Abstract
We examined accumulation, sequestration, elimination, and genetic variation for lead (Pb) loads within and between generations of Drosophila melanogaster. Flies were reared in control or leaded medium at various doses and tested for their Pb loads at different stages of development (larvae, eclosion, newly-eclosed adults, and mature adults). Pb loads were tested using Inductively Coupled Plasma Mass Spectrometry (ICP-MS). We found that D. melanogaster readily accumulated Pb throughout their lifespan and the levels of accumulation increased with Pb exposure in the medium. Wandering third-instar larvae accumulated more Pb than mature adults; this phenomenon may be due to elimination of Pb in the pupal cases during eclosion and/or depuration in adults post-eclosion. The accumulated Pb in mature adults was not transferred to F1 mature adult offspring. Using a set of recombinant inbred strains, we identified a quantitative trait locus for adult Pb loads and found that genetic variation accounted for 34% of the variance in Pb load. We concluded that D. melanogaster is a useful model organism for evaluating changes in Pb loads during development, as well as between generations. Furthermore, we found that genetic factors can influence Pb loads; this provides an essential foundation for evaluating phenotypic variation induced by the toxic effects of Pb.
Keywords: Pb load, accumulation, sequestration, elimination, genetic variation
1. Introduction
Lead (Pb) has been used by humans for thousands of years (Gilbert and Weiss, 2006) and this has caused widespread global contamination. The focus of Pb pollution remediation has been to target the most prevalent modes of exposure (i.e. Pb-based paint and leaded gasoline) to reduce the average blood Pb levels (BLLs) in humans. Legislation calling for a reduction of tetraethyl Pb in gasoline and restrictions on Pb-based residential paint has successfully helped to reduce the average BLLs in children over the last three decades (Jones et al., 2009).
Although efforts to decrease pollution and the average BLLs in U.S. children have been successful, Pb pollution is still a serious problem throughout the U.S. and across the world. Soil contamination downwind of smelters (Landrigan et al., 1975; Landrigan and Baker, 1981) and in urban gardens (Clark et al., 2008) contributes to potentially greater risk factors for children than direct exposure to Pb-based paint (Mielke and Reagan, 1998). In addition, industrial recycling (Huo et al., 2007), the use of Pb-based paint (Lin et al., 2009), leaded gasoline (Kaiser et al., 2001), and lax government regulation has caused widespread Pb pollution in soil and water in other countries (Falk, 2003).
Due to its widespread dissemination, exposure to Pb is a risk factor for wild populations of terrestrial and aquatic animals. Biomagnification of Pb has been demonstrated (Rubio-Franchini and Rico-Martinez, 2011) in invertebrate predators in both the field and the laboratory. This work indicates the importance of studying the accumulation of Pb in invertebrates for a comprehensive understanding of the ecological effects of Pb pollution. Across genera, terrestrial invertebrates accumulate metals and sequester these metals to various organs, particularly the digestive tissues, for storage-detoxification (Dallinger, 1993). Field research of wild populations of vertebrates and invertebrates can be challenging, making invertebrate laboratory model systems an attractive alternative.
Commonly known as the “fruit fly”, the vinegar fly (Drosophila melanogaster) is a commensal species that was initially developed by Charles W. Woodworth as a model system for genetic studies approximately a century ago (Abolaji et al., 2013). D. melanogaster is now commonly used in the biological sciences as an invertebrate alternative to mice and rat models. The advantages of using D. melanogaster as a model system include: 1) their short generation times; 2) ability to generate large sample sizes; 3) and ease in modeling long-term evolutionary changes (Abolaji et al., 2013; Burke and Rose, 2009; Rand, 2010; Rand et al., 2014). In addition, there is an extensive conservation of genes and developmental mechanisms between D. melanogaster and humans (Pandey and Nichols, 2011; Rubin et al., 2000).
Therefore, D. melanogaster is now gaining attention by environmental toxicologists as a suitable alternative model for toxicity testing (Abolaji et al., 2013; Rand, 2010; Rand et al., 2014). Several measurable endpoints have been suggested by others for the use of Drosophila as a toxicology model (Rand, 2010; Rand et al., 2014); these include, but are not limited to, lethality, morphology, behavioral traits, embryonic development, and targeted mechanistic studies. We have established D. melanogaster as a model system to investigate the behavioral, physiological, and genetic effects of chronic, developmental Pb exposure (He et al., 2009; Hirsch et al., 2003; Hirsch et al., 2009; Morley et al., 2003; Ruden et al., 2009).
Understanding the ability of D. melanogaster to sequester and eliminate Pb, as well as potential genetic predispositions, will provide a greater understanding of the effects of exposure to Pb in this model system. Previous studies have found that D. melanogaster readily accumulates Pb (Cohn et al., 1992; Hirsch et al., 2003), and that Pb is toxic for both larvae (Akins et al., 1992) and adults (Hirsch et al., 2003). In previous research, we identified a region of the Drosophila genome that is involved in Pb-dependent changes in locomotor activity (Hirsch et al., 2009) and in extensive changes in patterns of transcription induced by developmental Pb exposure (Ruden et al., 2009).
Therefore, the purpose of this study is to determine, for Drosophila melanogaster: 1) how Pb is accumulated, eliminated, and sequestered both within the same generation and subsequent generations, and 2) the extent of genetic variation influencing adult Pb loads. To accomplish our first aim, we quantified Pb loads at various stages of the life cycle (wandering third-instar larvae, pupal cases, newly-eclosed adults, and mature adults). We examined elimination of Pb loads post-eclosion by exposing individuals prior to eclosion or after eclosion. We monitored intergenerational transfer of Pb loads to the first generation of offspring. Lastly, we estimated heritability and mapped a quantitative trait locus in recombinant inbred strains exposed to Pb.
2. Materials and methods
2.1. Rearing
Medium used in all experiments was prepared with Carolina Biological Instant Drosophila Medium® (Formula 4–24), yeast, and distilled water (control medium) or lead acetate (PbAc) solution (leaded medium) in plastic vials (23 mL, 75 X 23.5 mm, polystyrene) capped with cotton.
Methods for rearing were derived from Hirsch et al. (2003). Canton-S wild type Drosophila melanogaster were maintained in control medium on a 12:12 light:dark cycle at 25 °C (± 0.5 °C). Groups of 20–30 mature adults laid eggs for 24 to 48 h in control or leaded medium before being discarded. Experimental animals were reared from egg stages and harvested at various points in their life cycle in either control or leaded medium, depending on the experiment.
The stages of the D. melanogaster life cycle are: 1) egg, 2) first-instar larva, 3) second-instar larva, 4) third-instar larva, 5) wandering third-instar larva, 6) white-eye pupa, 7) red-eye pupa, 8) newly-eclosed adult (emerged from pupal case), and 9) mature adult (Tyler, 2000). We used wandering third-instar larvae, red-eye pupae, newly-eclosed adults, and mature adults as developmental markers in these studies.
2.2. Pb loads in larvae
Larvae were reared in control or leaded medium (10, 40, 50, 75, 100, 250, or 500 μM PbAc) beginning in the egg stage. Wandering third-instar larvae were removed from the walls of plastic vials, floated in distilled water, and rolled on Kimwipes® to dry at collection.
2.3. Pb loads in pupal cases and newly-eclosed adults
Drosophila were reared in control or leaded medium (10, 50, 100, and 500 μM PbAc) from egg stage to collection. Pupae were collected at the red-eye pupal stage, just prior to eclosion. They were cleaned by rolling each pupa gently on Kimwipes® and allowed to eclose individually in vials. Newly-eclosed adults were collected and sexed over ice. Pupal cases and adults were each separated by sex and dose and coded so that each newly-eclosed adult could be matched with its pupal case.
2.4. Pb loads in adults exposed at various stages
Individuals were reared in control medium or leaded medium (10, 100, 250, or 500 μM PbAc) from either: 1) egg stage to eclosion, 2) eclosion to adult d six, or 3) from egg stage to adult d six. Individuals were either collected within six h of eclosion or at adult d six.
2.5. Intergenerational transmission of Pb loads
Mature adults were reared at 24 °C (± 0.5 °C) and given four d to mate and lay eggs in either control or leaded medium (250 μM PbAc) to rear the parental generation. The parental generation was reared in control or leaded medium from egg stage to eclosion. At eclosion, newly-eclosed adults were collected, sexed under carbon dioxide anesthesia within six h of eclosion, and placed on medium matching their pre-existing exposure. Individuals tested for parental generation Pb loads were housed 10 per vial in same-sex groups, raised to five d of age, and then transferred to control medium for 24 h to allow adults to clean excess Pb off their bodies. Adults were collected at adult d six and placed blindly into coded 15 mL polypropylene centrifuge tubes to determine the parental generation adult Pb load.
To rear the next generation of offspring, males and females were raised in same-sex groups of four flies per vial and transferred to control medium for 24 h at adult d five. At adult d six, males and females were mated within treatment group and allowed to lay eggs in control medium for four d. They were collected and placed blindly into coded 15 mL polypropylene centrifuge tubes.
Newly-eclosed males and females were collected within six h of eclosion, sexed under carbon dioxide anesthesia and housed in groups of 10 by sex and treatment. At adult d four, subjects were placed blindly into coded 15 mL polypropylene centrifuge tubes. This experiment was repeated three times.
2.6. Genetic variation in Pb loads
We used 72 recombinant inbred lines of D. melanogaster representing the “roo” lines derived from parental strains Oregon-R and Russian 2b (Gurganus et al., 1998). These lines vary in the presence or absence of site-specific DNA markers at approximately 80 sites throughout the genome. Flies were reared as described in Hirsch et al. (2009) in either control or leaded medium (250 μM PbAc). Flies were tested for Pb load at six d post-eclosion. Heritability of Pb load (the proportion of phenotypic variation caused by genetic differences among strains) was calculated as the among-lines component of variance divided by the total variance, as described in Hegmann and Possidente (1981).
The subjects described above were used for quantitative trait locus (QTL) analysis employing procedures described in Hirsch et al. (2009). Significant associations between the presence or absence of each DNA marker and mean Pb load per strain identifies a “quantitative trait locus” closely linked to the marker site with allelic variation that causes variation in Pb load.
2.7. Determination of Pb loads
All samples were coded so that Pb load processing could be done blind without knowledge of rearing history. Samples in all experiments were frozen at −20 °C in groups of 5–10 individuals per tube at collection and assayed using Inductively Coupled Plasma Mass Spectrometry (Union College, Schenectady, N.Y.) after treatment with nitric acid and hydrogen peroxide, as described in Hirsch et al. (2003). Each tube was treated in the same manner.
2.8. Data analysis
Data were normalized for the number of flies in each tube and expressed as ng of Pb per fly. We did not correct for weight differences between the sexes or within each sex. Differences in Pb loads between treated and control flies in each experiment were assessed by analysis of variance, using either SPSS or Instat, and adjusted using a Bonferroni correction. Generation and sex differences effects were analyzed in the same manner, except that sex and generation were additional fixed factors, where applicable. In the experiment testing intergenerational transfer of Pb loads, one-sample t-tests were done using SPSS to determine if average Pb loads were significantly different from zero. One replicate of the larval analysis was omitted in the experiment testing intergenerational transfer of Pb loads due to lost samples.
3. Results
3.1. Pb loads during development
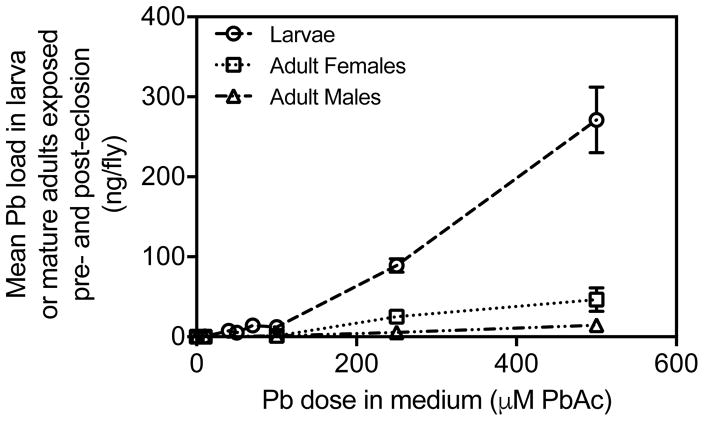
Pb loads were measured to test accumulation in wandering-third instar larvae and mature adults when developmentally exposed to control or leaded medium (Larvae: 0, 10, 40, 50, 75, 100, 250, or 500 μM PbAc; Mature Adults: 0, 10, 100, 250, or 500 μM PbAc). We found that D. melanogaster accumulated Pb at all developmental stages tested. Wandering third-instar larvae had higher Pb loads than mature adults exposed to Pb throughout their development (Fig. 1; Table 1).
Fig. 1.
Average Pb loads for each dose tested in ng/fly for wandering third-instar larvae and mature adults reared on control or leaded medium throughout development. Means ± standard error of mean shown (n = 8 larva, n = 3 control- treated adults, n = 3 Pb-treated adults).
Table 1.
Mean Pb loads during development. Mature adults exposed throughout development (both pre- and post-eclosion). Means (ng/fly) ± standard error of mean shown (n = 8 larva, n = 3 control-treated adults, n = 3 Pb-treated adults).
| Dose (μM PbAc) | Larva | Pupal Cases | Newly-eclosed adults | Mature Adult Females | Mature Adult Males |
|---|---|---|---|---|---|
| 0 | 0.009 ± 0.001 | 0.099 ± 0.02 | 0.04 ± 0.005 | 0.069 ± 0.031 | 0.0015 ± 0.003 |
| 10 | 0.42 ± 0.025 | 0.46 ± 0.03 | 0.12 ± 0.009 | 0.12 ± 0.012 | 0.056 ± 0.008 |
| 40 | 7.5 ± 0.67 | ||||
| 50 | 5.2 ± 0.71 | 2.77 ± 0.30 | 1.11 ± 0.14 | ||
| 75 | 14 ± 1.06 | ||||
| 100 | 12 ± 0.95 | 4.7 ± 0.38 | 3.36 ± 0.58 | 1.20 ± 0.63 | 1.24 ± 0.46 |
| 250 | 89 ± 8.49 | 25.06 ± 4.72 | 5.46 ± 0.75 | ||
| 500 | 271 ± 41.01 | 334.30 ± 39.43 | 8.44 ± 0.84 | 46.50 ± 14.72 | 14.43 ± 1.83 |
Wandering third-instar larvae showed a significant effect of Pb treatment (p < 0.0001; Fig. 1). Pb loads at low doses (10, 40, 50, 75 and 100 μM PbAc) were higher, but not significantly different, from controls. Pb loads of Pb-treated larvae were significantly greater than Pb loads for control-treated larvae when reared on 250 μM PbAc (p < 0.01) and 500 μM PbAc (p < 0.001).
Mature adults reared throughout their entire development (both pre- and post-eclosion) on control or leaded medium exhibited a significant effect of Pb treatment when sexes were combined (p < 0.0001; Fig. 1; Table 1). There was a sex by Pb interaction (p < 0.01), with higher Pb loads in females at both 250 and 500 μM PbAc. Similar to the pattern seen in larvae, adult Pb loads at 10 and 100 μM PbAc were higher than controls, but not significantly different (p > 0.05). At 250 and 500 μM PbAc, mean Pb loads were higher than controls in males (250 μM PbAc: p < 0.05; 500 μM PbAc: p < 0.0001), but Pb loads in females were only significantly different from controls at 500 μM PbAc (p < 0.01).
3.2. Elimination of Pb loads during eclosion
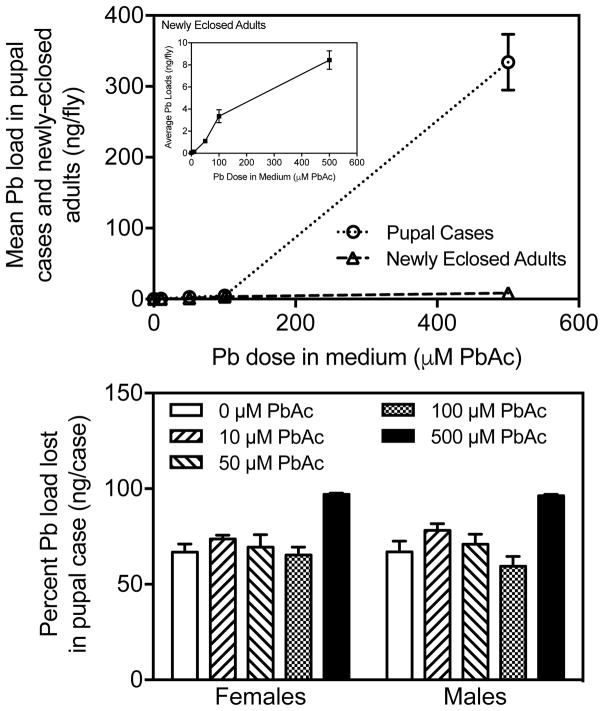
To determine if Pb loads were lost during eclosion, we measured the accumulation of Pb loads in pupal cases and newly eclosed adults during developmental exposure to control or leaded medium (10, 50, 100, or 500 μM PbAc). We found a significant effect (Fig. 2 Top; Table 1) of Pb treatment on Pb loads in pupal cases (p < 0.0001) and newly-eclosed adults (p < 0.0001) but not for sex in either pupal cases or adults. The amount of accumulated Pb in pupal cases from flies reared on 10 μM PbAc (mean 0.46 ng/pupal case ± 0.033 standard error [SE]), 50 μM PbAc (2.77 ± 0.299), and 100 μM PbAc (4.71 ± 0.376) was higher than controls (0.09 ± 0.018). The Pb loads in pupal cases were substantially higher (p < 0.0001) at 500 μM PbAc (334.30 ± 39.43) than all other doses (0, 10, 100 μM PbAc).
Fig. 2.
[Top] Average Pb loads for each dose tested in ng/fly for pupal cases and newly eclosed adults exposed prior to eclosion only. [Bottom] Percent Pb burden lost in pupal case during eclosion for each dose tested. Means ± standard error shown (n = 5–11 females, n = 6–12 males).
Pb loads in newly-eclosed adults remained low regardless of exposure level. There was an overall effect of Pb treatment on Pb loads in newly eclosed adults (p < 0.0001). Pb loads in control adults were significantly lower than Pb loads in both adults reared at 100 μM PbAc (p < 0.0001) and 500 μM PbAc (p < 0.0001).
There was substantially more Pb (at all doses tested) in pupal cases than in the newly-eclosed adults. Approximately 60 to 80% of Pb accumulated pre-eclosion was left behind in the discarded pupal case during eclosion at doses below 500 μM PbAc (Fig. 2 Bottom). At 500 μM PbAc, Pb was more efficiently eliminated during eclosion (97% in females, 96% in males).
3.3. Elimination of Pb loads post-eclosion
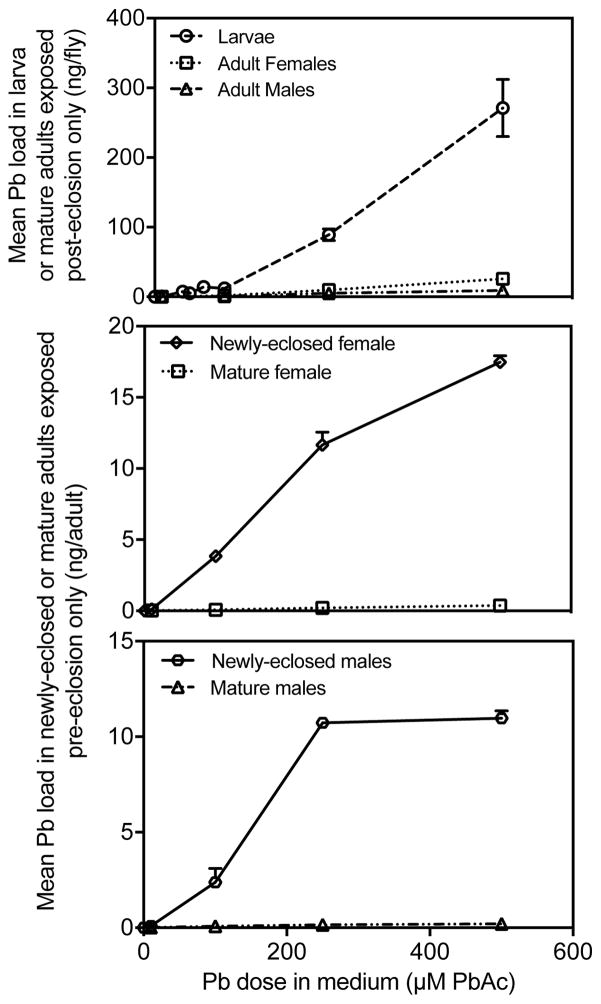
We tested elimination of Pb loads after eclosion in newly-eclosed adults or mature adults with varying developmental exposure (pre-eclosion only, post-eclosion only, or both pre- and post-eclosion) to control or leaded medium (10, 100, 250, or 500 μM PbAc). Pb loads were higher in wandering third-instar larvae (Fig. 3 Top) at all doses tested when compared to mature adults only exposed to Pb as adults (i.e. post-eclosion only). We compared the Pb loads in newly-eclosed adults and mature adults exposed to various doses pre-eclosion only (Fig. 3 Middle and Bottom). Pb loads were higher in newly-eclosed adults than in mature adults (Females: p < 0.0001; Males: p < 0.0001) when exposed prior to eclosion.
Fig. 3.
[Top] Mean Pb loads for each dose tested in ng/fly for wandering third-instar larvae and mature adults exposed post-eclosion only. Means ± standard error shown (n = 8 all larva, n = 4 control-treated adults, n = 6 all Pb-treated adults). [Middle and Bottom] Mean Pb loads in either female [middle] or male [bottom] newly eclosed adults or mature adults exposed pre-eclosion only. Means ± standard error (n = 4 control-treated adults, n = 6 Pb-treated adults).
3.4. Transmission of Pb loads to F1 generation
We tested the intergenerational transmission of Pb to the first generation of offspring by exposing parents (F0) to control or leaded (250 μM PbAc) medium from egg stages to adult d five and rearing offspring (F1 larvae and mature adults) in control medium.
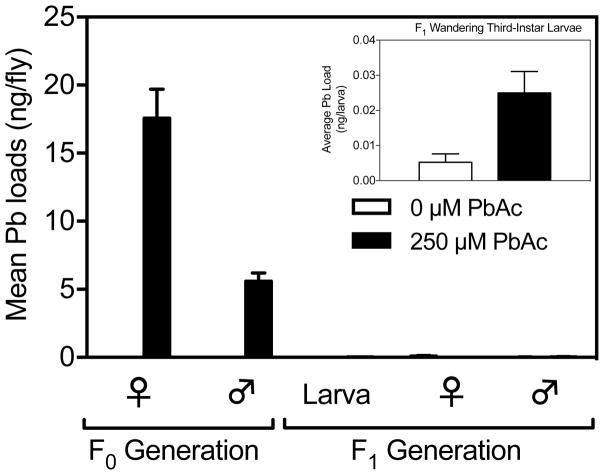
In the parental generation, adult mature males and females were collected and tested for their Pb loads. Both males (p < 0.0001) and females (p < 0.0001) had significantly higher Pb loads when developmentally exposed to 250 μM PbAc (Fig. 4; Table 2). Pb-treated females exhibited significantly greater (p < 0.0001) Pb loads compared to males: leaded females averaged 17.6 ng/female (± 2.12 SE) and males averaged 5.6 ng/male (± 0.61). These results may be reflective of size differences between males and females. Means for control males (0.002 ± 0.0007) and females (0.01 ± 0.004) were significantly different from zero (One-sample t-test: Males p < 0.05, Females p < 0.05). There were no significant replicate effects in males or females in the parental generation.
Fig. 4.
Intergenerational transmission of Pb loads to the first generation of offspring (F1). Means ± standard error (n = 15 F0 adults, n = 9–10 F1 larva, n = 13–14 F1 adults).
Table 2.
Intergenerational transmission of Pb loads from F0 mature adults to F1 larvae and F1 mature adults. Means (ng/fly) ± standard error of mean shown (n = 8 larva, n = 3 control-treated adults, n = 3 Pb-treated adults). Means ± standard error (n = 15 F0 adults, n = 9–10 F1 larva, n = 13–14 F1 adults).
| F0 Generation | F1 Generation | |||||
|---|---|---|---|---|---|---|
| Dose (μM PbAc) | Mature Females | Mature Males | Parental Dose (μM PbAc) | Larva | Mature Females | Mature Males |
| 0 | 0.01 ± 0.004 | 0.002 ± 0.0007 | 0 | 0.005 ± 0.002 | 0.10 ± 0.04 | 0.02 ± 0.004 |
| 250 | 17.57 ± 2.12 | 5.60 ± 0.61 | 250 | 0.02 ± 0.006 | 0.01 ± 0.002 | 0.03 ± 0.02 |
F1 larvae with either control or Pb-treated parents were reared in control medium from egg stages to wandering third-instar larval stage and tested for differences in Pb loads. The amount of Pb transferred to the F1 wandering third-instar larvae with Pb-treated parents (0.025 ± 0.006) was substantially lower than that found in the Pb-treated parents (Females: 17.6 ± 2.12; Males: 5.6 ± 0.61, Fig. 4; Table 2). There was a significant difference in Pb loads due to parental exposure to Pb (p < 0.0001, Fig. 4; Table 2); this is because Pb loads in F1 larvae with Pb-treated parents (0.025 ± 0.006) were higher than F1 larvae with control-treated parents (0.005 ± 0.002). Means for larvae with control-treated parents were not significantly different from zero, whereas means for larvae with Pb-treated parents were significantly higher than zero (p < 0.01). There was an overall effect of replicate for F1 larvae; however, the trend was the same between the replicates in this experiment.
Mature F1 females and males with either control-treated or Pb-treated parents were reared on clean medium from egg stages to adulthood and were tested for their Pb loads. Mean Pb loads in males with Pb-treated parents (0.03 ± 0.02) and females with Pb-treated parents (0.012 ± 0.002) were lower than their leaded parents (Pb-treated mothers: 17.6 ± 2.12; Pb-treated fathers: 5.6 ± 0.61, Fig. 4; Table 2). Pb loads in F1 males with Pb-treated parents and control-treated parents were not significantly different (mean F1 males with control-treated parents: 0.02 ± 0.004).
On the other hand, F1 females with Pb-treated parents had significantly (p < 0.05; Fig. 4; Table 2) lower Pb loads on average (0.012 ± 0.002) than F1 females with control-treated parents (0.103 ± 0.04). This is most likely due to variation in background levels of Pb in the media: four samples of F1 females with control-treated parents exhibited high levels of Pb (0.30, 0.22, 0.45, 0.18 ng/female). If these four samples are omitted from the data set, the mean Pb loads for F1 females with control-treated parents was much lower (0.02 ± 0.007). The difference in Pb loads between F1 females with control-treated parents and F1 females with Pb-treated parents was not significant (p > 0.05) when these samples were removed.
3.5. Genetic variation in Pb loads
We examined variation in Pb loads in males and females from each of 72 recombinant inbred lines (“roo” lines) exposed from egg stages to mature adults to control or leaded medium (250 μM PbAc). Mean Pb load per inbred line for Pb-treated males treated was 46 +/− 1.9 SE and ranged from 9.8 to 107 (p < 0.0001) among lines. Females treated with Pb averaged 92 +/− 3.5 and ranged from 17 to 229 among lines (p < 0.0001). The sex difference was significant (p < 0.0001), but there was no sex by strain interaction. The mean Pb load in control males was 0.1 +/− 0.02, whereas the mean Pb load in control females was 0.05 +/− 0.01 per line. There were no significant strain differences among lines in either sex in the controls. The heritability of Pb load was 0.32 in females, and 0.36 in males; this indicated that approximately 34% of the variation among strains for Pb load is caused by genetic variation among the roo lines.
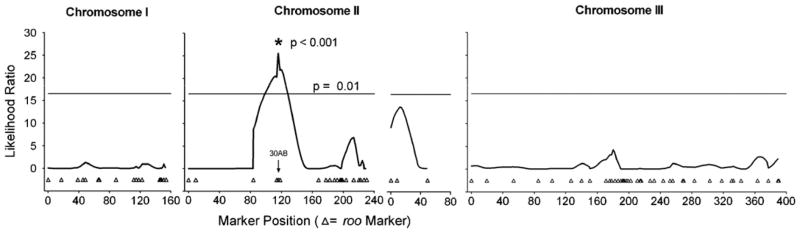
A single common significant QTL for variation in Pb load was observed for both males and females, located on the right arm of Chromosome II, cytological region 2–18 50C (p < 0.0001, Fig. 5). This QTL is responsible for 38% of the genetic variation in Pb load among strains. The heritability accounted for 813 pg2 of the 2,393 pg2 total phenotypic variance, with 309 pg2 of the genetic variance explained by the single QTL on Chromosome II. We searched for potential candidate genes in the 50C region by correlating differences in transcription levels between control- and Pb-treated adults for each line with the mean Pb load (methods described in Ruden et al. [2009] for 88 transcripts assayed in region 50C). We identified two candidate genes (CG6191-RA and CG8118) where strain differences for changes in transcription in response to Pb are significantly correlated with strain differences in Pb load (p < 0.05). CG6191-RA is associated with phagocytosis and regulation of cell division, and CG8118 is associated with Notch locus signaling pathway regulation of the nervous system (Ashburner et al., 2000).
Fig. 5.
Composite Interval Mapping for the Lead Load trait shows a significant QTL (p < 0.001) at approximately cytological region 50B on the left arm of chromosome two. The Y-axis is in Likelihood Ratio units, and the X-axis is in cM, representing the genetic map for chromosomes I, II and III.
4. Discussion
4.1. Pb loads throughout development
We found that D. melanogaster readily accumulated Pb throughout their life cycle, when exposed during development; these results are corroborated by other studies (Akins et al., 1992; Cohn et al., 1992; Hirsch et al., 2003; Massie et al., 1992; Peterson et al., 2017). The level of accumulation was dose-dependent and the amount of accumulation increased dramatically at concentrations above baseline after 100 μM PbAc. These results have important implications for biomagnification of Pb in the food chain, given that predators may feed on larval stages of prey.
The rate of accumulation was much greater in larvae than mature adults exposed to Pb throughout their development. Higher Pb loads in wandering third-instar larvae are the result of elimination of Pb loads both at eclosion and post-eclosion (when reared on clean medium). D. melanogaster appear to shed larval Pb loads by using the pupal case for storage-detoxification. Most of the Pb accumulated in the larval stage was left behind in the pupal case and therefore eliminated during eclosion. The relative effectiveness of elimination via the pupal case was greatest at the highest dose we tested (500 μM PbAc). This indicates that there may be a threshold for efficiency of elimination of Pb during eclosion, dependent upon dose exposure.
Similar metal accumulation of Pb in the exoskeleton has been found in other terrestrial invertebrates (garden snails: Beeby and Richmond, 1989; predatory Carabid beetles: Roberts and Johnson, 1978), but has never been reported (to our knowledge) in D. melanogaster. The treatment of the exoskeleton as a storage-excretion devise to eliminate Pb and other metals is well documented in invertebrates and has been correlated with replacement of calcium in the exoskeleton (Beeby, 1991; Roberts and Johnson, 1978). These results suggest that sequestration of Pb in the D. melanogaster pupal case during eclosion is an effective mechanism of Pb elimination, potentially mediated by its replacement of calcium.
We have found that sequestration of Pb in the pupal case may be effective mechanisms of elimination for individuals exposed to Pb. This mechanism, in combination with elimination of Pb loads post-eclosion through depuration, may explain higher accumulation of Pb loads in larvae than mature adults.
In addition to elimination at eclosion, Pb loads in wandering-third instar larvae are higher than mature adults exposed to Pb post-eclosion only. This shows that larvae accumulate more Pb than adults over the same period: it takes approximately four d for a fly reared in control medium (leaded flies take longer to develop [Cohn et al., 1992]) to reach the wandering-third instar larval stage (Tyler, 2000) and adults were reared on control or leaded medium for five d post-eclosion. In addition, adults appear capable of eliminating Pb post-eclosion.
Mature adults treated with 500 μM PbAc reared on clean medium post-eclosion had significantly lower Pb load when compared to newly-eclosed adults. This indicates that there is also a process of elimination occurring post-eclosion, given the opportunity to live in control medium. These results are consistent with findings showing that Orchesella cincta (Hairy-back Girdled Springtail) eliminate much of their Pb loads when placed on control medium (van Straalen and van Meerendonk, 1987).
Elimination of Pb loads post-eclosion may occur via sequestration of Pb in the intestinal tract. The Drosophila digestive tract contains several digestive tissues that are vital for nutritional physiology (Beeby, 1991; Dallinger, 1993; Lemaitre and Miguel-Aliaga, 2013). These organs “form a selective barrier” (Lemaitre and Miguel-Aliaga, 2013) to eliminate toxins and pathogens for metal accumulation and storage detoxification (Beeby, 1991; Dallinger, 1993; Lemaitre and Miguel-Aliaga, 2013). Wilson (2004) tested tissue sequestration in the digestive tract in both wandering third-instar larvae and mature adults. Larvae reared on leaded medium (100 or 500 μM PbAc) had proportionally higher Pb loads in their digestive tissues located in the abdomen compared to the abdomen of mature adults (Wilson, 2004). Larvae consume solid food (whereas adults ingest liquid) and have higher feeding rates than adults to optimize growth; this increases the amount of food transported to the midgut (Lemaitre and Miguel-Aliaga, 2013; Shanbhag and Tripathi, 2009). Therefore, higher Pb loads in larvae compared to mature adults (Wilson, 2004) may result from a higher proportion of leaded medium present in the digestive system of the larvae. In addition, sequestration of Pb to the digestive system in both larvae and adults (Wilson, 2004) may indicate active elimination of Pb via the digestive system, as well as storage-detoxification (Dallinger, 1993).
4.2. Transmission of Pb loads to F1 generation
We examined intergenerational transmission of Pb loads to the F1 generation. F0 mature adults exposed to leaded medium accumulated Pb throughout their development; this pattern is consistent with results shown in Fig. 1 and previous studies (Hirsch et al., 2003; Peterson et al., 2017). The mean Pb load for F0 females (17.57 ng/female ± 2.12 SE; Fig. 4; Table 2) was slightly smaller in comparison to results shown in Fig. 1 and Table 1 (25.06 ng/female ± 4.72 SE). This is most likely due to a difference in methods between the two experiments: in this experiment, adults were transferred to clean medium for 24 h prior to collection to allow the adults to groom excess Pb off their bodies. Therefore, slightly lower Pb loads in adults in this experiment may be due to Pb having been eliminated over the 24 h grooming period.
We found very low, but detectable Pb loads in the F1 wandering third-larvae. This indicates that some of the Pb accumulated in the parental female may be passed down to her offspring, presumably via the egg, as seen by low but detectable levels of Pb in the F1 larvae with Pb-treated parents.
Pb loads in F1 mature adults with Pb-treated parents were not significantly different from Pb loads in F1 mature adults with control-treated parents; this is consistent with a previous study (Peterson et al., 2017). We can conclude that any remaining Pb in the F1 larvae was eliminated by the time that offspring were reproductively viable as mature adults. Likely, Pb was eliminated in F1 offspring via mechanisms like those observed in previous experiments (i.e. at eclosion via the pupal case and rearing on clean medium after eclosion). Therefore, there was no significant intergenerational transmission of Pb to the first generation of mature offspring.
Few studies have explored transgenerational transmission (i.e. the effects of F0 exposure to environmental factors in the F3 generation, if the F1–F3 generations are not exposed [Skinner, 2008]) of Pb loads to subsequent generations of offspring; this is important given worldwide efforts to eliminate Pb pollution. Sen et al. (2015) found that maternal Pb exposure alters DNA methylation levels in neonatal blood. These results (Sen et al., A2015) suggest that Pb exposure may disrupt DNA methylation in fetal germ cells, which can, in turn, induce transgenerational epigenetic effects. Therefore, Pb exposure has the potential to cause long-term, multigenerational deformities in the absence of Pb pollution. Given that it is challenging to determine transgenerational epigenetic effects in humans or wildlife, D. melanogaster is a useful model to further delineate transgenerational epigenetic effects since Pb was eliminated by the first generation of mature adult offspring.
4.3. Genetic variation in Pb loads
Genetic variation significantly altered adult Pb load in response to developmental Pb exposure in D. melanogaster. A QTL on Chromosome II was a significant source of genetic variation affecting adult Pb accumulation, accounting for approximately one third of the genetic variation detected. Inbred lines used in this study accumulated, on average, substantially more Pb than the wild type strains tested here.
The homology between Drosophila and mammalian genomes (Mackay and Anholt, 2006) suggests that further analysis of such genetic variation may provide useful insights into mechanisms and risk for Pb loads in humans. It is notable that we observed no significant correlation among recombinant inbred lines between mean Pb load and Pb-induced changes in locomotor behavior (Hirsch et al., 2009). In addition, the major QTL in these lines mediating Pb-induced changes in locomotor behavior had no detectable effect on Pb loads. These observations suggest that mechanisms mediating accumulation of Pb are different from those underlying the effects of Pb on behavior. It is possible that the strains reached a threshold level for Pb-induced changes in locomotor activity regardless of their Pb load. This pattern suggests that BLLs in humans may be a good measure of prior exposure, but may not be as good a predictor of behavioral effects.
To our knowledge, this is the first evidence indicating genetic variation in Pb loads and identification of a specific region of the genome mediating genetic variation for this trait.
5. Conclusion
In conclusion, we have found that larvae accumulate substantially more Pb than adults, Pb is eliminated via the pupal case at eclosion, adults continue to eliminate Pb post-eclosion, and Pb loads are not transferred to the first generation of mature adult offspring. In addition, we found that genetic variation accounted for 34% of the variance in Pb load. Together these mechanisms may serve to minimize Pb exposure in the brain and to offspring, especially during the first d of adult life when experience plays an important role in the development of complex behaviors (Hirsch et al., 2003). The Drosophila model facilitates extending behavioral observations to include genetic (Hirsch et al., 2009; Ruden et al., 2009) and neural mechanisms (He et al., 2009; Morley et al., 2003) mediating these effects, as well as transgenerational effects of anthropogenic Pb.
HIGHLIGHTS.
D. melanogaster readily accumulated Pb throughout their lifespan and the levels of accumulation increased with Pb exposure in the medium.
Wandering third-instar larvae accumulated more Pb than mature adults.
The accumulated Pb in mature adults was not transferred to F1 mature adult offspring.
We identified a quantitative trait locus for adult Pb loads and found that genetic variation accounted for 34% of the variance in Pb load.
Acknowledgments
This work was supported by National Institute for Environmental Health Science (NIEHS) Grant (R01 ES012933), a WSU-NIEHS Center Grant (P30 ES020957), and an Income Fund Reimbursable Account sponsored by Dr. Helmut V.B. Hirsch and Dr. Helen Ghiradella. We thank the National Science Foundation for supporting instrumentation used for Pb analyses (Grant DUE-9952410, to Union College). We thank Dr. Helmut V.B. Hirsch and Dr. Helen Ghiradella (Department of Biological Sciences, University at Albany-State University of New York) for summer financial support for the first author. We would like to thank Dr. Gregory Lnenicka for reviewing the manuscript and providing invaluable feedback. We also thank Dr. Joanne Kehlbeck (Department of Chemistry, Union College), Dr. David Lawrence (Department of Environmental Health Sciences, University at Albany-State University of New York) and Dr. Robert Osuna (Department of Biological Sciences, University at Albany-State University of New York) for their advice and support, and Naomi Lima (Department of Biological Sciences, University at Albany-State University of New York) for her assistance in the laboratory. The authors have no conflicts of interest to report.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abolaji AO, Kamdem JP, Farombi EO, Rocha JBT. Drosophila melanogaster as a promising model organism in toxicological studies. Arch Bas App Med. 2013;1:33–38. [Google Scholar]
- Akins JM, Schroeder JA, Brower DL, Vasken Aposhian H. Evaluation of Drosophila melanogaster as an alternative animal model for studying the neurotoxicity of heavy metals. BioMet. 1992;5:111–120. doi: 10.1007/BF01062222. [DOI] [PubMed] [Google Scholar]
- Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium Nat Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeby A, Richmond L. The shell as a site of lead deposition in the snail Helix aspersa. Arch Environ Contam Toxicol. 1989;18:623–628. [Google Scholar]
- Beeby A. Toxic metal uptake and essential metal regulation in terrestrial invertebrates: A review. In: Newman MC, McIntosh AW, editors. Metal Exotoxicology Concepts & Applications. 1991. pp. 65–89. [Google Scholar]
- Burke MK, Rose MR. Experimental evolution with Drosophila. Am J Physiol Regul Integr Comp Physiol. 2009;296:1847–1854. doi: 10.1152/ajpregu.90551.2008. [DOI] [PubMed] [Google Scholar]
- Clark HF, Hausladen DM, Brabander DJ. Urban gardens: lead exposure, recontamination mechanisms, and implications for remediation design. Environ Res. 2008;107:312–319. doi: 10.1016/j.envres.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Cohn J, Widzowski DV, Cory-Slechta DA. Lead retards development of Drosophila melanogaster. Comp Biochem Physiol. 1992;102C:45–49. doi: 10.1016/0742-8413(92)90041-5. [DOI] [PubMed] [Google Scholar]
- Dallinger R. Strategies of metal detoxification in terrestrial invertebrates. In: Dallinger R, Rainbow S, editors. Ecotoxicology of Metals in Invertebrates. 1993. pp. 245–289. [Google Scholar]
- Falk H. International environmental health for the pediatrician: Case study of lead poisoning. Pediat. 2003;112:259–264. [PubMed] [Google Scholar]
- Gilbert SG, Weiss B. A rationale for lowering the blood lead action level from 10 to 2 μg/dL. NeuroTox. 2006;27:693–701. doi: 10.1016/j.neuro.2006.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurganus MC, Fry JD, Nuzhdin SV, Pasyukova EG, Lyman RF, Mackay TF. Genotype environment interaction at quantitative trait loci affecting sensory bristle number in Drosophila melanogaster. Genet. 1998;149:1883–98. doi: 10.1093/genetics/149.4.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He T, Hirsch HVB, Ruden DM, Lnenicka GA. Chronic lead exposure alters presynaptic calcium regulation and synaptic facilitation in Drosophila larvae. Neurotox. 2009;30:777–784. doi: 10.1016/j.neuro.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegmann JP, Possidente B. Estimating genetic correlations from inbred strains. Behav Genet. 1981;11:103–114. doi: 10.1007/BF01065621. [DOI] [PubMed] [Google Scholar]
- Hirsch HVB, Mercer J, Sambaziotis H, Huber M, Stark DT, Torno-Morley T, Hollocher K, Ghiradella H, Ruden DM. Behavioral effects of chronic exposure to low levels of lead in Drosophila melanogaster. Neurotox. 2003;24:435–442. doi: 10.1016/S0161-813X(03)00021-4. [DOI] [PubMed] [Google Scholar]
- Hirsch HVB, Possidente D, Averill S, Palmetto Despain T, Buytkins J, Thomas V, Goebel WP, Shipp-Hilts A, Wilson D, Hollocher K, Possidente B, Lnenicka G, Ruden DM. Variations at a quantitative trait locus (QTL) affect development of behavior in lead-exposed Drosophila melanogaster. NeuroTox. 2009;30:305–311. doi: 10.1016/j.neuro.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo X, Peng L, Xu X, Zheng L, Qiu B, Qi Z, Zhang B, Han D, Piao Z. Elevated blood lead levels of children in Guiyu, an electronic waste recycling town in China. Environ Health Perspect. 2007;115:1113–1117. doi: 10.1289/ehp.9697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RL, Homa DM, Meyer PA, Brody DJ, Caldwell KL, Pirkle JL, Brown MJ. Trends in blood lead levels and blood lead testing among US children aged 1 to 5 years, 1988–2004. Pediat. 2009;123:e376–e385. doi: 10.1542/peds.2007-3608. [DOI] [PubMed] [Google Scholar]
- Kaiser R, Henderson AK, Daley WR, Naughton M, Khan MH, Rahman M, Kieszak S, Rubin CH. Blood lead levels of primary school children in Dhaka, Bangladesh. Environ Health Perspect. 2001;109:563–566. doi: 10.1289/ehp.01109563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landrigan PJ, Gehlback SH, Rosenblum BF, Shoults JM, Candelaria RM, Barthel WF, Liddle JA, Smrek AL, Staehling NW, Sanders JDF. Epidemic lead absorption near an ore smelter: the role of particulate lead. New Eng J Med. 1975;292:123–129. doi: 10.1056/NEJM197501162920302. [DOI] [PubMed] [Google Scholar]
- Landrigan PJ, Baker EL. Exposure of children to heavy metals from smelters-epidemiology and toxic consequences. Environ Res. 1981;25:204–224. doi: 10.1016/0013-9351(81)90090-6. [DOI] [PubMed] [Google Scholar]
- Lemaitre B, Miguel-Aliaga I. The digestive tract of Drosophila melanogaster. Annu Rev Genomics Hum Genet. 2013;47:377–404. doi: 10.1146/annurev-genet-111212-133343. [DOI] [PubMed] [Google Scholar]
- Lin GZ, Peng RF, Chen Q, Wu ZG, Du L. Lead in housing paints: an exposure source still not taken seriously for children lead poisoning in China. Environ Res. 2009;109:1–5. doi: 10.1016/j.envres.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Mackay TFC, Anholt RRH. Of flies and man: Drosophila as a model for human complex traits. Annu Rev Genomics Hum Genet. 2006;7:339–367. doi: 10.1146/annurev.genom.7.080505.115758. [DOI] [PubMed] [Google Scholar]
- Massie HR, Aiello VR, Whitney SJP. Lead accumulation during aging of Drosophila and effect of dietary lead on life span. Age. 1992;15:47–49. [Google Scholar]
- Mielke HW, Reagan PL. Soil is an important pathway of human lead exposure. Environ Health Perspect. 1998;106:217–229. doi: 10.1289/ehp.98106s1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley EJ, Hirsch HVB, Hollocher K, Lnenicka GA. Effects of chronic lead exposure on the neuromuscular junction in Drosophila larvae. NeuroTox. 2003;24:35–41. doi: 10.1016/s0161-813x(02)00095-5. [DOI] [PubMed] [Google Scholar]
- Pandey UB, Nichols CD. Human disease models in Drosophila melanogaster and the role of the fly in therapeutic drug discovery. Pharmacol Rev. 2011;63:411–436. doi: 10.1124/pr.110.003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson EK, Yukilevich R, Kehlbeck J, LaRue KM, Ferraiolo K, Hollocher K, Hirsch HVB, Possidente B. Asymmetrical positive assortative mating induced by developmental lead (Pb2+) exposure in a model system, Drosophila melanogaster. Current Zoology. 2017 doi: 10.1093/cz/zox016. https://doi.org/10.1093/cz/zox016. [DOI] [PMC free article] [PubMed]
- Rand MD. Drosophotoxicology: The growing potential for Drosophila in neurotoxicology. Neurotoxicol Teratol. 2010;32:74. doi: 10.1016/j.ntt.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand MD, Montgomery SL, Prince L, Vorojeikina D. Developmental toxicity assays using the Drosophila model. Curr Protoc Toxicol. 2014;59:1.12.1–1.12.20. doi: 10.1002/0471140856.tx0112s59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RD, Johnson MS. Dispersal of heavy metals from abandoned mine workings and their transference through terrestrial food chains. Environ Pollut. 1978;16:293–310. [Google Scholar]
- Rubin GM, Yandell MD, Wortman JR, Gabor Miklos GL, Nelson CR, Hariharan IK, Fortini ME, Li PW, Apweiler R, Fleischmann W, Cherry JM, Henikoff S, Skupski MP, Misra S, Ashburner M, Birney E, Boguski MS, Brody T, Brokstein P, Celniker SE, Chervitz SA, Coates D, Cravchik A, Gabrielian A, Galle RF, Gelbart WM, George RA, Goldstein LSB, Gong F, Guan P, Harris NL, Hay BA, Hoskins RA, Li J, Li Z, Hynes RO, Jones SJM, Kuehl PM, Lemaitre B, Troy Littleton J, Morrison DK, Mungall C, O’Farrell PH, Pickeral OK, Shue C, Vosshall LB, Zhang J, Zhao Q, Zheng XH, Zhong F, Zhong W, Gibbs R, Venter JC, Adams MD, Lewis S. Comparative genomics of the eukaryotes. Sci. 2000;287:2204–2215. doi: 10.1126/science.287.5461.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio-Franchini I, Rico-Martinez R. Evidence of lead biomagnification in invertebrate predators from laboratory and field experiments. Environ Poll. 2011;159:1831–1835. doi: 10.1016/j.envpol.2011.03.021. [DOI] [PubMed] [Google Scholar]
- Ruden DM, Chen L, Possidente D, Possidente B, Rasouli P, Wang L, Lu X, Garfinkel MD, Hirsch HVB, Page GP. Genetical toxicogenomics in Drosophila identifies master-modulatory loci that are regulated by developmental exposure to lead. NeuroTox. 2009;30:898–914. doi: 10.1016/j.neuro.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen A, Heredia N, Senut MC, Land S, Hollocher K, Lu X, Dereski MO, Ruden DM. Multigenerational epigenetic inheritance in humans: DNA methylation changes associated with maternal exposure to lead can be transmitted to the grandchildren. Nat Sci Rep. 2015;5:14466. doi: 10.1038/srep14466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanbhag S, Tripathi S. Epithelial ultrastructure and cellular mechanisms of acid and base transport in the Drosophila midgut. J Exper Biol. 2009;212:1731–1744. doi: 10.1242/jeb.029306. [DOI] [PubMed] [Google Scholar]
- Skinner MK. What is an epigenetic transgenerational phenotype? F3 or F2. Reprod Toxicol. 2008;25:2–6. doi: 10.1016/j.reprotox.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler MS. Development of the fruit fly Drosophila melanogaster. In: Tyler MS, editor. Developmental Biology, a Guide for Experimental Study. 2000. pp. 8-1–8-27. [Google Scholar]
- Wilson DT. Doctoral Dissertation. State University of New York; Albany, Albany NY: 2004. The Development of Drosophila as an Animal Model for Studying the Behavioral Genetics of Lead Toxicology. [Google Scholar]
- van Straalen NM, van Meerendonk JH. Biological half-lives of lead in Orchesella cincta (L.) (Collembola) Bull Environ Contam Toxicol. 1987;38:213–219. doi: 10.1007/BF01606664. [DOI] [PubMed] [Google Scholar]