Abstract
The medicinal plant Centella asiatica has long been used to improve memory and cognitive function. We have previously shown that a water extract from the plant (CAW) is neuroprotective against the deleterious cognitive effects of amyloid-β (Aβ) exposure in a mouse model of Alzheimer’s disease, and improves learning and memory in healthy aged mice as well. This study explores the physiological underpinnings of those effects by examining how CAW, as well as chemical compounds found within the extract, modulate synaptic health in Aβ-exposed neurons.
Hippocampal neurons from amyloid precursor protein over-expressing Tg2576 mice and their wild-type (WT) littermates were used to investigate the effect of CAW and various compounds found within the extract on Aβ-induced dendritic simplification and synaptic loss. CAW enhanced arborization and spine densities in WT neurons and prevented the diminished outgrowth of dendrites and loss of spines caused by Aβ exposure in Tg2576 neurons. Triterpene compounds present in CAW were found to similarly improve arborization although they did not affect spine density. In contrast caffeoylquinic acid (CQA) compounds from CAW were able to modulate both of these endpoints, although there was specificity as to which CQAs mediated which effect.
These data suggest that CAW, and several of the compounds found therein, can improve dendritic arborization and synaptic differentiation in the context of Aβ exposure which may underlie the cognitive improvement observed in response to the extract in vivo. Additionally, since CAW, and its constituent compounds, also improved these endpoints in WT neurons, these results may point to a broader therapeutic utility of the extract beyond Alzheimer’s disease.
Keywords: Synaptic health, amyloid-β, Centella asiatica, neuroprotection
Introduction
Alzheimer’s disease (AD) affects more than 5 million people in the United States alone [1]. Amyloid-β (Aβ) peptide accumulates in the brain of AD patients [2] leading to the plaques that are the pathological hallmark of the disease. The amyloid hypothesis [3] suggests that this Aβ accumulation drives the neurodegeneration and synaptic loss that underlie cognitive decline. Decreased spine density, a reflection of synaptic loss, is widely reported in AD patients as well as animal models of AD [4, 5]. This loss of spines and dendritic complexity are prominent features in early-stage AD and correlate significantly with cognitive decline [6] supporting the idea that these endpoints represent the structural basis of cognitive dysfunction in AD.
The plant Centella asiatica (L) Urban (Apiaceae), also known as Gotu Kola, is used in traditional Chinese and Ayurvedic medicine to improve cognitive function and reverse cognitive impairments [7]. The neuroprotective and cognitive enhancing effects of Centella asiatica have been well-documented. Extracts of Centella asiatica have been shown to attenuate neurobehavioral and neurochemical effects of stroke [8], accelerate nerve regeneration [9], show antioxidant effects [10] and improve cognitive function in both human studies as well as in vivo models [11, 12]. Studies from our own lab have shown that a water extract of Centella asiatica (CAW) attenuates Aβ-induced cognitive impairments in the Tg2576 mouse model of AD [13]. These mice express a mutant form of human amyloid precursor protein (APP) leading to age-dependent Aβ accumulation in the hippocampus and cortex, and concomitant learning and memory deficits [14]. We found that two weeks of treatment with CAW in the drinking water normalized the behavioral deficits normally observed in aged Tg2576 animals [13]. Similarly we have also observed that CAW improves cognitive performance in aged wild-type (WT) animals as well, and this behavioral improvement was accompanied by increased synaptic gene expression in the brains of treated animals [15]. We have been able to recapitulate many of the beneficial effects of CAW using in vitro model systems.
We have seen that treatment with CAW protects against Aβ-induced cell death [16] as well as Aβ-induced mitochondrial dysfunction and oxidative stress in neuroblastoma cells [17]. However the effects of CAW on synaptic plasticity have yet to be examined. Additionally comparatively little is known about which compounds within the CAW extract mediate its beneficial effects. In this study we examine the effects of CAW, and several of the compounds contained therein, on dendritic morphology in hippocampal neurons isolated from WT and Tg2576 animals.
Materials and Methods
Aqueous extract of Centella asiatica
Dried Centella asiatica was purchased (Oregon’s Wild Harvest, GOT-03193c-OHQ01) and its identity was confirmed by comparing its thin layer chromatographic profile with that reported in the literature [18] and the Centella asiatica samples used in our previous studies [13, 16]. The water extract of Centella asiatica (CAW) was prepared by refluxing Centella asiatica (160g) with water (2000mL) for 2 hours, filtering the solution and freeze drying to yield a powder (~16–21g). Isolated neurons were treated with CAW at a concentration of 50ug/mL for 7 days.
The percent content of constituent compounds in CAW was assessed by HPLC coupled to UV detection (LC-UV) as previously described [16]. Briefly analysis was performed using an Agilent HPLC system coupled to a Surveyor Photodiode detector. An Agilent Eclipse Plus C8 column (4.6 × 150mm, 3.5 μ) with Eclipse Plus C8 guard column (4.5 × 12.5mm, 5μ) was used with a column temperature of 35°C. The column was eluted with a gradient of acetonitrile in water containing 0.05% acetic acid (acetonitrile increasing from 5% to 20% over 6 minutes, maintained at 20% from 6–12 minutes, then raised to 40% by minute 13 and 90% by minute 14, maintained at 90% until 17 minutes and returned to 5 % by minute 17.2 for equilibration at starting conditions by minute 20. Detection wavelengths were 205nm for triterpenes and 330nm for CQAs.
Caffeoylquinic acid (CQA) and triterpene treatment of primary neurons
The purified forms of 1,5-dicaffeoylquinic acid (1,5dCQA), isochlorogenic acid A (IsoA also called 3,5-dicaffeoylquinic acid), chlorogenic acid (CHLA), asiatic acid (AA), asiaticoside (AS), madecassic acid (MA) and madecassoside (MS) (Chromadex), were used to treat primary neurons. We have previously shown that IsoA and 1,5dCQA are two of the most abundant and potent diCQAs in CAW and are protective against Aβ toxicity in neuroblastoma cells [16]. CHLA was chosen as a representative monoCQA. Although CHLA did not elicit protection against Aβ toxicity in neuroblastoma cells [16] it is a metabolite of the diCQAs and therefore may be relevant in explaining the in vivo effects of the extract. The triterpene asiatic acid also has neuroprotective properties [19, 20]. The concentrations used were similar to their percent abundance in 50ug/mL CAW (Table 1), with the exception of AA and MA which were tested at higher concentrations equivalent to the molar concentrations of their glycoside counterpart (AS and MS respectively) since in vivo the glycosides would be metabolized into the aglycone form [21]
Table 1. Composition of CAW.
The weight by weight (w/w) % composition of each triterpene and three of the CQAs in the CAW mixture was determined from LC-UV analysis at 330nm.
| w/w % Composition in CAW | |
|---|---|
| Chlorogenic acid | 0.43% |
| Isochlorogenic acid A | 0.76% |
| 1,5 dicaffeoylquinic acid | 0.41% |
| Asiatic acid | 0.02% |
| Asiaticoside | 0.53% |
| Madecassic acid | Not detectable |
| Madecassoside | 0.84% |
Culture of primary hippocampal neurons
Embryonic Tg2576 mice and their WT littermates were used to generate primary neuronal cultures. The Tg2576 line expresses the human APPswe double mutation (K670N-M671L)[14], resulting in an accumulation of Aβ1-42 in the brain and the development of age-dependent Aβ plaques. Previous studies have shown that after several weeks in culture, neurons isolated from these animals display a dystrophic phenotype that includes simplified dendritic arborization and reduced spine density [22].
Hippocampal neurons were isolated from embryonic mice as previously described by Kaech and Banker [23]. All procedures were conducted in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the Portland VA Healthcare System (ACORP #3581-15). Briefly, embryos were harvested at 18 days of gestation and hippocampi isolated. For Sholl analysis of dendritic complexity neurons were plated on poly-l-lysine coated glass at 130,000 per coverslip in MEM media (GIBCO), 5% FBS (Atlanta Biologicals) and 0.6% glucose (Sigma Aldrich). After 4h, the coverslips were flipped into 60 mm dishes containing neural stem cell-derived glial cells (provided by Dr. Gary Banker, Jungers Center, OHSU) and maintained in 6 ml Neurobasal media (Gibco) supplemented with GlutaMAX (Gibco) and GS21 (Global Stem). Each dish was fed every week by removing 1 ml of the culture medium and adding 1 ml fresh Neurobasal media containing GlutaMAX plus GS21, with the first feed (at 5 DIV) containing 6 μM cytosine β-D-arabinofuranoside hydrochloride (AraC; Sigma-Aldrich). The fourth feed (19 DIV) also contained CAW (50μg/mL) or one of the following isolated compounds: CHLA (0.5μM), IsoA (0.75μM), 1,5dCQA (0.5μM), AA (0.5μM), AS (0.5μM), MA (1.5μM), MS (1.5μM). At 26 DIV, each coverslip was fixed in 4% paraformaldehyde (PFA) in PHEM buffer (60 mM PIPES, 25 mM HEPES, 10 mM EGTA, 2 mM MgCl2, pH 7.4). Coverslips were stained with Anti-MAP2B (Sigma-Aldrich #M4403; 3.3 μg/ml) and Goat anti-mouse IgG1-Cy3 (Jackson ImmunoResearch #115-165-205; 1.5μg/ml) and imaged with a Zeiss ApoTome2 microscope. Blinded Sholl analyses were performed using the Fiji platform [24] with the plug-in created by Ferreira et al. [25]. At least 180 cells were analyzed per treatment condition across 4–6 separate cultures.
For analysis of dendritic spines, 150,000 hippocampal neurons were electroporated with plasmids encoding Green Fluorescent Protein (eGFP) under the control of the CMV immediate-early enhancer and the chicken β-actin promoter [26] and plated onto dishes with poly-l-lysine coated coverslips containing 300,000 cortical neurons of the same genotype (plated in Neurobasal media containing GlutaMAX plus GS21 7 days prior to the addition of the hippocampal neurons). This strategy promoted robust synapse formation while maintaining the electroporated hippocampal neurons at a density that permitted the unambiguous visualization of non-intersecting dendritic segments. After 4 hr, coverslips were flipped into 60 mm dishes containing stem cell-derived glial cells as described above. Each dish was fed every week with 1 ml Neurobasal media plus GlutaMAX and GS21, with the first feed (at 5 DIV) containing AraC and the second feed (at 12 DIV) containing CAW or isolated compounds at the concentrations described above. Coverslips were then fixed in 4% PFA in PHEM buffer at 19 DIV and immunostained with anti-GFP (Life Technologies #A11122; 2μg/ml), detected with Alexa-488-conjugated goat anti-Rabbit secondary antibodies (Life Technologies #A11034; 2 μg/ml). The immunostained neurons were imaged using a Zeiss ApoTome2 microscope and blind quantification was performed using FIJI software. 17–20 images were collected from different neurons in each treatment group, and spines were quantified on at least 100 μm segments of dendrite length per image.
Statistics
Statistical significance was determined using one- and two-way analysis of variance. Bonferroni post-hoc tests were also conducted. Significance was defined as p ≤0.05. Analyses were performed using Excel or GraphPad Prism 6.
Results
CAW reverses Aβ-induced impairments in dendritic morphology in hippocampal neurons
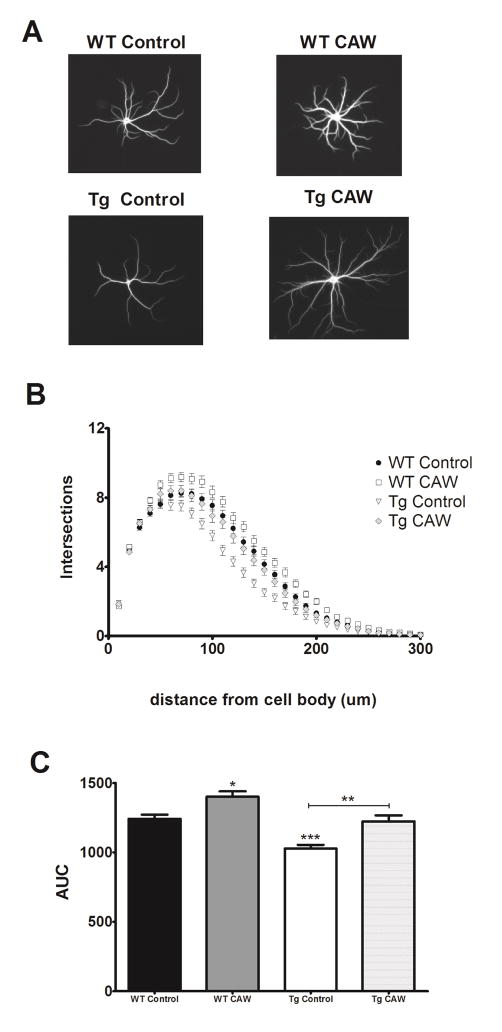
Due to their chronic production of Aβ1-42 cultured neurons from Tg2756 mice progressively develop neurodegenerative phenotypes including reduced dendritic complexity and decreased spine density relative to WT control neurons [22]. Our results were consistent with these findings showing that hippocampal Tg2576 neurons did in fact have substantially reduced dendritic complexity after 26 days in culture as compared to neurons from WT littermates (Figure 1A). CAW treatment normalized the simplified arborization in Tg2576 neurons (Figure 1B). Interestingly the extract also increased arborization in WT neurons above that observed in control levels (Figure 1C).
Figure 1. CAW increases dendritic arborization in Tg2576 and WT hippocampal neurons.
A) Representative images from each treatment group. B) Sholl analysis of the total number of dendritic branches of from Tg2576 and WT hippocampal neurons (n = 200–250 neurons per treatment condition). CAW treatment (50 ug/mL) increased dendritic complexity in WT control neurons, and restored the extent of dendritic arborization of Tg2576 neurons to control levels. C) CAW increased the cumulative arborization (as quantified by area under the curve (AUC)) in WT neurons and restored the cumulative arborization of Tg2576 neurons to control levels. *p<0.05; **p<0.01; ***p<0.001.
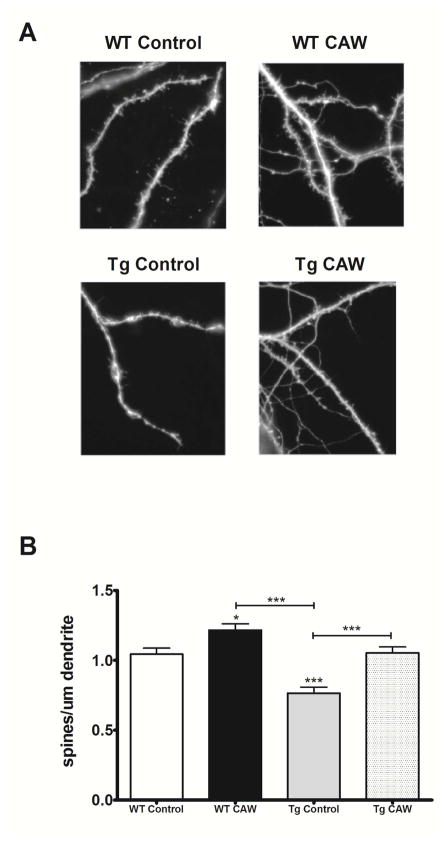
CAW had a similar effect on the number of dendritic spines in hippocampal neurons. Relative to WT control neurons spine density was markedly reduced in Tg2576 neurons after three weeks in culture (Figure 2A). This is again consistent with previous reports [22]. CAW treatment rescued this spine deficit in Tg2576 neurons to the same levels observed in WT controls (Figure 2B). Additionally, CAW also significantly increased spine density in WT neurons (Figure 2B).
Figure 2. CAW increases dendritic spine density in WT and Tg2576 hippocampal neurons.
A) Representative images from each treatment condition; B) CAW (50 ug/mL) increased the number of dendritic spines in WT neurons and restored spine density of Tg2576 neurons to control levels (n = 17–20 dendritic segments per treatment condition). *p<0.05; ***p<0.001.
Individual compounds from the CAW extract also reverse Aβ-induced impairments in dendritic morphology in hippocampal neurons
We have previously shown that diCQAs found in CAW, but not monoCQAs, nor triterpenes, can protect against Aβ toxicity in neuroblastoma cells [16]. Here we tested the effects of the CQAs IsoA, 1,5dCQA and CHLA as well as the triterpenes AA, AS, MA and MS on dendritic morphology in hippocampal neurons. Each compound was evaluated at a concentration similar to its percent composition in the dose of CAW tested with the exception of AA and MA. These triterpenes were negligible or undetectable in the extract (Table 1) but were tested at the same concentration as their glycosidic counterparts because of the potential for metabolism of AS and MS into AA and MA in vivo [21].
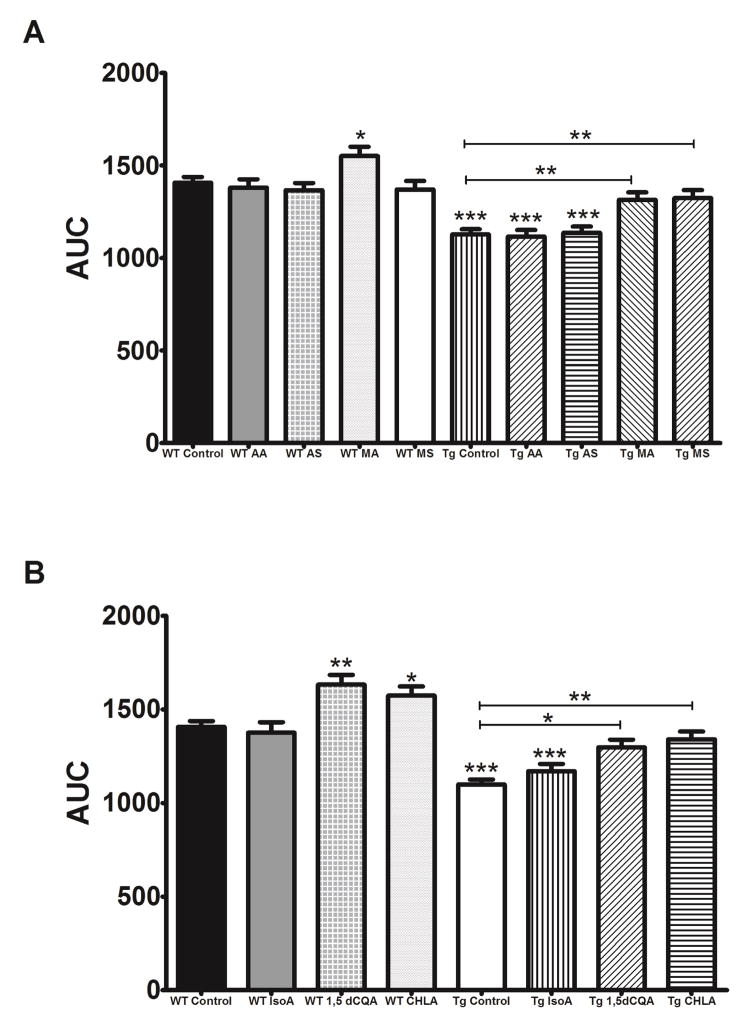
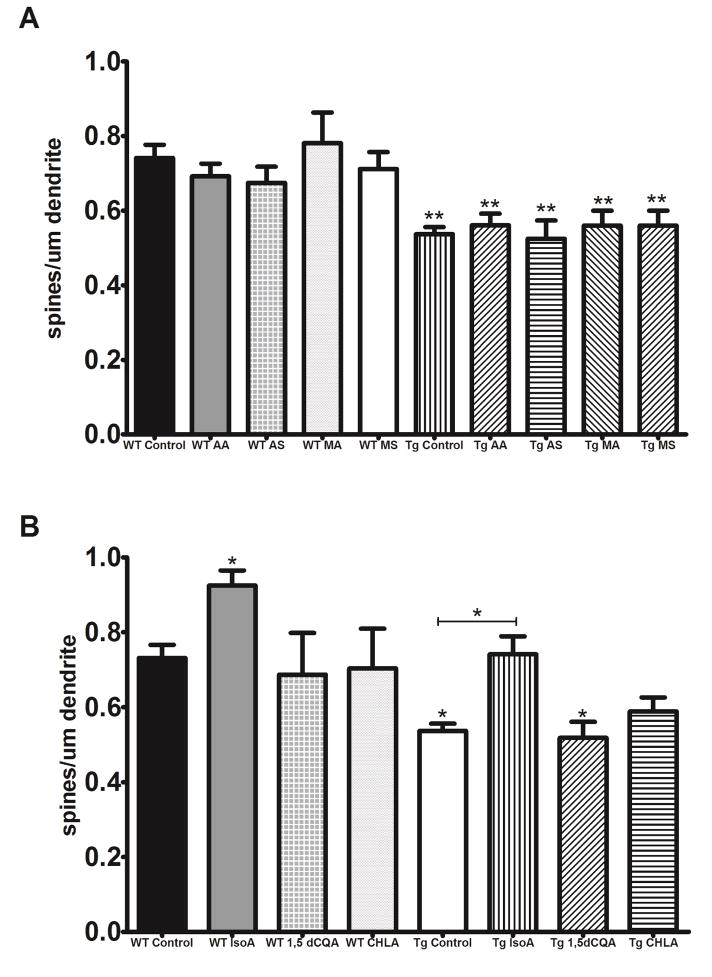
Both MA and MS reversed the deficits in dendritic arborization in Tg2576 neurons while AA and AS had no effect (Figure 3A). 1,5dCQA and CHLA, but not IsoA, were similarly able to attenuate the decreased arborization in Tg2576 neurons (Figure 3B). Notably, MA, 1,5dCQA and CHLA increased arborization in WT neurons as well (Figure 3A, 3B). When evaluating spine density we found that only Iso A was able to rescue the Aβ induced deficits (Figure 4B), neither the triterpenes nor the other CQAs tested improved spine density in Tg2576 neurons (Figure 4A, 4B). IsoA also significantly increased density in WT neurons (Figure 4B).
Figure 3. Individual compounds from CAW increase dendritic arborization in WT and Tg2576 hippocampal neurons.
A) MA (1μM) and MS (1μM) restored the cumulative arborization of Tg2576 hippocampal neurons to control levels and MA increased the cumulative arborization in WT neurons as well while AA (0.5uM) and AS (0.5uM) had no effect. (n = 180–250 neurons per treatment condition). *p<0.05; **p<0.01; ***p<0.001. B) 1,5dCQA (0.5μM) and CHLA (0.5μM) but not IsoA (0.75μM) increased cumulative dendritic arborization in WT control neurons, and restored the extent of dendritic arborization of Tg2576 neurons to control levels (n = 180–250 neurons per treatment condition). *p<0.05; **p<0.01; ***p<0.001.
Figure 4. IsoA increases dendritic spine density in WT and Tg2576 hippocampal neurons.
A) None of the triterpenes in CAW (AA [0.5μM], AS [0.5μM], MA [1μM] or MS [1μM]) had any effect on the spine density in either WT or Tg2576 neurons. (n = 17–20 dendritic segments per treatment condition). **p<0.01. B) IsoA (0.75 μM)) increased the number of dendritic spines in WT hippocampal neurons and restored spine density of Tg2576 neurons to control levels however neither dCQA1,5 (0.5μM) nor CHLA (0.5μM) had any effect on spine density in either genotype. (n = 17–20 dendritic segments per treatment condition). *p<0.05.
Discussion
The plant Centella asiatica has been used for centuries to improve memory and cognitive function [7]. We have previously demonstrated that the water extract of Centella asiatica (CAW) improves cognitive performance in aged Tg2576 mice [13] as well as aged WT mice [15]. This cognitive enhancement was accompanied by increases the expression of synaptic genes in the brains of treated animals [15] suggesting possible effects of CAW on synaptic plasticity. Here we directly evaluated the effects of CAW as well as individual compounds found in the extract on spine density and dendritic arborization in Aβ-exposed hippocampal neurons.
CAW restored Aβ-induced deficits in dendritic complexity and spine density in neurons from Tg2576 animals. However, evaluation of individual CQAs and triterpenes from the extract yielded mixed results. The effects of the CQAs differed between arborization and spine density with CHLA and 1,5dCQA restoring Aβ-induced deficits in arborization and IsoA reversing the deleterious effects of Aβ on spine density. We, and other groups, have previously demonstrated that CQAs are neuroprotective in neuroblastoma cells [16, 27] which is consistent with the improvements in neuronal health observed in this study in Tg2576 hippocampal neurons.
When assessing the triterpenes found within CAW, both MA and MS attenuated the diminished arborization in Tg2576 neurons. Interestingly none of the triterpenes improved the Aβ-induced reduction in spine density in these neurons. These results were somewhat unexpected given that we did not previously observe a protective effect of any of these triterpenes from Centella asiatica against Aβ toxicity in our previous work in neuroblastoma cells [16], however they are in line with reports of other triterpene compounds protecting against Aβ toxicity in different in vitro models [28, 29].
Interestingly we also saw an effect of CAW and some of its constituent compounds on these synaptic endpoints in WT neurons. These results support previous findings that juice from Centella asiatica increases arborization in the hippocampus of rats [30]. However, given the tight regulation of synaptic homeostasis in proper neuronal and synaptic activity [31, 32] these results raise the question of whether this would be functionally beneficial in vivo. Although more work is necessary to confirm that increases in arborization and spine density are in fact observed in the brains of WT animals, our previous work has demonstrated both increased synaptic gene expression and a cognitive-enhancing effect of CAW in healthy older animals [15] suggesting that if the extract does in fact modulate those endpoints in vivo the effects would not be deleterious. This is further supported by previous research that has demonstrated that mice treated with a CQA-rich extract show improved spatial learning which was attributed to increased synaptic formation in the hippocampus [33].
It is also notable that we saw effects of MA and MS but not of AA and AS since the compounds are structurally so similar. The same is true of IsoA and 1,5dCQA which we expected to yield similar results given that they are positional isomers. This could be because MA and MS were used at a higher concentration than AA and AS, and IsoA at a higher concentration than the 1,5dCQA, reflecting their relative composition in the entire CAW extract. It is possible similar effects would have been observed if higher concentrations of AA, AS and 1,5dCQA were tested. However, the fact that 1,5 dCQA but not IsoA increased arborization suggests that there may be a specificity of signaling interactions that does depend on chemical conformation. The work presented here, along with our previous studies, suggests that individual compounds from CAW elicit distinct biological effects both in the context of Aβ exposure as well as in healthy neurons (Table 2). While further research is needed to determine what accounts for these differential effects, these results underscore the benefit of using the complete extract rather than individual components in isolation.
Table 2. Biological effects of CAW and some of its chemical components.
A summary of the effects of CAW, as well as the triterpenes and CQAs tested, on Aβ toxicity in neuroblastoma cells and arborization and spine density in Tg2576 and WT neurons.
| Protects against Aβ toxicity in neuroblastoma cells* | Improves dendritic arborization in Tg2576 neurons | Improves dendritic spine density in Tg2576 neurons | Improves dendritic arborization in WT neurons | Improves dendritic spine density in WT neurons | |
|---|---|---|---|---|---|
| CAW | + | + | + | + | + |
| Chlorogenic Acid | − | + | − | + | − |
| Isochlorogenic Acid A | + | − | + | − | + |
| 1,5 dicaffeoylquinic acid | + | + | − | + | − |
| Asiatic acid | − | − | − | − | − |
| Asiaticoside | − | − | − | − | − |
| Madecassic acid | − | + | − | + | − |
| Madecassoside | − | + | − | − | − |
Gray et al. Journal of Alzheimer’s Disease, 2014
CAW is a complex mixture containing various other CQAs as well as many classes of compounds beyond triterpenes and CQAs evaluated in this study. The plant Centella asiatica, has been shown to contain other saponins, quinic or benzoic acid derivatives, flavonoids and several acetylenic compounds [34] and many of these types of compounds have been associated with increased spine density and improved cognitive function [35, 36]. Further research is necessary to determine if these compounds also participate in the effects of CAW on synaptic plasticity.
The exact mechanism by which CAW, or its chemical constituents, improves these dendritic endpoints also remains to be elucidated. The complex regulation of spine formation and arborization potentially implicates a variety of biological pathways and processes including histone modification, transcription factors, microRNAs, kinases, hormones and cell surface receptors [37, 38]. Although details on its molecular mechanisms are limited, Centella asiatica has been shown to activate ERK1/2 and AKT in neuroblastoma cells [39, 40], two pathways that have been shown to affect dendritic morphology as well [41, 42]. Studies are ongoing in our lab to evaluate signal transduction pathways affected by CAW and the role they play in the neuroprotective effects of the extract.
These findings demonstrate that CAW, and several of its constituent compounds, can increase synaptogenesis and arborization in isolated hippocampal neurons, changes that could underlie the cognitive enhancing effects of CAW observed in both aged Tg2576 and WT mice [13, 15]. Studies in our lab are underway to confirm whether oral treatment with CAW, or compounds found within the extract, increases spine density or arborization in the brains of Tg2576 or WT animals and whether these effects are related our previous findings regarding the mitochondrial effects of the extract [15, 17]. These future studies along with the observations presented here will help elucidate the therapeutic potential of CAW, which may extend beyond AD to conditions associated with cognitive impairment.
Highlights.
Centella asiatica attenuates Aβ-induced neuronal dystrophy in hippocampal neurons.
Individual compounds from the plant improve Aβ-induced abnormalities as well.
Centella asiatica also enhances arborization and spine density in healthy neurons.
Acknowledgments
This work was funded by NIH-NCCIH grant R01AT008099 (Soumyanath), NIH-NCCIH grant K99AT008831 (Gray) and a Department of Veterans Affairs Merit Review grant awarded to J. Quinn. The authors thank Dr. Gary Banker and Ms. Barbara Smoody for their support of these experiments. We gratefully acknowledge Dr. Stefanie Kaech and Ms. Aurelie Snyder for their assistance with the confocal imaging analysis performed in the Advanced Light Microscopy Core, Jungers Center, OHSU.
Abbreviations
- CAW
water extract of Centella asiatica
- AD
Alzheimer’s Disease
- CQA
caffeoylquinic acid
- IsoA
isocholorogenic acid A
- 1
5diCQA, 1,5-dicaffeoylquinic acid
- CHLA
chlorogenic acid
- AA
asiatic acid
- AS
asiaticoside
- MA
madecassic acid
- MS
madecasssoside
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hebert LESP, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 2003;60:1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- 2.Walsh DM, Selkoe DJ. Deciphering the molecular basis of memory failure in Alzheimer’s disease. Neuron. 2004;44:181–193. doi: 10.1016/j.neuron.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 3.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 4.Mavroudis IA, Fotiou DF, Manani MG, Njaou SN, Frangou D, Costa VG, Baloyannis SJ. Dendritic pathology and spinal loss in the visual cortex in Alzheimer’s disease: a Golgi study in pathology. Int J Neurosci. 2011;121:347–354. doi: 10.3109/00207454.2011.553753. [DOI] [PubMed] [Google Scholar]
- 5.Dickstein DL, Brautigam H, Stockton SD, Jr, Schmeidler J, Hof PR. Changes in dendritic complexity and spine morphology in transgenic mice expressing human wild-type tau. Brain Struct Funct. 2010;214:161–179. doi: 10.1007/s00429-010-0245-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baloyannis SJ. Dendritic pathology in Alzheimer’s disease. J Neurol Sci. 2009;283:153–157. doi: 10.1016/j.jns.2009.02.370. [DOI] [PubMed] [Google Scholar]
- 7.Shinomol GK, Muralidhara, Bharath MM. Exploring the role of “Brahmi” (Bocopa monnieri and Centella asiatica) in brain function and therapy. Recent Pat Endocr Metab Immune Drug Discov. 2011;5:33–49. doi: 10.2174/187221411794351833. [DOI] [PubMed] [Google Scholar]
- 8.Tabassum R, Vaibhav K, Shrivastava P, Khan A, Ejaz Ahmed M, Javed H, Islam F, Ahmad S, Saeed Siddiqui M, Safhi MM, Islam F. Centella asiatica attenuates the neurobehavioral, neurochemical and histological changes in transient focal middle cerebral artery occlusion rats. Neurol Sci. 2013;34:925–933. doi: 10.1007/s10072-012-1163-1. [DOI] [PubMed] [Google Scholar]
- 9.Soumyanath A, Zhong YP, Gold SA, Yu X, Koop DR, Bourdette D, Gold BG. Centella asiatica accelerates nerve regeneration upon oral administration and contains multiple active fractions increasing neurite elongation in vitro. Journal of Pharmacy & Pharmacology. 2005;57:1221–1229. doi: 10.1211/jpp.57.9.0018. [DOI] [PubMed] [Google Scholar]
- 10.Gupta R, Flora SJ. Effect of Centella asiatica on arsenic induced oxidative stress and mental distribution in rats. J Appl Toxicol. 2006;26:21322. doi: 10.1002/jat.1131. [DOI] [PubMed] [Google Scholar]
- 11.Wattanathorn J, Mator L, Muchimapura S, Tongun T, Pasuriwong O, Piyawatkul N, Yimtae K, Sripanidkulchai B, Singkhoraard J. Positive modulation of cognition and mood in the healthy elderly volunteer following the administration of Centella asiatica. Neurotoxicology. 2008;29:948–957. doi: 10.1016/j.jep.2007.11.038. [DOI] [PubMed] [Google Scholar]
- 12.Veerendra Kumar MH, Gupta YK. Effect of Centella asiatica on cognition and oxidative stress in an intracerebroventricular streptozotocin model of Alzheimer’s disease in rats. Clin Exp Pharmacol Physiol. 2003;30:336–342. doi: 10.1046/j.1440-1681.2003.03842.x. [DOI] [PubMed] [Google Scholar]
- 13.Soumyanath A, Zhang Y, Henson E, Wadsworth T, Bishop J, Gold BG, Quinn JF. Centella asiatica Extract Improves Behavioral Deficits in a Mouse Model of Alzheimer’s Disease: Investigation of a Possible Mechanism of Action. Int J Alzheimers Dis. 2012:381974. doi: 10.1155/2012/381974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 15.Gray NE, Harris CJ, Quinn JF, Soumyanath A. Centella asiatica modulates antioxidant and mitochondrial pathways and improves cognitive function in mice. Journal of Ethnopharmacol. 2016;180:78–86. doi: 10.1016/j.jep.2016.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gray NE, Morré J, Kelley J, Maier CS, Stevens JF, Quinn JF, Soumyanath A. Caffeoylquinic Acids in Centella asiatica Protect against Amyloid-β Toxicity. J Alzheimer’s Dis. 2014;40:359–373. doi: 10.3233/JAD-131913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gray NE, Sampath H, Zweig JA, Quinn JF, Soumyanath A. Centella asiatica Attenuates Amyloid-β-Induced Oxidative Stress and Mitochondrial Dysfunction. Journal of Alzheimer’s Disease. 2015;45:933–946. doi: 10.3233/JAD-142217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Günther B, Wagner H. Quantitative determination of triterpenes in extracts and phytopreparations of Centella asiatica (L.) urban. Phytomedicine. 1996;3:59–65. doi: 10.1016/S0944-7113(96)80011-0. [DOI] [PubMed] [Google Scholar]
- 19.Krishnamurthy RG, Senut MC, Zemke D, Min J, Frenkel MB, Greenberg EJ, Yu SW, Ahn N, Goudreau J, Kassab M, Panickar KS, Majid A. Asiatic acid, a pentacyclic triterpene from Centella asiatica, is neuroprotective in a mouse model of focal cerebral ischemia. Journal of Neuroscience Research. 2009;87:2541–2550. doi: 10.1002/jnr.22071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee MK, Kim SR, Sung SH, Lim D, Kim H, Choi H, Park HK, Je S, Ki YC. Asiatic acid derivatives protect cultured cortical neurons from glutamate-induced excitotoxicity. Research Communications in Molecular Pahtology & Pharmacology. 2000;108:75–86. [PubMed] [Google Scholar]
- 21.Rush WR, Murray GR, Graham DJM. The comparative steady-state bioavailability of the active ingredients of madecassol. Eur J Drug Metab Pharmacokinet. 1993;18:323–326. doi: 10.1007/BF03190180. [DOI] [PubMed] [Google Scholar]
- 22.Wu HY, Hudry E, Hashimoto T, Kuchibhotla K, Rozkalne A, Fan Z, Spires-Jones T, Xie H, Arbel-Ornath M, Grosskreutz CL, Bacskai BJ, Hyman BT. Amyloid beta induces the morphological neurodegenerative triad of spine loss, dendritic simplification, and neuritic dystrophies through calcineurin activation. J Neurosci. 2010;30:2636–2649. doi: 10.1523/JNEUROSCI.4456-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaech S, Banker G. Culturing hippocampal neurons. Nature Protocols. 2006;1:2406–2415. doi: 10.1038/nprot.2006.356. [DOI] [PubMed] [Google Scholar]
- 24.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open-source platform for biological-image analysis. Nature methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferreira T, Blackman AV, Oyrer J, Jayabal S, Chung AJ, Watt AJ, Sjostrom PJ, van Meyel DJ. Neuronal morphometry directly from bitmap images. Nature methods. 2014;11:982–984. doi: 10.1038/nmeth.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector… Gene. Gene. 1991;108:193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- 27.Tian X, Gao L, An L, Jiang X, Bai J, Huang J, Meng W, Zhao Q. Pretreatment of MQA, a caffeoylquinic acid derivative compound, protects against H2O2-induced oxidative stress in SH-SY5Y cells. Neurol Res. 2016;38:1079–1087. doi: 10.1080/01616412.2016.1245030. [DOI] [PubMed] [Google Scholar]
- 28.Sun Q, Jia N, Wang W, Jin H, Xu J, Hu H. Protective effects of astragaloside IV against amyloid beta1–42 neurotoxicity by inhibiting the mitochondrial permeability transition pore opening. PLoS One. 2014;9:e98866. doi: 10.1371/journal.pone.0098866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang YW, Tsai CW, Mong MC, Yin MC. Maslinic Acid Protected PC12 Cells Differentiated by Nerve Growth Factor against β-Amyloid-Induced Apoptosis. J Agric Food Chem. 2015;63:10243–10249. doi: 10.1021/acs.jafc.5b04156. [DOI] [PubMed] [Google Scholar]
- 30.Gadahad MR, Rao M, Rao G. Enhancement of hippocampal CA3 neuronal dendritic arborization by Centella asiatica (Linn) fresh leaf extract treatment in adult rats. J Chin Med Assoc. 2008;71:6–13. doi: 10.1016/s1726-4901(08)70066-2. [DOI] [PubMed] [Google Scholar]
- 31.Ramiro-Cortés Y, Hobbiss AF, Israely I. Synaptic competition in structural plasticity and cognitive function. Philos Trans R Soc Lond B Biol Sci. 2013;369:20130157. doi: 10.1098/rstb.2013.0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woolfrey KM, Srivastava DP. Control of Dendritic Spine Morphological and Functional Plasticity by Small GTPases. Neural Plast. 2016;2016:3025948. doi: 10.1155/2016/3025948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sasaki K, Han J, Shimozono H, Villareal MO, Isoda H. Caffeoylquinic acid-rich purple sweet potato extract, with or without anthocyanin, imparts neuroprotection and contributes to the improvement of spatial learning and memory of SAMP8 mouse. J Agric Food Chem. 2013;61:5037–5045. doi: 10.1021/jf3041484. [DOI] [PubMed] [Google Scholar]
- 34.Siddiqui BS, Aslam H, Ali ST, Khan S, Begum S. Chemical constituents of Centella asiatica. Journal of Asian natural products research. 2007;9:407–414. doi: 10.1080/10286020600782454. [DOI] [PubMed] [Google Scholar]
- 35.Torres-Pérez M, Tellez-Ballesteros RI, Ortiz-López L, Ichwan M, Vega-Rivera NM, Castro-García M, Gómez-Sánchez A, Kempermann G, Ramirez-Rodriguez GB. Resveratrol Enhances Neuroplastic Changes, Including Hippocampal Neurogenesis, and Memory in Balb/C Mice at Six Months of Age. PLoS One. 2015;10:e0145687. doi: 10.1371/journal.pone.0145687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tian M, Zeng Y, Hu Y, Yuan X, Liu S, Li J, Lu P, Sun Y, Gao L, Fu D, Li Y, Wang S, McClintock SM. 7, 8-Dihydroxyflavone induces synapse expression of AMPA GluA1 and ameliorates cognitive and spine abnormalities in a mouse model of fragile X syndrome. Neuropharmacology. 2015;89:43–53. doi: 10.1016/j.neuropharm.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 37.Jan YN, Jan LY. Branching out: mechanisms of dendritic arborization. Nat Rev Neurosci. 2010;11:316–328. doi: 10.1038/nrn2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Snigdha S, Prieto GA, Petrosyan A, Loertscher BM, Dieskau AP, Overman LE, Cotman CW. H3K9me3 Inhibition Improves Memory, Promotes Spine Formation, and Increases BDNF Levels in the Aged Hippocampus. J Neurosci. 2016;36:3611–3622. doi: 10.1523/JNEUROSCI.2693-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu Y, Cao Z, Khan I, Luo Y. Gotu Kola (Centella Asiatica) extract enhances phosphorylation of cyclic AMP response element binding protein in neuroblastoma cells expressing amyloid beta peptide. J Alzheimer’s Dis. 2008;13:341–349. doi: 10.3233/jad-2008-13311. [DOI] [PubMed] [Google Scholar]
- 40.Wanakhachornkrai O, Pongrakhananon V, Chunhacha P, Wanasuntronwong A, Vattanajun A. Neuritogenic effect of standardized extract of Centella asiatica ECa233 on human neuroblastoma cells. BMC Complement Altern Med. 2013;13:204. doi: 10.1186/1472-6882-13-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Majumdar D, Nebhan CA, Hu L, Anderson B, Webb DJ. An APPL1/Akt signaling complex regulates dendritic spine and synapse formation in hippocampal neurons. Mol Cell Neurosci. 2011;46:633–644. doi: 10.1016/j.mcn.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu D, Cao F, Sun S, Liu T, Feng S. Inhibition of the Ras/Raf/ERK1/2 Signaling Pathway Restores Cultured Spinal Cord-Injured Neuronal Migration, Adhesion, and Dendritic Spine Development. Neurochem Res. 2016;41:2086–2096. doi: 10.1007/s11064-016-1921-1. [DOI] [PubMed] [Google Scholar]