Abstract
Objective
A greater burden of physical function impairment occurs in HIV-infected adults; the impact of antiretroviral therapy (ART) initiation on muscle density (less dense = more fat), a measure of muscle quality, is unknown.
Design
AIDS Clinical Trials Group Study A5260s, a cardiometabolic substudy of A5257, randomized HIV-infected, ART-naïve adults to ritonavir-boosted atazanavir, darunavir, or raltegravir with tenofovir/emtricitabine backbone. Single-slice abdominal computed tomography (CT) scans from baseline and week 96 were reanalyzed for lean muscle area and density.
Methods
Two-sample t-tests described the differences between baseline and week 96 variables. Linear regression analysis was used to explore the role of a priori identified variables and potential confounders.
Results
Participants (n=235) were mostly men (90%); 31% were Black non-Hispanic; 21% were Hispanic. Over 96 weeks, small but significant increases were seen in oblique/transverse abdominal, rectus, and psoas muscles total area (range 0.21 to 0.83 cm2; p<0.05) but not the lean muscle component (all p ≥0.33). Significant decreases in overall density, consistent with increases in fat, were seen in all muscle groups (range −0.87 to –2.4 HU; p<0.01); for the lean muscle component, only decreases in oblique/transverse abdominal and rectus reached statistical significance (p<0.05). In multivariable analyses, Black race was associated with increased muscle density and female sex with decreased density; treatment arm was not associated with changes in mass or density.
Conclusions
The ART-associated increase in muscle area, regardless of regimen, is likely a reflection of increased fat within the muscle. The consequences of fatty infiltration of muscle on subsequent muscle function require further investigation.
Keywords: skeletal muscle, muscle density, HIV, aging, frailty
Introduction
Due to the success of antiretroviral treatment (ART), more than half of the individuals diagnosed with HIV in the United States are age 50 or older, and an estimated 70% in European countries will be over age 50 by 2030 [1]. Prior studies have shown that HIV-infected older adults are at an increased risk for frailty and physical function impairment compared to HIV-uninfected adults of similar age [2–5]. Physical function is determined, in part, by the quality and quantity of skeletal muscle. In healthy, HIV-uninfected adults, both muscle quality and quantity decline beginning in the fourth or fifth decade of life. With age, the decline in skeletal muscle quality is characterized by an accumulation of fat both around and within the muscle bundle. The increase in skeletal muscle fat can be measured non-invasively by lower density (Hounsfield units, HU) on computed tomography (CT) scan or by magnetic resonance imaging (MRI)[6], and is strongly correlated with lipid content by skeletal muscle biopsy [7]. Importantly, greater skeletal muscle fat in the thigh or the postural support muscles measured by CT scan are consistently strong predictors of worse physical function, and often stronger predictors of physical function than measures of skeletal muscle mass [8–12].
Changes in body fat with ART initiation are well-described, and large gains (up to 30% in the first 2 years) in visceral adiposity occur even with contemporary ART [13–15]. Few studies have described the changes in skeletal muscle fat that occur with HIV infection; to the best of our knowledge, no studies have described changes in skeletal muscle fat with ART initiation. Compared to HIV-uninfected men, HIV-infected men had lower density (greater fatty infiltration) of the mid-thigh muscle bundle and lower muscle quality with age, even after multivariable adjustment including VAT and SAT [16]. Among younger HIV-infected persons with body fat changes (previously referred to as lipodystrophy), decreased psoas muscle density was more strongly associated with insulin resistance than BMI, subcutaneous fat, lean body mass, or ART [17]. Exercise interventions among HIV-infected populations have led to significant improvement in muscle fat attenuation, providing evidence of the reversibility of these findings [18,19].
The goal of this analysis was to 1) determine the changes in skeletal muscle area and density that occur with initiation of ART among ART-naïve, HIV-infected adults, and 2) explore variables associated with these changes in muscle density. As visceral fat and total body weight increase with ART, we hypothesized that ART initiation would similarly be associated with an increase in muscle fat, a possible mechanism for physical function impairment.
Methods
AIDS Clinical Trials Group (ACTG) Study A5260s was a cardiometabolic substudy of A5257, in which HIV-infected, ART-naïve adults were randomized to receive ritonavir- boosted atazanavir (ATV/r), ritonavir-boosted darunavir (DRV/r), or raltegravir (RAL) with a backbone of tenofovir disoproxil fumarate /emtricitabine (TDF/FTC). Details of this study have been previously published [13]. Briefly, substudy participants had no known cardiovascular disease, diabetes, or uncontrolled thyroid disease, and did not receive lipid-lowering medications. The parent study and substudy were registered (NCT00811954 and NCT00851799) and were approved by the institutional review boards at participating sites. All participants provided written, informed consent.
Body Composition Measures
Body composition evaluations occurred at baseline and week 96. Single-slice CT scans at the L4–L5 level were used to quantify visceral adipose tissue (VAT). Scans were standardized and centrally read by blinded personnel at LA Biomed (Torrance, California; CT). Scans were then re-analyzed for muscle density at the University of Colorado with body-composition software that utilizes an interactive data language (IDL) platform (Excelis Visual Information Systems, Boulder, CO). IDL is a validated scientific programming language, used across disciplines to extract meaningful visualizations out of complex numerical data. The program is a semi-automatic segmentation technique based on CT density (HU) differences between adipose and other tissues. Images were read by a single investigator blinded to study arm, and approximately 5–10% of images were re-analyzed by a second, experienced research analysis technician to assure reproducibility within 5%. Skeletal muscle groups (psoas; combined oblique and transverse abdominal; rectus; spinalis) at the L4–5 site were segmented from surrounding adipose tissues and bone. Within each muscle group, the overall muscle area and density were obtained. Then the intermuscular fat (the fatty infiltration into the muscle) was identified as that muscle component with CT density less than or equal to 30 HU, and the cross-sectional area (CSA) and average HU of the lean component of muscle was determined.
Additional Clinical Characteristics
A clinical consequence of increased fat included metabolic syndrome, defined as the presence of 3 or more of the following: waist >40 inches (101.6cm) in men, >35 inches (88.9cm) in women; HDL-C <40 mg/dL in men or <50mg/dL in women or taking fish oil, niacin or fibrates; triglycerides ≥150 mg/dL or taking fish oil, niacin or fibrates; diastolic blood pressure (BP) ≥85 mmHg or systolic BP ≥130mmHg or taking anti-hypertensive medications; and fasting plasma glucose (FPG) ≥100 mg/dL or taking diabetes medications.
Statistical Analysis
Paired t-tests were used to describe the differences between baseline and week 96 for muscle area and density. Linear regression analysis was used to explore the role of a priori identified variables and potential confounders on the change in muscle area and density. Covariates considered during model building included age, sex, race/ethnicity (White, Black, Hispanic, other), hepatitis C infection (positive antibody), body mass index (BMI), CD4 T-cell count (baseline), and current smoking (smoking for muscle area analysis only). Change models were additionally adjusted for change in CD4 count (week 96 –week 0), change in BMI, and change in triglycerides. Variables with a p value <0.10 in univariate models were retained in the multivariate models. In addition, two sensitivity analyses were conducted. Due to concerns with collinearity with CD4 count, the addition of log HIV-1 RNA to the model was tested separately; the coefficients were minimally changed, thus these results are not presented. To assess the component of BMI that was most strongly related to changes in muscle mass or density, where change in BMI was significant in univariate models, we replaced change in BMI with % change in VAT. Statistical analyses were conducted in SAS v. 9.4 (SAS Institute, Cary, NC) and assumed a two-sided significance level of 0.05. No adjustment was made for multiple comparisons.
RESULTS
Two hundred and thirty-five (of 328) A5260s participants had paired week 0 and 96 CT scans available for re-analysis. The majority of participants were male (90%); 31% of participants were Black non-Hispanic and 21% were Hispanic (Table 1). At baseline, the median age was 36 (IQR 28–45) years, CD4 count was 349 cells/μL, and BMI was 24.5 kg/m2.
Table 1.
Baseline Characteristics of Participants with Available Paired CT Scans
| Baseline Characteristics (n=235) | N (%) or Median (IQR) |
|---|---|
| Age, years | 36 (28–45) |
| Male gender | 211 (90) |
| Race/Ethnicity | |
| White-non Hispanic | 101 (43) |
| Black-non Hispanic | 72 (31) |
| Hispanic | 50 (21) |
| Other | 12 (5) |
| Hepatitis C | 17 (7) |
| Current smoking | 83 (35) |
| Body mass index, kg/m2 | 24.5 (22.2–27.8) |
| CD4 count, cells/μL | 349 (190–459) |
| HIV-1 RNA level, log10 copies/mL | 4.56 (4.07–5.08) |
| Metabolic Syndrome (baseline) | 25 (11) |
| Metabolic Syndrome (week 96) | 39 (17) |
Changes in Muscle Area and Factors Associated with Changes
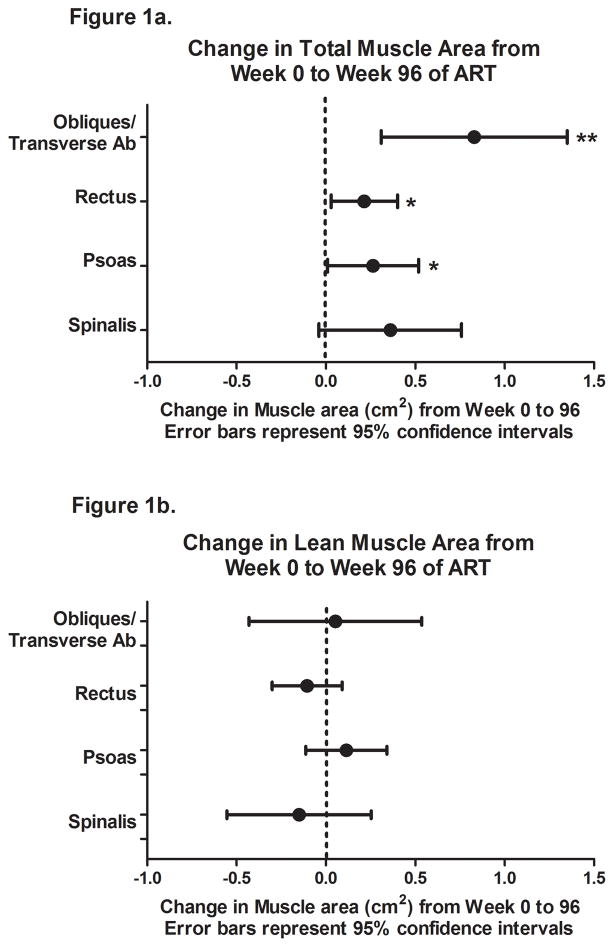
The total area of the oblique/transverse abdominal, rectus, and psoas muscle groups (including infiltrating intermuscular fat) increased significantly over 96 weeks, with a smaller, non-significant increase in the spinalis area (Figure 1a). When restricted to the lean muscle changes, week 96 area for all muscle groups were minimally different from baseline (Figure 1b, all p values ≥0.33).
Figure 1.
Change in total (a) and lean (b) muscle area from week 0 to 96 with antiretroviral initiation. * p<0.05, ** p <0.01, *** p<0.001
In multivariate analyses (Table 2), a greater increase in BMI was associated with significant increases in total muscle area of the oblique/transverse abdominal, rectus, psoas, and spinalis and greater increases in the lean area of the rectus and psoas muscles. Other associations were not consistent across muscle groups, and are detailed in Table 2. No differences by treatment arm were seen with changes in lean area of any of the muscle groups.
Table 2.
Variables associated with the change in total and lean muscle area with 96 weeks of ART
| Total Muscle Area | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| Coef | SE | p-value | Coef | SE | P-value | |
| Change in Oblique/Transverse Abdominal Total Area | ||||||
| BMI (baseline) | −0.11 | 0.06 | 0.04 | |||
| Change in BMI | 0.42 | 0.11 | 0.0001 | 0.38 | 0.12 | 0.001 |
| CD4 >200 cells (baseline) | −1.22 | 0.59 | 0.04 | |||
| Change in Rectus Total Area | ||||||
| Change in BMI | 0.28 | 0.04 | <0.0001 | 0.25 | 0.04 | <0.0001 |
| CD4 >200 cells (baseline) | −0.90 | 0.21 | <0.0001 | −0.40 | 0.20 | 0.05 |
| Triglycerides (baseline)* | 0.03 | 0.01 | 0.002 | 0.02 | 0.01 | 0.02 |
| Change in Psoas Total Area | ||||||
| BMI (baseline) | −0.07 | 0.03 | 0.009 | −0.05 | 0.02 | 0.03 |
| Change in BMI | 0.41 | 0.05 | <0.0001 | 0.39 | 0.05 | <0.0001 |
| CD4 >200 cells (baseline) | −0.87 | 0.29 | 0.003 | |||
| Triglycerides (baseline)* | 0.03 | 0.01 | 0.02 | 0.02 | 0.01 | 0.05 |
| Change in Spinalis Total Area | ||||||
| Change in BMI | 0.26 | 0.09 | 0.003 | 0.22 | 0.09 | 0.02 |
| CD4 >200 cells (baseline) | −1.02 | 0.45 | 0.03 | |||
| Smoking | 0.82 | 0.42 | 0.05 | 0.90 | 0.41 | 0.03 |
| Lean Muscle Area | Univariate | Multivariate | ||||
| Coef | SE | p-value | Coef | SE | P-value | |
| Change in Oblique/Transverse Abdominal Lean Area | ||||||
| Change in Triglycerides | −0.06 | 0.03 | 0.04 | |||
| Change in Rectus Lean Area | ||||||
| Age | −0.02 | 0.01 | 0.05 | −0.02 | 0.01 | 0.009 |
| Change in BMI | 0.18 | 0.04 | <0.0001 | 0.16 | 0.04 | 0.0003 |
| CD4 >200 cells (baseline) | −0.71 | 0.22 | 0.002 | |||
| Triglycerides (baseline)* | 0.02 | 0.01 | 0.02 | 0.02 | 0.01 | 0.03 |
| Change in Psoas Lean Area | ||||||
| Female | −0.68 | 0.38 | 0.07 | |||
| Race/Ethnicity (ref: white) | ||||||
| Black | 0.38 | 0.27 | 0.17 | |||
| Hispanic | 0.61 | 0.30 | 0.04 | |||
| Other | −0.34 | 0.54 | 0.52 | |||
| BMI (baseline) | −0.05 | 0.02 | 0.03 | |||
| Change in BMI | 0.35 | 0.04 | <0.0001 | 0.30 | 0.05 | <0.0001 |
| CD4 >200 cells (baseline) | −1.00 | 0.25 | <0.0001 | |||
| Triglycerides (baseline)* | 0.04 | 0.01 | 0.001 | 0.03 | 0.01 | 0.003 |
| Change in Spinalis Lean Area | ||||||
| Age | −0.05 | 0.02 | 0.02 | −0.04 | 0.02 | 0.03 |
| BMI (baseline) | −0.08 | 0.04 | 0.06 | |||
| CD4 >200 cells (baseline) | −1.10 | 0.46 | 0.02 | −0.89 | 0.47 | 0.05 |
| Triglycerides (baseline)* | 0.03 | 0.02 | 0.10 | |||
| Smoking | 0.81 | 0.43 | 0.06 | |||
Univariate models included age, female, race/ethnicity, hepatitis C, BMI, change in BMI, CD4 (baseline) >200 cells/μL, change in CD4 count, protease inhibitor (vs integrase strand transfer inhibitor), triglycerides, change in triglycerides; only variables with p<0.10 were included in the multivariate analyses and shown above;
per change by 10 units
BMI, body mass index
Percent (%) change in BMI was strongly correlated with % change in VAT (r=0.64, p<0.0001) and % change in SAT (r=0.75, p<0.0001). To further investigate whether the association between change in muscle area with BMI was explained primarily by change in VAT, % change in VAT was substituted for change in BMI for each model where this was significant in univariate analysis. Changes in total area of the oblique/transverse, rectus, and psoas muscles were significantly associated with % change in VAT, although the associations were weaker than with change in BMI. Change in total spinalis, lean rectus, and lean psoas area were not associated with % change in VAT in multivariate models.
Changes in Muscle Density and Factors Associated with Muscle Density Change
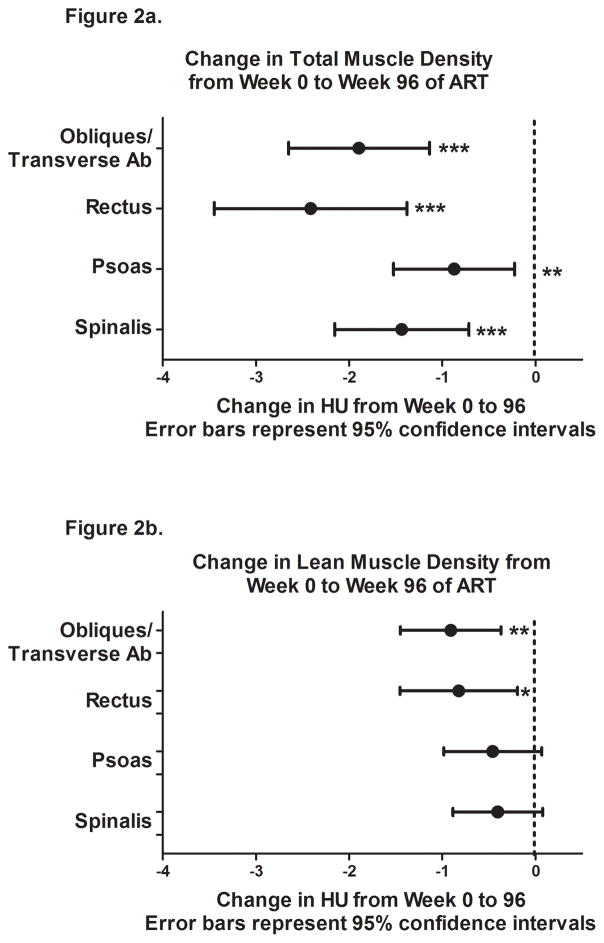
From baseline to week 96, the overall muscle density decreased significantly, with the greatest differences in the rectus muscle followed by the oblique/transverse abdominal, spinalis, and psoas (Figure 2a). When restricted to the lean muscle, the density of the oblique/transverse abdominal and rectus decreased significantly, with smaller non-significant decreases in the psoas and spinalis (Figure 2b).
Figure 2.
Change in total (a) and lean (b) muscle density (HU) from week 0 to 96 with antiretroviral initiation. * p<0.05, ** p <0.01, *** p<0.001
In multivariate analyses, Black race was significantly associated with less decrease in overall muscle density of the oblique/transverse abdominal and psoas, and increased lean muscle density of the oblique/transverse abdominal, rectus, psoas, and spinalis (Table 3). Female sex was associated with larger decreases in overall muscle density of the psoas and lean muscle density of the rectus. A larger increase in BMI from week 0 to 96 was associated with larger decreases in oblique/transverse abdominal overall density, but increases in lean muscle density of the psoas. Greater baseline triglycerides were associated with increased lean muscle density of the spinalis, but not with other muscle groups. Of note, treatment arm was not associated with significant changes in overall or lean muscle density of any of the measured muscle groups (all p≥0.26). When % change in VAT was substituted for change in BMI, % change in VAT was associated only with total oblique/traverse abdominal muscle density.
Table 3.
Variables associated with the change in total and lean muscle density over 96 weeks
| Total Muscle Density | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| Coef | SE | p-value | Coef | SE | P-value | |
| Change in Oblique/Transverse Abdominal Muscle Density (HU) | ||||||
| Race/Ethnicity (ref white) | ||||||
| Black | 2.25 | 0.90 | 0.01 | 2.42 | 0.89 | 0.007 |
| Hispanic | 0.07 | 1.01 | 0.94 | 0.35 | 1.00 | 0.72 |
| Other | 0.61 | 1.78 | 0.73 | 0.08 | 1.76 | 0.97 |
| Change in BMI | −0.49 | 0.16 | 0.003 | −0.51 | 0.16 | 0.002 |
| Change in Psoas Muscle Density (HU) | ||||||
| Female | −2.10 | 1.08 | 0.05 | −2.08 | 1.09 | 0.05 |
| Race/Ethnicity (ref white) | ||||||
| Black | 1.70 | 0.78 | 0.03 | 1.74 | 0.77 | 0.03 |
| Hispanic | 0.49 | 0.87 | 0.57 | 0.39 | 0.87 | 0.66 |
| Other | 0.40 | 1.54 | 0.79 | 0.77 | 1.53 | 0.62 |
| Change in BMI | 0.26 | 0.14 | 0.07 | |||
| CD4 >200 cells (baseline) | −1.71 | 0.74 | 0.02 | |||
| Change in Spinalis Muscle Density (HU) | ||||||
| CD4 >200 cells (baseline) | −1.34 | 0.82 | 0.10 | |||
| Lean Muscle Density | Univariate | Multivariate | ||||
| Coef | SE | p-value | Coef | SE | P-value | |
| Change in Oblique/Transverse Abdominal Lean Muscle Density (HU) | ||||||
| Race/Ethnicity (ref white) | ||||||
| Black | 1.94 | 0.64 | 0.003 | |||
| Hispanic | 0.06 | 0.72 | 0.93 | |||
| Other | 0.48 | 1.26 | 0.70 | |||
| Change in Rectus Lean Muscle Density (HU) | ||||||
| Female | −2.61 | 1.08 | 0.02 | −3.41 | 1.09 | 0.002 |
| Race/Ethnicity (ref white) | ||||||
| Black | 1.32 | 0.75 | 0.08 | 1.58 | 0.74 | 0.03 |
| Hispanic | −0.43 | 0.84 | 0.61 | −0.09 | 0.83 | 0.91 |
| Other | 1.74 | 1.48 | 0.24 | 2.28 | 1.45 | 0.12 |
| BMI (baseline) | 0.12 | 0.07 | 0.08 | 0.17 | 0.07 | 0.02 |
| Change in Psoas Lean Muscle Density (HU) | ||||||
| Female | −1.70 | 0.88 | 0.05 | |||
| Race/Ethnicity (ref white) | ||||||
| Black | 1.61 | 0.63 | 0.01 | 1.61 | 0.62 | 0.01 |
| Hispanic | 0.14 | 0.70 | 0.84 | −0.01 | 0.69 | 1.00 |
| Other | 0.28 | 1.24 | 0.82 | 0.23 | 1.22 | 0.85 |
| Change in BMI | 0.38 | 0.11 | 0.0008 | 0.31 | 0.12 | 0.009 |
| CD4 >200 cells (baseline) | −1.40 | 0.60 | 0.02 | |||
| Change in Spinalis Lean Muscle Density (HU) | ||||||
| Race/Ethnicity (ref white) | ||||||
| Black | 1.16 | 0.58 | 0.05 | 1.31 | 0.58 | 0.03 |
| Hispanic | 0.27 | 0.64 | 0.67 | −0.03 | 0.64 | 0.96 |
| Other | −0.03 | 1.14 | 0.98 | −0.29 | 1.13 | 0.80 |
| CD4 >200 cells (baseline) | −1.04 | 0.55 | 0.06 | |||
| Triglycerides (baseline)* | 0.01 | 0.00 | 0.05 | 0.06 | 0.03 | 0.02 |
Univariate models included age, female, race/ethnicity, hepatitis C, BMI, change in BMI, CD4 (baseline) >200 cells/μL, change in CD4 count, protease inhibitor (vs integrase strand transfer inhibitor), triglycerides, change in triglycerides; only variables with p<0.10 were included in the multivariate analyses and shown above
BMI, body mass index
per change of 10 units
Clinical Factors Correlated with Change in Muscle Density
Lastly, to explore clinical implications of change in muscle density on metabolic markers, we measured correlation coefficients between change in total and lean muscle density and change in glucose, insulin, and VAT. There was a weak but significant correlation (r −0.13, p=0.0499) between the change in glucose and change in the lean muscle density of the oblique/transverse abdominal, and moderate correlations between change in VAT and change in total and lean muscle density of the oblique/transverse abdominal muscles (r= −0.289, p<0.0001 and r= −0.210 and p=0.0013, respectively). Other relationships between change in glucose, insulin, or VAT were small and non-significant (data not shown). Lastly, changes in muscle density did not differ between participants with or without metabolic syndrome (all p≥0.32).
Discussion
Multiple prior studies have shown that ART initiation is associated with small increases in lean body mass, and greater changes in total body weight and VAT [20–22]. The 1.5–3% gain in trunk skeletal muscle area measured by CT scan in the current analysis is comparable to the previously published DXA measured increase of 1.8% in total body lean mass from this study population [13]. While observed gains in lean body mass with ART initiation are often assumed to represent a return to health, the findings of our current study suggest that the increase in muscle area is explained by an increase in fat content within muscle, rather than an improvement in high-quality, dense skeletal muscle. Indeed, when intermuscular fat was excluded, increases in muscle area were no longer seen. Furthermore, both the overall muscle and lean muscle density decreased with 96 weeks of ART, with no difference seen between ART arms. Although the study did not include an untreated HIV control group, our results suggest that ART initiation and return to health is associated with greater fat within the trunk skeletal muscles.
First, one may question whether changes in muscle density have clinical relevance, particularly when analyzing trunk muscles rather than larger muscle groups in the thigh. Of the muscles included on the single-slice CT scan, the rectus is a trunk flexor, spinalis are trunk extensors, the psoas is the strongest of the hip flexors, and the oblique both rotate and flex the trunk. In combination, these core trunk muscles are particularly important in everyday activities, contribute to balance, and provide compensatory support in fall prevention [23,24]. With the caveat that much of the existing data of CT-based muscle density is derived from older adults in the Health Aging and Body Composition (ABC) Study (aged 70–80 years), several analyses support the clinical relevance of CT-measured muscle fat area and density of trunk muscles in association with physical function and falls [7–12,24,25]. Furthermore, one study from Health ABC found that trunk muscle attenuation explained more of the variance in physical function than thigh muscle attenuation or area [9].
The change in skeletal muscle density (range −0.87 to –2.4 HU, Figure 2a) observed in our cohort is of a similar magnitude as that observed between comparison groups of interest or with interventions: psoas muscle density differed by 4–8% or 2–5 HU between HIV-infected individuals with or without lipodystrophy [17]. In an intervention of metformin (n=14) versus metformin with exercise (n=10) in HIV-infected participants, metformin alone was associated with a decline of 1 HU, versus an increase of 2 HU in the combined group [18]. In a separate intervention of healthy post-menopausal women, exercise with or without hormone replacement therapy resulted in small (1.2 to 1.5 HU) improvements in most thigh muscle compartments compared to control, and the change in posterior thigh muscles correlated with an improvement in running speed [26]. Lastly, greater trunk muscle density was associated with improvements in a Short Performance Battery test (β-coefficient range 0.50 to 1 HU) or measures of postural sway (β-coefficient range 0.30 to 6.8 HU) [27]. These studies suggest that the observed changes in muscle density with ART initiation may be clinically relevant.
The factors associated with changes in muscle fat identify at-risk populations for targeted interventions, or suggest potential mechanisms for fatty muscle infiltration. Key findings across multiple muscle groups in our study were the strong associations between increased fatty muscle infiltration (decreased HU) among women, and decreased fatty muscle infiltration (increased HU) among Black participants following 96 weeks of ART. In prior cross-sectional studies, HIV-infected women had significantly greater intermuscular adipose tissue measured by MRI compared to HIV-infected men from two separate cohorts [28,29]; no data on differences in intramuscular adipose tissue change were reported. Furthermore, sex differences on ART-associated changes in skeletal muscle mass are infrequently described and conflicting: in a subset of participants from randomized ART initiation study, ACTG A5224s, no gender differences in lean body mass changes were found [30]. An observational study found a 2.0 kg annual increase in lean body mass among women vs annual decreased lean mass (−0.32 kg) among men, but did not reach statistical significance [31]; in another study by the same cohort, ART use was associated with greater appendicular lean mass among men but not women [32]. Although few associations between muscle mass and physical function have been evaluated in HIV, poorer physical function among HIV-uninfected women could be explained by differences in body composition measures including both muscle density and fat mass [33].
Muscle fat and muscle area differences by race have also been previously described, but not consistent with our results. Indeed, Black race among both men and women tends to be associated with lower muscle density [7,34], without racial differences in the association between muscle density or area and physical function [34]. Data on race differences in muscle density with interventions are limited. The observed differences by race and sex may be explained by confounding factors not included in this exploratory analysis including physical activity, nutrition, or concomitant medications.
Several limitations should be noted. First, physical function or strength assessments were not obtained, thus the direct clinical impact of muscle area and density on physical function cannot be established. We could not control for differences in exercise between subgroups. Additionally, CT scans of the thigh would have complemented the findings in the trunk muscles but were not obtained. The study population included few women and was mostly less than 50 years old, thus the study results may not be generalizable to other populations. Furthermore, the age differences in muscle mass and density may have been more pronounced with a wider age variety of participants. Finally, although there were a large numbers of analyses performed without adjustment, but the magnitude and consistency of the findings across muscle groups reduce the possibility of a chance finding.
In summary, initiation of ART among HIV-infected persons was associated with an increase in truncal skeletal muscle area, which is likely a reflection of increased fat within the muscle rather than an increase in high quality skeletal muscle. We were unable to detect differences in skeletal muscle fat infiltration by ART type in the current cohort; rather, changes in skeletal muscle fat were more closely associated with race and sex. Future studies should seek to understand reasons for race or sex differences, such as differences in physical activity, diet, hormonal changes, or genetic factors. Increased fatty muscle has been associated with weakness, falls, and a decline in physical activity among older adults; similar clinical significance should be established among HIV-infected middle-aged and older adults. Lastly, interventions such as diet and exercise should be investigated as potential therapies to limit fat accumulation within skeletal muscle and prevent long-term functional and metabolic complications.
Acknowledgments
This research was supported by the National Institutes of Health, National Institute on Aging (K23 AG050260 to KME), National Institute of Allergy and Infectious Diseases (K23 AI110532 to JEL and K24 AI120834 to TTB and AI068636 and UM1 AI106701) and supported by National Institute of Mental Health (NIMH), National Institute of Dental and Craniofacial Research (NIDCR). This research also was supported by the National Institutes of Health (HL095132, HL095126, AI69471, AI56933, and OD010569) from the National Heart, Lung, and Blood Institute, the National Institute of Allergy and Infectious Diseases, and the Office of the Director. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health. Additional support was provided through the Colorado Translational Research Imaging Center. Pharmaceutical support was provided by Bristol-Myers Squibb Company, Gilead Sciences, Inc., Merck & Co., Inc., and Tibotec Therapeutics.
JSC, JHS, GAM, and TTB developed and led the main study protocol (A5260s). KME, SF, FM, AS, JEL, GAM, and TTB developed and led the current analysis. SF conducted the data analysis with input from all authors. KME wrote the first manuscript draft. All authors reviewed and edited the manuscript.
References
- 1.Smit M, Brinkman K, Geerlings S, et al. Future challenges for clinical care of an ageing population infected with HIV: a modelling study. Lancet Infect Dis. 2015;15:810–8. doi: 10.1016/S1473-3099(15)00056-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Althoff KN, Jacobson LP, Cranston RD, et al. Age, Comorbidities, and AIDS Predict a Frailty Phenotype in Men Who Have Sex With Men. J Gerontol A Biol Sci Med Sci. 2013 doi: 10.1093/gerona/glt148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piggott DA, Varadhan R, Mehta SH, et al. Frailty, Inflammation, and Mortality Among Persons Aging With HIV Infection and Injection Drug Use. J Gerontol A Biol Sci Med Sci. 2015;70:1542–7. doi: 10.1093/gerona/glv107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Desquilbet L, Jacobson LP, Fried LP, et al. HIV-1 infection is associated with an earlier occurrence of a phenotype related to frailty. J Gerontol A Biol Sci Med Sci. 2007;62:1279–86. doi: 10.1093/gerona/62.11.1279. [DOI] [PubMed] [Google Scholar]
- 5.Erlandson KM, Allshouse AA, Jankowski CM, et al. Comparison of functional status instruments in HIV-infected adults on effective antiretroviral therapy. HIV Clin Trials. 2012;13:324–34. doi: 10.1310/hct1306-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol. 1998;85:115–22. doi: 10.1152/jappl.1998.85.1.115. [DOI] [PubMed] [Google Scholar]
- 7.Goodpaster BH, Kelley DE, Thaete FL, He J, Ross R. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J Appl Physiol. 2000;89:104–10. doi: 10.1152/jappl.2000.89.1.104. [DOI] [PubMed] [Google Scholar]
- 8.Goodpaster BH, Carlson CL, Visser M, et al. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol. 2001;90:2157–65. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- 9.Hicks GE, Simonsick EM, Harris TB, et al. Trunk muscle composition as a predictor of reduced functional capacity in the health, aging and body composition study: the moderating role of back pain. J Gerontol A Biol Sci Med Sci. 2005;60:1420–4. doi: 10.1093/gerona/60.11.1420. [DOI] [PubMed] [Google Scholar]
- 10.Hicks GE, Simonsick EM, Harris TB, et al. Cross-sectional associations between trunk muscle composition, back pain, and physical function in the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2005;60:882–7. doi: 10.1093/gerona/60.7.882. [DOI] [PubMed] [Google Scholar]
- 11.Visser M, Goodpaster BH, Kritchevsky SB, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60:324–33. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- 12.Visser M, Kritchevsky SB, Goodpaster BH, et al. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J Am Geriatr Soc. 2002;50:897–904. doi: 10.1046/j.1532-5415.2002.50217.x. [DOI] [PubMed] [Google Scholar]
- 13.McComsey GA, Moser C, Currier J, et al. Body Composition Changes After Initiation of Raltegravir or Protease Inhibitors: ACTG A5260s. Clin Infect Dis. 2016;62:853–62. doi: 10.1093/cid/ciw017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McComsey GA, Kitch D, Sax PE, et al. Peripheral and central fat changes in subjects randomized to abacavir-lamivudine or tenofovir-emtricitabine with atazanavir-ritonavir or efavirenz: ACTG Study A5224s. Clin Infect Dis. 2011;53:185–96. doi: 10.1093/cid/cir324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erlandson KM, Taejaroenkul S, Smeaton L, et al. A Randomized Comparison of Anthropomorphic Changes With Preferred and Alternative Efavirenz-Based Antiretroviral Regimens in Diverse Multinational Settings. Open Forum Infect Dis. 2015;2:ofv095. doi: 10.1093/ofid/ofv095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Natsag J, Erlandson KM, Sellmeyer DE, et al. HIV Infection Is Associated with Increased Fatty Infiltration of the Thigh Muscle with Aging Independent of Fat Distribution. PLoS One. 2017;12:e0169184. doi: 10.1371/journal.pone.0169184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Torriani M, Hadigan C, Jensen ME, Grinspoon S. Psoas muscle attenuation measurement with computed tomography indicates intramuscular fat accumulation in patients with the HIV-lipodystrophy syndrome. J App Physiol. 2003;95:1005–10. doi: 10.1152/japplphysiol.00366.2003. [DOI] [PubMed] [Google Scholar]
- 18.Driscoll SD, Meininger GE, Ljungquist K, et al. Differential effects of metformin and exercise on muscle adiposity and metabolic indices in human immunodeficiency virus-infected patients. J Clin Endocrinol Metab. 2004;89:2171–8. doi: 10.1210/jc.2003-031858. [DOI] [PubMed] [Google Scholar]
- 19.Dolan SE, Frontera W, Librizzi J, et al. Effects of a supervised home-based aerobic and progressive resistance training regimen in women infected with human immunodeficiency virus: a randomized trial. Arch Intern Med. 2006;166:1225–31. doi: 10.1001/archinte.166.11.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mallon PW, Miller J, Cooper DA, Carr A. Prospective evaluation of the effects of antiretroviral therapy on body composition in HIV-1-infected men starting therapy. AIDS. 2003;17:971–9. doi: 10.1097/00002030-200305020-00005. [DOI] [PubMed] [Google Scholar]
- 21.Mulligan K, Parker RA, Komarow L, et al. Mixed patterns of changes in central and peripheral fat following initiation of antiretroviral therapy in a randomized trial. J Acquir Immunodefic Syndr. 2006;41:590–7. doi: 10.1097/01.qai.0000214811.72916.67. [DOI] [PubMed] [Google Scholar]
- 22.Pozniak AL, Gallant JE, DeJesus E, et al. Tenofovir disoproxil fumarate, emtricitabine, and efavirenz versus fixed-dose zidovudine/lamivudine and efavirenz in antiretroviral-naive patients: virologic, immunologic, and morphologic changes--a 96-week analysis. J Acquir Immunodefic Syndr. 2006;43:535–40. doi: 10.1097/01.qai.0000245886.51262.67. [DOI] [PubMed] [Google Scholar]
- 23.Granacher U, Lacroix A, Muehlbauer T, Roettger K, Gollhofer A. Effects of core instability strength training on trunk muscle strength, spinal mobility, dynamic balance and functional mobility in older adults. Gerontology. 2013;59:105–13. doi: 10.1159/000343152. [DOI] [PubMed] [Google Scholar]
- 24.Granacher U, Gollhofer A, Hortobagyi T, Kressig RW, Muehlbauer T. The importance of trunk muscle strength for balance, functional performance, and fall prevention in seniors: a systematic review. Sports Med. 2013;43:627–41. doi: 10.1007/s40279-013-0041-1. [DOI] [PubMed] [Google Scholar]
- 25.Suri P, Kiely DK, Leveille SG, Frontera WR, Bean JF. Trunk muscle attributes are associated with balance and mobility in older adults: a pilot study. PMR. 2009;1:916–24. doi: 10.1016/j.pmrj.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taaffe DR, Sipila S, Cheng S, Puolakka J, Toivanen J, Suominen H. The effect of hormone replacement therapy and/or exercise on skeletal muscle attenuation in postmenopausal women: a yearlong intervention. Clin Physiol Funct Imaging. 2005;25:297–304. doi: 10.1111/j.1475-097X.2005.00628.x. [DOI] [PubMed] [Google Scholar]
- 27.Anderson DE, Quinn E, Parker E, et al. Associations of Computed Tomography-Based Trunk Muscle Size and Density With Balance and Falls in Older Adults. J Gerontol A Biol Sci Med Sci. 2016;71:811–6. doi: 10.1093/gerona/glv185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scherzer R, Shen W, Heymsfield SB, et al. Intermuscular adipose tissue and metabolic associations in HIV infection. Obesity. 2011;19:283–91. doi: 10.1038/oby.2010.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dodell GB, Kotler DP, Engelson ES, et al. Intermuscular and subcutaneous adipose tissue distributions differ in HIV+ versus HIV-men and women. International journal of body composition research. 2009;7:73–8. [PMC free article] [PubMed] [Google Scholar]
- 30.Grant PM, Kitch D, McComsey GA, et al. Long-term body composition changes in antiretroviral-treated HIV-infected individuals. AIDS. 2016;28:2805–2813. doi: 10.1097/QAD.0000000000001248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McDermott AY, Terrin N, Wanke C, Skinner S, Tchetgen E, Shevitz AH. CD4+ cell count, viral load, and highly active antiretroviral therapy use are independent predictors of body composition alterations in HIV-infected adults: a longitudinal study. Clin Infect Dis. 2005;41:1662–70. doi: 10.1086/498022. [DOI] [PubMed] [Google Scholar]
- 32.McDermott AY, Shevitz A, Knox T, Roubenoff R, Kehayias J, Gorbach S. Effect of highly active antiretroviral therapy on fat, lean, and bone mass in HIV-seropositive men and women. Am J Clin Nutr. 2001;74:679–86. doi: 10.1093/ajcn/74.5.679. [DOI] [PubMed] [Google Scholar]
- 33.Tseng LA, Delmonico MJ, Visser M, et al. Body composition explains sex differential in physical performance among older adults. J Gerontol A Biol Sci Med Sci. 2014;69:93–100. doi: 10.1093/gerona/glt027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lang T, Cauley JA, Tylavsky F, et al. Computed tomographic measurements of thigh muscle cross-sectional area and attenuation coefficient predict hip fracture: the health, aging, and body composition study. J Bone Miner Res. 2010;25:513–9. doi: 10.1359/jbmr.090807. [DOI] [PMC free article] [PubMed] [Google Scholar]