Abstract
Nitric oxide (NO) regulates platelet activation by cGMP-dependent mechanisms and by mechanisms that are not completely defined. Platelet activation includes exocytosis of platelet granules, releasing mediators that regulate interactions between platelets, leukocytes, and endothelial cells. Exocytosis is mediated in part by N-ethylmaleimide-sensitive factor (NSF), an ATPase that disassembles complexes of soluble NSF attachment protein receptors. We now demonstrate that NO inhibits exocytosis of dense granules, lysosomal granules, and α-granules from human platelets by S-nitrosylation of NSF. Platelets lacking endothelial NO synthase show increased rolling on venules, increased thrombosis in arterioles, and increased exocytosis in vivo. Regulation of exocytosis is thus a mechanism by which NO regulates thrombosis.
Keywords: α-granules, nitric oxide
Nitric oxide (NO) plays a major role in vascular homeostasis, regulating vascular tone, leukocyte trafficking, and platelet adhesion and aggregation (1–4). NO regulates thrombosis in part by inhibition of platelet adhesion and aggregation (2, 5, 6). One well described pathway by which NO regulates platelet activation is mediated by guanylate cyclase (7–24). NO activates guanylate cyclase in platelets, leading to an increase in cGMP (25–27). Targets of cGMP in platelets include cGMP-regulated phosphodiesterases (PDEs) PDE2 and PDE5, cGMP-dependent protein kinase type Iβ, and perhaps cAMP-dependent protein kinases as well (28). The cGMP pathway mediates many of the effects of NO on platelet activation, such as inhibition of platelet aggregation. Thus, cGMP is a major mediator of NO signal transduction in platelets.
NO also regulates platelets by cGMP-independent pathways. For example, certain NO donors inhibit platelet aggregation in a cGMP-independent manner (12, 29–31). Furthermore, NO activates platelet ADP-ribosyltransferase in a cGMP-independent manner (32). Finally, NO inhibits Ca2+ mobilization in platelets independent of cGMP (33). Thus, NO inhibits platelet activation by both cGMP-dependent and less well characterized cGMP-independent pathways.
Exocytosis of platelet granules is an important process by which platelets mediate thrombosis and vascular inflammation. The three types of platelet granules: dense granules, α-granules, and lysosomal granules, contain molecules that are released into the blood or are translocated to the platelet surface after exocytosis. Dense granules, the first to be released upon activation, contain small molecules such as serotonin, ATP and ADP, which are involved in platelet recruitment and activation. Contents of the α-granule include von Willebrand factor, P-selectin, and β-thromboglobulin, which promote platelet adherence, aggregation, and rolling on vessel walls. Lysosomal granules are the last to be released and contain degradative enzymes (34, 35).
Platelet granule exocytosis is mediated by a set of proteins that regulate vesicle trafficking (35–38). Soluble N-ethylmaleimide-sensitive factor (NSF) attachment receptors (SNAREs) determine the specificity of the fusion reaction between a granule and its target membrane. SNAREs, localized on vesicles (v-SNAREs) and on target membranes (t-SNAREs) interact with each other, forming stable SNARE complexes, and localize the vesicle to a particular target membrane, bringing the two membranes into apposition. The SNAREs expressed in platelets include syntaxin-2, syntaxin-4, soluble NSF-attachment protein (SNAP)-23, vesicle-associated membrane protein (VAMP)-3, and VAMP-8 (39, 40).
NSF and its adapter protein, α-SNAP, are also necessary for platelet granule exocytosis (41, 42). NSF interacts with SNARE complexes by means of α-SNAP, hydrolyzes ATP, and disassembles the SNARE complex (43, 44). NSF was originally identified as a protein necessary for intracisternal Golgi transport whose activity was sensitive to N-ethylmaleimide (NEM) (45). NEM alkylates cysteine residues, and cysteine residues are also a common site of posttranslational NO modification (46, 47). Others have shown that NO regulates platelet granule release by unknown mechanisms (48, 49). We recently showed that NO regulates vascular inflammation by controlling endothelial release of von Willebrand factor (50). We now demonstrate that NO regulates platelet granule exocytosis by reversibly modifying NSF.
Materials and Methods
Platelet Collection, Purification, and Treatment. Normal healthy blood donors were recruited. Subjects were excluded if they had used aspirin or a nonsteroidal antiinflammatory agent 10 days before the blood draw. Blood was collected by venipuncture into sodium citrate anticoagulant tubes. Whole blood was centrifuged at 180 × g for 15 min to isolate the top layer of platelet-rich plasma (PRP).
Diethylamine NONOate (DEA-NONOate) (Cayman Chemical) was used as a NO donor. DEA-NONOate was diluted in 0.01 M NaOH and incubated with PRP for 10 min. l-nitroarginine methyl ester (l-NAME) (Sigma) was used to inhibit endogenous platelet NOS. PRP was incubated with 1 mM l-NAME for 20 min. For studies using N-acetylcysteine (NAC) (Sigma), PRPs were first incubated with DEA-NONOate for 10 min, and then incubated with 1 mM NAC for 10 min. To inhibit platelet guanylate cyclase, 1-H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) was solubilized in DMSO and PBS (DMSO not exceeding 20 μM), and then 0, 0.1, 1.0, and 10 μM ODQ (Sigma) was incubated with diluted PRP 15 min before NO donor addition.
Wild-type Vs. Endothelial NO Synthase (eNOS) Null Platelet Granule Exocytosis. Platelets were isolated from mice as described by others (50, 51). Washed platelets were diluted 1:20 in Tyrode's buffer and incubated with control or thrombin (Sigma) for 15 min. FITC-labeled anti-mouse P-selectin antibody (BD Pharmingen) was added and incubated for 20 min. Cells were fixed with 1% formalin and analyzed by FACS.
Assays of Exocytosis. Exocytosis was analyzed by FACS. PRP was diluted 1:20 in cation-free Tyrode's buffer. After incubation with the NO donor, 15 μM of the platelet activator, thrombin receptor activating-peptide (TRAP)-6 (Bachem), or buffer was added, and the mixture was incubated for 15 min. PRP (200 μl) was added to 10 μl of a phycoerytherin-labeled antibody to CD62P (P-selectin), CD63 (GP53) (BD Transduction Laboratories), or an isotype control for 20 min. PRP was then fixed in 1% formalin. Surface P-selectin and GP53 translocation was measured by FACS analysis (FACSCalibur, Becton Dickinson).
Exocytosis was also analyzed by an ELISA. β-Thromboglobulin release into the supernatant was measured by using an ELISA (Diagnostica Stago, Parsippany, NJ).
Dense-granule exocytosis was quantified by measuring ATP release from activated platelets, using a Chrono-Lume (Chrono-Log, Havertown, PA) luciferase-binding detection assay to measure luciferin-luciferase binding to ATP.
Permeabilization of Platelets. PRP was collected, pelleted at 500 × g for 2.5 min, and suspended in buffer as described (41). Briefly, 20 μl of platelets were added to 60 μl of buffer and 10 μg/ml saponin (Sigma) to permeabilize the platelets. Antibody (300 μg/ml) to IgG or to NSF (Santa Cruz Biotechnology) was added to the membrane permeable platelets and incubated for 20 min. Platelets were then activated by addition of 100 mM CaCl2.
NSF Interaction with Syntaxin-4 Assays. The interaction of NSF and syntaxin-4 was studied in vitro and ex vivo. In vitro interaction studies used recombinant His6-NSF, His6-α-SNAP, and GST-syntaxin-4. Recombinant proteins were expressed in bacteria and purified. Recombinant NSF (0.5 μg) was incubated with DEA-NONOate for 10 min and then added to equal amounts of α-SNAP and GST-syntaxin-4. The incubation buffer was 4 mM Hepes/0.1 M NaCl/1 mM EDTA/3.5 mM CaCl2/0.5% Nonidet P-40. Either 10 mM ATP or ATP-γS with 20 mM MgCl2 was added to some samples along with 50 μl of binding buffer, and 20 μl of 50% glutathione-Sepharose beads. The mixture was incubated for1hat 4°C, washed in binding buffer, and boiled for 3 min with SDS sample buffer. Samples were fractionated on 4–15% precast gels (Bio-Rad) and immunoblotted.
Studies of S-Nitrosylated NSF. The nitrosylated cysteine immunoprecipitation studies examined endogenous NSF in wild-type mouse platelets. Platelets were isolated by using buffers and methods described (50, 51) and pooled from wild-type mice. Equal aliquots of platelets were incubated with control, 0, 10, 100, or 1,000 μM DEA-NONOate or 1 μM A23187 (Sigma), or 5 mM l-NAME for 10 min. Platelets were then pelleted by centrifugation at 3,000 × g for 15 min, and lysed in NETN lysis buffer. The lysate was incubated with a monoclonal antibody to nitrosocysteine (AG Scientific) and protein G (Sigma) for 4 h. Samples were washed, SDS sample buffer was added, boiled for 3 min, and fractionated on a 4–15% gel (Bio-Rad). Samples were immunoblotted with monoclonal antibody to NSF (BD Transduction Laboratories).
NSF Addition to Permeabilized Platelets. Platelets were permeabilized as described above, incubated with 1 mM DEA-NONOate or control. Platelets were then incubated with control, 1.5 μg of recombinant NSF, or NSF incubated with 1 mM DEA-NONOate. Platelets were stimulated or not stimulated with 5 μM TRAP and 25 μM Ca2+, and exocytosis was measured by FACS for surface expression of P-selectin.
Platelet Granule Exocytosis in Shed Blood. The distal 3 mm of the tail of anesthetized wild-type and eNOS null mice were amputated and immersed into Tyrode's buffer with 30 units/ml heparin. Blood shed from the amputated tail was collected for 30 sec, and antibody to P-selectin was added for 20 min. Samples were fixed with 1% formalin and analyzed by FACS for surface P-selectin expression.
Intravital Microscopy. Intravital microscopy was performed as described by others (50, 51). Platelets were isolated and purified from wild-type or eNOS-/- mice (The Jackson Laboratory) and incubated for 20 min with 1 μM calcein-AM (Molecular Probes). Wild-type mice were anesthetized with ketamine (80 mg/kg) and xylazine (13 mg/kg) and then injected intravenously with 5 × 107 platelets for the rolling study or 1 × 108 platelets for the thrombosis study. Mesentery was exteriorized, venules (120–150 μm in diameter) or arterioles (60–80 μm in diameter) were selected, and the mouse mesentery was prepared on an inverted fluorescent microscope (Nikon). Endothelial damage was induced by the addition of 250 μM FeCl3 to venules or 500 μM FeCl3 to arterioles, and images of platelet rolling or thrombus formation were captured with a digital camera (Retiga). Platelet rolling was determined by counting the number of platelets that remained transiently within a frame for the 30-ms collection time. Time to formation of the first thrombus >10 μm in diameter was recorded.
Results
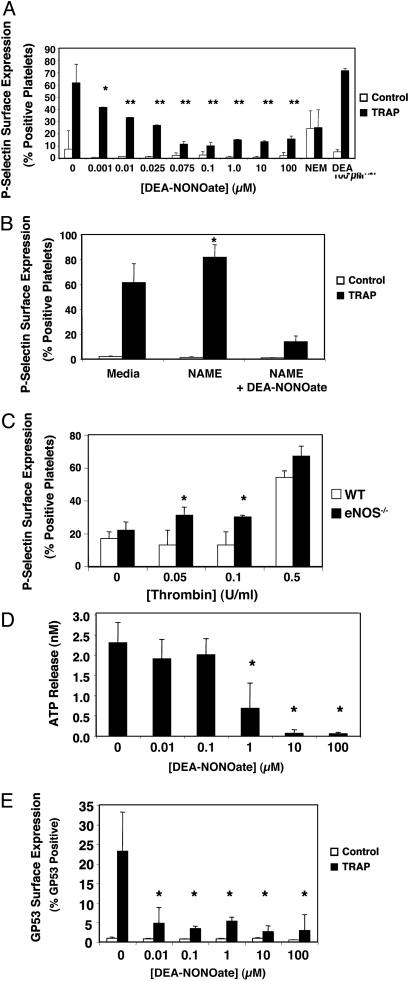
NO Inhibition of Platelet Granule Exocytosis. To explore the effects of NO on platelet granule exocytosis, we harvested human platelets, added exogenous NO or control, and then activated platelets with TRAP. Platelet exocytosis of α-granules was measured by FACS analysis of surface translocation of P-selectin. The NO donor DEA-NONOate inhibits TRAP activation of platelet α-granule exocytosis in a dose-dependent manner (Fig. 1A). As little as 10 nM NO inhibits 50% of α-granule exocytosis.
Fig. 1.
NO inhibition of platelet granule exocytosis. (A) Exogenous NO inhibits α-granule exocytosis: P-selectin. Human platelets were incubated with DEA-NONOate for 15 min and treated with 15 μM TRAP, and the amount of P-selectin on the surface was measured by FACS. (n = 6 ± SD, *, P = 0.05; **, P < 0.01 vs. TRAP without NO donor.) (B) Endogenous NO inhibits α-granule exocytosis. Human platelets were pretreated with 1 mM l-NAME for 15 min to inhibit endogenous NOS and treated with 15 μM TRAP, and the amount of P-selectin on the surface was measured by FACS. (n = 3–6 ± SD, *, P < 0.01 vs. 0 mM l-NAME.) (C) Endogenous NO inhibits α-granule exocytosis. Platelets from wild-type and eNOS-/- mice were treated with control or thrombin, and the amount of P-selectin on the surface was measured by FACS. (n = 3 ± SD, *, P < 0.01 vs. WT.) (D) NO inhibits dense-granule exocytosis. Human platelets were pretreated with DEA-NONOate for 15 min and treated with 15 μM TRAP, and the amount of released ATP was measured by chemiluminescence. (n = 3 ± SD, *, P < 0.01 vs. 0 μM.) (E) NO inhibits lysosomal granule release. Human platelets were pretreated with increasing amounts of DEA-NONOate for 15 min and treated with 15 μM TRAP, and the amount of gp53 (CD63) on the surface was measured by FACS. (n = 3 ± SD, *, P = < 0.01 vs. 0 μM.)
We next studied the effect of endogenously produced NO on platelet granule exocytosis. We incubated human platelets with the NOS inhibitor l-NAME, activated the platelets, and measured P-selectin as before. l-NAME inhibition of NOS increases granule exocytosis, suggesting that endogenous NO decreases exocytosis (Fig. 1B). We then studied murine platelets from mice lacking eNOS. We incubated murine platelets from wild-type and eNOS-/- mice with increasing amounts of thrombin and measured the translocation of P-selectin. At low doses of thrombin endogenous NO inhibits granule exocytosis (Fig. 1C).
We also examined the effect of exogenous NO on exocytosis of dense granules and lysosomal granules in human platelets. NO decreases exocytosis of dense granules (Fig. 1D) and lysosomal granules (Fig. 1E). These data show that exogenous and endogenous NO inhibits exocytosis of all three types of platelet granules.
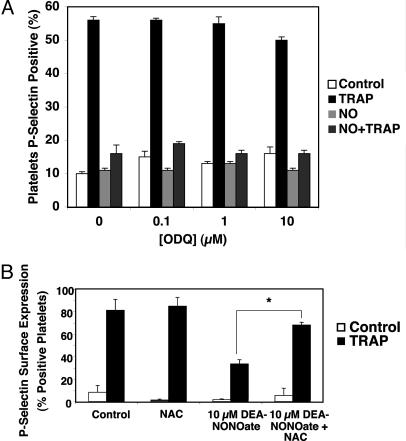
NO Inhibition Is Independent of cGMP. Previous studies demonstrate that NO inhibits platelet aggregation in a guanylate cyclase-dependent manner. To examine the role of cGMP in mediating NO inhibition of platelet granule exocytosis, we pretreated human platelets with buffer or 0, 0.1, 1.0, or 10 μM ODQ, an inhibitor of guanylate cyclase. We then treated the platelets with 10 μM DEA-NONOate. Finally, we stimulated platelets with control or TRAP and measured P-selectin surface translocation. ODQ does not block NO inhibition of exocytosis (Fig. 2A). These results suggest that NO inhibits granule exocytosis through a mechanism independent of guanylate cyclase.
Fig. 2.
NO inhibition of platelet α-granule exocytosis is independent of cGMP. (A) Inhibition of guanylate cyclase does not affect NO inhibition of exocytosis. Human platelets were pretreated with ODQ for 10 min, then treated with 10 μM DEA-NONOate for 15 min and finally treated with control or 15 μM TRAP, and the amount of P-selectin on the surface was measured by FACS. (n = 3 ± SD.) (B) The denitrosylating agent NAC reverses NO inhibition of platelet granule exocytosis. Human platelets were pretreated with 10 μM DEA-NONOate for 10 min, then treated with 1 mM N-acetyl-cysteine for 10 min and finally treated with control or 15 μM TRAP, and the amount of P-selectin on the surface was measured by FACS. (n = 3 ± SD.)
To explore the role of S-nitrosylation in mediating NO inhibition of platelet granule exocytosis, we pretreated human platelets with control or DEA-NONOate for 10 min and then treated platelets with the denitrosylating agent NAC for 10 min. Finally, we stimulated platelets with control or TRAP and measured P-selectin surface translocation. NAC reverses the ability of NO to inhibit granule exocytosis (Fig. 2B). These results support the hypothesis that NO inhibits platelet granule exocytosis in part through S-nitrosylation of unknown proteins.
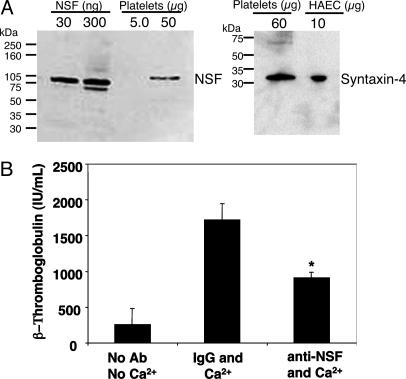
NSF Regulates Platelet Granule Exocytosis. NSF has been shown to regulate intracellular trafficking and exocytosis. To examine whether NSF is a target of NO, we first confirmed that platelets contain NSF (Fig. 3A). Platelets also contain syntaxin-4, a SNARE family member that interacts with NSF and regulates exocytosis of granules in a variety of cell types (Fig. 3A).
Fig. 3.
NSF regulates platelet granule exocytosis. (A) Human platelets contain NSF and syntaxin-4. Lysates of human platelets, recombinant NSF, or human aortic endothelial cells (HAEC) were immunoblotted with antibody to NSF (Left) or syntaxin-4 (Right). (B) NSF regulates α-granule release: antibody inhibition. Human platelets were permeabilized, incubated with antibody, and activated with Ca2+. β-Thromboglobulin release was measured by ELISA. (n = 4–6 ± SD, *, P < 0.05 vs. IgG and Ca2+.)
We next verified that NSF regulates granule exocytosis in platelets. We permeabilized human platelets with saponin, added antibody to NSF to inhibit it, activated the platelets with Ca2+, and measured α-granule exocytosis by using an ELISA for β-thromboglobulin. Antibody to NSF decreases granule exocytosis (Fig. 3B). Thus, human platelets contain NSF, and NSF regulates α-granule exocytosis.
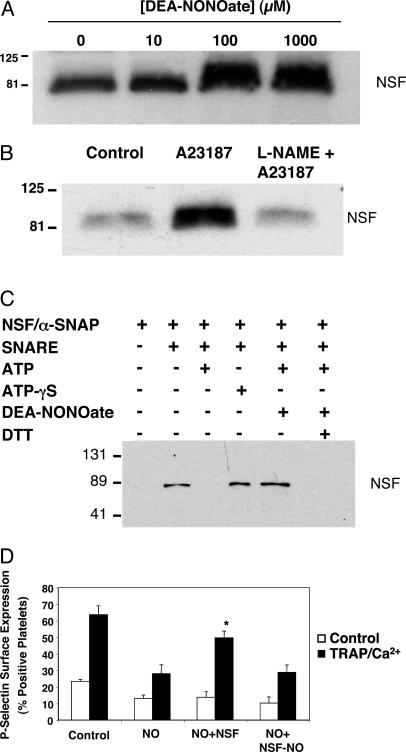
NO Inhibits NSF. We next demonstrated that NSF within platelets can be nitrosylated. Platelets from wild-type mice were isolated and incubated with 0, 10, 100, or 1,000 μM DEA-NONOate or control, lysates were immunoprecipitated with antibody to nitrosocysteine, and fractions were immunoblotted with antibody to NSF. Exogenous NO donors nitrosylate NSF ex vivo (Fig. 4A). To demonstrate that endogenous NO can nitrosylate NSF, we treated platelets with the Ca2+ ionophore A23187 with the NOS inhibitor l-NAME, followed by immunoprecipitation with antibody to nitrosocysteine residues and immunoblotting for NSF as above. A23187 increases S-nitrosylation of NSF, a phenomenon inhibited by l-NAME (Fig. 4B). Thus, exogenous and endogenous NO can S-nitrosylate NSF.
Fig. 4.
NO inhibits NSF regulation of platelet granule exocytosis. (A) NSF is a target of exogenous NO: dose–response. Murine platelets were harvested, and equal numbers were incubated with control or DEA-NONOate, lysed, immunoprecipitated with antibody to nitrosocysteine, and immunoblotted with antibody to NSF. NSF is nitrosylated in a dose-dependent manner. (Each step was repeated three times with similar results.) (B) NSF is a target of endogenous NO. Murine platelets were harvested, preincubated with control or l-NAME 5 mM, treated with control or 1 μM Ca2+ ionophore, lysed, immunoprecipitated with antibody to nitrosocysteine, and immunoblotted with antibody to NSF. (C) NO inhibits NSF separation from syntaxin-4 in vitro. Recombinant His6-NSF was pretreated or not pretreated with DEA-NONOate and incubated with a-SNAP and GST-SNARE fusion polypeptides expressed in platelets. ATP or ATP-gS was added, and the mixture was precipitated with glutathione-Sepharose. Precipitated proteins were immunoblotted with antibody to the NSF tag. (D) NSF is a target of NO. Platelets were permeabilized, exposed to 1 mM DEA-NONOate, and then incubated with recombinant NSF or nitrosylated NSF. Platelets were then stimulated or not stimulated with TRAP and Ca2+, and exocytosis was measured by FACS. (n = 3 ± SD, *, P < 0.05 for NO plus NSF vs. NO plus NSF-NO.)
NSF regulates exocytosis in part by interacting with and disassembling SNARE complexes. To explore the effect of NO on the interaction of NSF and SNARE molecules, we prepared recombinant His6-NSF, His6-α-SNAP, GST-syntaxin-4, GST-VAMP-3, and GST-SNAP-23. We pretreated NSF with DEA-NONOate, and then added α-SNAP, syntaxin-4, VAMP-3, and SNAP-23. The mixture was incubated for 1 h, precipitated with glutathione-Sepharose beads, and precipitants were immunoblotted with antibody to NSF. ATP-γS increases the interaction between NSF and syntaxin-4, as expected (Fig. 4C). ATP enables NSF to separate from syntaxin-4, also as expected (Fig. 4C). However, NO blocks the ability of NSF to separate from syntaxin-4, even in the presence of ATP (Fig. 4C). Finally, DTT restores the ability of NSF to disassemble the SNARE complex (Fig. 4C). These data suggest that NO reversibly blocks the ability of NSF to disassemble a SNARE complex.
To demonstrate that NSF is a target of NO in platelets, we permeabilized platelets as above, pretreated the platelets with NO donors, and then added either recombinant NSF or S-nitrosylated NSF to the platelets. The platelets were activated with TRAP and Ca2+, and exocytosis was measured by FACS analysis of P-selectin expression. NO inhibits α-granule exocytosis as expected (Fig. 4D). Adding NSF to the platelets restores exocytosis (Fig. 4D). However, S-nitrosylated NSF is incapable of reversing the effects of NO. These data demonstrate that NSF is a target of NO in platelets.
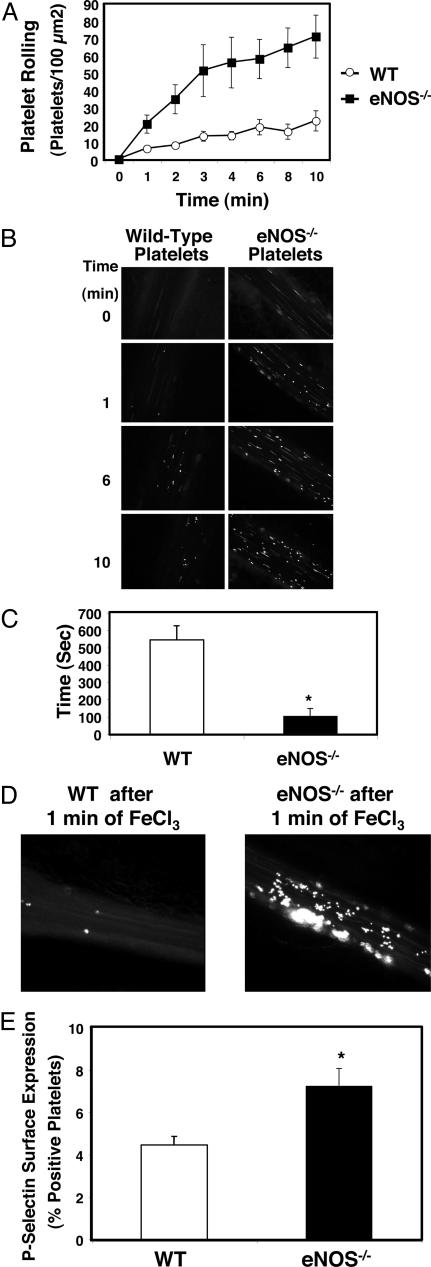
NO Regulates Platelet Function in Vivo. We explored the effect of endogenous NO in regulating platelet activation in vivo, using digital imaging of intravital microscopy. Platelets were isolated from eNOS-/- or wild-type mice, fluorescently labeled, and injected intravenously into wild-type mice. Mesenteric venules were damaged with ferric chloride (FeCl3), and the number of rolling platelets was counted over time. Platelets from mice deficient in eNOS demonstrate increased rolling compared with platelets from wild-type mice (Fig. 5 A and B).
Fig. 5.
NO inhibits platelet adherence and aggregation in vivo. (A) Platelet rolling. Wild-type mice were anesthetized and injected with calcein-AM-labeled platelets from wild-type (WT) or eNOS-/- mice. The mesentery was externalized, and venules 120–150 μm in diameter were treated with 250 mM FeCl3. Platelet rolling on venules was imaged with a digital fluorescent camera (n = 7–8 ± SEM). (B) Digital fluorescence images of platelet rolling. Wild-type mice were anesthetized and injected with calcein-AM-labeled platelets from wild-type or eNOS-/- mice. The mesentery was externalized, and venules 120–150 μm in diameter were treated with 250 mM FeCl3. Platelet rolling on venules was imaged with a digital fluorescent camera 0, 1, 6, and 10 min after FeCl3 treatment. (Representative images from n = 7–8.) (C) Thrombosis. Mice were injected with labeled platelets as above. The mesentery was externalized, and arterioles 50–80 μm in diameter were treated with 500 mM FeCl3. The time to formation of the first thrombus >10 μm in size was recorded (n = 5 ± SD). (D) Digital fluorescent images of thrombosis. Wild-type and eNOS-/- mice were injected with labeled wild-type platelets, arterioles were treated with FeCl3 as above, and a digital fluorescent camera was used to visualize formation of the first thrombus >10 μm in size. (E) Exocytosis in shed platelets. The distal 3 mm of wild-type and eNOS-/- mice tails were amputated, and shed blood was collected. Platelets were analyzed for surface P-selectin expression by FACS. Shed platelets from eNOS-/- have increased exocytosis as measured by P-selectin expression compared with WT mice. (n = 3 ± SD, *, P < 0.002 for WT vs. eNOS-/-.)
We also examined the role of endogenous NO in regulating platelet thrombosis in vivo. Fluorescently labeled eNOS-/- and wild-type platelets were injected into wild-type mice, and thrombosis in arterioles was induced by superfusion of FeCl3. Arterioles were imaged and the time to the formation of stable thrombi of >10 μm was recorded. Platelets deficient in eNOS exhibit a more rapid formation of thrombi compared with platelets from wild-type mice (Fig. 5 C and D).
Finally, we measured the effect of endogenous NO on platelet exocytosis in vivo. Platelets were collected from wild-type and eNOS-/- mice during trail bleeding and analyzed for surface expression of P-selectin by FACS. The presence of eNOS is associated with a decrease in platelet exocytosis (Fig. 5E).
These murine data show that NO regulates platelet rolling and thrombosis in vivo, and that NO regulates platelet exocytosis in vivo. Taken together, our data suggest that one mechanism by which NO regulates platelet function is by inhibition of platelet exocytosis.
Discussion
The major finding of our study is that NO inhibits platelet granule exocytosis by S-nitrosylating NSF and inhibiting the ability of NSF to disassemble the SNARE complex. Others have shown that NSF regulates the disassembly of the SNARE complex, a step necessary for exocytosis in a wide variety of biological systems (36, 42, 52). Reed and coworkers (39, 41) and Whiteheart and coworkers (53) have shown that NSF and SNARE molecules regulate platelet exocytosis. Our data show that NO covalently modifies NSF in platelets, thereby blocking exocytosis.
Extensive previous work of others has emphasized that NO inhibits platelet activation by cGMP-dependent mechanisms (7–24). The NO signaling pathways involving cGMP regulate platelet aggregation, a process that is mediated in part by gpIIb/IIIa conformational changes. We have identified an additional NO signaling pathway that regulates platelet exocytosis by a cGMP-independent process, S-nitrosylation of NSF. Taken together, these studies suggest that NO regulates platelets by at least two distinct pathways.
We first demonstrate that exogenous and endogenous NO inhibits platelet granule exocytosis (Fig. 1). We then confirmed that NSF regulates platelet granule exocytosis, and then showed that the disassembly of the SNARE complex is inhibited by NO modification of NSF (Figs. 3 and 4). Importantly, addition of NSF to platelets incubated with NO restores exocytosis, demonstrating that NO modification of NSF inhibits NSF activity in platelets (Fig. 4D). These data show that NSF is a target of NO in platelets. We also show that NO derived from endogenous eNOS regulates platelet function and granule exocytosis in vivo (Fig. 5).
Our previous work shows that NO inhibits endothelial exocytosis (50). Others have suggested that NO may increase exocytosis in platelets. For example, Busse and Fleming and coworkers (54) have shown that NO mediates insulin-triggered platelet exocytosis. However, the NO donor used in this study was a long-acting donor applied for a brief time. Nonetheless, insulin and thrombin may regulate exocytosis by a set of distinct pathways. Our current work also shows that NO regulates exocytosis in a cGMP-independent manner: ODQ does not affect NO inhibition of granule release (Fig. 2 A). Recent data suggest that cGMP pathways may stimulate platelet activation: platelet aggregation is decreased in cGMP-dependent protein kinase knockout mice (55). Thus, NO and cGMP may play multiple roles in the regulation of platelet activation.
NO regulates thrombosis in animals and humans (1, 2, 5). For example, Freedman et al. (6) demonstrated that bleeding times were decreased in eNOS null mice. An interesting aspect of their study is that the investigators also found that bleeding times in wild-type mice transfused with platelets lacking eNOS were markedly decreased compared with mice transfused with wild-type platelets. Thus, in addition to endothelial derived eNOS, platelet-derived eNOS regulates thrombosis. Our data suggest an explanation for this phenomenon: NO inhibits platelet granule exocytosis.
However, this previous study suggested that endogenous NO does not regulate P-selectin surface expression, in contrast to our results. This discrepancy can be resolved by considering the agonists and the concentrations used. Our study found that endogenous NO exerts its inhibitory effects at low levels of thrombin stimulation, not at maximal stimulatory doses of thrombin (Fig. 1C). In addition, other studies have demonstrated that platelet granule exocytosis responses may differ, depending on the agonist used to stimulate platelets (56).
Bleeding disorders such as Hemansky–Pudlak syndrome and Griscelli syndrome are caused by defects in platelet granule biogenesis and exocytosis, further demonstrating the fundamental importance of platelet exocytosis to hemostasis. The contribution of α-granules to thrombosis in vivo has recently been further underscored by Walther et al. (56) who showed that platelets lacking serotonin had impaired α-granule exocytosis ex vivo and decreased adhesion in vivo. Endogenous NO inhibition of platelet granule exocytosis is a mechanism to regulate thrombosis. NSF and SNARE molecules may be important new targets for novel therapies to treat bleeding and thrombotic diseases including, venous thrombosis, stroke, and myocardial infarction.
Acknowledgments
This work was supported by National Institutes of Health Grants R01 HL63706, R01 HL074061, P01 HL65608, and P01 HL56091; American Heart Association Grant EIG 0140210N; the Ciccarone Center; the John and Cora H. Davis Foundation (to C.J.L.); and National Institutes of Health Grants RR07002 and HL074945 (to C.N.M.).
Author contributions: C.N.M., R.B.S., W.M.B., and C.J.L. designed research; C.N.M., K.M., K.C., M.Y., and W.B. performed research; C.N.M., J.L.M., W.M.B., and N.F. contributed new reagents/analytic tools; C.N.M., R.J.A.M., and C.J.L. analyzed data; and C.N.M. and C.J.L. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: NSF, N-ethylmaleimide-sensitive factor; SNARE, soluble NSF attachment receptor; DEA-NONOate, diethylamine NONOate; VAMP, vesicle-associated membrane protein; SNAP, soluble NSF-attachment protein; PRP, platelet-rich plasma; l-NAME, l-nitroarginine methyl ester; NAC, N-acetylcysteine; ODQ, 1-H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one; eNOS, endothelial NO synthase; TRAP, thrombin receptor-activating peptide.
References
- 1.Moncada, S., Palmer, R. M. & Higgs, E. A. (1991) Pharmacol. Rev. 43, 109-142. [PubMed] [Google Scholar]
- 2.Loscalzo, J. (2001) Circ. Res. 88, 756-762. [DOI] [PubMed] [Google Scholar]
- 3.Michel, T. & Feron, O. (1997) J. Clin. Invest. 100, 2146-2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cirino, G., Fiorucci, S. & Sessa, W. C. (2003) Trends Pharmacol. Sci. 24, 91-95. [DOI] [PubMed] [Google Scholar]
- 5.Radomski, M. W., Palmer, R. M. & Moncada, S. (1987) Lancet 2, 1057-1058. [DOI] [PubMed] [Google Scholar]
- 6.Freedman, J. E., Sauter, R., Battinelli, E. M., Ault, K., Knowles, C., Huang, P. L. & Loscalzo, J. (1999) Circ. Res. 84, 1416-1421. [DOI] [PubMed] [Google Scholar]
- 7.Moro, M. A., Russel, R. J., Cellek, S., Lizasoain, I., Su, Y., Darley-Usmar, V. M., Radomski, M. W. & Moncada, S. (1996) Proc. Natl. Acad. Sci. USA 93, 1480-1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McDonald, L. J. & Murad, F. (1995) Adv. Pharmacol. 34, 263-275. [DOI] [PubMed] [Google Scholar]
- 9.Loscalzo, J. (1992) Am. J. Cardiol. 70, 18B-22B. [DOI] [PubMed] [Google Scholar]
- 10.Ignarro, L. J. (1990) Pharmacol. Toxicol. (Copenhagen) 67, 1-7. [DOI] [PubMed] [Google Scholar]
- 11.Gordge, M. P., Hothersall, J. S. & Noronha-Dutra, A. A. (1998) Br. J. Pharmacol. 124, 141-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsikas, D., Ikic, M., Tewes, K. S., Raida, M. & Frolich, J. C. (1999) FEBS Lett. 442, 162-166. [DOI] [PubMed] [Google Scholar]
- 13.Mendelsohn, M. E., O'Neill, S., George, D. & Loscalzo, J. (1990) J. Biol. Chem. 265, 19028-19034. [PubMed] [Google Scholar]
- 14.Pigazzi, A., Heydrick, S., Folli, F., Benoit, S., Michelson, A. & Loscalzo, J. (1999) J. Biol. Chem. 274, 14368-14375. [DOI] [PubMed] [Google Scholar]
- 15.Severina, I. S. (1998) Biochemistry 63, 794-801. [PubMed] [Google Scholar]
- 16.Radomski, M. W. & Moncada, S. (1993) Adv. Exp. Med. Biol. 344, 251-264. [DOI] [PubMed] [Google Scholar]
- 17.Ignarro, L. J. (1989) Pharm. Res. 6, 651-659. [DOI] [PubMed] [Google Scholar]
- 18.Ignarro, L. J. (1989) FASEB J. 3, 31-36. [DOI] [PubMed] [Google Scholar]
- 19.Furchgott, R. F. & Vanhoutte, P. M. (1989) FASEB J. 3, 2007-2018. [PubMed] [Google Scholar]
- 20.Marletta, M. A., Tayeh, M. A. & Hevel, J. M. (1990) Biofactors 2, 219-225. [PubMed] [Google Scholar]
- 21.Rubanyi, G. M. (1991) J. Cell. Biochem. 46, 27-36. [DOI] [PubMed] [Google Scholar]
- 22.Luscher, T. F. (1992) Br. J. Clin. Pharmacol. 34, Suppl. 1, 29S-35S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Michel, T. & Smith, T. W. (1993) Am. J. Cardiol. 72, 33C-38C. [DOI] [PubMed] [Google Scholar]
- 24.Ignarro, L. J. (1999) Biosci. Rep. 19, 51-71. [DOI] [PubMed] [Google Scholar]
- 25.Radomski, M. W., Palmer, R. M. & Moncada, S. (1987) Biochem. Biophys. Res. Commun. 148, 1482-1489. [DOI] [PubMed] [Google Scholar]
- 26.Mellion, B. T., Ignarro, L. J., Ohlstein, E. H., Pontecorvo, E. G., Hyman, A. L. & Kadowitz, P. J. (1981) Blood 57, 946-955. [PubMed] [Google Scholar]
- 27.Busse, R., Luckhoff, A. & Bassenge, E. (1987) Naunyn-Schmiedeberg's Arch. Pharmacol. 336, 566-571. [DOI] [PubMed] [Google Scholar]
- 28.Feil, R., Lohmann, S. M., de Jonge, H., Walter, U. & Hofmann, F. (2003) Circ. Res. 93, 907-916. [DOI] [PubMed] [Google Scholar]
- 29.Pawloski, J. R., Swaminathan, R. V. & Stamler, J. S. (1998) Circulation 97, 263-267. [DOI] [PubMed] [Google Scholar]
- 30.Sogo, N., Magid, K. S., Shaw, C. A., Webb, D. J. & Megson, I. L. (2000) Biochem. Biophys. Res. Commun. 279, 412-419. [DOI] [PubMed] [Google Scholar]
- 31.Beghetti, M., Sparling, C., Cox, P. N., Stephens, D. & Adatia, I. (2003) Am. J. Physiol. 285, H637-H642. [DOI] [PubMed] [Google Scholar]
- 32.Brune, B. & Lapetina, E. G. (1990) Arch. Biochem. Biophys. 279, 286-290. [DOI] [PubMed] [Google Scholar]
- 33.Pernollet, M. G., Lantoine, F. & Devynck, M. A. (1996) Biochem. Biophys. Res. Commun. 222, 780-785. [DOI] [PubMed] [Google Scholar]
- 34.Harrison, P. & Cramer, E. M. (1993) Blood Rev. 7, 52-62. [DOI] [PubMed] [Google Scholar]
- 35.Furie, B., Furie, B. C. & Flaumenhaft, R. (2001) Thromb. Haemostasis 86, 214-221. [PubMed] [Google Scholar]
- 36.Jahn, R., Lang, T. & Sudhof, T. C. (2003) Cell 112, 519-533. [DOI] [PubMed] [Google Scholar]
- 37.Rothman, J. E. (1994) Nature 372, 55-63. [DOI] [PubMed] [Google Scholar]
- 38.Reed, G. L., Fitzgerald, M. L. & Polgar, J. (2000) Blood 96, 3334-3342. [PubMed] [Google Scholar]
- 39.Polgar, J., Chung, S. H. & Reed, G. L. (2002) Blood 100, 1081-1083. [DOI] [PubMed] [Google Scholar]
- 40.Flaumenhaft, R., Croce, K., Chen, E., Furie, B. & Furie, B. C. (1999) J. Biol. Chem. 274, 2492-2501. [DOI] [PubMed] [Google Scholar]
- 41.Polgar, J. & Reed, G. L. (1999) Blood 94, 1313-1318. [PubMed] [Google Scholar]
- 42.Whiteheart, S. W., Rossnagel, K., Buhrow, S. A., Brunner, M., Jaenicke, R. & Rothman, J. E. (1994) J. Cell Biol. 126, 945-954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.May, A. P., Whiteheart, S. W. & Weis, W. I. (2001) J. Biol. Chem. 276, 21991-21994. [DOI] [PubMed] [Google Scholar]
- 44.Tagaya, M., Wilson, D. W., Brunner, M., Arango, N. & Rothman, J. E. (1993) J. Biol. Chem. 268, 2662-2666. [PubMed] [Google Scholar]
- 45.Block, M. R., Glick, B. S., Wilcox, C. A., Wieland, F. T. & Rothman, J. E. (1988) Proc. Natl. Acad. Sci. USA 85, 7852-7856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stamler, J. S., Singel, D. J. & Loscalzo, J. (1992) Science 258, 1898-1902. [DOI] [PubMed] [Google Scholar]
- 47.Stamler, J. S. (1994) Cell 78, 931-936. [DOI] [PubMed] [Google Scholar]
- 48.Michelson, A. D., Benoit, S. E., Furman, M. I., Breckwoldt, W. L., Rohrer, M. J., Barnard, M. R. & Loscalzo, J. (1996) Am. J. Physiol. 270, H1640-H1648. [DOI] [PubMed] [Google Scholar]
- 49.Broekman, M. J., Eiroa, A. M. & Marcus, A. J. (1991) Blood 78, 1033-1040. [PubMed] [Google Scholar]
- 50.Matsushita, K., Morrell, C. N., Cambien, B., Yang, S. X., Yamakuchi, M., Bao, C., Hara, M. R., Quick, R. A., Cao, W., O'Rourke, B., et al. (2003) Cell 115, 139-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cambien, B., Bergmeier, W., Saffaripour, S., Mitchell, H. A. & Wagner, D. D. (2003) J. Clin. Invest. 112, 1589-1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sollner, T., Bennett, M. K., Whiteheart, S. W., Scheller, R. H. & Rothman, J. E. (1993) Cell 75, 409-418. [DOI] [PubMed] [Google Scholar]
- 53.Lemons, P. P., Chen, D. & Whiteheart, S. W. (2000) Biochem. Biophys. Res. Commun. 267, 875-880. [DOI] [PubMed] [Google Scholar]
- 54.Randriamboavonjy, V., Schrader, J., Busse, R. & Fleming, I. (2004) J. Exp. Med. 199, 347-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li, Z., Xi, X., Gu, M., Feil, R., Ye, R. D., Eigenthaler, M., Hofmann, F. & Du, X. (2003) Cell 112, 77-86. [DOI] [PubMed] [Google Scholar]
- 56.Walther, D. J., Peter, J. U., Winter, S., Holtje, M., Paulmann, N., Grohmann, M., Vowinckel, J., Alamo-Bethencourt, V., Wilhelm, C. S., Ahnert-Hilger, G. & Bader, M. (2003) Cell 115, 851-862. [DOI] [PubMed] [Google Scholar]