Abstract
Background
Elastic-scattering spectroscopy (ESS) can assess in vivo and in real-time the scattering and absorption properties of tissue related to underlying pathologies.
Objectives
To evaluate the potential of ESS for differentiating neoplastic from non-neoplastic polyps during colonoscopy.
Design
Pilot study, retrospective data analysis.
Setting
Academic practice.
Patients
A total of 83 patients undergoing screening/surveillance colonoscopy.
Interventions
ESS spectra of 218 polyps (133 non-neoplastic, 85 neoplastic) were acquired during colonoscopy. Spectral data were correlated to the classification of biopsy samples by 3 GI pathologists. High-dimensional methods were used to design diganostic algorithms.
Main Outcome Measurements
Diagnostic performance of ESS.
Results
Analysis of spectra from polyps of all sizes (N=218) resulted in a sensitivity of 91.5%, specificity of 92.2%, and accuracy of 91.9% with a high-confidence rate of 90.4%. Restricting analysis to polyps <1cm (n=179) resulted in a sensitivity of 87.0%, specificity of 92.1%, and accuracy of 90.6% with a high-confidence rate of 89.3%. Analysis of polyps ≤5 mm (n=157) resulted in a sensitivity of 86.8%, specificity of 91.2%, and accuracy of 90.1% with a high-confidence rate of 89.8%.
Limitations
Sample size, retrospective validation used to obtain performance estimates.
Conclusions
Results indicate that ESS permits accurate, real-time classification of polyps as neoplastic or non-neoplastic. ESS is a simple, low cost, clinically robust method with minimal impact on procedure flow, especially when integrated into standard endoscopic biopsy tools. Performance on polyps ≤ 5mm indicates that ESS may, in theory, achieve PIVI performance thresholds. ESS may one day prove to be a useful tool used in endoscopic screening and surveillance of colorectal cancer.
Keywords: spectrum analysis, spectroscopy, endoscopy, colorectal neoplasm
Introduction
In the United States, colorectal cancer (CRC) is the second leading cause of cancer death, with nearly 140,000 new cases and 50,000 deaths annually in recent years (1). Currently, the effectiveness of colonoscopic CRC prevention hinges on detecting and removing all polyps for histopathological evaluation (2). However, a large proportion (up to ~50%) of polyps removed will turn out to have been hyperplastic. Hyperplastic polyps carry negligible malignant potential especially if they are located in the distal colon and are < 5mm in size (3–6). Thus, the current practice of resecting all polyps detected at colonoscopy for subsequent histopathological assessment is inherently inefficient and adds time, cost, and incremental risk of bleeding and perforation (7, 8), to a high-demand, high-volume screening procedure. There is a great need for simple, rapid, widely deployable, low-cost methods for “smart” in situ assessment of polyp histology. Reliable polyp histology in real time would enable the more cost-effective “resect and discard” approach proposed for the management of small polyps with low malignant potential (9, 10). Several technologies for the endoscopic assessment of the malignant potential of a polyp have been investigated. These include standard white light endoscopy (WLE), high-definition WLE (9, 11, 12), electronic/virtual chromoendoscopy (13–21), and endomicroscopy (22–27).
Reflectance spectroscopy, specifically elastic-scattering spectroscopy (ESS) has shown promise for distinguishing pathologies in hollow organs lined by an epithelium (28) as well as in cystic and solid tissues including the breast, lymph nodes (29, 30), prostate (31), and thyroid (32, 33). In the gastrointestinal tract, ESS appears to be sensitive to dysplasia in the esophagus (34–39) and to a variety of colonic pathologies including neoplasia (40–45).
ESS is mediated by fiberoptic probes with specialized optical geometries and is sensitive to the absorption spectra of major native chromophores (e.g., oxy-/deoxy-hemoglobin) and, more importantly, to scattering properties that are directly influenced by the micromorphology of cells and tissues in proximity to the probe tip. ESS spectra derive from the wavelength-dependent optical scattering efficiency (and the effects of changes in the scattering angular probability) caused by the optical index gradients of cellular and subcellular structures. Thus, unlike Raman and fluorescence spectroscopies, ESS provides largely microstructural rather than biochemical information. Structural features like nuclear size, crowding, chromaticity, and chromatin granularity, as well as mitochondrial and organellar size and density, influence ESS spectra. These structural features are, to differing degrees, standard components of a histopathological assessment for neoplasia, but ESS is a real-time, point-source measurement that senses tissue morphology semi-quantitatively without actually rendering a microscopic image. In addition, because of its inherent simplicity and ease of miniaturization, ESS is low cost, clinically robust, and has minimal impact on procedure flow, especially when integrated into standard endoscopic biopsy tools like forceps and snares (38).
In this manuscript, we investigate the potential of ESS as in vivo and real-time technology for the endoscopic assessment of polyp histology. Specifically, we examine retrospectively the performance of ESS for distinguishing neoplastic from non-neoplastic polyps during colonoscopy. Unlike previous studies that have used larger blunt ESS probes to classify polyps, among other colonic pathologies (40), the present study uses a different ESS probe architecture that is designed specifically for clinical practicality by installing the optics into biopsy tools that can be integrated seamlessly into procedure flow.
Methods
Instrumentation
The ESS system and probes have been described previously (38, 43, 45). Briefly, the ESS optical biopsy forceps consist of 2 identical adjacent fibers with 200-μm cores (1 for illumination, the other for detection), with a numerical aperture of 0.22 in air. The center-to- center separation between the fibers is ~250 μm. With this probe configuration, a tissue depth of about 350 μm and a tissue volume ≤0.2 mm3 is interrogated. By halving the diameter of the illumination fiber (now 200μm) compared to the one our group used previously within a dedicated 2.5 mm diameter, Teflon-jacketed blunt ESS probe (36, 37, 40), the new miniaturized ESS optics can be integrated into the very tools used to perform polypectomy. As such, biopsy forceps were constructed with a hollow central channel extending into the space between the jaws (ESCO Medical Instruments, Stony Brook, NY) capable of accommodating a 0.470 mm outer diameter of the hypotube encasing the fiber probe (Figure 1a). As in previously described ESS designs, the optical fibers from the forceps connect to the ESS system, which consists of a broadband light source (pulsed Xenon arc lamp: LS-1130-3, Perkin Elmer, Waltham, Massachusetts), a built-in computer with custom software, a spectrometer (S2000, Ocean Optics, Inc., Dunedin, Florida), microcontroller board, and power supply, all housed in a clinically friendly, compact enclosure (Optimum Technologies, Inc., Southbridge, Mass). Immediately preceding each procedure, the ESS forceps and spectrometer were calibrated for system response by measuring reflectance from a spectrally flat diffuse reflectance standard (Spectralon®, Labsphere Inc., North Sutton, New Hampshire), permitting correction for spectral variations of the light source, spectrometer, fiber transmission, and fiber coupling. It should be noted that the light source for our endoscopes (Olympus 100 series with EVIS EXERA I & II systems), like all continuous wave light sources, does not interfere with ESS readings, as background illumination is measurable and subtracted automatically from the ESS measurement.
Figure 1.
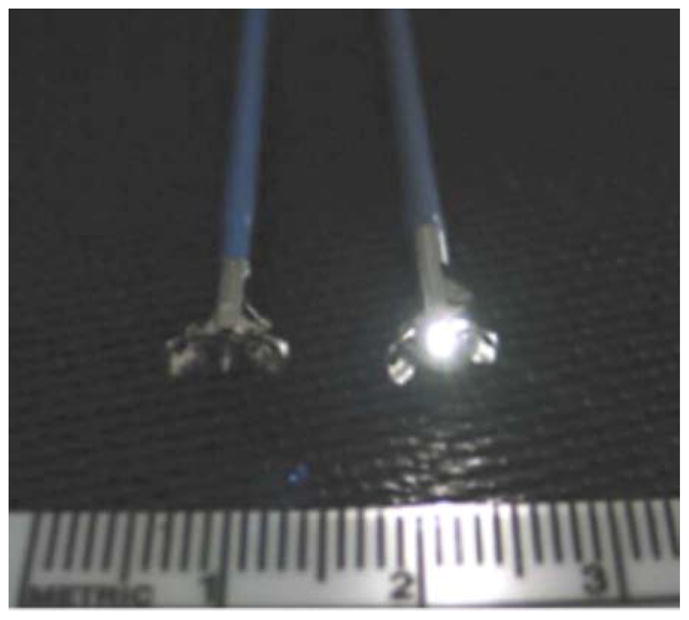
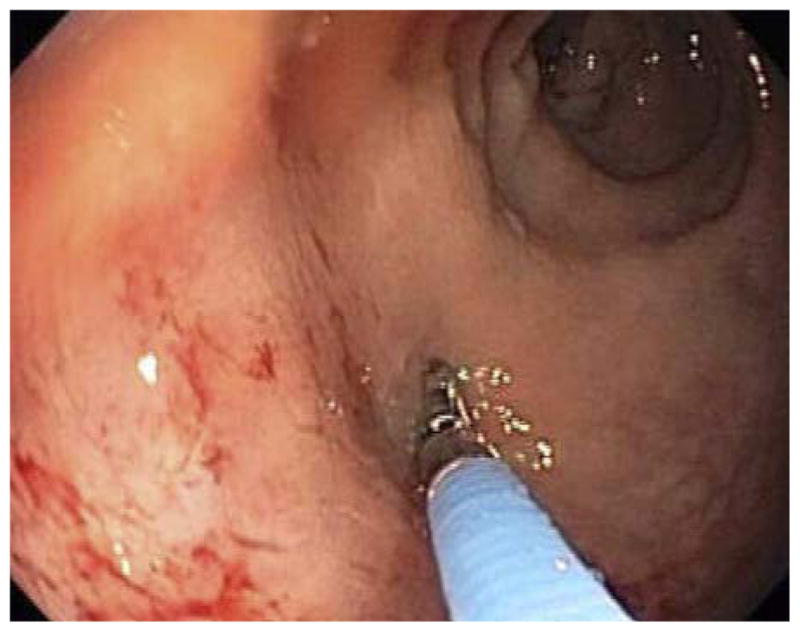
(a) Side by side comparison of standard biopsy forceps (left) and forceps with integrated dual fiber probe for ESS (right). (b) Use of ESS integrated forceps for polyp assessment.
Clinical Measurements
Data collection was performed as part of an IRB-approved clinical study at the Boston University Medical Campus (BUMC) and VA Boston Healthcare System (VABHS), Boston, Massachusetts. Subjects were recruited from individuals undergoing CRC screening/surveillance colonoscopies at VABHS. This study was designed to gauge the performance of ESS for the identification of neoplasia associated with sporadic colorectal cancer and, as such, patients at increased risk for developing CRC including those with genetic predispositions and/or chronic colonic inflammation were excluded. All endoscopic examinations and biopsies were clinically indicated and performed as they normally would be by GI attendings and/or supervised trainees alike. The only instruction given to endoscopists was to position the integrated optical forceps in the open position in gentle contact with the center or apex mucosa of the identified polyp or suspicious lesion for spectral data acquisition (Figure 1b). Five ESS measurements were taken in rapid succession (within ~3 seconds) after which the forceps jaws were closed immediately to obtain a physical biopsy. By using forceps with integrated ESS optics, precise co-registration of optical readings and physical biopsies was assured. Three specialist gastrointestinal pathologists, using predefined standard histopathological criteria of H&E stained sections, reviewed each endoscopic pinch biopsy independently, blinded to endoscopic and spectroscopic findings. For a particular polyp, the “optical biopsies” were then correlated to the consensus classification by histopathology. ESS polyp readings from 83 patients (218 polyps - 85 neoplastic, 133 non-neoplastic), collected during 58 routine surveillance (70%) and 25 screening colonoscopies (30%), were used for the analysis. Overall, baseline characteristics were representative of the demographics of the study population served by the VA Boston Healthcare System, as summarized in Table 1.
Table 1.
Patient Demographics
| Age (median, min–max) | 61, 39–84 |
|---|---|
| Gender | |
| Male (%) | 81 (98%) |
| Race/Ethnicity | |
| White (%) | 74 (89%) |
| African American (%) | 6 (7%) |
| Hispanic (%) | 2 (3%) |
| Other (%) | 1 (1%) |
Data Analysis
All ESS spectra were pre-processed automatically before spectral analysis, whereby the five measurements acquired from each polyp were averaged, smoothed, and cropped to encompass 126 wavelength bands spanning 330 nm to 760 nm (Figure 2). These spectra were then normalized to enhance spectral shape, not relative intensities. To classify measured spectra, we used a previously described approach to creating diagnostic algorithms based on multidimensional pattern-recognition/machine learning (43). Briefly, the classification algorithm uses an ensemble of decision rules obtained from different spectral regions, each incorporating the high/low-confidence decision paradigm. Application of this paradigm results in 3 possible outcomes: A polyp is classified as neoplastic or non-neoplastic if the decision is made in high-confidence; the third outcome is that no decision is rendered if the result is deemed to have been made in low-confidence. The level of confidence is assessed during the training phase, where the algorithm identifies and learns heterogeneous features among the neoplastic and non-neoplastic labeled training samples that lead to uncertain classification. Thus, the algorithm establishes a decision rule where decisions on samples made with uncertain features are deemed to have been made with low-confidence, whereas decisions on samples outside this area are considered to have been made with high-confidence.
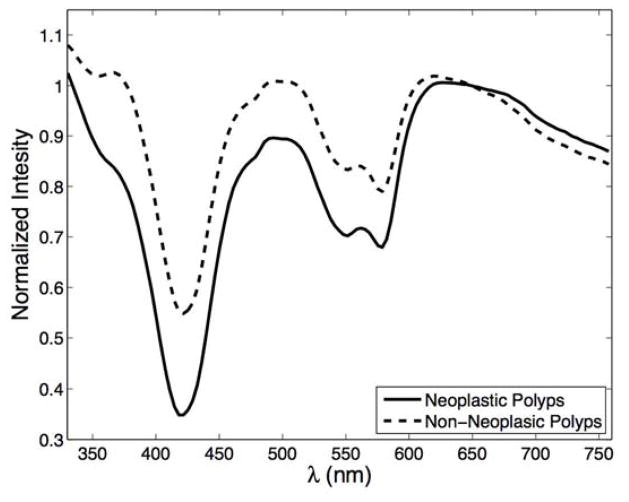
Figure 2.
Representative ESS spectra for neoplastic polyps (solid line) and non-neoplastic polyps (dashed line).
Statistical Analysis
Leave-1-patient-out cross-validation was used to obtain classification performance estimates. We used sensitivity, specificity, and overall accuracy as the primary diagnostic performance measures based on a given high-confidence decision rate (HCR), defined as the number of high-confidence decisions over the total number of decisions. Exact binomial confidence intervals of 95% are provided with reported performance estimates. We determined post hoc that the overall accuracy (the performance statistic of greatest interest) would be estimated with a precision (95% confidence interval) of ± 5% given a sample size of about 200 polyps. Taking into consideration a target high-confidence decision rate of about 90%, a sample size of about 220 polyps would be needed to estimate the overall accuracy with the aforementioned level of precision.
Results
A total of 218 polyps from 83 patients were interrogated optically with ESS and used for analysis. Of these, 133 were non-neoplastic by histopathological consensus (80 hyperplastic, 53 normal, i.e., polypoid colonic mucosa, no diagnostic abnormality) and 85 were found to be neoplastic (80 tubular adenomas, 4 with villous elements/high-grade dysplasia, 1 adenocarcinoma). Table 2 summarizes the polyp distribution by histology and size.
Table 2.
Distribution of polyp characteristics
| Histology | ||||||
|---|---|---|---|---|---|---|
| Non-Neoplastic | Neoplastic | |||||
| Normal (n=53) | HP (n=80) | TA (n=80) | Villous Elements/HGD (n=4) | CA (n=1) | ||
| Size | ≥ 1 cm (n=39) | 0 | 2 | 32 | 4 | 1 |
| 6–9 mm (n=22) | 3 | 11 | 8 | 0 | 0 | |
| ≤ 5 mm (n=157) | 50 | 67 | 40 | 0 | 0 | |
| ≤5mm, Rectosigmoid (n=85) | 21 | 54 | 10 | 0 | 0 | |
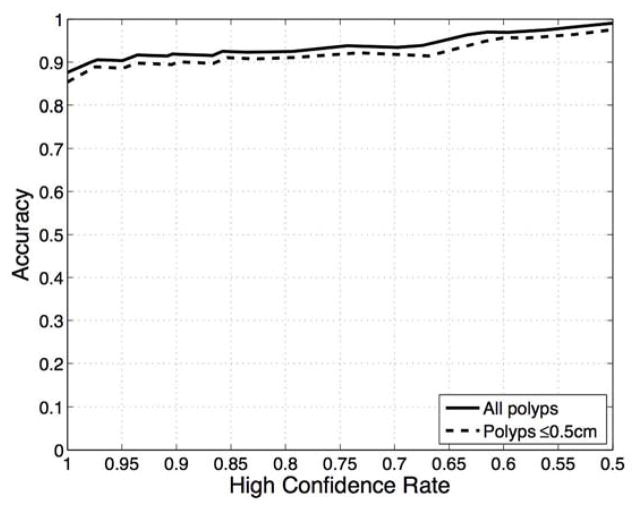
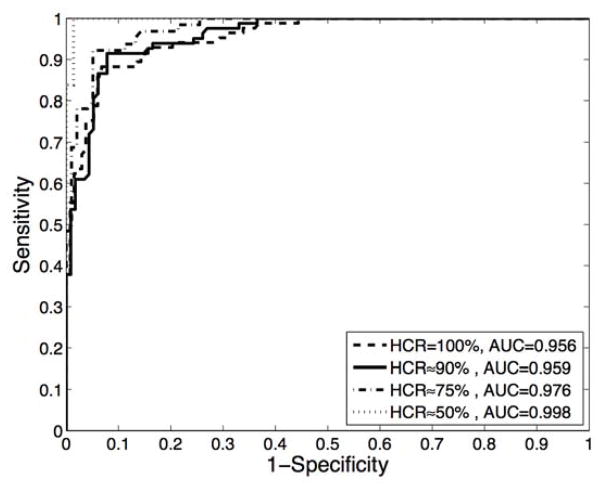
As incorporated in the diagnostic algorithm used to classify the ESS spectra obtained from polyps, the high-confidence decision rate becomes a design parameter that reflects the trade-off between the performance achieved with high-confidence decisions versus the number with low-confidence decisions. Figure 3a shows the relationship between HCR and accuracy for all polyps in our study and for those polyps ≤ 5mm, where it is observed that as the HCR decreases, the performance of the algorithm improves. Thus, as seen in the ROC curves shown in Figure 3b, improved classification performance can be achieved by relaxing the confidence level of the decisions at the expense of having a greater number of low-confidence decisions. The results presented in this section correspond to a high-confidence rate of approximately 90%, as this was the lowest HCR that was associated with an accuracy ≥ 90% for polyps ≤ 5mm.
Figure 3.
(a) Accuracy as a function of the high-confidence rate for all polyps (solid line) and polyps ≤ 5mm (dashed line). (b) ROC curves obtained at different high-confidence rate values.
Performance results from retrospective validation of the diagnostic algorithm at a about 90% HCR level are summarized in Table 3 for high-confidence decisions as well as for all decisions, i.e., both high and low-confidence decisions. Analysis of ESS measurements of all colonic polyps yielded a sensitivity of 91.5%, specificity of 92.2%, and an accuracy of 91.9% at a high-confidence rate of 90.4% for distinguishing neoplastic from non-neoplastic polyps. Similar performance levels were observed when restricting the analysis to polyps < 1cm, with a sensitivity of 87.0%, specificity of 92.1%, and an accuracy of 90.6% at a high-confidence rate of 89.3%. For all diminutive polyps (≤ 5mm), ESS achieved a sensitivity of 86.8%, specificity of 91.3%, and an accuracy of 90.1% at a high-confidence rate of 89.8%. For diminutive rectosigmoid polyps, ESS achieved a sensitivity of 88.9%, specificity of 91.2%, and an accuracy of 90.9% at a high-confidence rate of 90.6%. Table 4 displays the classification results broken down by polyp size and consensus histopathological diagnosis. There are several encouraging findings regarding the use of ESS for detecting neoplastic polyps. First, ESS displays very good performance for classifying polyps > 5mm with an accuracy of 96.4% (54/56) and a high-confidence rate of 91.8% (56/61). Second, performance for diminutive polyps ≤ 5mm is only slightly lower with an accuracy of 90.1% with high-confidence rate of 89.8%. In addition, advanced adenomas, defined as adenomas 1cm or greater, or with villous elements/high-grade dysplasia, were classified with an accuracy of 97.2% (35/36) and a high-confidence rate of 97.3% (36/37), outperforming the accuracy of 87.0% (40/46) and high-confidence rate of 95.6% (46/48) observed for non-advanced adenomas.
Table 3.
Performance of ESS for differentiating neoplastic from non-neoplastic polyps based on size
| Sensitivity, % (95% CI) | Specificity, % (95% CI) | Accuracy, % (95% CI) | NPV, % (95% CI) | HCR % (95% CI) | ||
|---|---|---|---|---|---|---|
| All Polyps (N = 218) | High-Confidence Decisions | 91.5 (83.2–96.5) | 92.2 (85.6–96.4) | 91.9 (87.1–95.3) | 93.8 (87.7–97.5) | 90.4 (85.7–93.9) |
| All Decisions | 89.4 (80.8–95.0) | 86.5 (79.5–91.8) | 87.6 (82.5–91.7) | 92.7 (86.7–96.2) | ||
| Polyps < 1 cm (n = 179) | High-Confidence Decisions | 87.0 (73.7–95.1) | 92.1 (85.5–96.3) | 90.6 (85.0–94.7) | 94.6 (88.6–98.0) | 89.3 (83.9–93.5) |
| All Decisions | 83.3 (69.8–92.5) | 86.3 (79.2–91.6) | 85.5 (79.4–90.3) | 93.4 (87.4–97.1) | ||
| Polyps ≤ 5 mm (n = 157) | High-Confidence Decisions | 86.8 (71.9–95.6) | 91.3 (84.1–95.9) | 90.1 (83.9–94.7) | 95.0 (89.5–98.5) | 89.8 (84.0–94.1) |
| All Decisions | 82.5 (67.2–92.7) | 86.3 (78.7–92.0) | 85.3 (78.8–90.5) | 93.5 (87.1–97.4) | ||
| Rectosigmoid Polyps ≤ 5 mm (n = 85) | High-Confidence Decisions | 88.9 (51.8–99.7) | 91.2 (81.8–96.7) | 90.9 (82.2–96.3) | 98.4 (91.5–99.9) | 90.6 (82.3–95.8) |
| All Decisions | 80.0 (44.4–97.5) | 86.7 (76.8–93.4) | 85.9 (76.6–92.5) | 97.0 (86.9–99.6) | ||
Table 4.
Breakdown of correctly classified polyps, in high-confidence, by size and histology. Number of low-confidence decisions is shown in parenthesis.
| Histology | ||||||
|---|---|---|---|---|---|---|
| Non-Neoplastic | Neoplastic | |||||
| Normal | HP | TA | Villous Elements/HGD | CA | ||
| Size | ≥ 1cm | n/a | 1/1 (1) | 30/31 (1) | 4/4 | 1/1 |
| 6–9 mm | 3/3 (0) | 8/8 (3) | 7/8 (0) | n/a | n/a | |
| ≤ 5mm | 41/45 (5) | 53/58 (9) | 33/38 (2) | n/a | n/a | |
| ≤5 mm, Rectosigmoid (n = 85) | 20/21 (0) | 42/47 (7) | 8/9 (1) | n/a | n/a | |
Discussion
In the current retrospective study, we sought to assess the potential of ESS as a platform for the real-time diagnosis of neoplastic polyps during colonoscopy. The results of this feasibility study indicate that ESS can accurately distinguish neoplastic from non-neoplastic polyps. Our findings are in line with previous work that has explored the use of light-scattering spectroscopy for polyp classfication (40, 41, 44). In particular, our results highlight that the thin fiberoptics integrated into biopsy forceps afford sufficient signal-to-noise permitting them to perform as well if not better than the larger, dedicated blunt probes previously described (40).
Other studies using a variety of different endoscopic technologies have shown promise for the real-time assessment of polyp histology. Rex et al. demonstrated an accuracy of 94% with a HCR of 82% for classifying polyps of all sizes with narrow-band imaging (NBI; Olympus America, Center Valley, PA), and an accuracy of 93% with a HCR of 80% for diminutive polyps (16). Ladabaum et al. found an average accuracy of 81% with a HCR of 80% for 12 community-based endoscopists who used NBI to assess the histology of diminutive polyps following ex vivo and practice-based learning programs (14). Recently, Rastogi et al. conducted a study assessing the impact of computer-based teaching modules on polyp prediction accuracy with NBI by non-expert users (15). The resultant prediction accuracies for diminutive polyps ranged from 86%–97% with a HCR of 71%–85% for a group that included non-expert community and academic physicians as well as expert endoscopists. Other electronic/virtual chromoendoscopy methods that have shown promise include Fuji Intelligent Color Enhancement (FICE; Fujinon Inc., Saitama, Japan), with reported accuracies of 90% (18), and 85% (19) using high magnification systems, and I-Scan (Pentax Inc., Hamburg, Germany), with reported accuracies of about 80% (20), and 93% with an 80% HCR for classifying diminutive polyps (21). Endomicroscopy has also been studied for the real-time assessment of polyp histology. Shahid et al. obtained an accuracy of 80% for assessing the histology of diminutive polyps using probe-based confocal laser endomicroscopy (pCLE; Mauna Kea Technologies, Paris, France) (25) and accuracies of 79% and 83% using pCLE to assess polyp histology in real-time as well as offline, respectively (24). In contrast, Kuiper et al. reported accuracies of 78% and 84% with a HCR of 33% and 44% when 2 expert pCLE users assessed videos offline (23). Additionally, using high-resolution microendoscopy Parikh et al. reported an overall accuracy of 94% when assessing histology of polyps of any size and of 95% for diminutive polyps (27). The performance of ESS in the present report, i.e. an accuracy of 92% at a HCR of 90% for polyps of all sizes and an accuracy of 90% at a HCR of 90% for diminutive polyps, is promising when compared to those reported for other endoscopic technologies.
The incorporation of a high-confidence decision paradigm into real-time polyp classification has enabled improved diagnostic performance and reliability of other endoscopic approaches. As described above, electronic/virtual chromoendoscopy and confocal laser endomicroscopy have realized improved polyp classification performance when the analysis is restricted to decisions made in high-confidence (14–16, 21, 23). Our results follow that trend, as shown in Table 3. In our classification paradigm, the high-confidence decision rate is a variable parameter that determines the trade-off between the system’s accuracy and the number of low-confidence decisions. For clinical practice, this trade-off would require a cost-benefit analysis to determine the optimal HCR and performance characteristics given the predicted usage costs and management options for low-confidence decisions. At present, polyps classified with low-confidence would require resection and histopathological assessment per current standards of care. As such, greater cost savings would be achieved by operating at a target level where a threshold diagnostic accuracy is achieved with the highest possible HCR.
The performance obtained on polyps ≤ 5mm is of particular interest as it directly relates to the American Society for Gastrointestinal Endoscopy (ASGE) Preservation and Incorporation of Valuable Endoscopic Innovations (PIVI) guidelines for the real-time endoscopic assessment for the histology of diminutive colorectal polyps (10). The PIVI guidelines establish 2 performance benchmarks for polyps ≤ 5 mm that an endoscopic technology must meet to be considered a standard of care to enable a resect-and-discard and/or leave-behind approach. The first is that, when used with high-confidence, the technology used on polyps ≤ 5mm combined with histopathology of polyps > 5mm should provide a ≥90% agreement in the resurveillance interval compared to decisions based on pathology alone. We found that ESS can classify polyps ≤ 5mm with an accuracy of 90.1%. Retrospective prediction of post-polypectomy intervals using accepted surveillance guidelines (46) translates into an agreement with pathology of 90%. With a 90% HCR, a resurveillance interval would not be able to be predicted in 10% of patients due to low-confidence decisions, but these results suggest that ESS could meet the PIVI threshold for a resect and discard approach using high confidence readings. Second, in order to leave a ≤ 5mm rectosigmoid hyperplastic polyp behind, high-confidence readings should have ≥ 90% negative predictive value (NPV) for adenomatous histology. Our results indicate that the histology of diminutive rectosigmoid polyps was assessed with a NPV of 98% which would meet the PIVI threshold. Although the prevalence of rectosigmoid adenomas in the our current dataset (12%) is lower than the 19% to 40% in other studies (14, 47, 48), the observed sensitivity and specificity of about 90% would, nevertheless, likely still result in a NPV ≥ 90% at higher prevalence levels. These results are encouraging as it may position ESS as a viable platform for real-time histological assessment of polyps in clinical practice.
ESS as practiced in the present study would be disarmingly simple to use and reassuringly familiar to most endoscopists. There would be no additional endoscopic technique to master beyond standard polypectomy, the system would report high-confidence polyp histology to the operator automatically in well under 1 sec, there is no interpretation learning curve or operator bias involved, and there would be minimal impact on endoscopic work flow. If the performance of ESS observed in this retrospective training-study is confirmed by prospective testing, the fact that it requires no operator interpretation could make it an appealing alternative for deployment in academic and community settings alike. In addition, ESS could serve an adjunctive role for other minimally obtrusive, readily accessible, and high-performing technologies like NBI where operator factors may lead to decreased performance outside of academic centers (14, 15). The combination of endoscopic imaging modalities with ESS could help to increase the HCR for the real-time histology of polyps. Another advantage of ESS forceps is the observed performance on diminutive polyps, where other probe-based techniques appear to have been less accurate likely due to issues of probe stability and mis-targeting of biopsies (23, 25).
In summary, we have demonstrated that elastic-scattering spectroscopy is a potentially viable endoscopic technology for the accurate endoscopic assessment of polyp histology. Our overarching aim was to assess the feasibility of using ESS as an optical tool to differentiate neoplastic from non-neoplastic polyps, and we believe that feasibility and usability have been demonstrated successfully. In particular, the performance observed with polyps ≤ 5mm suggests strongly that ESS may meet, and even exceed, the PIVI guidelines for adoption in clinical practice. This said, our results should be assessed in the context of the inherent limitations of a retrospective study design and a relatively small sample size dominated by male subjects. A data-driven approach was used to analyze acquired ESS spectra and, as such, performance would benefit from a larger cohort of patients, permitting algorithms to better learn and test diagnostic patterns. In this context, ongoing studies seek to build upon the presently accrued number of subjects, and to move forward with a prospective data analysis and study design. Another limitation of the study is the absence of sessile serrated adenomas (SSA) in the analysis. For algorithm training, ESS measurements require association with index pathology and SSAs present a unique challenge in that they are morphologically similar to hyperplastic polyps (non-neoplastic in our scheme) yet clinically can be premalignant (i.e. neoplastic). This duality has a high likelihood of confounding algorithm training and retrospective validation. As such, subjects with SSAs were excluded from this pilot phase of the study. Future prospective validation of the present algorithm will specifically address how our approach classifies SSAs, and we suspect that an algorithm specific to this class of polyps will be required. Finally, additional study of the features that inform ESS optical diagnoses should also lead to improved instrumentation, more specific diagnostic algorithms and improved performance.
In conclusion, our study indicates that ESS holds promise for the real-time classification of polyps as neoplastic or non-neoplastic, and could be an endoscopic assessment technology that conforms to the PIVI guidelines. ESS, especially when integrated into familiar tissue sampling tools, provides a minimally obtrusive, real-time, in situ assessment of colonic lesions, and may prove to be an essential tool in the endoscopic prevention of colorectal cancer.
Take-Home Message.
Elastic-scattering spectroscopy (ESS) is a low-cost, dye-free technology that permits accurate, real-time differentiation of neoplastic from non-neoplastic polyps. Because it is mediated by ultra-thin disposable optical fibers, ESS can be integrated into familiar tissue-sampling tools that are unobtrusive to clinical flow.
ESS requires no interpretation of observed histology by the operator obviating issues of a learning curve and interobserver agreement.
Acknowledgments
This work was supported in part by VA CSR&D MERIT Award 1I01CX000347, NIH/NCI grant U54 CA10467, and the Wallace H. Coulter Foundation and the Center for the Integration of Medicine & Innovative Technology (CIMIT).
Acronyms
- ASGE
American Society for Gastrointestinal Endoscopy
- BUMC
Boston University Medical Campus
- CLE
Confocal laser endomicroscopy
- CRC
Colorectal cancer
- ESS
Elastic-scattering spectroscopy
- HCR
High-confidence decision rate
- NBI
Narrow band imaging
- NPV
negative predictive value
- PIVI
Preservation and Incorporation of Valuable Endoscopic Innovations
- VABHS
VA Boston Healthcare System
- WLE
White light endoscopy
Footnotes
Author Contributions:
Eladio Rodriguez-Diaz, Ph.D.: conception and design; analysis and interpretation of the data; drafting of the article.
Qin Huang, M.D.: analysis and interpretation of the data.
Sandra Cerda, M.D.: analysis and interpretation of the data.
Michael J. O’Brien, M.D.: analysis and interpretation of the data.
Irving J. Bigio, Ph.D.: conception and design; critical revision of the article for important intellectual content.
Satish K. Singh, M.D.: conception and design; drafting of the article; critical revision of the article for important intellectual content; final approval of the article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Eladio Rodriguez-Diaz, Department of Medicine, Section of Gastroenterology, Boston University School of Medicine Medical Service, Gastroenterology Unit, VA Boston Healthcare System, Boston MA
Qin Huang, Department of Pathology, VA Boston Healthcare System, Boston MA
Sandra R. Cerda, Department of Pathology and Laboratory Medicine, Boston University School of Medicine
Michael J. O’Brien, Department of Pathology and Laboratory Medicine, Boston University School of Medicine
Irving J. Bigio, Department of Biomedical Engineering, College of Engineering, Boston University Department of Medicine, Section of Gastroenterology, Boston University School of Medicine
Satish K. Singh, Department of Medicine, Section of Gastroenterology, Boston University School of Medicine Department of Biomedical Engineering, College of Engineering, Boston University Medical Service, Gastroenterology Unit, VA Boston Healthcare System, Boston MA
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Rex DK, Johnson DA, Anderson JC, et al. American college of gastroenterology guidelines for colorectal cancer screening 2009 [corrected] Am J Gastroenterol. 2009;104:739–750. doi: 10.1038/ajg.2009.104. [DOI] [PubMed] [Google Scholar]
- 3.Butterly LF, Chase MP, Pohl H, et al. Prevalence of clinically important histology in small adenomas. Clin Gastroenterol Hepatol. 2006;4:343–348. doi: 10.1016/j.cgh.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 4.Gupta N, Bansal A, Rao D, et al. Prevalence of advanced histological features in diminutive and small colon polyps. Gastrointest Endosc. 2012;75:1022–1030. doi: 10.1016/j.gie.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 5.Huang CS, O’brien MJ, Yang S, et al. Hyperplastic polyps, serrated adenomas, and the serrated polyp neoplasia pathway. Am J Gastroenterol. 2004;99:2242–2255. doi: 10.1111/j.1572-0241.2004.40131.x. [DOI] [PubMed] [Google Scholar]
- 6.Lieberman D, Moravec M, Holub J, et al. Polyp size and advanced histology in patients undergoing colonoscopy screening: Implications for CT colonography. Gastroenterology. 2008;135:1100–1105. doi: 10.1053/j.gastro.2008.06.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ko CW, Riffle S, Shapiro JA, et al. Incidence of minor complications and time lost from normal activities after screening or surveillance colonoscopy. Gastrointest Endosc. 2007;65:648–656. doi: 10.1016/j.gie.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 8.Rabeneck L, Paszat LF, Hilsden RJ, et al. Bleeding and perforation after outpatient colonoscopy and their risk factors in usual clinical practice. Gastroenterology. 2008;135:1899–1906. 1906.e1. doi: 10.1053/j.gastro.2008.08.058. [DOI] [PubMed] [Google Scholar]
- 9.Ignjatovic A, East JE, Suzuki N, et al. Optical diagnosis of small colorectal polyps at routine colonoscopy (detect inspect characterise resect and discard; DISCARD trial): A prospective cohort study. Lancet Oncol. 2009;10:1171–1178. doi: 10.1016/S1470-2045(09)70329-8. [DOI] [PubMed] [Google Scholar]
- 10.Rex DK, Kahi C, O’Brien M, et al. The american society for gastrointestinal endoscopy PIVI (preservation and incorporation of valuable endoscopic innovations) on real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc. 2011;73:419–422. doi: 10.1016/j.gie.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 11.Rastogi A, Keighley J, Singh V, et al. High accuracy of narrow band imaging without magnification for the real-time characterization of polyp histology and its comparison with high-definition white light colonoscopy: A prospective study. Am J Gastroenterol. 2009;104:2422–2430. doi: 10.1038/ajg.2009.403. [DOI] [PubMed] [Google Scholar]
- 12.Sikka S, Ringold DA, Jonnalagadda S, et al. Comparison of white light and narrow band high definition images in predicting colon polyp histology, using standard colonoscopes without optical magnification. Endoscopy. 2008;40:818–822. doi: 10.1055/s-2008-1077437. [DOI] [PubMed] [Google Scholar]
- 13.Gupta N, Bansal A, Rao D, et al. Accuracy of in vivo optical diagnosis of colon polyp histology by narrow-band imaging in predicting colonoscopy surveillance intervals. Gastrointest Endosc. 2012;75:494–502. doi: 10.1016/j.gie.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Ladabaum U, Fioritto A, Mitani A, et al. Real-time optical biopsy of colon polyps with narrow band imaging in community practice does not yet meet key thresholds for clinical decisions. Gastroenterology. 2013;144:81–91. doi: 10.1053/j.gastro.2012.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rastogi A, Rao DS, Gupta N, et al. Impact of a computer-based teaching module on characterization of diminutive colon polyps by using narrow-band imaging by non-experts in academic and community practice: A video-based study. Gastrointest Endosc. 2014;79:390–398. doi: 10.1016/j.gie.2013.07.032. [DOI] [PubMed] [Google Scholar]
- 16.Rex DK. Narrow-band imaging without optical magnification for histologic analysis of colorectal polyps. Gastroenterology. 2009;136:1174–1181. doi: 10.1053/j.gastro.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 17.Singh R, Bhat YM, Thurairajah PH, et al. Is narrow band imaging superior to high-definition white light endoscopy in the assessment of diminutive colorectal polyps? J Gastroenterol Hepatol. 2013;28:472–478. doi: 10.1111/jgh.12098. [DOI] [PubMed] [Google Scholar]
- 18.Pohl J, Nguyen-Tat M, Pech O, et al. Computed virtual chromoendoscopy for classification of small colorectal lesions: A prospective comparative study. Am J Gastroenterol. 2008;103:562–569. doi: 10.1111/j.1572-0241.2007.01670.x. [DOI] [PubMed] [Google Scholar]
- 19.Kim YS, Kim D, Chung SJ, et al. Differentiating small polyp histologies using real-time screening colonoscopy with fuji intelligent color enhancement. Clin Gastroenterol Hepatol. 2011;9:744–749. e1. doi: 10.1016/j.cgh.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 20.Schachschal G, Mayr M, Treszl A, et al. Endoscopic versus histological characterisation of polyps during screening colonoscopy. Gut. 2014;63:458–465. doi: 10.1136/gutjnl-2013-304562. [DOI] [PubMed] [Google Scholar]
- 21.Lee CK, Lee SH, Hwangbo Y. Narrow-band imaging versus i-scan for the real-time histological prediction of diminutive colonic polyps: A prospective comparative study by using the simple unified endoscopic classification. Gastrointest Endosc. 2011;74:603–609. doi: 10.1016/j.gie.2011.04.049. [DOI] [PubMed] [Google Scholar]
- 22.Buchner AM, Shahid MW, Heckman MG, et al. Comparison of probe-based confocal laser endomicroscopy with virtual chromoendoscopy for classification of colon polyps. Gastroenterology. 2010;138:834–842. doi: 10.1053/j.gastro.2009.10.053. [DOI] [PubMed] [Google Scholar]
- 23.Kuiper T, van den Broek FJ, van Eeden S, et al. Feasibility and accuracy of confocal endomicroscopy in comparison with narrow-band imaging and chromoendoscopy for the differentiation of colorectal lesions. Am J Gastroenterol. 2012;107:543–550. doi: 10.1038/ajg.2012.14. [DOI] [PubMed] [Google Scholar]
- 24.Shahid MW, Buchner AM, Raimondo M, et al. Accuracy of real-time vs. Blinded offline diagnosis of neoplastic colorectal polyps using probe-based confocal laser endomicroscopy: A pilot study. Endoscopy. 2012 doi: 10.1055/s-0031-1291589. [DOI] [PubMed] [Google Scholar]
- 25.Shahid MW, Buchner AM, Heckman MG, et al. Diagnostic accuracy of probe-based confocal laser endomicroscopy and narrow band imaging for small colorectal polyps: A feasibility study. Am J Gastroenterol. 2012;107:231–239. doi: 10.1038/ajg.2011.376. [DOI] [PubMed] [Google Scholar]
- 26.Ussui VM, Wallace MB. Confocal endomicroscopy of colorectal polyps. Gastroenterol Res Pract. 2012;2012:545679. doi: 10.1155/2012/545679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parikh ND, Perl D, Lee MH, et al. In vivo diagnostic accuracy of high-resolution microendoscopy in differentiating neoplastic from non-neoplastic colorectal polyps: A prospective study. Am J Gastroenterol. 2014;109:68–75. doi: 10.1038/ajg.2013.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mourant JR, Bigio IJ, Boyer J, et al. Spectroscopic diagnosis of bladder cancer with elastic light scattering. Lasers in Surgery and Medicine. 1995;17:350–357. doi: 10.1002/lsm.1900170403. [DOI] [PubMed] [Google Scholar]
- 29.Bigio IJ, Bown SG, Briggs G, et al. Diagnosis of breast cancer using elastic-scattering spectroscopy: Preliminary clinical results. Journal of Biomedical Optics. 2000;5:221–228. doi: 10.1117/1.429990. [DOI] [PubMed] [Google Scholar]
- 30.Johnson KS, Chicken DW, Pickard DC, et al. Elastic scattering spectroscopy for intraoperative determination of sentinel lymph node status in the breast. Journal of Biomedical Optics. 2004;9:1122–1128. doi: 10.1117/1.1802191. [DOI] [PubMed] [Google Scholar]
- 31.A’amar OM, Liou L, Rodriguez-Diaz E, et al. Comparison of elastic scattering spectroscopy with histology in ex vivo prostate glands: Potential application for optically guided biopsy and directed treatment. Lasers Med Sci. 2012 doi: 10.1007/s10103-012-1245-6. [DOI] [PubMed] [Google Scholar]
- 32.Rosen J, Suh H, Giordano N, et al. Preoperative discrimination of benign from malignant disease in thyroid nodules with indeterminate cytology using elastic light-scattering spectroscopy. IEEE Transactions on Biomedical Engineering. 2013 doi: 10.1109/TBME.2013.2267452. [DOI] [PubMed] [Google Scholar]
- 33.Suh H, A’amar O, Rodriguez-Diaz E, et al. Elastic light-scattering spectroscopy for discrimination of benign from malignant disease in thyroid nodules. Ann Surg Oncol. 2010 doi: 10.1245/s10434-010-1452-y. [DOI] [PubMed] [Google Scholar]
- 34.Amelink A, Haringsma J, Sterenborg HJ. Noninvasive measurement of oxygen saturation of the microvascular blood in barrett’s dysplasia by use of optical spectroscopy. Gastrointest Endosc. 2009;70:1–6. doi: 10.1016/j.gie.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 35.Georgakoudi I, Jacobson BC, Van Dam J, et al. Fluorescence, reflectance, and light-scattering spectroscopy for evaluating dysplasia in patients with barrett’s esophagus. Gastroenterology. 2001;120:1620–1629. doi: 10.1053/gast.2001.24842. [DOI] [PubMed] [Google Scholar]
- 36.Lovat L, Bown S. Elastic scattering spectroscopy for detection of dysplasia in barrett’s esophagus. Gastrointest Endosc Clin N Am. 2004;14:507–17. ix. doi: 10.1016/j.giec.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 37.Lovat LB, Johnson K, Mackenzie GD, et al. Elastic scattering spectroscopy accurately detects high grade dysplasia and cancer in barrett’s oesophagus. Gut. 2006;55:1078–1083. doi: 10.1136/gut.2005.081497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodriguez-Diaz E, Bigio IJ, Singh SK. Integrated optical tools for minimally invasive diagnosis and treatment at gastrointestinal endoscopy. Robot Comput Integr Manuf. 2011;27:249–256. doi: 10.1016/j.rcim.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu Y, Fearn T, Mackenzie G, et al. Elastic scattering spectroscopy for detection of cancer risk in barrett’s esophagus: Experimental and clinical validation of error removal by orthogonal subtraction for increasing accuracy. J Biomed Opt. 2009;14:044022. doi: 10.1117/1.3194291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dhar A, Johnson KS, Novelli MR, et al. Elastic scattering spectroscopy for the diagnosis of colonic lesions: Initial results of a novel optical biopsy technique. Gastrointestinal Endoscopy. 2006;63:257–261. doi: 10.1016/j.gie.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 41.Ge Z, Schomacker KT, Nishioka NS. Identification of colonic dysplasia and neoplasia by diffuse reflectance spectroscopy and pattern recognition techniques. Applied Spectroscopy. 1998;52:833–839. [Google Scholar]
- 42.Mourant JR, Bigio IJ, Boyer J, et al. Elastic scattering spectroscopy as a diagnostic tool for differentiating pathologies in the gastrointestinal tract: Preliminary testing. Journal of Biomedical Optics. 1996;1:192. doi: 10.1117/12.231372. [DOI] [PubMed] [Google Scholar]
- 43.Rodriguez-Diaz E, Castanon DA, Singh SK, et al. Spectral classifier design with ensemble classifiers and misclassification-rejection: Application to elastic-scattering spectroscopy for detection of colonic neoplasia. Journal of Biomedical Optics. 2011;16:67009. doi: 10.1117/1.3592488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zonios G, Perelman LT, Backman V, et al. Diffuse reflectance spectroscopy of human adenomatous colon polyps in vivo. Applied Optics. 1999;38:6628–6637. doi: 10.1364/ao.38.006628. [DOI] [PubMed] [Google Scholar]
- 45.Rodriguez-Diaz E, Atkinson C, Jepeal LI, et al. Elastic scattering spectroscopy as an optical marker of inflammatory bowel disease activity and subtypes. Inflamm Bowel Dis. 2014 doi: 10.1097/MIB.0000000000000058. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: A joint guideline from the american cancer society, the US multi-society task force on colorectal cancer, and the american college of radiology. CA Cancer J Clin. 2008;58:130–160. doi: 10.3322/CA.2007.0018. [DOI] [PubMed] [Google Scholar]
- 47.Pickhardt PJ, Choi JR, Hwang I, et al. Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. New England Journal of Medicine. 2003;349:2191–2200. doi: 10.1056/NEJMoa031618. [DOI] [PubMed] [Google Scholar]
- 48.Graser A, Stieber P, Nagel D, et al. Comparison of CT colonography, colonoscopy, sigmoidoscopy and faecal occult blood tests for the detection of advanced adenoma in an average risk population. Gut. 2009;58:241–248. doi: 10.1136/gut.2008.156448. [DOI] [PubMed] [Google Scholar]