Abstract
Background
Growth differentiation factor‐15 (GDF‐15) is related to major bleeding when measured at initial presentation in patients with acute coronary syndromes (ACSs) treated with dual antiplatelet therapy. It is unknown whether follow‐up measurements provide additional information. The objective of this study was to investigate whether GDF‐15 measured 1 month after an ACS provides additional information beyond the baseline levels with regard to the risk of major bleeding.
Methods and Results
GDF‐15 was measured at baseline and at 1 month after an ACS in 4049 patients included in the PLATelet inhibition and patient Outcomes (PLATO) trial. The association between 1‐month GDF‐15 level and non–coronary artery bypass grafting surgery‐related major bleeding was assessed by a multivariable Cox model, adjusting for baseline GDF‐15, age, anemia, impaired renal function, history of gastrointestinal bleeding, and sex. Elevated GDF‐15 (>1800 ng/L) at 1 month was associated with an increased risk of non‐coronary artery bypass grafting‐related major bleeding (3.9% versus 1.2%; hazard ratio, 3.38; 95% CI, 1.89–6.06), independent of baseline GDF‐15. Patients who had elevated GDF‐15 levels at baseline and subsequent nonelevated GDF‐15 at 1 month had a similar risk as patients who had nonelevated levels at both measurements.
Conclusions
GDF‐15 at 1 month after an ACS is related to the risk of bleeding during DAPT and provides additional information on the bleeding risk beyond baseline GDF‐15 levels. GDF‐15 levels may therefore be useful as part of decision support concerning long‐term antithrombotic treatment in patients post‐ACS.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT00391872.
Keywords: biomarker, bleeding, ischemic heart disease
Subject Categories: Biomarkers
Introduction
Antithrombotic therapy is a cornerstone in the management of patients with acute coronary syndrome (ACS). Intense platelet inhibition in the acute phase and for 1 year after percutaneous coronary intervention (PCI) is associated with decreased rates of ischemic events, but at the cost of higher rates of bleeding.1, 2, 3 Recent studies have shown further reduction in ischemic events by dual antiplatelet therapy (DAPT) beyond 1 year after an ACS and PCI.4, 5, 6 Patients with ACS are, however, heterogeneous regarding the risks for both ischemic and bleeding events. The most recent European Society of Cardiology non‐ST‐elevation ACS guidelines state that more‐intense and prolonged P2Y12 inhibition may be considered, taking into account the individual ischemic and bleeding risks.7 However, how these risks should be assessed and balanced during DAPT has not been specified so far.
In order to facilitate the estimation of bleeding risk, we have been searching for circulating biomarkers associated with bleeding during antithrombotic treatment. Recently, we identified that plasma level of growth differentiation factor‐15 (GDF‐15), a member of the transforming growth factor‐beta superfamily, was independently associated with, and contributed to, estimation of the risk of major bleeding events in ACS on DAPT when measured at the time of initial presentation.8 The association between the GDF‐15 level and risk of major bleeding has also been verified in patients with atrial fibrillation receiving different types of oral anticoagulant therapy.9
In the present study, we hypothesized that a repeated measurement of GDF‐15 level 1 month after an ACS might provide additional information with regard to risk of major bleeding beyond the GDF‐15 level at the index event.
Methods
Study Population
The PLATelet inhibition and patient Outcomes (PLATO) trial was a randomized, double‐blind trial comparing ticagrelor with clopidogrel on background aspirin treatment in patients with ACS, with 6 to 12 months of follow‐up. It adhered to the Declaration of Helsinki and was approved by ethical review boards. All patients gave their written informed consent to participate. Ticagrelor treatment was associated with reductions in ischemic events, including mortality, as compared with clopidogrel. The design and overall results have been previously published.1, 10 Eligibility criteria of the trial are found in Table S1.
A biomarker substudy was also part of the study program that aimed to include all patients at randomization (16 876 of the 18 624 randomized patients were included). In addition, repeated blood sampling in a subset of around 4000 patients also at 1 month after randomization was prespecified in the biomarker substudy. In the present study, we included patients who had samples available for GDF‐15 both at randomization and at 1 month (n=4049). See Figure S1 for a flow chart of the patient selection. The first and last authors had full access to the data.
Growth Differentiation Factor‐15
Samples were obtained by direct venipuncture at randomization and at 1 month. Plasma was frozen in aliquots and stored at −70°C until central analysis at the Uppsala Clinical Research Center laboratory. Levels of GDF‐15 in plasma were determined with Elecsys electrochemiluminescence immunoassay on a Cobas Immunoanalyzer system (Roche Diagnostics, Rotkreuz, Switzerland). The analytical details of the assay have been published previously.11 Based on previous studies, elevated level of GDF‐15 was defined as >1800 ng/L.11, 12, 13
Outcome
For this study, the end point of interest was major bleeding not related to coronary artery bypass grafting (CABG) surgery. The PLATO definition of non‐CABG‐related major bleeding included both life‐threatening bleeding (fatal, intracranial, or intrapericardial bleeding; hypovolemic shock or severe hypotension attributed to bleeding and requiring vasopressors or surgery; and decline in hemoglobin of >50 g/L) and other major bleeding (bleeding leading to significant disability; decline in hemoglobin of 30–50 g/L). As a secondary end point in this study, we also assessed the composite of cardiovascular death/myocardial infarction (MI)/stroke.
End points were assessed from the landmark set at the 1‐month follow‐up visit, that is, outcomes between randomization and 1 month were not considered.
All events were adjudicated by an independent central adjudication committee.
Statistical Analysis
Patient characteristics are presented as medians and interquartile range (IQR) for continuous variables and as percentage and number for categorical variables.
Log2‐transformed levels of GDF‐15 at baseline and 1 month are presented as box plots, and the individual relative change in GDF‐15 from baseline to 1 month is presented as a waterfall plot (ie, an ordered bar plot, where each bar represents an individual patient's relative change in biomarker level from baseline to 1 month).
To explore the relationship between GDF‐15 levels at baseline and at 1 month, we fitted Cox models with GDF‐15 level entered as restricted cubic splines (unadjusted). The predicted event rates up until 330 days after baseline, and 300 days after the 1‐month visit, are plotted in relation to GDF‐15 concentration at each time point. The x‐axis was truncated at the 1st percentile of baseline GDF‐15 at the lower end and at the 99th percentile at the higher end.
GDF‐15 levels (log2 transformed) at 1 month were modeled in an ordinary least squares model, including the following covariates: log2‐transformed baseline GDF‐15, age, sex, smoking status, diabetes mellitus, ACS management strategy (invasive or noninvasive), hypertension, heart failure, baseline hemoglobin, baseline estimated glomerular filtration rate (eGFR), history of gastrointestinal bleeding, and history of peripheral arterial disease, where continuous covariates were entered as restricted cubic splines to account for nonlinearities. Each covariate's contribution to the model was calculated as the partial Chi2 statistic minus the covariate's degrees of freedom.
Kaplan–Meier estimated event rates for non‐CABG‐related major bleeding are plotted from a 1‐month landmark up to 300 days according to GDF‐15 elevation status at 1 month. Similar Kaplan–Meier curves from 1 month according to GDF‐15 at 1 month stratified by baseline GDF‐15 elevation status are presented as well.
Risk of non‐CABG‐related major bleeding in relation to GDF‐15 at 1 month was also assessed in a Cox model adjusted for baseline GDF‐15 elevation (>1800 ng/L), age (<75 years versus ≥75 years), impaired renal function (eGFR <50 mL/min per 1.73 m2), history of gastrointestinal bleeding, and sex, stratified on presence of anemia at baseline (defined as baseline hemoglobin <130 g/L in men, <120 g/L in women). Results are presented as estimated hazard ratio (HR) and 95% CIs. We assessed the model's discriminatory performance using Harrell's C index and compared it with the C index of a model where 1‐month GDF‐15 was excluded.
The statistical software R (version 3.3.2; R Foundation for Statistical Computing, Vienna, Austria) was used for all analyses.
Results
Clinical Characteristics
Clinical characteristics at randomization are presented in Table. A majority of patients were of male sex, and risk factors such as habitual smoking, hypertension, and diabetes mellitus were highly prevalent. Approximately one fifth had a history of MI and 12% a history of previous PCI. When adjusting these rates to include events occurring after randomization, including PCI during the initial hospitalization, 89% had suffered an MI and 74% had a previous PCI procedure.
Table 1.
Clinical Characteristics
| Characteristic | Total Study Population | Patients With Non‐CABG‐Related Major Bleeding From Baseline During Follow‐up | Patients Without Non‐CABG‐Related Major Bleeding From Baseline During Follow‐up | |||
|---|---|---|---|---|---|---|
| (n=4049) | (n=149) | (n=3900) | ||||
| Age, median (IQR) | 61 | (54–70) | 68 | (60–74) | 61 | (53–70) |
| Male sex | 70% | (2849) | 59% | (88) | 71% | (2761) |
| Randomized treatment: Ticagrelor | 50% | (2018) | 52% | (77) | 50% | (1954) |
| Habitual smoker | 37% | (1509) | 32% | (48) | 37% | (1461) |
| Hypertension | 65% | (2650) | 68% | (102) | 65% | (2548) |
| Diabetes mellitus | 22% | (883) | 26% | (39) | 22% | (844) |
| Previous heart failure | 5% | (219) | 7% | (11) | 5% | (208) |
| Peripheral arterial disease | 6% | (251) | 9% | (13) | 6% | (238) |
| Chronic kidney disease | 8% | (312/3908) | 17% | (25) | 8% | (287) |
| MI before index event | 20% | (794) | 19% | (28) | 20% | (766) |
| Previous MI/Index event=MI | 89% | (3611) | 87% | (129) | 89% | (3482) |
| PCI before index event | 12% | (472) | 14% | (21) | 12% | (451) |
| Previous PCI/in‐hospital PCI | 74% | (2981) | 83% | (124) | 73% | (2857) |
| Baseline hemoglobin (g/L), median (IQR) | 142 | (132–151) | 136 | (124–145) | 142 | (132–151) |
| Baseline eGFR (mL/min per 1.73 m2), median (IQR) | 84 | (67–101) | 73.7 | (55.5–94.3) | 84.4 | (67.3–101.4) |
CABG indicates coronary artery bypass graft surgery; eGFR, estimated glomerular filtration rate; IQR, interquartile range; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Growth Differentiation Factor‐15 Levels at Baseline and 1 Month
Median GDF‐15 level at baseline was 1509 ng/L (IQR, 1127–2106) and at 1 month 1381 ng/L (IQR, 1036–1927). GDF‐15 levels were similar in ticagrelor‐ and clopidogrel‐treated patients both at baseline and at 1 month (data not shown). In Figure 1A, log2‐transformed GDF‐15 levels at baseline and 1 month are presented, and, in Figure 1B, the individual relative change in GDF‐15 is shown. Even though GDF‐15 levels were marginally lower at 1 month at the population level, there were patients with substantial relative increases in GDF‐15 and, likewise, patients with substantial relative decreases.
Figure 1.
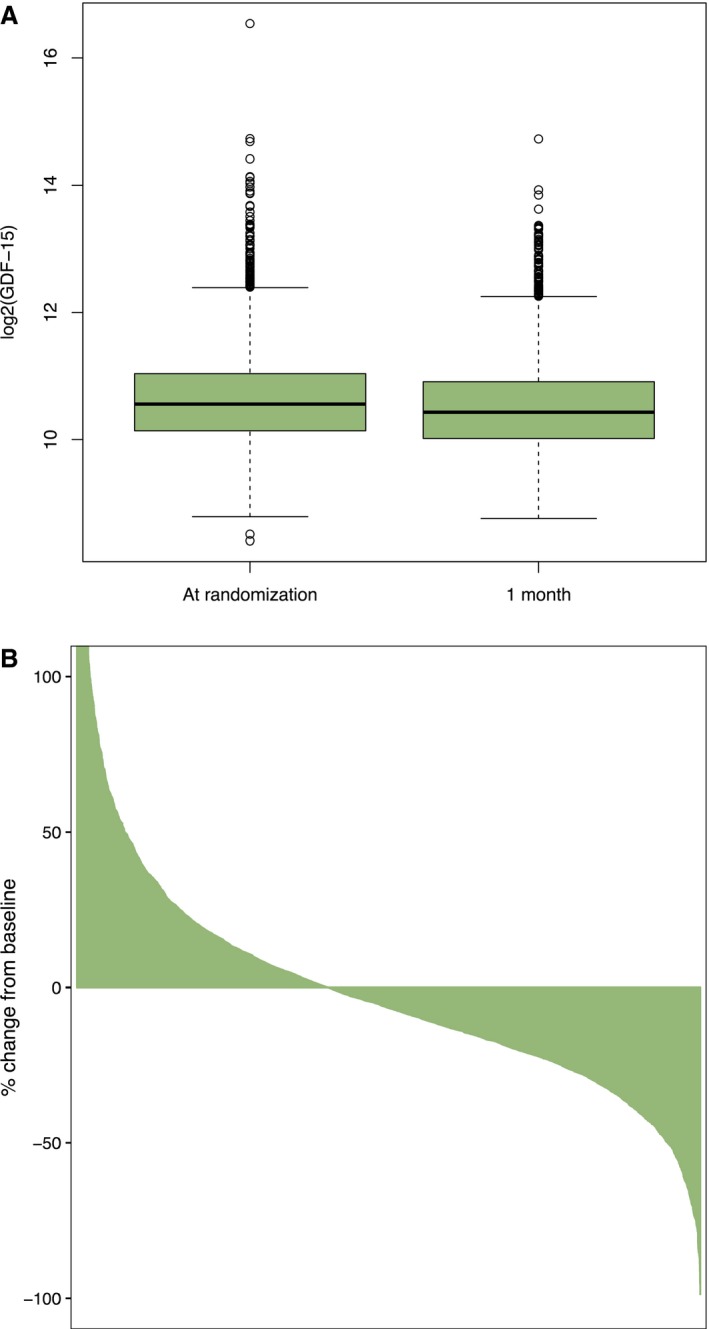
GDF‐15 at baseline and at 1 month. A, Log2‐transformed GDF‐15 levels. B, Waterfall plot indicating relative change in GDF‐15 levels between baseline and 1 month (please note that data are truncated at ∼+100%; a few patients had >100% relative increase in GDF‐15). The plot is an ordered bar plot, where each patient's % change in GDF‐15 from baseline to 1 month is plotted. Although GDF‐15 levels were only slightly lower on the population level (A), some patients had substantial relative changes in GDF‐15 (B). GDF‐15 indicates growth differentiation factor‐15.
When modeling 1‐month GDF‐15 levels with an ordinary least squares model, the most important predictors were: baseline GDF‐15 levels, age, diabetes mellitus, and eGFR (Figure S2), and this model explained 59% of the variance in 1‐month GDF‐15.
Major Bleeding in Relation to Growth Differentiation Factor‐15 Levels
From 1 month onward, there were 71 events of non‐CABG‐related major bleeding. Increased GDF‐15 was associated with a higher probability of non‐CABG‐related major bleeding during follow‐up, both when assessed from baseline and from the 1‐month follow‐up visit (Figure 2). The spline plot also indicated that the selected 1800 ng/L cutoff could separate patients at high risk of bleeding from those with low risk, given that the slope of the curves tended to increase at around that point.
Figure 2.
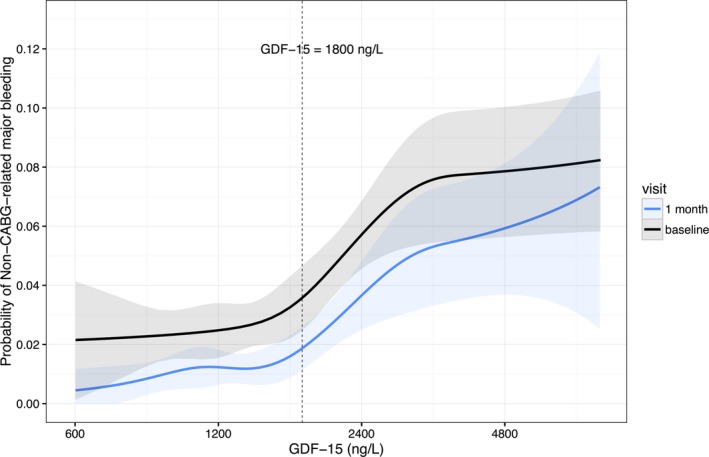
Probability of non‐CABG‐related major bleeding in relation to GDF‐15 assessed from baseline (black) and from the 1‐month follow‐up visit (blue). The dashed line indicates a GDF‐15 level of 1800 ng/L, which was used as a cutoff to define GDF‐15 elevation in this study. CABG indicates coronary artery bypass grafting. CABG indicates coronary artery bypass grafting; GDF‐15 indicates growth differentiation factor‐15.
In patients with GDF‐15 levels above 1800 ng/L at 1 month, 3.9% experienced non‐CABG‐related major bleeding from 1 month up to 300 days thereafter, as compared with 1.2% in patients with nonelevated GDF‐15 at 1 month (Figure 3). In the multivariable analysis, elevated GDF‐15 at 1 month was associated with a 3‐fold increased risk of non‐CABG‐related major bleeding (HR, 3.38; 95% CI, 1.89–6.06). The C index was 0.67, compared with 0.63 in a model excluding 1‐month GDF‐15. There was also an increased incidence of the composite end point cardiovascular death/MI/stroke in patients with elevated GDF‐15 (9.2% in those with GDF‐15 >1800 ng/L at 1 month, compared with 4.7% for patients with nonelevated GDF‐15).
Figure 3.
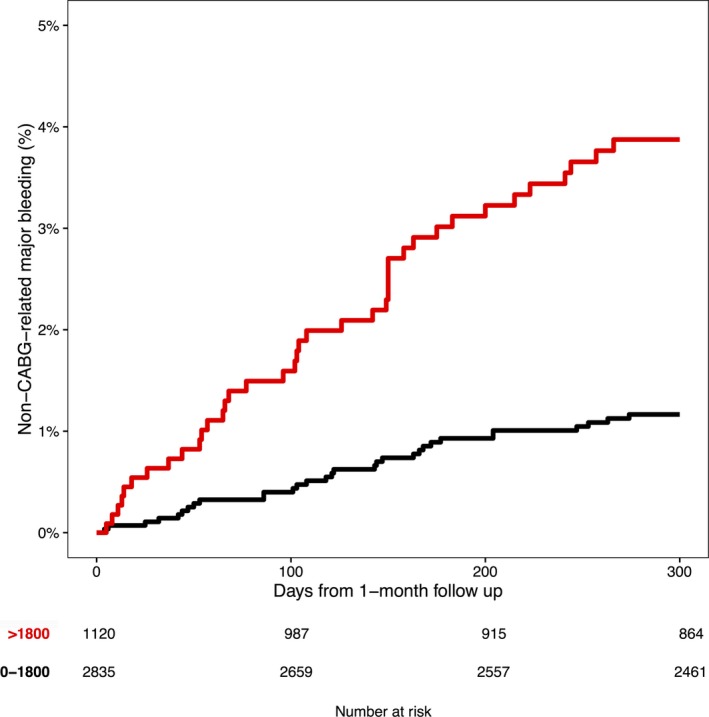
Kaplan‐Meier estimates of non‐CABG‐related major bleeding from the 1‐month follow‐up visit according to 1‐month GDF‐15 levels (ng/L). CABG indicates coronary artery bypass grafting.
Patients who had nonelevated baseline GDF‐15 levels and subsequent elevation of GDF‐15 at 1 month were at increased risk for non‐CABG‐related major bleeding, compared with patients who had nonelevated values at both measurements. In those who had elevated baseline GDF‐15 and subsequent nonelevated GDF‐15, the risk of non‐CABG‐related major bleeding from 1 month onward was similar as in patients who had nonelevated levels at both measurements (Figure 4).
Figure 4.
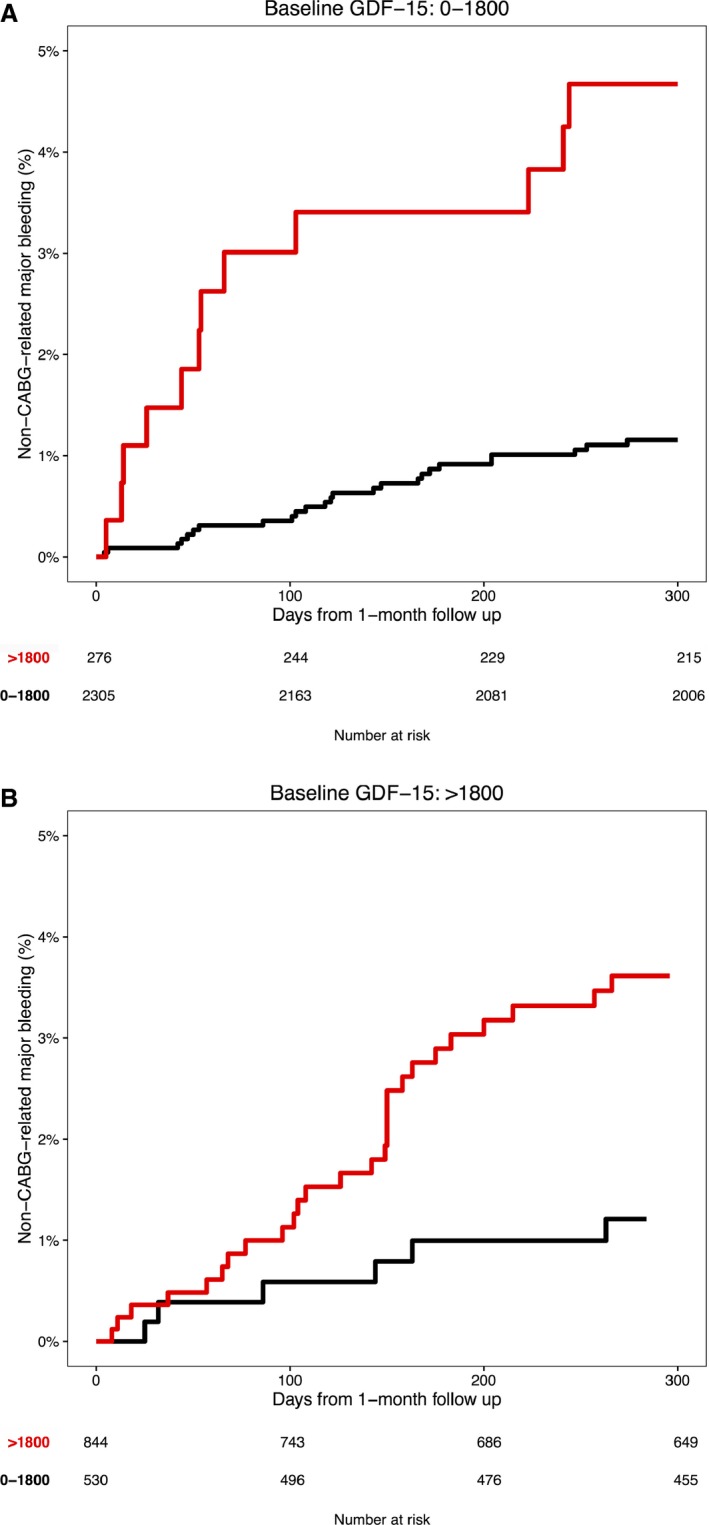
Kaplan–Meier estimates of non‐CABG‐related major bleeding from the 1‐month follow‐up visit according to 1‐month GDF‐15 levels in (A) patients with nonelevated (≤1800 ng/L) baseline GDF‐15 levels and (B) patients with elevated (>1800 ng/L) baseline GDF‐15 levels. CABG indicates coronary artery bypass grafting.
Discussion
The main finding in this study was that the GDF‐15 level at 1 month post‐ACS provided additional information regarding risk of non‐CABG‐related major bleeding beyond that of GDF‐15 levels obtained in the acute phase. When adjusting for baseline characteristics, including baseline GDF‐15 level, an elevated level of GDF‐15 at 1 month was associated with a 3‐fold increased risk of non‐CABG‐related major bleeding during DAPT after an ACS.
Previous studies have demonstrated an association between GDF‐15 level and risk of bleeding both in patients with ACS treated with DAPT and in those with atrial fibrillation treated with oral anticoagulation, when measuring GDF‐15 once at the initial presentation.8, 9 The present findings validate the level of GDF‐15 as a useful indicator of risk of bleeding beyond the acute setting, and indicate that GDF‐15 might be a useful marker for monitoring bleeding risk in patients with coronary artery disease on antithrombotic treatment.
GDF‐15 is, however, also a biomarker associated with other cardiovascular outcomes. It is independently related to the composite of recurrent ischemic events and mortality in ACS,8, 12, 14 as well as in stable coronary artery disease.15 GDF‐15 is also associated with increased risk of the composite of MI and death in the general population.16, 17, 18 Furthermore, it is associated with both myocardial16 and renal dysfunction.19 Therefore, information on the GDF‐15 level should preferably be used in multivariable clinical prediction models as one of several risk indicators when balancing the risk of bleeding and risk of ischemic events in patients on antithrombotic treatments.
The possible mechanisms underlying the independent association between GDF‐15 and cardiovascular events have been proposed to be related to its association with inflammation,20 oxidative stress,21 endothelial and myocardial dysfunction,16 atherosclerosis, and aging.22 In response to tissue injury like MI, there is a further elevation of GDF‐15 that might be related to further activation of inflammatory activity and myocardial stress.23, 24 With regard to the more recently discovered bleeding association, the mechanism might still be related to frailty and cellular aging.24 However, GDF‐15 knockout mice show accelerated thrombus formation compared with wild type, and, in vitro, GDF‐15 has been shown to inhibit platelet integrin activation,25 which provides a possible pathophysiological link between the observed association between GDF‐15 levels and bleeding in patients treated with antithrombotic and/or anticoagulant therapies.
Currently, there is intense discussion on the duration and intensity of DAPT and/or the combination of platelet inhibition with oral anticoagulation in patients with coronary artery disease. In this situation, there is often a delicate balance between ischemic risk and bleeding risk that has to be taken into account, as expressed in recent guidelines.7 In a substudy of the DAPT trial, a risk score to guide these decisions was proposed, although this used age as the only indicator of bleeding risk without providing any estimate of the discriminatory ability of the final model.26 In the present study, the bleeding signal captured by GDF‐15 levels provided incremental information beyond age and other clinical factors, both before start of treatment in the acute phase and when repeated during DAPT treatment in the chronic phase.
Limitations
There are several limitations to this work. First, patients with biomarkers at 1 month were a subset of all patients in the biomarker substudy of PLATO. Second, with a landmark analysis at 1 month, the risk of time‐dependent confounding is acknowledged. Third, there were relatively few bleeding events, allowing for adjustment for few variables. Fourth, the exclusion criteria of the PLATO trial included known risk factors for bleeding (eg, oral anticoagulant therapy and thrombocytopenia), which precluded adjustment for these factors in the present study.
Conclusion
GDF‐15 level measured at 1 month after an ACS is related to risk of bleeding during DAPT and provides additional information on bleeding risk beyond the level of GDF‐15 at baseline and other clinical risk indicators of bleeding. GDF‐15 level may therefore be useful to support decision making on the intensity and duration of long‐term antithrombotic treatment in patients with coronary artery disease. Further prospective studies are, though, warranted to assess whether availability of GDF‐15 levels at presentation and during follow‐up after an ACS will improve clinical outcomes.
Sources of Funding
This research was supported by AstraZeneca, who funded the PLATO trial. Roche Diagnostics supported this research by providing the GDF‐15 assay free of charge.
Disclosures
Lindholm reports institutional research grants from AstraZeneca and GlaxoSmithKline and lecture fees from AstraZeneca. Hagström reports expert committee member, lecture fees, and institutional research grant from Sanofi and Amgen; institutional research grants from AstraZeneca and GlaxoSmitKline; and expert committee member for Ariad and MSD. James reports institutional research grant, honoraria, and consultant/advisory board fee from AstraZeneca; institutional research grant and consultant/advisory board fee from Medtronic; institutional research grants and honoraria from The Medicines Company; and consultant/advisory board fees from Janssen and Bayer. Becker reports scientific advisory board member for Janssen, Ionis Pharmaceuticals, and AstraZeneca and safety review committee member for Portola. Cannon reports grants and personal fees from Amgen, Arisaph, Boehringer Ingelheim, Bristol‐Myers Squibb, Merck, and Takeda; personal fees from AstraZeneca, GlaxoSmithKline, Kowa, Lipimedix, Pfizer, Regeneron, Sanofi, and Janssen; and grants from Daiichi‐Sankyo, Janssen. Himmelmann reports being an employee of AstraZeneca. Katus reports personal fees from AstraZeneca, Bayer Vital, and Roche Diagnostics. Maurer reports honoraria/advisory board fees from AstraZeneca, Boehringer Ingelheim, Roche, Amgen, and MSD. López‐Sendón reports personal fees from Boehringer Ingelheim; grants from Bayer and GlaxoSmithKline; and grants and personal fees from Novartis, Servier, Pfizer, Menarini, and Sanofi. Steg reports research grant and speaking, or consulting fees from Merck, Sanofi, and Servier, and speaking or consulting fees from Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol‐Myers Squibb, CSL‐Behring, Daiichi Sankyo, GlaxoSmithKline, Janssen, Lilly, Novartis, Pfizer, Regeneron, and The Medicines Company. Storey reports institutional research grants, consultancy fees, honoraria, and travel support from AstraZeneca; consultancy fees from Aspen, PlaqueTec, The Medicines Company, ThermoFisher Scientific, Correvio, and Bayer; and travel support from Medtronic. Wallentin reports institutional research grants, consultancy fees, lecture fees, and travel support from Bristol‐Myers Squibb/Pfizer, AstraZeneca, GlaxoSmithKline, and Boehringer Ingelheim; institutional research grants from Merck & Co and Roche; consultancy fees from Abbott; and holds 2 patents involving GDF‐15.
Supporting information
Table S1. Eligibility Criteria of the PLATO Trial
Figure S1. Selection of study population.
Figure S2. Importance of variables included in the ordinary least squares model for GDF‐15 levels at 1 month, where importance is measured as Chi2—df.
Acknowledgment
We thank Ebba Bergman, PhD, at Uppsala Clinical Research Center, for editorial support.
(J Am Heart Assoc. 2017;6:e005580 DOI: 10.1161/JAHA.117.005580.)28411246
References
- 1. Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, Mahaffey KW, Scirica BM, Skene A, Steg PG, Storey RF, Harrington RA. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–1057. [DOI] [PubMed] [Google Scholar]
- 2. Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann F‐J, Ardissino D, De Servi S, Murphy SA, Riesmeyer J, Weerakkody G, Gibson CM, Antman EM; TRITON‐TIMI 38 Investigators . Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–2015. [DOI] [PubMed] [Google Scholar]
- 3. Mehta SR, Yusuf S, Peters RJ, Bertrand ME, Lewis BS, Natarajan MK, Malmberg K, Rupprecht H, Zhao F, Chrolavicius S, Copland I, Fox KA; Clopidogrel in Unstable angina to prevent Recurrent Events trial (CURE) Investigators . Effects of pretreatment with clopidogrel and aspirin followed by long‐term therapy in patients undergoing percutaneous coronary intervention: the PCI‐CURE study. Lancet. 2001;358:527–533. [DOI] [PubMed] [Google Scholar]
- 4. Mauri L, Kereiakes DJ, Yeh RW, Driscoll‐Shempp P, Cutlip DE, Steg PG, Normand S‐LT, Braunwald E, Wiviott SD, Cohen DJ, Holmes DR Jr, Krucoff MW, Hermiller J, Dauerman HL, Simon DI, Kandzari DE, Garratt KN, Lee DP, Pow TK, Ver Lee P, Rinaldi MJ, Massaro JM. Twelve or 30 months of dual antiplatelet therapy after drug‐eluting stents. N Engl J Med. 2014;371:2155–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bonaca MP, Bhatt DL, Cohen M, Steg PG, Storey RF, Jensen EC, Magnani G, Bansilal S, Fish MP, Im K, Bengtsson O, Oude Ophuis T, Budaj A, Theroux P, Ruda M, Hamm C, Goto S, Spinar J, Nicolau JC, Kiss RG, Murphy SA, Wiviott SD, Held P, Braunwald E, Sabatine MS. Long‐term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372:1791–1800. [DOI] [PubMed] [Google Scholar]
- 6. Udell JA, Bonaca MP, Collet J‐P, Lincoff AM, Kereiakes DJ, Costa F, Lee CW, Mauri L, Valgimigli M, Park S‐J, Montalescot G, Sabatine MS, Braunwald E, Bhatt DL. Long‐term dual antiplatelet therapy for secondary prevention of cardiovascular events in the subgroup of patients with previous myocardial infarction: a collaborative meta‐analysis of randomized trials. Eur Heart J. 2016;37:390–399. [DOI] [PubMed] [Google Scholar]
- 7. Roffi M, Patrono C, Collet J‐P, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, Gencer B, Hasenfuss G, Kjeldsen K, Lancellotti P, Landmesser U, Mehilli J, Mukherjee D, Storey RF, Windecker S, Baumgartner H, Gaemperli O, Achenbach S, Agewall S, Badimon L, Baigent C, Bueno H, Bugiardini R, Carerj S, Casselman F, Cuisset T, Erol Ç, Fitzsimons D, Halle M, Hamm C, Hildick‐Smith D, Huber K, Iliodromitis E, James S, Lewis BS, Lip GY, Piepoli MF, Richter D, Rosemann T, Sechtem U, Steg PG, Vrints C, Luis Zamorano J. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST‐Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:267–315. [DOI] [PubMed] [Google Scholar]
- 8. Hagström E, James SK, Bertilsson M, Becker RC, Himmelmann A, Husted S, Katus HA, Steg PG, Storey RF, Siegbahn A, Wallentin L; PLATO Investigators . Growth differentiation factor‐15 level predicts major bleeding and cardiovascular events in patients with acute coronary syndromes: results from the PLATO study. Eur Heart J. 2016;37:1325–1333. [DOI] [PubMed] [Google Scholar]
- 9. Wallentin L, Hijazi Z, Andersson U, Alexander JH, De Caterina R, Hanna M, Horowitz JD, Hylek EM, Lopes RD, Asberg S, Granger CB, Siegbahn A; ARISTOTLE Investigators . Growth differentiation factor 15, a marker of oxidative stress and inflammation, for risk assessment in patients with atrial fibrillation: insights from the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial. Circulation. 2014;130:1847–1858. [DOI] [PubMed] [Google Scholar]
- 10. James S, Akerblom A, Cannon CP, Emanuelsson H, Husted S, Katus H, Skene A, Steg PG, Storey RF, Harrington R, Becker R, Wallentin L. Comparison of ticagrelor, the first reversible oral P2Y(12) receptor antagonist, with clopidogrel in patients with acute coronary syndromes: rationale, design, and baseline characteristics of the PLATelet inhibition and patient Outcomes (PLATO) trial. Am Heart J. 2009;157:599–605. [DOI] [PubMed] [Google Scholar]
- 11. Wallentin L, Lindholm D, Siegbahn A, Wernroth L, Becker RC, Cannon CP, Cornel JH, Himmelmann A, Giannitsis E, Harrington RA, Held C, Husted S, Katus HA, Mahaffey KW, Steg PG, Storey RF, James SK. Biomarkers in relation to the effects of ticagrelor in comparison with clopidogrel in non‐ST‐elevation acute coronary syndrome patients managed with or without in‐hospital revascularization: a substudy from the Prospective Randomized Platelet Inhibition and Patient Outcomes (PLATO) trial. Circulation. 2014;129:293–303. [DOI] [PubMed] [Google Scholar]
- 12. Wollert KC, Kempf T, Peter T, Olofsson S, James S, Johnston N, Lindahl B, Horn‐Wichmann R, Brabant G, Simoons ML, Armstrong PW, Califf RM, Drexler H, Wallentin L. Prognostic value of growth‐differentiation factor‐15 in patients with non‐ST‐elevation acute coronary syndrome. Circulation. 2007;115:962–971. [DOI] [PubMed] [Google Scholar]
- 13. Wollert KC, Kempf T, Lagerqvist B, Lindahl B, Olofsson S, Allhoff T, Peter T, Siegbahn A, Venge P, Drexler H, Wallentin L. Growth differentiation factor 15 for risk stratification and selection of an invasive treatment strategy in non ST‐elevation acute coronary syndrome. Circulation. 2007;116:1540–1548. [DOI] [PubMed] [Google Scholar]
- 14. Lindholm D, James SK, Bertilsson M, Becker RC, Cannon CP, Giannitsis E, Harrington RA, Himmelmann A, Kontny F, Siegbahn A, Steg PG, Storey RF, Velders MA, Weaver WD, Wallentin L. Biomarkers and coronary lesions predict outcomes after revascularization in non‐ST‐elevation acute coronary syndrome. Clin Chem. 2017;63:573–584. [DOI] [PubMed] [Google Scholar]
- 15. Hagström E, Held C, Stewart RAH, Aylward PE, Budaj A, Cannon CP, Koenig W, Krug‐Gourley S, Mohler ER III, Steg PG, Tarka E, Östlund O, White HD, Siegbahn A, Wallentin L. Growth differentiation factor 15 predicts all‐cause morbidity and mortality in stable coronary heart disease. Clin Chem. 2017;63:325–333. [DOI] [PubMed] [Google Scholar]
- 16. Lind L, Wallentin L, Kempf T, Tapken H, Quint A, Lindahl B, Olofsson S, Venge P, Larsson A, Hulthe J, Elmgren A, Wollert KC. Growth‐differentiation factor‐15 is an independent marker of cardiovascular dysfunction and disease in the elderly: results from the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) Study. Eur Heart J. 2009;30:2346–2353. [DOI] [PubMed] [Google Scholar]
- 17. Wallentin L, Zethelius B, Berglund L, Eggers KM, Lind L, Lindahl B, Wollert KC, Siegbahn A. GDF‐15 for prognostication of cardiovascular and cancer morbidity and mortality in men. PLoS One. 2013;8:e78797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rohatgi A, Patel P, Das SR, Ayers CR, Khera A, Martinez‐Rumayor A, Berry JD, McGuire DK, de Lemos JA. Association of growth differentiation factor‐15 with coronary atherosclerosis and mortality in a young, multiethnic population: observations from the Dallas Heart Study. Clin Chem. 2012;58:172–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ho JE, Hwang S‐J, Wollert KC, Larson MG, Cheng S, Kempf T, Vasan RS, Januzzi JL, Wang TJ, Fox CS. Biomarkers of cardiovascular stress and incident chronic kidney disease. Clin Chem. 2013;59:1613–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bonaterra GA, Zügel S, Thogersen J, Walter SA, Haberkorn U, Strelau J, Kinscherf R. Growth differentiation factor‐15 deficiency inhibits atherosclerosis progression by regulating interleukin‐6‐dependent inflammatory response to vascular injury. J Am Heart Assoc. 2012;1:e002550 DOI: 10.1161/JAHA.112.002550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schlittenhardt D, Schober A, Strelau J, Bonaterra GA, Schmiedt W, Unsicker K, Metz J, Kinscherf R. Involvement of growth differentiation factor‐15/macrophage inhibitory cytokine‐1 (GDF‐15/MIC‐1) in oxLDL‐induced apoptosis of human macrophages in vitro and in arteriosclerotic lesions. Cell Tissue Res. 2004;318:325–333. [DOI] [PubMed] [Google Scholar]
- 22. Eggers KM, Kempf T, Wallentin L, Wollert KC, Lind L. Change in growth differentiation factor 15 concentrations over time independently predicts mortality in community‐dwelling elderly individuals. Clin Chem. 2013;59:1091–1098. [DOI] [PubMed] [Google Scholar]
- 23. Unsicker K, Spittau B, Krieglstein K. The multiple facets of the TGF‐β family cytokine growth/differentiation factor‐15/macrophage inhibitory cytokine‐1. Cytokine Growth Factor Rev. 2013;24:373–384. [DOI] [PubMed] [Google Scholar]
- 24. Wollert KC, Kempf T, Wallentin L. Growth differentiation factor 15 as a biomarker in cardiovascular disease. Clin Chem. 2017;63:140–151. [DOI] [PubMed] [Google Scholar]
- 25. Rossaint J, Vestweber D, Zarbock A. GDF‐15 prevents platelet integrin activation and thrombus formation. J Thromb Haemost. 2013;11:335–344. [DOI] [PubMed] [Google Scholar]
- 26. Yeh RW, Secemsky EA, Kereiakes DJ, Normand S‐LT, Gershlick AH, Cohen DJ, Spertus JA, Steg PG, Cutlip DE, Rinaldi MJ, Camenzind E, Wijns W, Apruzzese PK, Song Y, Massaro JM, Mauri L. Development and validation of a prediction rule for benefit and harm of dual antiplatelet therapy beyond 1 year after percutaneous coronary intervention. JAMA. 2016;315:1735–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Eligibility Criteria of the PLATO Trial
Figure S1. Selection of study population.
Figure S2. Importance of variables included in the ordinary least squares model for GDF‐15 levels at 1 month, where importance is measured as Chi2—df.


