Publisher's Note: There is an Inside Blood Commentary on this article in this issue.
Key Points
Genetically accurate xenografts of CMML are achievable with near 100% frequency in NSGS mice.
Robust human engraftment and overt phenotypes of CMML and JMML xenografts here facilitate preclinical therapeutic evaluation in vivo.
Abstract
Chronic myelomonocytic leukemia (CMML) and juvenile myelomonocytic leukemia (JMML) are myelodysplastic syndrome (MDS)/myeloproliferative neoplasm (MPN) overlap disorders characterized by monocytosis, myelodysplasia, and a characteristic hypersensitivity to granulocyte-macrophage colony-stimulating factor (GM-CSF). Currently, there are no available disease-modifying therapies for CMML, nor are there preclinical models that fully recapitulate the unique features of CMML. Through use of immunocompromised mice with transgenic expression of human GM-CSF, interleukin-3, and stem cell factor in a NOD/SCID-IL2Rγnull background (NSGS mice), we demonstrate remarkable engraftment of CMML and JMML providing the first examples of serially transplantable and genetically accurate models of CMML. Xenotransplantation of CD34+ cells (n = 8 patients) or unfractionated bone marrow (BM) or peripheral blood mononuclear cells (n = 10) resulted in robust engraftment of CMML in BM, spleen, liver, and lung of recipients (n = 82 total mice). Engrafted cells were myeloid-restricted and matched the immunophenotype, morphology, and genetic mutations of the corresponding patient. Similar levels of engraftment were seen upon serial transplantation of human CD34+ cells in secondary NSGS recipients (2/5 patients, 6/11 mice), demonstrating the durability of CMML grafts and functionally validating CD34+ cells as harboring the disease-initiating compartment in vivo. Successful engraftments of JMML primary samples were also achieved in all NSGS recipients (n = 4 patients, n = 12 mice). Engraftment of CMML and JMML resulted in overt phenotypic abnormalities and lethality in recipients, which facilitated evaluation of the JAK2/FLT3 inhibitor pacritinib in vivo. These data reveal that NSGS mice support the development of CMML and JMML disease-initiating and mature leukemic cells in vivo, allowing creation of genetically accurate preclinical models of these disorders.
Introduction
Chronic myelomonocytic leukemia (CMML) and juvenile myelomonocytic leukemia (JMML) are clonal myeloid disorders that exhibit features of both myelodysplastic syndromes (MDS) and myeloproliferative neoplasms (MPNs) and are classified by the World Health Organization (WHO) under the rubric of MDS/MPN overlap syndromes.1 Both disorders are characterized by bone marrow (BM) dysplasia and cytopenias accompanied by proliferation of cells of the monocytic and granulocytic lineages resulting in a peripheral monocytosis.2-4 JMML occurs in children, and >90% of patients harbor germline or somatic mutations resulting in constitutive activation of RAS/MAPK signaling, including mutations in NF1, CBL, NRAS, KRAS, or PTPN11.5-8 These mutations are present in the initiating clone7,8 and result in a characteristic granulocyte-macrophage colony-stimulating factor (GM-CSF) hypersensitivity.9 In contrast, CMML is a disease of the elderly with highly recurrent mutations in SRSF2,10-14 ASXL1,12,13,15 and TET2.12,13,16 Each of these 3 genes are mutated in 40% to 60% of CMML patients and usually present in the dominant clone,13 but they are rarely mutated in JMML.5-8 Despite the genetic differences between CMML and JMML, these diseases share a similar GM-CSF hypersensitivity17 and overlapping mutations that converge on RAS/MAPK signaling (due to mutations in NRAS, KRAS, CBL, and JAK2 in CMML).12,13
The discovery of recurrent mutations in CMML has provided greater insight into the molecular pathogenesis of the disease and motivated the creation of genetically engineered mouse models (GEMMs) to mimic these genetic events. However, currently existing GEMMs of highly recurrent mutations found in CMML, alone or in combination, do not fully recapitulate the unique immunophenotypic and morphological features of CMML.18-22 In addition, these models do not accurately represent the clinical heterogeneity or clonal genetic composition of CMML.13 Finally, there are no passageable cell line models of CMML or secondary acute myeloid leukemia (AML) derived from CMML that carry the aforementioned genomic abnormalities, which occur at high frequency in CMML. The lack of such models presents barriers to deciphering CMML pathophysiology and developing effective therapies for CMML.
Currently, the median survival of CMML is 34 months, and there are no approved therapies that impact the natural history of this lethal disease.2,3,12 These facts make development of robust preclinical models for CMML imperative. To this end, great improvements in xenograft mouse strains have recently facilitated successful xenotransplantation of leukemia subtypes that were previously difficult to engraft. Such improvements in immunocompromised mice include development of NOD/SCID-IL2Rγnull (NSG) and NOD/RAG-IL2Rγnull (NRG) mouse strains (reviewed recently by Doulatov et al and Goyama et al23,24), which are devoid of lymphoid cells and harbor additional defects in innate immunity enabling more robust engraftment of human hematopoietic cells as well as T-cell development compared with prior immunocompromised mouse strains. Despite these advances, Philadelphia chromosome–negative chronic myeloid neoplasms such as CMML remain difficult to engraft, motivating the development of second-generation strains of NSG and NRG mice, which include transgenic expression of human myelosupportive cytokines that are normally non–cross-reactive between mouse and human cells. The second-generation strains that are derived from NSG and NRG mice include NSGS25 mice, which express human stem cell factor, GM-CSF, and interleukin-3 (IL-3) from a transgenic locus, as well as MITRG/MISTRG mice, which express thrombopoietin and macrophage colony-stimulating factor in addition to IL-3 and GM-CSF.26 NSGS and MISTRG mice have already demonstrated improved engraftment of myeloid cells and myeloid cell output of normal CD34+ cells as well as subtypes of AML that previously engrafted at low rates in NSG mice.26-30 However, robust xenotransplantation models are still lacking for several chronic myeloid neoplasms, particularly MDS31-33 and CMML. Given the known hypersensitivity of CMML and JMML to GM-CSF, we evaluated engraftment of CMML and JMML in NSGS mice here and identified highly robust engraftment of CD34+ cells as well as mononuclear cells (MNCs) from BM and peripheral blood (PB) from CMML and JMML patients. The ensuing serially transplantable grafts result in diseases that are remarkably similar to the morphology, immunophenotype, and genetic characteristics seen in primary patients. Moreover, the overt phenotypes identified in recipient NSGS mice using the xenotransplantation methods here facilitated evaluation of preclinical therapeutics, which are greatly needed for CMML and JMML.
Methods
Patient samples
Diagnostic BM or PB aspirates were obtained from a total of 18 distinct CMML patients, including 8 CMML patients treated at Memorial Sloan Kettering Cancer Center (MSKCC) and 10 from Moffitt Cancer Center as well as 4 JMML patients treated at the University of California, San Francisco (UCSF). Patient samples were collected after obtaining written informed consent. The use of human materials was approved by the Institutional Review Board of MSKCC, the Moffitt Cancer Center Scientific Review Committee, the University of South Florida Institutional Review Board, and UCSF, in accordance with the Declaration of Helsinki. Detailed patient characteristics are listed in Figure 1B, Figure 4A, and supplemental Figure 3B, available on the Blood Web site.
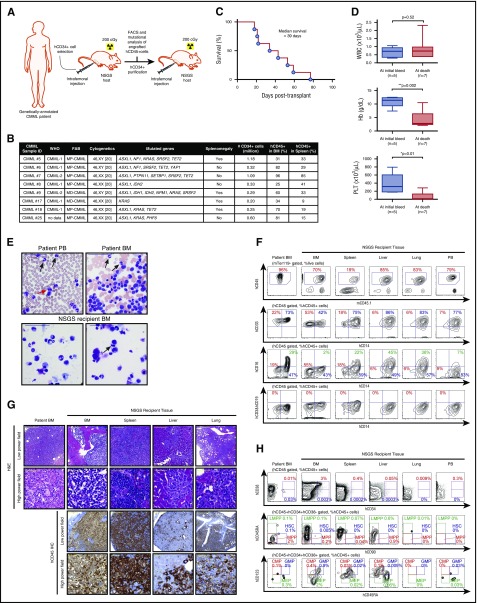
Figure 1.
Robust engraftment of CMML patient samples into NSGS mice. (A) Schema of method for engraftment of human CD34+ (hCD34+) cells from the BM of CMML patients into sublethally irradiated NSGS mice and serial transplantation. Each patient sample was injected into 1 NSGS-recipient mouse. (B) Clinical and genetic characteristics of CMML patient samples successfully engrafted along with number of cells transplanted per recipient mouse and chimerism of hCD45+ cells in BM and spleen of NSGS mice, French-American-British (FAB) classification. (C) Kaplan-Meier survival curve of recipient NSGS mice engrafted with CD34+ cells from CMML patients (n = 8). (D) White blood cell (WBC) count, hemoglobin (Hb), and platelet (PLT) count of recipient NSGS mice at initial bleed (2 weeks posttransplantation) and at time of moribund state (the mean value is represented by the line inside the box ± standard deviation represented by lines above and below the box; *P < .05, **P < .01; number of individual mice is represented in the figure). (E) Cytomorphology of primary patient PB and BM (top) and corresponding NSGS-recipient mouse BM xenografted from the same patient (bottom). Black arrows indicate dysplastic neutrophils; red arrow indicates binucleated erythroid precursor; scale: 50 μm. (F) Immunophenotype of a representative CMML patient BM (left column) and tissues from a corresponding recipient mouse xenografted from the same patient (the percentages listed represent the percent of cells within the gate described above each plot). (G) Hematoxylin and eosin (H&E) as well as IHC stain for hCD45+ cells in primary patient BM (left column) and corresponding NSGS tissues (scale bars for low-power field: 200 μm, original magnification ×100; scale bars for high-power field: 50 μm, original magnification ×1600). (H) Flow cytometric analysis for hematopoietic stem and progenitor compartments of BM, spleen, liver, lung, and PB from the same NSGS-recipient mouse (the percentages represent the percent of cells within hCD45+ cells). CMP, common myeloid progenitor; FACS, fluorescence-activated cell sorting; GMP, granulocyte/macrophage progenitor; HSC, hematopoietic stem cell; LMPP, lymphoid-primed multipotent progenitor; MEP, megakaryocyte/erythroid progenitor; MPP, multipotent progenitor.
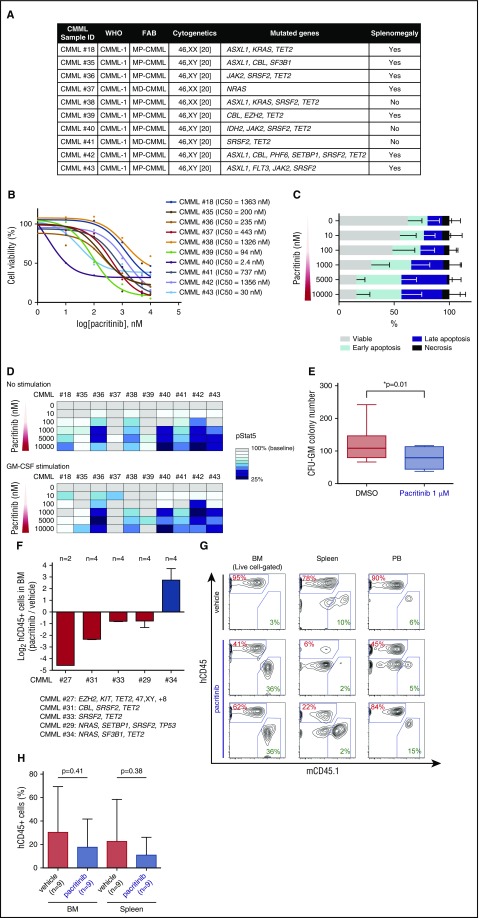
Figure 4.
Evaluation of the efficacy of pacritinib in CMML patient samples in vitro and in vivo. (A) Clinical and genetic characteristics of CMML patient samples used for pacritinib treatment in vitro and in vivo. (B) BM samples from CMML patients were cultured with pacritinib for 48 hours, and the half maximal inhibitory concentration was calculated for each patient sample based on the cell viability after drug exposure (n = 10). (C) Percentages of viable, early-apoptotic, late-apoptotic, and necrotic cells based on 4′,6-diamidino-2-phenylindole and Annexin V flow cytometric analysis of CMML BM MNCs treated with vehicle or pacritinib at the indicated doses in vitro (shown are mean percentages ± standard deviation across 10 patient samples). (D) Heatmap representation of phospho-STAT5 levels in CMML BM MNCs by intracellular flow cytometry after treatment with pacritinib at the indicated doses for 1 hour (top; median data across 10 patients is shown). Cells were also prestimulated with GM-CSF at 10 ng/mL for 15 minutes after incubation with pacritinib (bottom; n = 10). Levels of phospho-STAT5 in cells treated with pacritinib were evaluated considering the level of phospho-STAT5 in cells treated with dimethyl sulfoxide (DMSO) as 100% in each patient sample. (E) Colony numbers after 14 days of CMML BM MNCs seeded in methylcellulose with 1 μM pacritinib or dimethyl sulfoxide (CFU-GM, colony forming unit-granulocyte macrophage; median value is shown represented by the line inside the box ± standard deviation represented by lines above and below the box; n = 7 patient samples; *P < .05). (F) Log2 ratio of human CD45 (hCD45)+ cells in the BM of pacritinib- vs vehicle-treated PDX models from 5 CMML patients (n = 2 or 4 mice per patient). Genetic information for each patient is shown in supplemental Figure 3B. (G) Representative flow cytometric analysis of hCD45 in NSGS recipients xenografted with CMML BM MNCs following 7 days of oral administration of vehicle or 150 mg/kg/d pacritinib (mCD45.1: mouse CD45.1). (H) Comparison of hCD45 chimerism in the BM and spleen NSGS-recipient mice treated with either vehicle or pacritinib (mean value ± standard deviation and number of individual mice are represented in the figure).
Xenotransplantation
All animal experiments were carried out in accordance with institutional guidelines approved by MSKCC and Moffitt Cancer Center. Six- to 10-week-old NSGS (stock no. 013062) mice were purchased from the Jackson Laboratory and bred at each center under pathogen-free conditions. For xenotransplantation, mice were sublethally irradiated (200-250 cGy) 3 to 24 hours before transplantation followed by direct intrafemoral (as described previously34) or tail vein injection. CD34+ cells were purified from viably frozen patient BM MNCs by immunomagnetic selection (Miltenyi Biotec). Viably frozen BM or PB MNCs were used as source of cells for xenografts using unfractionated BM or PB MNCs. Complete blood count analysis was performed on PB collected from submandibular bleeding or cardiac puncture posteuthanasia using a Procyte Dx Hematology Analyzer (IDEXX Veterinary Diagnostics). Detailed methods for flow cytometric analysis, colony forming assays, histology, and in vitro and in vivo drug studies are noted in the supplemental Methods.
Mutational analysis
Genomic analysis of patient BM MNCs and fluorescence-activated cell sorting–purified hCD45+ cells from corresponding xenografts was performed using a targeted next-generation sequencing assay. This assay sequences all coding regions of 585 genes known to be recurrently mutated in leukemias, lymphomas, and solid tumors (the MSKCC HemePACT assay). Sequence library construction and bioinformatics analysis of mutations and copy number alterations were performed as previously described.35
Statistics
Prism Version 6 software (GraphPad) was used for statistical analysis. Data are presented as the mean ± standard deviation. Statistical analysis was performed using the 2-tailed Student t test for comparison of 2 groups to determine the level of significance.
Results
Xenotransplantation of CD34+ cells into NSGS mice results in robust engraftment of human CMML
Prior genomic analysis of CMML patients has demonstrated that clonal genetic alterations in CMML leukemic cells are present in the CD34+ CD38− cell fraction.13 Although xenotransplantation studies evaluating disease-initiating cells have not been performed previously for CMML, analogous studies in MDS33 and CML36,37 identified that disease-initiating cells are restricted to this same CD34+ CD38− population. Given this, we first evaluated the ability of CD34+ BM MNCs to give rise to CMML in vivo.
Direct intrafemoral injection of 0.2 to 1.18 × 106 cells from 8 CMML patients into sublethally irradiated NSGS mice gave rise to human hematopoietic cell engraftment (marked by hCD45 positivity) in 100% (8/8) of cases where ≥0.2 × 106 cells were injected per mouse (Figure 1A-B; supplemental Table 1). Engraftment levels based on hCD45+ cells harvested from engrafted mice at weeks 3 to 11 ranged from 25% to 96% in BM and 9% to 85% in spleen. This method of xenotransplantation resulted in lethality of recipient mice at a median of 39 days (Figure 1C). Death of NSGS-recipient mice was heralded by the development of anemia and thrombocytopenia. Mean hemoglobin and platelet counts at initial bleed were 11.4 g/dL and 316 × 103/μL, respectively, which fell to 2.8 g/dL and 12 × 103/μL at the time of death (P = .002 and 0.01, respectively, Student t test; Figure 1D).
We next evaluated the morphology and immunophenotype of PB and BM cells from recipient NSGS mice relative to samples directly taken from the corresponding patient. NSGS-recipient CMML xenografts reliably demonstrated dysplastic neutrophils and erythroid precursors as were present in patient samples (Figure 1E). In addition, the majority of cells engrafted in mice were of myeloid lineage with no lymphoid (hCD19+ or hCD3+) cell engraftment based on flow cytometric analysis of BM, spleen, and PB MNCs in recipient NSGS mice (Figure 1F; supplemental Figure 1A; supplemental Table 1). The vast majority of engrafted cells were hCD33+ or hCD33+/hCD14+ double-positive cells, the latter of which represent human monocytes. Importantly, human myeloid and monocytic cells robustly infiltrated non–hematopoietic organs in NSGS-recipient mice occupying 34% to 97% and 11% to 83% of total nucleated cells in the liver and lung, respectively, at the time of death (Figure 1F; supplemental Figure 1B). Recently, it was demonstrated that CMML patients have increased proportions of classical monocytes (CD14+/CD16−) at the expense of other monocyte fractions.30 Although the CMML patient–derived xenografts (PDXs) did not totally recapitulate the skewed repartition of monocyte subsets seen in CMML patients, a large proportion of hCD45+ cells were CD14+/CD16− classical monocytes in BM, PB, spleen, liver, and lung of recipient NSGS mice (Figure 1F; supplemental Figure 1A). Morphologic analysis, as well as immunohistochemical (IHC) staining for hCD45, revealed massive infiltration of leukemic cells in the BM, spleen, liver, and lung (Figure 1G; supplemental Figure 1C).
We next evaluated the composition and frequency of immunophenotypic human hematopoietic stem and progenitor cells (HSPCs) in CMML PDX models. At the time of death, evaluation of CD34+/CD38− and CD34+/CD38+ HSPC subsets across tissues revealed the presence of immunophenotypically -defined HSPCs in the BM (Figure 1H; supplemental Figure 1D).
CMML-initiating cells display long-term self-renewal capacity in serial transplantation assays
Despite advances in immunocompromised hosts and xenotransplantation methods, demonstrating serial transplantability of human hematopoietic cells from normal adult CD34+ cells or MDS patient samples has remained a major challenge.31,32 Even cotransplantation of mesenchymal stromal cells with MDS CD34+ cells fails to generate >5% hCD45+ cells in secondarily transplanted recipients.31 Moreover, prior work has suggested that human grafts may be less durable in NSGS mice than in NSG mice and less efficient in serial transplantation to secondary recipient mice.24,25 To test serial transplantability of CMML primary xenografts, hCD34+ cells were purified from primary recipients at the time of death and 0.2 to 0.5 × 106 cells per recipient were transplanted into sublethally irradiated secondary NSGS recipients (Figure 1A; supplemental Figure 2; supplemental Table 1). Serial transplantation of 5 patient samples was attempted. Two out of these 5 patient samples successfully engrafted in 6/11 secondary recipient mice with a level of hCD45+ cell engraftment that was >30% of the total MNCs in the BM. In contrast, the other 3 patient samples did not engraft (hCD45+ cells <1% of the BM MNCs) in the rest of 5 mice by day 201 to 219 posttransplant. Furthermore, this engraftment was composed of cells of human myeloid origin at the time of death (supplemental Figure 2A). In fact, the level of hCD45+ engraftment in primary vs secondary recipients was indistinguishable (supplemental Figure 2B). Serial transplantation using this method resulted in lethality of secondary recipients with a median of 99 days, and death was heralded by anemia and thrombocytopenia as was seen in primary recipients (supplemental Figure 2C-D). Moreover, immunophenotypically defined HSPCs38 were present in the BM of secondary recipients with a similar multipotent progenitor and CMP predominance, as was seen in primary recipients (supplemental Figure 2E).
Successful generation of CMML xenografts using unfractionated BM or PB MNCs
Given limitations in BM aspirate sampling and the labor intensity of HSPC purification as well as intrafemoral injection, we next tested the engraftment of unfractionated BM MNCs into NSGS mice via tail vein injection. Xenotransplantation of 2 to 4 × 106 BM MNCs were injected per sublethally irradiated NSGS-recipient mouse (supplemental Figure 3A). Ten CMML patients (listed in supplemental Figure 3B) distinct from those used for CD34+ cell transplants were injected into 38 recipients (supplemental Table 1). As with the CD34+ cell xenograft method, transplantation of BM MNCs resulted in lethality of recipient NSGS mice with a median of 72 days heralded by anemia and thrombocytopenia relative to saline-injected NSGS control recipients (supplemental Figure 3C-D). In addition, robust engraftment of hCD45+ cells was observed in flow cytometry analysis (supplemental Figure 3E). Morphological analysis of xenografted recipient mice revealed robust myeloid engraftment in BM, spleen, and liver, which were of human origin based on hCD45 IHC staining (supplemental Figure 3F). Moreover, morphologic features identified in patient BM biopsies (atypical eosinophils) were observed in the corresponding NSGS-recipient mice, suggesting that the PDX model is capable of recapitulating unique clinical features of disease (supplemental Figure 3F).
Given the success of the transplantation of BM MNCs, we next tested the engraftment of unfractionated PB MNCs into NSGS mice in direct comparison. We engrafted 2 to 4 × 106 BM MNCs or 2 to 4 × 106 PB MNCs from 2 patients into 10 and 6 NSGS recipients, respectively (supplemental Figure 4A). Here again engraftment of PB MNCs using this method resulted in robust human cell engraftment in BM, spleen, and PB, at levels indistinguishable from those generated using BM MNCs as a source (supplemental Figure 4B). Comparison of the levels of hCD45+ chimerism in BM, spleen, and PB across the 3 methods of xenotransplantation studied here revealed that each method resulted in comparable engraftment in the PB of recipient mice using CD34+, BM MNCs, or PB MNCs as source (Figure 2A). However, intrafemoral injection of CD34+ cells resulted in higher levels of engraftment in the BM (median 70% hCD45+ cells) compared with tail vein injection of BM or PB MNCs (median of 28% and 28% hCD45+ cells [P = .02 and P = .04], respectively; Student t test). Conversely, tail vein injection of BM or PB MNCs resulted in higher human chimerism in recipient spleens (mean value of 59% and 59% hCD45+ cells, respectively) compared with intrafemoral injection of hCD34+ cells (mean value of 29% hCD45+ cells; P = .02 and P = .04 relative to BM or PB MNCs, respectively, Student t test).
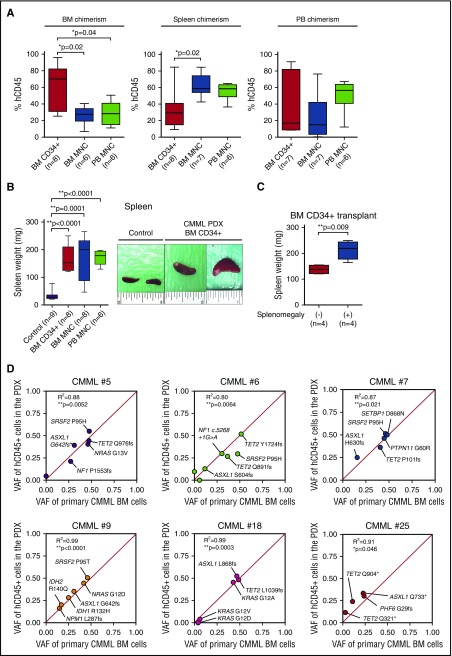
Figure 2.
Phenotypic and genetic evaluation of CMML xenografts. (A) Comparison of human CD45 (hCD45) chimerism in the BM, spleen, and PB of NSGS-recipient mice. Recipient NSGS mice were engrafted with purified BM CD34+ cells, BM MNCs, or PB MNCs (the mean value is represented by the line inside the box ± standard deviation represented by lines above and below the box; *P < .05; number of individual mice is represented in the figure). (B) Spleen weights of NSGS-recipient mice engrafted with purified BM CD34+ cells, BM MNCs, or PB MNCs from CMML patients and control mice (left). NSGS-recipient mice that were injected with <0.2 million hCD34+ cells and showed no evidence of engraftment of hCD45+ cells at day 201 posttransplant were used as controls (the mean value is represented by the line inside the box ± standard deviation represented by lines above and below the box; *P < .05, **P < .01; number of individual mice is represented in the figure). Representative photographs of spleens from mice injected with BM CD34+ cells and control mice are shown on the right (each photograph was taken with an inch ruler). (C) Spleen weights of NSGS-recipient mice engrafted with CD34+ cells from CMML patients with (n = 4) or without (n = 4) splenomegaly (the mean value is represented by the line inside the box ± standard deviation represented by lines above and below the box; **P < .01). (D) Variant allele frequency (VAF) of genomic DNA from BM MNCs from CMML patients (x-axis) and corresponding purified hCD45+ cells from engrafted NSGS mice at end stage (y-axis). The correlation of patient and xenograft VAF is measured in each case by the coefficient of determination (R2) and P values calculated by PRISM 6.
Despite modest differences in proportions of human chimerism across xenotransplantation methods, each method resulted in hepatosplenomegaly of engrafted mice (Figure 2B; supplemental Figure 1E). Although splenomegaly is a common cause of morbidity in CMML, it is only observed in a proportion of patients.2,3 Interestingly, xenografts generated from CMML patients with clinical evidence of splenomegaly had significantly larger spleens than those successfully generated from patients without clinical splenomegaly (P = .009 using hCD34+ cells as a source, Student t test; Figure 2C; supplemental Figure 4C). There was no difference in histology and survival between NSGS-recipient mice engrafted with BM MNCs and PB MNCs (supplemental Figure 4D-E).
Given the above correlation of phenotype in patients and corresponding xenografts, we next sought to compare additional clinical phenotypes across CMML patients and their PDX models. As mentioned earlier, despite having a nearly universally poor outcome, CMML is marked by disease heterogeneity with several prognostication classification systems for the disease. The 2008 WHO has stratified CMML into CMML-1 (<5% or <10% blasts in PB or BM, respectively) and CMML-2 (5%-19% or 10%-19% blasts in PB or BM, respectively).1,4 In addition, the French-American-British stratification of CMML groups the disease into myelodysplastic-CMML (MD-CMML) or myeloproliferative-CMML (MP-CMML) based on the absence or presence of leukocytosis (WBC > 13 000/μL), respectively.4 Although engraftment was achieved with both MD-CMML and MP-CMML (n = 3 and 14 patients, respectively), as well as CMML-1 and CMML-2 (n = 14 and 3 patients, respectively) in any of the 3 transplantation methods here (Figure 1B; supplemental Figure 3B), we could not make any firm conclusions regarding correlation of clinical subtype of CMML and likelihood of engraftment because of the small number of samples.
Clonal composition of CMML PDX models closely resembles those found in CMML patients
Although the above morphologic and immunophenotypic analyses suggested successful CMML engraftment, detection of human hematopoietic cells alone does not prove successful engraftment of human disease-initiating cells because normal residual HSCs can also engraft and repopulate in mice. Thus, we performed quantitative mutational analysis of CMML xenografts and compared mutations present in xenografted human cells to those directly from the corresponding patients. Mutational analysis of DNA purified from hCD45+ cells from 6 PDX models and total BM MNCs from the corresponding patient revealed striking similarity in both the mutations present as well as their allelic ratios (Figure 2D; supplemental Table 2). Targeted mutational analysis of 585 genes recurrently mutated in cancer (including all genes reported as mutated in human myeloid neoplasms) revealed near perfect correlation of VAF across primary patient and xenografted leukemias. In addition to confirming genetic accuracy of the xenografts created, these data also revealed that CMML xenografts nearly perfectly recapitulated the clonal architecture of CMML with 5/6 patients having at least 1 clonal mutated gene present at ∼50% VAF and 3 to 7 subclonal mutated genes present at <40% VAF (Figure 2D; supplemental Table 2).
Successful generation of JMML xenografts in NSGS mice
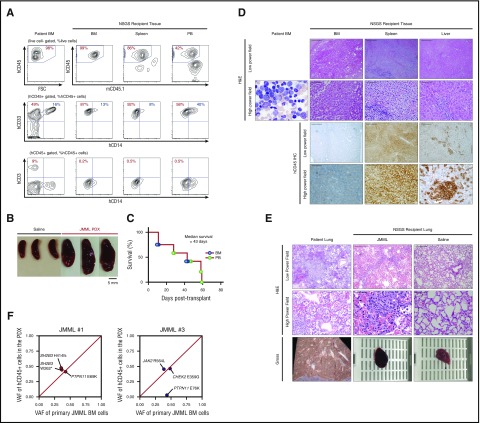
Given the robust engraftment of CMML in NSGS mice demonstrated above, we next evaluated engraftment of JMML in NSGS mice. As mentioned earlier, JMML is a MDS/MPN characterized by an overproduction of monocytes that infiltrate tissues and are marked by a near 100% frequency of mutations that activate RAS/MAPK signaling.5,7,8 Unlike CMML, for which serially transplantable and genetically accurate PDX models have not been generated previously, a serially transplantable JMML model has recently been described in RAG-IL2Rγnull mice.39 Tail vein injection of unfractionated BM (2.2-4.1 × 106) or PB MNCs (1.3-2.0 × 106) from 4 JMML patients resulted in robust myelomonocytic engraftment with splenomegaly in 7 out of 12 NSGS recipients (3 out of 4 patients, n = 6 BM and n = 6 PB) (Figure 3A-B; supplemental Table 1) and lethality of recipient mice at a median of 43 days (Figure 3C). Moreover, histological analysis of BM, spleen, and liver revealed human myelomonocytic engraftment similar to the disease infiltration pattern seen in the corresponding patient (Figure 3D). For example, 1 illustrative JMML patient (JMML no. 1) presented at 35 months of life with a WBC of 57.1 × 103/μL, an absolute monocyte count of 7.4 × 103/μL, circulating myeloid precursors, a fetal hemoglobin of 49%, and profound hepatosplenomegaly. Sequencing of his BM MNCs revealed somatic mutations in PTPN11 (p.E69K) and SH2B3 (p.W262* and p.H413fs). The patient was sequentially treated with chemotherapy and received a stem cell transplant but relapsed 34 days posttransplant with worsening splenomegaly and respiratory distress, suggesting an extremely aggressive clinical course. The patient died 45 days posttransplant because of respiratory failure, and the autopsy revealed an atypical acute and organizing diffuse alveolar damage due to hemorrhage, leukemic infiltration, and infectious pneumonitis. The corresponding NSGS recipients also had an aggressive phenotype with a median survival of 11 days, the shortest of any JMML PDX generated here. Strikingly, the PDX model also demonstrated leukemic infiltration accompanied by hemorrhage due to alveolar damage, a feature not seen in other NSGS mice xenografted with other patient samples here, suggesting that the model is capable of recapitulating unique clinical features of disease (Figure 3E). The clonal architecture of JMML no. 1 was well preserved in the xenograft, whereas that of JMML no. 3 was not fully recapitulated in the xenograft (Figure 3F; supplemental Table 2). In serial transplantation of unfractionated spleen cells from JMML no. 1 PDX, samples successfully engrafted in 3/3 secondary recipient mice. Secondary transplantation resulted in an aggressive phenotype with a short latency and a level of hCD45+ cell engraftment >55% of the total MNCs in the BM (supplemental Figure 5A-B) along with splenomegaly and alveolar hemorrhage as observed in the recipients of initial transplantation (supplemental Figure 5C-E).
Figure 3.
Establishment of PDX of JMML. (A) Immunophenotype of patient BM MNCs (left column) and tissues from a representative recipient mouse xenografted from the same patient (the percentages listed represent the percent of cells within the gate described above each plot; hCD45, human CD45; mCD45.1, mouse CD45.1). (B) Splenomegaly in JMML PDX mice when compared with spleens from NSGS mice injected with saline. (C) Kaplan-Meier survival curve of recipient NSGS mice engrafted with BM MNCs (n = 6) or PB MNCs (n = 6) from JMML patients (n = 4). (D) H&E and immunohistochemistry for hCD45+ cells in patient BM and the BM, spleen, and liver of xenografted BM MNCs from the same patient (scale bars for low-power field: 200 μm, original magnification ×100; scale bars for high-power field: 50 μm, original magnification ×1600). (E) H&E of 1 illustrative patient with lung hemorrhage at autopsy and the corresponding JMML PDX and saline-injected mouse for control (scale bars for low-power field: 200 μm, original magnification ×100; scale bars for high-power field: 50 μm, original magnification ×1600). (F) VAF of genomic DNA from BM MNCs from JMML patients (x-axis) and corresponding purified hCD45+ cells from engrafted NSGS mice at end stage (y-axis).
JAK2/FLT3 inhibition reduces CMML clonogenicity in vitro and leukemic burden in vivo
The hypersensitivity of CMML and JMML to GM-CSF has resulted in efforts to therapeutically target these diseases by targeting the GM-CSF receptor and/or its downstream signaling intermediates in the RAS/MAPK/PI3K, JAK/STAT, and NF-κB signaling pathways. Given promising results from our recent phase 1 clinical trial of the JAK1/2 inhibitor ruxolitinib in CMML,40 we decided to test the utility of the CMML preclinical models for evaluation of the JAK2/FLT3 inhibitor pacritinib.41,42 In addition to its capacity to potently inhibit JAK2 and FLT3, pacritinib is an attractive therapeutic because of its inhibitory effects on other deregulated pathways in CMML, such as those associated with CSF1R and IRAK1, both targets of pacritinib.43-45 Short-term culture of BM MNCs from CMML patients (Figure 4A; supplemental Table 1) in vehicle or increasing doses of pacritinib revealed effective CMML cell killing upon 48 hours of pacritinib exposure with a median half maximal inhibitory concentration of 339 nM (range 2.4-1363 nM) across 10 individual CMML patients (Figure 4B). Cell death occurred via apoptosis as was evident from dose-dependent increases in apoptotic and necrotic cells (Figure 4C). Pacritinib effectively inhibited phosphorylation of STAT5 (pSTAT5) with or without exposure to GM-CSF (Figure 4D). Finally, in vitro evaluation in colony forming assays of BM MNCs from CMML patients revealed effective inhibition of colony formation of CMML cells upon exposure to pacritinib (Figure 4E).
Although pacritinib was effective in inhibiting in vitro CMML growth and GM-CSF stimulation, we observed significant heterogeneity across patients with respect to pacritinib sensitivity, which is generally not expected in homogenous GEMMs in vivo.46,47 To determine if our PDX models could recapitulate the heterogeneity in sensitivity seen in vitro, we evaluated the effects of pacritinib on disease maintenance in vivo using the previously generated CMML PDX models. Seven days after injection of BM MNCs from 5 CMML patients into NSGS recipients, mice were treated with 7 days of pacritinib at 150 mg/kg/d or vehicle via oral gavage. Following 7 days of treatment, mice were sacrificed, and the proportion of human leukemic cells in BM, spleen, and PB was evaluated. Pacritinib was effective in reducing hCD45+ cells in each tissue compartment by day 50 posttransplant in 4 out of 5 patients, showing evidence of heterogeneity in response to in vivo pacritinib treatment as seen in vitro (Figure 4F-H).
Discussion
Given the severity of the diseases and limitations of currently available treatments, it is our priority here to develop better preclinical models to forward the improvement of future therapeutic interventions for both CMML and JMML. Moreover, CMML is associated with morbidity because of transfusion-dependent cytopenias and symptomatic splenomegaly (the latter of which occurs in ∼25% of CMML patients).2,3 Severe anemia and thrombocytopenia occur in ∼50% and 40% of CMML patients, respectively.2,3 Finally, extramedullary involvement and myelomonocytic infiltration are well established to occur in CMML and commonly reported in skin, lymph nodes, and other extramedullary sites in patients. Currently, no GEMMs of CMML reproduce the entire spectrum of disease manifestations while maintaining genetic fidelity to human CMML. To this end, we demonstrate here the generation of highly robust xenotransplantation models of CMML and JMML that accurately recapitulate these disease phenotypes in vivo. Although we did not see differences in survival in PDX models based on clinical classifications of the disease (CMML-1 vs CMML-2 or MD-CMML vs MP-CMML) using any of the 3 xenograft methods, greater numbers of distinct patient samples xenografted across larger numbers of recipient mice will be important to address whether NSGS CMML xenografts recapitulate outcomes predicted by CMML classification systems. It may also be helpful to determine if there is any correlation between the percentage of CD34+ cells in unfractionated BM or PB MNCs and likelihood of successful xenotransplantation of CMML and JMML in the future.
The phenotypic effects seen here with CMML xenotransplantation over a relatively short timeframe are unique given that previously published xenograft models of MDS31-33 and aggressive MPNs such as myelofibrosis48 do not result in overt phenotypic effects despite engraftment with human diseased cells. The high levels of human engraftment achieved here across numerous tissues with resulting lethality, cytopenias, and hepatosplenomegaly render these models very useful in preclinical evaluation of novel therapeutic approaches for CMML. The usefulness in evaluation of drug efficacy was demonstrated here with the evaluation of JAK2/FLT3 inhibition with pacritinib in vivo. The in vivo results combined with the effectiveness of pacritinib in reducing pSTAT5 levels in response to GM-CSF stimulation in vitro further confirm the importance of targeting GM-CSF cytokine stimulation as a therapeutic approach in CMML.
Of note, 1 prior study attempted xenotransplantation of CMML cells using NOD/SCID mice as well as a strain of NOD/SCID mice with transgenic expression of human GM-CSF.49 Interestingly, engraftment of hCD33+ cells was seen only in NOD/SCID mice expressing human GM-CSF, whereas no CMML patient samples engrafted in parental NOD/SCID mice. Nevertheless, hCD33+ engraftment never exceeded 30% in that study, and verification of engraftment of diseased vs normal cells was not performed.49 In addition, serial transplantability was not tested, and overt disease phenotypes were not reported in recipient mice. In contrast, here we identified that NSGS CMML PDX accurately recapitulated the recurrent mutations identified in the corresponding patients, a result that confirms bona fide engraftment of diseased cells. Moreover, the high VAF of mutations identified in total hCD45+ cells here without further subfractionation prior to sequencing confirms the high frequency of leukemic cells generated in these models. Finally, the fact that clonal and subclonal genetic alterations were preserved following xenotransplantation make these models very useful for evaluation of the effects of in vivo therapy on clonal composition of the disease.
In addition to use in preclinical evaluation of therapeutics, the CMML and JMML xenotransplantation methods demonstrated here will be very useful in clarifying the functional cellular origins of these disorders using primary patient materials. Unlike MDS or MPNs, HSPCs have not previously been demonstrated to initiate CMML using in vivo xenotransplantation assays. Here we demonstrate the serial transplantability of CD34+ cells from CMML patients in NSGS mice, whereas the CD34− compartment lacked disease transplantability. These results further confirm that the CD34+ cell compartment contains disease-initiating capacity in CMML, consistent with prior genomic analysis of purified cell subsets from CMML patients.
Acknowledgments
This work is supported by grants from the Aplastic Anemia and MDS International Foundation and the Lauri Strauss Leukemia Foundation (A.Y.); the Leukemia and Lymphoma Society Special Fellow Award (S.C.-W.L.); the American Society of Hematology Senior Research Training Award for Fellows (J.T. and B.H.D.); the New York State Council on Graduate Medical Education Empire Clinical Research Investigator Program Fellowship (B.H.D. and W.Y.); grants from the Moffitt Cancer Center, the American Society of Hematology, and the Edward P. Evans Foundation (E.P.); and grants from the Edward P. Evans Foundation, the Department of Defense Bone Marrow Failure Research Program (BM150092 and W81XWH-12-1-0041), the National Institutes of Health, National Heart, Lung, and Blood Institute (R01 HL128239), the Josie Robertson Investigator Program, an award from the Starr Foundation (I8-A8-075), the Leukemia and Lymphoma Society, and the Pershing Square Sohn Cancer Research Alliance (O.A.-W.).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: A.Y., M.E.B., A.V., Y.M., H.Z., S.C.-W.L., C.L., S.X.L., M.B., J.T., Q.Z., Y.Z., S.Y., Y.R.C., X.J.Z., B.H.D., W.Y., M.F.B., E.P., and O.A.-W. performed the experiments and analyzed data; K.F., S.N., A.F.L., M.L.L., V.K., and E.S. collected patient material and clinical data; and A.Y., M.E.B., E.P., and O.A.-W. wrote the manuscript.
Conflict-of-interest disclosure: E.P. received research funding from Cell Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Eric Padron, Malignant Hematology Program,H. Lee Moffitt Cancer Center and Research Institute, 12902 Magnolia Dr, Tampa, FL 33612; e-mail: eric.padron@moffitt.org; and Omar Abdel-Wahab, Human Oncology and Pathogenesis Program and Leukemia Service, Department of Medicine, Memorial Sloan Kettering Cancer Center, 1275 York Ave, New York, NY 10065; e-mail: abdelwao@mskcc.org.
References
- 1.Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391-2405. [DOI] [PubMed] [Google Scholar]
- 2.Itzykson R, Fenaux P, Solary E. Chronic myelomonocytic leukemia: myelodysplastic or myeloproliferative? [published correction appears in Best Pract Res Clin Haematol. 2014;27(1):79 ]. Best Pract Res Clin Haematol. 2013;26(4):387-400. [DOI] [PubMed] [Google Scholar]
- 3.Padron E, Steensma DP. Cutting the cord from myelodysplastic syndromes: chronic myelomonocytic leukemia-specific biology and management strategies. Curr Opin Hematol. 2015;22(2):163-170. [DOI] [PubMed] [Google Scholar]
- 4.Savona MR, Malcovati L, Komrokji R, et al. ; MDS/MPN International Working Group. An international consortium proposal of uniform response criteria for myelodysplastic/myeloproliferative neoplasms (MDS/MPN) in adults. Blood. 2015;125(12):1857-1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Locatelli F, Niemeyer CM. How I treat juvenile myelomonocytic leukemia. Blood. 2015;125(7):1083-1090. [DOI] [PubMed] [Google Scholar]
- 6.Chang TY, Dvorak CC, Loh ML. Bedside to bench in juvenile myelomonocytic leukemia: insights into leukemogenesis from a rare pediatric leukemia. Blood. 2014;124(16):2487-2497. [DOI] [PubMed] [Google Scholar]
- 7.Stieglitz E, Taylor-Weiner AN, Chang TY, et al. The genomic landscape of juvenile myelomonocytic leukemia. Nat Genet. 2015;47(11):1326-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caye A, Strullu M, Guidez F, et al. Juvenile myelomonocytic leukemia displays mutations in components of the RAS pathway and the PRC2 network. Nat Genet. 2015;47(11):1334-1340. [DOI] [PubMed] [Google Scholar]
- 9.Emanuel PD, Bates LJ, Castleberry RP, Gualtieri RJ, Zuckerman KS. Selective hypersensitivity to granulocyte-macrophage colony-stimulating factor by juvenile chronic myeloid leukemia hematopoietic progenitors. Blood. 1991;77(5):925-929. [PubMed] [Google Scholar]
- 10.Yoshida K, Sanada M, Shiraishi Y, et al. Frequent pathway mutations of splicing machinery in myelodysplasia. Nature. 2011;478(7367):64-69. [DOI] [PubMed] [Google Scholar]
- 11.Patnaik MM, Lasho TL, Finke CM, et al. Spliceosome mutations involving SRSF2, SF3B1, and U2AF35 in chronic myelomonocytic leukemia: prevalence, clinical correlates, and prognostic relevance. Am J Hematol. 2013;88(3):201-206. [DOI] [PubMed] [Google Scholar]
- 12.Itzykson R, Kosmider O, Renneville A, et al. Prognostic score including gene mutations in chronic myelomonocytic leukemia. J Clin Oncol. 2013;31(19):2428-2436. [DOI] [PubMed] [Google Scholar]
- 13.Itzykson R, Kosmider O, Renneville A, et al. Clonal architecture of chronic myelomonocytic leukemias. Blood. 2013;121(12):2186-2198. [DOI] [PubMed] [Google Scholar]
- 14.Meggendorfer M, Roller A, Haferlach T, et al. SRSF2 mutations in 275 cases with chronic myelomonocytic leukemia (CMML). Blood. 2012;120(15):3080-3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gelsi-Boyer V, Trouplin V, Adélaïde J, et al. Mutations of polycomb-associated gene ASXL1 in myelodysplastic syndromes and chronic myelomonocytic leukaemia. Br J Haematol. 2009;145(6):788-800. [DOI] [PubMed] [Google Scholar]
- 16.Delhommeau F, Dupont S, Della Valle V, et al. Mutation in TET2 in myeloid cancers. N Engl J Med. 2009;360(22):2289-2301. [DOI] [PubMed] [Google Scholar]
- 17.Padron E, Painter JS, Kunigal S, et al. GM-CSF-dependent pSTAT5 sensitivity is a feature with therapeutic potential in chronic myelomonocytic leukemia. Blood. 2013;121(25):5068-5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim E, Ilagan JO, Liang Y, et al. SRSF2 mutations contribute to myelodysplasia by mutant-specific effects on exon recognition. Cancer Cell. 2015;27(5):617-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abdel-Wahab O, Gao J, Adli M, et al. Deletion of Asxl1 results in myelodysplasia and severe developmental defects in vivo. J Exp Med. 2013;210(12):2641-2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klinakis A, Lobry C, Abdel-Wahab O, et al. A novel tumour-suppressor function for the Notch pathway in myeloid leukaemia. Nature. 2011;473(7346):230-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moran-Crusio K, Reavie L, Shih A, et al. Tet2 loss leads to increased hematopoietic stem cell self-renewal and myeloid transformation. Cancer Cell. 2011;20(1):11-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quivoron C, Couronné L, Della Valle V, et al. TET2 inactivation results in pleiotropic hematopoietic abnormalities in mouse and is a recurrent event during human lymphomagenesis. Cancer Cell. 2011;20(1):25-38. [DOI] [PubMed] [Google Scholar]
- 23.Doulatov S, Notta F, Laurenti E, Dick JE. Hematopoiesis: a human perspective. Cell Stem Cell. 2012;10(2):120-136. [DOI] [PubMed] [Google Scholar]
- 24.Goyama S, Wunderlich M, Mulloy JC. Xenograft models for normal and malignant stem cells. Blood. 2015;125(17):2630-2640. [DOI] [PubMed] [Google Scholar]
- 25.Nicolini FE, Cashman JD, Hogge DE, Humphries RK, Eaves CJ. NOD/SCID mice engineered to express human IL-3, GM-CSF and Steel factor constitutively mobilize engrafted human progenitors and compromise human stem cell regeneration. Leukemia. 2004;18(2):341-347. [DOI] [PubMed] [Google Scholar]
- 26.Rongvaux A, Willinger T, Martinek J, et al. Development and function of human innate immune cells in a humanized mouse model. Nat Biotechnol. 2014;32(4):364-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wunderlich M, Chou FS, Link KA, et al. AML xenograft efficiency is significantly improved in NOD/SCID-IL2RG mice constitutively expressing human SCF, GM-CSF and IL-3. Leukemia. 2010;24(10):1785-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ellegast JM, Rauch PJ, Kovtonyuk LV, et al. inv(16) and NPM1mut AMLs engraft human cytokine knock-in mice. Blood. 2016;128(17):2130-2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saito Y, Ellegast JM, Rafiei A, et al. Peripheral blood CD34+ cells efficiently engraft human cytokine knock-in mice. Blood. 2016;128(14):1829-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Selimoglu-Buet D, Wagner-Ballon O, Saada V, et al. ; Francophone Myelodysplasia Group. Characteristic repartition of monocyte subsets as a diagnostic signature of chronic myelomonocytic leukemia. Blood. 2015;125(23):3618-3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Medyouf H, Mossner M, Jann JC, et al. Myelodysplastic cells in patients reprogram mesenchymal stromal cells to establish a transplantable stem cell niche disease unit. Cell Stem Cell. 2014;14(6):824-837. [DOI] [PubMed] [Google Scholar]
- 32.Mossner M, Jann JC, Wittig J, et al. Mutational hierarchies in myelodysplastic syndromes dynamically adapt and evolve upon therapy response and failure. Blood. 2016;128(9):1246-1259. [DOI] [PubMed] [Google Scholar]
- 33.Pang WW, Pluvinage JV, Price EA, et al. Hematopoietic stem cell and progenitor cell mechanisms in myelodysplastic syndromes. Proc Natl Acad Sci USA. 2013;110(8):3011-3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chung YR, Kim E, Abdel-Wahab O. Femoral bone marrow aspiration in live mice. J Vis Exp. 2014;(89):e51660. [DOI] [PMC free article] [PubMed]
- 35.Cheng DT, Mitchell TN, Zehir A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): a hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn. 2015;17(3):251-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eisterer W, Jiang X, Christ O, et al. Different subsets of primary chronic myeloid leukemia stem cells engraft immunodeficient mice and produce a model of the human disease. Leukemia. 2005;19(3):435-441. [DOI] [PubMed] [Google Scholar]
- 37.Gerber JM, Qin L, Kowalski J, et al. Characterization of chronic myeloid leukemia stem cells. Am J Hematol. 2011;86(1):31-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Majeti R, Park CY, Weissman IL. Identification of a hierarchy of multipotent hematopoietic progenitors in human cord blood. Cell Stem Cell. 2007;1(6):635-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krombholz CF, Aumann K, Kollek M, et al. Long-term serial xenotransplantation of juvenile myelomonocytic leukemia recapitulates human disease in Rag2-/-γc-/- mice. Haematologica. 2016;101(5):597-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Padron E, Dezern A, Andrade-Campos M, et al. ; Myelodysplastic Syndrome Clinical Research Consortium. A multi-institution phase I trial of ruxolitinib in patients with chronic myelomonocytic leukemia (CMML). Clin Cancer Res. 2016;22(15):3746-3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poulsen A, William A, Blanchard S, et al. Structure-based design of oxygen-linked macrocyclic kinase inhibitors: discovery of SB1518 and SB1578, potent inhibitors of Janus kinase 2 (JAK2) and Fms-like tyrosine kinase-3 (FLT3). J Comput Aided Mol Des. 2012;26(4):437-450. [DOI] [PubMed] [Google Scholar]
- 42.William AD, Lee AC, Blanchard S, et al. Discovery of the macrocycle 11-(2-pyrrolidin-1-yl-ethoxy)-14,19-dioxa-5,7,26-triaza-tetracyclo[19.3.1.1(2,6).1(8,12)]heptacosa-1(25),2(26),3,5,8,10,12(27),16,21,23-decaene (SB1518), a potent Janus kinase 2/fms-like tyrosine kinase-3 (JAK2/FLT3) inhibitor for the treatment of myelofibrosis and lymphoma. J Med Chem. 2011;54(13):4638-4658. [DOI] [PubMed] [Google Scholar]
- 43.Obba S, Hizir Z, Boyer L, et al. The PRKAA1/AMPKα1 pathway triggers autophagy during CSF1-induced human monocyte differentiation and is a potential target in CMML. Autophagy. 2015;11(7):1114-1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rhyasen GW, Bolanos L, Fang J, et al. Targeting IRAK1 as a therapeutic approach for myelodysplastic syndrome. Cancer Cell. 2013;24(1):90-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singer JW, Al-Fayoumi S, Ma H, Komrokji RS, Mesa R, Verstovsek S. Comprehensive kinase profile of pacritinib, a nonmyelosuppressive Janus kinase 2 inhibitor. J Exp Pharmacol. 2016;8:11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mullally A, Lane SW, Ball B, et al. Physiological Jak2V617F expression causes a lethal myeloproliferative neoplasm with differential effects on hematopoietic stem and progenitor cells. Cancer Cell. 2010;17(6):584-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koppikar P, Abdel-Wahab O, Hedvat C, et al. Efficacy of the JAK2 inhibitor INCB16562 in a murine model of MPLW515L-induced thrombocytosis and myelofibrosis. Blood. 2010;115(14):2919-2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang X, Prakash S, Lu M, et al. Spleens of myelofibrosis patients contain malignant hematopoietic stem cells. J Clin Invest. 2012;122(11):3888-3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramshaw HS, Bardy PG, Lee MA, Lopez AF. Chronic myelomonocytic leukemia requires granulocyte-macrophage colony-stimulating factor for growth in vitro and in vivo. Exp Hematol. 2002;30(10):1124-1131. [DOI] [PubMed] [Google Scholar]