SUMMARY
Autophagy degrades cytoplasmic components and is important for development and human health. Although autophagy is known to be influenced by systemic intercellular signals, the proteins that control autophagy are largely thought to function within individual cells. Here we report that Drosophila Macroglobulin complement-related (Mcr), a complement orthologue, plays an essential role during developmental cell death and inflammation by influencing autophagy in neighboring cells. This function of Mcr involves the immune receptor Draper, suggesting a relationship between autophagy and the control of inflammation. Interestingly, Mcr function in epithelial cells is required for macrophage autophagy and migration to epithelial wounds, a Draper-dependent process. This study reveals, unexpectedly, that complement-related from one cell regulates autophagy in neighboring cells via an ancient immune signaling program.
Keywords: autophagy, programmed cell death, complement, immune receptor signaling
Etoc
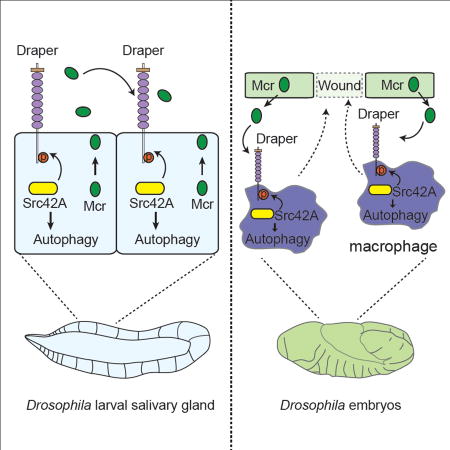
A complement orthologue in Drosophila plays an essential role during developmental cell death and inflammation by influencing autophagy in neighboring cells.
INTRODUCTION
Autophagy is a conserved process that cells use to degrade their own cytoplasmic components by delivery to lysosomes (Mizushima and Komatsu, 2011). Autophagy ensures intracellular quality control and is associated with diseases such as cancer, immune disorders and neurodegeneration (Mizushima et al., 2008). Most autophagy studies have focused on cell survival under nutrient limiting conditions, but it has also been associated with cell death (Baehrecke, 2005). The Drosophila larval salivary gland undergoes steroid-triggered cell death during development and is an excellent model to study autophagy in dying cells. Both autophagy (Atg) genes and caspases are required for larval salivary gland degradation (Berry and Baehrecke, 2007), but how autophagy is regulated during cell death is poorly understood.
Macroautophagy (hereafter autophagy) involves the recruitment of cytoplasmic cargoes to forming double membrane vesicles known as autophagosomes (Mizushima and Komatsu, 2011). Autophagosomes fuse with lysosomes to form autolysosomes where cargoes are degraded. Each step in this vesicle trafficking process is controlled by core Atg proteins that are conserved from yeast to humans (Mizushima and Komatsu, 2011). Most Atg proteins and their regulators were identified through pioneering studies of the single cell yeast Saccharomyces cerevisiae (Harding et al., 1995; Thumm et al., 1994; Tsukada and Ohsumi, 1993), and little is known about systemic factors that signal between different cells to control autophagy within the bodies of multicellular animals.
Autophagy appears to be influenced by systemic body-wide signals, but the proteins that control autophagy are largely thought to function within individual cells. For example, nutrient deprivation was shown to induce autophagy in both flies and mice (Kuma et al., 2004; Scott et al., 2004). Animal starvation leads to decreased growth factors and insulin-like peptide levels, and it is assumed that this decrease in insulin is the systemic signal that leads to increased autophagy because of the well-known role for the class I PI3-Kinase and mTOR nutrient sensing pathways that are both downstream of insulin and inhibit autophagy (Arico et al., 2001; Scott et al., 2004). Secreted tumor necrosis factor (TNF) is also thought to stimulate autophagy during infection (Levine et al., 2011). However, the best evidence for TNF-regulated autophagy in animals is based on the use of blocking antibodies in the context of tissue damage and infection (Cadwell et al., 2010), and genetic evidence in support of TNF serving as a systemic regulator of autophagy in animals is lacking. The most direct evidence for a cell non-autonomous signal that regulates autophagy comes from studies of pathogen-stimulated autophagy in host cells, where pathogen and host receptor interactions triggered an Atg-dependent phagocytic program (Joubert et al., 2009; Sanjuan et al., 2007). Although extracellular metabolites have been shown to influence autophagy (Eng et al., 2010), little is known about how proteins from one cell activate autophagy in a different cell within an animal.
The complement system is best known as a regulator of inflammation and immune clearance of pathogens (Janeway et al., 2001). Complement proteins exist as inactive protease zymogens that become activated to opsonize pathogens to facilitate clearance by engulfment, and these factors are conserved from invertebrate organisms to humans (Williams and Baxter, 2014). Recent work suggests more diverse roles for complement, including roles in male fertility in mosquitoes (Pompon and Levashina, 2015) and in synapse pruning and a model of Alzheimer’s disease in mice (Hong et al., 2016; Stevens et al., 2007). Therefore, complement may possess diverse biological functions within animals.
The complement related mcr gene was identified as a factor that is required for phagocytosis of the fungal pathogen Candida albicans in Drosophila (Stroschein-Stevenson et al., 2006). Here we show that mcr is necessary for autophagy but not caspase activity during Drosophila salivary gland degradation during development. Unlike most known regulators of autophagy, mcr functions in a cell non-autonomous manner to regulate autophagy in neighboring cells within the dying salivary gland. Interestingly, mcr appears to function upstream of the conserved immune receptor Draper, a factor that functions in a cell autonomous manner to regulate autophagy in dying salivary gland cells. Surprisingly, mcr does not influence either nutrient deprivation-induced autophagy in the fat body or developmentally programmed autophagy in the dying midgut of Drosophila. Rather, mcr is required for autophagy in embryonic macrophages where Draper is known to be required for an inflammatory response to epithelial wounds. Remarkably, this requirement for mcr is in the embryonic epidermis, indicating that this complement related molecule also functions in a novel cell non-autonomous manner to regulate autophagy and migration to wounds. Moreover, the addition of recombinant Mcr protein to an embryonic macrophage-derived cell line is sufficient to induce autophagy that depends on draper and multiple Atg genes. These studies reveal an unexpected role for complement in the regulation of autophagy in neighboring cells that depends on an ancient immune signaling program.
RESULTS
mcr functions in a caspase-independent manner during salivary gland cell death
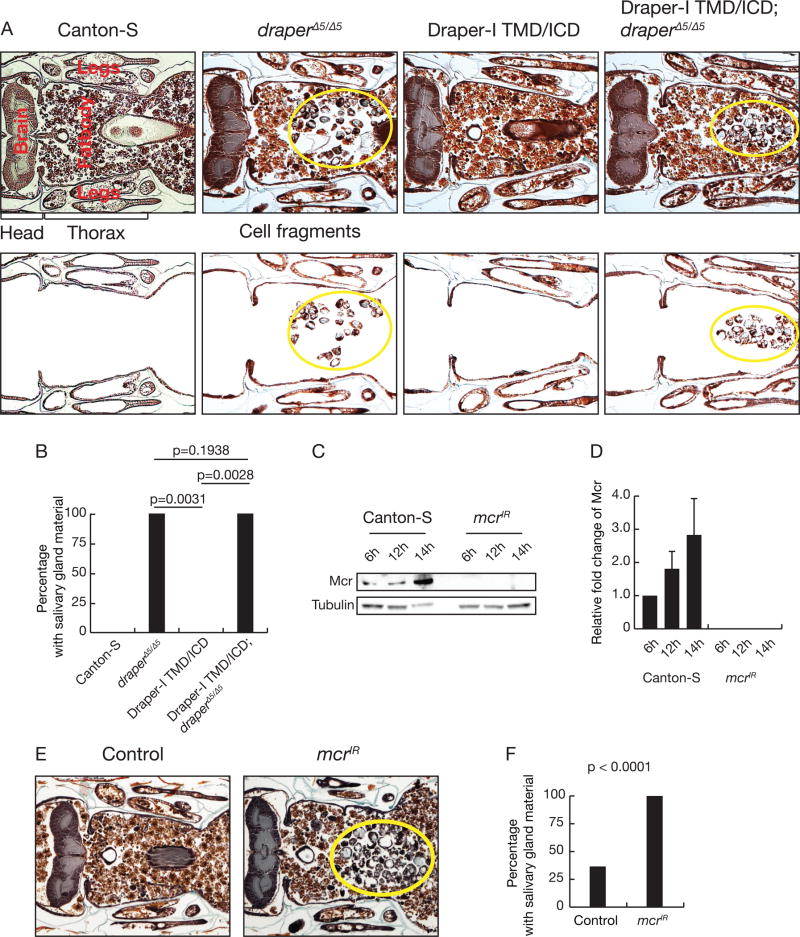
The immune receptor Draper is required for autophagy during salivary gland degradation where it functions upstream of Atg genes (McPhee et al., 2010). This suggests that the Draper receptor, which mediates phagocyte recognition of dying cells (Freeman et al., 2003; MacDonald et al., 2006), may sense an extracellular signal to control autophagy within dying cells. To test this model, we investigated the role of the Draper extracellular domain. We found that the extracellular domain of Draper was required for salivary gland degradation (Figures 1A and 1B). Significantly, loss of the only reported Draper ligand, Pretaporter (Prtp) (Kuraishi et al., 2009), that is expressed in salivary glands did not affect salivary gland degradation (Figures S1A–1C), suggesting an unknown extracellular factor activates Draper-dependent autophagy.
Figure 1. The Draper-I extracellular domain and Mcr are required for salivary gland cell degradation.
(A) Wild-type Canton-S animals (n = 20), draper null mutant animals (n = 22), animals that express salivary gland-specific expression of Drpr-I lacking the extracellular domain (n = 8), and drpr null mutants with salivary gland-specific expression of Drpr-I lacking the extracellular domain (n = 26) analyzed by histology for the presence of salivary gland material (yellow circles) 24h after puparium formation. The images on the bottom emphasize salivary gland cellular fragments with other tissues removed.
(B) Quantification of data from (A). Statistical significance: Chi-square test.
(C) Western blot analyses of Mcr and Tubulin 6h, 12h, and 14h after puparium formation in salivary gland extracts from control and mcr knockdown animals.
(D) Quantification of data from (C). All samples are normalized to Tubulin and plotted relative to their respective 6h samples. Error bars, mean ± SEM; n=3.
(E) Control (n = 27) and salivary gland-specific mcr knockdown animals (n = 24) analyzed by histology for the presence of salivary gland material (yellow circles) at 24h after puparium formation.
(F) Quantification of data from (E). Statistical significance: Chi-square test.
See also Figure S1.
Mcr, a conserved complement-related protein (Williams and Baxter, 2014), is expressed in salivary glands and increases following the rise in steroid that triggers cell death 12 hours after puparium formation (Figures 1C and 1D). This prompted us to consider if mcr is required for larval salivary gland degradation. We screened for persistence of salivary gland material 8 hours after this tissue is normally degraded (Jiang et al., 1997; Lee and Baehrecke, 2001), at 24 hours after puparium formation. We expressed an upstream activating sequence (UAS)-promoted double-stranded inverse repeat construct designed to target mcr (UAS-mcrIR) with the salivary gland-specific fkh-Gal4 driver, which depleted Mcr protein levels in salivary glands (Figures 1C and 1D). Significantly more mcrIR-expressing animals possessed persistent salivary gland cell fragments compared to control animals (Figures 1E and 1F). Targeting a different region of mcr also impaired salivary gland degradation compared to controls (Figures S1D and 1E).
Mcr binds to the surface of the fungus Candida albicans to promote phagocytosis and clearance (Stroschein-Stevenson et al., 2006). Therefore, Mcr could also function in phagocytic blood cells to mediate salivary gland degradation. However, driving UAS-mcrIR expression in blood cells did not lead to a defect in salivary gland clearance (Figures S2A and S2B). Salivary gland destruction requires cell growth arrest and an increase in the steroid hormone 20-hydroxyecdysone (Berry and Baehrecke, 2007; Jiang et al., 1997). Experiments with a cell growth reporter indicated that the salivary gland degradation defect in mcr knockdown animals was not due to a failure in cell growth arrest (Figures S2C). In addition, reduced mcr function failed to alter the steroid response factors EcR and BR-C (Figures S2D–G). These data indicate that mcr is required for larval salivary gland clearance, does not alter either cell growth or steroid signaling, and functions tissue-autonomously in salivary glands during degradation.
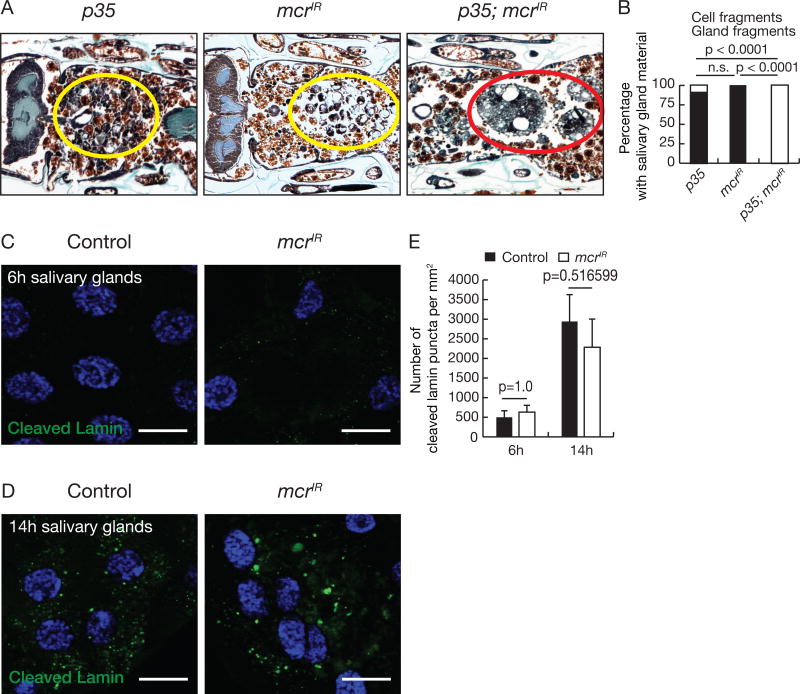
Caspases and autophagy function additively in the clearance of larval salivary glands (Berry and Baehrecke, 2007). Inhibition of caspases leads to the persistence of condensed cellular fragments, while blocking autophagy results in the persistence of vacuolated cell fragments. Simultaneous blockade of both caspases and autophagy leads to robust morphological preservation of large salivary gland fragments. We found that while salivary gland-specific expression of the caspase inhibitor p35 led to the persistence of condensed cell fragments, mcrIR expression led to the presence of vacuolated cell fragments (Figures 2A and 2B), similar to autophagy mutant phenotypes. In addition, simultaneous expression of both p35 and mcrIR in salivary glands led to persistence of multi-cell salivary gland tissue fragments (Figures 2A and 2B), indicating that Mcr functions additively with caspases. Furthermore, similar to autophagy mutants, mcr knockdown in salivary glands did not influence the degradation of the caspase substrate nuclear Lamin (Martin and Baehrecke, 2004) (Figures 2C–E). Combined, these results indicate that Mcr does not influence caspases, and suggests that Mcr signals in the autophagy branch of salivary gland degradative pathways.
Figure 2. mcr functions in an additive manner with caspases during salivary gland degradation.
(A) Animals with salivary gland-specific p35 expression (n = 16), mcr knockdown (n = 24), and p35 expression plus mcr knockdown (n = 25) analyzed by histology for the presence of salivary gland material at 24h after puparium formation. Yellow circles, cell fragments; red circle, salivary gland fragments.
(B) Quantification of data from (A). Statistical significance: Chi-square test.
(C and D) Salivary glands dissected 6h (C) and 14h after puparium formation (D) from control animals and those with salivary gland-specific mcr knockdown, stained with anti-cleaved Lamin antibody (green) and Hoechst (blue). Scale bars, 50 µm.
(E) Quantification of data from (C and D). Error bars, mean ± SEM; n = 12 (control 6h), n = 10 (control 14h), n = 10 (mcrIR 6h), n = 14 (mcrIR 6h). Statistical significance: Student’s t-test.
See also Figure S2.
mcr is necessary for autophagy in neighboring cells during salivary gland cell death
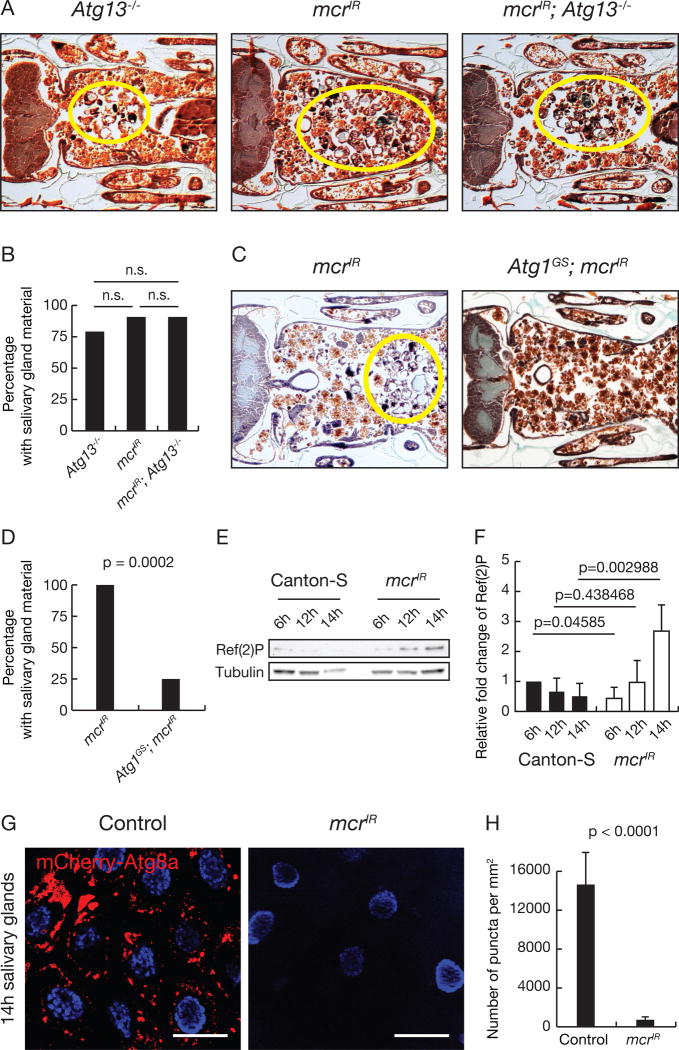
We next tested whether an Atg gene mutant enhances the mcrIR salivary gland persistence phenotype. Animals with either knockdown of mcr, Atg13−/− mutant animals, or knockdown of mcr in Atg13−/− mutant animals all possess similar salivary gland cell fragment phenotypes (Figures 3A and 3B), consistent with mcr functioning in the autophagy pathway. Atg1 misLin et al. expression is sufficient to induce autophagy in salivary glands and other tissues (Berry and Baehrecke, 2007; Chang et al., 2013; Scott et al., 2007), and suppressed the salivary gland clearance defect caused by reduced mcr function (Figures 3C and 3D). These data indicate that Mcr regulates autophagy and functions upstream of Atg1.
Figure 3. Mcr regulates autophagy during salivary gland cell degradation.
(A) Atg13 null mutants (n = 14), salivary gland-specific mcr knockdown (n = 21), and Atg13 null mutants with salivary gland-specific mcr knockdown (n = 11), analyzed by histology for the presence of salivary gland material (yellow circles) at 24h after puparium formation.
(B) Quantification of data from (A). Statistical significance: Chi-square test.
(C) Animals with salivary gland-specific mcr knockdown (n = 24) and those with salivary gland-specific mcr knockdown expressing Atg1 (n = 24) analyzed by histology for salivary gland material (yellow circle) at 24h after puparium formation.
(D) Quantification of data from (C). Statistical significance: Chi-square test.
(E) Western blot analysis of Ref(2)P and Tubulin 6h, 12h, and 14h after puparium formation in salivary gland extracts from control and salivary gland-specific mcr knockdown animals.
(F) Quantification of data from (E). Ref(2)P in control and mcrIR samples normalized to Tubulin and plotted relative to their respective 6h sample levels. Error bars, mean ± SEM; n=3. Statistical significance: Student’s t-test.
(G) mCherry-Atg8a puncta in control salivary glands (n = 18) and salivary gland with tissue-specific mcr knockdown (n = 24). Scale bars, 50 µm.
(H) Quantification of data from (G). Error bars, mean ± SEM. Statistical significance: Student’s t-test.
We then asked whether mcr influences autophagy markers. The cargo receptor Ref(2)P (p62 in mammals) is degraded by autophagy (Nezis et al., 2008), and we analyzed if mcr loss influences Ref(2)P levels during salivary gland degradation. Unlike wild-type animals, which have decreased Ref(2)P levels as salivary glands activate autophagy prior to cell death, mcr knockdown in salivary glands resulted in Ref(2)P accumulation (Figures 3E and 3F). We expressed mcrIR in all salivary gland cells and analyzed mCherry-Atg8a autophagy reporter activity (Denton et al., 2012). Consistent with a role for Mcr in autophagy, we found that salivary glands that express mcrIR possessed significantly fewer mCherry-Atg8a puncta compared to controls (Figures 3G and 3H). These data indicate that Mcr is required for autophagy in salivary gland cells.
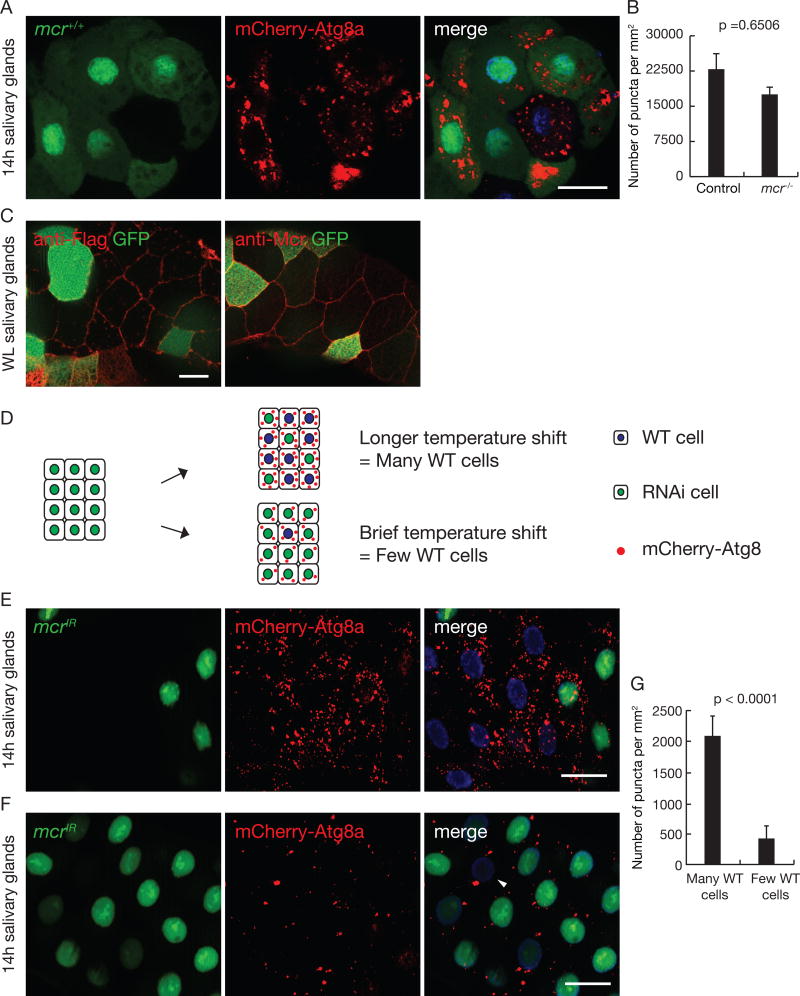
Although extracellular signaling molecules have been suggested to regulate autophagy, genetic evidence in support of such an extracellular signal is limited. Given that Mcr appears to be a secreted protein (Stroschein-Stevenson et al., 2006), it could regulate autophagy activity in neighboring cells in a non-autonomous manner. To test this hypothesis, we produced mosaic salivary glands with mcr mutant cell clones and compared mCherry-Atg8a autophagy reporter puncta formation between wild-type control (that express GFP) and mcr mutant cells (lacking GFP). To our surprise, unlike Atg gene mutants and other known autophagy regulators, including Draper, which function in a cell autonomous manner, mCherry-Atg8a puncta were present in both mcr mutant cells and neighboring control cells (Figures 4A and 4B). Similar results were obtained with mcr knockdown (Figure S3A). Consistent with these data, we detected Flag-tagged Mcr protein multiple cell distances away from cells where it was expressed in a pattern that is similar to endogenous Mcr (Figure 4C), and similar immune reactivity was not detected in salivary glands lacking Flag-tagged Mcr (Figure S3B). These data support a model whereby secreted Mcr functions in a cell non-autonomous manner to regulate autophagy in salivary gland cell neighbors.
Figure 4. Mcr regulates autophagy in a cell non-autonomous manner in salivary glands.
(A) Salivary glands dissected 14h after puparium formation containing a loss-of-function mcrEY07421 mutant cell clone (lacking GFP), imaged for mCherry-Atg8a (red), GFP (green) and Hoechst (blue). Scale bars, 50 µm.
(B) Quantification of data from (A). Error bars, mean ± SEM; n = 24 (control), n = 19 (mcr −/−). Statistical significance: Student’s t-test.
(C) Salivary glands expressing Mcr-flag specifically in GFP-marked cells at wandering third instar larval stage were dissected and stained with antibodies against Flag (left) and Mcr (right). Scale bars, 20 µm.
(D) Strategy for Gal80 flip-in induction of salivary glands with variable numbers of mcrIR expressing salivary gland cells.
(E and F) Salivary glands dissected 14h after puparium formation and imaged for mCherry-Atg8a puncta (red) after varying the numbers of mcrIR-expressing cells (green, GFP-positive). Following temperature shift, Gal80 is expressed in a subset of cells, repressing Gal4 activation of UAS-mcrIR. There are more mCherry-Atg8a puncta in salivary glands with many wild-type cells (lacking green GFP) (E), compared to glands with one or two wild-type cells (arrow head, F). Scale bars, 50 µm.
(G) Quantification of data from (E) and (F). Error bars, mean ± SEM; n = 12 (many WT cells), n = 12 (few WT cells). Statistical significance: Student’s t-test.
See also Figure S3.
To further examine the cell non-autonomous function of Mcr, we used the temperature-sensitive Gal80 system to express mcrIR in a subset of salivary gland cells. By varying the temperature shift length we produced salivary glands with varying numbers of wild-type and mcrIR-expressing cells (Figure 4D). In contrast to controls that lack both mCherry-Atg8a reporter puncta and Mcr protein on the cell cortex (Figures S3C and S3D), following temperature shift, we observed mCherry-Atg8a autophagy reporter puncta at 14 hours after puparium formation, and the number of puncta was associated with the number of control cells lacking GFP (Figures 4E – 4G). Under these conditions, Mcr protein was detected on the cortex of salivary gland cells (Figure S3E). Taken together, these data provide further support for the model that Mcr can function in a cell non-autonomous manner to regulate autophagy in neighboring cells.
mcr regulates autophagy via immune receptor signaling in both salivary glands and during macrophage inflammatory response to wounds
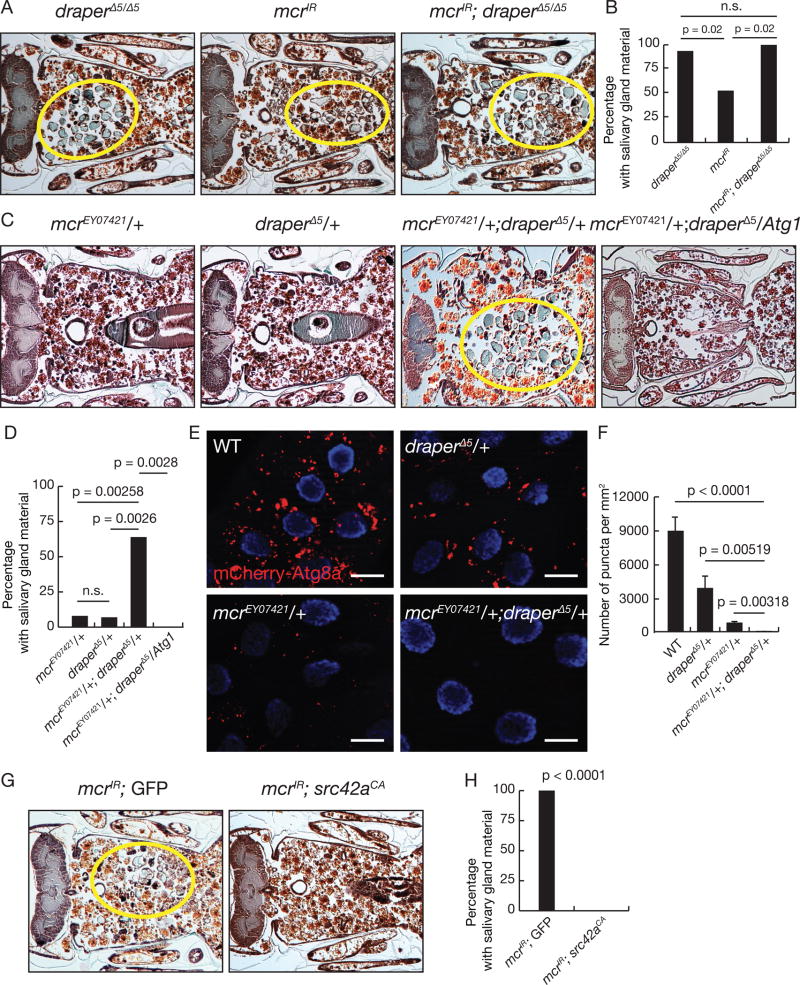
Draper functions upstream of Atg proteins (McPhee et al., 2010). Surprisingly, the complement molecule C1q was recently shown to bind Megf10, the most similar protein to Draper in mice (Iram et al., 2016). We therefore explored the possibility that Mcr might act upstream of Draper, potentially as a Draper ligand to activate salivary gland degradation and autophagy. We first expressed mcrIR in the salivary glands of draper null animals, and found there was no clear difference in the cell fragment phenotypes of either draper null mutant animals, expression of mcrIR in salivary glands, or combined draper mutant and mcrIR knockdown animals (Figures 5A and 5B), indicating that mcr functions in the same pathway as draper. Moreover, animals with combined loss of one allele of both mcrEY07421 and draperΔ5 possessed a significant defect in salivary gland clearance compared to control animals with allelic loss of either mcrEY07421 or draperΔ5 at 24 hours after puparium formation (Figures 5C and 5D). Significantly, expression of Atg1 in the salivary glands of animals with combined loss of one allele of both mcrEY07421 and draperΔ5 suppressed the defect in salivary gland clearance at 24 hours after puparium formation (Figures 5C and 5D). In addition, mcrEY07421 draperΔ5 trans-heterozygous animals possessed significantly fewer mCherry-Atg8a puncta in their salivary gland cells at 14 hours after puparium formation compared to control animals (Figures 5E and 5F). These data support a strong genetic relationship between Mcr and Draper. If Mcr is upstream of Draper, activation of signaling events downstream of Draper should bypass the need for Mcr. The protein kinase Src42A phosphorylates Draper and is required for downstream clearance of dying salivary glands (McPhee et al., 2010; Ziegenfuss et al., 2008). We found that expression of constitutively active Src42A (Src42ACA) was sufficient to suppress the mcrIR knockdown salivary gland clearance defect (Figures 5G and 5H). Taken together, these data argue strongly that Mcr and Draper are in the same genetic pathway, and that Mcr functions upstream of activation of the Draper receptor.
Figure 5. Mcr functions with Draper to activate autophagy and salivary gland degradation.
(A) drpr null mutants (n = 20), salivary gland-specific mcr knockdown (n = 32), and drpr mutants with salivary gland-specific mcr knockdown (n = 24) analyzed by histology for the presence of salivary gland material (yellow circles) at 24h after puparium formation.
(B) Quantification of data from (A). Statistical significance: Chi-square test.
(C) Animals lacking one allele of mcrEY07421 (n = 23), one allele of drprΔ5 (n = 27), one allele of both mcrEY07421 and drprΔ5 (n = 22), and one allele of both mcrEY07421 and drprΔ5 with expression of Atg1 in salivary glands (n = 18) analyzed by histology for the presence of salivary gland material (yellow circles) at 24h after puparium formation.
(D) Quantification of data from (C). Statistical significance: Chi-square test.
(E) mCherry-Atg8a puncta in salivary glands of wild type (WT) animals, animals lacking one allele of drprΔ5, one allele of mcrEY07421, and one allele of both mcrEY07421 and drprΔ5. Scale bars, 50 µm.
(F) Quantification of data from (E). Error bars, mean ± SEM; n ≥ 18. Statistical significance: Student’s t-test.
(G) Animals with either salivary gland-specific knockdown of mcr (n=31) or salivary gland-specific knockdown of mcr with expression of constitutively active src42A (n=12) analyzed by histology for the presence of salivary gland material (yellow circles) at 24h after puparium formation.
(H) Quantification of data from (G). Statistical significance: Chi-square test.
See also Figure S4.
Draper is required for activation of autophagy in salivary glands, but not for starvation-induced autophagy in the larval fat body (McPhee et al., 2010). This prompted us to investigate whether mcr is required for autophagy in other cell contexts. Reduced mcr function in either the larval fat body of starved animals (Scott et al., 2004) or the developing larval intestinal midgut (Chang et al., 2013) failed to influence autophagy (Figures S4A – S4D). In addition, starved larvae did not exhibit a significant change in Mcr levels in the fat body (Figures S4E and S4F).
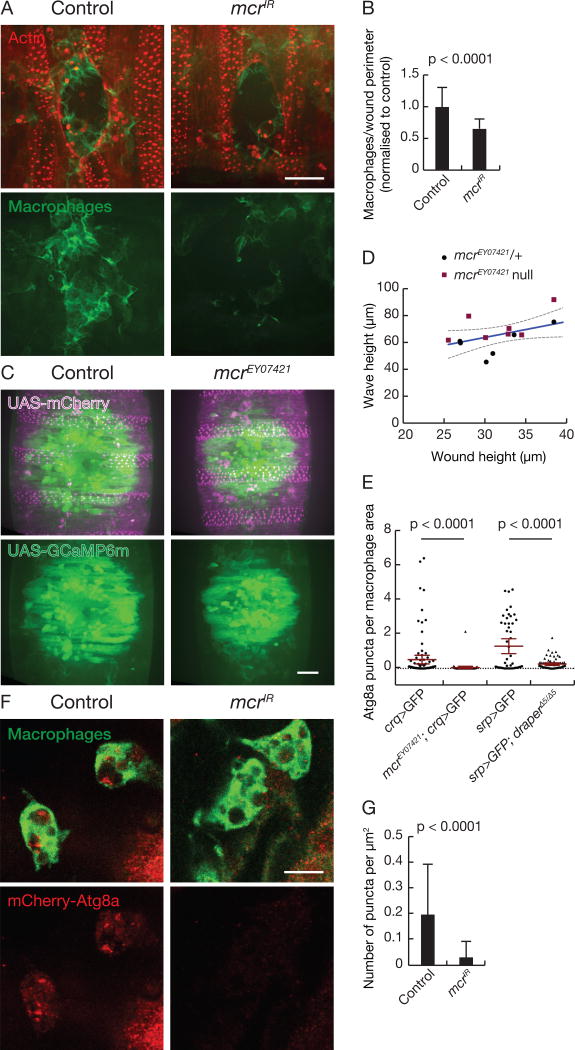
We next explored whether mcr is required for macrophage migration to epithelial wounds in the Drosophila embryo where both Draper and Src42A are required (Evans et al., 2015; Weavers et al., 2016). We knocked down mcr specifically in epithelial cells (Figure S5A) and analyzed macrophage recruitment to laser-induced epithelial wounds. Remarkably, reduced mcr function in the epithelial cells resulted in the recruitment of significantly fewer macrophages to wounds (Figures 6A and 6B), indicating that Mcr mediates an efficient inflammatory response to damage. Importantly, reduced mcr function failed to impact either macrophage number (Figure S5B), the calcium wave associated with wounding (Figures 6C and 6D), or wound closure rate (Figure S5C). These results prompted us to examine if embryonic macrophages possess mCherry-Atg8a autophagy reporter puncta. Not only did these cells possess autophagy reporter puncta, but both mcr and draper mutant embryonic macrophages possessed significantly fewer mCherry-Atg8a puncta (Figure 6E). Moreover, embryos with knockdown of mcr specifically in epithelial cells possessed significantly fewer mCherry-Atg8a puncta in their macrophages than controls (Figures 6F and 6G). These data indicate that Mcr mediates the inflammatory response of macrophages to wounds, and that this cell migration is associated with Mcr regulation of autophagy.
Figure 6. Mcr is required in a cell non-autonomous manner for macrophage recruitment to wounds.
(A) Epithelial-driven mcrIR reduces recruitment of stage 15 embryonic macrophages to laser-induced wounds compared to control. Z-projection of macrophage recruitment to laser-induced wounds 60 minutes post-wounding. Macrophages (green) can be seen around the wound edge of the epithelium (red). Scale bar, 20 µm.
(B) Number of macrophages at the wound edge per µm of wound perimeter normalized to control (n ≥ 22). Error bars, SD; Statistical significance: unpaired t-test with Welsh correction.
(C) Wound-induced calcium wave is not affected in mcr mutants. Still images of calcium wave (green) around the wound edge of the embryonic epithelium (magenta) immediately after wounding. Scale bar 20 µm.
(D) Wound height versus height of calcium wave was calculated for both heterozygous embryos (black circles) and homozygous mcr mutant embryos (red squares).
(E) mCherry-Atg8a puncta in stage 15 embryonic macrophages are reduced in mcr and drpr mutants. Number of mCherry-Atg8a puncta per macrophage cell normalized to cell area (n ≥ 49 macrophages and n ≥ 4 embryos per treatment). Error bars, 95% CI; Statistical significance: Kolmogorov-Smirnov test.
(F) Epithelial-driven mcrIR reduces mCherry-Atg8a puncta in stage 15 embryonic macrophages. Single Z-slice of macrophages (green) and mCherry-Atg8a puncta (red) in control and epithelial-driven mcrIR. Scale bar, 10 µm.
(G) Number of mCherry-Atg8a puncta per cell normalized to cell area (n ≥ 226 macrophages, n ≥ 10 embryos). Error bars, SD; Statistical significance: Mann-Whitney test.
See also Figure S5.
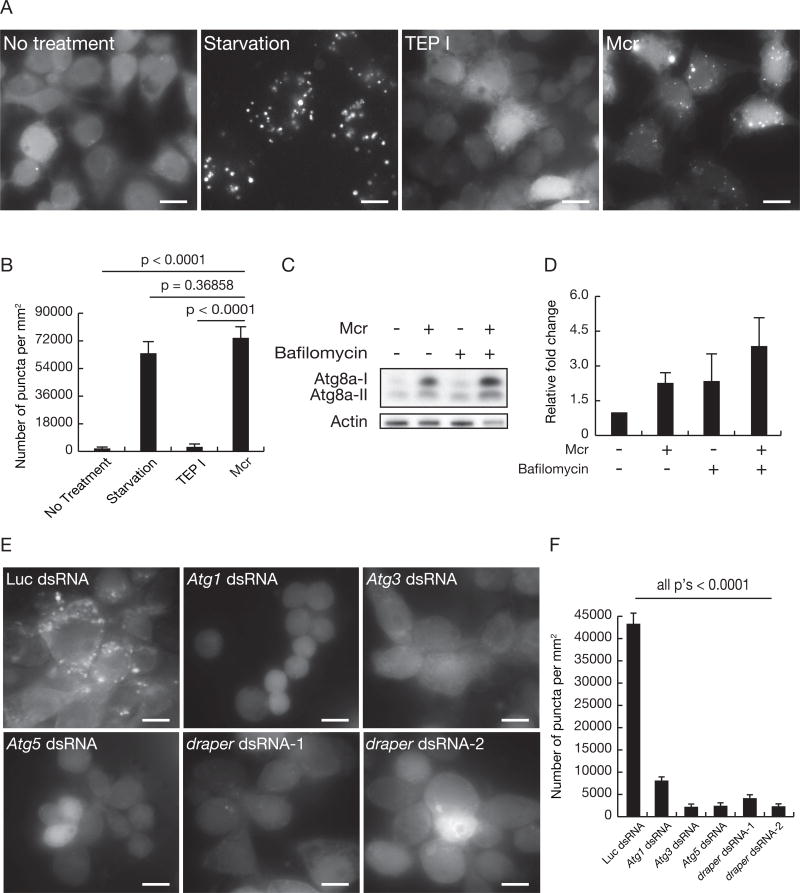
To determine whether application of Mcr to individual cells is sufficient to induce Atg8a puncta and activate autophagy, we used Drosophila embryonic macrophage-derived S2R+ cells that possess a stable GFP-Atg8a autophagy reporter construct that is sensitive to nutrient deprivation (Anding and Baehrecke, 2015). Significantly, the addition of recombinant Mcr protein into culture medium was sufficient to induce similar numbers of GFP-Atg8a puncta as cells cultured under nutrient limiting conditions (Figures 7A and 7B). By contrast, addition of recombinant mosquito TEP1, a known orthologue of complement C3, did not induce GFP-Atg8a puncta (Figures 7A and 7B). The effect of Mcr appears to represent activation of autophagy, since the addition of both Mcr and the lysosome inhibitor Bafilomycin resulted in increased abundance of Atg8a-II compared to addition of either Mcr or Bafilomycin alone (Figures 7C and 7D).
Figure 7. Mcr is sufficient for induction of autophagy in S2 cells.
(A) S2R+ cells stably expressing GFP-Atg8a were either untreated (no treatment), cultured with serum free-medium (starvation), treated with 50 µg (110nM) TEP I protein (TEP I) or treated with 50 µg (82nM) Mcr protein (Mcr). GFP-Atg8a puncta were assessed at 20h. Representative images from three independent experiments are shown. Scale bars, 10 µm.
(B) Quantification of data from (A). Error bars, mean ± SEM; Statistical significance: Student’s t-test.
(C) Western immuno-blots of Atg8a and Actin in extracts of S2R+ cells stably expressing GFP-Atg8a and treated with either 82nM Mcr, 100nM bafilomycin A1, or both.
(D) Quantification of data from (C). All samples are normalized to Actin and plotted relative to no treatment samples. Error bars, mean ± SEM.
(E) S2R+ cells with stable expression of GFP-Atg8a were treated with dsRNAs against either Luciferase, Atg1, Atg3, Atg5 or drpr and then treated with 50 µg (82nM) Mcr protein. GFP-Atg8a puncta were assessed at 20h. Representative images from three independent experiments are shown. Scale bars, 10 µm.
(F) Quantification of data from (E). Error bars, mean ± SEM; Statistical significance: Student’s t-test.
See also Figure S4.
To determine if the formation of GFP-Atg8a puncta in S2R+ cells by addition of Mcr is dependent on Atg genes, we used double stranded (ds)RNA to decrease the function of multiple Atg genes. We found that knockdown of either Atg1, Atg3 or Atg5 suppressed the formation of GFP-Atg8a puncta in S2R+ cells after addition of Mcr (Figures 7E and 7F). Finally, consistent with the Draper receptor receiving the signal to activate autophagy, we found that two distinct dsRNAs that target draper also inhibited Mcr induction of GFP-Atg8a puncta in S2R+ cells (Figures 7E and 7F). Taken together, these results indicate that Mcr functions in a cell nonautonomous manner through Draper to activate autophagy in multiple cell contexts.
DISCUSSION
Much is known about the regulation of autophagy, including that a conserved group of core Atg proteins function within individual cells to control this process (Mizushima and Komatsu, 2011). Although body-wide signals are thought to control autophagy, particularly during nutrient restriction, direct genetic evidence in support of systemic activators of autophagy is limited. Our findings reveal a novel function for the complement related protein Mcr in the control of autophagy in neighboring cells. Mcr appears to signal through the immune receptor Draper to regulate autophagy during programmed cell death and the inflammatory response of macrophages to wounds, but not during nutrient deprivation-induced autophagy in the fat body. Therefore, this study highlights a mechanism by which autophagy is controlled in distinct cell contexts within an animal.
The mechanisms that underlie the cell-specific roles of autophagy must vary. Although it is logical that the core Atg proteins function in diverse cell types to control this conserved catabolic process, multiple mechanisms likely account for differences in autophagy. For example, different signaling and regulatory pathways (Chang et al., 2013; McPhee et al., 2010; Nelson et al., 2014; Tracy et al., 2016), recruitment of distinct autophagic cargoes (Stolz et al., 2014), different rates of autophagy (Mizushima and Komatsu, 2011), and other regulatory mechanisms may account for cell context-specific autophagy programs. Here we describe an autophagic program that is controlled by Mcr and Draper, proteins that have been implicated in inflammation (MacDonald et al., 2006; Stroschein-Stevenson et al., 2006). Significantly we show that mcr is neither required for nutrient deprivation-induced autophagy, nor for developmentally programmed autophagy in dying intestine cells. By contrast, mcr is required for autophagy in both developmentally programmed cell death of salivary glands and inflammation associated with embryonic epithelial wound healing. The identification of such specific regulators of autophagy in distinct cell contexts may be important, as this concept is at the foundation of precise modulation of autophagy for therapeutic purposes.
The relationship between cells that die by apoptosis and inflammation has been extensively studied (Ravichandran and Lorenz, 2007). Dying cells release pro-inflammatory factors, such as cytokines, and present eat me signals that facilitate inflammatory macrophage removal of dying cells. Draper, the Drosophila orthologue of the C. elegans CED-1, is a well-known engulfment receptor that functions in the recognition of apoptotic cells (MacDonald et al., 2006; Zhou et al., 2001). By contrast, dying Drosophila salivary gland cells do not appear to be eaten by phagocytes (Martin and Baehrecke, 2004; McPhee et al., 2010), and do not require Pretaporter, the only known ligand of Draper (Kuraishi et al., 2009), for salivary gland clearance. Rather, dying salivary glands use autophagy, at least in part, to facilitate self-degradation (Berry and Baehrecke, 2007). Therefore, it is particularly interesting that the inflammatory proteins Mcr and Draper function to mediate inter-and intra-cellular activation of autophagy during salivary gland degradation. Mcr appears to signal from one cell to another via the immune receptor Draper that activates a cell autonomous autophagy program.
Autophagy is also known to influence inflammation and the immune response. The impacts of autophagy on inflammation can be multifaceted, and can include modulation of proinflammatory signaling as well as influencing secretion of immune mediators (Deretic et al., 2013). Furthermore, autophagy influences infection by clearance of pathogens by either xenophagy (Levine et al., 2011) or LC3-associated phagocytosis (Sanjuan et al., 2007). By contrast, this study implicates autophagy in the regulation of inflammatory response to sterile wounds in the Drosophila embryo. Remarkably, Mcr from the wounded epidermis is required to activate autophagy in and migration of macrophages to the injury. Therefore, this study highlights the possibility that the program to control autophagy in the dying salivary gland has similarities to the inflammatory response during wound healing. It is possible that these seemingly different cell types use a common program to control autophagy without similar cellular consequences. Alternatively, autophagy could have common purposes in salivary glands and macrophages that could be important for efficient wound healing and regeneration of tissues. Although dying salivary gland cells are clearly not migratory, numerous tissue changes are occurring in the forming adult tissues at this stage in development, and it is possible that autophagy may contribute to this tissue formation by providing metabolic substrates. In addition, it is possible that during this developmental period with extensive tissue remodeling, including that within the intestine, autophagy in the salivary gland somehow helps to prevent infection.
The relationship between Mcr and Draper is likely an ancient mechanism for activation of the Draper immune receptor, as C1q has recently been shown to activate Megf10 in mammals (Iram et al., 2016). Complement has been most studied in the context of pathogen clearance, but recent studies also highlight the importance of complement in other contexts (Kolev et al., 2014), including roles in microglial synapse pruning and in a mouse model of Alzheimer’s disease (Hong et al., 2016; Stevens et al., 2007). This study highlights the potential role of autophagy in complement-associated processes. Since autophagy machinery has been associated with both neurodegeneration (Mizushima et al., 2008) and immune disorders, for example through the function of non-canonical LC3-associated phagocytosis (Martinez et al., 2016), it will be interesting to determine if complement and autophagy are associated in human diseases.
STAR Methods
Detailed methods are provided in the online version of this paper and include the following:
KEY RESOURCES TABLE
CONTACT FOR REAGENT AND RESOURCE SHARING
EXPERIMENTAL MODELS AND SUBJECT DETAILS
Drosophila strains
METHOD DETAILS
Protein Extracts and Western Blotting
Histology
Immunolabeling and microscopy
Induction of cell clones
Starvation of larvae
Live imaging of Drosophila blood cells
S2R+ cell culture and RNAi
Recombinant Protein Expression and Purification
QUANTIFICATION AND STATISTICAL ANALYSES
STAR Methods
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents may be directed to, and will be fulfilled by the corresponding author Eric H. Baehrecke (Eric.Baekrecke@umassmed.edu)
EXPERIMENTAL MODELS AND SUBJECT DETAILS
Drosophila strains
Fly crosses and experiments were performed at 25°C unless noted otherwise. We used Canton- S as the wild-type control. For loss of function studies, we used FRT40A, mcrEY07421 (Hall et al., 2014), drprΔ5 (MacDonald et al., 2006), Atg13Δ74 (Chang and Neufeld, 2009), prtpΔ2 (Kuraishi et al., 2009). We used the following Vienna Drosophila RNAi Center (VDRC) stocks: UAS-mcrIR VDRC Transformant ID (TID) 100197, UAS-mcrIR VDRC TID 2785. The sequences used for VDRC knockdown strains are available for each TID at http://stockcenter.vdrc.at/control/main. For mis-expression studies, we used UAS-p35 (Hay et al., 1994), UAS-Atg1GS10797 (Scott et al., 2007), UAS-src42aca (Tateno et al., 2000), UAS-Drpr-I-NSS::TMD/ICD. For clonal misexpression and RNAi studies we used yw hsFlp; +; Act>CD2>Gal4 (“>” is FRT site), UAS-GFP (nls), yw hsFlp; pmCherry-Atg8a; Act>CD2>Gal4, UAS-GFP (nls) and Sp/Cyo; TubP>stop>Gal80/ Tb (Bohm et al., 2010). mCherry-Atg8a was used as a marker for autophagy (Denton et al., 2012), and tGPH was used as an activity reporter of phosphatidylinositol-3,4,5-P3 (Britton et al., 2002). To obtain flies containing Flag-tagged Mcr, we inserted the entire coding region of Mcr cDNA isolated from clone LD23292 (Drosophila Genomics Resource Center, Bloomington, IN, USA) into pTWF vector from Drosophila Gateway vectors and we generated transgenic flies following standard procedures (Rubin and Spradling, 1982). Six fly lines carrying the transgene on the second or third chromosome were established, and one with it on the third chromosome was used.
METHOD DETAILS
Protein Extracts and Western Blotting
Salivary glands were dissected from wild-type Canton-S and mcrIR animals staged 6, 12, and 14 hour after puparium formation at 25°C. Salivary gla nds were homogenized in Laemmli buffer (0.1% glycerol, 2% SDS, 0.125 M Tris [pH 6.8], 0.05% β-mercaptoethanol, and 0.05% bromophenol blue) and boiled for 5 min at 100°C. Equal amounts of proteins were separated on 4%–15% SDS polyacrylamide gels. Proteins were transferred to 0.45 µm Immobilon-P membranes (Millipore) according to standard procedures. Blots were stripped with low-pH stripping buffer (25 mM glycine-HCl, pH 2, 15[w/v] SDS) between antibodies. We used guinea pig anti-Mcr (1:500, Robert Ward), mouse anti-ecdysone receptor (1:500, Developmental Studies Hybridoma Bank), mouse anti-Broad Complex (1:100, Developmental Studies Hybridoma Bank), rat anti-Ref(2)P (1:5000, Harald Stenmark), rabbit anti-Atg8 (1:2000, Gabor Juhasz), rat anti-pretaporter (1:500, Yoshinobu Nakanishi), mouse anti-actin (1:100, Developmental Studies Hybridoma Bank) and mouse anti-β-Tubulin (1:50, Developmental Studies Hybridoma Bank) primary antibodies. Three independent biological repeats were performed.
Histology
Flies were maintained at 25°C and aged to 24 hours after puparium formation. For paraffin sections and light microscopy, whole pupae were fixed in FAAG (80% ethanol, 4% Formaldehyde, 5% Acetic Acid, 1% Glutaraldehyde) overnight at 4°C, embedded in paraffin, sectioned, stained with Weigert’s Hematoxylin and Pollack Trichrome, and examined using a Zeiss Axiophot II microscope.
Immunolabeling and microscopy
For immunohistochemistry, salivary glands were dissected from animals staged relative to puparium formation at 25°C, fixed in 4% paraformald ehyde overnight at 4°C, blocked in PBS, 1% BSA and 0.1% Tween-20 (PBSBT) for 2 hours at room temperature, and incubated with primary antibodies overnight at 4°C. We used rabbit anti-cleaved-Lamin (Asp 230) (1:500, Cell Signaling Technology), guinea pig anti-Mcr (1:200, Robert Ward) antibodies, and mouse anti-Flag (1:200, Sigma-Aldrich). Following incubation with primary antibodies, salivary glands were washed for 4 × 30 min in PBSBT, incubated with appropriate secondary antibodies for 2 hours at room temperature, and washed for 1 hour in PBSBT. Salivary glands were mounted in Vectashield (Vector Laboratories) and examined using a Zeiss Axiophot II microscope. For mCherry-Atg8a and tGPH analyzes, salivary glands were dissected from animals staged relative to puparium formation at 25°C, fixed in 4% paraformaldehyde containing 2 µM Hoechst stain for 15 min at room temperature, washed in PBS, and mounted in PBS. mCherry-Atg8a puncta were quantified using Zeiss Automeasure software.
Embryos were fixed in 4% formaldehyde and devitellinized before washed with PAT (1% BSA and 0.1% Triton X-100 in PBS). Embryos were then transferred to fresh PAT containing primary antibody and rolled overnight at 4°C. After futher washes in PAT, embryos were incubated for 2 hours at room temperature in fresh PAT containing secondary antibody. After further washes in PBT, embryos were mounted on slides using Vectashield and visualized by confocal microscopy. We used rat anti-mCherry (1:500, Cappel), purified mouse anti-Fascin (1:200 clone sn7C, Developmental Studies Hybridoma Bank) and guinea pig anti-Mcr (1:200, Robert Ward) as primary antibodies with goat anti-rat-CF594 (Sigma-Aldrich), goat anti-mouse- FITC (Jackson Immunoresearch Laboratories) and goat anti-guineapig-AlexaFluor647 (Molecular Probes) used as secondary antibodies, respectively.
Induction of cell clones
To induce RNAi-expressing cell clones in Drosophila tissues, we obtained an overnight egg lays at 25°C, and temperature shifted embryos at 37°C for 30 min. To induce mcrEY07421 null mutant cell clones, we crossed yw hsFlp; FRT40A, ubiquitin (ubi)-GFP (nls); pmCherry-Atg8a virgins to w; FRT40A, mcrEY07421 males. We obtained 6 hour egg lays at 25 °C, and following the egg lay, temperature shifted embryos at 37 °C for 1 hour. To induce Gal80 flip-in clones, we crossed yw hsFlp; mcrIR; TubP>stop>Gal80 virgins to w; pmCherry-Atg8a; fkh-Gal4, UAS-GFP (nls) males. We obtained an overnight egg lay at 25 °C, and following the egg lay, temperature shifted embryos at 37 °C for 1 hour.
Starvation of larvae
We either allowed early third instar larvae to remain in the food (fed) or transferred them from food to 20% sucrose (starved) in PBS for 4 hours.
Live imaging of Drosophila blood cells
For live imaging experiments, stage 15 embryos were collected from overnight apple juice agar plates and mounted on slides in a minimal volume of 10S Voltalef oil (VWR), following dechorionation in bleach for 1 min and extensive washing in water. All imaging was carried out at room temperature. For quantification of wound responses, epithelial wounds were induced using a nitrogen-pumped Micropoint ablation laser tuned to 435 nm (Andor Technologies). Embryos were then imaged at 60 min post-wounding using a 40× oil immersion objective lens on a PerkinElmer UltraView spinning disc microscope. To quantify blood cell responses to wounds, the number of blood cells in contact with/inside the wound edge was determined from z-stack images. Wound size was determined from red channel images in ImageJ. The number of macrophages was then divided by the wound perimeter and the value normalized to the appropriate control.
S2R+ cell culture and RNAi
Drosophila S2R+ cells (Anding and Baehrecke, 2015) were cultured in Schneider’s medium (Gibco) supplemented with 10% fetal bovine serum (FBS, Gibco), 1% Glut-MAX (Gibco), and 0.2% Penicillin-Streptomycin (Pen-Strep). S2R+ cells were plated into 96-well plates at a density of 30,000 cells per well in a culture volume of 150 µl per well. dsRNA was added to a final concentration of 13 µg/ml, and the cells were incubated for four days at 25 °C to allow for depletion of the corresponding gene product.
Recombinant Protein Expression and Purification
Recombinant proteins were produced using the Bac-to-Bac™ baculovirus expression system (Thermo Fisher). A. gambiae TEP1 was cloned into pFastbac1 (Invitrogen) by using RseII/SpeI restriction sites, including the native signal peptide and a C-terminal 6×His tag. Protein was expressed in High Five cells (Invitrogen) grown to a density of 1.2 million/ml in Excel-405 medium (JRH Biosciences) and infected with virus [multiplicity of infection (moi) ≈ 1.0]. Protein was expressed for 80–90 hours at 27 °C. The cells were spun down at 3,300 × g, and the supernatant was concentrated to 1/10 the starting volume by using a tangential concentrator (Millipore). Concentrated medium was dialyzed in PBS (pH 7.4) for 12–16 hours at 4 °C. The dialyzed medium was loaded onto Talon resin (Clontech), washed with 10 CV (100ml) wash buffer [20mM Tris (pH 7.8)/250 mM NaCl] and eluted by a step gradient [250 mM imidazole (pH 7.8)]. The eluate was immediately desalted on a HiTrap 26/10 column (GE Healthcare) equilibrated with Q buffer [20 mM Tris (pH 8.0)/100 mM NaCl]. Desalted protein was loaded onto a MonoQ column (GE Healthcare) and eluted with a linear gradient from 100–600 mM NaCl. Further purification was achieved by gel filtration (Superdex 200; GE Healthcare) equilibrated with S buffer [20 mM Hepes (pH 7.5)/100 mM NaCl], followed by cation exchange on a MonoS column (GE Healthcare). Pure TEP1 protein eluted at ≈ 200 mM NaCl. The entire Mcr ectodomain (sMcr) including the native signal peptide (aa 1–1725 of DGRC cDNA clone LD23292) was subcloned into pFastbac1 with a C-terminal 6×His tag. Protein was secreted by T. ni cells cultured in ESF-921 media (Expression Systems LLC) and harvested at 72 hpi. Following concentration and diafiltration in 0.2 M NaCl, 20 mM Tris pH 7.8, sMcr-6xHis was purified on Co-Talon™ (Clontech) and eluted with a gradient on 0–250 mM imidazole. The crude eluate was purified to homogeneity by anion exchange chromatography on MonoQ 10/10 (GE Healthcare) with 20 mM Tris pH 8.5, 80–600 mM NaCl, and size-exclusion chromatography Superdex200 16/60 (GE Healthcare) with 20 mM Hepes pH 7.5, 150 mM NaCl. The purified protein was concentrated to >1 mg/ml with 20% w/w glycerol, aliquots flash-frozen in liquid nitrogen and stored at −80 °C until use.
QUANTIFICATION AND STATISTICAL ANALYSES
All experiments were performed independently at least three times. For GFP-Atg8a puncta quantification, at least 15 random images were chosen and the number of cells with GFP-positive puncta were counted. An average of 80 cells were examined for each group, and P values were calculated using a two-tailed unpaired t-test. For animal studies, sample sizes were determined empirically based on previous studies to ensure appropriate statistical power. No animals were excluded from statistical analyses, the experiments were not randomized, and the investigators were not blinded.
Supplementary Material
(A) Western blot analyses of Prtp and Tubulin protein levels in salivary glands isolated from wild-type (Canton-S) animals 6h, 12h, and 14h after puparium formation.
(B) Control animals lacking one allele of prtpΔ2 (n = 10) and prtp null mutants (n = 15) analyzed by histology for the presence of salivary gland material at 24h after puparium formation.
(C) Quantification of data from (B). Statistical significance: Chi-square test.
(D) A mcr-RNAi line that targets a different sequence exhibits the same phenotype by histological analyses 24h after puparium formation as Figure 1E and F. Control (n = 26), mcr knockdown (n = 26), salivary gland material (yellow circles). Bottom images: salivary gland cellular fragments without other tissues.
(E) Quantification of data from (D). Statistical significance: Chi-square test.
(A) Control (n = 24) and blood cell-specific knockdown of mcr (n = 27) analyzed by histology for the presence of salivary gland material at 24h after puparium formation.
(B) Quantification of data from (A). Statistical significance: Chi-square test.
(C) Salivary gland (sg) tGPH analyses in feeding larvae and 14h after puparium formation in control (feeding, n = 17, 14h, n = 14) and salivary gland-specific knockdown of mcr (feeding, n = 15, 14h, n = 27) animals. Scale bars, 50 µm.
(D) EcR and Tubulin protein levels in salivary gland extracts isolated from control and salivary gland-specific mcr knockdown animals at 6h, 12h, and 14h after puparium formation.
(E) Quantification of data from (D). All samples are normalized to Tubulin and plotted relative to their respective 6h samples. Error bars, mean ± SEM; n=3. Statistical significance: Student’s t-test.
(F) BR-C and Tubulin protein levels in salivary gland extracts isolated from control and salivary gland-specific mcr knockdown animals at 6h, 12h, and 14h after puparium formation.
(G) Quantification of data from (F). All samples are normalized to Tubulin and plotted relative to their respective 6h samples. Error bars, mean ± SEM; n=3. Statistical significance: Student’s t-test.
(A) Salivary glands expressing mCherry-Atg8a in all cells, and mcrIR specifically in GFP-marked cells at 14h after puparium formation, imaged for mCherry-Atg8a puncta (red), GFP (green) and Hoechst (blue). n = 20. Scale bars, 50 µm.
(B) Wandering larval (WL) salivary glands were dissected from wild-type animals (Canton-S) and stained with anti-Flag (left) and anti-Mcr (right) antibodies. Scale bars, 20 µm.
(C) The tubP>stop>Gal80 flip-in system functions to restrict Gal4 expression. In flies without temperature shift, mcrIR and GFP are expressed in all salivary gland cells and there are no mCherry-Atg8a puncta at 14h after puparium formation. Nuclei are stained with Hoechst (blue). n = 16. Scale bars, 50 µm.
(D and E) Wandering larval (WL) salivary glands were dissected from animals either without (D, n = 18) or with (E, n = 22) temperature shift, and stained with anti-Mcr antibody (red) and Hoechst (blue). Scale bars, 50 µm.
(A) Fat body expressing mCherry-Atg8a in all cells, and mcrIR specifically in GFP-marked clone cells. Third instar larvae were starved for 4h and fat bodies were dissected and imaged for mCherry-Atg8a (red) and GFP (green). Representative images are shown. n = 11. Scale bars, 50 µm.
(B) mCherry-Atg8a was expressed in the fat body of control and those with fat body-specific mcr knockdown. Third instar larvae were starved for 4h and fat bodies were dissected and imaged for mCherry-Atg8a (red). Representative images are shown. Scale bars, 50 µm.
(C) Quantification of data from (B). Atg8a puncta were quantified using Zeiss Automeasure software. Error bars, mean ± SEM; control (n = 11), mcrIR (n = 17). Statistical significance: Student’s t-test.
(D) Midgut expressing mCherry-Atg8a in all cells, and mcrIR specifically in GFP-marked clone cells. Midguts were dissected from animals at puparium formation (0h) and imaged for mCherry-Atg8a (red) and GFP (green). Representative images are shown. n = 12. Scale bars, 50 µm.
(E) Mcr and Tubulin levels in fatbodies isolated from feeding and starved 2nd instar larvae.
(F) Quantification of data from (E). All samples are normalized to Tubulin. Error bars, mean ± SEM; n=3. Statistical significance: Student’s t-test.
(A) Analyses of mcr knockdown efficiency in epithelial cells. Control and mcrIR stage 15 embryos were immunostained for Mcr, showing a significant reduction in overall levels of Mcr following RNAi knockdown. Scale bar, 20 µm.
(B) Macrophage numbers are unaffected in epithelial-driven mcrIR animals (n ≥ 24).
(C) Mcr has no effect on wound closure at stage 15. Control (n = 10, black circles) and mcrEY07421 (n = 7, red squares) wound perimeter was measured every 10 min for 1 h and normalized to the 5 min post-wound perimeter. Second order polynomial fit, preferred model one curve fits both sets of data
Highlights.
Macroglobulin complement-related (Mcr) functions in salivary gland cell death
Mcr regulates cell death by influencing autophagy in neighboring cells
Mcr is required in epithelial cells for macrophage migration to epithelial wounds
Mcr function in epithelial cells is required for macrophage autophagy
Acknowledgments
We thank T. Fortier, M. Freeman, S. Hall, G. Juhasz, Y. Nakanishi, D. Schafer, M. Sieber, A. Spradling, R. Ward, the Baehrecke laboratory, the Bloomington Stock Center, the Vienna Drosophila Resource Center and the Developmental Studies Hybridoma Bank for flies, antibodies, advice, and technical support. This work was supported by R01GM079431 to EHB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SUPPLEMENTAL INFORMATION
Supplemental Information includes five figures and can be found online at.
AUTHOR CONTRIBUTIONS
L.L., F.S.L.M.R., C.K., A.C., R.H.G.B., W.W. and E.H.B. designed the experiments. All experiments were performed by L.L., F.S.L.M.R., C.K.., and A.C., M.L. provided transgenic flies, L.L., C.K. and E.H.B. wrote the manuscript and all authors commented on it.
References
- Anding AL, Baehrecke EH. Vps15 is required for stress induced and developmentally triggered autophagy and salivary gland protein secretion in Drosophila. Cell Death Differ. 2015;22:457–464. doi: 10.1038/cdd.2014.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arico S, Petiot A, Bauvy C, Dubbelhuis PF, Meijer AJ, Codogno P, Ogier-Denis E. The tumor suppressor PTEN positively regulates macroautophagy by inhibiting the phosphatidylinositol 3-kinase/protein kinase B pathway. J Biol Chem. 2001;276:35243–35246. doi: 10.1074/jbc.C100319200. [DOI] [PubMed] [Google Scholar]
- Baehrecke EH. Autophagy: dual roles in life and death? Nature Reviews Mol Cell Biol. 2005;6:505–510. doi: 10.1038/nrm1666. [DOI] [PubMed] [Google Scholar]
- Baxter RH, Chang CI, Chelliah Y, Blandin S, Levashina EA, Deisenhofer J. Structural basis for conserved complement factor-like function in the antimalarial protein TEP1. Proc Natl Acad Sci U S A. 2007;104:11615–11620. doi: 10.1073/pnas.0704967104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry DL, Baehrecke EH. Growth arrest and autophagy are required for salivary gland cell degradation in Drosophila. Cell. 2007;131:1137–1148. doi: 10.1016/j.cell.2007.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohm RA, Welch WP, Goodnight LK, Cox LW, Henry LG, Gunter TC, Bao H, Zhang B. A genetic mosaic approach for neural circuit mapping in Drosophila. Proc Natl Acad Sci U S A. 2010;107:16378–16383. doi: 10.1073/pnas.1004669107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton JS, Lockwood WK, Li L, Cohen SM, Edgar BA. Drosophila's insulin/PI3-kinase pathway coordinates cellular metabolism with nutritional conditions. Dev Cell. 2002;2:239–249. doi: 10.1016/s1534-5807(02)00117-x. [DOI] [PubMed] [Google Scholar]
- Cadwell K, Patel KK, Maloney NS, Liu TC, Ng AC, Storer CE, Head RD, Xavier R, Stappenbeck TS, Virgin HW. Virus-plus-susceptibility gene interaction determines Crohn's disease gene Atg16L1 phenotypes in intestine. 2010;141:1135–1145. doi: 10.1016/j.cell.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang T-K, Shravage BV, Hayes SD, Powers CM, Simin RT, Harper JW, Baehrecke EH. Uba1 functions in Atg7- and Atg3-independent autophagy. Nat Cell Biol. 2013;15:1067–1078. doi: 10.1038/ncb2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YY, Neufeld TP. An Atg1/Atg13 complex with multiple roles in TOR-mediated autophagy regulation. Mol Biol Cell. 2009;20:2004–2014. doi: 10.1091/mbc.E08-12-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton D, Chang TK, Nicolson S, Shravage B, Simin R, Baehrecke EH, Kumar S. Relationship between growth arrest and autophagy in midgut programmed cell death in Drosophila. Cell Death Differ. 2012;19:1299–1307. doi: 10.1038/cdd.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deretic V, Saitoh T, Akira S. Autophagy in infection, inflammation and immunity. Nat Rev Immunol. 2013;13:722–737. doi: 10.1038/nri3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta S, Baehrecke EH. Warts is required for PI3K-regulated growth arrest, autophagy, and autophagic cell death in Drosophila. Curr Biol. 2008;18:1466–1475. doi: 10.1016/j.cub.2008.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng CH, Yu K, Lucas J, White E, Abraham RT. Ammonia derived from glutaminolysis is a diffusible regulator of autophagy. Sci Signal. 2010;3:ra31. doi: 10.1126/scisignal.2000911. [DOI] [PubMed] [Google Scholar]
- Evans IR, Rodrigues FS, Armitage EL, Wood W. Draper/CED-1 mediates an ancient damage response to control inflammatory blood cell migration in vivo. Curr Biol. 2015;25:1606–1612. doi: 10.1016/j.cub.2015.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman MR, Delrow J, Kim J, Johnson E, Doe CQ. Unwrapping glial biology: Gcm target genes regulating glial development, diversification, and function. Neuron. 2003;38:567–580. doi: 10.1016/s0896-6273(03)00289-7. [DOI] [PubMed] [Google Scholar]
- Hall S, Bone C, Oshima K, Zhang L, McGraw M, Lucas B, Fehon RG, Ward RE. Macroglobulin complement-related encodes a protein required for septate junction organization and paracellular barrier function in Drosophila. Development. 2014;141:889–898. doi: 10.1242/dev.102152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding TM, Morano KA, Scott SV, Klionsky DJ. Isolation and characterization of yeast mutants in the cytoplasm to vacuole protein targeting pathway. J Cell Bio. 1995;131:591–602. doi: 10.1083/jcb.131.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay BA, Wolff T, Rubin GM. Expression of baculovirus P35 prevents cell death in Drosophila. Development. 1994;120:2121–2129. doi: 10.1242/dev.120.8.2121. [DOI] [PubMed] [Google Scholar]
- Hong S, Beja-Glasser VF, Nfonoyim BM, Frouin A, Li S, Ramakrishnan S, Merry KM, Shi Q, Rosenthal A, Barres BA, et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science. 2016;352:712–716. doi: 10.1126/science.aad8373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iram T, Ramirez-Ortiz Z, Byrne MH, Coleman UA, Kingery ND, Means TK, Frenkel D, El Khoury J. Megf10 Is a Receptor for C1Q That Mediates Clearance of Apoptotic Cells by Astrocytes. J Neurosci. 2016;36:5185–5192. doi: 10.1523/JNEUROSCI.3850-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway CA, P T, Walport M, Shlomchik MJ. Immunobiology, The Immune System in Health and Disease. 5. New York: Garland Science; 2001. [Google Scholar]
- Jiang C, Baehrecke EH, Thummel CS. Steroid regulated programmed cell death during Drosophila metamorphosis. Development. 1997;124:4673–4683. doi: 10.1242/dev.124.22.4673. [DOI] [PubMed] [Google Scholar]
- Joubert PE, Meiffren G, Grégoire IP, Pontini G, Richetta C, Flacher M, Azocar O, Vidalain PO, Vidal M, Lotteau V, et al. Autophagy induction by the pathogen receptor CD46. Cell Host Microbe. 2009;6:354–366. doi: 10.1016/j.chom.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Kolev M, Le Friec G, Kemper C. Complement--tapping into new sites and effector systems. Nat Rev Immunol. 2014;14:811–820. doi: 10.1038/nri3761. [DOI] [PubMed] [Google Scholar]
- Kuma A, Hatano M, Matsui M, Yamamoto A, Nakaya H, Yoshimori T, Ohsumi Y, Tokuhisa T, Mizushima N. The role of autophagy during the early neonatal starvation period. Nature. 2004;432:1032–1036. doi: 10.1038/nature03029. [DOI] [PubMed] [Google Scholar]
- Kuraishi T, Nakagawa Y, Nagaosa K, Hashimoto Y, Ishimoto T, Moki T, Fujita Y, Nakayama H, Dohmae N, Shiratsuchi A, et al. Pretaporter, a Drosophila protein serving as a ligand for Draper in the phagocytosis of apoptotic cells. EMBO J. 2009;28:3868–3878. doi: 10.1038/emboj.2009.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C-Y, Baehrecke EH. Steroid regulation of autophagic programmed cell death during development. Development. 2001;128:1443–1455. doi: 10.1242/dev.128.8.1443. [DOI] [PubMed] [Google Scholar]
- Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature. 2011;469:323–335. doi: 10.1038/nature09782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald JM, Beach MG, Porpiglia E, Sheehan AE, Watts RJ, Freeman MR. The Drosophila cell corpse engulfment receptor Draper mediates glial clearance of severed axons. Neuron. 2006;50:869–881. doi: 10.1016/j.neuron.2006.04.028. [DOI] [PubMed] [Google Scholar]
- Martin DN, Baehrecke EH. Caspases function in autophagic cell death in Drosophila. Development. 2004;131:275–284. doi: 10.1242/dev.00933. [DOI] [PubMed] [Google Scholar]
- Martinez J, Cunha LD, Park S, Yang M, Lu Q, Orchard R, Li QZ, Yan M, Janke L, Guy C, et al. Noncanonical autophagy inhibits the autoinflammatory, lupus-like response to dying cells. Nature. 2016;533:115–119. doi: 10.1038/nature17950. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- McPhee CK, Logan MA, Freeman MR, Baehrecke EH. Activation of autophagy during cell death requires the engulfment receptor Draper. Nature. 2010;465:1093–1096. doi: 10.1038/nature09127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N, Komatsu M. Autophagy: Renovation of Cells and Tissues Cell. 2011;147:728–741. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muro I, Berry DL, Huh JR, Chen CH, Huang H, Yoo SJ, Guo M, Baehrecke EH, Hay BA. The Drosophila caspase Ice is important for many apoptotic cell deaths and for spermatid individualization, a nonapoptotic process. Development. 2006;133:3305–3315. doi: 10.1242/dev.02495. [DOI] [PubMed] [Google Scholar]
- Nelson C, Ambros V, Baehrecke EH. miR-14 regulates autophagy during developmental cell death by targeting ip3-kinase 2. Mol Cell. 2014;56:376–388. doi: 10.1016/j.molcel.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nezis IP, Simonsen A, Sagona AP, Finley K, Gaumer S, Contamine D, Rusten TE, Stenmark H, Brech A. Ref(2)P, the Drosophila melanogaster homologue of mammalian p62, is required for the formation of protein aggregates in adult brain. J Cell Biol. 2008;180:1065–1071. doi: 10.1083/jcb.200711108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompon J, Levashina EA. A New Role of the Mosquito Complement-like Cascade in Male Fertility in Anopheles gambiae. PLoS Biol. 2015;13:e1002255. doi: 10.1371/journal.pbio.1002255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravichandran KS, Lorenz U. Engulfment of apoptotic cells: signals for a good meal. Nat Rev Immunol. 2007;7:964–974. doi: 10.1038/nri2214. [DOI] [PubMed] [Google Scholar]
- Rubin GM, Spradling AC. Genetic transformation of Drosophila with transposable element vectors. Science. 1982;218:348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- Sanjuan MA, Dillon CP, Tait SW, Moshiach S, Dorsey F, Connell S, Komatsu M, Tanaka K, Cleveland JL, Withoff S, et al. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature. 2007;450:1253–1257. doi: 10.1038/nature06421. [DOI] [PubMed] [Google Scholar]
- Scott RC, Juhász G, Neufeld TP. Direct induction of autophagy by Atg1 inhibits cell growth and induces apoptotic cell death. Curr Biol. 2007;17:1–11. doi: 10.1016/j.cub.2006.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott RC, Schuldiner O, Neufeld TP. Role and regulation of starvation-induced autophagy in the Drosophila fat body. Dev Cell. 2004;7:167–178. doi: 10.1016/j.devcel.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- Stolz A, Ernst A, Dikic I. Cargo recognition and trafficking in selective autophagy. Nat Cell Biol. 2014;16:495–501. doi: 10.1038/ncb2979. [DOI] [PubMed] [Google Scholar]
- Stroschein-Stevenson SL, Foley E, O'Farrell PH, Johnson AD. Identification of Drosophila gene products required for phagocytosis of Candida albicans. PLoS Biol. 2006;4:e4. doi: 10.1371/journal.pbio.0040004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateno M, Nishida Y, Adachi-Yamada T. Regulation of JNK by Src during Drosophila development. Science. 2000;287:324–327. doi: 10.1126/science.287.5451.324. [DOI] [PubMed] [Google Scholar]
- Thumm M, Egner R, Koch B, Schlumpberger M, Straub M, Veenhuis M, Wolf DH. Isolation of autophagocytosis mutants of Saccharomyces cerevisiae. FEBS Lett. 1994;349:275–280. doi: 10.1016/0014-5793(94)00672-5. [DOI] [PubMed] [Google Scholar]
- Tracy K, Velentzas PD, Baehrecke EH. Ral GTPase and the exocyst regulate autophagy in a tissue-specific manner. EMBO Rep. 2016;17:110–121. doi: 10.15252/embr.201541283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukada M, Ohsumi Y. Isolation and characterization of autophagy-defective mutants of Saccharomyces cerevisiae. FEBS Lett. 1993;333:169–174. doi: 10.1016/0014-5793(93)80398-e. [DOI] [PubMed] [Google Scholar]
- Weavers H, Evans IR, Martin P, Wood W. Corpse Engulfment Generates a Molecular Memory that Primes the Macrophage Inflammatory Response. Cell. 2016;165:1658–1671. doi: 10.1016/j.cell.2016.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M, Baxter R. The structure and function of thioester-containing proteins in arthropods. Biophys Rev. 2014;6:261–272. doi: 10.1007/s12551-014-0142-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Hartwieg E, Horvitz HR. CED-1 is a transmembrane receptor that mediates cell corpse engulfment in C. elegans. Cell. 2001;104:43–56. doi: 10.1016/s0092-8674(01)00190-8. [DOI] [PubMed] [Google Scholar]
- Ziegenfuss JS, Biswas R, Avery MA, Sheehan AE, Hog K, Yeung Y-G, Stanley ER, Freeman MR. Draper-dependent glial phagocytic activity is mediated by Src and Syk family kinase signaling. Nature. 2008;453:935–939. doi: 10.1038/nature06901. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Western blot analyses of Prtp and Tubulin protein levels in salivary glands isolated from wild-type (Canton-S) animals 6h, 12h, and 14h after puparium formation.
(B) Control animals lacking one allele of prtpΔ2 (n = 10) and prtp null mutants (n = 15) analyzed by histology for the presence of salivary gland material at 24h after puparium formation.
(C) Quantification of data from (B). Statistical significance: Chi-square test.
(D) A mcr-RNAi line that targets a different sequence exhibits the same phenotype by histological analyses 24h after puparium formation as Figure 1E and F. Control (n = 26), mcr knockdown (n = 26), salivary gland material (yellow circles). Bottom images: salivary gland cellular fragments without other tissues.
(E) Quantification of data from (D). Statistical significance: Chi-square test.
(A) Control (n = 24) and blood cell-specific knockdown of mcr (n = 27) analyzed by histology for the presence of salivary gland material at 24h after puparium formation.
(B) Quantification of data from (A). Statistical significance: Chi-square test.
(C) Salivary gland (sg) tGPH analyses in feeding larvae and 14h after puparium formation in control (feeding, n = 17, 14h, n = 14) and salivary gland-specific knockdown of mcr (feeding, n = 15, 14h, n = 27) animals. Scale bars, 50 µm.
(D) EcR and Tubulin protein levels in salivary gland extracts isolated from control and salivary gland-specific mcr knockdown animals at 6h, 12h, and 14h after puparium formation.
(E) Quantification of data from (D). All samples are normalized to Tubulin and plotted relative to their respective 6h samples. Error bars, mean ± SEM; n=3. Statistical significance: Student’s t-test.
(F) BR-C and Tubulin protein levels in salivary gland extracts isolated from control and salivary gland-specific mcr knockdown animals at 6h, 12h, and 14h after puparium formation.
(G) Quantification of data from (F). All samples are normalized to Tubulin and plotted relative to their respective 6h samples. Error bars, mean ± SEM; n=3. Statistical significance: Student’s t-test.
(A) Salivary glands expressing mCherry-Atg8a in all cells, and mcrIR specifically in GFP-marked cells at 14h after puparium formation, imaged for mCherry-Atg8a puncta (red), GFP (green) and Hoechst (blue). n = 20. Scale bars, 50 µm.
(B) Wandering larval (WL) salivary glands were dissected from wild-type animals (Canton-S) and stained with anti-Flag (left) and anti-Mcr (right) antibodies. Scale bars, 20 µm.
(C) The tubP>stop>Gal80 flip-in system functions to restrict Gal4 expression. In flies without temperature shift, mcrIR and GFP are expressed in all salivary gland cells and there are no mCherry-Atg8a puncta at 14h after puparium formation. Nuclei are stained with Hoechst (blue). n = 16. Scale bars, 50 µm.
(D and E) Wandering larval (WL) salivary glands were dissected from animals either without (D, n = 18) or with (E, n = 22) temperature shift, and stained with anti-Mcr antibody (red) and Hoechst (blue). Scale bars, 50 µm.
(A) Fat body expressing mCherry-Atg8a in all cells, and mcrIR specifically in GFP-marked clone cells. Third instar larvae were starved for 4h and fat bodies were dissected and imaged for mCherry-Atg8a (red) and GFP (green). Representative images are shown. n = 11. Scale bars, 50 µm.
(B) mCherry-Atg8a was expressed in the fat body of control and those with fat body-specific mcr knockdown. Third instar larvae were starved for 4h and fat bodies were dissected and imaged for mCherry-Atg8a (red). Representative images are shown. Scale bars, 50 µm.
(C) Quantification of data from (B). Atg8a puncta were quantified using Zeiss Automeasure software. Error bars, mean ± SEM; control (n = 11), mcrIR (n = 17). Statistical significance: Student’s t-test.
(D) Midgut expressing mCherry-Atg8a in all cells, and mcrIR specifically in GFP-marked clone cells. Midguts were dissected from animals at puparium formation (0h) and imaged for mCherry-Atg8a (red) and GFP (green). Representative images are shown. n = 12. Scale bars, 50 µm.
(E) Mcr and Tubulin levels in fatbodies isolated from feeding and starved 2nd instar larvae.
(F) Quantification of data from (E). All samples are normalized to Tubulin. Error bars, mean ± SEM; n=3. Statistical significance: Student’s t-test.
(A) Analyses of mcr knockdown efficiency in epithelial cells. Control and mcrIR stage 15 embryos were immunostained for Mcr, showing a significant reduction in overall levels of Mcr following RNAi knockdown. Scale bar, 20 µm.
(B) Macrophage numbers are unaffected in epithelial-driven mcrIR animals (n ≥ 24).
(C) Mcr has no effect on wound closure at stage 15. Control (n = 10, black circles) and mcrEY07421 (n = 7, red squares) wound perimeter was measured every 10 min for 1 h and normalized to the 5 min post-wound perimeter. Second order polynomial fit, preferred model one curve fits both sets of data