Abstract
Objective
To examine the change in use of hypnotics and/or sedatives after gastric bypass surgery or intensive lifestyle modification in adults with obesity.
Methods
Adults with obesity who underwent gastric bypass surgery or initiated intensive lifestyle modification between 2007 and 2012 were identified through the Scandinavian Obesity Surgery Registry and a Swedish commercial weight loss database. The two cohorts were matched on BMI, age, sex, education, history of hypnotics and/or sedatives use, and treatment year (surgery n = 20,626; lifestyle n = 11,973; 77% women, mean age 41 years, mean BMI 41 kg/m2). The proportion of participants with filled hypnotics and/or sedatives prescriptions was compared yearly for 3 years.
Results
In the matched treatment cohorts, 4% had filled prescriptions for hypnotics and/or sedatives during the year before treatment. At 1 year follow‐up, following an average weight loss of 37 kg and 18 kg in the surgery and intensive lifestyle cohorts, respectively, this proportion had increased to 7% in the surgery cohort but remained at 4% in the intensive lifestyle cohort (risk ratio 1.7; 95% CI: 1.4‐2.1); at 2 years, the proportion had increased to 11% versus 5% (risk ratio 2.0; 95% CI: 1.7‐2.4); and at 3 years, it had increased to 14% versus 6% (risk ratio 2.2; 95% CI: 1.9‐2.6).
Conclusions
Gastric bypass surgery was associated with increased use of hypnotics and/or sedatives compared with intensive lifestyle modification.
Introduction
Between 1980 and 2014, the worldwide prevalence of obesity increased rapidly to 10.8% in men and 14.9% in women and in Sweden to 21.4% in men and 18.6% in women 1. The corresponding prevalences for class III obesity (BMI ≥ 40 kg/m2) were 0.6%, 1.6%, 0.9%, and 1.5%, respectively 1. This is concerning because obesity affects various aspects of health 2, including sleep 3.
Obesity has been associated with sleep disorders such as obstructive sleep apnea, insomnia, and restless leg syndrome 3. Perhaps as a consequence, individuals with obesity have also been found to use more hypnotics and/or sedatives than individuals without obesity 4. Weight loss interventions have been shown to improve a range of sleep parameters, mostly those associated with obstructive sleep apnea 5, 6, but their effect on use of hypnotics and sedatives is unclear. This is an important area to address, as use of hypnotics and sedatives has been associated with vehicle accidents 7, fall‐related injuries 8, cognitive decline 9, and mortality 10.
Using Swedish nationwide and virtually complete registers, we aimed to assess the effect of weight loss through gastric bypass surgery versus intensive lifestyle modification on use of hypnotics and/or sedatives in adults with obesity. We hypothesized that the use of hypnotics and sedatives would decrease following weight loss due to improvements in obesity‐related sleep disorders and to a greater degree after gastric bypass surgery than intensive lifestyle modification, due to the higher magnitude of weight loss after surgery.
Methods
This study included individuals from the Scandinavian Obesity Surgery Registry (SOReg), which is a nationwide prospective registry of bariatric surgery patients, and the Itrim Health Database, which is a registry of individuals who underwent weight loss through a low‐ or very‐low‐calorie diet (LCD/VLCD) with lifestyle modification 11, 12, 13, 14. Individuals were linked to the nationwide Swedish Prescribed Drug Register, health registers at the National Board of Health and Welfare, and Statistics Sweden using the Swedish personal identity number, a unique identifier for each Swedish resident.
All analyses were conducted on deidentified data, and the study was approved by the regional ethics review board in Stockholm, Sweden. Participants were given the option to opt out of the registries.
Data sources
SOReg
SOReg is a nationwide registry for patients who undergo bariatric surgery in Sweden 13. It is currently estimated to cover approximately 99% of all bariatric surgeries, including both public and private provision. Data on various health factors, including BMI, are collected as part of clinical practice and recorded electronically.
The Itrim Health Database
The Itrim Health Database contains health information on participants of a commercially available intensive lifestyle modification program in Sweden. Baseline and quarterly follow‐up data on various health factors, including BMI, were collected from 35 Itrim centers across Sweden, utilizing the same information technology platform.
The Prescribed Drug Register
The Prescribed Drug Register records all filled prescriptions in Sweden. It contains detailed individual‐level information on the date, type, and amount of prescriptions filled. We accessed data on prescriptions registered between July 1, 2005, and September 30, 2015.
Other registers
Data on age, sex, education, and emigrations were collected for each individual from the Longitudinal Integration Database for Health Insurance and Labor Market Studies, the Education Register, and the Total Population Register 15 at Statistics Sweden. Data on hospital visits and deaths were available through linkage with the National Patient Register and the Causes of Death Register at the National Board of Health and Welfare.
Inclusion and exclusion criteria
We restricted the study population to individuals who were at least 18 years old, with a BMI between 30 and 50 kg/m2 at the start of treatment. SOReg patients undertaking nongastric bypass surgery were excluded (2%). As we required each individual to have prescription data from 2 years prior to 3 years after initiation of treatment, we restricted the study population to those who initiated treatment between July 1, 2007, and September 30, 2012. Individuals who emigrated after treatment initiation were excluded from the study (SOReg: n = 126, Itrim: n = 54).
Matching
Individuals from SOReg and Itrim were matched on treatment year and the set of covariates identified through a directed acyclic graph (Supporting Information Figure S1) 16 built using DAGitty v.2.3 (DAGitty software, Utrecht, Netherlands) 17. The minimal sufficient adjustment set for producing an unbiased estimate of the total effect of bariatric surgery on sleep problems included age, sex, socioeconomic status, baseline BMI, and sleep problems prior to treatment. We used data on education levels as a proxy for socioeconomic status 18.
Coarsened exact matching techniques were used, which involves categorizing values of each matching factor into substantively meaningful groups (Supporting Information Table S1) upon which exact matching was then performed 19. To minimize loss of information, we allowed matching strata to include different numbers of surgery patients and intensive lifestyle modification participants.
Exposures
Patients in the surgery cohort (from SOReg) underwent gastric bypass. Participants in the intensive lifestyle treatment cohort (from Itrim) underwent a 3‐month dietary weight loss phase facilitated by LCD or VLCD, followed by a 9‐month weight maintenance phase (Supporting Information Methods S1) 11. The choice of LCD or VLCD was based on the participants' baseline BMI, personal preference, and contraindication status. Treatment with LCD/VLCD produces a greater degree of weight loss than other nonsurgical treatments 11, 20 and was selected as the comparator cohort due to presence of obesity and intention to lose weight.
Outcome and follow‐up
The main outcome was hypnotics and/or sedatives prescriptions filling, identified via the nationwide Prescribed Drug Register through the World Health Organization Anatomical Therapeutic Chemical (ATC) codes under N05C (Supporting Information Table S2). Medications were excluded if their primary indication included health conditions other than sleep problems, such as mental health disorders (clomethiazole [N05CM02] and Valerianae radix [N05CM09]). Midazolam (N05CD08) was excluded as it is indicated for preoperative sedation. Collectively, the excluded medications constituted 0.26% of all sleep medication prescriptions in the matched data set throughout the entire study period.
The categorical outcome was defined as the proportion of individuals with at least one filled prescription of the selected medications in a given year. The continuous outcome was defined as annual mean treatment dose of hypnotics and/or sedatives, calculated as follows:
where defined daily doses (DDDs) refers to the daily dose of a particular medication recommended by the World Health Organization 21.
Individuals were followed from treatment initiation until death or end of follow‐up, whichever came first. All individuals in the current data set had at least 3 years follow‐up. We also created a second matched data set composed of the subgroup of individuals with 5 years follow‐up.
Covariates
Baseline weight and height measurements were used to calculate BMI. Poor mental health was defined as a history of inpatient stays or outpatient visits for psychiatric disorders (ICD10: F00‐F99) and/or prescribed medications for mental health disorders (ATC codes: N06, N05A, and N05B). In the surgery cohort, information on use of continuous positive airway pressure (CPAP) was obtained during clinical examination at baseline. History of inpatient stays and outpatient visits for any cause were identified through the National Patient Register, and prescription history was identified from the Prescribed Drug Register.
Statistical analysis
We compared the proportion of individuals with filled hypnotics and/or sedatives prescriptions post treatment in the surgery and intensive lifestyle cohorts using a generalized linear model with log link, assuming a binomial distribution (or Poisson in the event of nonconvergence). Analyses were also performed using linear regression to estimate the mean between‐cohort difference in annual treatment dose in individuals with filled hypnotic and/or sedative prescriptions prior to treatment. All analyses were weighted to take into account the different sizes of matching strata.
Subgroup analysis
Subgroup analyses and treatment interaction tests were performed by baseline age, sex, education level, BMI, and mental health status.
Within‐group analysis
To assess dose‐response relationship between BMI change and outcomes, we repeated the analyses comparing outcomes by tertiles of 1‐year %BMI change separately for the surgery and the intensive lifestyle cohorts. In the surgery cohort, we also investigated the outcome in patients with versus without CPAP at baseline (CPAP use data were not available in the intensive lifestyle cohort).
Sensitivity analysis
We repeated the main analysis in the matched data set, additionally adjusting for the original matching variables (continuous age, continuous BMI, cumulative DDDs within 2 years prior to treatment, and treatment year), indicators of poor mental health, and history of health care contacts from 1 year to 2 years prior to treatment (measured through history of inpatient stays, outpatient visits, and filled prescription for any medications). Health care contacts in the year immediately prior to treatment (from 0 to 1 year prior to treatment) were not considered because they may be unusually high during that year, especially in the surgery cohort.
All analyses were performed using SAS version 9.4 (SAS Institute, Cary, North Carolina) and Stata version 14 (StataCorp, College Station, Texas). A statistically significant finding was defined as a two‐sided P value of < 0.05.
Results
Study population
Before matching, there were 24,291 individuals in the surgery cohort and 13,095 individuals in the intensive lifestyle treatment cohort. After matching, the numbers were 20,626 (85% of the starting sample) and 11,973 (91% of the starting sample), respectively (Supporting Information Figure S2).
Baseline characteristics
Before matching, surgery patients were more likely to be younger, have lower education, have higher BMI, and have filled hypnotics and/or sedatives prescriptions than intensive lifestyle participants. After matching, discrepancies across treatment cohorts were no longer detected, except for BMI, which remained 0.5 kg/m2 (95% CI: 0.4‐0.6; P < 0.001) higher in the surgery than the intensive lifestyle cohort (Table 1). In the matched sample, surgery patients were also more likely to have poor mental health and to have had health care contacts prior to treatment compared with the intensive lifestyle participants.
Table 1.
Baseline characteristics
| Before matching | After matching | |||||
|---|---|---|---|---|---|---|
| Matching factor |
Surgery (n = 24,291) |
Intensive lifestyle (n = 13,095) |
Mean difference or risk ratio (95% CI) |
Surgery (n = 20,626) |
Intensive lifestyle (n = 11,973) |
Mean difference or risk ratio (95% CI) |
| Mean age (y) | 41.3 (10.9) | 46.0 (12.0) | −4.7 (−5.0 to 4.5) | 41.2 (10.7) | 41.3 (11.1) | −0.1 (−0.3 to 0.0) |
| Men, n (%) | 5,715 (23.5) | 3,071 (23.5) | 1.0 (1.0 to 1.0) | 4,791 (23.2) | 2,781 (23.2) | Exactly matched |
| University education, n (%) | 5,173 (21.4) | 6,203 (47.6) | 0.4 (0.4 to 0.5) | 4,514 (21.9) | 2,620 (21.9) | Exactly matched |
| Mean BMI at screening (kg/m2) | 41.5 (4.0) | 34.5 (3.8) | 7.0 (6.9 to 7.1) | 41.4 (4.0) | 40.9 (4.2) | 0.5 (0.4 to 0.6) |
| Filled hypnotics/sedatives prescriptions at 0‐1 y pretreatment, n (%) | 3,543 (14.6) | 1,185 (9.1) | 1.6 (1.5 to 1.7) | 826 (4.0) | 479 (4.0) | Exactly matched |
| Mean treatment dose (DDD) | 317 (452) | 188 (313) | 129 (101 to 156) | 70 (91) | 67 (90) | 3 (−1 to 7) |
| Filled hypnotics and/or sedatives prescriptions between 1‐2 y pretreatment, n (%) | 3,529 (14.6) | 1,174 (9.0) | 1.6 (1.5 to 1.7) | 883 (4.3) | 513 (4.3) | Exactly matched |
| Mean treatment dose (DDD) | 288 (429) | 190 (352) | 97 (70 to 124) | 65 (83) | 66 (84) | 0 (‐5 to 4) |
| Other covariates | ||||||
| Use of continuous positive airway pressure, n (%) | 2,223 (9.2) | NA | NR | 1,831 (8.9) | NA | NR |
| Indicators of poor mental health, n (%) | 7,187 (29.6) | 2,293 (17.5) | 1.7 (1.6 to 1.8) | 4,797 (23.3) | 1,806 (15.1) | 1.5 (1.4 to 1.7) |
| Health care contacts 1‐2 y pretreatment | ||||||
| Outpatient visits, n (%) | 14,289 (58.8) | 5,136 (39.2) | 1.5 (1.5 to 1.5) | 11,609 (56.3) | 4,737 (39.6) | 1.4 (1.3 to 1.5) |
| Inpatient stays, n (%) | 3,483 (14.3) | 1,274 (9.7) | 1.5 (1.4 to 1.6) | 2,688 (13.0) | 1,238 (10.3) | 1.3 (1.1 to 1.4) |
| Filled prescription for any medications, n (%) | 21,778 (89.7) | 10,577 (80.8) | 1.1 (1.1 to 1.1) | 18,241 (88.4) | 9,751 (81.4) | 1.1 (1.1 to 1.1) |
All mean values are presented with standard deviations.
Mean difference is for continuous variables, and risk ratio is for categorical values.
All data were weighted to take into account different sizes of matching strata.
Indicators of poor mental health: inpatient stay or outpatient visit listing a mental health diagnosis, or filled prescriptions for mental health indications.
Proportions of individuals with filled hypnotics and/or sedatives prescriptions stratified by levels of matching factors in the unmatched data set are shown in Supporting Information Figure S6.
Abbreviations: DDDs, defined daily doses; NA, not available; NR, not relevant.
Weight loss and hypnotics and/or sedatives use after surgery and intensive lifestyle modification
The mean 1‐year weight loss was 37 kg in the surgery and 18 kg in the intensive lifestyle cohort (mean difference 19 kg, 95% CI: 18‐20; P < 0.001). The three most commonly prescribed hypnotics and sedatives were zopiclone, zolpidem, and propiomazine, accounting for 96% of all prescriptions in both treatment cohorts throughout the study period (Supporting Information Table S2 and Supporting Information Figure S3).
During follow‐up, the risk of having filled hypnotics and/or sedatives prescriptions was higher in the surgery than the intensive lifestyle cohort, and the risk ratio increased with longer follow‐up, peaking at 3 years (risk ratio 2.2, 95% CI: 1.9‐2.6; P < 0.001; Figure 1). In the second matched data set, the proportion continued to increase for up to 5 years of follow‐up (Figure 1).
Figure 1.
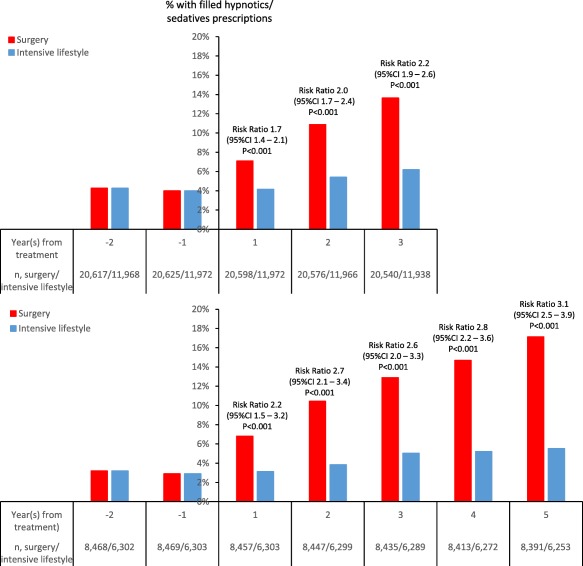
Proportion of patients with filled hypnotics and/or sedatives prescriptions in the matched study population with 3 years of follow‐up (upper panel) and in the subgroup with 5 years of follow‐up (lower panel). Risk ratios (95% CI) apply to the between‐cohort differences at each follow‐up time point. Baseline characteristics of subgroups with 5 years of follow‐up are shown in Supporting Information Table S3. The proportion of patients with filled hypnotics and/or sedatives prescriptions in the unmatched study population is shown in Supporting Information Figure S4. [Color figure can be viewed at wileyonlinelibrary.com]
Among those with filled hypnotics and/or sedatives prescriptions prior to treatment, mean treatment dose increased more in the surgery than the intensive lifestyle cohort, with a mean difference at 3 years of 57 DDDs (95% CI: 39‐75; P < 0.001; Figure 2). In the second matched data set, the mean difference continued to increase for up to 5 years of follow‐up (Figure 2).
Figure 2.
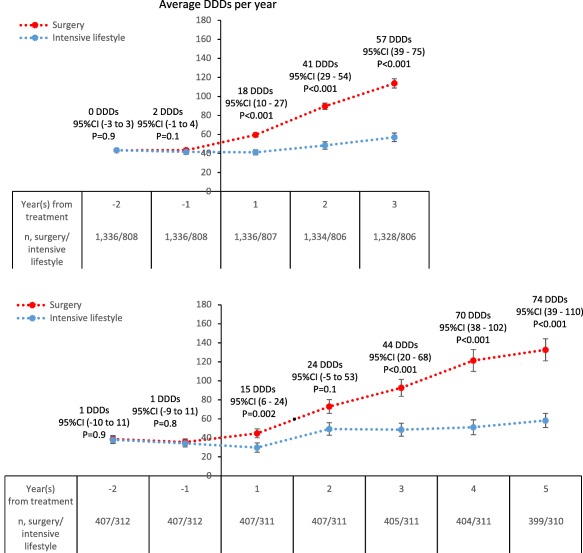
Mean treatment dose among those with filled hypnotics and/or sedatives prescriptions at 1 and/or 2 years prior to treatment, in the matched study population with 3 years of follow‐up (upper panel) and in the subgroup with 5 years of follow‐up (lower panel). Error bars are 95% CI. Mean differences (95% CI) apply to the between‐cohort differences at each follow‐up time point. Baseline characteristics of subgroups with 5 years of follow‐up are shown in Supporting Information Table S3. Mean treatment dose among those with filled hypnotics and/or sedatives prescriptions in the unmatched study population are shown in Supporting Information Figure S5. Abbreviations: DDDs, defined daily doses. [Color figure can be viewed at wileyonlinelibrary.com]
Adjustment for the original matching variables prior to categorization (Supporting Information Table S1) and/or indicators of poor mental health and/or history of health care contacts before treatment resulted in ≤ 0.2 change for risk ratios and ≤ 2 DDD change for mean differences, which remained statistically significant at all time points.
Subgroup analyses
In 12 out of 14 subgroups investigated, surgery patients were at greater risk of having filled hypnotics and/or sedatives prescriptions than intensive lifestyle participants during follow‐up (Figure 3). Within each level of educational attainment, the risk was increased in the surgery compared to the intensive lifestyle cohort. We found a statistically significant interaction by baseline BMI, but there was no clear dose‐response relationship.
Figure 3.
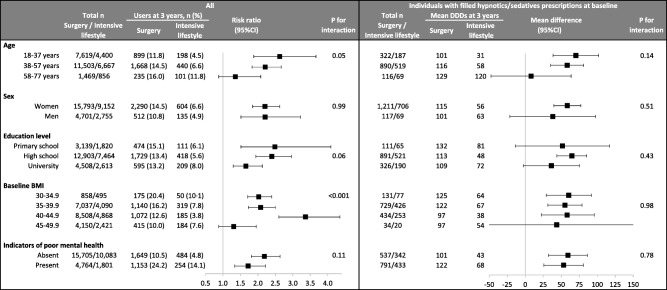
Use of hypnotics and/or sedatives in the two treatment cohorts at 3‐year follow‐up, stratified by baseline characteristics, categorically in the overall matched study population (left) and continuously among those with filled hypnotics and/or sedatives prescriptions at 1 and/or 2 years prior to treatment (right). Abbreviation: DDDs, defined daily doses.
In individuals with filled hypnotics and/or sedatives prescriptions prior to baseline, we did not find any treatment‐subgroup interactions regarding dose, potentially due to low power (Figure 3).
Within‐group analyses
Percent BMI change
No dose‐response relationship was found for 1‐year %BMI change and the outcome in either the surgery or the intensive lifestyle cohort (Figure 4).
Figure 4.
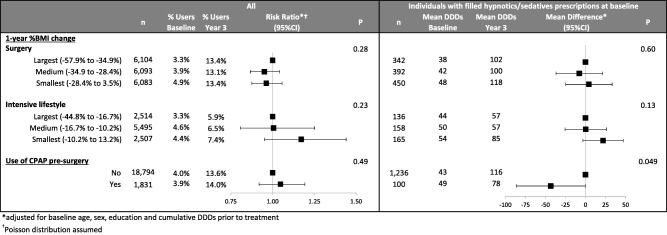
Within‐treatment cohort analysis of the relationship of BMI change and of presurgical use of CPAP on the categorical outcome (with filled hypnotics and/or sedative prescriptions, Yes/No) in the overall matched study population (left) and for the continuous outcome (mean treatment dose, DDDs) among those with filled hypnotics and/or sedatives prescriptions at 1 and/or 2 years prior to treatment (right). *Adjusted for baseline age, sex, education, and cumulative DDDs prior to treatment. †Poisson distribution assumed. Abbreviations: CPAP, continuous positive airway pressure; DDDs, defined daily doses.
Baseline use of CPAP (surgery only)
The risk of having filled hypnotics and/or sedatives prescriptions 3 years after treatment did not differ by baseline use of CPAP. Among those with filled hypnotics and/or sedatives prescriptions at baseline, mean treatment dose increased more in patients without than with CPAP at baseline (Figure 4).
Discussion
We found higher use of hypnotics and/or sedatives following gastric bypass compared with intensive lifestyle modification at 3‐ and 5‐year follow‐ups, and this difference was present in 12 out of 14 subgroups investigated. There was no evidence in either treatment cohort for a dose‐response relationship between %BMI change at 1 year and change in use of hypnotics and/or sedatives at 3 years.
Our finding of an increased use of hypnotics and/or sedatives after gastric bypass is consistent with that from an uncontrolled 2‐year follow‐up study of 165 Norwegian patients 22 and from a Swedish study comparing 3,139 gastric bypass patients with 31,390 general population controls over 4 years of follow‐up 23. Note that the Swedish study did not match for baseline BMI.
Because we did not find evidence for a dose‐response relationship between %BMI change and change in use of hypnotics and/or sedatives, nor any change after substantial nonsurgically induced weight loss, it is likely that the increased use was driven by the undertaking of gastric bypass surgery and not by weight loss per se. While we cannot identify the cause of this phenomenon through our study, a number of plausible explanations can be identified in the literature. One possibility is “addiction transfer,” whereby patients stop overeating for anxiety relief but acquire other compulsive disorders such as alcoholism or substance abuse after bariatric surgery 24. The mechanism is unclear, and whether compulsive eating behavior prior to surgery can be considered as addiction remains debatable 25, but this phenomenon is continually being reported in bariatric surgery patients 24. One way to test this hypothesis is through a subgroup analysis by baseline mental health status. In this study, there was no interaction between treatment cohort and baseline mental health status; i.e., use of sleep medication during follow‐up was higher in the surgery cohort compared with the intensive lifestyle cohort, regardless of the baseline mental health status. Perhaps a more specific marker of poor mental health or addictive behavior is needed.
Another plausible explanation is that gastric bypass increases the risk of alcoholism and substance abuse 26, 27, 28, 29, which in turn leads to poor sleep 30, 31. Malabsorption after gastric bypass may explain the increased mean treatment dose among baseline users, but not the uptake of new users after surgery 32. One could also argue that hypnotics and/or sedatives use could increase after surgery due to patients having more frequent contact with clinicians at follow‐up than intensive lifestyle participants. However, this is unlikely to be the full explanation, given the continuous uptake of new users even at 3 to 5 years follow‐up, when health care contacts for postoperative care have presumably decreased. In addition, we observed greater dose increases in individuals in the surgery than the lifestyle cohort, among those using hypnotics and/or sedatives at baseline, and these individuals already have an established contact with a physician.
In the presence of obstructive sleep apnea, most hypnotics and/or sedatives are to be used with caution 33, 34. It is possible that clinicians start to prescribe more hypnotics and/or sedatives to treat residual sleep problems following substantial improvement in severity of obstructive sleep apnea post bariatric surgery (recently shown in another study using SOReg data 35), as use of hypnotics and/or sedatives is no longer contraindicated. If this was the case, we should observe a greater increase in hypnotics and sedatives prescription fillings among baseline CPAP users than in those without CPAP. Instead, we found a lower mean treatment dose at 3 years after surgery among CPAP users than in those without CPAP at baseline, although both cohorts increased their use compared to baseline. Also, at baseline, the use of hypnotics and/or sedatives did not differ between those with and without CPAP. It seems that despite published warnings, hypnotics and sedatives are still often used in patients with CPAP, potentially to aid with sleep and improve compliance 36, 37, 38, 39.
Our study utilized nationwide registry data, which implies minimum loss to follow‐up for the outcome (0.5% due to emigrations), large sample size, and high generalizability within the Swedish population, which is predominantly Caucasian. Use of hypnotics and/or sedatives was assessed through filled prescriptions recorded in the Prescribed Drug Register, providing better accuracy than self‐reported data. We included participants in an intensive lifestyle modification program as our comparator cohort, which addressed previous concerns over lack of studies on bariatric surgery with comparator interventions producing sufficient weight loss 40. The average magnitude of 1‐year weight loss from the intensive lifestyle cohort in this study (18 kg) is substantially larger than other conventional treatment comparators in previous bariatric surgery studies (max 8 kg) 20.
There are several limitations to this study. Even though we minimized the overlap between sleep and mental health problems by excluding medications with shared indications for mental health problems, we cannot control the off‐label use of the remaining medications, and there is often more than one reason to prescribe a certain medication. Nevertheless, even if use of hypnotics or sedatives is not a perfect marker for sleep problems, our finding of increased hypnotics and sedatives after bariatric surgery remains a clinically important observation. Also, our estimates were robust to adjustments for baseline mental health.
This study was not a randomized controlled trial and is therefore prone to confounding. We attempted to minimize confounding by matching on covariates identified through a directed acyclic graph, but we cannot prove if our diagram is true and complete. There may be other inherent (personality) differences between the surgery and intensive lifestyle treatment cohorts that we do not capture, which may contribute to the observed differences. For instance, before matching, intensive lifestyle participants had higher levels of education, likely representing a more health‐conscious population who may be more resistant to pharmacological aid such as hypnotics or sedatives. In our analysis, we aimed to capture this difference in health‐seeking behavior between the treatment cohorts by adjusting for history of health care contacts. The estimates changed by ≤ 0.2 for risk ratios and ≤ 2 DDDs for mean differences, but this may not be sufficient. However, surgery increased the risk of having filled hypnotics and/or sedatives prescriptions compared with intensive lifestyle in all education level subgroups.
The surgery cohort in our current study only included gastric bypass. Our results may not be generalizable to other procedure types.
Conclusion
We found that the use of hypnotics and/or sedatives increased following gastric bypass, and the increase continued for up to 5 years. We saw no change in use of hypnotics and/or sedatives in the intensive lifestyle treatment group. Future studies are needed to identify the underlying mechanisms and assess whether these results may be observed after other bariatric or even nonbariatric surgeries. Our findings indicate the need for sleep medication monitoring and management following gastric bypass to prevent uptake of new users and continuous increase in the mean treatment dose of hypnotics and sedatives after gastric bypass.
Supporting information
Supporting Information Appendixs.
Acknowledgments
We would like to thank John Dixon, PhD, and psychiatrist Johan Reutfors, MD, PhD, whom we consulted during the project development stage. We would also like to thank Cheryl Albright, PhD, for her help editing the manuscript. None of these individuals received compensation for contributions to this article.
Funding agencies: Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award number R01DK105948 and by the Vetenskapsrådet/Swedish Research Council under grant number 2013‐3770. This work was also supported by a Monash Postgraduate Travel Grant Award, Baker Heart and Diabetes Institute travel support, Deakin University travel support, and the Harold‐Mitchell Travel Award. WLN is supported by a Monash Graduate Scholarship, a Monash International Post‐graduate Research Scholarship, and a Baker Bright Sparks Top‐Up Scholarship. JES is funded by an NHMRC Senior Research Fellowship. AP is supported by an NHMRC Development Fellowship. Authors of this study were also supported by the Swedish Research Council (MN: K2014‐99X‐22495‐01‐3; JS: 2010–1078). The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funder/sponsor.
Disclosure: IN is the previous director of the Scandinavian Obesity Surgery Registry. JO is the current director. CM, MN, and JS report receiving consulting fees for participation in the scientific advisory committee of Itrim. Further, MN has received research grants from Pfizer, Cambridge Weight Plan, Novo Nordisk, and Astra Zeneca; royalty payments from Studentlitteratur for coauthoring chapters in a Swedish textbook on obesity; and lecture or consulting fees from Pfizer, Sanofi‐Aventis, Roche, and Strategic Health Resources. AP and JES have participated in a Novo Nordisk advisory board. The other authors declared no conflict of interest.
Author contributions: WLN and MN drafted the manuscript and carried out the statistical analysis. All authors critically revised the manuscript for important intellectual content and contributed to and approved the final version. They are the guarantors. WLN, MN, GB, AP, and JES conceived and designed the study. MN acquired the data, provided administrative, technical, and material support, and supervised the study. MN and GB had full access to all of the data in the study. WLN and MN take full responsibility for the integrity of the data and the accuracy of the data analysis as well as the final decision to submit for publication.
Contributor Information
Winda L. Ng, Email: winda.liviya@baker.edu.au
Martin Neovius, Email: martin.neovius@ki.se.
References
- 1. NCD Risk Factor Collaboration . Trends in adult body‐mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population‐based measurement studies with 19.2 million participants. Lancet 2016;387:1377‐1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Visscher TL, Seidell JC. The public health impact of obesity. Annu Rev Public Health 2001;22:355‐375. [DOI] [PubMed] [Google Scholar]
- 3. Hargens TA, Kaleth AS, Edwards ES, Butner KL. Association between sleep disorders, obesity, and exercise: a review. Nat Sci Sleep 2013;5:27‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vozoris NT, Leung RS. Sedative medication use: prevalence, risk factors, and associations with body mass index using population‐level data. Sleep 2011;34:869‐874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Greenburg DL, Lettieri CJ, Eliasson AH. Effects of surgical weight loss on measures of obstructive sleep apnea: a meta‐analysis. Am J Med 2009;122:535‐542. [DOI] [PubMed] [Google Scholar]
- 6. Araghi MH, Chen YF, Jagielski A, et al. Effectiveness of lifestyle interventions on obstructive sleep apnea (OSA): systematic review and meta‐analysis. Sleep 2013;36:1553‐1562, 62A‐62E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Engeland A, Skurtveit S, Morland J. Risk of road traffic accidents associated with the prescription of drugs: a registry‐based cohort study. Ann Epidemiol 2007;17:597‐602. [DOI] [PubMed] [Google Scholar]
- 8. Woolcott JC, Richardson KJ, Wiens MO, et al. Meta‐analysis of the impact of 9 medication classes on falls in elderly persons. Arch Intern Med 2009;169:1952‐1960. [DOI] [PubMed] [Google Scholar]
- 9. Glass J, Lanctot KL, Herrmann N, Sproule BA, Busto UE. Sedative hypnotics in older people with insomnia: meta‐analysis of risks and benefits. BMJ 2005;331:1169. doi:10.1136/bmj.38623.768588.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hausken AM, Skurtveit S, Tverdal A. Use of anxiolytic or hypnotic drugs and total mortality in a general middle‐aged population. Pharmacoepidemiol Drug Saf 2007;16:913‐918. [DOI] [PubMed] [Google Scholar]
- 11. Hemmingsson E, Johansson K, Eriksson J, Sundstrom J, Neovius M, Marcus C. Weight loss and dropout during a commercial weight‐loss program including a very‐low‐calorie diet, a low‐calorie diet, or restricted normal food: observational cohort study. Am J Clin Nutr 2012;96:953‐961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Johansson K, Sundstrom J, Marcus C, Hemmingsson E, Neovius M. Risk of symptomatic gallstones and cholecystectomy after a very‐low‐calorie diet or low‐calorie diet in a commercial weight loss program: 1‐year matched cohort study. Int J Obes (Lond) 2014;38:279‐284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hedenbro JL, Naslund E, Boman L, et al. Formation of the Scandinavian Obesity Surgery Registry, SOReg. Obes Surg 2015;25:1893‐1900. [DOI] [PubMed] [Google Scholar]
- 14. Sundstrom J, Bruze G, Ottosson J, Marcus C, Naslund I, Neovius M. Weight loss and heart failure: a nationwide study of gastric bypass surgery versus intensive lifestyle treatment. Circulation 2017;135:1577‐1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ludvigsson JF, Almqvist C, Bonamy AE, et al. Registers of the Swedish total population and their use in medical research. Eur J Epidemiol 2016;31:125‐136. [DOI] [PubMed] [Google Scholar]
- 16. Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology 1999;10:37‐48. [PubMed] [Google Scholar]
- 17. Textor J, Hardt J, Knuppel S. DAGitty: a graphical tool for analyzing causal diagrams. Epidemiology 2011;22:745. doi:10.1097/EDE.0b013e318225c2be [DOI] [PubMed] [Google Scholar]
- 18. Galobardes B, Shaw M, Lawlor DA, Lynch JW, Davey Smith G. Indicators of socioeconomic position (part 1). J Epidemiol Community Health 2006;60:7‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Blackwell M, Iacus S, King G, Porro G. cem: coarsened exact matching in Stata. Stata J 2009;9:524‐546. [Google Scholar]
- 20. Gloy VL, Briel M, Bhatt DL, et al. Bariatric surgery versus non‐surgical treatment for obesity: a systematic review and meta‐analysis of randomised controlled trials. BMJ 2013;347:f5934. doi:10.1136/bmj.f5934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. WHO Collaborating Centre for Drug Statistics Methodology web site . ATC/DDD Index. http://www.whocc.no/atc_ddd_index/. Updated May 9, 2017.
- 22. Hanvold SE, Loken EB, Paus SF, et al. Great health benefits but no change in employment or psychopharmaceutical drug use 2 years after Roux‐en‐Y gastric bypass. Obes Surg 2015;25:1672‐1679. [DOI] [PubMed] [Google Scholar]
- 23. Backman O, Stockeld D, Rasmussen F, Naslund E, Marsk R. Alcohol and substance abuse, depression and suicide attempts after Roux‐en‐Y gastric bypass surgery. Br J Surg 2016;103:1336‐1342. [DOI] [PubMed] [Google Scholar]
- 24. Blum K, Bailey J, Gonzalez AM, et al. Neuro‐genetics of Reward Deficiency Syndrome (RDS) as the root cause of “addiction transfer”: a new phenomenon common after bariatric surgery. J Genet Syndr Gene Ther 2011;2012:S2‐001. doi:10.4172/2157-7412.S2-001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sogg S. Alcohol misuse after bariatric surgery: epiphenomenon or “Oprah” phenomenon? Surg Obes Relat Dis 2007;3:366‐368. [DOI] [PubMed] [Google Scholar]
- 26. Ostlund MP, Backman O, Marsk R, et al. Increased admission for alcohol dependence after gastric bypass surgery compared with restrictive bariatric surgery. JAMA Surg 2013;148:374‐377. [DOI] [PubMed] [Google Scholar]
- 27. Svensson PA, Anveden A, Romeo S, et al. Alcohol consumption and alcohol problems after bariatric surgery in the Swedish obese subjects study. Obesity (Silver Spring) 2013;21:2444‐2451. [DOI] [PubMed] [Google Scholar]
- 28. King WC, Chen JY, Mitchell JE, et al. Prevalence of alcohol use disorders before and after bariatric surgery. JAMA 2012;307:2516‐2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Conason A, Teixeira J, Hsu CH, Puma L, Knafo D, Geliebter A. Substance use following bariatric weight loss surgery. JAMA Surg 2013;148:145‐150. [DOI] [PubMed] [Google Scholar]
- 30. Brower KJ. Alcohol's effects on sleep in alcoholics. Alcohol Res Health 2001;25:110‐125. [PMC free article] [PubMed] [Google Scholar]
- 31. Mahfoud Y, Talih F, Streem D, Budur K. Sleep disorders in substance abusers: how common are they? Psychiatry (Edgmont) 2009;6:38‐42. [PMC free article] [PubMed] [Google Scholar]
- 32. Miller AD, Smith KM. Medication and nutrient administration considerations after bariatric surgery. Am J Health Syst Pharm 2006;63:1852‐1857. [DOI] [PubMed] [Google Scholar]
- 33. FASS.se Allmänheten web site. http://www.fass.se/LIF/home/index.jsp.
- 34. Food and Drug Administration. FDA Label Search. http://labels.fda.gov/ingredientname.cfm
- 35. Sundbom M, Hedberg J, Marsk R, et al. Substantial decrease in comorbidity 5 years after gastric bypass: a population‐based study from the Scandinavian Obesity Surgery Registry. Ann Surg 2017;265:1166‐1171. [DOI] [PubMed] [Google Scholar]
- 36. Bradshaw DA, Ruff GA, Murphy DP. An oral hypnotic medication does not improve continuous positive airway pressure compliance in men with obstructive sleep apnea. Chest 2006;130:1369‐1376. [DOI] [PubMed] [Google Scholar]
- 37. Series F, Workshop P. Can improving sleep influence sleep‐disordered breathing? Drugs 2009;69(Suppl 2):77‐91. [DOI] [PubMed] [Google Scholar]
- 38. Berry RB, Patel PB. Effect of zolpidem on the efficacy of continuous positive airway pressure as treatment for obstructive sleep apnea. Sleep 2006;29:1052‐1056. [DOI] [PubMed] [Google Scholar]
- 39. Lettieri CJ, Collen JF, Eliasson AH, Quast TM. Sedative use during continuous positive airway pressure titration improves subsequent compliance: a randomized, double‐blind, placebo‐controlled trial. Chest. 2009;136:1263‐1268. [DOI] [PubMed] [Google Scholar]
- 40. Ludwig DS, Ebbeling CB, Livingston EH. Surgical vs lifestyle treatment for type 2 diabetes. JAMA 2012;308:981‐982. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Appendixs.


