Abstract
Differentiated cells of the corneal epithelium are converted to hair, along with their associated stem cells, then interfollicular epidermis, by means of a multistep process triggered by dermal developmental signals. The committed basal cells of the adult corneal epithelium dedifferentiate under the control of signals from an associated embryonic hair-forming dermis, likely Wnts, and revert to a limbal basal cell phenotype. This initial process involves the down-regulation of Pax6 and the loss of expression of corneal-specific keratins and the induction of basal keratinocyte markers. These dedifferentiated cells are able to reinduce dermal condensations, which in turn induce the formation of hair follicles from cells that have lost Pax6 expression, by means of a Noggin-dependent mechanism. An epidermis is subsequently formed by cells derived from the newly segregated hair stem cells.
Keywords: cornea, dermal–epidermal interactions, Wnt, β-catenin, Noggin
Transdifferentiation, or cell fate change, is rare in mammals, where adult cells are not generally capable of forming cell types from other tissues or lineages, although it is well established in urodele and zebrafish (reviewed in refs. 1–3). It is possible, however, that the developmental potential of mammalian cells might be enhanced by exposing them to developmental signals. Indeed, adult corneal epithelial cells are able to give rise to hairs and interfollicular epidermis under the influence of an embryonic dermis (4). The mechanism by which this transdifferentiation occurs is the subject of the present study.
Dedifferentiation of committed cells in mammals to produce multipotent precursor cells has been demonstrated in vitro for oligodendrocyte precursor cells (5) and muscle cells (6). Evidence of plasticity in adult cells in vivo involves the ability of stem cells from various tissues to become differentiated cells of other tissues when exposed to different niche conditions, such as neural stem cells transforming into cells of the hemopoietic system (7) and hemopoietic stem cells giving rise to liver, gut, lung, and skin (8). The degree of adult stem cell plasticity is still controversial, however, with the possibility that cell fusion (9) and contamination of the starting population might account for some of the cases (reviewed in refs. 10–15).
It has long been known that the developmental phenotype of an epidermis is determined chiefly by the identity of its associated mesenchymal tissue (16), so that the presence and number of the appendages produced by an epidermis, for example, pelage hairs vs. vibrissae vs. sweat glands, is dependent on the origin of the dermis. Recent work has highlighted the importance of the Wnt/β-catenin/Lef (17–21), bone morphogenetic protein (BMP)/Noggin (20, 22), and Sonic hedgehog (SHH) (23, 24) pathways in this complex interplay. Moreover hair follicle formation can be induced de novo in adult interfollicular epidermis by dermal papilla cells (25) or by direct activation of Lef/β-catenin signaling (26, 27). It has, however, not been possible to determine which cells are responding to the inductive signals because the stem cells in the interfollicular epidermis are dispersed throughout the K5 and K14 expressing basal layer (reviewed in ref. 28–30) and give rise to transient amplifying cells that, after limited division, migrate upwards and terminally differentiate, replacing the expression of K5 and K14 with the differentiation-specific K1 and K10. Another population of multipotent stem cells is located in the bulge region of the hair follicle and constitutes a reservoir that participates not only in the regular cycling of the hair follicle, but can also reform the interfollicular epidermis (28, 29, 31–33).
In contrast, the stem cells of the adult cornea are segregated in the limbus, a ring of tissue around the central cornea, and give rise to transient amplifying progeny that migrate centripetally to replace the cells shed in the central cornea (34, 35). The cells of the basal layer of the limbus have a number of characteristics that distinguish them from the differentiating cells of the central cornea; in particular, they express the basal keratins K5 and K14, and not the corneal-type keratins K3 and K12, which are, in contrast, expressed in all layers of the central cornea (36–38). A major difference between corneal and skin keratinocytes is that the corneal epithelium derives from a Pax6-expressing ectoderm and its development and maintenance is dependent on Pax6 activity (reviewed in refs. 39–41). Pax6 is a paired homeodomain containing nuclear transcription factor that controls many downstream genes in eye morphogenesis, including K12 (42), and defects in Pax6 can lead to severe defects in eye development and maintenance (43).
Adult central corneal epithelium, which is comprised of differentiating cells and contains no stem cells, can be reprogrammed to become hairs and interfollicular epidermis under the influence of an embryonic hair-forming dermis (4). This means that committed transient amplifying, or differentiating, cells are able to transdifferentiate into cells of another ectodermal lineage. Using this cornea-to-epidermis model system we examined the potential role of the Wnt/β-catenin and Noggin/BMP signaling pathways, known to be involved in epidermal morphogenesis, as well as the kinetics and distribution of the homeobox transcription factor Pax6, which is essential for corneal identity, as well as differentiation-specific markers such as K3/K12 for corneal keratinocytes, K5/K14 for basal keratinocytes, and K10 for epidermal keratinocytes. From these data, we are able to show that this transdifferentiation process consists of a number of discrete, sequential steps. These steps include an initial activation, followed by dedifferentiation, probably under the control of a general Wnt dermal signal, wherein all of the basal corneal epithelial cells adopt a phenotype resembling that of the basal layer of the limbus. The next step involves these newly competent cells responding to a second set of dermal signals, including Noggin, to form hair placodes and finally mature hair. In a subsequent step, cells from these hairs, presumably newly formed bulge stem cells, are then able to migrate out to give rise to a complete epidermis.
Materials and Methods
Animals. OF1 and athymic nude mice were from Iffa Credo, rabbits were from Elevage Scientifique des Dombes (Romans, France). Noggin heterozygous knockout mice were a generous gift from R. Harland (University of California, Berkeley) and C. Niehrs (University of Heidelberg, Heidelberg, Germany). During graft surgery, nude mice were anesthetized by i.p. injection of valium and imalgene. All animal procedures were performed according to the French Animal Protection and Health Ministry, authorization number 04622 (to D.D.).
Recombination of Mouse Embryonic Dermis and Rabbit Central Cornea. Recombinants were performed between wild-type mouse embryonic dermis and adult rabbit central corneal epithelium as described (4). Briefly, the dermis from the upper lip of embryonic day (E) 12.5 or the back of E14.5 mouse embryos was obtained by means of protease treatment. The rabbit central cornea was dissected, leaving a 3-mm border of transparent cornea attached to the limbus to ensure that no limbal cells were included, and the epithelium was dissociated from the underlying stroma by treatment with EDTA.
Mice heterozygous for the Noggin knockout were mated, and embryos were collected at E14.5. Homozygous embryos (Noggin-/-) were determined initially on the basis of phenotypic characteristics (44). Heterozygous embryos (Noggin+/-), which are phenotypically indistinguishable from wild-type, were identified on the basis of β-galactosidase staining. These assignments were confirmed by PCR analysis of genomic DNA. The dermis and epidermis were separated, and recombinants were performed as above.
The recombinants were grafted under the kidney capsule of nude mice and recovered after various time points between 1 and 30 days and embedded in OCT compound before cryosectioning for immunofluorescence or immunohistochemistry.
Immunofluorescence Analysis. OCT compound-embedded samples were sectioned (8 μm) and stored at -80°C. Primary antibodies were detected with a secondary antibody directed against the appropriate species labeled with Alexa Fluor 488 or Alexa Fluor 546 (Molecular Probes). Keratin antibodies used in this study were as follows: rat monoclonal anti-keratin 10; mouse monoclonal anti-keratin 3 (AE5) and anti-keratin 5 (AE14) (generous gifts from T. T. Sun, New York University, New York) (45); guinea pig anti-K14 (a generous gift from L. Langbein, German Cancer Research Centre, Heidelberg, Germany); and rabbit anti-keratin 6, 16, and 17 (generous gifts from P. A. Coulombe, Johns Hopkins University, Baltimore) (46). We also used rabbit anti-Pax6 (Chemicon); rabbit anti-lef 1 [a generous gift from R. Grosschedl (University of Munich, Munich) and E. Fuchs (The Rockefeller University, New York)]; and mouse monoclonal and rabbit β-catenin (Sigma). For double labeling with mouse antibodies and mouse anti-keratin 12 antibody (36), an Alexa Fluor 456-coupled anti-keratin 12 antibody was made by using the Alexa Fluor protein labeling kit (Molecular Probes). Nuclei were routinely counterstained with Hoechst 33258 or DAPI. For the study of mitotic activity we used mouse monoclonal anti-Ki67 (MM1) (NovoCastra, Newcastle, U.K.), as well as BrdUrd labeling.
In Situ Hybridization. Mouse and rabbit genomic DNA was isolated by using TRIzol (GIBCO/BRL). Mouse genomic DNA was randomly biotin-labeled by using the Bioprime DNA labeling system (Life Technologies, Grand Island, NY). OCT compound sections were fixed in 4% paraformaldehyde and permeabilized with liquid N2 before hybridization with labeled mouse genomic DNA (gDNA) with unlabeled rabbit gDNA as a competitor. The probe was revealed by using streptavidin coupled to FITC, and nuclei were counterstained with DAPI (47).
Results
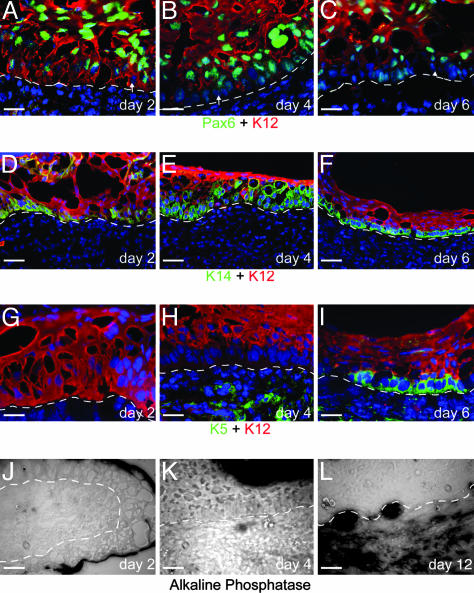
Dedifferentiation of the Recombinant Epithelial Basal Layer. Because only the central corneal epithelium was used in these experiments, all of the cells in the recombined epithelium initially express the corneal keratins K3 and K12 as well as nuclear Pax6, but not detectable levels of K5 and K14 (See Supporting Text and Fig. 6 A–C, which are published as supporting information on the PNAS web site). By day 2 after recombination, the cells at the dermal-epidermal junction reorganize to form a basal layer in which the level of Pax6 expression decreases and its subcellular localization is shifted, occurring primarily in the cytoplasm (Fig. 1A). At this stage, all cell layers of the epithelium still express K12 (Fig. 1 A, D, and G). Subsequently, the cells of the basal layer begin to down-regulate and, by day 4, have lost K12 expression (Fig. 1 B, E, and H) as well as decreased Pax6. In the K12-expressing cells of the suprabasal layers, by contrast, Pax6 is still strongly expressed in the nucleus (Fig. 1B). This downregulation and relocalization of Pax6 continues, and, by day 6, it is lost in patches in the basal layer (Fig. 1C).
Fig. 1.
Dedifferentiation of the basal layer of the recombined corneal epithelium. (A–C) Pax6 protein expression decreases and becomes cytoplasmic in the basal layer (arrows) as early as day 2 (A) after recombination when all epithelial cells still express K12. Pax6 expression in the basal layer continues to decline at day 4 (B), which at this stage also down-regulates the expression of K12. By day 6 (C), the basal layer no longer expresses K12 and has lost the expression of Pax6 in patches (arrowhead). (D–F) K14 expression is turned on by day 2 (D), primarily in the basal layer, although some suprabasal cells are K14-positive. By day 4 (E)to6(F), the expression of K14 becomes progressively restricted to the K12-negative basal layer. (G–I) K5 is not expressed soon after recombination (day 2, G), or even when K12 expression is lost in the basal layer (bl) by day 4 (H). At day 6 (I), K5 expression is turned on in the K12-negative basal layer. Green background staining in the dermis is the result of the secondary anti-mouse antibody staining nonspecifically in the mouse-derived dermis. (J–L) Dermal condensations, as measured by alkaline phosphatase activity, are no longer present at day 1 (J) but begin to appear at day 4 (K) and are strongly present by day 12 (L). e, epithelium; d, dermis. [Scale bars: 20 μm (A–C and G–I) and 50 μm (D–F and J–L).]
As early as day 2, cells, primarily in the basal layer, begin to express K14 (Fig. 1D). By day 4, K14 expression becomes restricted to the basal layers of the epithelium (Fig. 1E) and, by day 6, is limited to the K12-negative basal layer (Fig. 1F). K5 is induced after its normal partner, K14; no K5 expression is seen at day 2 (Fig. 1G), whereas, at day 4, there is little K5 expression in the epithelial basal layer (Fig. 1H). By 6 days, the basal layer of the epithelium strongly expresses both K5 and K14 and is negative for K12 (Fig. 1 F and I). The cells of the corneal epithelium in contact with the dermis thus lose their main differentiated central corneal characteristics and adopt a phenotype resembling the less differentiated basal layer of the limbus. An interesting point concerns the uncoupled down regulation of the corneal-type keratin pair. At day 2, all of the cells of the epithelium express both K12 and K3 (Fig. 7A, which is published as supporting information on the PNAS web site). The down-regulation of K12 in the basal layer precedes that of K3 by a significant period, i.e., by day 5–8, the basal layer has lost expression of K12 but still expresses K3 (Fig. 7 B and C). Expression of K3 is finally lost in the cells that form the hair pegs (Fig. 4A).
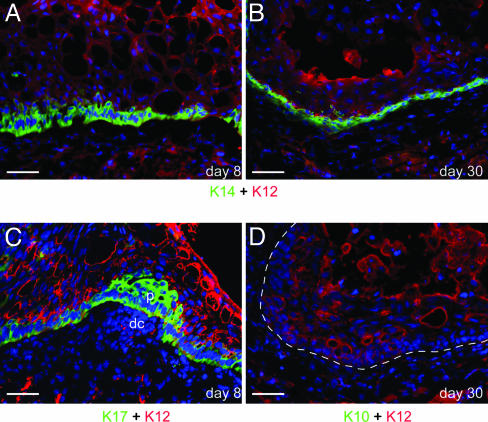
Fig. 4.
Embryonic dermis lacking Noggin fails to support the formation of hair follicles and epidermis. At day 8, the Noggin-/- (A) recombinants have a K14-positive, K12-negative basal layer with the suprabasal layers being K12-positive and K14-negative. By day 30 (B), the epithelium still has only a basal layer of K14-positive, K12-negative cells with no evidence of even hair peg formation. At day 8 (C), recombinants have a K17-positive, K12-negative basal layer and show dermal condensations (dc) and placodes (p). At day 30 (D), no K10 expression is detected in the Noggin-/- recombinants. (Scale bars: 20 μm.)
The Dermal Signal Responsible for the Dedifferentiation of the Epithelial Basal Cells Is Not Generated by Dermal Condensations. At E14.5, the mouse back dermis has dermal condensations that are a source of signals for hair formation (16, 48) and which, in turn, receive signals from the epidermis that are essential for their continued maintenance (49). In the absence of these signals, the dermal condensations do not form. One of the diagnostic characteristics of the dermal condensations is strong alkaline phosphatase activity (reviewed in ref. 50). In early time points after recombination, there is no histological evidence of dermal condensations, and no alkaline phosphatase activity is detected in the dermis (Fig. 1J). After 4–6 days, weak alkaline phosphatase activity appears in single or small clumps of cells in the dermis adjacent to the epithelium (Fig. 1K), indicating that the dermal condensations have begun to reform. At later time points (day 12, Fig. 1L), dermal condensations are formed underlying the epithelium. The reorganization of the dermis is thus contingent on the dedifferentiation of the epithelial basal layer.
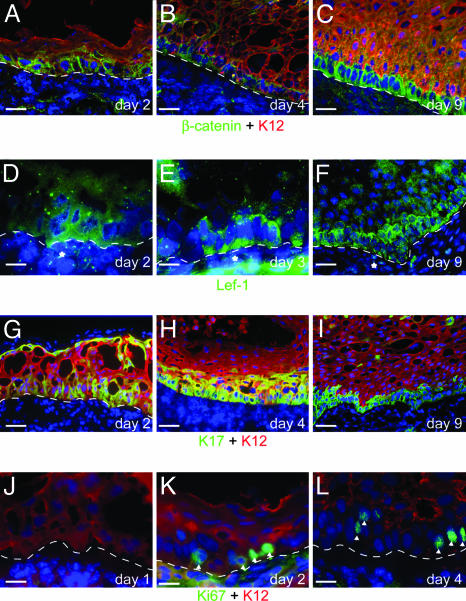
β-Catenin, Lef-1, and K17 Protein Expression Is Increased in the Epithelial Cells That Respond to the Dermal Signal. At day 2 after the recombination, there is a strong increase in the level of nonmembrane-associated β-catenin protein in the cells of the lower layers of the epithelium (Fig. 2A). In the suprabasal layers, the amount of β-catenin protein is considerably lower and confined to the cell membrane. By day 4, the cytoplasmic β-catenin staining becomes increasingly restricted to the basal layer (Fig. 2B), and, by day 9, elevated β-catenin is found only in the basal layer (Fig. 2C).
Fig. 2.
The expression levels of β-catenin, Lef-1, and K17 are coordinately increased in the dedifferentiating cells. (A–C) The levels of non-membrane-bound β-catenin in the recombined epithelium are greatly increased from day 2, predominantly in the basal layer (A and B). By 9 days, the elevated levels of β-catenin are restricted to the K12-negative basal layer (C). (D–F) Lef-1 expression is seen in both the epithelium and dermis in the recombinants. In the epidermis, high levels of Lef-1 protein are seen primarily in the basal layer, starting from day 2 (D) but continue to be expressed at day 3 (E) and 9 (F). In the dermis, the Lef-1 staining is concentrated in groups of cells adjacent to the epidermis (*). (G–I) K17 is expressed in the epithelium from at least day 2 (G) both in basal and suprabasal layers. At later time points (H and I), it becomes progressively restricted to the K12-negative basal layer. Cell division in the recombinants occurs in cells that are undergoing dedifferentiation. On day 1 (J), no cells in the epithelium are positive for the cell division marker Ki67 whereas, by day 2 (K), numerous cells are Ki67-positive (arrowheads), primarily in the basal layer. At later stages (L), the Ki67-positive cells are restricted to the K12-negative basal layer. [Scale bars: 20 μm (A–C and F–L) and 50 μm (D and E).]
Concurrent with the increased levels of β-catenin is an increase in the expression of Lef-1 (Fig. 2 D–F). Expression of Lef-1 can also be detected in some cells of the adjacent dermis, and, at day 7–9, these positive cells might be associated with clumps of Lef-1-positive cells in the basal layer.
Keratin K17 can be induced by Wnt/β-catenin signaling (51) and is up-regulated in activated keratinocytes, as well as being constitutively expressed in the basal layer of the limbal epithelium (Fig. 6D). K17 expression appears as early as day 1, primarily in the basal layer of the recombined epithelium, similarly to K14. At day 2, strong expression of K17 is present in the lower layers, with isolated expression in suprabasal layers (Fig. 2G). As with K14 expression, K17 expression becomes progressively restricted to the lower layers (Fig. 2H) and finally (Fig. 2I) to the K12-negative basal layer. Keratins K6 and K16, which are also stress-related keratins (46), are expressed in patches throughout the recombinant epithelium (data not shown).
Induction of Cell Division in the Recombined Corneal Epithelium. Ki67 is a marker of cells that are actively dividing and is poorly expressed in the rabbit central cornea, especially in the suprabasal layers (data not shown). One day after the recombination, there are no Ki67-positive cells detectable in the epithelium (Fig. 2 J), but, at day 2, Ki67-positive cells appear, mainly in the newly organized basal layer (Fig. 2K), indicating that cells have entered into the cell cycle. These cells are still positive for K12. At later time points (Fig. 2L), the number of Ki67-positive cells increases, and these dividing cells become restricted to the K12-negative basal layer. Another method of looking at cell division is by means of BrdUrd labeling of newly synthesized DNA. Using this method (see Supporting Text), we confirm that cell division occurs in the dedifferentiating basal epithelial cells (Fig. 7 D–F).
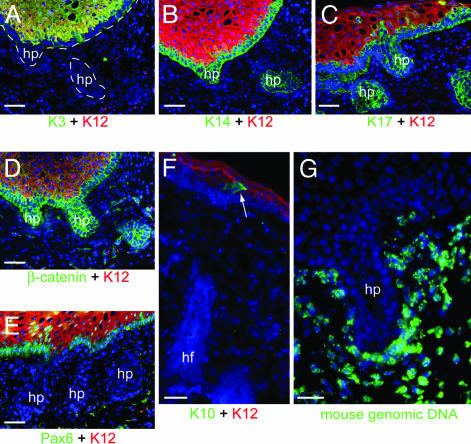
Interfollicular Epidermis Is Formed from the Dedifferentiated Cells of the Corneal Epithelium by Means of Cells Originating from the Newly Formed Hair Follicles. The first hair pegs are visible as projections of K12- and K3-negative, K14- and K17-positive basal cells into the upper dermis by day 7–8 (Fig. 3 A–C). The cells of the hair pegs have high levels of cytoplasmic β-catenin (Fig. 3D) and no Pax6 expression (Fig. 3E). As the hair follicles develop, they start to differentiate into distinct layers including a K16- and K17-positive outer root sheath and K6irs and K6hf-positive inner root sheath (data not shown). The base of the follicles develops into a bulb in which a dermal papilla is enclosed, which shows strong alkaline phosphatase activity and nuclear Lef-1 (data not shown).
Fig. 3.
Formation of hair pegs and follicles by the rabbit corneal epithelium. From ≈8 days, the epithelium begins to form hair pegs (hp). These pegs are negative for K12 and K3 (A) and positive for K14 (B) and K17 (C) and have high levels of β-catenin expression (D). They have also lost Pax6 expression (E). After the formation of the hair follicles (hf), K10-positive cells appear (arrow) at the junction of the hair follicle and the corneal epithelium (F). In situ hybridization using mouse genomic DNA shows no mouse DNA in the rabbit epithelial cells, even in hair pegs (G). [Scale bars: 20 μm (A–F) and 25 μm (G).]
Cells expressing the interfollicular suprabasal keratin K10 are detected at the junction of the newly formed hair follicles and the epithelium (Figs. 3F and 7J). Subsequently, K10-positive cells migrate out and displace the remaining K12-positive cells of the corneal epithelium and go on to form a fully differentiated, stratified epidermis with distinct granular anuclear cornified layers, as previously shown (4).
Dedifferentiation of the Rabbit Corneal Epithelium Is Not Due to Cell Fusion. The rabbit and mouse tissues can easily be distinguished based on the staining pattern of their nuclei, mouse nuclei having a punctuate appearance when stained with Hoechst or DAPI whereas, in rabbit nuclei, the DNA is homogenously distributed (52). In >120 grafts examined, based on the DNA staining pattern, the epithelial compartment was always composed exclusively of rabbit cells, both at early stages and after the formation of hair and epidermis. To definitively preclude the possibility that cell fusion induced the dedifferentiation of the rabbit cells, we carried out in situ hybridization using labeled mouse genomic DNA as a probe and unlabeled rabbit genomic DNA as a competitor. There was no labeling of any nuclei in the recombined epithelium from at least day 2, even in de novo hair pegs formed at 13 days (Fig. 3G), indicating that no mouse–rabbit cell fusion has occurred.
Noggin Expression in the Dermis Is Not Required for the Initial Dedifferentiation of Corneal Keratinocytes but Is Essential for Hair Formation and the Subsequent Appearance of K10-Expressing Cells. Mice in which Noggin, a secreted BMP agonist, has been homozygously knocked out die before birth at around E18 to -19 (44, 53). Although hair placodes are formed at E14.5 and onwards, they are reduced in number, and Noggin-/- skin is unable to support the postnatal growth of hair follicles (22, 54). When Noggin deficient (-/-) or heterozygous (+/-) (Fig. 7 G–J) embryonic dermis is recombined with adult corneal epithelium, both undergo the same initial dedifferentiation stages as recombinants with the wild-type dermis. Thus, at 8 days, epithelium combined with a Noggin-/- dermis shows a basal layer that has lost K12 expression and started to express K14 (Fig. 4A) and K17 (Fig. 4C). Dermal condensations are found in the Noggin-deficient dermis (Fig. 4C). After 4 weeks, however, whereas the +/- recombinants have developed mature hair follicles and K10-positive cells (Fig. 7J), the -/- recombinants were arrested at the initial dedifferentiation step, with a K14-positive (Fig. 4B) basal layer but no hair pegs or K10-positive cells (Fig. 4D).
Discussion
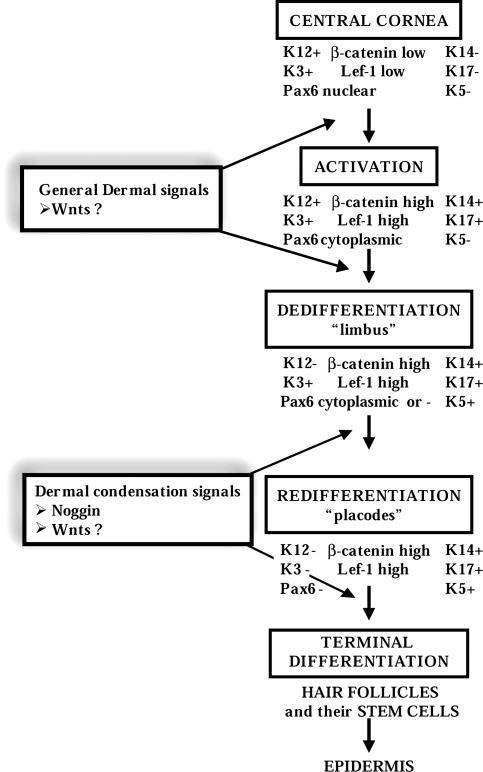
In this work we are able to elucidate the mechanism involved in the transdifferentiation of central corneal epithelium into hairs and then interfollicular epidermis. From the detailed analysis of the time course of events, and the molecular markers involved, it is apparent that there are several distinct steps in this reprogramming (Fig. 5). As such, this process corresponds to a model whereby committed cells change their fate by means of an initial dedifferentiation step in response to certain environmental stimuli, and these cells are then able to undergo a different differentiation program. In this sense, it resembles a “dedifferentiation model” (55) rather than direct transdifferentiation. No published cases of “direct transdifferentiation,” however, have shown cells that simultaneously coexpress two sets of terminal differentiation markers, implying that some degree of dedifferentiation is a general mechanism even in so-called direct transdifferentiation.
Fig. 5.
Major steps in the transdifferentiation of rabbit corneal epithelium into hair and epidermis, outlining the individual steps visible by using the expression of corneal, limbal, and epidermal markers. Note that the epidermis is not directly derived from the dedifferentiated basal layer but secondarily from Pax6- hair follicles.
Step 1: Down-Regulation of Pax6 and Dedifferentiation of the Corneal Epithelial Basal Cell May Result from a General Wnt Signal from the Dermis. Shortly after recombination, there is a large increase in the levels of cytoplasmic β-catenin, particularly in the basal layer of the epithelium, most likely due to β-catenin stabilization by means of Wnt signaling. The lack of observed nuclear β-catenin staining is likely due to the masking effect observed when using frozen sections (56). Lef-1 protein expression is also up-regulated in these basal cells. In addition, K17, which has been shown to be regulated by β-catenin/Lef-1 signaling (51), is also highly expressed in these cells. This result strongly suggests that Wnt signaling is implicated in this initial dedifferentiation. More direct evidence of Wnt involvement would be desirable, but currently the lack of a dermis-specific promoter precludes the use of dermal Wnt knockouts or dermal-specific expression of Wnt antagonists such as Dickkopf-1 (17).
Another early event in this step is the down-regulation and redistribution to the cytoplasm of Pax6. In the recombinants, K12 expression is turned off in cells where Pax6 is relocalized to the cytoplasm and hence cannot act as a transcriptional activator. In normal adult cornea, this cytoplasmic localization of Pax6 occurs only in the basal layer of the limbus. Interestingly, K3 is not coordinately down-regulated with K12 but is lost only later in the process. This sequence mirrors the situation during development where K12 is expressed earlier than K3 (36). The loss of K12 thus presumably results directly from the loss of Pax6 expression whereas that of K3 is likely due to a secondary mechanism.
The activation of the corneal epithelial cells is accompanied by a reentry of the cells into the cell cycle, which is restricted to the dedifferentiating cells of the basal layer. The relatively high proportion of Ki67-positive cells seen at day 2 plus the fact that many K12-negative, K14-positive cells are BrdUrd-positive shows that the dedifferentiated basal layer is not derived from a small number of stem cells but that many, possibly all, cells in the basal layer of the central cornea are able to undergo this reprogramming. The basal layer of the recombined epithelium thus turns off expression of Pax6 and K12 and turns on that of K17 and K5/14, adopting a phenotype resembling that of the cells in the limbal basal layer probably under the guidance of a general Wnt signal from the embryonic dermis.
Step 2: Induction and Formation of Hair by a Dialogue Involving Noggin and Wnts Between the Dedifferentiated Epithelial Cells and the Dermis. During the initial phase of dedifferentiation dermal condensations are lost, suggesting that the corneal epithelium lacks the signals to induce and maintain them. They are subsequently reformed, and this delay seems to correspond to the time required by the cells of the basal layer of the corneal epithelium to dedifferentiate and express a factor required for condensation induction and maintenance. Our results thus support a model of epidermal appendage differentiation whereby a competent dermis provides a general signal to instruct the epidermis to start appendage formation (57). In turn, the epidermis self-organizes into placodes, by means of an activator-inhibitor model (58), which then induce the formation of dermal condensations.
The reformed dermal condensations are then able to support the formation of hair pegs from the dedifferentiated basal layer. Interestingly, Pax6 expression is lost in cells forming the hair peg, although it is retained in other areas. The down-regulation of Pax6 might thus be a prerequisite for hair and interfollicular epidermis development, but whether the loss of Pax6 is a result of loss of signals from the stroma that maintain its epithelial expression or by means of a direct inhibition of its expression remains to be determined. These hair pegs then give rise to hair follicles consisting exclusively of cells originating from rabbit corneal cells with no fusion occurring between mouse and rabbit cells.
When Noggin-/- dermis is used, no hairs are formed, and the recombinants are arrested at the first step of the process, despite the fact that dermal condensations reappear. The second step, i.e., the formation of hair pegs and follicles, thus mirrors normal embryonic development where inhibition of epidermally expressed BMPs by Noggin expressed in the dermal condensations is required for the formation of hair pegs (20, 22), but not for the formation of the dermal condensations and associated placodes (44, 53). The increase in β-catenin and the expression of Lef-1 in the hair pegs strongly imply that the Wnt signaling pathway is also required for this second step.
Step 3: An Interfollicular Epidermis Is Formed by Cells Originating from the Hair Follicle, Implicating Newly Formed Hair Stem Cells. We confirm that cells expressing K10, a marker for differentiating keratinocytes in interfollicular epidermis (45), appear initially at the junction of the newly formed hair follicles and the remaining corneal epithelium only when the follicles have undergone extensive development (4). These cells displace the corneal epithelium and give rise to a mature interfollicular epidermis that is thus derived by means of an intermediary step of hair follicle formation by means of a mechanism similar to that which occurs in reepithelialization after wounding (59, 60) and not directly from the dedifferentiated, limbal-type basal layer. This delay underlines the fact that corneal cells do not directly transdifferentiate into epidermal cells but must first undergo a dedifferentiation step. The fact that the Noggin-deficient recombinants do not form either hair follicles or interfollicular epidermis confirms that, in this model, the hair follicles are the source of the cells that become the epidermis. This fact implies that the corneal-derived hair follicles contain a source of multipotent cells similar to those usually found in the bulge region.
In conclusion, our results show that epidermal cells have been induced from differentiated corneal cells by developmental signals from an embryonic dermis. This reprogramming occurs by means of a multistep process that involves dedifferentiation, followed by redifferentiation into hair follicles together with associated multipotent stem cells.
Supplementary Material
Acknowledgments
We thank P. Coulombe, E. Fuchs, R. Grosschedl, L. Langbein, and T. T. Sun for the generous gifts of antibodies and R. Harland and C. Niehrs for the generous gift of the Noggin knockout mice. We thank M. Raff for his insightful commentary, G. Chevalier for expert assistance, C. Jolly and C. Vourc'h for assistance with the in situ hybridization, and B. Peyrusse for iconography. This research was supported by a grant from the Sociéte de Recherche Dermatologique, a Ministère des Affaires Etrangères fellowship (to D.J.P.), and a Fondation pour la Recherche Médicale fellowship (to Y.Y.).
Author contributions: D.J.P. and D.D. designed research; D.J.P. and Y.Y. performed research; D.J.P. and D.D. analyzed data; and D.J.P. and D.D. wrote the paper.
Abbreviations: BMP, bone morphogenetic protein; En, embryonic day n.
References
- 1.Tanaka, E. M. (2003) Curr. Opin. Genet. Dev. 13, 497-501. [DOI] [PubMed] [Google Scholar]
- 2.Nye, H. L., Cameron, J. A., Chernoff, E. A. & Stocum, D. L. (2003) Dev. Dyn. 226, 280-294. [DOI] [PubMed] [Google Scholar]
- 3.Brockes, J. P. & Kumar, A. (2002) Nat. Rev. Mol. Cell. Biol. 3, 566-574. [DOI] [PubMed] [Google Scholar]
- 4.Ferraris, C., Chevalier, G., Favier, B., Jahoda, C. A. & Dhouailly, D. (2000) Development (Cambridge, U.K.) 127, 5487-5495. [DOI] [PubMed] [Google Scholar]
- 5.Kondo, T. & Raff, M. (2000) Science 289, 1754-1757. [DOI] [PubMed] [Google Scholar]
- 6.Odelberg, S. J., Kollhoff, A. & Keating, M. T. (2000) Cell 103, 1099-1109. [DOI] [PubMed] [Google Scholar]
- 7.Bjornson, C. R., Rietze, R. L., Reynolds, B. A., Magli, M. C. & Vescovi, A. L. (1999) Science 283, 534-537. [DOI] [PubMed] [Google Scholar]
- 8.Krause, D. S., Theise, N. D., Collector, M. I., Henegariu, O., Hwang, S., Gardner, R., Neutzel, S. & Sharkis, S. J. (2001) Cell 105, 369-377. [DOI] [PubMed] [Google Scholar]
- 9.Vassilopoulos, G. & Russell, D. W. (2003) Curr. Opin. Genet. Dev. 13, 480-485. [DOI] [PubMed] [Google Scholar]
- 10.Goodell, M. A. (2003) Curr. Opin. Hematol. 10, 208-213. [DOI] [PubMed] [Google Scholar]
- 11.Alison, M. R., Poulsom, R., Otto, W. R., Vig, P., Brittan, M., Direkze, N. C., Preston, S. L. & Wright, N. A. (2003) J. Cell Sci. 116, 599-603. [DOI] [PubMed] [Google Scholar]
- 12.Tsai, R. Y., Kittappa, R. & McKay, R. D. (2002) Dev. Cell 2, 707-712. [DOI] [PubMed] [Google Scholar]
- 13.Vescovi, A., Gritti, A., Cossu, G. & Galli, R. (2002) Cells Tissues Organs 171, 64-76. [DOI] [PubMed] [Google Scholar]
- 14.Prockop, D. J. (2003) J. Cell Biol. 160, 807-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raff, M. (2003) Annu. Rev. Cell Dev. Biol. 19, 1-22. [DOI] [PubMed] [Google Scholar]
- 16.Dhouailly, D. (1977) in Frontiers of Matrix Biology, ed. Robert, L. (Karger, Basel), Vol. 4, pp. 86-121. [Google Scholar]
- 17.Andl, T., Reddy, S. T., Gaddapara, T. & Millar, S. E. (2002) Dev. Cell 2, 643-653. [DOI] [PubMed] [Google Scholar]
- 18.DasGupta, R., Rhee, H. & Fuchs, E. (2002) J. Cell Biol. 158, 331-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huelsken, J., Vogel, R., Erdmann, B., Cotsarelis, G. & Birchmeier, W. (2001) Cell 105, 533-545. [DOI] [PubMed] [Google Scholar]
- 20.Jamora, C., DasGupta, R., Kocieniewski, P. & Fuchs, E. (2003) Nature 422, 317-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Merrill, B. J., Gat, U., DasGupta, R. & Fuchs, E. (2001) Genes Dev. 15, 1688-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Botchkarev, V. A., Botchkareva, N. V., Sharov, A. A., Funa, K., Huber, O. & Gilchrest, B. A. (2002) J. Invest. Dermatol. 118, 3-10. [DOI] [PubMed] [Google Scholar]
- 23.St.-Jacques, B., Dassule, H. R., Karavanova, I., Botchkarev, V. A., Li, J., Danielian, P. S., McMahon, J. A., Lewis, P. M., Paus, R. & McMahon, A. P. (1998) Curr. Biol. 8, 1058-1068. [DOI] [PubMed] [Google Scholar]
- 24.Chiang, C., Swan, R. Z., Grachtchouk, M., Bolinger, M., Litingtung, Y., Robertson, E. K., Cooper, M. K., Gaffield, W., Westphal, H., Beachy, P. A. & Dlugosz, A. A. (1999) Dev. Biol. 205, 1-9. [DOI] [PubMed] [Google Scholar]
- 25.Reynolds, A. J. & Jahoda, C. A. (1992) Development (Cambridge, U.K.) 115, 587-593. [DOI] [PubMed] [Google Scholar]
- 26.Lo Celso, C., Prowse, D. M. & Watt, F. M. (2004) Development (Cambridge, U.K.) 131, 1787-1799. [DOI] [PubMed] [Google Scholar]
- 27.Gat, U., DasGupta, R., Degenstein, L. & Fuchs, E. (1998) Cell 95, 605-614. [DOI] [PubMed] [Google Scholar]
- 28.Watt, F. M. (2002) J. Dermatol. Sci. 28, 173-180. [DOI] [PubMed] [Google Scholar]
- 29.Potten, C. S. & Booth, C. (2002) J. Invest. Dermatol. 119, 888-899. [DOI] [PubMed] [Google Scholar]
- 30.Lavker, R. M. & Sun, T. T. (2000) Proc. Natl. Acad. Sci. USA 97, 13473-13475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blanpain, C., Lowry, W. E., Geoghegan, A., Polak, L. & Fuchs, E. (2004) Cell 118, 635-648. [DOI] [PubMed] [Google Scholar]
- 32.Fuchs, E., Merrill, B. J., Jamora, C. & DasGupta, R. (2001) Dev. Cell 1, 13-25. [DOI] [PubMed] [Google Scholar]
- 33.Oshima, H., Rochat, A., Kedzia, C., Kobayashi, K. & Barrandon, Y. (2001) Cell 104, 233-245. [DOI] [PubMed] [Google Scholar]
- 34.Lavker, R. M., Tseng, S. C. & Sun, T. T. (2004) Exp. Eye Res. 78, 433-446. [DOI] [PubMed] [Google Scholar]
- 35.Rama, P., Bonini, S., Lambiase, A., Golisano, O., Paterna, P., De Luca, M. & Pellegrini, G. (2001) Transplantation 72, 1478-1485. [DOI] [PubMed] [Google Scholar]
- 36.Chaloin-Dufau, C., Sun, T. T. & Dhouailly, D. (1990) Cell Differ. Dev. 32, 97-108. [DOI] [PubMed] [Google Scholar]
- 37.Schermer, A., Galvin, S. & Sun, T. T. (1986) J. Cell Biol. 103, 49-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun, T. T. & Lavker, R. M. (2004) J. Invest. Dermatol. Symp. Proc. 9, 202-207. [DOI] [PubMed] [Google Scholar]
- 39.Gehring, W. J. (2002) Int. J. Dev. Biol. 46, 65-73. [PubMed] [Google Scholar]
- 40.Pichaud, F. & Desplan, C. (2002) Curr. Opin. Genet. Dev. 12, 430-434. [DOI] [PubMed] [Google Scholar]
- 41.Simpson, T. I. & Price, D. J. (2002) BioEssays 24, 1041-1051. [DOI] [PubMed] [Google Scholar]
- 42.Liu, J. J., Kao, W. W. & Wilson, S. E. (1999) Exp. Eye Res. 68, 295-301. [DOI] [PubMed] [Google Scholar]
- 43.Hanson, I. M. (2003) Pediatr. Res. 54, 791-796. [DOI] [PubMed] [Google Scholar]
- 44.Brunet, L. J., McMahon, J. A., McMahon, A. P. & Harland, R. M. (1998) Science 280, 1455-1457. [DOI] [PubMed] [Google Scholar]
- 45.Sun, T. T., Eichner, R., Nelson, W. G., Vidrich, A. & Woodcock-Mitchell, J. (1983) Curr. Probl. Dermatol. 11, 277-291. [DOI] [PubMed] [Google Scholar]
- 46.McGowan, K. & Coulombe, P. A. (1998) Subcell. Biochem. 31, 173-204. [PubMed] [Google Scholar]
- 47.Lichter, P., Tang, C. J., Call, K., Hermanson, G., Evans, G. A., Housman, D. & Ward, D. C. (1990) Science 247, 64-69. [DOI] [PubMed] [Google Scholar]
- 48.Botchkarev, V. A. & Kishimoto, J. (2003) J. Invest. Dermatol. Symp. Proc. 8, 46-55. [DOI] [PubMed] [Google Scholar]
- 49.Kishimoto, J., Burgeson, R. E. & Morgan, B. A. (2000) Genes Dev. 14, 1181-1185. [PMC free article] [PubMed] [Google Scholar]
- 50.Paus, R., Muller-Rover, S., Van Der Veen, C., Maurer, M., Eichmuller, S., Ling, G., Hofmann, U., Foitzik, K., Mecklenburg, L. & Handjiski, B. (1999) J. Invest. Dermatol. 113, 523-532. [DOI] [PubMed] [Google Scholar]
- 51.McGowan, K. M. & Coulombe, P. A. (1998) J. Cell Biol. 143, 469-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cunha, G. R. & Vanderslice, K. D. (1984) Stain Technol. 59, 7-12. [DOI] [PubMed] [Google Scholar]
- 53.McMahon, J. A., Takada, S., Zimmerman, L. B., Fan, C. M., Harland, R. M. & McMahon, A. P. (1998) Genes Dev. 12, 1438-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Botchkarev, V. A., Botchkareva, N. V., Roth, W., Nakamura, M., Chen, L. H., Herzog, W., Lindner, G., McMahon, J. A., Peters, C., Lauster, R., et al. (1999) Nat. Cell Biol. 1, 158-164. [DOI] [PubMed] [Google Scholar]
- 55.Frisen, J. (2002) Neuron 35, 415-418. [DOI] [PubMed] [Google Scholar]
- 56.Tsuji, H., Ishida-Yamamoto, A., Takahashi, H. & Iizuka, H. (2001) J. Dermatol. Sci. 27, 170-177. [DOI] [PubMed] [Google Scholar]
- 57.Olivera-Martinez, I., Viallet, J. P., Michon, F., Pearton, D. J. & Dhouailly, D. (2004) Int. J. Dev. Biol. 48, 107-115. [DOI] [PubMed] [Google Scholar]
- 58.Jiang, T. X., Widelitz, R. B., Shen, W. M., Will, P., Wu, D. Y., Lin, C. M., Jung, H. S. & Chuong, C. M. (2004) Int. J. Dev. Biol. 48, 117-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Taylor, G., Lehrer, M. S., Jensen, P. J., Sun, T. T. & Lavker, R. M. (2000) Cell 102, 451-461. [DOI] [PubMed] [Google Scholar]
- 60.Miller, S. J., Burke, E. M., Rader, M. D., Coulombe, P. A. & Lavker, R. M. (1998) J. Invest. Dermatol. 110, 13-19. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.