinical events such as hospitalization for heart failure, arrhythmia, and mortality.4, 8, 10, 14, 15 In the setting of severe heart failure from PPCM, the low prevalence of LGE might inform decision making when contemplating advanced heart failure therapies such as mechanical circulatory support as a bridge to recovery and heart transplantation.
Our work has limitations. Patients had a 13‐week window for the baseline CMR scan so the extent of initial disease might not be fully captured in this cohort. Given the multicenter multivendor setting, there was variable CMR expertise and limited availability of advanced pulse sequences such as parametric mapping.31, 32, 33 Advances in CMR quantification of myocardial tissue parameters such as T1 mapping and extracellular volume fraction measurement,25 which appear prognostically relevant in other disease states,26, 34, 35, 36 were not available at most centers. CMR services continue to evolve and expand but still vary across centers. Yet, this work represents the first prospective multicenter study employing LGE in the United States, and we believe these data represent a significant advance in understanding PPCM. Second, lower participant enrollment for the CMR portion of the study also diminished statistical power to detect associations. For example, we lacked the statistical power to examine the relationship between death and LGE since only 2 deaths occurred. Still, we believe the absence of LGE in women with PPCM who died represents an important hypothesis‐generating clinical observation. Third, CMR scanning was not always acquired in the immediate postpartum period, and so some women had already exhibited significant functional recovery on their baseline scan. Since participation in the IPAC study required an ejection fraction <45% by echocardiography to meet enrollment criteria, we believe these women had PPCM with early recovery.
Conclusion
Women with PPCM exhibit a very low prevalence of focal irreversible myocardial damage detectable by LGE. Accordingly, most patients exhibited significant improvements in their LVEF with favorable changes noted in left ventricular mass and volumes. Baseline ejection fraction was not associated with LGE, whereas 6‐month LVEF was associated with LGE in the minority who experienced persistent systolic dysfunction.
Sources of Funding
This work was funded by the National Heart, Lung, and Blood Institute 1RC1HL102429‐01.
Disclosures
Dr Schelbert has served on advisory boards for Bayer Healthcare and Merck for matters unrelated to peripartum cardiomyopathy and has received contrast material donated by Bracco Diagnostics for research purposes. The remaining authors have no disclosures to report.
Supporting information
Table S1. Pulse Sequence Parameters for Cardiac Magnetic Resonance Imaging
Appendix S1. IPAC Investigators
Acknowledgments
We thank the women who volunteered to participate in this work.
(J Am Heart Assoc. 2017;6:e005472. DOI: 10.1161/JAHA.117.005472.)
This manuscript was handled independently by Christopher M. Kramer, MD, as a guest editor.
References
- 1. McNamara DM, Elkayam U, Alharethi R, Damp J, Hsich E, Ewald G, Modi K, Alexis JD, Ramani GV, Semigran MJ, Haythe J, Markham DW, Marek J, Gorcsan J III, Wu WC, Lin Y, Halder I, Pisarcik J, Cooper LT, Fett JD. Clinical outcomes for peripartum cardiomyopathy in North America: results of the IPAC Study (Investigations of Pregnancy‐Associated Cardiomyopathy). J Am Coll Cardiol. 2015;66:905–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Elkayam U. Clinical characteristics of peripartum cardiomyopathy in the United States: diagnosis, prognosis, and management. J Am Coll Cardiol. 2011;58:659–670. [DOI] [PubMed] [Google Scholar]
- 3. Haghikia A, Rontgen P, Vogel‐Claussen J, Schwab J, Westenfeld R, Ehlermann P, Berliner D, Podewski E, Hilfiker‐Kleiner D, Bauersachs J. Prognostic implication of right ventricular involvement in peripartum cardiomyopathy: a cardiovascular magnetic resonance study. ESC Heart Fail. 2015;2:139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gulati A, Jabbour A, Ismail TF, Guha K, Khwaja J, Raza S, Morarji K, Brown TD, Ismail NA, Dweck MR, Di Pietro E, Roughton M, Wage R, Daryani Y, O'Hanlon R, Sheppard MN, Alpendurada F, Lyon AR, Cook SA, Cowie MR, Assomull RG, Pennell DJ, Prasad SK. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA. 2013;309:896–908. [DOI] [PubMed] [Google Scholar]
- 5. Mouquet F, Lions C, de Groote P, Bouabdallaoui N, Willoteaux S, Dagorn J, Deruelle P, Lamblin N, Bauters C, Beregi JP. Characterisation of peripartum cardiomyopathy by cardiac magnetic resonance imaging. Eur Radiol. 2008;18:2765–2769. [DOI] [PubMed] [Google Scholar]
- 6. Arora NP, Mohamad T, Mahajan N, Danrad R, Kottam A, Li T, Afonso LC. Cardiac magnetic resonance imaging in peripartum cardiomyopathy. Am J Med Sci. 2014;347:112–117. [DOI] [PubMed] [Google Scholar]
- 7. Renz DM, Rottgen R, Habedank D, Wagner M, Bottcher J, Pfeil A, Dietz R, Kivelitz D, Elgeti T. New insights into peripartum cardiomyopathy using cardiac magnetic resonance imaging. Rofo. 2011;183:834–841. [DOI] [PubMed] [Google Scholar]
- 8. Wong TC, Piehler KM, Zareba KM, Lin K, Phrampus A, Patel A, Moon JC, Ugander M, Valeti U, Holtz JE, Fu B, Chang C‐CH, Mathier M, Kellman P, Butler J, Gheorghiade M, Schelbert EB. Myocardial damage detected by late gadolinium enhancement cardiovascular magnetic resonance is associated with subsequent hospitalization for heart failure. J Am Heart Assoc. 2013;2:e000416 DOI: 10.1161/JAHA.113.000416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cheong BY, Muthupillai R, Wilson JM, Sung A, Huber S, Amin S, Elayda MA, Lee VV, Flamm SD. Prognostic significance of delayed‐enhancement magnetic resonance imaging: survival of 857 patients with and without left ventricular dysfunction. Circulation. 2009;120:2069–2076. [DOI] [PubMed] [Google Scholar]
- 10. Klem I, Shah DJ, White RD, Pennell DJ, van Rossum AC, Regenfus M, Sechtem U, Schvartzman PR, Hunold P, Croisille P, Parker M, Judd RM, Kim RJ. Prognostic value of routine cardiac magnetic resonance assessment of left ventricular ejection fraction and myocardial damage: an international, multicenter study. Circ Cardiovasc Imaging. 2011;4:610–619. [DOI] [PubMed] [Google Scholar]
- 11. Bello D, Shah DJ, Farah GM, Di Luzio S, Parker M, Johnson MR, Cotts WG, Klocke FJ, Bonow RO, Judd RM, Gheorghiade M, Kim RJ. Gadolinium cardiovascular magnetic resonance predicts reversible myocardial dysfunction and remodeling in patients with heart failure undergoing beta‐blocker therapy. Circulation. 2003;108:1945–1953. [DOI] [PubMed] [Google Scholar]
- 12. Masci PG, Doulaptsis C, Bertella E, Del Torto A, Symons R, Pontone G, Barison A, Droogne W, Andreini D, Lorenzoni V, Gripari P, Mushtaq S, Emdin M, Bogaert J, Lombardi M. Incremental prognostic value of myocardial fibrosis in patients with non‐ischemic cardiomyopathy without congestive heart failure. Circ Heart Fail. 2014;7:448–456. [DOI] [PubMed] [Google Scholar]
- 13. Gao P, Yee R, Gula L, Krahn AD, Skanes A, Leong‐Sit P, Klein GJ, Stirrat J, Fine N, Pallaveshi L, Wisenberg G, Thompson TR, Prato F, Drangova M, White JA. Prediction of arrhythmic events in ischemic and dilated cardiomyopathy patients referred for implantable cardiac defibrillator: evaluation of multiple scar quantification measures for late gadolinium enhancement magnetic resonance imaging. Circ Cardiovasc Imaging. 2012;5:448–456. [DOI] [PubMed] [Google Scholar]
- 14. Klem I, Weinsaft JW, Bahnson TD, Hegland D, Kim HW, Hayes B, Parker MA, Judd RM, Kim RJ. Assessment of myocardial scarring improves risk stratification in patients evaluated for cardiac defibrillator implantation. J Am Coll Cardiol. 2012;60:408–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Almehmadi F, Joncas SX, Nevis I, Zahrani M, Bokhari M, Stirrat J, Fine NM, Yee R, White JA. Prevalence of myocardial fibrosis patterns in patients with systolic dysfunction: prognostic significance for the prediction of sudden cardiac arrest or appropriate implantable cardiac defibrillator therapy. Circ Cardiovasc Imaging. 2014;7:593–600. [DOI] [PubMed] [Google Scholar]
- 16. Kim RJ, Fieno DS, Parrish TB, Harris K, Chen EL, Simonetti O, Bundy J, Finn JP, Klocke FJ, Judd RM. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation. 1999;100:1992–2002. [DOI] [PubMed] [Google Scholar]
- 17. Kim RJ, Albert TS, Wible JH, Elliott MD, Allen JC, Lee JC, Parker M, Napoli A, Judd RM. Performance of delayed‐enhancement magnetic resonance imaging with gadoversetamide contrast for the detection and assessment of myocardial infarction: an international, multicenter, double‐blinded, randomized trial. Circulation. 2008;117:629–637. [DOI] [PubMed] [Google Scholar]
- 18. Schelbert EB, Fonarow GC, Bonow RO, Butler J, Gheorghiade M. Therapeutic targets in heart failure: refocusing on the myocardial interstitium. J Am Coll Cardiol. 2014;63:2188–2198. [DOI] [PubMed] [Google Scholar]
- 19. Maceira AM, Prasad SK, Khan M, Pennell DJ. Normalized left ventricular systolic and diastolic function by steady state free precession cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2006;8:417–426. [DOI] [PubMed] [Google Scholar]
- 20. Fontana M, Treibel TA, Martinez‐Naharro A, Rosmini S, Kwong RY, Gillmore JD, Hawkins PN, Moon JC. A case report in cardiovascular magnetic resonance: the contrast agent matters in amyloid. BMC Med Imaging. 2017;17:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kawel N, Nacif M, Santini F, Liu S, Bremerich J, Arai AE, Bluemke DA. Partition coefficients for gadolinium chelates in the normal myocardium: comparison of gadopentetate dimeglumine and gadobenate dimeglumine. J Magn Reson Imaging. 2012;36:733–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kellman P, Arai AE, McVeigh ER, Aletras AH. Phase‐sensitive inversion recovery for detecting myocardial infarction using gadolinium‐delayed hyperenhancement. Magn Reson Med. 2002;47:372–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schelbert EB, Hsu LY, Anderson SA, Mohanty BD, Karim SM, Kellman P, Aletras AH, Arai AE. Late gadolinium‐enhancement cardiac magnetic resonance identifies postinfarction myocardial fibrosis and the border zone at the near cellular level in ex vivo rat heart. Circ Cardiovasc Imaging. 2010;3:743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schelbert EB, Cao JJ, Sigurdsson S, Aspelund T, Kellman P, Aletras AH, Dyke CK, Thorgeirsson G, Eiriksdottir G, Launer LJ, Gudnason V, Harris TB, Arai AE. Prevalence and prognosis of unrecognized myocardial infarction determined by cardiac magnetic resonance in older adults. JAMA. 2012;308:890–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moon JC, Messroghli DR, Kellman P, Piechnik SK, Robson MD, Ugander M, Gatehouse PD, Arai AE, Friedrich MG, Neubauer S, Schulz‐Menger J, Schelbert EB. Myocardial T1 mapping and extracellular volume quantification: a Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement. J Cardiovasc Magn Reson. 2013;15:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schelbert EB, Piehler KM, Zareba KM, Moon JC, Ugander M, Messroghli DR, Valeti US, Chang CH, Shroff SG, Diez J, Miller CA, Schmitt M, Kellman P, Butler J, Gheorghiade M, Wong TC. Myocardial fibrosis quantified by extracellular volume is associated with subsequent hospitalization for heart failure, death, or both across the spectrum of ejection fraction and heart failure stage. J Am Heart Assoc. 2015;4:e002613 DOI: 10.1161/JAHA.115.002613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gonzalez JA, Kramer CM. Role of imaging techniques for diagnosis, prognosis and management of heart failure patients: cardiac magnetic resonance. Curr Heart Fail Rep. 2015;12:276–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ricciardi MJ, Wu E, Davidson CJ, Choi KM, Klocke FJ, Bonow RO, Judd RM, Kim RJ. Visualization of discrete microinfarction after percutaneous coronary intervention associated with mild creatine kinase‐MB elevation. Circulation. 2001;103:2780–2783. [DOI] [PubMed] [Google Scholar]
- 29. Felker GM, Shaw LK, O'Connor CM. A standardized definition of ischemic cardiomyopathy for use in clinical research. J Am Coll Cardiol. 2002;39:210–218. [DOI] [PubMed] [Google Scholar]
- 30. McCrohon JA, Moon JC, Prasad SK, McKenna WJ, Lorenz CH, Coats AJ, Pennell DJ. Differentiation of heart failure related to dilated cardiomyopathy and coronary artery disease using gadolinium‐enhanced cardiovascular magnetic resonance. Circulation. 2003;108:54–59. [DOI] [PubMed] [Google Scholar]
- 31. Piehler KM, Wong TC, Puntil KS, Zareba KM, Lin K, Harris DM, Deible CR, Lacomis JM, Czeyda‐Pommersheim F, Cook SC, Kellman P, Schelbert EB. Free‐breathing, motion‐corrected late gadolinium enhancement is robust and extends risk stratification to vulnerable patients. Circ Cardiovasc Imaging. 2013;6:423–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Giri S, Chung YC, Merchant A, Mihai G, Rajagopalan S, Raman SV, Simonetti OP. T2 quantification for improved detection of myocardial edema. J Cardiovasc Magn Reson. 2009;11:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xue H, Greiser A, Zuehlsdorff S, Jolly MP, Guehring J, Arai AE, Kellman P. Phase‐sensitive inversion recovery for myocardial T1 mapping with motion correction and parametric fitting. Magn Reson Med. 2013;69:1408–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wong TC, Piehler K, Meier CG, Testa SM, Klock AM, Aneizi AA, Shakesprere J, Kellman P, Shroff SG, Schwartzman DS, Mulukutla SR, Simon MA, Schelbert EB. Association between extracellular matrix expansion quantified by cardiovascular magnetic resonance and short‐term mortality. Circulation. 2012;126:1206–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wong TC, Piehler K, Kang IA, Kadakkal A, Kellman P, Schwartzman DS, Mulukutla SR, Simon MA, Shroff SG, Kuller LH, Schelbert EB. Myocardial extracellular volume fraction quantified by cardiovascular magnetic resonance is increased in diabetes and associated with mortality and incident heart failure admission. Eur Heart J. 2014;35:657–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Banypersad SM, Fontana M, Maestrini V, Sado DM, Captur G, Petrie A, Piechnik SK, Whelan CJ, Herrey AS, Gillmore JD, Lachmann HJ, Wechalekar AD, Hawkins PN, Moon JC. T1 mapping and survival in systemic light‐chain amyloidosis. Eur Heart J. 2014;36:244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Pulse Sequence Parameters for Cardiac Magnetic Resonance Imaging
Appendix S1. IPAC Investigators


