Introduction
According to a recent Scientific Statement of the American Heart Association (AHA), salt sensitivity of blood pressure (BP) is a trait in which BP “changes parallel to changes in salt intake.”1 It is said that salt sensitivity is “a risk factor for cardiovascular mortality and morbidity, independent of and as powerful as BP.”1 Although the criteria for identifying salt sensitivity are not standardized, it has been estimated that 30% to 50% of hypertensive humans are salt sensitive and ≈25% of normotensive humans are salt sensitive.2, 3 According to the AHA Scientific Statement, salt sensitivity of BP has become “an issue of clinical importance because the phenotype carries prognostic implications potentially as strong as those of traditional cardiovascular risk factors.”1 Various methods of testing for salt sensitivity have been applied in research settings; however, tests for salt sensitivity that are useful in routine clinical practice have yet to be identified.
Advances in understanding of the mechanisms and clinical significance of salt sensitivity have long been hampered by the lack of standardization in the methods used for assessing salt sensitivity.4 In this analysis, we discuss the main research methods of testing for salt sensitivity and present current views on how best to assess this complex phenotype, including many views that were not addressed by the recent AHA Scientific Statement. To broaden the scientific consideration, we also present alternative perspectives on the contention that salt sensitivity is a risk factor for cardiovascular mortality “independent of and as powerful as BP.”1
Is There a Scientifically Superior Method of Testing for Salt Sensitivity? Consideration of the AHA View
The AHA provides some brief recommendations1 on how to assess salt sensitivity with 2 different types of short‐term protocols: (1) “outpatient dietary protocols” requiring a total time of ≈2 weeks to directly measure BP responses to changes in dietary intake of salt and (2) an “inpatient acute protocol,” which might be viewed as an indirect test of salt sensitivity, requiring a total time of only ≈3 days to measure BP responses to furosemide and simultaneous dietary salt restriction in subjects who have been intravenously and orally administered salt beforehand.5, 6
In published studies with either outpatient dietary protocols or the furosemide‐based inpatient acute protocol, a subject is classified as salt sensitive if the protocol causes mean arterial pressure (MAP) to change by more than an arbitrary cutoff chosen by the investigators. The AHA Scientific Statement provides data that “exemplifies the need to choose arbitrary cutoffs for the magnitude of BP change used to classify subjects as salt sensitive.”1 Unfortunately, as noted by de Leeuw and Kroon, “the magnitude of the response above which pressure is considered to be salt‐sensitive varies enormously among studies.”7 Although the limitations of using arbitrary and wide‐ranging cutoffs for assessing continuous traits like salt sensitivity are well known,4, 7 investigators rely on such cutoffs when discussing the biological and demographic characteristics of salt‐sensitive subjects.1 The AHA Scientific Statement does not include recommendations on specific cutoffs and on which particular protocol provides the best current approach for identifying hypertensive or normotensive subjects with salt sensitivity.
According to the AHA Scientific Statement, “there is no evidence base to determine best research practices in terms of measurement of salt sensitivity of BP in humans.”1 Thus, the AHA does not provide guidance on whether a particular testing protocol for salt sensitivity is scientifically preferred and does not really distinguish between the recommended outpatient dietary protocols and the furosemide‐based inpatient acute protocol with respect to their accuracy and reproducibility in assessing BP salt sensitivity. Hence, the Scientific Statement does not propose or indicate that 1 of the methods discussed in the AHA recommendations, or any other method, be considered as the current reference method for assessing salt sensitivity.
Is There a Scientifically Superior Method of Testing for Salt Sensitivity? Consideration of an Alternative View
Per the view of many investigators, the evidence base demonstrates that a carefully performed dietary protocol is scientifically superior to other protocols, including the furosemide‐based, inpatient acute protocol, for assessing salt sensitivity of BP. Specifically, the position of many investigators is that a carefully controlled dietary protocol (without any diuretic treatment) which includes a 1‐week period of low salt intake, and a 1‐week period of high salt intake, is the “gold‐standard method” for assessing salt sensitivity.8, 9, 10, 11, 12 Other investigators may not use the term gold‐standard method, but they note that “the most reliable method to measure salt sensitivity is the blood pressure response to a change in dietary salt intake”13 and refer to such dietary testing as “the standard reference procedure.”14 Here, we use the term “reference method” or “preferred method” rather than the term gold‐standard method.
Table lists the main features of the dietary protocol that many investigators refer to as the preferred approach for assessing salt sensitivity. Considering the views and study results of a variety of investigators discussed below, we recommend that the current reference method of testing for salt sensitivity be based on a dietary protocol with these features. The dietary protocol can be performed on an inpatient or outpatient basis. We believe that a carefully performed inpatient dietary protocol (not furosemide‐based) is likely to deliver highly reproducible results comparable to a carefully performed outpatient dietary protocol. However, we focus more on the outpatient‐type dietary protocol because of its greater evidence base with respect to studies of reproducibility and prediction of cardiovascular risk.
Table 1.
Candidate Reference Method of Testing for Salt Sensitivity
| Dietary protocol with the following features: |
| 1‐week period of low salt intake of no more than 50 mmol NaCl/day |
| 1‐week period of high salt intake of ≈250 mmol NaCl/daya |
| Order of administration of different salt diets may vary per study objective |
| Prescription and monitoring of well‐characterized diets throughout entire studyb |
| Multiple measurements of 24‐hour urine Na+ excretion to confirm NaCl intake |
| BP measurements based on a highly reproducible salt sensitivity test protocolc |
| Cutoff to classify normotensives as salt sensitive: MAP change ≥3 to 5 mm Hgd |
| Cutoff to classify hypertensives as salt sensitive: MAP change ≥8 to 10 mm Hgd |
BP indicates blood pressure; MAP, mean arterial pressure.
For double‐blind, placebo‐controlled testing of the BP effects of changes in salt intake, the high salt intake and the placebo can be administered in unmarked capsules.
Because potassium,15 nitrate,16, 17 and other dietary factors can affect BP, the contents of the diets should be carefully described for each study phase and contents should not be varied unless required as part of the study objective. Based on the diets that were used in studies of protocols with demonstrated high reproducibility for classifying subjects as salt sensitive,18, 19, 20 a dietary potassium intake in the range of 60 to 80 mmol/day could be recommended.
Details of BP measurement techniques and the BP cutoffs used in test protocols reported to be highly reproducible can be found in the supplemental table (Table S1) and in publications by Sharma et al,18 Overlack et al,19 and Draaijer et al.20
The specific cutoffs in these ranges should be prespecified. If the high salt intake amount happens to be somewhat lower than the target salt intake of 250 mmol/day, the cutoff may be based on the lower number in the recommended cut‐off range. If the amount of salt administered is very close to, or somewhat above, the target salt intake of 250 mmol/day, the cutoff may be based on the higher number in the recommended cut‐off range.
Some of the features of the dietary test protocol described in Table, including the specified levels of salt intake, were mentioned in the AHA recommendations for outpatient dietary protocols.1 However, in contrast to the AHA recommendations, the recommendations in Table: (1) designate a candidate reference method; (2) specify the BP cutoffs for use in identifying salt sensitivity; and (3) do not require that the period of high salt intake precede the period of low salt intake in the test protocol. We next discuss these issues in further detail and provide information demonstrating superior reproducibility of the preferred dietary protocol compared with other protocols for classifying subjects as salt sensitive. In addition, we discuss how the evidence base demonstrates that salt sensitivity diagnosed by the preferred dietary protocol, but not salt sensitivity diagnosed by the furosemide‐based inpatient acute protocol, is an independent risk factor for time to a cardiovascular event.
Cutoffs for Classifying Subjects as Salt Sensitive in the Proposed Reference Method
When testing normotensive subjects with the proposed reference protocol and the physiological levels of salt intake described in Table, the cutoff for classifying someone with salt sensitivity is considered to be a change in MAP of at least 3 to 5 mm Hg in response to the change in salt intake18, 19, 21; when testing hypertensive subjects, the classification cutoff is generally considered to be a change in MAP of at least 8 to 10 mm Hg.20, 22, 23 When ambulatory BP monitors have been used in careful dietary studies of hypertensive subjects, the cutoff for diagnosis has been considered to be a change in the 24‐hour measurement of MAP of ≈5 mm Hg.11
Reproducibility of Dietary Protocols to Test for Salt Sensitivity
When the preferred dietary protocol with the features described in Table is performed in either normotensive18, 19 or hypertensive subjects,20 the reproducibility of the protocol for classifying subjects as salt sensitive or as non‐salt‐sensitive is very high (>90%; here we use the term reproducibility to mean test‐retest repeatability; Figure). We believe that these studies are critical in that they provide strong evidence of excellent reproducibility of the preferred dietary protocol in classifying subjects for salt‐sensitivity status. The reproducibility was high when the different salt diets were given in random order19, 20 and when the low‐salt diet was given before the high‐salt diet.18 This high level of reproducibility for identifying subjects with salt sensitivity has not been documented with any other test protocol.
Figure 1.
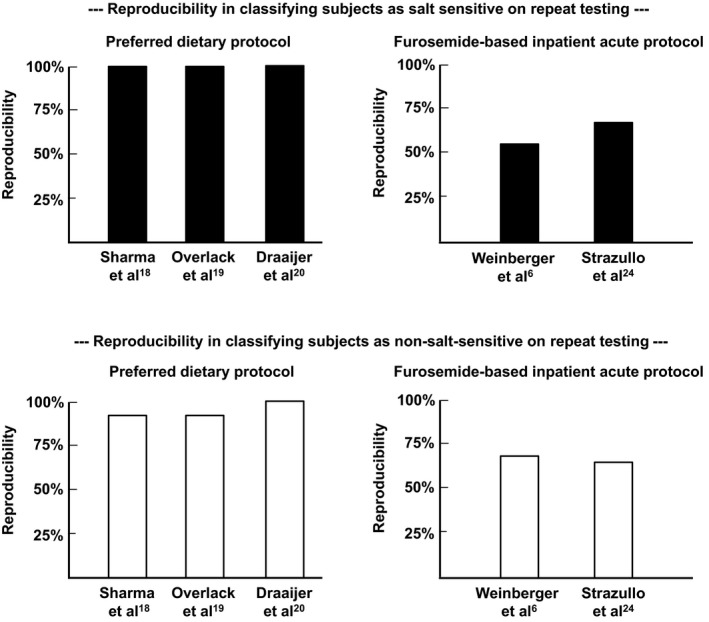
Reproducibility of different test protocols for classifying subjects as salt sensitive or non‐salt‐sensitive with repeat testing. Top panel, test‐retest repeatability of different protocols for classifying subjects as salt sensitive (denoted by solid bars). Bottom panel, test‐retest repeatability for classifying subjects as non‐salt‐sensitive (denoted by open bars). The features of the preferred dietary protocol are summarized in Table and detailed in the supplement (Table S1). The features of the furosemide‐based inpatient acute protocol are described in references by Weinberger et al5, 6 and Strazullo et al.24
It should be noted that dietary protocols appear to have poor reproducibility in classifying subjects for salt sensitivity when a standardized diet is not carefully prescribed throughout the entire study,25, 26 or when the amounts of salt administered in the low‐salt phase and high‐salt phase27, 28 do not approximate the amounts recommended in Table and in the AHA Scientific Statement.1 Characteristics of dietary protocols showing excellent reproducibility in classifying subjects as salt sensitive, and those showing poor reproducibility, are shown in a detailed table in the supplement (Table S1).
According to the GenSalt investigators,12 a testing method with features similar to the preferred dietary protocol showed evidence of reproducible results when subjects were given a repeat test even 4.5 years after the original test. However, the correlation coefficients of the BP changes between those widely separated repeat tests were not high, and the investigators did not attempt to classify the subjects for salt sensitivity per specific cutoffs. It is possible that even if investigators use a test protocol with high reproducibility over a short period, an individual's BP response to a change in salt intake may vary over a period of years because of changes in age, environmental factors, or lifestyle or changes in the character of the disorder itself.
When Testing for Salt Sensitivity in Dietary Protocols, Should the Period of High Salt Intake Precede the Period of Low Salt Intake?
The recommendations of the AHA Scientific Statement suggest that in dietary protocols testing for salt sensitivity, the high‐salt‐intake period should precede the low‐salt‐intake period.1 The AHA Scientific Statement suggests that dietary protocols start with a high‐salt‐intake period to uniformly suppress activity of the renin‐angiotensin‐aldosterone system (RAAS) and thereby minimize baseline variability in the hormonal system that regulates plasma sodium concentrations and arterial BP.1 According to the AHA Statement, such suppression of RAAS activity by high salt intake “may confer more uniformity to the subsequent response to the low salt intake.”1 However, whereas baseline variability in activity of the RAAS may be decreased with high salt intake, variability in nitric oxide (NO) activity or sympathetic nerve activity may be increased (because salt‐sensitive subjects can respond differently to a high salt intake than resistant subjects with respect to changes in activity of the NO system29, 30, 31 and sympathetic nervous system32, 33). The rationale for starting dietary protocols with a high‐salt diet would seem to assume that when investigating biological responses to changes in salt intake, it is more important to suppress initial variability in the RAAS than it is to suppress initial variability in other systems that contribute to the regulation of BP.
We believe that investigators should make decisions about the sequential order of the low‐salt‐intake and high‐salt‐intake periods based on the study objectives. If the objective is to identify subjects who are salt sensitive, then randomized administration of the different salt diets is reasonable. When different salt diets have been administered in random order, the reproducibility of the preferred type of dietary protocol for classifying subjects as salt sensitive has been shown to be very high.19, 20 If the objective is to study the mechanisms whereby salt loading increases BP, then the period of low salt intake could precede the period of high salt intake, provided that a proper time control is included. To study the mechanisms whereby salt restriction lowers BP, then the period of high salt intake could precede the period of low salt intake. We caution against drawing conclusions about disturbances that mediate salt‐induced increases in BP, from studies of the BP‐lowering effects of salt depletion.
Use of Short‐Term Dietary Protocols to Estimate the Prevalence of Salt Sensitivity
Some investigators believe that short‐term dietary protocols that test for salt sensitivity “may underestimate the phenomenon” because “they miss slow salt‐sensitive responders” that would be detected in dietary protocols of longer duration.34 For example, Hamlyn and Blaustein contend that “to properly capture the true BP response” in dietary intervention trials, each period of low salt intake and high salt intake should be of 3 months in duration.34 However, this view appears to be at odds with the study results summarized by Aburto et al, suggesting that the reductions in BP that occur with reduced sodium intake in trials of less than 3 months in duration may actually be greater than those that occur in trials of more than 3 months in duration.35 Moreover, even if short‐term dietary protocols (<3 months) reveal fewer cases of BP salt sensitivity than long‐term dietary protocols (>3 months), this does not address the utility of a diagnosis of BP salt sensitivity made with the short‐term dietary protocol for: (1) predicting increased risk for adverse cardiovascular events or (2) investigating the mechanisms whereby increases in salt intake induce increases in BP.
Use of Unphysiological Amounts of Salt in Protocols to Test for Salt Sensitivity
Some investigators have studied or modeled the effects of extreme changes in salt intake on BP (>1000 mmol/day).36, 37 Extreme, unphysiological increases in salt intake appear to cause substantial increases in BP in nearly all normal individuals.36 We recommend against the use of extreme, unphysiological changes in salt intake for assessing salt sensitivity because of the unclear relevance of such conditions to salt‐induced changes in BP in real life. The levels of salt intake described in the proposed reference method in Table, and in the methodological recommendations of the AHA, are within the amounts consumed by humans in nonexperimental circumstances.1
Surrogate Methods of Testing for Salt Sensitivity as Alternatives to the Preferred Dietary Protocol
Although the proposed reference protocol provides a highly reproducible, direct method of testing for BP salt sensitivity, it also requires considerable time and resources to perform and its use is generally limited to specialized research settings. Accordingly, there is major interest in identifying quick and relatively inexpensive surrogate (indirect) methods of testing for salt sensitivity.13 A simple and reliable surrogate test would facilitate research on the mechanisms and consequences of salt sensitivity and could also be evaluated for use in routine clinical practice. To assess the performance of surrogate tests for identifying salt sensitivity, it is necessary to compare them against a reference method, or against a surrogate method that has been vetted by comparisons with the reference method, imperfect as it may be. Various investigators have developed surrogate tests of salt sensitivity. However, as discussed in the AHA Scientific Statement, most surrogate tests have considerable limitations or have not been extensively studied and require further validation.1 Of the tests discussed, the furosemide‐based “inpatient acute protocol”5, 6 is the most extensively studied surrogate method of assessing salt sensitivity. Although the furosemide‐based inpatient acute protocol is too complicated for use in routine clinical practice, it has often been used in clinical research settings.
Is the Furosemide‐Based “Inpatient Acute Protocol” Useful for Measurement of BP Salt Sensitivity?
The AHA Scientific Statement1 provides specific recommendations on how to perform the furosemide‐based “inpatient acute protocol” for assessing BP salt sensitivity, but does not provide recommendations for performing other surrogate methods of testing for salt sensitivity. This may suggest that the furosemide‐based inpatient acute protocol, performed per the AHA recommendations, is a useful method for identifying subjects with salt sensitivity. To assist in understanding potential pitfalls of the furosemide‐based inpatient acute protocol for identifying salt‐sensitive subjects, we next discuss concerns about the accuracy and reproducibility of this method. We also discuss questions regarding the utility of this protocol for investigating mechanisms of salt sensitivity and for predicting cardiovascular outcomes.
The well‐known inpatient acute protocol, derived from the work of Weinberger et al,5, 6 does not directly measure BP responsiveness to changes only in salt intake. Rather, it measures responsiveness to the BP‐lowering effects of furosemide and simultaneous dietary salt restriction in subjects who have been intravenously and orally administered salt beforehand.5, 6 In this inpatient acute protocol, which could also be referred to as a furosemide‐sensitivity test, individuals are classified as “salt sensitive” if their MAP decreases by 10 mm Hg or more in response to the combination of furosemide and dietary salt restriction.5, 6
How Reproducible is the Furosemide‐Based Inpatient Acute Protocol for Classifying Individuals as Salt Sensitive?
In referring to studies of the furosemide‐based inpatient acute protocol by Weinberger and Fineberg,6 the AHA Scientific Statement notes that the correlation between the changes in MAP occurring with repeat tests was 0.56, and that 4 of 28 subjects changed their status from salt sensitive to salt resistant or vice versa, suggesting “modest reproducibility” of the protocol.1 However, this information does not provide a complete picture of the very limited reproducibility of the protocol with respect to classifying subjects as salt sensitive. In the study cited, it appears that only ≈55% of the subjects deemed to be salt sensitive in the first test were classified as salt sensitive on the repeat test (on the repeat test, ≈45% of the salt‐sensitive subjects appear to have become classified as either “indeterminate” or “salt resistant”).6 This constitutes poor reproducibility of the inpatient acute protocol for classifying subjects as salt sensitive based on the 10‐mm‐Hg cutoff used by Weinberger et al.
Investigators using a classification scheme for salt sensitivity different from the one used by Weinberger et al have also reported results showing limited reproducibility of the furosemide‐based inpatient acute protocol.24 As shown in Figure, the reproducibility of a diagnosis of salt sensitivity made with the furosemide‐based inpatient acute protocol, using the cutoffs of either Weinberger and Fineberg6 or of other investigators,24 appears substantially lower than the reproducibility of a diagnosis made with the preferred type of dietary protocol.18, 19, 20
How Accurate Is the Furosemide‐Based Inpatient Acute Protocol for Classifying Subjects as Salt Sensitive?
Because the furosemide‐based inpatient acute protocol has poor reproducibility in classifying subjects as salt sensitive, and does not measure the effects of only changes in salt intake on BP, we next address the question: How accurate is the furosemide‐based inpatient acute protocol for determining whether an individual has BP salt sensitivity?
Multiple studies are available in which individuals tested with the furosemide‐based inpatient acute protocol were also tested with a carefully performed, direct dietary protocol for salt sensitivity,8, 9, 11, 24, 38 that is, the type of protocol that many investigators consider to be the preferred test for assessing salt sensitivity (Table).8, 9, 10, 11, 12 The AHA Scientific Statement refers to several of these studies and notes that comparisons between results obtained using acute protocols and “slower dietary sodium intake” protocols “were consistently significant.”1 However, the general comment that the comparisons between the 2 protocols “were consistently significant” does not address the accuracy of the furosemide‐based inpatient acute protocol for identifying subjects with BP salt sensitivity as judged by the preferred dietary protocol.
Considering all of the comparative studies together,8, 9, 11, 24, 38 the furosemide‐based inpatient acute protocol appears to have an accuracy of only ≈65% to 75% for assessing salt sensitivity (where accuracy is defined as the number of true positives and true negatives divided by the total number of individuals tested). Thus, the furosemide‐based inpatient acute protocol is characterized by limited accuracy and poor reproducibility with respect to determining whether a subject is, in fact, salt sensitive.
In the inpatient acute protocol,5, 6 it should be kept in mind that the effects of changes in oral salt intake on mechanisms regulating BP are susceptible to potential confounding by effects of furosemide and intravenous salt loading. For example, furosemide itself not only has effects on Na+, K+, 2Cl− cotransporters in the renal tubule, but it also has effects on Na+, K+, 2Cl− cotransporters in the vasculature.39, 40 In addition, administration of salt by the intravenous route may have different biological effects than administration of salt by the more physiological oral route.
Is Salt Sensitivity Diagnosed by the Furosemide‐Based Inpatient Acute Protocol or by the Preferred Dietary Protocol an Independent Risk Factor for Cardiovascular Morbidity or Mortality?
Studies by Morimoto et al provide evidence that in hypertensive subjects, a diagnosis of salt sensitivity made with the preferred dietary protocol is an independent risk factor for time to a major cardiovascular event.41 According to the AHA Scientific Statement, “more definitive proof of an independent role for salt sensitivity of BP as a cardiovascular risk factor was provided by Weinberger and colleagues”42 who used the furosemide‐based inpatient acute protocol for diagnosis of salt sensitivity. However, the retrospective cohort study of Weinberger et al42 examined mortality risk (not cardiovascular risk) was exploratory in nature and yielded inconsistent results across different mortality analyses. In that study, in which the furosemide‐based, inpatient acute protocol was used as the surrogate test for salt sensitivity, logistic regression analysis indicated that such “salt sensitivity” might be a risk factor for death.42 However, because of the known limitations of logistic regression analysis, the investigators also performed a Cox proportional hazards analysis. In the Cox analysis, salt sensitivity assessed by the furosemide‐based inpatient acute protocol was not an independent predictor of time to death.42
The AHA Statement notes that “the novel observation” in the study of Weinberger et al was that “the survival curves of salt resistant hypertensive subjects and salt sensitive normotensive subjects were not significantly different.”1 However, no information was provided in the study of Weinberger et al42 on the power of the statistical analysis for detecting differences in survival curves between those 2 patient subgroups. Because the effects on mortality of salt sensitivity, as judged by the furosemide‐based inpatient acute protocol, were inconsistent across the different analyses by Weinberger et al,42 and because of the exploratory nature of retrospective cohort studies, the results of the Weinberger mortality studies with the inpatient acute protocol are not definitive.
We are unaware of any study demonstrating that a diagnosis of salt sensitivity with the furosemide‐based inpatient acute protocol is an independent risk factor for time to cardiovascular death or to a cardiovascular event. The evidence to date indicates that salt sensitivity judged by a careful dietary protocol, but not salt sensitivity judged by the furosemide‐based inpatient acute protocol, may well be an independent risk factor for time to a cardiovascular event. It remains to be established whether salt sensitivity judged by any type of protocol is an independent risk factor for time to death from cardiovascular causes (or from other causes).
The Impact of Protocol Selection on Understanding Mechanisms of Salt Sensitivity
The mechanisms of salt sensitivity are the subject of ongoing controversy and discussion.1, 29, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52 Whereas a considerable degree of controversy stems from differing views on the interpretation of specific studies or theories, it can also be related to differences between the methods used to identify subjects with salt sensitivity. For example, studies with the furosemide‐based protocol indicate that during the salt restriction phase, salt‐sensitive hypertensive subjects undergo similar or greater increases in plasma catecholamines than do salt‐resistant hypertensive subjects.5, 53, 54, 55 However, studies with the preferred type of dietary protocol indicate that during the salt restriction phase, salt‐sensitive hypertensive subjects undergo smaller increases in plasma and urinary catecholamines than do salt‐resistant hypertensive subjects.32, 33
The method of testing for salt sensitivity may also influence understanding of the demographics of salt sensitivity. For example, studies with the furosemide‐based protocol in normotensive subjects5 indicated that “the frequency of salt sensitivity among blacks was similar to that seen among whites.”2 However, studies with the preferred type of dietary protocol have indicated that salt sensitivity is more common in normotensive blacks than in normotensive whites.56 This raises the question: In studies with a furosemide‐based protocol, how should one interpret mechanistic and demographic findings pertaining to salt sensitivity if the observations have not been checked and confirmed in salt sensitivity studies with the preferred dietary protocol? Given the need for confirmatory testing, it is unclear how studies with the furosemide‐based inpatient acute protocol advance understanding of the mechanisms and demographics of salt sensitivity beyond the knowledge gained from studies with the preferred dietary protocol.
Conclusions
According to the recent scientific statement from the AHA, salt sensitivity of BP is a common disorder that “carries prognostic implications potentially as strong as those of traditional cardiovascular risk factors.”1 Advances in understanding the mechanisms and consequences of salt sensitivity remain hampered by a lack of standardization of the protocols and criteria used to identify individuals with this disorder. Among the various methods of testing for salt sensitivity, the carefully controlled dietary protocol described herein provides the highest test‐retest repeatability for identifying salt‐sensitive subjects. Many investigators, ourselves included, consider such a dietary protocol to be the current reference method of testing for salt sensitivity. The reference dietary protocol requires considerable time and resources and is intended for use in research settings. Tests for salt sensitivity that are useful in routine clinical practice have yet to be identified. The most widely used surrogate test for salt sensitivity is a furosemide‐based, inpatient acute protocol that includes potentially confounding features and demonstrates inferior test‐retest repeatability and questionable accuracy for identifying subjects with salt sensitivity. Other surrogate tests for salt sensitivity have been described, but have undergone only limited tests of validation. Salt sensitivity, as judged by the preferred dietary test protocol, but not by the furosemide‐based protocol or any other kind of protocol, has been demonstrated to be an independent risk factor for time to a cardiovascular event. It remains to be determined whether salt sensitivity judged by any type of testing protocol is an independent risk factor for time to death from cardiovascular causes or from other causes. Finally, even if a robust test for salt sensitivity is developed that can be easily performed and readily introduced into clinical practice, prospective studies will be required to determine whether the routine use of such a test would have beneficial effects on clinical outcomes.
Sources of Funding
Sources of funding related to this work included support from the National Center for Research Resources, M0 RR‐00079, US Public Health Service. Additional support was provided by a Praemium Academiae award of the Czech Academy of Sciences to Pravenec, National Institutes of Health/National Heart, Lung, and Blood Institute grant RO1‐HL64230, and gifts from the Saw Island Foundation, the Antel Foundation, and the Maier Family Foundation.
Disclosures
None.
Supporting information
Table S1. Features of Dietary Protocols That Have Been Tested for Reproducibility in Classifying Subjects as SS or SR
J Am Heart Assoc. 2017;6:e005653 DOI: 10.1161/JAHA.117.005653.28365569
References
- 1. Elijovich F, Weinberger MH, Anderson CA, Appel LJ, Bursztyn M, Cook NR, Dart RA, Newton‐Cheh CH, Sacks FM, Laffer CL. Salt sensitivity of blood pressure: a scientific statement from the American Heart Association. Hypertension. 2016;68:e7–e46. [DOI] [PubMed] [Google Scholar]
- 2. Weinberger MH. Salt sensitivity of blood pressure in humans. Hypertension. 1996;27:481–490. [DOI] [PubMed] [Google Scholar]
- 3. Kotchen TA, Cowley AW Jr, Frohlich ED. Salt in health and disease–a delicate balance. N Engl J Med. 2013;368:1229–1237. [DOI] [PubMed] [Google Scholar]
- 4. Sullivan JM. Salt sensitivity. Definition, conception, methodology, and long‐term issues. Hypertension. 1991;17:I61–I68. [DOI] [PubMed] [Google Scholar]
- 5. Weinberger MH, Miller JZ, Luft FC, Grim CE, Fineberg NS. Definitions and characteristics of sodium sensitivity and blood pressure resistance. Hypertension. 1986;8:II127–II134. [DOI] [PubMed] [Google Scholar]
- 6. Weinberger MH, Fineberg NS. Sodium and volume sensitivity of blood pressure. Age and pressure change over time. Hypertension. 1991;18:67–71. [DOI] [PubMed] [Google Scholar]
- 7. de Leeuw PW, Kroon AA. Salt and sensitivity. Hypertension. 2013;62:461–462. [DOI] [PubMed] [Google Scholar]
- 8. Sharma AM, Schorr U, Cetto C, Distler A. Dietary v intravenous salt loading for the assessment of salt sensitivity in normotensive men. Am J Hypertens. 1994;7:1070–1075. [DOI] [PubMed] [Google Scholar]
- 9. Galletti F, Ferrara I, Stinga F, Iacone R, Noviello F, Strazzullo P. Evaluation of a rapid protocol for the assessment of salt sensitivity against the blood pressure response to dietary sodium chloride restriction. Am J Hypertens. 1997;10:462–466. [DOI] [PubMed] [Google Scholar]
- 10. Galletti F, Strazzullo P. The blood pressure‐salt sensitivity paradigm: pathophysiologically sound yet of no practical value. Nephrol Dial Transplant. 2016;31:1386–1391. [DOI] [PubMed] [Google Scholar]
- 11. de la Sierra A, Giner V, Bragulat E, Coca A. Lack of correlation between two methods for the assessment of salt sensitivity in essential hypertension. J Hum Hypertens. 2002;16:255–260. [DOI] [PubMed] [Google Scholar]
- 12. Gu D, Zhao Q, Chen J, Chen JC, Huang J, Bazzano LA, Lu F, Mu J, Li J, Cao J, Mills K, Chen CS, Rice T, Hamm LL, He J. Reproducibility of blood pressure responses to dietary sodium and potassium interventions: the GenSalt study. Hypertension. 2013;62:499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Felder RA, White MJ, Williams SM, Jose PA. Diagnostic tools for hypertension and salt sensitivity testing. Curr Opin Nephrol Hypertens. 2013;22:65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Iatrino R, Manunta P, Zagato L. Salt sensitivity: challenging and controversial phenotype of primary hypertension. Curr Hypertens Rep. 2016;18:70. [DOI] [PubMed] [Google Scholar]
- 15. Kanbay M, Bayram Y, Solak Y, Sanders PW. Dietary potassium: a key mediator of the cardiovascular response to dietary sodium chloride. J Am Soc Hypertens. 2013;7:395–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Khatri J, Mills CE, Maskell P, Odongerel C, Webb AJ. It is rocket science—why dietary nitrate is hard to beet! Part I: twists and turns in the realisation of the nitrate‐nitrite‐NO pathway. Br J Clin Pharmacol. 2017;83:129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mills CE, Khatri J, Maskell P, Odongerel C, Webb AJ. It is rocket science—why dietary nitrate is hard to beet! Part II: further mechanisms and therapeutic potential of the nitrate‐nitrite‐NO pathway. Br J Clin Pharmacol. 2017;83:140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sharma AM, Schattenfroh S, Kribben A, Distler A. Reliability of salt‐sensitivity testing in normotensive subjects. Klin Wochenschr. 1989;67:632–634. [DOI] [PubMed] [Google Scholar]
- 19. Overlack A, Ruppert M, Kolloch R, Gobel B, Kraft K, Diehl J, Schmitt W, Stumpe KO. Divergent hemodynamic and hormonal responses to varying salt intake in normotensive subjects. Hypertension. 1993;22:331–338. [DOI] [PubMed] [Google Scholar]
- 20. Draaijer P, de Leeuw P, Maessen J, van Hooff J, Leunissen K. Salt‐sensitivity testing in patients with borderline hypertension: reproducibility and potential mechanisms. J Hum Hypertens. 1995;9:263–269. [PubMed] [Google Scholar]
- 21. Schmidlin O, Forman A, Leone A, Sebastian A, Morris RC Jr. Salt sensitivity in blacks: evidence that the initial pressor effect of NaCl involves inhibition of vasodilatation by asymmetrical dimethylarginine. Hypertension. 2011;58:380–385. [DOI] [PubMed] [Google Scholar]
- 22. Kawasaki T, Delea CS, Bartter FC, Smith H. The effect of high‐sodium and low‐sodium intakes on blood pressure and other related variables in human subjects with idiopathic hypertension. Am J Med. 1978;64:193–198. [DOI] [PubMed] [Google Scholar]
- 23. Bigazzi R, Bianchi S, Baldari D, Sgherri G, Baldari G, Campese VM. Microalbuminuria in salt‐sensitive patients. A marker for renal and cardiovascular risk factors. Hypertension. 1994;23:195–199. [DOI] [PubMed] [Google Scholar]
- 24. Strazzullo P, Galletti F, Dessi‐Fulgheri P, Ferri C, Glorioso N, Malatino L, Mantero F, Manunta P, Semplicini A, Ghiadoni L, Zoccali C. Prediction and consistency of blood pressure salt‐sensitivity as assessed by a rapid volume expansion and contraction protocol. Salt‐Sensitivity Study Group of the Italian Society of Hypertension. J Nephrol. 2000;13:46–53. [PubMed] [Google Scholar]
- 25. Gerdts E, Lund‐Johansen P, Omvik P. Reproducibility of salt sensitivity testing using a dietary approach in essential hypertension. J Hum Hypertens. 1999;13:375–384. [DOI] [PubMed] [Google Scholar]
- 26. Mattes RD, Falkner B. Salt‐sensitivity classification in normotensive adults. Clin Sci (Lond). 1999;96:449–459. [PubMed] [Google Scholar]
- 27. Obarzanek E, Proschan MA, Vollmer WM, Moore TJ, Sacks FM, Appel LJ, Svetkey LP, Most‐Windhauser MM, Cutler JA. Individual blood pressure responses to changes in salt intake: results from the DASH‐Sodium trial. Hypertension. 2003;42:459–467. [DOI] [PubMed] [Google Scholar]
- 28. Zoccali C, Mallamaci F, Cuzzola F, Leonardis D. Reproducibility of the response to short‐term low salt intake in essential hypertension. J Hypertens. 1996;14:1455–1459. [DOI] [PubMed] [Google Scholar]
- 29. Morris RC, Schmidlin O, Sebastian A, Tanaka M, Kurtz TW. Vasodysfunction that involves renal vasodysfunction, not abnormally increased renal retention of sodium, accounts for the initiation of salt‐induced hypertension. Circulation. 2016;137:881–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fujiwara N, Osanai T, Kamada T, Katoh T, Takahashi K, Okumura K. Study on the relationship between plasma nitrite and nitrate level and salt sensitivity in human hypertension: modulation of nitric oxide synthesis by salt intake. Circulation. 2000;101:856–861. [DOI] [PubMed] [Google Scholar]
- 31. Fang Y, Mu JJ, He LC, Wang SC, Liu ZQ. Salt loading on plasma asymmetrical dimethylarginine and the protective role of potassium supplement in normotensive salt‐sensitive Asians. Hypertension. 2006;48:724–729. [DOI] [PubMed] [Google Scholar]
- 32. Campese VM, Romoff MS, Levitan D, Saglikes Y, Friedler RM, Massry SG. Abnormal relationship between sodium intake and sympathetic nervous system activity in salt‐sensitive patients with essential hypertension. Kidney Int. 1982;21:371–378. [DOI] [PubMed] [Google Scholar]
- 33. Yatabe MS, Yatabe J, Yoneda M, Watanabe T, Otsuki M, Felder RA, Jose PA, Sanada H. Salt sensitivity is associated with insulin resistance, sympathetic overactivity, and decreased suppression of circulating renin activity in lean patients with essential hypertension. Am J Clin Nutr. 2010;92:77–82. [DOI] [PubMed] [Google Scholar]
- 34. Hamlyn JM, Blaustein MP. Salt sensitivity, endogenous ouabain and hypertension. Curr Opin Nephrol Hypertens. 2013;22:51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Aburto NJ, Ziolkovska A, Hooper L, Elliott P, Cappuccio FP, Meerpohl JJ. Effect of lower sodium intake on health: systematic review and meta‐analyses. BMJ. 2013;346:f1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Luft FC, Rankin LI, Bloch R, Weyman AE, Willis LR, Murray RH, Grim CE, Weinberger MH. Cardiovascular and humoral responses to extremes of sodium intake in normal black and white men. Circulation. 1979;60:697–706. [DOI] [PubMed] [Google Scholar]
- 37. Clemmer JS, Pruett WA, Coleman TG, Hall JE, Hester RL. Mechanisms of blood pressure salt sensitivity: new insights from mathematical modeling. Am J Physiol Regul Integr Comp Physiol. 2016. Available at: http://ajpregu.physiology.org/content/early/2016/12/09/ajpregu.00353.2016. Accessed March 17, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Weinberger MH, Stegner JE, Fineberg NS. A comparison of two tests for the assessment of blood pressure responses to sodium. Am J Hypertens. 1993;6:179–184. [PubMed] [Google Scholar]
- 39. Orlov SN, Koltsova SV, Kapilevich LV, Gusakova SV, Dulin NO. NKCC1 and NKCC2: the pathogenetic role of cation‐chloride cotransporters in hypertension. Genes Dis. 2015;2:186–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chipperfield AR, Harper AA. Chloride in smooth muscle. Prog Biophys Mol Biol. 2000;74:175–221. [DOI] [PubMed] [Google Scholar]
- 41. Morimoto A, Uzu T, Fujii T, Nishimura M, Kuroda S, Nakamura S, Inenaga T, Kimura G. Sodium sensitivity and cardiovascular events in patients with essential hypertension. Lancet. 1997;350:1734–1737. [DOI] [PubMed] [Google Scholar]
- 42. Weinberger MH, Fineberg NS, Fineberg SE, Weinberger M. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension. 2001;37:429–432. [DOI] [PubMed] [Google Scholar]
- 43. Hall JE. Renal dysfunction, rather than non‐renal vascular dysfunction, mediates salt‐induced hypertension. Circulation. 2016;137:894–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kurtz TW, DiCarlo SE, Morris RC. Logical issues with the pressure natriuresis theory of chronic hypertension. Am J Hypertens. 2016;29:1325–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lohmeier TE, Pruett WA. Illogical critiques of the pressure natriuresis theory of chronic hypertension. Am J Hypertens. 2016;29:1332–1334. [DOI] [PubMed] [Google Scholar]
- 46. Kurtz TW, Dominiczak AF, DiCarlo SE, Pravenec M, Morris RC. Molecular based mechanisms of Mendelian forms of salt‐dependent hypertension: questioning the prevailing theory. Hypertension. 2015;65:932–941. [DOI] [PubMed] [Google Scholar]
- 47. Kurtz TW, DiCarlo SE, Pravenec M, Schmidlin O, Tanaka M, Morris RC. An alternative hypothesis to the widely held view that renal excretion of sodium accounts for resistance to salt‐induced hypertension. Kidney Int. 2016;90:965–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Averina VA, Othmer HG, Fink GD, Osborn JW. A mathematical model of salt‐sensitive hypertension: the neurogenic hypothesis. J Physiol. 2015;593:3065–3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pettersen KH, Bugenhagen SM, Nauman J, Beard DA, Omholt SW. Arterial stiffening provides sufficient explanation for primary hypertension. PLoS Comput Biol. 2014;10:e1003634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Leenen FH. The central role of the brain aldosterone‐“ouabain” pathway in salt‐sensitive hypertension. Biochim Biophys Acta. 2010;1802:1132–1139. [DOI] [PubMed] [Google Scholar]
- 51. Blaustein MP, Leenen FH, Chen L, Golovina VA, Hamlyn JM, Pallone TL, Van Huysse JW, Zhang J, Wier WG. How NaCl raises blood pressure: a new paradigm for the pathogenesis of salt‐dependent hypertension. Am J Physiol Heart Circ Physiol. 2012;302:H1031–H1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Blaustein MP, Chen L, Hamlyn JM, Leenen FH, Lingrel JB, Wier WG, Zhang J. Pivotal role of alpha2 Na+ pumps and their high affinity ouabain binding site in cardiovascular health and disease. J Physiol. 2016;594:6079–6103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Laffer CL, Elijovich F. Differential predictors of insulin resistance in nondiabetic salt‐resistant and salt‐sensitive subjects. Hypertension. 2013;61:707–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Elijovich F, Laffer CL, Amador E, Gavras H, Bresnahan MR, Schiffrin EL. Regulation of plasma endothelin by salt in salt‐sensitive hypertension. Circulation. 2001;103:263–268. [DOI] [PubMed] [Google Scholar]
- 55. Laffer CL, Bolterman RJ, Romero JC, Elijovich F. Effect of salt on isoprostanes in salt‐sensitive essential hypertension. Hypertension. 2006;47:434–440. [DOI] [PubMed] [Google Scholar]
- 56. Morris RC Jr, Sebastian A, Forman A, Tanaka M, Schmidlin O. Normotensive salt sensitivity—effects of race and dietary potassium. Hypertension. 1999;33:18–23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Features of Dietary Protocols That Have Been Tested for Reproducibility in Classifying Subjects as SS or SR


