Abstract
Background
Diabetes mellitus (DM) is a critical medical problem that can make people more likely to develop infectious complications, even sepsis. However, the influence of DM on the outcomes of septic patients is still controversial. Thus, we conducted the present meta-analysis to investigate whether DM worsens outcomes of septic patients.
Material/Methods
We searched studies from PubMed, Embase, and Cochrane Library databases from 1966 to July 1, 2016. The primary outcome we chose was 28-day or 30-day mortality or in-hospital mortality.
Results
Our meta-analysis of 10 enrolled studies performed between 2000 and 2016 shows that the mortality rate of septic patients with DM was slightly lower than that of non-diabetic patients (risk ratio [RR]=0.97, 95% confidence interval [CI]: 0.96 to 0.98, P<0.00001). On the other hand, septic patients with DM had a shorter hospital stay (weighted mean difference (WMD)=−2.27, 95% CI: −4.11 to −0.44, P=0.01), a higher incidence rate of AKI (RR=1.56, 95% CI: 1.25 to 1.95, P<0.001), and a similar incidence of respiratory dysfunction (RR=0.86, 95% CI: 0.71 to 1.04, P=0.11) compared with those without DM.
Conclusions
The results from the meta-analysis suggest that DM does not impair the outcome of patients with sepsis, and the incidence of acute kidney injury increases dramatically in septic patients with DM. Due to the limitations of the analysis, more well-designed trials are still necessary.
MeSH Keywords: Acute Kidney Injury; Diabetes Mellitus, Type 2; Mortality; Outcome Assessment (Health Care); Respiratory System Abnormalities; Sepsis
Background
All over the world, diabetes mellitus (DM) is a common metabolic disorder, described as a state of persistent hyperglycemia. It can be divided into 2 types based on the pathogenesis. The prevalence of DM is rising both in America and China [1,2]. Without appropriate control of blood glucose, various complications occur in different organs and systems of diabetic patients [3].
Sepsis occurs along with numerous kinds of infections, complicated by multi-organ dysfunction or even shock without effective treatment. The occurrence of multi-organ dysfunction or shock has become the most common cause of mortality and morbidity in intensive care units. Over the last few decades, hospital mortality from sepsis has ranged from 25% to 80% [4].
DM increases the risk of developing infectious complications, including sepsis [5]. Based on preclinical studies, DM is known to interact with multiple components of the innate immune system, and also has some inhibitory effects on the adaptive immune system [6,7]. Among patients with sepsis, around 20% have DM [8]. Although the mortality of sepsis is declining due to great improvements in septic treatment and nursing, sepsis still remains a critical problem, especially for diabetic patients. However, the influence of DM on the outcomes of sepsis is still controversial [9–18]. Thus, we conducted a meta-analysis to analyze all the relevant studies and explore whether DM worsens the outcomes of septic patients.
Material and Methods
Search strategy
We searched Medline (PubMed), Embase, and the Cochrane Library for articles associated with the desired context. The keywords we used as search terms were ‘diabetes’ or ‘diabetic’ or ‘hyperglycemia’, ‘sepsis’ or ‘septic’ or ‘septicemia’ and ‘mortality’ or ‘outcome’ or ‘death’. The detailed search strategies are shown in Supplementary file: search strategy. All databases were searched for articles published from inception until July 1, 2016. No language restriction or publication date restriction was imposed for the search.
Study selection
Studies exploring the relationship between DM and outcomes (including mortality and complications) of patients were enrolled. Studies were excluded if they included pediatric patients, or they did not include the comparison between patients with DM and without DM, or they were cross-sectional or epidemiologic studies. Two investigators independently reviewed the articles followed by face-to-face discussion on some disagreements. Duplicates were removed at the beginning of reviewing and full-text articles were selected for assessment from the remaining articles by screening the titles and abstract according to the following criteria: 1. they were retrospective or prospective cohort studies of human subjects and the study population were over 15 years old; 2. the studies contained a group of diabetic patients with sepsis and a comparable group of non-diabetic patients as the control group; 3. the primary outcome of 30-day or in-hospital mortality was reported in both groups. After that, animal experiments, case reports, and cross-sectional or epidemiologic studies were excluded. The Newcastle-Ottawa scale was used to assess the methodological quality of included studies [19], and if any study got 6 or more points of a total 9 points, we defined it as high quality.
Data extraction
Variables studied included the first author, publication year, the type of study, research country, the number of included hospitals and participants, the severity of sepsis, the type of DM, and outcomes using a self-designed data extraction table (Table 1). Primary outcome was either 28-day or 30-day mortality or in-hospital mortality. Moreover, we chose 3 secondary outcomes: length of hospital stay, the incidence of acute kidney injury (AKI), and respiratory dysfunction. The primary and secondary outcomes were defined according to the original author’s definition.
Table 1.
Characteristics of the studies included in the meta-analysis.
| Author | Year | Type of study | Country | Hospitals | Population | Severity of sepsis | Type of diabetes | Primary outcome | Secondary outcomes | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Length of hospital stay | Acute kidney injury | Respiratory dysfun-ction | |||||||||
| Moss [9] | 2000 | A prospective cohort study | America | 4 | 113 | Septic shock | Not mentioned | In-hospital mortality | No | No | Yes |
| Moutzouri [10] | 2008 | A prospective cohort study | Greece | 1 | 64 | Severe sepsis or septic shock | 2 | In-hospital mortality | No | No | No |
| Stegenga [11] | 2010 | A retrospective cohort study | 11 countries | 164 | 830 | Severe sepsis or septic shock | Not mentioned | mortality at day 28 | Yes | No | No |
| Schuetz [12] | 2011 | A retrospective cohort study | America | 2 | 7754 | All sepsis | Not mentioned | In-hospital mortality | No | No | No |
| Yang [13] | 2011 | A retrospective cohort study | Singapore | 1 | 9221 | All sepsis | 2 | In-hospital mortality | Yes | Yes | Yes |
| Schuetz [14] | 2012 | A prospective cohort study | America | 1 | 1849 | All sepsis | 1+2 | In-hospital mortality | No | No | No |
| Chang [15] | 2012 | A prospective cohort study | China, Taiwan | 1 | 16497 | Severe sepsis or septic shock | 2 | In-hospital mortality | Yes | Yes | Yes |
| Al-Dorzi [16] | 2012 | A retrospective cohort study | Canada, USA and Saudi Arabia | 28 | 8670 | Septic shock | Not mentioned | In-hospital mortality | No | No | No |
| Venot [17] | 2015 | A prospective cohort study | France | 12 | 1064 | Severe sepsis or septic shock | Not mentioned | In-hospital mortality | Yes | Yes | No |
| De Miguel-Yanes [18] | 2015 | A retrospective cohort study | Spain | 95% hospital of Spain | 217280 | All sepsis | 2 | In-hospital mortality | Yes | No | No |
Statistical analysis
Review Manager Version 5.2 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012) was used for statistical analysis. Results are expressed by Forest plots, using relative risk (RR) with 95% confidence intervals (CI) for dichotomous data and weighted mean difference (WMD) with 95% CI for continuous data. The statistical heterogeneity among the studies was calculated and assessed according to the Mantel-Haenszel chi-square test and the I2 test. I2 <25%, 25%< I2 <50%, 50%< I2 <75%, and I2 >75% indicated no, low, moderate, and high statistical heterogeneity, respectively. In addition, P-value <0.1 of the chi-square test was considered as a statistically significant heterogeneity. For all analysis, random-effects model analysis using the Mantel-Haenszel method was used if a significant heterogeneity was noticed. Otherwise, the fixed-effects model analysis using the Mantel-Haenszel method was used. A formal analysis of publication bias was based on a funnel plot, and a predefined subgroup analysis was undertaken to explore the effect of DM on patients with all stages of sepsis and patients with severe sepsis and septic shock. A p-value <0.05 was considered statistically significant.
Results
Study selection
A total of 4398 records were identified according to the initial search strategy. From these records, 1433 duplicates were removed. We eliminated 2930 records from the remaining 2965 records by screening titles and abstracts, and full-text assessment was conducted in the last 35 records. Ten studies that satisfied the selection criteria were chosen. The study flow diagram, including the reasons for exclusion of studies, is shown in Figure 1.
Figure 1.
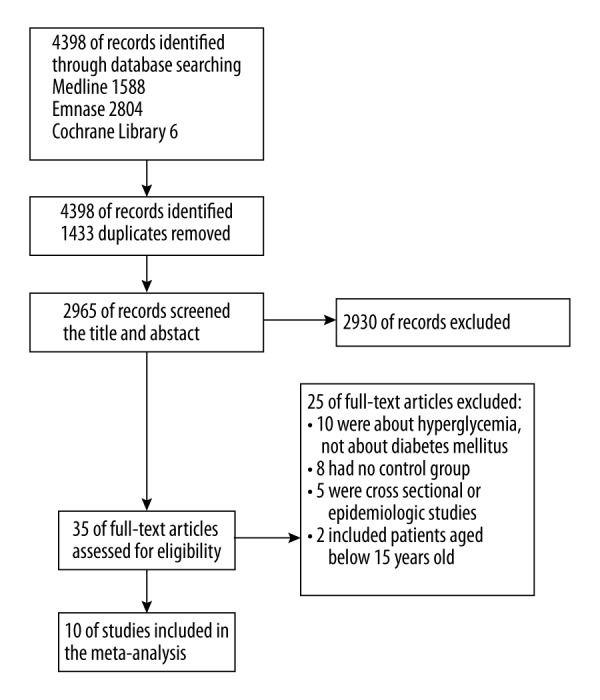
Study flow diagram. A total of 10 studies were included in the meta-analysis.
Characteristics of included studies
The characteristics of included studies are shown in Table 1. All the studies were published between 2000 and 2015, and a total of 26 3342 septic patients consisting of 63 361 diabetic patients and 19 9981 non-diabetic patients were included. The sources of infection in the included studies were not limited to any specific organs or systems. There are 5 retrospective cohort studies and 5 prospective cohort studies. Six of the included studies were multicenter trials and 6 studies only enrolled patients with severe sepsis or septic shock, while the others contained patients with sepsis of all stages. Five studies did not mention the type of DM. The level of Hgb A1c was only measured in 1 enrolled study [10]; however, the level of HgbA1c in diabetic patients was within normal limits in the study. All of the studies reported primary outcome, either 28- or 30-day mortality or in-hospital mortality, among which 4 studies reported length of hospital stay and 3 studies reported the incidence of AKI and respiratory dysfunction. In all the studies, preexisting DM was identified by the medical history of each patient, while patients without DM or with undeterminable DM status were considered non-diabetic. Moreover, the definition of sepsis was based on a known or suspected site of infection according to clinical assessment, plus obvious signs of systemic inflammation. If the sepsis-induced dysfunction of at least 1 organ or system occurred, we defined it as severe sepsis or septic shock. The Newcastle-Ottawa scale scores of each study included are shown in Table 2.
Table 2.
Newcastle-Ottawa scale (NOS) scores of the studies included in the meta-analysis.
| Author | Year | A | B | C | D | E | F | G | H | NOS Score |
|---|---|---|---|---|---|---|---|---|---|---|
| Moss | 2000 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 7 |
| Moutzouri | 2008 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 7 |
| Stegenga | 2010 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Schuetz | 2011 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 6 |
| Yang | 2011 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 6 |
| Schuetz | 2012 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 6 |
| Chang | 2012 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 6 |
| Al-Dorzi | 2012 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 6 |
| Venot | 2015 | 1 | 1 | 1 | 1 | 2 | 1 | 0 | 1 | 8 |
| De Miguel-Yanes | 2015 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 6 |
A – Representativeness of the exposed cohort; B – selection of the non-exposed cohort; C – ascertainment of exposure; D – demonstration that outcome of interest was not present at start of study; E – comparability of cohorts on the basis of the design or analysis; F – assessment of outcome; G – was follow-up long enough for outcomes to occur?; H – adequacy of follow-up of cohorts.
Primary outcome
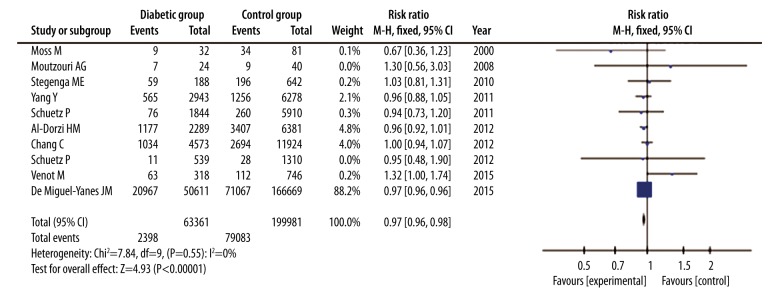
In all these included studies, a total of 103 051 patients died within 28 or 30 days or before hospital discharge, among which were 23 968 diabetic patients and 79 083 non-diabetic patients. Overall mortality of all the studies was 37.8% in diabetic patients and 39.5% in non-diabetic patients. According to the Forest plot, mortality in diabetic patients was slightly lower than that in non-diabetic patients (RR=0.97, 95% CI: 0.96 to 0.98, P<0.00001), with no heterogeneity among the studies (I2=0%; P=0.55) (Figure 2). A fixed-effect model analysis using the Mantel-Haenszel method was used due to the low heterogeneity, and sensitivity analysis was not performed.
Figure 2.
Forest plot of mortality of septic patients with DM versus those without DM. Fixed-effects model analysis using the Mantel-Haenszel method was used for meta-analysis. Risk ratios are shown with 95% CI.
Publication bias
A funnel plot was used to assess the publication bias in the meta-analysis of the influence of DM on mortality of patients with sepsis. As can be seen from the plot, there was no evidence of significant funnel plot asymmetry (Figure 3).
Figure 3.
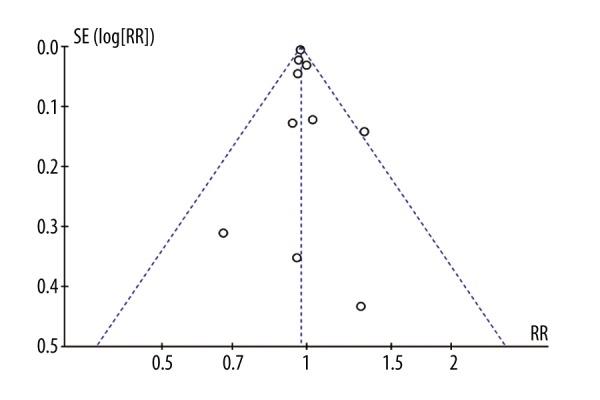
Funnel plot of the meta-analysis.
Subgroup analysis
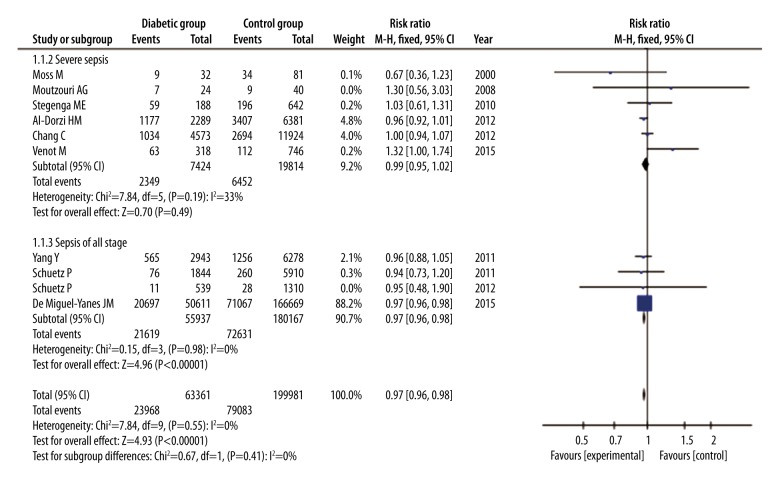
To explore the effect of DM in different stages of sepsis, we conducted a subgroup analysis. Of the 10 studies, 6 were categorized into a group of severe sepsis or sepsis shock and the other 4 studies were categorized into a group of sepsis of all stages. In the former group, 27 238 patients were involved in, 7424 of which were patients with DM. Based on the Forest plot, there were no statistical differences in mortality between diabetic patients and non-diabetic patients (RR=0.99, 95% CI: 0.95 to 1.02, P=0.49), with a low heterogeneity (I2=33%, P=0.19) (Figure 4). On the other hand, 236 104 patients were involved in the latter group and there were 55 937 patients with DM and 180 167 patients with no DM. We found that mortality in diabetic patients was slightly lower than in non-diabetic patients (RR=0.97, 95% CI: 0.96 to 0.98, P<0.00001), without heterogeneity (I2=0%, P=0.98) (Figure 4), which is similar to the analysis of all studies. All analyses were based on a fixed-effects model analysis using the Mantel-Haenszel method.
Figure 4.
Forest plot of a subgroup analysis to explore influence of DM on mortality of patients with different stages. Fixed-effects model analysis using the Mantel-Haenszel method was used for meta-analysis. Risk ratios are shown with 95% CI.
Secondary outcome
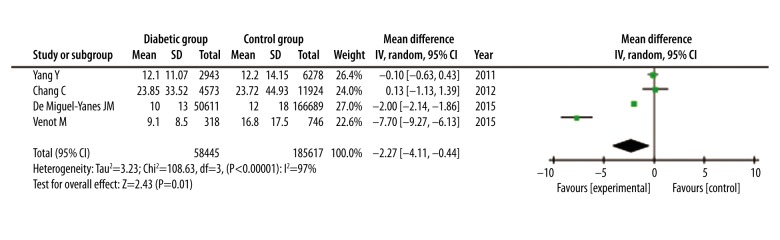
Length of hospital stay was reported in 4 studies with a total of 244 062 patients. The average length of hospital stay ranged from 9.1 days to 23.9 days, and the standard deviation (SD) ranged from 8.5 days to 33.5 days in diabetic patients. Average length of hospital stay ranged from 12 days to 23.7 days, and SD ranged from 14.2 days to 45.0 days in non-diabetic patients. According to the Forest plot, septic patients with DM had shorter hospital stays than those without DM (WMD=−2.27, 95% CI: −4.11 to −0.44, P=0.01). Because of high heterogeneity (I2=97%; P<0.00001), a random-effects model analysis using the Mantel-Haenszel method was applied (Figure 5).
Figure 5.
Forest plot of length of hospital stay of septic patients with DM versus those without DM. Random-effects model analysis using the Mantel-Haenszel method was used for meta-analysis. Weighted mean differences are shown with 95% CI.
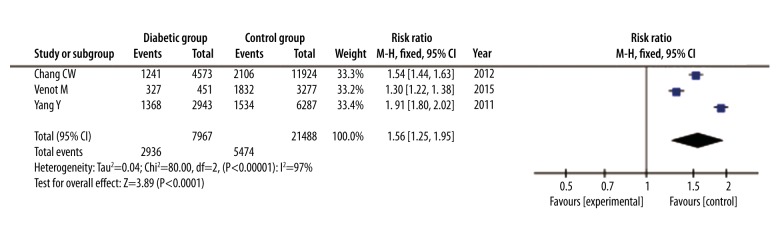
The incidence of AKI was reported in only 3 studies with a total of 29 455 patients. The incidence of AKI was reported for 2936 of 7967 diabetic patients and for 5474 of 21 488 non-diabetic patients. We found a higher incidence rate of AKI in diabetic patients than in non-diabetic patients (RR=1.56, 95% CI: 1.25 to 1.95, P<0.001). A random-effects model analysis using the Mantel-Haenszel method was used based on the high heterogeneity (I2=97%; P<0.00001) (Figure 6).
Figure 6.
Forest plot of incidence of AKI of septic patients with DM versus those without DM. Random-effects model analysis using the Mantel-Haenszel method was used for meta-analysis. Risk ratios are shown with 95% CI.
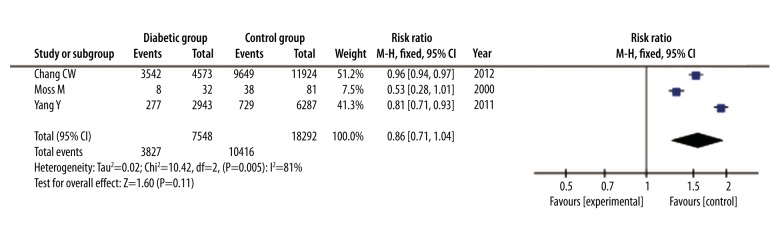
The presence of respiratory dysfunction was reported in 3 studies with a total of 25 840 patients. Respiratory dysfunction occurred in 3827 of 7548 diabetic patients and in 10416 of 18292 non-diabetic patients. There was no significant difference in incidence of respiratory dysfunction between the 2 groups (RR=0.86, 95% CI: 0.71 to 1.04, P=0.11). Due to high heterogeneity, random-effects model analysis using the Mantel-Haenszel method was used (I2=81%; P=0.005) (Figure 7).
Figure 7.
Forest plot of incidence of respiratory dysfunction of septic patients with DM versus those without DM. Random-effects model analysis using the Mantel-Haenszel method was used for meta-analysis. Risk ratios are shown with 95% CI.
Discussion
Our study is the first meta-analysis to focus on the influence of DM on the outcomes of septic patients. The principle result from our analysis is that septic patients with DM do not have a higher mortality than those without DM. In contrast, DM may help patients recover from sepsis, especially from early-stage sepsis. In terms of secondary outcomes, septic patients with DM have shorter hospital stays and have higher incidence of AKI than those without DM. The incidence of respiratory dysfunction in diabetic patients is similar to that of non-diabetic patients.[7]
We investigated the effect of DM as a protective factor on mortality in patients with sepsis. Although DM increases the risk of infection or sepsis in most people, DM does not increase the risk of mortality once infection or sepsis has occurred. The mechanisms responsible for this result deserve to be explored further. Several studies have demonstrated that sepsis is associated with the activation of inflammation and coagulation [20–22], and the activation of coagulation accounts for a large proportion of deaths. Moreover, an included study [11] measured several markers of coagulation and fibrinolysis to evaluate the influence of DM on the coagulation pathway in sepsis. In comparing the diabetic and non-diabetic cohorts, no difference was found in procoagulation markers, including prothrombin time (PT), activated partial thromboplastin times (APTT), thrombin-antithrombin complexes, and D-Dimer, nor in anticoagulant markers, including protein S and protein C. A similar result was reported in another study [23], in which a cohort of diabetic patients with gram-negative sepsis caused by B. pseudomallei was compared with a healthy cohort, demonstrating that DM is associated with abnormal coagulation and fibrinolysis to a certain extent. However, these abnormalities are not remarkable compared with the larger abnormalities caused by sepsis. In terms of inflammation cytokines, it has been reported that levels of plasma interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF alpha) are increased in patients with DM [24,25], whereas a downregulation of basal cytokine production could be noticed in blood cells in type 2 diabetes patients. In the included study mentioned above [11], the plasma concentrations of IL-6 and TNF alpha were also measured and no differences were found between the 2 groups of patients with sepsis.
Moreover, other potential mechanisms were explored. De Jong[15] et al. [26] found that patients with gram-negative sepsis caused by B. pseudomallei have abundant plasma protease factor VII-activating protease (FSAP) activation, which is a plasma hyaluronic acid-binding protein 2 significantly associated with the stage of sepsis. However, the study found that the existence of DM does not influence the extent of FSAP activation, which also supports our result from the analysis above.
Short-term hyperglycemia is an independent risk factor for mortality in patients without a past medical history of DM [27–29]. The blood glucose level of patients with DM is usually higher than that of healthy people, which may make them better able to tolerate the impact of short-term hyperglycemia when sepsis occurs. Conversely, when non-diabetic patients with sepsis experience short-term hyperglycemia, circulating inflammation cytokine levels increase significantly [30] and induce more inflammatory response, which can be lethal. Thus, septic patients with DM could have lower mortality than those without DM, as shown from the results above.
However, DM has negative effects on host immunity. The functions of leucocytes, especially polymorphonuclear leukocytes (PMN), are impaired by hyperglycemia. It was reported by several studies [31,32] that membrane fluidity of PMN were significantly lower in diabetic patients, resulting in the decrease of multiple functions, such as impaired migration, reduced phagocytosis, and intracellular killing capacity, as well as altered chemotaxis. In addition, a recent study reported that patients with DM have multiple downregulated miRNAs involving various signaling pathways, including hematopoietic cell lineage, MAPK signaling pathway, and Fc gamma R-mediated phagocytosis [33]. The impairment of the host immune system may account for the result of Venot et al. [17], which show higher mortality in diabetic patients. However, the overall result of the meta-analysis indicated that DM may be slightly more beneficial than harmful for septic patients. Because the variables were not matched well in these included studies, more well-designed trials are needed.
There are few studies on the influence of DM on secondary outcomes of patients with sepsis, but we still found some useful information. In terms of AKI, all the 3 studies included reported a higher incidence in diabetic patients, and 1 of them excluded patients with chronic kidney disease and prerenal AKI. DM can impair renal function by destroying kidney tissue, and hyperglycemia due to DM severely increased activation of NF-kappa B and oxidant levels with the stages of sepsis, according to an animal experiment, in addition to the injury [34], which leads to a much higher incidence of AKI in diabetic patients. On the contrary, no significant difference was found in the incidence of respiratory dysfunction between the 2 groups from the analysis of these 3 studies. Nevertheless, 2 of the 3 studies showed a protective function of DM, which is similar to the result of another meta-analysis [35]. The mechanism by which DM affects respiratory function has not been reported. We found no study specifically focussed on length of hospital stay. The Forest plot shows that half of the studies found no difference between patients with and without DM, and the other half reported a shorter length of stay. However, because DM can impair wound healing, the result is not very convincing and may be due to excellent care management, considering the importance of care management for patient outcomes [36]. Nevertheless, the included studies did not mention this aspect.
The main limitation of our meta-analysis is that only 1 study totally matched diabetic patients to patients without DM on confounding factors such as age, sex, sources of infection, and APACHE II scores. Lack of matching for these confounding factors may have influenced the results to a certain degree. Only 4 studies reporting the level of blood glucose and no study reported the severity of DM, which may have generated bias in the analysis. In addition, the treatment of all the patients in the meta-analysis was decided by different physicians. Our results may have been influenced by the long study period (16 years) and the changes in diagnostic capabilities and treatment modalities over that time period. Two trials enrolled in the meta-analysis did not mention the age of patients, but were retained because the heterogeneity of primary outcome was kept low and we had no specific evidence to exclude them. In contrast, Esper et al. [37] also did not mention the age of patients, but including it in our meta-analysis would have increased the heterogeneity; therefore, we decided to exclude it after discussion.
Conclusions
Our meta-analysis shows that DM is not associated with adverse outcomes of patients with sepsis, and it may even be beneficial. This analysis summarizes the available data and provides insight into the association. However, more well-designed studies will help clarify the relationship further, considering the limitations of the meta-analysis. In addition, septic patients with DM have a higher incidence of AKI and a shorter hospital stay. Thus, when sepsis occurs in diabetic patients, renal function should be assessed promptly.
Supplementary File: Search strategy
Search strategy in PubMed.
| #1 | diabetes[Title/Abstract] |
| #2 | diabetic[Title/Abstract] |
| #3 | hyperglycemia[Title/Abstract] |
| #4 | #1 or #2 or #3 |
| #5 | sepsis[Title/Abstract] |
| #6 | septic[Title/Abstract] |
| #7 | septicemia[Title/Abstract] |
| #8 | #5 or #6 or #7 |
| #9 | mortality[Title/Abstract] |
| #10 | outcome[Title/Abstract] |
| #11 | death[Title/Abstract] |
| #12 | #9 or #10 or #11 |
| #13 | #4 and #8 and #12 |
Search strategy in Embase.
| #1 | ‘diabetes’: ab,ti |
| #2 | ‘diabetic’: ab,ti |
| #3 | ‘hyperglycemia’: ab,ti |
| #4 | #1 or #2 or #3 |
| #5 | ‘sepsis’: ab,ti |
| #6 | ‘septic’: ab,ti |
| #7 | ‘septicemia’: ab,ti |
| #8 | #5 or #6 or #7 |
| #9 | ‘mortality’: ab,ti |
| #10 | ‘outcome’: ab,ti |
| #11 | ‘death’: ab,ti |
| #12 | #9 or #10 or #11 |
| #13 | #4 and #8 and #12 |
Search strategy in Cochrane Library.
| #1 | ‘diabetes’ |
| #2 | ‘diabetic’ |
| #3 | ‘hyperglycemia’ |
| #4 | #1 or #2 or #3 |
| #5 | ‘sepsis’ |
| #6 | ‘septic’ |
| #7 | ‘septicemia’ |
| #8 | #5 or #6 or #7 |
| #9 | ‘mortality’ |
| #10 | ‘outcome’ |
| #11 | ‘death’ |
| #12 | #9 or #10 or #11 |
| #13 | #4 and #8 and #12 |
Footnotes
Source of support: This study was supported by a grant from the National Natural Science Foundation of China (81571881)
References
- 1.Davies J, Wang H, Jia W. China Diabetes Society 2016: A call for papers. Lancet Diabetes Endocrinol. 2016;4(1):14–15. doi: 10.1016/S2213-8587(15)00465-9. [DOI] [PubMed] [Google Scholar]
- 2.Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity – related health risk factors, 2001. JAMA. 2003;289(1):76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 3.Gregg EW, Li Y, Wang J, et al. Changes in diabetes-related complications in the United States, 1990–2010. N Engl J Med. 2014;370(16):1514–23. doi: 10.1056/NEJMoa1310799. [DOI] [PubMed] [Google Scholar]
- 4.Angus DC, Wax RS. Epidemiology of sepsis: an update. Crit Care Med. 2001;29(7 Suppl):S109–16. doi: 10.1097/00003246-200107001-00035. [DOI] [PubMed] [Google Scholar]
- 5.Muller LM, Gorter KJ, Hak E, et al. Increased risk of common infections in patients with type 1 and type 2 diabetes mellitus. Clin Infect Dis. 2005;41(3):281–88. doi: 10.1086/431587. [DOI] [PubMed] [Google Scholar]
- 6.Delamaire M, Maugendre D, Moreno M, et al. Impaired leucocyte functions in diabetic patients. Diabet Med. 1997;14(1):29–34. doi: 10.1002/(SICI)1096-9136(199701)14:1<29::AID-DIA300>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 7.Spatz M, Eibl N, Hink S, et al. Impaired primary immune response in type-1 diabetes. Functional impairment at the level of APCs and T-cells. Cell Immunol. 2003;221(1):15–26. doi: 10.1016/s0008-8749(03)00043-1. [DOI] [PubMed] [Google Scholar]
- 8.Investigators N-SS, Finfer S, Chittock DR, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360(13):1283–97. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 9.Moss M, Guidot DM, Steinberg KP, et al. Diabetic patients have a decreased incidence of acute respiratory distress syndrome. Crit Care Med. 2000;28(7):2187–92. doi: 10.1097/00003246-200007000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Moutzouri AG, Athanassiou GA, Dimitropoulou D, et al. Severe sepsis and diabetes mellitus have additive effects on red blood cell deformability. J Infect. 2008;57(2):147–51. doi: 10.1016/j.jinf.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Stegenga ME, Vincent JL, Vail GM, et al. Diabetes does not alter mortality or hemostatic and inflammatory responses in patients with severe sepsis. Crit Care Med. 2010;38(2):539–45. doi: 10.1097/CCM.0b013e3181c02726. [DOI] [PubMed] [Google Scholar]
- 12.Schuetz P, Jones AE, Howell MD, et al. Diabetes is not associated with increased mortality in emergency department patients with sepsis. Ann Emerg Med. 2011;58(5):438–44. doi: 10.1016/j.annemergmed.2011.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang Y, Salam ZH, Ong BC, Yang KS. Respiratory dysfunction in patients with sepsis: protective effect of diabetes mellitus. Am J Crit Care. 2011;20(2):e41–47. doi: 10.4037/ajcc2011391. [DOI] [PubMed] [Google Scholar]
- 14.Schuetz P, Kennedy M, Lucas JM, et al. Initial management of septic patients with hyperglycemia in the noncritical care inpatient setting. Am J Med. 2012;125(7):670–78. doi: 10.1016/j.amjmed.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Chang CW, Kok VC, Tseng TC, et al. Diabetic patients with severe sepsis admitted to intensive care unit do not fare worse than non-diabetic patients: A nationwide population-based cohort study. PLoS One. 2012;7(12):e50729. doi: 10.1371/journal.pone.0050729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Dorzi HM, Tamim H, Bouchama A, et al. Presentation and outcome of diabetic patients admitted with septic shock. American Journal of Respiratory and Critical Care Medicine Conference: American Thoracic Society International Conference, ATS. 2012;185 (no pagination) [Google Scholar]
- 17.Venot M, Weis L, Clec’h C, et al. Acute kidney injury in severe sepsis and septic shock in patients with and without diabetes mellitus: A multicenter study. PLoS One. 2015;10(5):e0127411. doi: 10.1371/journal.pone.0127411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Miguel-Yanes JM, Mendez-Bailon M, Jimenez-Garcia R, et al. Trends in sepsis incidence and outcomes among people with or without type 2 diabetes mellitus in Spain (2008–2012) Diabetes Res Clin Pract. 2015;110(3):266–75. doi: 10.1016/j.diabres.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 19.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 20.Levi M, van der Poll T, Buller HR. Bidirectional relation between inflammation and coagulation. Circulation. 2004;109(22):2698–704. doi: 10.1161/01.CIR.0000131660.51520.9A. [DOI] [PubMed] [Google Scholar]
- 21.Annane D, Bellissant E, Cavaillon JM. Septic shock. Lancet. 2005;365(9453):63–78. doi: 10.1016/S0140-6736(04)17667-8. [DOI] [PubMed] [Google Scholar]
- 22.Kinasewitz GT, Yan SB, Basson B, et al. Universal changes in biomarkers of coagulation and inflammation occur in patients with severe sepsis, regardless of causative micro-organism [ISRCTN74215569] Crit Care. 2004;8(2):R82–90. doi: 10.1186/cc2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koh GCKW, Meijers JCM, Maude RR, et al. Diabetes does not influence activation of coagulation, fibrinolysis or anticoagulant pathways in Gram-negative sepsis (melioidosis) Thromb Haemost. 2011;106(6):1139–48. doi: 10.1160/TH11-07-0504. [DOI] [PubMed] [Google Scholar]
- 24.Pickup JC, Chusney GD, Thomas SM, Burt D. Plasma interleukin-6, tumour necrosis factor alpha and blood cytokine production in type 2 diabetes. Life Sci. 2000;67(3):291–300. doi: 10.1016/s0024-3205(00)00622-6. [DOI] [PubMed] [Google Scholar]
- 25.Kado S, Nagase T, Nagata N. Circulating levels of interleukin-6, its soluble receptor and interleukin-6/interleukin-6 receptor complexes in patients with type 2 diabetes mellitus. Acta Diabetol. 1999;36(1–2):67–72. doi: 10.1007/s005920050147. [DOI] [PubMed] [Google Scholar]
- 26.de Jong HK, Koh GCKW, Bulder I, et al. Diabetes-independent increase of factor VII-activating protease activation in patients with Gram-negative sepsis (melioidosis) J Thromb Haemost. 2015;13(1):41–46. doi: 10.1111/jth.12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krinsley JS. Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients. Mayo Clin Proc. 2003;78(12):1471–78. doi: 10.4065/78.12.1471. [DOI] [PubMed] [Google Scholar]
- 28.Leonidou L, Mouzaki A, Michalaki M, et al. Cytokine production and hospital mortality in patients with sepsis-induced stress hyperglycemia. J Infect. 2007;55(4):340–46. doi: 10.1016/j.jinf.2007.05.177. [DOI] [PubMed] [Google Scholar]
- 29.Jimenez-Ibanez EO, Castillejos-Lopez M, Hernandez A, et al. High mortality associated with hyperglycemia, neutrophilia, and lymphopenia in critically ill patients. Tohoku J Exp Med. 2012;226(3):213–20. doi: 10.1620/tjem.226.213. [DOI] [PubMed] [Google Scholar]
- 30.Yu WK, Li WQ, Li N, Li JS. Influence of acute hyperglycemia in human sepsis on inflammatory cytokine and counterregulatory hormone concentrations. World J Gastroenterol. 2003;9(8):1824–27. doi: 10.3748/wjg.v9.i8.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valerius NH, Eff C, Hansen NE, et al. Neutrophil and lymphocyte function in patients with diabetes mellitus. Acta Med Scand. 1982;211(6):463–67. doi: 10.1111/j.0954-6820.1982.tb01983.x. [DOI] [PubMed] [Google Scholar]
- 32.Masuda M, Murakami T, Egawa H, Murata K. Decreased fluidity of polymorphonuclear leukocyte membrane in streptozocin-induced diabetic rats. Diabetes. 1990;39(4):466–70. doi: 10.2337/diab.39.4.466. [DOI] [PubMed] [Google Scholar]
- 33.Cui Y, Chen W, Chi J, Wang L. Comparison of transcriptome between type 2 diabetes mellitus and impaired fasting glucose. Med Sci Monit. 2016;22:4699–706. doi: 10.12659/MSM.896772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uyanik A, Unal D, Uyanik MH, et al. The effects of polymicrobial sepsis with diabetes mellitus on kidney tissues in ovariectomized rats. Renal Failure. 2010;32(5):592–602. doi: 10.3109/08860221003759478. [DOI] [PubMed] [Google Scholar]
- 35.Gu WJ, Wan YD, Tie HT, et al. Risk of acute lung injury/acute respiratory distress syndrome in critically ill adult patients with pre-existing diabetes: A meta-analysis. PLoS One. 2014;9(2):e90426. doi: 10.1371/journal.pone.0090426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ciccone MM, Aquilino A, Cortese F, et al. Feasibility and effectiveness of a disease and care management model in the primary health care system for patients with heart failure and diabetes (Project Leonardo) Vasc Health Risk Manag. 2010;6:297–305. doi: 10.2147/vhrm.s9252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Esper AM, Moss M, Martin GS. The effect of diabetes mellitus on organ dysfunction with sepsis: An epidemiological study. Crit Care. 2009;13(1):R18. doi: 10.1186/cc7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Search strategy in PubMed.
| #1 | diabetes[Title/Abstract] |
| #2 | diabetic[Title/Abstract] |
| #3 | hyperglycemia[Title/Abstract] |
| #4 | #1 or #2 or #3 |
| #5 | sepsis[Title/Abstract] |
| #6 | septic[Title/Abstract] |
| #7 | septicemia[Title/Abstract] |
| #8 | #5 or #6 or #7 |
| #9 | mortality[Title/Abstract] |
| #10 | outcome[Title/Abstract] |
| #11 | death[Title/Abstract] |
| #12 | #9 or #10 or #11 |
| #13 | #4 and #8 and #12 |
Search strategy in Embase.
| #1 | ‘diabetes’: ab,ti |
| #2 | ‘diabetic’: ab,ti |
| #3 | ‘hyperglycemia’: ab,ti |
| #4 | #1 or #2 or #3 |
| #5 | ‘sepsis’: ab,ti |
| #6 | ‘septic’: ab,ti |
| #7 | ‘septicemia’: ab,ti |
| #8 | #5 or #6 or #7 |
| #9 | ‘mortality’: ab,ti |
| #10 | ‘outcome’: ab,ti |
| #11 | ‘death’: ab,ti |
| #12 | #9 or #10 or #11 |
| #13 | #4 and #8 and #12 |
Search strategy in Cochrane Library.
| #1 | ‘diabetes’ |
| #2 | ‘diabetic’ |
| #3 | ‘hyperglycemia’ |
| #4 | #1 or #2 or #3 |
| #5 | ‘sepsis’ |
| #6 | ‘septic’ |
| #7 | ‘septicemia’ |
| #8 | #5 or #6 or #7 |
| #9 | ‘mortality’ |
| #10 | ‘outcome’ |
| #11 | ‘death’ |
| #12 | #9 or #10 or #11 |
| #13 | #4 and #8 and #12 |