Abstract
Chronic lymphocytic leukemia (CLL) is a heterogeneous disease and has a highly variable clinical course with survival ranging from a couple of months to several decades. MicroRNAs (miRNAs), small non-coding RNAs that regulate transcription and translation of genes, have been found to be involved in CLL initiation, progression and resistance to therapy. In addition, they can be used as prognostic biomarkers and as targets for novel therapies.
In this review, we describe the association between miRNAs and the cytogenetic aberrations commonly found in CLL, as well as with other prognostic factors. We describe the presence of miRNAs as extracellular entities in the plasma and serum of CLL patients and discuss their role in resistance to therapy. Finally, we will explore the potential of targeted miRNA therapy for the treatment of CLL, with a special emphasis on MRX34, the first miRNA mimic that is currently being evaluated for clinical use.
MicroRNAs (miRNAs) are small, non-coding RNAs of 19–25 base pairs long. Their main function is the regulation of gene expression by either mRNA degradation or inhibition of translation, but other functions, such as mRNA stabilization, translational activation and RNA decoy, have been described as well1. By now, it is well established that miRNAs are important in almost all cellular processes, including differentiation, proliferation, cell cycle regulation and apoptosis, processes that are deregulated in human cancers2. It was in chronic lymphocytic leukemia (CLL), the most common adult leukemia in the Western world, that the first miRNAs involved in human diseases were described. We showed that a cluster containing miR-15a and miR-16-1 was frequently deleted or downregulated in CLL, and that this downregulation correlated with allelic loss at 13q14, a region deleted in the majority of CLL cases3. After this initial report, many more studies correlated abnormal miRNA expression with cancer, resulting in more than 16,000 publications so far.
In this review, we will describe the association between certain miRNAs and the cytogenetic aberrations commonly found in CLL, as well as with other prognostic factors. Furthermore, we will discuss their importance as circulating miRNAs in CLL and their role in resistance to chemotherapeutic agents that are used to treat CLL. Finally, we will describe the potential of targeted miRNA therapy for the treatment of CLL.
miRNAs associated with common cytogenetic aberrations in CLL
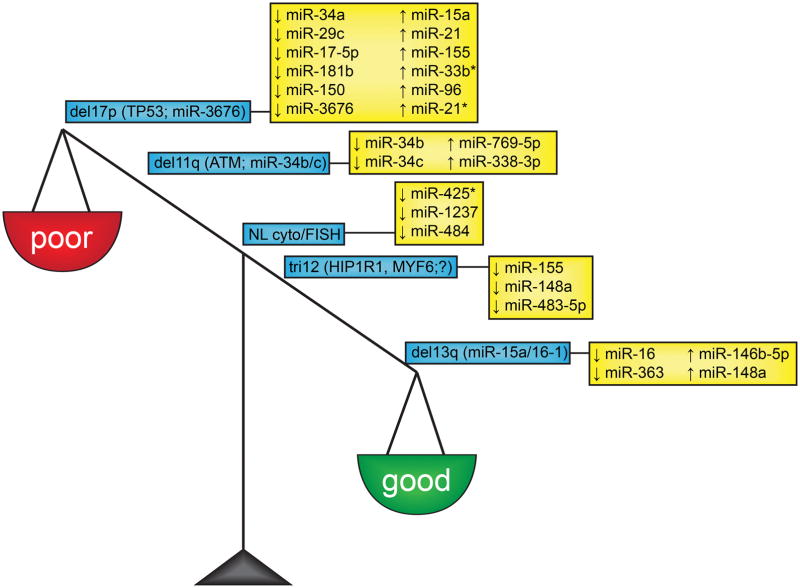
The prognosis and outcome of patients with CLL is highly variable and has been found to be largely dependent on cytogenetic abnormalities that occur in the tumors. The majority of patients with CLL (~80%) can be categorized in five distinct cytogenetic prognostic subgroups: deletion of the long arm of chromosome 13 (del13q), deletion of the long arm of chromosome 11 (del11q), deletion of the short arm of chromosome 17 (del17), trisomy of chromosome 12 (tri12) and normal cytogenetics and normal fluorescent in situ hybridization analyses (NL cyto/FISH). For some abnormalities, the target protein-coding or non-coding gene has been identified (Figure 1), while others are still under intensive investigation to unravel the biological significance of the aberrations.
Figure 1. MicroRNAs associated with common cytogenetic aberrations in CLL.
CLL patients an be categorized in different cytogenetic subgroups based on prognosis: del13 is a good prognostic factor, tri12 and NL cyto/FISH are intermediate prognostic factors and del11q and del17p are associated with poor prognosis. The protein-coding and non-coding genes between brackets in the blue boxes represent targets of the cytogenetic aberration; the miRNAs highlighted in the yellow boxes represent miRNAs that have been associated with the specific cytogenetic aberration.
Del13q is the most common cytogenetic abnormality (~55% of patients) and a good prognostic factor (median survival of 133 months)4. Detailed analyses of the deleted 13q14 region failed to demonstrate the presence of any protein-coding tumor suppressor gene in this region5–8. However, further investigation revealed the presence of a cluster containing two small non-coding miRNAs, miR-15a and miR-16-13. Analysis of the expression of these miRNAs showed that both miR-15a and miR-16-1 were deleted or downregulated in the majority of CLL patients3. The miR-15a/16-1 cluster functions as a tumor suppressor by targeting various oncogenes, of which B-cell CLL/lymphoma 2 (BCL2) and myeloid cell leukemia 1 (MCL1) are the most important in CLL9,10. The role of miR-15a/16-1 in CLL has been reviewed elsewhere11,12. Recently, downregulation of miR-16 and miR-363 and upregulation of miR-146b-5p and miR-148a were found to be associated with del13q CLL13.
Del11q occurs in ~18% of CLL patients and is associated with poor response to treatment and shorter progression free survival (median survival of 79 months)4,14. 11q is the genomic locus of ataxia-telangiectasia mutated (ATM), which is the major target of this deletion as mutations in ATM correlate with poor prognosis in CLL15. Often located in the 11q commonly deleted region is the miR-34b and miR-34c cluster. These miRNAs are targets of the tumor suppressor p53 (TP53)16, which is often deregulated or mutated in CLL. miR-34b/c expression is significantly lower in CLL patients with del11q17 and besides being deleted in CLL, miR-34b/c is also often epigenetically inactivated through hypermethylation18,19. Furthermore, an inverse correlation has been found between the presence of del11q and miR-34b/c hypermethylation, suggesting that there are different modes of silencing miR-34b/c that are independent of each other18. One of the main targets of the miR-34b/c cluster is 70 kD zeta-associated protein (ZAP70), a well-known prognostic factor in CLL20. Microarray miRNA profiling revealed a significant upregulation of two other miRNAs, miR-769-5p and miR-338-3p in CLL patients with del11q when compared to CLLs from other cytogenetic subgroups13.
Del17p is the poorest prognostic factor, and is characterized by a median survival of 32 months and poor response to therapy4. This region harbors TP53, the most commonly deregulated (either by deletion or mutation) tumor suppressor in human cancers21. Mraz and colleagues compared the expression of 35 miRNAs in CLL samples with TP53 abnormalities with wild type TP53 CLL samples, and found that three miRNAs, miR-34a, miR-29c and miR-17-5p were significantly downregulated in CLL samples carrying TP53 abnormalities22. It has been shown that the miR-34 family is regulated by TP53 through direct interaction20,23. Furthermore, TP53 is involved in a regulatory feedback loops with miR-15a/16-120. Also, our group identified miR-15a, miR-21, miR-34a, miR-155 and miR-181b as being differentially expressed between CLL patients with del17p versus CLLs with normal 17p and normal karyotype24. When comparing CLL cases with del17p with CLL cases without this aberration, Negrini and colleagues found a significant downregulation of miR-34a and miR-150 and upregulation of miR-33b*, miR-96 and miR-21* in del17p cases13. Finally, miR-3676, located at 17p13 and co-deleted with TP53 in CLL, has been found to directly target the T-cell leukemia/lymphoma 1 (TCL1) oncogene25, which is involved in CLL cell survival, as well as the pathogenesis of leukemias26. miR-3676 is significantly downregulated in four cytogenetic subgroups (del11q, del13q, del17p and normal karyotype and normal FISH analysis (NL cyto/FISH)) compared with normal CD19+ B-cells, and is mutated in 1% of CLL patients25.
Approximately 18% of patients do not have any apparent cytogenetic aberrations, as being evidenced by a NL cyto/FISH. In ~ 16% of CLL patients a trisomy of chromosome 12 (tri12) is found. Both are intermediate prognostic factors with median survival of 111 months and 114 months, respectively4, but the genetic defects associated with both subgroups remain poorly understood. In the instance of tri12, there might be a gene dosage effect of a candidate oncogene, due to the presence of the extra chromosome 12. However, such a gene has not been identified so far14. A small gene expression study comparing four CLL samples with tri12 with 16 CLL controls identified four genes whose expression is significantly associated with tri12: Huntingtin interacting protein 1 related (HIP1R), myogenic factor 6 (MYF6), purinergic receptor P2Y, G-protein coupled, 14 (P2RY14) and cluster of differentiation 200 (CD200), but only HIP1R and MYF6 are located on chromosome 1227. With regard to miRNA expression, CLL patients with tri12 showed significantly reduced expression of miR-155, miR-148a and miR-483-5p when compare to CLLs from other cytogenetic subgroups13. In CLL patients with NL cyto/FISH, miR-425*, miR-1237 and miR-484 were found to be downregulated13.
Genome-wide miRNA expression analysis of these five main cytogenetic subgroups identified 32 miRNAs that were able to discriminate del13q, NL cyto/FISH, tri12, del11q and del17p subtypes of CLL28. In addition, it was shown that disease progression in del17p cases was strongly associated with low expression of miR-223, miR-29b/c and miR-181 family, unmutated immunoglobulin heavy chain variable region (IGHV) and low expression of ZAP70, two prognostic factors in CLL28 (see next section).
miRNAs associated with other prognostic factors in CLL
Besides the presence of cytogenetic aberrations, there are several other markers that can be used to predict the prognosis of CLL patients, including the mutational status of IGHV, expression levels of ZAP70, CD38 expression levels and expression of beta-2-microglobulin (B2M). Typically, the presence of unmutated IGHV genes, high expression of ZAP70 (>20%), high levels of CD38 (≥30%) and elevated B2M expression levels (>2x upper limit of normal, ULN) are indicators for poor prognosis29. Several groups have established miRNAs and miRNA signatures associated with these prognostic factors in CLL.
In 2005, our group performed genome-wide miRNA expression analysis and identified a 13 miRNA signature consisting of miR-15a, miR-195, miR-221, miR-23b, miR-155, miR-223, miR-29a-2, miR-24-1, miR-29b-2, miR-146, miR-16-1, miR-16-2 and miR-29c that was able to differentiate CLL cases with low ZAP70 expression from those with high ZAP70 expression, and CLL cases with unmutated IGHV from those with mutated IGHV30. Several of the miRNAs from this original signature could be confirmed, including increased expression of miR-15a31,32 and miR-1631, and decreased expression of miR-29a31 and miR-29b33,34 as being associated with unmutated IGHV, and decreased levels of miR-29c32,33,35,36 and miR-22332,33,35–37 as being associated with unmutated IGHV and with increased expression of ZAP70, CD38 and B2M. One miRNA that was not included in the original signature, but that was repeatedly reported to correlate with poor prognostic markers (unmutated IGHV and increased expression of ZAP70, CD38 and B2M) is miR-150. MiR-150 is generally found to be downregulated in poor prognosis patients32,33,38, but Li and colleagues found that increased expression of miR-150 was associated with high expression of ZAP7035. In addition, a recent study found opposite prognostic significance of cellular and serum circulating miR-150 in CLL, where decreased levels of miR-150 in CLL B-cells, but increased levels of miR-150 plasma levels were associated with tumor burden, disease aggressiveness and poor prognostic factors, such as unmutated IGHV and increased expression of ZAP70, CD38 and B2M39.
A few miRNA-based scoring systems have been proposed to aid prognosis and stratification of CLL survival. Stamatopoulos and colleagues40 developed a quantitative PCR (qPCR) score based on the individual prognostic markers ZAP70, lipoprotein lipase (LPL) and miR-29c to predict overall survival. The score ranges from 0 (most favorable prognosis) to 3 (most unfavorable prognosis) and the presence of a poor prognostic factor (high expression of ZAP70 or LPL, and low miR-29c expression) increases the score with 1 point. This qPCR score was able to significantly predict treatment-free survival and overall survival by dividing patients into 3 groups (score 0/3, 1–2/3 and 3/3). Rossi and colleagues24 developed a 21FK score based on expression of miR-21, FISH and karyotype that stratifies patients according to overall survival. The score ranges from 0 (low risk) to 2 (high risk) and also here, a poor prognostic factor (high miR-21, del17p on FISH or karyotype) increased the score with 1 point. When comparing the power of 21FK score with this of the classic prognostic factors, such as B2M, ZAP70, IGHV, and CD38, the score was found to be the best performer.
Circulating miRNAs in CLL
Most studies focused on miRNA expression analysis in CLL B-cells, but circulating miRNAs can also be detected in plasma and serum of CLL samples. In fact, the number of circulating miRNAs found in CLL plasma samples was almost one third higher compared with normal, control plasma41. A series of 14 plasma miRNAs (of which miR-150, miR-150*, miR-29a, miR-135a* and miR-195 were the most differentially expressed) were able to discriminate CLL samples from normal plasma, multiple myeloma and hairy cell leukemia41. When the expression of these differentially expressed miRNAs was analyzed in the corresponding CLL B-cells, the obtained profiles were distinct and clearly different, suggesting that circulating miRNAs may be released by other cell types besides CLL B-cells. In addition, a higher number of miRNAs could be detected in plasma of ZAP70+ CLL samples than in ZAP70− CLL samples, and different miRNAs were expressed. For example, miR-19b and miR-144* levels were higher in ZAP70+ samples, while expression of miR-205, miR-29a and miR-652 was higher in ZAP70− CLL plasma samples41.
miRNA plasma levels can also predict response to CLL therapy as evidenced by miR-155 expression levels in CLL samples collected before treatment was initiated. In these samples, miR-155 was found to be significantly higher expressed in patients who fail to achieve a complete remission, when compared to those experiencing a complete response42.
miRNAs and CLL therapy resistance
Although CLL is generally an indolent disease, a significant number of patients show an aggressive clinical course with resistance to therapy or relapse after initial treatment. That resistance to therapy is a significant medical issue in CLL is underlined by the fact that the 5-year progression-free survival of patients receiving the standard of care chemotherapy-based fludarabine, cyclophosphamide and rituximab (FCR) treatment is less than 50%43. When looking at chemotherapy-refractory CLL cases, 30–40% seem to be caused by deletions and/or mutations of the tumor suppressor TP5344, while approximately one-third have a del11q/ATM. In the remaining cases, the cause for therapy refraction still remains unclear. As miRNAs, including miR-34, miR-155 and miR-181, have been demonstrated to be involved in chemoresistance and therapy refraction in many types of cancer, including CLL, resistance in these cases might be explained by abnormal expression of miRNAs.
MiR-34a is a tumor suppressor miRNA, which is downregulated in CLL cases with del17p and/or mutated TP5322,45, the poorest prognosis subgroup characterized by poor response to therapy. In addition, it is expressed at significantly lower levels in fludarabine-refractory CLL than in CLL cases without refractory disease and this was irrespective of the 17p/TP53 status44. In TP53 wild-type patients, miR34a is expressed at variable levels, which prompted Asslaber and colleagues to study the correlation of a single nucleotide polymorphism (SNP309) in the intronic promoter of MDM2, a gene upstream of TP5345. They found that patients with the GG genotype showed significantly lower expression of miR-34a when compared to patients with the TT genotype, and that these low levels of miR-34a were associated with shorter time to treatment. Upregulation or reintroduction of miR-34a induces the pro-apoptotic Bax and cell cycle regulator p2144, as well as apoptosis45. Therefore, miR-34a is a promising candidate for targeted anti-cancer therapy, which will be discussed in more detail in the next section.
The miR-181 family, and more precisely miR-181a and miR-181b, have been found to be involved in therapy resistance in CLL as well. We showed that miR-181a is downregulated in therapy-refractory CLL and that low expression of miR-181b in therapy-refractory cases predicts treatment-free survival24. Moreover, CLL patients with progressive disease show decreasing levels of miR-181b expression, whereas in patients with stable disease miR-181b levels remained constant28,46. In contrast, miR-181a and miR-221 were found to be upregulated and miR-29a was found to be downregulated in pretreatment samples of fludarabine-resistant CLL patients as compared to sensitive patients47. Finally, reintroduction of miR-181a and miR-181b enhances drug sensitivity in primary CLL cell cells through direct targeting of the anti-apoptotic genes BCL2, MCL1 and X-linked inhibitor of apoptosis, E3 ubiquitin protein ligase (XIAP)48.
miR-155 is a well-known oncogenic miRNA that is overexpressed and associated with poor prognosis in many types of cancer49. In addition, miR-155 has been demonstrated to be involved in chemoresistance50,51. Recently, it was suggested that Toll-like receptor 9 (TLR9) stimulation leads to protection of CLL cells from fludarabine-induced apoptosis in patients bearing adverse prognostic factors, and that this is marked by upregulation of miR-155-3p, the strand that is generally degraded52.
To establish a miRNA signature associated with fludarabine resistance, Ferracin et al compared the expression profiles of 723 human miRNAs before and 5 days after fludarabine treatment in a set of patients, which either responded or were refractory to fludarabine treatment53. They found that high expression of miR-148a, miR-221 and miR-21 was able to differentiate refractory from sensitive CLL samples. Knock-down of miR-221 and miR-21 in vitro increased caspase activity, suggesting that those miRNAs may be involved in the development of fludarabine resistance.
miRNA-based targeted therapy for the treatment of CLL
miRNAs are very attractive targets for novel therapeutics due to their prevalence in physiological processes and widespread involvement in human diseases, including cancer. Given that miRNAs can either function as a tumor suppressor gene or an oncogene54, targeted strategies are based on either re-expression of downregulated tumor suppressor miRNAs or silencing of oncogenic miRNAs (Figure 2; reviewed by1,2,55). There are several advantages associated with the use of miRNAs as therapeutic tools: they exist as short sequences, can be easily chemically modified and are able to target multiple genes that are involved in different signal transduction pathways56. The latter can also be a challenge, as modulating aberrantly expressed miRNAs may result in the deregulation of pathways that were not affected in the first place, and this can be accompanied by off-target effects. This is especially true for miRNA replacement therapy, as reintroduction of a miRNA may lead to supra-physological conditions in which the normal targets are saturated or overloaded, redirecting the excess of miRNAs to secondary targets57. The main major challenge, however, remains successful in vivo delivery. The ideal delivery system should be efficient, stable, safe and tumor-specific. Introduction of naked RNA molecules in the human body leads to rapid degradation, which makes encapsulation in some kind of carrier necessary. In this regard, nanocarriers seem to be promising delivery vehicles for oligonucleotide-based therapeutics (reviewed by58).
Figure 2. Therapeutic strategies to target oncogenic microRNAs (or oncomiRs) and tumor suppressor microRNAs.
(A) Under normal conditions, miRNAs repress their mRNA targets resulting in reduced expression of proteins. (B) When an oncomiR is overexpressed, its tumor suppressor target is blocked, resulting in inhibition of protein expression. An oncomiR can be targeted by the introduction of miRNA inhibitors, which prohibit miRNA-based targeting and results in re-expression of the tumor suppressor target (marked in red). (C) When a tumor suppressor miRNA is downregulated, its oncogenic target loses its repression, resulting in overexpression of the oncoprotein. Re-expression of a miRNA mimic will block the translation of the oncogene, resulting in normal levels of protein expression (marked in red). Verliezen
One very promising miRNA to be used as a therapeutic target in CLL is miR-34a. It is a key tumor suppressor downregulated in a plethora of human cancers, including neuroblastoma, glioblastoma and cancers of the ovary, colon, liver, lung, breast, prostate, pancreas, kidney, bladder, skin, esophagus, cervix and urothelium (reviewed by59,60). As already mentioned in the previous section, in CLL, miR-34a is downregulated in cases with del17p and/or mutated TP5322,45, which makes sense since miR-34a is a major downstream target of TP5323. In addition, low expression of miR-34a is associated with worse prognosis and fludarabine-refractory disease44. Re-expression of miR-34a in primary CLL patient cells resulted in a significant increase in apoptosis when wild-type TP53 was expressed, but not when TP53 was attenuated61. The widespread involvement of miR-34a in human cancer makes it an ideal candidate for miRNA replacement therapy. In fact, MRX34, containing a miR-34a mimic, is the first miRNA mimic to enter clinical trials and is currently being evaluated in a multicenter Phase I study in patients with liver cancer or those with liver metastases from other cancers, as well as in patients with hematological malignancies, including CLL (ClinicalTrials.gov identifier NCT01829971).
MRX34 (Mirna Therapeutics) is a double-stranded miR-34 mimic, which is encapsulated in SMARTICLES (ionizable liposomes that form particles of ~120 nm in diameter; Marina Biotech, Bothell, WA) for safe and efficient delivery60. Preclinical in vivo experiments in an orthotopic mouse model of hepatocellular carcinoma showed significant tumor regression and prolonged survival without notable drug-related side effects upon treatment with MRX3462,63. A more than 100-fold increase in miR-34 expression was detected in liver tumor cells and resulted in reduced expression of miR-34 oncogenic targets64. Currently, MRX34 is being tested in a Phase I clinical trial with the primary objectives to establish the maximum tolerated dose and the recommended Phase II dose in a variety of cancers. For CLL patients, MRX34 is administered intravenously and a treatment schedule of five days in a row with two weeks off in 21-day cycles is being evaluated (ClinicalTrials.gov Identifier NCT01829971). The secondary objectives are to investigate safety, tolerability and pharmacokinetic profile, as well as to assess any biological activity and clinical outcomes. Interim data on safety and preliminary efficacy for 52 patients were recently released and showed that MRX34 has a manageable safety profile. Main treatment emergent adverse events consisted of infusion reactions (such as fever, chills, nausea, vomiting, back and flank pain) and fatigue, diarrhea, headache, dehydration, elevation of liver enzymes, decreased albumin, hyponatremia, lymphopenia, thrombocytopenia, and neutropenia64. The Phase I study is expected to be completed by the end of 2015.
Discussion and future directions
CLL is a heterogeneous disease and has a highly variable clinical course with survival ranging from a couple of months to several decades. A number of biomarkers with prognostic value have been identified, such as cytogenetic abnormalities (del13q, tri12, del11q, NL cyto/FISH and del17p), IGHV mutation status, ZAP70 expression, B2M expression and CD38 expression, but the underlying molecular mechanisms are not always that well-defined. Over recent years, miRNAs have been identified that can contribute to CLL initiation, progression and resistance to therapy, or can be used as biomarkers. High-throughput techniques, such as microarrays and RNA-sequencing, make it possible to generate a massive amount of data and identify a large number of differentially expressed miRNAs. Based on the platforms and techniques being used, the outcome can greatly vary, making it hard to reproduce the results. However, several miRNAs, such as miR-34a, miR-155, miR-29c and miR-223 keep popping up, regardless of the applied techniques and platforms. These may be further evaluated as “true” biomarkers or disease contributors, and led to the first clinical trials using a miRNA mimic for miR-34a in CLL. So far, MRX34 is the only miRNA-based therapeutics ready for clinical evaluation in cancer; however, a miR-122 inhibitor used to treat patients with chronic hepatitis C virus (HCV) infection has been found to be safe and efficient in a Phase 2a clinical trial65. Although the preliminary results of MRX34 are promising and the non-coding RNA research community is getting excited about the use of a first miRNA mimic in the clinic, there is still a long way to go before this new drug can be successfully used for the treatment of patients with cancer.
Acknowledgments
Dr Calin is The Alan M. Gewirtz Leukemia & Lymphoma Society Scholar. Work in Dr. Calin’s laboratory is supported in part by the NIH/NCI grants 1UH2TR00943-01 and 1 R01 CA182905-01, the UT MD Anderson Cancer Center SPORE in Melanoma grant from NCI (P50 CA093459), a Developmental Research Award by Leukemia SPORE P50 CA100632, Aim at Melanoma Foundation and the Miriam and Jim Mulva research funds, the Brain SPORE (2P50CA127001), the Center for radiation Oncology Research Project, the Center for Cancer Epigenetics Pilot project, a 2014 Knowledge GAP MDACC grant, a CLL Moonshot pilot project, the UT MD Anderson Cancer Center Duncan Family Institute for Cancer Prevention and Risk Assessment, a SINF grant in colon cancer, the Laura and John Arnold Foundation, the RGK Foundation and the Estate of C. G. Johnson, Jr,.
References
- 1.Van Roosbroeck K, Pollet J, Calin GA. miRNAs and long noncoding RNAs as biomarkers in human diseases. Expert review of molecular diagnostics. 2013;13:183–204. doi: 10.1586/erm.12.134. [DOI] [PubMed] [Google Scholar]
- 2.Berindan-Neagoe I, del Monroig PC, Pasculli B, Calin GA. MicroRNAome genome: a treasure for cancer diagnosis and therapy. CA: a cancer journal for clinicians. 2014;64:311–36. doi: 10.3322/caac.21244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calin GA, Dumitru CD, Shimizu M, et al. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:15524–9. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dohner H, Stilgenbauer S, Benner A, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. The New England journal of medicine. 2000;343:1910–6. doi: 10.1056/NEJM200012283432602. [DOI] [PubMed] [Google Scholar]
- 5.Bullrich F, Fujii H, Calin G, et al. Characterization of the 13q14 tumor suppressor locus in CLL: identification of ALT1, an alternative splice variant of the LEU2 gene. Cancer research. 2001;61:6640–8. [PubMed] [Google Scholar]
- 6.Mertens D, Wolf S, Schroeter P, et al. Down-regulation of candidate tumor suppressor genes within chromosome band 13q14. 3 is independent of the DNA methylation pattern in B-cell chronic lymphocytic leukemia. Blood. 2002;99:4116–21. doi: 10.1182/blood.v99.11.4116. [DOI] [PubMed] [Google Scholar]
- 7.Migliazza A, Bosch F, Komatsu H, et al. Nucleotide sequence, transcription map, and mutation analysis of the 13q14 chromosomal region deleted in B-cell chronic lymphocytic leukemia. Blood. 2001;97:2098–104. doi: 10.1182/blood.v97.7.2098. [DOI] [PubMed] [Google Scholar]
- 8.Rondeau G, Moreau I, Bezieau S, et al. Comprehensive analysis of a large genomic sequence at the putative B-cell chronic lymphocytic leukaemia (B-CLL) tumour suppresser gene locus. Mutat Res. 2001;458:55–70. doi: 10.1016/s0027-5107(01)00219-6. [DOI] [PubMed] [Google Scholar]
- 9.Calin GA, Cimmino A, Fabbri M, et al. MiR-15a and miR-16-1 cluster functions in human leukemia. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:5166–71. doi: 10.1073/pnas.0800121105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cimmino A, Calin GA, Fabbri M, et al. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:13944–9. doi: 10.1073/pnas.0506654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pekarsky Y, Croce CM. Role of miR-15/16 in CLL. Cell death and differentiation. 2015;22:6–11. doi: 10.1038/cdd.2014.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calin GA, Croce CM. Chronic lymphocytic leukemia: interplay between noncoding RNAs and protein-coding genes. Blood. 2009;114:4761–70. doi: 10.1182/blood-2009-07-192740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Negrini M, Cutrona G, Bassi C, et al. microRNAome expression in chronic lymphocytic leukemia: comparison with normal B-cell subsets and correlations with prognostic and clinical parameters. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014;20:4141–53. doi: 10.1158/1078-0432.CCR-13-2497. [DOI] [PubMed] [Google Scholar]
- 14.Parker TL, Strout MP. Chronic lymphocytic leukemia: prognostic factors and impact on treatment. Discovery medicine. 2011;11:115–23. [PubMed] [Google Scholar]
- 15.Stankovic T, Skowronska A. The role of ATM mutations and 11q deletions in disease progression in chronic lymphocytic leukemia. Leukemia & lymphoma. 2014;55:1227–39. doi: 10.3109/10428194.2013.829919. [DOI] [PubMed] [Google Scholar]
- 16.Corney DC, Flesken-Nikitin A, Godwin AK, Wang W, Nikitin AY. MicroRNA-34b and MicroRNA-34c are targets of p53 and cooperate in control of cell proliferation and adhesion-independent growth. Cancer research. 2007;67:8433–8. doi: 10.1158/0008-5472.CAN-07-1585. [DOI] [PubMed] [Google Scholar]
- 17.Balatti V, Pekarky Y, Rizzotto L, Croce CM. miR deregulation in CLL. Advances in experimental medicine and biology. 2013;792:309–25. doi: 10.1007/978-1-4614-8051-8_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deneberg S, Kanduri M, Ali D, et al. microRNA-34b/c on chromosome 11q23 is aberrantly methylated in chronic lymphocytic leukemia. Epigenetics : official journal of the DNA Methylation Society. 2014;9:910–7. doi: 10.4161/epi.28603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang LQ, Kwong YL, Wong KF, et al. Epigenetic inactivation of mir-34b/c in addition to mir-34a and DAPK1 in chronic lymphocytic leukemia. Journal of translational medicine. 2014;12:52. doi: 10.1186/1479-5876-12-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fabbri M, Bottoni A, Shimizu M, et al. Association of a microRNA/TP53 feedback circuitry with pathogenesis and outcome of B-cell chronic lymphocytic leukemia. Jama. 2011;305:59–67. doi: 10.1001/jama.2010.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suzuki K, Matsubara H. Recent advances in p53 research and cancer treatment. J Biomed Biotechnol. 2011;2011:978312. doi: 10.1155/2011/978312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mraz M, Malinova K, Kotaskova J, et al. miR-34a, miR-29c and miR-17-5p are downregulated in CLL patients with TP53 abnormalities. Leukemia. 2009;23:1159–63. doi: 10.1038/leu.2008.377. [DOI] [PubMed] [Google Scholar]
- 23.He L, He X, Lim LP, et al. A microRNA component of the p53 tumour suppressor network. Nature. 2007;447:1130–4. doi: 10.1038/nature05939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rossi S, Shimizu M, Barbarotto E, et al. microRNA fingerprinting of CLL patients with chromosome 17p deletion identify a miR-21 score that stratifies early survival. Blood. 2010;116:945–52. doi: 10.1182/blood-2010-01-263889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balatti V, Rizzotto L, Miller C, et al. TCL1 targeting miR-3676 is codeleted with tumor protein p53 in chronic lymphocytic leukemia. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:2169–74. doi: 10.1073/pnas.1500010112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bottoni A, Calin GA. MicroRNAs as main players in the pathogenesis of chronic lymphocytic leukemia. MicroRNA. 2014;2:158–64. doi: 10.2174/2211536602666131126002337. [DOI] [PubMed] [Google Scholar]
- 27.Porpaczy E, Bilban M, Heinze G, et al. Gene expression signature of chronic lymphocytic leukaemia with Trisomy 12. European journal of clinical investigation. 2009;39:568–75. doi: 10.1111/j.1365-2362.2009.02146.x. [DOI] [PubMed] [Google Scholar]
- 28.Visone R, Rassenti LZ, Veronese A, et al. Karyotype-specific microRNA signature in chronic lymphocytic leukemia. Blood. 2009;114:3872–9. doi: 10.1182/blood-2009-06-229211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. The New England journal of medicine. 2005;352:804–15. doi: 10.1056/NEJMra041720. [DOI] [PubMed] [Google Scholar]
- 30.Calin GA, Ferracin M, Cimmino A, et al. A MicroRNA signature associated with prognosis and progression in chronic lymphocytic leukemia. The New England journal of medicine. 2005;353:1793–801. doi: 10.1056/NEJMoa050995. [DOI] [PubMed] [Google Scholar]
- 31.Marton S, Garcia MR, Robello C, et al. Small RNAs analysis in CLL reveals a deregulation of miRNA expression and novel miRNA candidates of putative relevance in CLL pathogenesis. Leukemia. 2008;22:330–8. doi: 10.1038/sj.leu.2405022. [DOI] [PubMed] [Google Scholar]
- 32.Papakonstantinou N, Ntoufa S, Chartomatsidou E, et al. Differential microRNA profiles and their functional implications in different immunogenetic subsets of chronic lymphocytic leukemia. Molecular medicine. 2013;19:115–23. doi: 10.2119/molmed.2013.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fulci V, Chiaretti S, Goldoni M, et al. Quantitative technologies establish a novel microRNA profile of chronic lymphocytic leukemia. Blood. 2007;109:4944–51. doi: 10.1182/blood-2006-12-062398. [DOI] [PubMed] [Google Scholar]
- 34.Zhu DX, Miao KR, Fang C, et al. Aberrant microRNA expression in Chinese patients with chronic lymphocytic leukemia. Leukemia research. 2011;35:730–4. doi: 10.1016/j.leukres.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 35.Li S, Moffett HF, Lu J, et al. MicroRNA expression profiling identifies activated B cell status in chronic lymphocytic leukemia cells. PloS one. 2011;6:e16956. doi: 10.1371/journal.pone.0016956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stamatopoulos B, Meuleman N, Haibe-Kains B, et al. microRNA-29c and microRNA-223 down-regulation has in vivo significance in chronic lymphocytic leukemia and improves disease risk stratification. Blood. 2009;113:5237–45. doi: 10.1182/blood-2008-11-189407. [DOI] [PubMed] [Google Scholar]
- 37.Zhou K, Yi S, Yu Z, et al. MicroRNA-223 expression is uniformly down-regulated in B cell lymphoproliferative disorders and is associated with poor survival in patients with chronic lymphocytic leukemia. Leukemia & lymphoma. 2012;53:1155–61. doi: 10.3109/10428194.2011.642303. [DOI] [PubMed] [Google Scholar]
- 38.Mraz M, Chen L, Rassenti LZ, et al. miR-150 influences B-cell receptor signaling in chronic lymphocytic leukemia by regulating expression of GAB1 and FOXP1. Blood. 2014;124:84–95. doi: 10.1182/blood-2013-09-527234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stamatopoulos B, Van Damme M, Crompot E, et al. Opposite prognostic significance of cellular and serum circulating microRNA-150 in Chronic Lymphocytic Leukemia patients. Molecular medicine. 2015 doi: 10.2119/molmed.2014.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stamatopoulos B, Meuleman N, De Bruyn C, et al. A molecular score by quantitative PCR as a new prognostic tool at diagnosis for chronic lymphocytic leukemia patients. PloS one. 2010:5. doi: 10.1371/journal.pone.0012780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moussay E, Wang K, Cho JH, et al. MicroRNA as biomarkers and regulators in B-cell chronic lymphocytic leukemia. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:6573–8. doi: 10.1073/pnas.1019557108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferrajoli A, Shanafelt TD, Ivan C, et al. Prognostic value of miR-155 in individuals with monoclonal B-cell lymphocytosis and patients with B chronic lymphocytic leukemia. Blood. 2013;122:1891–9. doi: 10.1182/blood-2013-01-478222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Badoux XC, Keating MJ, Wang X, et al. Fludarabine, cyclophosphamide, and rituximab chemoimmunotherapy is highly effective treatment for relapsed patients with CLL. Blood. 2011;117:3016–24. doi: 10.1182/blood-2010-08-304683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zenz T, Mohr J, Eldering E, et al. miR-34a as part of the resistance network in chronic lymphocytic leukemia. Blood. 2009;113:3801–8. doi: 10.1182/blood-2008-08-172254. [DOI] [PubMed] [Google Scholar]
- 45.Asslaber D, Pinon JD, Seyfried I, et al. microRNA-34a expression correlates with MDM2 SNP309 polymorphism and treatment-free survival in chronic lymphocytic leukemia. Blood. 2010;115:4191–7. doi: 10.1182/blood-2009-07-234823. [DOI] [PubMed] [Google Scholar]
- 46.Visone R, Veronese A, Balatti V, Croce CM. MiR-181b: new perspective to evaluate disease progression in chronic lymphocytic leukemia. Oncotarget. 2012;3:195–202. doi: 10.18632/oncotarget.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moussay E, Palissot V, Vallar L, et al. Determination of genes and microRNAs involved in the resistance to fludarabine in vivo in chronic lymphocytic leukemia. Molecular cancer. 2010;9:115. doi: 10.1186/1476-4598-9-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu DX, Zhu W, Fang C, et al. miR-181a/b significantly enhances drug sensitivity in chronic lymphocytic leukemia cells via targeting multiple anti-apoptosis genes. Carcinogenesis. 2012;33:1294–301. doi: 10.1093/carcin/bgs179. [DOI] [PubMed] [Google Scholar]
- 49.Jurkovicova D, Magyerkova M, Kulcsar L, et al. miR-155 as a diagnostic and prognostic marker in hematological and solid malignancies. Neoplasma. 2014;61:241–51. doi: 10.4149/neo_2014_032. [DOI] [PubMed] [Google Scholar]
- 50.Chen L, Jiang K, Jiang H, Wei P. miR-155 mediates drug resistance in osteosarcoma cells via inducing autophagy. Experimental and therapeutic medicine. 2014;8:527–32. doi: 10.3892/etm.2014.1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yu DD, Lv MM, Chen WX, et al. Role of miR-155 in drug resistance of breast cancer. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine. 2015;36:1395–401. doi: 10.1007/s13277-015-3263-z. [DOI] [PubMed] [Google Scholar]
- 52.Fonte E, Apollonio B, Scarfo L, et al. In vitro sensitivity of CLL cells to fludarabine may be modulated by the stimulation of Toll-like receptors. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:367–79. doi: 10.1158/1078-0432.CCR-12-1922. [DOI] [PubMed] [Google Scholar]
- 53.Ferracin M, Zagatti B, Rizzotto L, et al. MicroRNAs involvement in fludarabine refractory chronic lymphocytic leukemia. Molecular cancer. 2010;9:123. doi: 10.1186/1476-4598-9-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Garzon R, Calin GA, Croce CM. MicroRNAs in Cancer. Annual review of medicine. 2009;60:167–79. doi: 10.1146/annurev.med.59.053006.104707. [DOI] [PubMed] [Google Scholar]
- 55.Redis RS, Berindan-Neagoe I, Pop VI, Calin GA. Non-coding RNAs as theranostics in human cancers. Journal of cellular biochemistry. 2012;113:1451–9. doi: 10.1002/jcb.24038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ling H, Fabbri M, Calin GA. MicroRNAs and other non-coding RNAs as targets for anticancer drug development. Nat Rev Drug Discov. 2013;12:847–65. doi: 10.1038/nrd4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Musilova K, Mraz M. MicroRNAs in B-cell lymphomas: how a complex biology gets more complex. Leukemia. 2015 doi: 10.1038/leu.2014.351. [DOI] [PubMed] [Google Scholar]
- 58.Wicki A, Witzigmann D, Balasubramanian V, Huwyler J. Nanomedicine in cancer therapy: Challenges, opportunities, and clinical applications. Journal of controlled release : official journal of the Controlled Release Society. 2015;200C:138–57. doi: 10.1016/j.jconrel.2014.12.030. [DOI] [PubMed] [Google Scholar]
- 59.Misso G, Di Martino MT, De Rosa G, et al. Mir-34: a new weapon against cancer? Molecular therapy Nucleic acids. 2014;3:e194. doi: 10.1038/mtna.2014.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bader AG. miR-34 - a microRNA replacement therapy is headed to the clinic. Frontiers in genetics. 2012;3:120. doi: 10.3389/fgene.2012.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Merkel O, Asslaber D, Pinon JD, Egle A, Greil R. Interdependent regulation of p53 and miR-34a in chronic lymphocytic leukemia. Cell cycle. 2010;9:2764–8. [PubMed] [Google Scholar]
- 62.Bader AG, Daige CL, Kelnar K, et al. Preclinical data of a microRNA-based therapy for hepatocellular carcinoma. Annual AACR Conference; Chicago, IL. 2012. [Google Scholar]
- 63.Daige CL, Priddy L, Kelnar K, et al. The development of a miRNA-based therapeutic candidate for hepatocellular carcinoma. AACR-NCI-EORTC International Conference: Molecular Targets and Cancer Therapeutics; San Francisco, CA. 2011. [Google Scholar]
- 64.Mirna Therapeutics Presents Interim Phase 1 Data on First-in-Class microRNA-34 mimic, MRX34, at the 26th EORTC-NCI-AACR Symposium on Molecular Targets and Cancer Therapeutics; 2014. [Accessed 03/10/2015]. at http://www.mirnarx.com. [Google Scholar]
- 65.Janssen HL, Reesink HW, Lawitz EJ, et al. Treatment of HCV infection by targeting microRNA. The New England journal of medicine. 2013;368:1685–94. doi: 10.1056/NEJMoa1209026. [DOI] [PubMed] [Google Scholar]