Abstract
Background
Understanding the flow of patients through the continuum of HIV care is critical to determine how best to intervene so that the proportion of HIV-infected persons who are on antiretroviral treatment and virally suppressed is as large as possible.
Methods
Using immunological and virological data from the Centers for Disease Control and Prevention and the North American AIDS Cohort Collaboration on Research and Design from 2009–2012, we estimated the distribution of time spent in and dropout probability from each stage in the continuum of HIV care. We used these estimates to develop a queueing model for the expected number of patients found in each stage of the cascade.
Results
HIV-infected individuals spend an average of about 3.1 months following HIV diagnosis before being linked to care, or dropping out of that stage of the continuum with a probability of 8%. Those who link to care wait an additional 3.7 months on average before getting their second set of laboratory results (indicating engagement in care) or dropping out of care with probability of almost 6%. Those engaged in care spent an average of almost one year before achieving viral suppression on antiretroviral therapy or dropping out with average probability 13%. For patients who achieved viral suppression, the average time suppressed on ART was an average of 4.5 years.
Conclusions
Interventions should be targeted to more rapidly identifying newly infected individuals, and increasing the fraction of those engaged in care that achieves viral suppression.
Keywords: HIV continuum of care, queueing model, operations research, antiretroviral therapy, viral suppression
Introduction
Of the roughly 1.2 million people living with HIV in the United States (US), it is estimated that only 325,000 have an undetectable viral load as a result of successful antiretroviral therapy (ART).1 Achieving viral suppression requires several steps: 1) diagnosis; 2) linkage to care; 3) engagement in care; 4) viral suppression on ART. Failure at any one of these stages represents overall failure, that is, a failure to achieve viral suppression. This sequence of steps is commonly referred to as the HIV care continuum (HIV CC). The HIV CC has become a widely used framework to describe HIV treatment success at national, state and local levels.2
In the US, the Centers for Disease Control and Prevention (CDC) estimates the HIV CC using a prevalence approach, in which the percentages of individuals in each stage of care are calculated using the consistent denominator of the total estimated number of people infected with HIV.1 Other approaches to estimating the HIV CC include using data submitted to the National HIV Surveillance System (NHSS) to estimate those tested for HIV, and reported CD4+ T-lymphocyte (CD4) and/or viral load (VL) tests as proxy measures to estimate linkage to care and engagement in care, and reported VL tests <200 copies/mL to estimate successful viral suppression. To estimate those linked to care, CDC calculated the number of persons with HIV diagnosed within a given year who have at least one CD4 or VL test within 3 months after their HIV diagnosis.3 Engagement in care has been defined by two or more CD4 or VL tests at least three months apart during a calendar year.3 Finally, in the context of clinical care data, the continuum is typically represented in bar graphs that present the percentage of HIV+ individuals in or beyond each stage of care, conditional on the previous stage, e.g., among those who know their serostatus who are in care.4
There are several limitations to these approaches used to estimate the HIV CC. These include the incompleteness of data collected by state and local health departments and compiled by the CDC, the limited sensitivity and specificity of laboratory tests as a proxy for linkage and engagement in care, and the movement of HIV+ individuals between jurisdictions.5,6 Another deficiency is the cross-sectional representation of the HIV CC, for this static picture is only a snapshot of the distribution of patients across the continuum.7 Finally, and perhaps most importantly, these approaches do not capture how people living with HIV move through the HIV CC. The path from an HIV diagnosis to successful viral suppression can be thought of as a series of waits in a series of lines. We do not simply want to know how many individuals are in each stage of the treatment cascade; we also want to know long people spend in and how many people are lost after each stage. Given that the overwhelming majority of new HIV infections are transmitted by individuals not in care, understanding the flow of patients through the continuum, and bottlenecks along their path, is critical for developing intervention strategies that will most effectively increase the number of HIV-infected persons who are in care and virally suppressed.8
In this paper, we describe the development of a queueing model of the care continuum. The model parameters are estimated using continuum data from CDC, additional data from the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) and the existing literature. While more complex versions of the HIV CC exist where patients can cyclically engage, disengage and reengage in HIV care, processes alternately called “churn” or accessing the continuum through a “side door”, our model captures the essential features of patients’ passage through the HIV CC by estimating patients’ delay in transit from one care stage to the next along with the probability of dropping out after each stage of care. 9,10 Estimating the parameters of the HIV CC in this serial manner (without “churn”) means that our model will underestimate the true fraction of infected persons who eventually achieve viral suppression.
Methods
Queueing Model
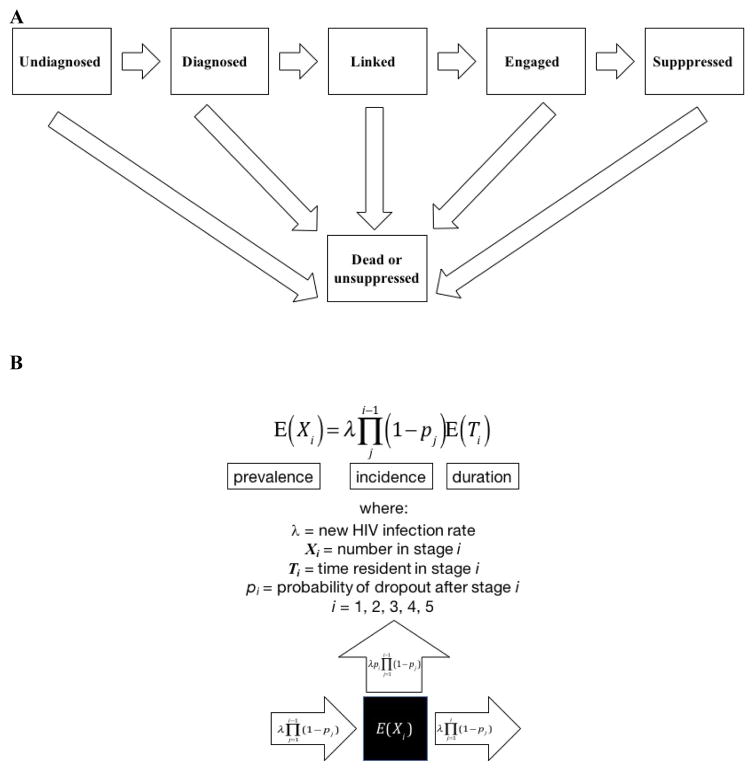
The basic features of our queueing model are based on Little’s Law, which states that the average number of individuals in a system is the product of the arrival rate and the average waiting time in the system, or in epidemiological terms, prevalence equals incidence times duration.11,12 The individuals in our model are HIV-infected individuals, and waiting times correspond to the time spent in different stages of care. Our model consists of adjacent queues linked in series to denote transit from the infected but undiagnosed state to diagnosis, linkage to care, engagement in care, and successful viral suppression (Figure 1A). The initial input into the HIV+ but undiagnosed state is represented by λ, the aggregate rate of new HIV infections. The expected number of individuals residing in the ith stage (E(Xi), Figure 1B) is the new infection rate (λ) times the product of the progression rates out of all preceding states in the continuum ( where pj is the probability of dropping out following stage j) multiplied by the expected time resident in stage i (E(Ti)). In other words, presence in one stage of the HIV CC is conditional upon progressing through the previous stages.
Figure 1.
Figure 1A is a schematic representation of the HIV continuum of care as a queueing process from infection through to viral suppression. Those leaving the queue in this model are either virally unsuppressed or dead. Figure 1B provides the equations associated with each stage in the continuum, which include input into and progression and dropout from each stage.
Schematic representation of the queuing model and associated equations.
Data
To estimate parameters in the model, we used data from the CDC and 17 longitudinal cohorts of patients with HIV infection associated with NA-ACCORD. The data from CDC included persons aged ≥ 13 years with HIV infection diagnosed in 2009. Individuals were followed for their CD4 or VL tests from the diagnosis date until December 31, 2012, which was the administrative censoring date. The CDC data were not a comprehensive national sample but represent a census of individuals with HIV from California (Los Angeles County and San Francisco only), the District of Columbia, Hawaii, Illinois, Indiana, Iowa, Louisiana, Michigan, Missouri, New Hampshire, New York, North Dakota, South Carolina, West Virginia and Wyoming. The CDC supplied data on the time from diagnosis to first CD4 or VL test; time from first CD4 or VL test to the second CD4 or VL test; and time from the second CD4 or VL test until a VL test below 200 copies/mL. These data were used as proxies for time to linkage to care, engagement in care, and initiation of ART, respectively, following the CDC guidance on using surveillance data to monitor national care and prevention objectives.
To estimate the mean time individuals are virally suppressed in the last stage of the model, we used data from NA-ACCORD to determine time from first undetectable VL test to virologic failure (i.e., the first detectable VL test on therapy). This basic model does not account for those individuals who may initially fail first-line ART, but are suppressed on a second-line regimen. Those who initially fail first-line ART and have experienced a first detectable viral load, were counted as failing viral suppression - and therefore, regardless of rapidity of initiating second-line ART, these individuals were considered unsuppressed. To model the time virally suppressed, we obtained data on 8845 individuals, aged > 18 years, pooled from the longitudinal studies that are part of NA-ACCORD. These data provide a count of the NA-ACCORD subjects who initiated ART any time between January 1, 2009 and December 31, 2011 and sums all those who were virally suppressed in any given month until the end of 2011.
Finally, to estimate the probability that an HIV-infected individual drops out of the continuum before diagnosis, and the mean time from infection until either HIV diagnosis or dropout (whichever comes first), we used figures from the literature. Karon et al reported that the proportion of previously untested HIV-infected persons with a concurrent first HIV test and AIDS diagnosis was 0.23.13 We employed this proportion as our estimate of the probability that a newly infected individual will drop out of the continuum before an HIV diagnosis (in the absence of AIDS). The analysis in Karon et al also implies that newly infected persons remain undiagnosed for 2.8 years on average before either developing AIDS or testing positive for HIV before developing AIDS. Comparable estimates have been obtained in more recent studies.13,14 We therefore estimate the mean residency time from infection until either HIV diagnosis or dropout as 2.8 years.
Survival Models and Likelihood Functions
We used two survival models to estimate likelihood functions for the time individuals spent in each stage and whether or not individuals progressed to the next stage in the HIV CC, with censoring due either to dropout or failure to progress within the observation periods of the data. The first survival model was based on exponential distributions, thus the hazard rates for progression into the next stage or dropout were assumed to be constant over time. This leads to a simple competing risks model with constant stage-specific probabilities of dropping out of the HIV CC. The second model was a proportional hazards model based on Weibull distributions, thus the hazard rates for progression to the next stage or dropping out were power functions assumed proportional to each other. This also resulted in constant stage-specific probabilities of dropout. For each stage, the parameters of these models were estimated via maximum likelihood (Appendix). Standard deviations for each parameter were calculated using the Delta method.15 We compared the likelihood ratios for the exponential and Weibull models for each stage.
Results
We report the results from the Weibull model, which provided a significantly better fit to the data than the simpler exponential model (results for the exponential model are included in the Appendix in Table S1). The expected time from diagnosis to the first CD4/VL test or dropout (whichever occurs first) in the Weibull model, indicating how long an individual was diagnosed but not yet linked to care, was 3.1 months (95% CI: 3.0–3.2). The time to the second CD4/VL test (a proxy for being engaged in care) or dropout was 3.6 months (95% CI: 3.6–3.7). In the subsequent stage, the expected time from the second CD4/VL test to the first suppressed VL test (indicating someone was still engaged in care, and either not on ART or not yet suppressed) or dropout was 14.6 months (95% CI: 13.3–15.9) in the Weibull model. The duration of viral suppression, as measured by the time from the first undetectable to the first detectable VL test was 36.6 months (95% CI: 35.6–37.6). The estimated probability that an individual with diagnosed HIV drops out of the HIV CC before being linked to care was 0.079 (95% CI: 0.074–0.083) for the Weibull. Of those linked to care, the estimated probability of dropping out before being engaged in care 0.056 (95% CI: 0.052–0.060). Of those engaged in care, the estimated probability an individual drops out before achieving an initial viral suppression was 0.094 (95% CI: 0.078–0.11) for the Weibull. In all cases, the Weibull model provided a significantly better fit to the data than the simpler exponential model (see Table 1; all likelihood ratio chi-square tests are significant at p < 0.0001).
Table 1.
Estimated Expected Stage Occupancy Times and Dropout Fractions in the HIV Continuum of Care
Table 1 provides the expected stage occupancy times and dropout fractions in the HIV continuum of care with associated 95% confidence intervals and log likelihoods for the Weibull model, respectively, for each stage. In all cases, the Weibull model offers a better fit to the data (compared to the exponential) and all likelihood ratio chi-square tests are significant at p < 0.0001.
| Stage | Weibull |
|---|---|
| Diagnosed (before 1st CD4/VL test) | |
| Mean Time in Stage in months | 3.1 |
| 95% Confidence Interval | 2.98–3.24 |
| Dropout Fraction | 0.079 |
| 95% Confidence Interval | 0.074–0.083 |
| Log Likelihood | −27267 |
| Chi-square (versus exponential) | 7620 |
|
| |
| Linked to Care (before 2nd CD4/VL test) | |
| Mean Time in Stage in months | 3.6 |
| 95% Confidence Interval | 3.55–3.73 |
| Dropout Fraction | 0.056 |
| 95% Confidence Interval | 0.052–0.060 |
| Log Likelihood | −28857 |
| Chi-square (versus exponential) | 1556 |
|
| |
| Engaged in Care (before undetectable VL test) | |
| Mean Time in Stage in months | 14.6 |
| 95% Confidence Interval | 13.32–15.86 |
| Dropout Fraction | 0.094 |
| 95% Confidence Interval | 0.078–0.11 |
| Log Likelihood | −33078 |
| Chi-square (versus exponential) | 1340 |
|
| |
| Viral Load Suppressed to Unsuppressed (length of viral suppression) | |
| Mean Time in Stage in months | 36.6 |
| 95% Confidence Interval | 35.58–37.57 |
| Log Likelihood | −10733 |
| Chi-square (versus exponential) | 1228 |
Discussion
To date, scholarship on the continuum of care has focused on reporting proportions of persons who drop out at each stage of the pathway that leads from case detection to successful viral suppression. The queueing model presented in this paper aims to enrich our understanding of patient experience by estimating how much time individuals – both those who drop out and those who progress -- can expect to spend in a given stage of the pathway.
The traditional cross-sectional description of the continuum of care suggests at which stage there are barriers in providing care and treatment for HIV-infected individuals. The queueing model estimated herein provides more insights in terms of the difficulties of being in a given stage as regards the probability of achieving viral suppression. In fact, this model offers a way to identify the weakest link in the continuum of care and where an emphasis should be placed in developing interventions to improve treatment and prevention outcomes. The basic formula for the expected number of individuals in a given stage in the continuum shown in Figure 1B contains the term (1 − pj), which is the complement of the probability of dropout after stage j; in other words (1 − pj) is the probability of progression to the next stage. In the most basic terms, it is where this probability is the smallest that efforts would be best directed, where flow through the continuum is most constricted. In this instance, using the data from CDC and NA-ACCORD, the model suggests that the drop-out while being in care is the highest, with 9% (Weibull) leaving the continuum at this point, but the time in this stage is also the most protracted, with it taking 14.6 months (Weibull) to transit out and achieve a suppressed viral load.
This model implies that speeding progression through (i.e., shortening stage occupancy times), and reducing the probability of dropout from each stage are complementary strategies to improve treatment and prevention outcomes in HIV. Speeding progress through the continuum and reducing the probability of dropout are two different, though related, operational tasks. The first task requires efforts to accelerate progression toward the suppressed stage (i.e., encouraging left-to-right flow along the continuum as represented in figure 1A). Examples might include novel efforts to identify infected but undiagnosed individuals and get them into care or initiating ART immediately upon diagnosis. The second task involves efforts to reduce loss to follow up. Relevant interventions might include the use of patient navigators, peer counseling, directed youth case management, buprenorphine or methadone treatment for opioid-dependent patients, supporting emergency medication coverage, treatment education, transportation, housing assistance, and support groups for patients.16–18 To develop an intervention most relevant to the results presented here, one would need to know more about what is delaying diagnosis and viral suppression and what leads patients to drop out of care in these final stages of the continuum.
However, maximizing the probability of progression to the next stage in real-world settings may require larger expenditures. To understand how to allocate resources most efficiently to best improve patient outcomes would require understanding the costs of increasing the probability of progression and equivalently reducing the dropout probability as well as the effectiveness of interventions designed for these purposes for each stage. While few interventions have been described in terms of their per-patient costs and effectiveness, there are a limited number of health economic evaluations of interventions particularly in the later stage of the continuum of care.19 New studies have examined the economic impact of a larger set of interventions, but on their impact on infections averted, not on the stage-specific transitions or dropout probabilities along the continuum.20 Thus, it is difficult to provide a quantitative analysis of resources needed to improve outcomes and further research is needed to provide the necessary estimates for the parameters.
Our model has other clinical implications. For instance, while the dropout rate and transition time from a second CD4 or VL test to a suppressed one are of the greatest magnitude when compared to the other stages, the average time from diagnosis to an initial assessment of a patient’s immunological and virological status by a CD4 or VL test is approximately 3 months in US. Other research has demonstrated that about 20–25% of new diagnoses in the US are infections at advanced disease with less than 200 CD4+ T-lymphocytes/mm3 or an opportunistic infection.14 In this setting, even relatively short delays in linkage to care have heightened health risks for patients who are already severely immunocompromised, a disproportionate impact on new infections, and are associated with increased mortality for patients.8, 21, 22 Finally, in the Weibull model, the combined time during which patients know their HIV serostatus but are not virally suppressed (i.e., from diagnosis to undetectable viral load test) is close to two years (21.3 months) and the combined probability of dropout is 22.9%. This indicates that many HIV+ patients may be in contact with the health care system, but “stuck” in it, with delays that present considerable risks to them and their sexual partners and with almost a quarter of them dropping out of the system while ostentibly in care.
Our model is the first representation of the treatment continuum using a queueing model, but it is a basic one. One weakness of our model is that it does not account for people exiting the continuum during the period of observation and returning to it in a subsequent time period, which has been called a side door into care.23 To build a more complex model, longitudinal data on CD4 and VL tests for individual patients are needed and may soon be available from the sources we have used (i.e., as HIV surveillance data including CD4 and VL test results become available for more years). One of the goals of this research is to provide a tool to guide decision making with the data that are easily available to local officials. Even if our model fails to capture the complicated trajectories of patients entering, exiting and entering the continuum of care on numerous occasions, the results we show here can be thought of as a lower bound estimate for the expected number of patients who achieve viral suppression. That is, since we are making the assumption that patients, once they leave the continuum, are not returning to it, our results represent a worst-case scenario.
Our model also does not capture important heterogeneity in patient behavior or demographic characteristics. For example, it does not reflect what would happen if there were two classes of individuals with separate hazards, such as one high-risk group that became unsuppressed early on and another low-risk group that had consistent viral suppression over the long-term nor does it depict the trajectories for specific populations, such as young men-who-have-sex-with-men, people of color, or people who use drugs, which would provide a more specific picture of the bottlenecks in the continuum. However, our goal in this paper was to model mean passage times through the continuum and including analyses of mixture distributions for waiting times is beyond the scope of this paper.
Our model also assumes that incidence is relatively stable.1 However, incidence is not stable among certain populations in the US, and is increasing in young men-who-have-sex-with men (MSM) and geographical areas such as in the American South, although more recent research has suggested decreases in new HIV diagnoses overall.24–27 In these situations, a more complicated model can be developed in which the rate of new infections is a function of time.
Understanding the HIV care continuum is a vital part of ensuring optimal care for persons living with HIV and reducing HIV transmission from those who are not virally suppressed.8 The three threats to successful suppression on ART, “late diagnosis, sluggish linkage to care, and fleeting retention” are all temporal phenomena that are compromising patient health and efforts to control the HIV epidemic in the US, where up to 70% of people living with HIV do not yet have their virus under control .1, 28 Our model deepens our knowledge of how the continuum operates across time, but there is more work to do to understand how to best intervene to improve patient outcomes, particularly among different demographic groups, which may have different trajectories through the continuum. While the diagnosis of bottlenecks (i.e., long stage occupancy times before viral suppression) in the HIV continuum has been a focus of research including ours, there is a need for economic analyses to clarify the costs and effectiveness of interventions targeting the HIV CC to efficiently allocate resources. However, the insights offered by our model have policy implications that can be helpful to clinicians and program managers now by describing where patients get stuck in the systems of care and where they are most likely to leave the system altogether.
Supplementary Material
Acknowledgments
Research funding: Gregg S. Gonsalves was supported by a Public Health Services and Systems Research Award for Predoctoral and Postdoctoral Scholars in Public Health Delivery, from the University of Kentucky Research Foundation. A. David Paltiel is supported by NIMH R01 MH105203 and NIDA R01DA015612.
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the U.S. Centers for Disease Control and Prevention.
Meetings at which parts of this research were presented: Keeneland Conference 2015: Using Public Health Research to Build an Effective, Efficient, and Equitable System, 20–22 April 2015, Lexington, KY.
Contributor Information
Gregg S. Gonsalves, Department of Epidemiology of Microbial Diseases, Yale School of Public Health, New Haven, CT, USA.
A. David Paltiel, Department of Health Policy and Management, Yale School of Public Health, New Haven, CT, USA.
Paul D. Cleary, Department of Health Policy and Management, Yale School of Public Health, New Haven, CT, USA.
Michael John Gill, Department of Medicine, University of Calgary, Alberta, Canada.
Mari M. Kitahata, Professor of Medicine, Center for AIDS Research, University of Washington, Seattle, WA, USA.
Peter F. Rebeiro, Department of Medicine, Vanderbilt University School of Medicine, Nashville, TN, USA.
Michael J. Silverberg, Kaiser Permanente Northern California, Oakland, CA, USA.
Michael Horberg, Kaiser Permanente Mid-Atlantic Permanente Research Institute, Rockville, Maryland, USA.
Alison G Abraham, Department of Ophthalmology, Johns Hopkins School of Medicine, Baltimore, MD, USA.
Keri N. Althoff, Department of Epidemiology, Johns Hopkins University, Baltimore, MD, USA.
Richard Moore, Johns Hopkins University, Baltimore, MD, USA.
Ronald J. Bosch, Center for Biostatistics in AIDS Research, Harvard School of Public Health, Boston, MA, USA.
H. Irene Hall, Division of HIV/AIDS Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention, Atlanta, GA, USA.
Edward H. Kaplan, Yale School of Management, New Haven, CT, USA.
References
- 1.Bradley H, Hall HI, Wolitski RJ, et al. Vital Signs: HIV diagnosis, care, and treatment among persons living with HIV--United States, 2011. MMWR Morb Mortal Wkly Rep. 2014;63(47):1113–1117. [PMC free article] [PubMed] [Google Scholar]
- 2.Mugavero MJ, Amico KR, Horn T, Thompson MA. The state of engagement in HIV care in the United States: from cascade to continuum to control. Clin Infect Dis. 2013;57(8):1164–1171. doi: 10.1093/cid/cit420. [DOI] [PubMed] [Google Scholar]
- 3.Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 US dependent areas—2010. HIV Surveill Suppl Rep. 2012;17(3) [Google Scholar]
- 4.Horberg MA, Hurley LB, Klein DB, et al. The HIV care cascade measured over time and by age, sex, and race in a large national integrated care system. AIDS Patient Care STDs. 2015;29(11):582–590. doi: 10.1089/apc.2015.0139. [DOI] [PubMed] [Google Scholar]
- 5.Lesko CR, Sampson LA, Miller WC, et al. Measuring the HIV care continuum using public health surveillance data in the United States. [Review] J Acquir Immune Defic Syndr. doi: 10.1097/QAI.0000000000000788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rebeiro PF, Althoff KN, Lau B, et al. Laboratory measures as proxies for primary care encounters: implications for quantifying clinical retention among HIV-infected adults in North America. Am J Epidemiol. 2015;182(11):952–960. doi: 10.1093/aje/kwv181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gardner EM, McLees MP, Steiner JF, del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52(6):793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Skarbinski J, Rosenberg E, Paz-Bailey G, et al. Human immunodeficiency virus transmission at each step of the care continuum in the United States. JAMA Intern Med. 2015;175(4):588–596. doi: 10.1001/jamainternmed.2014.8180. [DOI] [PubMed] [Google Scholar]
- 9.Gill MJ, Krentz HB. Unappreciated epidemiology: the churn effect in a regional HIV care programme. Int J STD AIDS. 2009;20(8):540–544. doi: 10.1258/ijsa.2008.008422. [DOI] [PubMed] [Google Scholar]
- 10.Miller WC, Lesko CR, Powers KA. The HIV care cascade: simple concept, complex realization. Sex Transm Dis. 2014;41(1):41–42. doi: 10.1097/OLQ.0000000000000081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Little JD. A proof for the queuing formula: L= λ W. [Accessed September 19, 2015];Oper Res. 1961 9(3):383–387. http://pubsonline.informs.org/doi/abs/10.1287/opre.9.3.383. [Google Scholar]
- 12.Freeman J, Hutchison GB. Prevalence, incidence and duration. [Accessed April 4, 2016];Am J Epidemiol. 1980 112(5):707–723. doi: 10.1093/oxfordjournals.aje.a113043. http://aje.oxfordjournals.org/content/112/5/707. [DOI] [PubMed] [Google Scholar]
- 13.Karon JM, Song R, Brookmeyer R, Kaplan EH, Hall HI. Estimating HIV incidence in the United States from HIV/AIDS surveillance data and biomarker HIV test results. Stat Med. 2008;27(23):4617–4633. doi: 10.1002/sim.3144. [DOI] [PubMed] [Google Scholar]
- 14.Hall HI, Song R, Szwarcwald CL, Green T. Brief Report: Time from infection with the human immunodeficiency virus to diagnosis, United States. JAIDS J Acquir Immune Defic Syndr. 2015;69(2):248–251. doi: 10.1097/QAI.0000000000000589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cox C. Encyclopedia of Biostatistics. John Wiley & Sons, Ltd; 2005. [Accessed November 4, 2015]. Delta method. http://onlinelibrary.wiley.com/doi/10.1002/0470011815.b2a15029/abstract. [Google Scholar]
- 16.Shah M, Risher K, Berry SA, Dowdy DW. The epidemiologic and economic impact of improving HIV testing, linkage, and retention in care in the United States. Clin Infect Dis. 2016;62(2):220–229. doi: 10.1093/cid/civ801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burns DN, DeGruttola V, Pilcher CD, et al. Toward an endgame: finding and engaging people unaware of their HIV-1 infection in treatment and prevention. [Accessed February 9, 2015];AIDS Res Hum Retroviruses. 2014 30(3):217–224. doi: 10.1089/aid.2013.0274. http://online.liebertpub.com/doi/abs/10.1089/AID.2013.0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pilcher C, Hatano H, Dasgupta A, et al. Providing same day, observed ART to newly diagnosed HIV+ outpatients is associated with improved virologic suppression. 2015 Jul; pag.ias2015.org.
- 19.Nosyk B, Krebs E, Eyawo O, Min JE, Barrios R, Montaner JSG. Cost-effectiveness analysis along the continuum of HIV care: how can we optimize the effect of HIV treatment as prevention programs? Curr HIV/AIDS Rep. 2014;11(4):468–478. doi: 10.1007/s11904-014-0227-7. [DOI] [PubMed] [Google Scholar]
- 20.Jain KM, Maulsby C, Brantley M, et al. Cost and cost threshold analyses for 12 innovative US HIV linkage and retention in care programs. AIDS Care. 2016;0(0):1–6. doi: 10.1080/09540121.2016.1164294. [DOI] [PubMed] [Google Scholar]
- 21.Crowe SM, Carlin JB, Stewart KI, Lucas CR, Hoy JF. Predictive value of CD4 lymphocyte numbers for the development of opportunistic infections and malignancies in HIV-infected persons. [Accessed May 3, 2016];JAIDS J Acquir Immune Defic Syndr. 1991 4(8):770–776. http://journals.lww.com/jaids/Abstract/1991/08000/Predictive_Value_of_CD4_Lymphocyte_Numbers_for_the.5.aspx. [PubMed] [Google Scholar]
- 22.Hanna DB, Pfeiffer MR, Torian LV, Sackoff JE. Concurrent HIV/AIDS diagnosis increases the risk of short-term HIV-related death among persons newly diagnosed with AIDS, 2002–2005. [Accessed February 21, 2016];AIDS Patient Care STDs. 2008 22(1):17–28. doi: 10.1089/apc.2007.0042. http://online.liebertpub.com/doi/abs/10.1089/apc.2007.0042. [DOI] [PubMed] [Google Scholar]
- 23.Hallett TB, Eaton JW. A side door into care cascade for HIV-infected patients? [Accessed September 19, 2015];JAIDS J Acquir Immune Defic Syndr. 2013 63:S228–S232. doi: 10.1097/QAI.0b013e318298721b. http://journals.lww.com/jaids/Fulltext/2013/07012/A_Side_Door_Into_Care_Cascade_for_HIV_Infected.20.aspx. [DOI] [PubMed] [Google Scholar]
- 24.Beyrer C, Sullivan P, Sanchez J, et al. The increase in global HIV epidemics in MSM. AIDS. 2013;27(17):2665–2678. doi: 10.1097/01.aids.0000432449.30239.fe. [DOI] [PubMed] [Google Scholar]
- 25.Reif S, Geonnotti KL, Whetten K. HIV Infection and AIDS in the Deep South. Am J Public Health. 2006;96(6):970–973. doi: 10.2105/AJPH.2005.063149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson A, Hall H, Hu X, Lansky A, Holtgrave DR, Mermin J. Trends in diagnoses of HIV infection in the united states, 2002–2011. JAMA. 2014;312(4):432–434. doi: 10.1001/jama.2014.8534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frieden TR, Foti KE, Mermin J. Applying public health principles to the HIV epidemic — how are we doing? N Engl J Med. 2015;373(23):2281–2287. doi: 10.1056/NEJMms1513641. [DOI] [PubMed] [Google Scholar]
- 28.Mascolini M. The three biggest HIV problems in the United States: Late testing, late care, and early dropout. Research Initiative: Treatment Action! 2011;16:1–73. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.