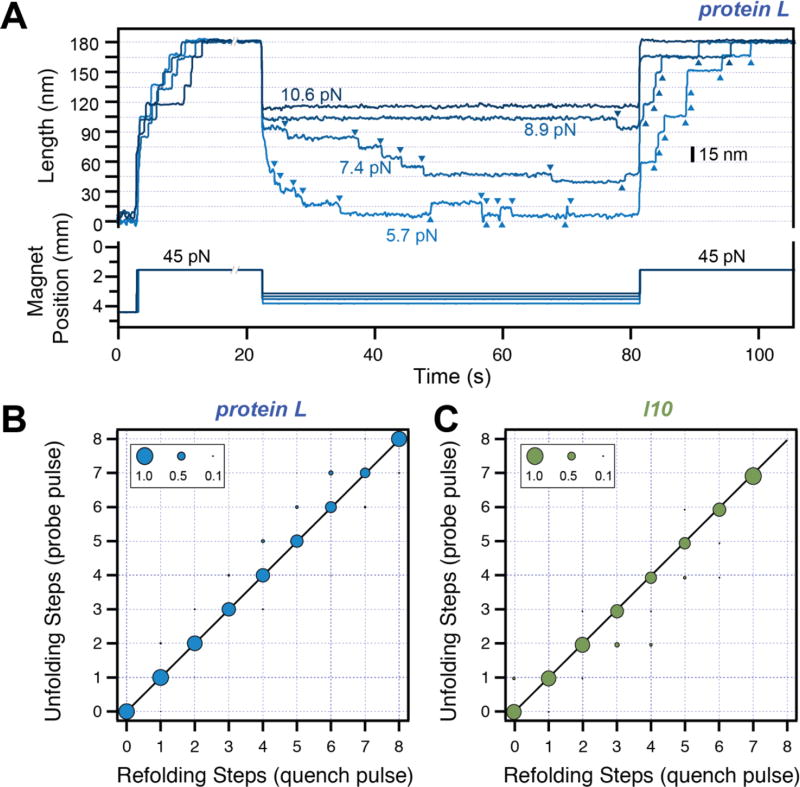
Figure 3. Refolding under force.
A) Typical traces measured with magnetic tweezers. Protein L is exposed to a high-force pulse (45 pN –fingerprint pulse), where tethering of a single protein construct is confirmed by unfolding all its domains. The force is then quenched to different low values, where the protein domains refold with a characteristic step size and force-dependent kinetics. A final high-force probe pulse is used to determine the number of refolded domains at low force. B) and C) Number of unfolding steps in the probe pulse as a function of refolding steps in the quench pulse for protein L (B) and titin I10 (C). The one-to-one correspondence indicates that the folded tertiary structure forms immediately after the collapse steps for these two proteins.