ABSTRACT
Background: A wealth of research has reported on the anti-obesity effects of green tea extract (GTE). Although browning of white adipose tissue (WAT) has been reported to attenuate obesity, no study has disclosed the effects of GTE on browning in Sprague Dawley rats.
Objectives: The aims of the study were to investigate the effects of GTE on anti-obesity and browning, and their underlying mechanisms.
Methods: Four groups of rats (n=10/group) were used including a normal diet with vehicle treatment, and a high-energy diet (HED) with vehicle or GTE by oral gavage at 77.5 or 155 mg/kg/day for 8 weeks. Body weight, fat accumulation, and serum biochemical parameters were used to evaluate obesity. The gene expressions were analyzed using RT-qPCR and western blotting.
Results: GTE modulated HED-induced body weight, fat accumulation, and serum levels of triacylglycerol, total cholesterol, low-density lipoprotein, free fatty acids, aspartate aminotransferase, and alanine aminotransferase. Moreover, GTE enhanced the serum high-density lipoprotein. Most importantly, the biomarkers of beige adipose tissue were up-regulated in WAT in GTE-given groups. GTE induced genes involved in different pathways of browning, and reduced transducin-like enhancer protein-3 in WAT.
Conclusion: Our results suggest that GTE may improve obesity through inducing browning in HED-fed rats.
Abbreviations: ALT: Alanine transaminase; AST: Aspartate transaminase; BAT: Brown adipose tissue; BMP-7: Bone morphogenetic protein-7; BW: Body weight; CIDEA: Cell death activator; CPT-1: Carnitine palmitoyltransferase-1; EFP: Epididymal fat pad; FFA: Free fatty acid; FGF-21: Fibroblast growth factor-21; GTE: Green tea extract; HDL: High-density lipoprotein; HED: high-energy diet; LDL: Low-density lipoprotein; MFP: Mesenteric fat pad; PGC-1α: Activates PPAR-γ coactivator-1; PPAR-γ: Peroxisome proliferator-activated receptor-γ; PRDM-16: PR domain containing 16; RFP: Renal fat pad; SD: Sprague Dawley; TC: Total cholesterol; TG: Triacylglycerol; TLE-3: Transducin-like enhancer protein-3: UCP-1: Uncoupling protein-1; WAT: White adipose tissue.
KEYWORDS: Anti-obesity, beige adipose tissue, browning of white adipose tissue, green tea extract, pathways of browning
Introduction
Obesity is one of the major risk factors for pathological disorders, including diabetes, hypertension, atherosclerosis, and cancer [1,2]. The prevalence of obesity is estimated to increase by 7% and 10% among men and women, respectively, by 2020 [3]. Therefore, to resolve the issue of obesity is an important requirement for human health. Several studies reported that food and food ingredients are good targets for improving obesity disorders, leading to the investigation of their anti-obesity mechanisms [4–6]. For example, green tea and its extracts were suggested to reduce body weight (BW) through inducing apoptosis, inhibiting adipogenesis, preventing energy uptake, enhancing lipolysis, elevating fatty acid oxidation-related genes, and increasing energy expenditure [7,8]. However, the effect of green tea on the browning of white adipose tissues (WAT) of Sprague Dawley (SD) rats is still unreported.
Nowadays, three types of adipose tissue are known, including WAT, brown adipose tissue (BAT), and beige adipose tissue. WAT is optimized to store energy in large lipid droplets for later use [9], and the accumulation of body fat in WAT leads to both hypertrophy and hyperplasia of white adipocytes [10]. These changes are associated with obesity-related diseases such as type-2 diabetes and an inflammatory response [1,11]. In contrast, BAT generates energy mostly in the form of heat [12], and promoting BAT activities was reported to prevent obesity [13]. Therefore, BAT was suggested as a potential target of anti-obesity therapy [14]. Recently, increasing research has focused on the other type of adipose tissue, beige adipose tissue, because beige adipose tissue was also identified as another potential candidate for obesity treatment due to its capacity to burn glucose and fat to produce heat [15]. Beige adipose tissue, traditionally seen as WAT, is a new form of adipose tissue and can be transformed from WAT [16]. Although beige adipose tissue and WAT are derived from Pax7− and Myf5− precursor cells and express different gene signatures from BAT [17–19], beige adipose tissue is similar to BAT in that it has more mitochondria and higher expressions of thermogenesis and lipolysis genes [20].
Several genes of different pathways were suggested to regulate the browning of WAT. Peroxisome proliferator-activated receptor-γ (PPAR-γ) is the master transcriptional regulator of fat differentiation, and in concert with the PR domain containing-16 (PRDM-16) activates PPAR-γ coactivator-1α (PGC-1α) to induce brown-selective genes [21–23]. Browning is also driven by the pathways involving either fibroblast growth factor-21 (FGF-21) or bone morphogenetic protein-7 (BMP-7) [24,25]. An increase of FGF-21 in WAT stabilizes PGC-1α to enhance browning [24]. Moreover, EWS/YBX-1/BMP-7 pathway was suggested to mediate browning [25]. Conversely, transducin-like enhancer protein-3 (TLE-3), which suppresses brown-selective genes and induces white-selective genes, is involved in a negative regulation pathway of the browning process [26]. Differences between beige adipose tissue and WAT are observed in genes regulating them and also in mitochondrial and fatty acid oxidation-related genes. Compared to WAT, beige adipose tissue presents higher levels of uncoupling protein-1 (UCP-1), cell death activator (CIDEA), and carnitine palmitoyltransferase-1 (CPT-1) [18,27,28]. Enhancement of these genes transforms WAT into fat-oxidizing and energy-expending machines [29]. The aim of this study was to investigate the effects of green tea extract (GTE) on anti-obesity and browning of WAT in SD rats fed a high-energy diet (HED). Additionally, we analyzed the gene expressions following GTE treatment to evaluate GTE-induced pathways of browning of WAT. Therefore, the present study could demonstrate new insights into the anti-obesity mechanism induced by GTE.
Materials and methods
Animals and diets
Male SD rats (6 weeks old, ~215 g) were purchased from a local supplier (LASCO, Taipei, Taiwan) and singly housed during the study. Rats were given seven days to acclimate to their new environment under standard laboratory conditions (a 12/12-h light/dark cycle, 22 ~ 24°C, 40%~60% humidity). All rats were fed ad libitum with the diets purchased from a local supplier. Ten rats given a normal diet (see Table 1 for ingredient composition) comprised the control group (C). The other 30 rats were fed an HED (see Table 1 for ingredient composition) and randomly and equally divided into three groups, including an HE group (given the HED), a 1X group (given the HED and 77.5 mg/kg/day of GTE), and a 2X group (given the HED and 155 mg/kg/day of GTE). For humans, giving 750 mg/day of GTE was reported to protect against obesity [30]. The rat dose was converted from a human equivalent dose based on the body surface area by the following formula from the US Food and Drug Administration: assuming a human weight of 60 kg, the dose for rat is 750 mg/day÷60 kg × 6.2 = 77.5 mg/kg/day; the conversion coefficient of 6.2 was used to account for differences between rats and humans. The GTE powder (Hong-Zhong Biotechnology, Tainan, Taiwan) was produced from green tea by standard procedures with a certificate of the analytical methods, and contained 83.5% total catechins, 38.5% epigallocatechin gallate (EGCG), 96.6% total polyphenols, and 1.8% caffeine (analyzed by HPLC). GTE was dissolved in 1 ml of distilled water and orally administrated to the 1X and 2X groups by gavage at 09:00 every day for eight weeks, while 1 ml of distilled water was given to the C and HE groups instead. Food and water intake levels were recorded every day, and BWs were measured weekly. Animals were sacrificed by CO2 at the end of the eighth week. All animal experiments were performed in accordance with the protocol (IACUC-10,303) approved by the Institutional Animal Care and Use Committee (IACUC) of Shih Chien University.
Table 1.
Ingredient composition of the diets fed to rats.
| Ingredient composition | Normal diet | High-energy diet |
|---|---|---|
| Corn starch | 46.23% | 4.50% |
| Dextrin | 15.38% | 1.49% |
| Casein-vitamin free | 13.89% | 13.96% |
| Sucrose | 9.92% | 27.89% |
| Fructose | 0.00% | 19.91% |
| Powdered cellulose | 4.96% | 4.70% |
| Soybean Oil | 3.97% | 21.90% |
| AIN 93M Mineral Mix | 3.47% | 3.47% |
| AIN 93 Vitamin Mix | 0.99% | 0.99% |
| Choline bitartrate | 0.23% | 0.23% |
| L-Cystine | 0.17% | 0.17% |
| t-Butylhydroquinone | 0.79% | 0.79% |
| kcal/g | 3.8 | 4.50 |
Adipose tissues sampling
WATs from mesenteric, epididymal, and perirenal sites were collected, rinsed with PBS, and then weighed immediately. All samples were stored at −80°C until the beginning of analysis.
Serum biochemical parameters
After eight hours of starving, rat blood was collected and serum was obtained by centrifugation at 3500 g for 10 minutes at 4°C. Plasma triacylglycerol (TG), total cholesterol (TC), low-density lipoprotein (LDL), high-density lipoprotein (HDL), alanine transaminase (ALT), and aspartate transaminase (AST) were measured using a Hitachi 7080 analyzer (Hitachi, Japan). The free fatty acid (FFA) concentration was determined by an enzymatic colorimetric assay according to the manual of a commercial kit (Boehringer Mannheim, Germany).
Determination of the adipocyte size of WAT
Epididymal fat pads (EFPs) were fixed in 4% paraformaldehyde (pH 7.2) at 4°C for 16 hours. EFP samples were dehydrated in absolute ethanol, cleared in xylene, and then embedded in paraffin. After cutting the paraffin into 4-μm sections, sections were stained with Harris hematoxylin and counterstained with eosin, and digital images were taken under light microscopy (Nikon, Japan). To analyze the digital images, the area, approximate diameter, perimeter, and shape factor were measured in 10 adipocytes (five slides for each rat, 10 rats per group). The analysis of these results was performed using the Sigma ScanPro4 program (Sigma, USA).
RNA extraction and quantitative RT-PCR (RT-qPCR)
RNA was extracted using an RNeasy Mini kit (Qiagen, Germany), and 500 ng of total RNA of each sample was reverse-transcribed with an iScript cDNA Synthesis Kit (Bio-Rad, USA) according to instructions of the manufacturer. A qPCR was performed in a MyiQ Single-Color Real-Time PCR Detection System (Bio-Rad), and sequences of gene-specific primers (Purigo, Taiwan) are shown in Table 2. β-actin was used as an internal control for normalizing messenger (m)RNA levels of the tested genes.
Table 2.
Primer sequences used in the RT-qPCR.
| Gene | Gene accession numbers | Forward primers | Reverse primers |
|---|---|---|---|
| PPAR-γ | NM_013124.3 | GACCTCTCTGTGATGGATGAC | TCGCACTTTGGTATTCTTGGA |
| PRDM-16 | NM_001291029.1 | GCAGACCCTGTGGGAGTCCTGAAA | GCTCCCCTGTGTGTGTCCTCAGAT |
| BMP-7 | NM_001191856.2 | CGCTCCAAGACTCCAAAGAA | GGTCTCGGAAGCTAACATACAG |
| FGF-21 | NM_130752.1 | CAACAACCAGATGGAACTCTCTA | GGTACACATTGTATCCGTCCTT |
| PGC-1α | AY237127.1 | GCCGGAGCAATCTGAGTTAT | GATCACCAAACAGCCGTAGA |
| TLE-3 | NM_053400.1 | GATAGGCAGATGGACAGACAAG | GAGAAGATGGAGCAGAGAAACC |
| UCP-1 | NM_012682.2 | AGGGTTTGCGCCTTCTTT | GGGACTTCATCAGCTCTTTCTT |
| CPT-1 | NM_031559.2 | CGGAGCCAGGAGATATAGATAGA | GAATCTGACTGGGTGGGATTAG |
| CIDEA | NM_001170467.1 | GGACACAGAGGAGTTCTTTCAG | CGAAGGTGACTCTGGCTATTC |
| Leptin | NM_013076.3 | GGTTTCGTGGTGCTGACTAA | CACATCCTGTTCCGACTCTTAC |
| Adiponectin | BC092565.1 | AAGTCTGGCTCCAAGTGTATG | GGTAGAGAAGGAAGCCTGTAAAT |
| β-actin | NM_031144.3 | ACAGGATGCAGAAGGAGATTAC | ACAGTGAGGCCAGGATAGA |
Western blot determination of UCP-1 and β-actin
EFPs were homogenized in RIPA lysis buffer containing protease inhibitors (Sigma) for 30 min and then sonicated at 4°C. After centrifuged (16 000 × g for 10 minutes at 4°C), the supernatant was collected. The concentration of protein was measured by the Bradford method with a protein assay kit (Bio-Rad). The protein from the lysate was loaded at 40 μg, separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis, and then transferred onto nitrocellulose membranes (Millipore, USA). The blots were hybridized with primary antibodies against UCP-1 (Proteintech Group, USA) and β-actin (Cell Signalling Technology, USA). The horseradish peroxidase activity was detected by an enhanced chemiluminescence system (UVP BioSpectrum Imaging System, Upland, USA) following incubation with the horseradish peroxidase-conjugated secondary antibodies (Cell Signalling Technology). The values were obtained by dividing the density of the band of UCP-1 by that of β-actin from the same sample.
Statistical analyses
Data were analyzed by a one-way analysis of variance (ANOVA) with Tukey’s post-hoc test. All results are presented as the mean ± standard error of the mean. p values < 0.05 were considered significant.
Results
Food intake, BW, and the feed conversion efficiency
The effect of GTE on obesity was investigated using male SD rats with HED-induced obesity. Weekly food intake of each rat was approximately 140 ~ 173 g, and no difference was seen among all groups (Table 3). The average BW did not significantly differ between groups at week 0. From the 2nd week to the end of the trial, the HE group had significantly higher BW than the other groups. During the period of the study, average BW gains were 221.2 ± 17.7, 306.3 ± 19.6, 246.5 ± 26.4, and 241.0 ± 20.9 in the C, HE, 1X, and 2X groups, respectively (Figure 1). Although the weight of total food intake per rat was not different between the groups, the food conversion efficiency (Increase of BW / Total calories intake) was higher in the HE group than the C, 1X, and 2X groups (Table 3). The 1X group showed slightly higher BW and food conversion efficiency than the 2X group, but the difference was not statistically significant. Group C with the lowest average BW gain indicated that obesity successfully occurred in HED-fed rats.
Table 3.
Effects of GTE on the body weight, diet intake, food efficiency, and serum biochemical parameters in HED-fed rats.
| C | HE | 1X | 2X | |
|---|---|---|---|---|
| Total food intake (g)/rat | 1386.7 ± 13.4a | 1382.0 ± 18.9a | 1377.9 ± 18.0a | 1376.2 ± 19.1a |
| Increase of BW/rat | 221.2 ± 17.7a | 306.3 ± 19.6c | 252.5 ± 24.7b | 247.2 ± 16.6b |
| FCE (%) | 15.9 ± 1.3a | 22.2 ± 1.4c | 18.4 ± 1.8b | 17.9 ± 1.3b |
| TG (mg/dl) | 44.3 ± 3.0a | 79.0 ± 10.3c | 55.1 ± 7.5b | 52.1 ± 3.9b |
| TC (mg/dl) | 42.0 ± 6.4a | 69.5 ± 7.8d | 61.2 ± 6.6 c | 53.3 ± 7.5 b |
| LDL (mg/dl) | 5.5 ± 0.7a | 7.8 ± 1.2b | 6.3 ± 1.6a | 6.0 ± 0.9a |
| HDL (mg/dl) | 20.1 ± 4.0c | 13.4 ± 1.4a | 16.0 ± 1.3b | 16.1 ± 1.5b |
| FFA (μM) | 3.2 ± 1.1a | 4.9 ± 0.9b | 3.9 ± 0.5a | 3.5 ± 0.4a |
| AST (U/L) | 126.9 ± 39.4a | 224.1 ± 79.3b | 171.9 ± 13.7a | 163.1 ± 15.2a |
| ALT (U/L) | 33.1 ± 4.6a | 58.6 ± 9.7c | 47.9 ± 6.7b | 43.1 ± 11.6b |
Control, vehicle control; HE, HED vehicle control; 1X, HED with 77.5 mg/kg/day of GTE; 2X, HED with 155 mg/kg/day of GTE. All values are the mean ± SEM (n = 10 rats/group). Different superscript letters (a, b, c) indicate a significant difference at p < 0.05 by a one-way ANOVA with Tukey’s post-hoc test. BW, body weight; FCE, food conversion efficiency (Increase of BW/Total calories intake); TG, triacylglycerol; TC total cholesterol; FFA, free fatty acids; AST, aspartate transaminase; ALT, alanine transaminase.
Figure 1.

Body weights in eight weeks. C; vehicle control, HE; HED vehicle control, 1X; HED with 77.5 mg/kg/day of GTE, 2X; HED with 155 mg/kg/day of GTE. Data are the mean ± SEM (n = 10 rats/group).
Plasma biochemical parameters
Plasma biochemistry was evaluated to assess the anti-obesity effects of GTE in HED-fed SD rats. Group C had lower TG, TC, LDL, FFA, AST, and ALT than the HE group, and lower TG, TC, and ALT than the 1X and 2X groups. The HDL level was highest in the C group (Table 3). GTE treatment resulted in significant decreases in TG, TC, LDL, FFA, AST, ALT, and FFA, and an increase of HDL in both the 1X and 2X groups compared to the HE group. Moreover, a lower concentration of TC was found in the 2X group than in the 1X group (Table 3).
Fat accumulation and compositions of WAT
Effects of GTE on fat accumulation were studied by analyzing the weight of WAT and the size of adipocytes. Weights of epididymal, perirenal, and mesenteric WATs were lower in the C, 1X, and 2X groups than in the HE group. Compared to the C group, the 1X group had higher weights of EFPs and renal fat pads (RFPs), and only RFPs were heavier in the 2X group. The body fat percentage of control rats was lower than those of HE and 1X rats, and both 1X and 2X rats had a lower body fat percentage than HE rats (Table 4). In addition, adipocytes of the EFPs were much smaller in rats of the 1X and 2X groups than in rats of the HE group (Figure 2).
Table 4.
Effects of GTE on the mass of the EFP, RFP, MFP, and total body fat in HED-fed rats.
| C | HE | 1X | 2X | |
|---|---|---|---|---|
| EFP (g) | 6.6 ± 0.9a | 12.4 ± 1.9c | 8.9 ± 1.5b | 7.5 ± 2.0ab |
| RFP (g) | 10.9 ± 1.a | 20.0 ± 1.7c | 14.8 ± 2.2b | 13.6 ± 1.8b |
| MFP (g) | 5.6 ± 1.2a | 9.7 ± 1.6b | 6.9 ± 1.3a | 6.3 ± 1.5a |
| Body fat percentage (%) | 5.1 ± 0.6a | 8.0 ± 0.9c | 6.6 ± 0.8b | 6.0 ± 0.8ab |
Control, vehicle control; HE, HED vehicle control; 1X, HED with 77.5 mg/kg/day of GTE; 2X, HED with 155 mg/kg/day of GTE. All values are the mean ± SEM (n = 10 rats/group). Different superscript letters (a, b, c) indicate a significant difference at p < 0.05 by a one-way ANOVA with Tukey’s post-hoc test.
Figure 2.
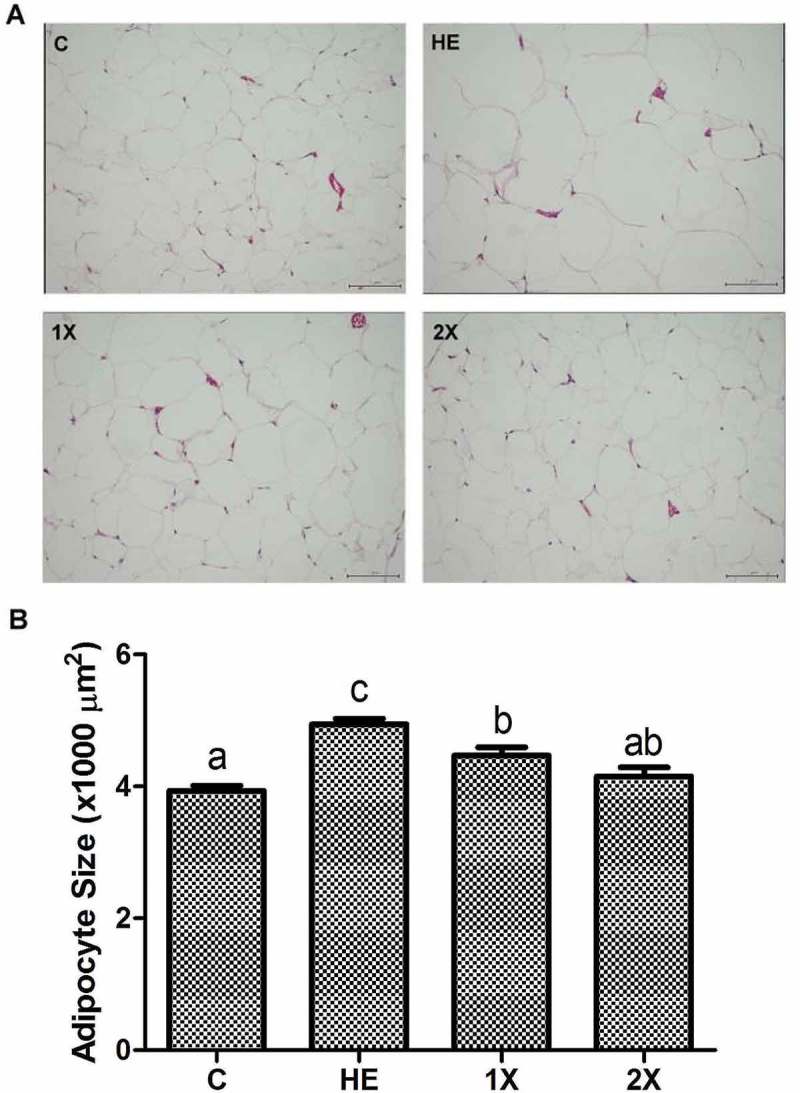
Histological analysis of epididymal fat pads stained with hematoxylin-eosin. C; vehicle control, HE; HED vehicle control, 1X; HED with 77.5 mg/kg/day of GTE, 2X; HED with 155 mg/kg/day of GTE. (a) Sections of epididymal fat pads fixed and stained with hematoxylin-eosin to visualize adipocytes (original magnification ×200). (b) Adipocytes were analyzed with an image analysis system and quantified. All values are the mean ± SEM (n = 10 rats/group). Different superscript letters (a, b, c) indicate a significant difference at p < 0.05 by a one-way ANOVA with Tukey’s post-hoc test. Scale bar = 50μM.
Leptin and adiponectin mRNA expressions
Since our purpose was to understand the anti-obesity mechanism of GTE in rats fed with a HED, the expressions of mRNA were compared in three HED fed groups. mRNA expressions of leptin and adiponectin were measured by an RT-qPCR. Leptin was higher in the HE group than in the 1X and 2X groups, and adiponectin was higher in the 1X and 2X groups than in the HE group. Although the 2X group showed higher expression of adiponectin than the 1X group, the difference was not significant (Figure 3).
Figure 3.
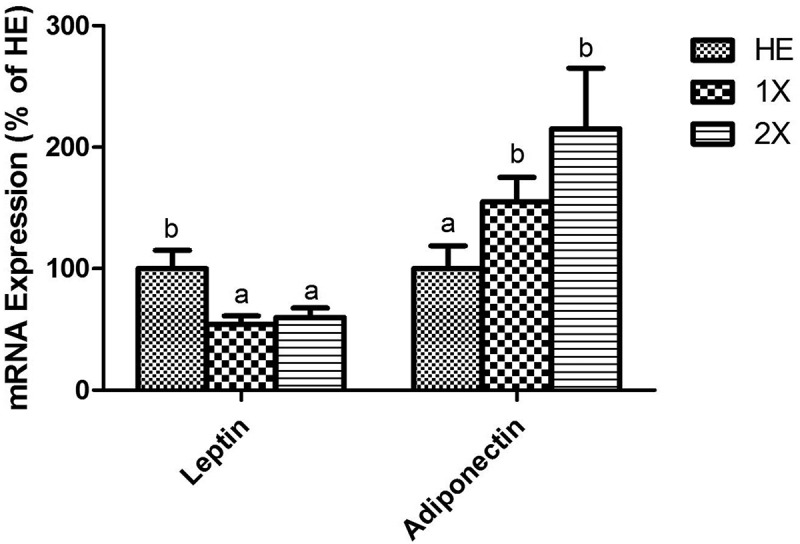
Expressions of leptin and adiponectin in the EFPs. HE; HED vehicle control, 1X; HED with 77.5 mg/kg/day of GTE, 2X; HED with 155 mg/kg/day of GTE. All values are the mean ± SEM (n = 10 rats/group). Different superscript letters (a and b) indicate a significant difference at p < 0.05 by a one-way ANOVA with Tukey’s post-hoc test.
Expressions of beige-regulating and thermogenic genes in EFPs
Several genes that regulate the transformation of white into beige adipose tissue and thermogenesis were measured by an RT-qPCR to evaluate the effect of GTE on the acquisition of BAT and beige adipose tissue characteristics by white adipocytes. PPAR-γ, PRDM-16, BMP-7, FGF-21, and PGC-1α, which drive development of beige adipose tissue, were significantly higher in the 1X and 2X groups than in the HE group. Moreover, TLE-3, which suppresses brown-selective genes and induces white-selective genes, was downregulated in the 1X and 2X groups (Figure 4(a)). Consistently, the biomarkers of browning, including UCP-1, CPT-1, and CIDEA, were upregulated in the 1X and 2X groups. Expressions of these genes did not differ between the 1X and 2X groups (Figure 4(b)). Similar to the result of mRNA expression, the protein levels of UCP-1 were significantly higher in the GTE-treated groups than the HE group and were not different between the 1X and 2X groups (Figure 4(c,d)).
Figure 4.
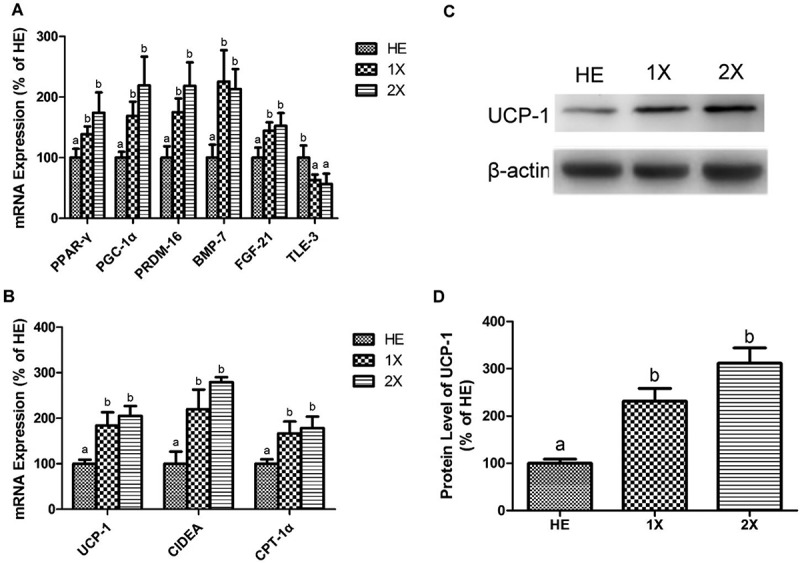
Expressions of genes related to beige transformation and thermogenesis in the EFPs. HE; HED vehicle control, 1X; HED with 77.5 mg/kg/day of GTE, 2X; HED with 155 mg/kg/day of GTE. Genes related to (a) beige transformation and (b) thermogenesis were detected by an RT-qPCR. Representative blots (c) and protein levels (d) of UCP-1 were detected using western blotting. All values are the mean ± SEM (n = 10 rats/group). Different superscript letters (a and b) indicate a significant difference at p < 0.05 by a one-way ANOVA with Tukey’s post-hoc test.
Discussion
The anti-obesity mechanisms of GTE became an important topic in nutritional and food science research because beneficial effects of GTE against obesity were demonstrated in human and animal models [7,8,31]. In the present study, we provide evidence that GTE significantly improved obesity phenomena, such as BW, fat weight, the adipocyte size, and plasma chemical parameters in rats with HED-induced obesity. The reduction of leptin and induction of adiponectin in the study also indicated that GTE modulated obesity in rats. Moreover, genes upregulated in beige adipose tissue were higher in the 1X and 2X groups compared to the HE group; and TLE-3, a beige adipose tissue transformation suppressor [26], was downregulated in the 1X and 2X groups. To our knowledge, this is the first paper revealing that GTE alone can induce the biomarkers of beige adipose tissue and the multiple pathways of browning that involve either PPAR-γ, PRDM-16, FGF-21, BMP-7, or TLE-2 associated with a prevention of HED-induced obesity in SD rats.
Beige adipose tissue is thought to be a potential target of anti-obesity therapy due to its capacity for oxidizing lipids and dissipating energy [20,32]. In addition, the browning of WAT was reported to be highly correlated with the anti-obesity effect without an increase in BAT function. For example, transgenic expression of PRDM-16 in WAT promoted the development of beige adipose tissue and resistance to obesity without enhancing the BAT mass [33]. Moreover, UCP-1 overexpression in adipose tissue suppressed obesity [13]. Since PRDM-16 and UCP-1 are highly expressed in beige adipose tissue, an increase of beige adipose tissue should resist weight gain [23]. Therefore, determining the level of browning of WAT could help assess the anti-obesity effect of GTE. Browning is regulated by several pathways. PPAR-γ, PRDM-16, and FGF-21, belonging to two different pathways, induced PGC-1α to enhance expressions of genes involved in thermogenesis and fatty acid oxidation, such as UCP-1 [34], CIDEA [35,36], and CPT-1 [37,38]. Moreover, BMP-7 was a member of EWS/YBX-1/BMP-7 pathway, which drives browning by upregulating UCP-1 and downregulating the WAT-specific marker, TCF21 [39]. In contrast, TLE-3, an inhibitor of PRDM-16, suppresses the browning of WAT [26]. In the present study, GTE significantly induced PPAR-γ, PRDM-16, PGC-1α, BMP-7, and FGF-21, and reduced TLE-3, which was associated with enhancement of the browning biomarkers, UCP-1, CIDEA, and CPT-1, in the 1X and 2X groups compared to the HE group. These results revealed that WAT seemed to transform to beige adipose tissue within eight weeks when SD rats were given GTE daily. Sae-tan et al. [40] reported that a combination of decaffeinated green tea and voluntary exercise induced genes related to browning in mice fed high fat diets, but the browning effect was not observed when the mice were given only green tea [40]. Since exercise had already been linked to browning [41], the role of green tea in regulation of browning was still a puzzle. Moreover, they did not provided any physiological or weight data following treatments. Herein, we provide the first evidence that GTE alone was sufficient to induce browning-related genes in WAT, which was accompanied by a significant anti-obesity effect in HED-fed rats.
Browning is defined as a process that the expression of UCP-1 increases in WAT [26]. Browning has been observed in EFP and used for investigating browning in many previous studies [21,34,40,42–44]. Therefore, the gene expressions of EFP are undoubtedly important to evaluate if GTE induces browning in this study. Our results indicate that GTE could be able to induce browning, because the GTE up-regulated the biomarkers of beige and the pathways of browning in EFP. Since EFP was suggested to be least able to induce cold-derived browning [15] and to predominantly contain white adipocytes [41], browning may also occur in subcutaneous, renal, and mesenteric WATs post the treatment of GTE. However, more experiments are needed to understand the effect of GTE on browning in other WATs.
PPAR-γ has tissue-specific effects [45]. In adipocytes, PPAR-γ is known for its anti-obesity capability and insulin sensitization. PPAR-γ enhances fatty acid uptake and adipogenesis in WAT to reverse insulin resistance [46], but upregulation of PPAR-γ does not necessarily cause an increase in BW [47,48]. Moreover, Tian et al. [49] demonstrated that green tea polyphenols induced the extracellular signal-regulated kinase (ERK)1/2-PPAR-γ-adiponectin pathway followed by reduction of fat deposits [49]. Therefore, it is not surprising that a PPAR-γ agonist was reported to have anti-diabetes and anti-obesity capabilities [50]. For the anti-obesity effect, PPAR-γ can improve HED-induced obesity through browning of WAT and also upregulating adiponectin. Adiponectin is known to increase fatty acid oxidation and insulin sensitivity and prevent obesity and inflammation [51–53]. In the present study, we showed that both PPAR-γ and adiponectin increased following GTE treatment of HED-fed rats. Although the concentration of circulating adiponectin was not determined, an increase of circulating adiponectin was probable following GTE treatment due to the higher mRNA levels of adiponectin in WAT of the GTE-treated groups than the HE group. Circulating adiponectin is mainly secreted by adipocyte, and positive correlation was shown between the mRNA level of adiponectin and the concentration of circulating adiponectin [49,54–57]. Moreover, the increase of PPAR-γ mRNA in WAT was indicated to be associated with an increase of circulating adiponectin in the previous studies [49,58]. Thus, the results suggest that GTE might induce anti-obesity, anti-inflammatory, and insulin sensitization effects of adiponectin via upregulation of PPAR-γ in adipocytes. For detailing the systemic effect of GTE via adiponectin, a further study, such as to measure the concentration of circulating adiponectin, should be performed in the future.
In the present study, the C group was designed for evaluating if obesity was successfully induced by HED in the rats. Moreover, the results of the groups fed with a HED (HE, 1X, and 2X groups) were sufficient to support that GTE could induce browning-related genes and limit weight gain in HED-fed rats. Therefore, the gene expressions in WAT were not analyzed. Although gene expressions of the C group were not determined, GTE seemed to prevent the down-regulation of browning-related genes in HED-fed rats because lower mRNA expressions of browning-related genes in WAT were reported in the previous studies [40,59,60]. However, further study is necessary to investigate effects of GTE in the rats fed with different diets.
The anti-obesity effects of GTE are usually evaluated by physical differences in the bodies between rats given and those not given GTE. The GTE used in the current study significantly reduced increases in BW, WAT, and adipocyte size. The results indicated that obesity was modulated when HED-fed rats were given GTE. Furthermore, GTE-administered rats had lower levels of TG, TC, LDL, and FFA, and a higher level of HDL in serum. Increasing TG, TC, LDL, and FFA and decreasing HDL are frequently associated with cardiovascular disease, which is one of the obesity-related diseases. Since high serum FFA levels were observed in obese and diabetic patients [61] and promote hepatic steatosis, the GTE may prevent hepatic steatosis by reducing FFA levels. Preventing hepatic steatosis was also supported by our observation that GTE-administered rats showed lower serum ALT and AST levels which were correlated with hepatic steatosis and injury in obese mice [62]. Therefore, GTE could improve HED-induced obesity and might prevent its derivative diseases.
The effects of functional foods are influenced by the dose administered [63–69]. Overdosing results in toxicity [63,64], and an inadequate dose leads to low efficiency [65–69]. A high dose of GTE (EGCG > 100 mg/kg/day) increases serum ALT and AST levels due to induction of liver toxicity [64]. Since lower levels of ALT and AST were shown in the 1X and 2X groups compared to the HE group, neither dose of GTE used in this study appeared to cause liver injury. To understand the dose effect, we treated HED-fed rats with two different doses of GTE. Previous studies reported that receiving GTE at 750 mg/day significantly reduced the BW of human subjects [30,70]. According to a formula from the US Food and Drug Administration, 77.5 mg/kg/day of GTE for an SD rat is equivalent to 750 mg/day for a 60-kg human. Therefore, 77.5 and 155 mg/kg/day of GTE were used in the current study. Our results showed that when HED-fed rats were given 155 mg/kg/day of GTE (2X group), their BW, fat weight, plasma chemical parameters, and adipocyte size were slightly better than those of rats given 77.5 mg/kg/day of GTE (1X group); however, no statistical difference in these obesity parameters was shown between the 1X and 2X groups. Similarly, expressions of beige-related genes also are higher in the 2X group but did not statistically differ between the 1X and 2X groups. Therefore, 77.5 and 155 mg/kg/day of GTE seemed to be efficient doses for an anti-obesity effect, but we cannot exclude that the anti-obesity effects may be enhanced by a higher dose of GTE.
In summary, our study presents that GTE alone is sufficient to promote the biomarkers of beige adipose tissue and multiple pathways of browning in WAT and to safely improve HED-induced obesity in SD rats.
Biography
Li-Han Chen conducted research and wrote the paper; Chung-Tiang Liang, Ching-Hung Chan, and Meng-Han Fan conducted research; Yi-Wen Chien wrote the paper. Hui-Yu Huang designed the research, supervised the project, and wrote the paper. All authors discussed the results and contributed to the manuscript.
Funding Statement
Funds were raised by the authors.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Kopelman PG. Obesity as a medical problem. Nature. 2000;404:635–10. [DOI] [PubMed] [Google Scholar]
- [2].Visscher TL, Seidell JC. The public health impact of obesity. Annu Rev Public Health. 2001;22:355–375. [DOI] [PubMed] [Google Scholar]
- [3].Bibbins-Domingo K, Chertow GM, Coxson PG, et al. Projected effect of dietary salt reductions on future cardiovascular disease. N Engl J Med. 2010;362:590–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Choi KM, Lee YS, Shin DM, et al. Green tomato extract attenuates high-fat-diet-induced obesity through activation of the ampk pathway in c57bl/6 mice. J Nutr Biochem. 2013;24:335–342. [DOI] [PubMed] [Google Scholar]
- [5].Bhaswant M, Poudyal H, Mathai ML, et al. Green and black cardamom in a diet-induced rat model of metabolic syndrome. Nutrients. 2015;7:7691–7707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Okuda MH, Zemdegs JC, de Santana AA, et al. Green tea extract improves high fat diet-induced hypothalamic inflammation, without affecting the serotoninergic system. J Nutr Biochem. 2014;25:1084–1089. [DOI] [PubMed] [Google Scholar]
- [7].Huang J, Wang Y, Xie Z, et al. The anti-obesity effects of green tea in human intervention and basic molecular studies. Eur J Clin Nutr. 2014;68:1075–1087. [DOI] [PubMed] [Google Scholar]
- [8].Rains TM, Agarwal S, Maki KC. Antiobesity effects of green tea catechins: a mechanistic review. J Nutr Biochem. 2011;22:1–7. [DOI] [PubMed] [Google Scholar]
- [9].Scherer PE. Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes. 2006;55:1537–1545. [DOI] [PubMed] [Google Scholar]
- [10].Shepherd PR, Gnudi L, Tozzo E, et al. Adipose cell hyperplasia and enhanced glucose disposal in transgenic mice overexpressing glut4 selectively in adipose tissue. J Biol Chem. 1993;268:22243–22246. [PubMed] [Google Scholar]
- [11].Haslam DW, James WP. Obesity. Lancet. 2005;366:1197–1209. [DOI] [PubMed] [Google Scholar]
- [12].Klaus S. Functional differentiation of white and brown adipocytes. Bioessays. 1997;19:215–223. [DOI] [PubMed] [Google Scholar]
- [13].Kopecky J, Clarke G, Enerback S, et al. Expression of the mitochondrial uncoupling protein gene from the ap2 gene promoter prevents genetic obesity. J Clin Invest. 1995;96:2914–2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Cypess AM, Kahn CR. Brown fat as a therapy for obesity and diabetes. Curr Opin Endocrinol Diabetes Obes. 2010;17:143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Nedergaard J, Cannon B. The browning of white adipose tissue: some burning issues. Cell Metab. 2014;20:396–407. [DOI] [PubMed] [Google Scholar]
- [16].Vitali A, Murano I, Zingaretti MC, et al. The adipose organ of obesity-prone c57bl/6j mice is composed of mixed white and brown adipocytes. J Lipid Res. 2012;53:619–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wu J, Bostrom P, Sparks LM, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Seale P, Bjork B, Yang W, et al. Prdm16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sharp LZ, Shinoda K, Ohno H, et al. Human bat possesses molecular signatures that resemble beige/brite cells. PLoS One. 2012;7:e49452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wu J, Cohen P, Spiegelman BM. Adaptive thermogenesis in adipocytes: is beige the new brown? Genes Dev. 2013;27:234–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Petrovic N, Walden TB, Shabalina IG, et al. Chronic peroxisome proliferator-activated receptor gamma (ppargamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, ucp1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem. 2010;285:7153–7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bostrom P, Wu J, Jedrychowski MP, et al. A pgc1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med. 2013;19:1252–1263. [DOI] [PubMed] [Google Scholar]
- [24].Fisher FM, Kleiner S, Douris N, et al. Fgf21 regulates pgc-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012;26:271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Seale P. Transcriptional regulatory circuits controlling brown fat development and activation. Diabetes. 2015;64:2369–2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Villanueva CJ, Vergnes L, Wang J, et al. Adipose subtype-selective recruitment of tle3 or prdm16 by ppar gamma specifies lipid storage versus thermogenic gene programs. Cell Metabo. 2013;17:423–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kajimura S, Seale P, Kubota K, et al. Initiation of myoblast to brown fat switch by a prdm16-c/ebp-beta transcriptional complex. Nature. 2009;460:1154–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Rosell M, Kaforou M, Frontini A, et al. Brown and white adipose tissues: intrinsic differences in gene expression and response to cold exposure in mice. Am J Physiol Endocrinol Metab. 2014;306:E945–E964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Orci L, Cook WS, Ravazzola M, et al. Rapid transformation of white adipocytes into fat-oxidizing machines. P Natl Acad Sci USA. 2004;101:2058–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Auvichayapat P, Prapochanung M, Tunkarnnerdthai O, et al. Effectiveness of green tea on weight reduction in obese thais: a randomized, controlled trial. Physiol Behav. 2008;93:486–491. [DOI] [PubMed] [Google Scholar]
- [31].Xu Y, Zhang M, Wu T, et al. The anti-obesity effect of green tea polysaccharides, polyphenols and caffeine in rats fed with a high-fat diet. Food Funct. 2015;6:297–304. [DOI] [PubMed] [Google Scholar]
- [32].Kajimura S, BM Spiegelman, Seale P. Brown and beige fat: physiological roles beyond heat generation. Cell Metabo. 2015;22:546–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Seale P, Conroe HM, Estall J, et al. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest. 2011;121:96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Cao L, Choi EY, Liu XL, et al. White to brown fat phenotypic switch induced by genetic and environmental activation of a hypothalamic-adipocyte axis. Cell Metabo. 2011;14:324–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Servera M, López N, Serra F, et al. Expression of “brown-in-white” adipocyte biomarkers shows gender differences and the influence of early dietary exposure. Genes Nutr. 2014;9:372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kleiner S, Mepani RJ, Laznik D, et al. Development of insulin resistance in mice lacking pgc-1alpha in adipose tissues. Proc Natl Acad Sci USA. 2012;109:9635–9640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wu J, Jun HJ, McDermott JR. Formation and activation of thermogenic fat. Trends Genet. 2015;31:232–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Vega RB, Huss JM, Kelly DP. The coactivator pgc-1 cooperates with peroxisome proliferator-activated receptor alpha in transcriptional control of nuclear genes encoding mitochondrial fatty acid oxidation enzymes. Mol Cell Biol. 2000;20:1868–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Elsen M, Raschke S, Tennagels N, et al. Bmp4 and bmp7 induce the white-to-brown transition of primary human adipose stem cells. Am J Physiol Cell Physiol. 2014;306:C431–440. [DOI] [PubMed] [Google Scholar]
- [40].Sae-Tan S, Rogers CJ, Lambert JD. Decaffeinated green tea and voluntary exercise induce gene changes related to beige adipocyte formation in high fat-fed obese mice. J Funct Foods. 2015;14:210–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Bartelt A, Heeren J. Adipose tissue browning and metabolic health. Nat Rev Endocrinol. 2014;10:24–36. [DOI] [PubMed] [Google Scholar]
- [42].Shahid M, Javed AA, Chandra D, et al. Iex-1 deficiency induces browning of white adipose tissue and resists diet-induced obesity. Sci Rep. 2016;6:24135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].JH Shin, SH Lee, YN Kim, et al. Ahnak deficiency promotes browning and lipolysis in mice via increased responsiveness to beta-adrenergic signalling. Sci Rep. 2016;6:23426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].BM Owen, Ding X, DA Morgan, et al. Fgf21 acts centrally to induce sympathetic nerve activity, energy expenditure, and weight loss. Cell Metab. 2014;20:670–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Ahmadian M, Suh JM, Hah N, et al. Ppar gamma signaling and metabolism: the good, the bad and the future. Nature Medicine. 2013;19:557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Ahmadian M, Duncan RE, Sul HS. The skinny on fat: lipolysis and fatty acid utilization in adipocytes. Trends Endocrin Met. 2009;20:424–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Sugii S, Olson P, Sears DD, et al. Ppar gamma activation in adipocytes is sufficient for systemic insulin sensitization. P Natl Acad Sci USA. 2009;106:22504–22509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Kersten S. Peroxisome proliferator activated receptors and obesity. Eur J Pharmacol. 2002;440:223–234. [DOI] [PubMed] [Google Scholar]
- [49].Tian C, Ye XL, Zhang R, et al. Green tea polyphenols reduced fat deposits in high fat-fed rats via erk1/2-ppar gamma-adiponectin pathway. PLoS One. 2013;8:53796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Bhattarai BR, Kafle B, Hwang JS, et al. Novel thiazolidinedione derivatives with anti-obesity effects: dual action as ptp1b inhibitors and ppar-gamma activators. Bioorg Med Chem Lett. 2010;20:6758–6763. [DOI] [PubMed] [Google Scholar]
- [51].Yamauchi T, Kamon J, Minokoshi Y, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating amp-activated protein kinase. Nat Med. 2002;8:1288–1295. [DOI] [PubMed] [Google Scholar]
- [52].Fruebis J, Tsao T-S, Javorschi S, et al. Proteolytic cleavage product of 30-kda adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. P Natl Acad Sci USA. 2001;98:2005–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Bell-Anderson KS, Aouad L, Williams H, et al. Coordinated improvement in glucose tolerance, liver steatosis and obesity-associated inflammation by cannabinoid 1 receptor antagonism in fat aussie mice. Int J Obesity. 2011;35:1539–1548. [DOI] [PubMed] [Google Scholar]
- [54].Iwaki M, Matsuda M, Maeda N, et al. Induction of adiponectin, a fat-derived antidiabetic and antiatherogenic factor, by nuclear receptors. Diabetes. 2003;52:1655–1663. [DOI] [PubMed] [Google Scholar]
- [55].Yamauchi T, Kamon J, Waki H, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–946. [DOI] [PubMed] [Google Scholar]
- [56].Barnea M, Shamay A, Stark AH, et al. A high-fat diet has a tissue-specific effect on adiponectin. And related enzyme expression. Obesity. 2006;14:2145–2153. [DOI] [PubMed] [Google Scholar]
- [57].Arita Y, Kihara S, Ouchi N, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Bioph Res Co. 1999;257:79–83. [DOI] [PubMed] [Google Scholar]
- [58].Soares FLP, Matoso RD, Teixeira LG, et al. Gluten-free diet reduces adiposity, inflammation and insulin resistance associated with the induction of ppar-alpha and ppar-gamma expression. J Am Coll Nutr. 2013;24:1105–1111. [DOI] [PubMed] [Google Scholar]
- [59].Fromme T, Klingenspor M. Uncoupling protein 1 expression and high-fat diets. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1–8. [DOI] [PubMed] [Google Scholar]
- [60].Rocha-Rodrigues S, Rodriguez A, Gouveia AM, et al. Effects of physical exercise on myokines expression and brown adipose-like phenotype modulation in rats fed a high-fat diet. Life Sci. 2016;165:100–108. [DOI] [PubMed] [Google Scholar]
- [61].Shepherd PR, Kahn BB. Glucose transporters and insulin action–implications for insulin resistance and diabetes mellitus. N Engl J Med. 1999;341:248–257. [DOI] [PubMed] [Google Scholar]
- [62].Fiorini RN, Donovan JL, Rodwell D, et al. Short-term administration of (-)-epigallocatechin gallate reduces hepatic steatosis and protects against warm hepatic ischemia/reperfusion injury in steatotic mice. Liver Transpl. 2005;11:298–308. [DOI] [PubMed] [Google Scholar]
- [63].Hasler CM. Functional foods: their role in disease prevention and health promotion. Food Technol-Chicago. 1998;52:63–70. [Google Scholar]
- [64].Galati G, Lin A, Sultan AM, et al. Cellular and in vivo hepatotoxicity caused by green tea phenolic acids and catechins. Free Radical Bio Med. 2006;40:570–580. [DOI] [PubMed] [Google Scholar]
- [65].Cabrera C, Artacho R, Gimenez R. Beneficial effects of green tea–a review. J Am Coll Nutr. 2006;25:79–99. [DOI] [PubMed] [Google Scholar]
- [66].Lattanzio V, Kroon PA, Linsalata V, et al. Globe artichoke: a functional food and source of nutraceutical ingredients. J Funct Foods. 2009;1:131–144. [Google Scholar]
- [67].Alissa EM, Ferns GA. Functional foods and nutraceuticals in the primary prevention of cardiovascular diseases. J Nutr Metab. 2012;2012:569486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Chadwick R, Henson S, Moseley B, et al. Functional foods. Vol. 20 New York (NY): Springer Science & Business Media; 2003. [Google Scholar]
- [69].Wasser SP, Akavia E. Regulatory issues of mushrooms as functional foods and dietary supplements: safety and efficacy In: Cheung PCK, editor. Mushrooms as functional foods. New York (NY): Wiley; 2008. p. 199–221. [Google Scholar]
- [70].Thavanesan N. The putative effects of green tea on body fat: an evaluation of the evidence and a review of the potential mechanisms. Brit J Nutr. 2011;106:1297–1309. [DOI] [PubMed] [Google Scholar]


