Abstract
Background
Poorer virologic response to nevirapine vs efavirenz-based antiretroviral therapy (ART) has been reported in adult systematic reviews and pediatric studies.
Methods
We compared drug discontinuation and viral load (VL) response in ART-naïve Ugandan/Zimbabwean children ≥3 years of age initiating ART with clinician-chosen nevirapine vs efavirenz in the ARROW trial. Predictors of suppression <80, <400 and <1000 copies/ml at 36, 48 and 144 weeks were identified using multivariable logistic regression with backwards elimination (p=0.1).
Results
445(53%) children received efavirenz and 391(47%) nevirapine. Children receiving efavirenz were older (median 8.6 years vs 7.5 nevirapine, p<0.001) and had higher CD4% (12% vs 10%, p=0.05) but similar pre-ART VL (p=0.17). The initial non-nucleoside-reverse-transcriptase-inhibitor (NNRTI) was permanently discontinued for adverse events in 7/445 (2%) children initiating efavirenz vs 9/391 (2%) initiating nevirapine (p=0.46); at switch to second-line in 17 vs 23, for tuberculosis in 0 vs 26, for pregnancy in 6 vs 0, and for other reasons in 15 vs 5. Early (36-48 week) virologic suppression <80 copies/ml was superior with efavirenz, particularly in children with higher pre-ART VL (p=0.0004); longer-term suppression was superior with nevirapine in older children (p=0.05). Early suppression was poorer in the youngest and oldest children, regardless of NNRTI (p=0.02); longer-term suppression was poorer in those with higher pre-ART VL regardless of NNRTI (p=0.05). Results were broadly similar for <400 and <1000 copies/ml.
Conclusion
Short-term VL suppression favored efavirenz, but long-term relative performance was age-dependent, with better suppression in older children with nevirapine, supporting WHO’s recommendation that nevirapine remain an alternative NNRTI.
Keywords: HIV, children, antiretroviral therapy, viral load, NNRTI
Introduction
Globally, >3 million children/adolescents are living with HIV, >90% in sub-Saharan Africa(1). WHO guidelines recommend children/adolescents ≥3 years initiate two nucleoside-reverse-transcriptase-inhibitors (NRTI) plus one non-nucleoside-reverse-transcriptase-inhibitor (NNRTI). The preferred NNRTI is efavirenz (with nevirapine an alternative) based on systematic reviews indicating better viral load (VL) response(2) and short-term toxicity(3). However, pediatric nevirapine-based fixed-dose-combinations are widespread in resource-limited settings(4); understanding whether nevirapine is associated with poorer virologic response in children initiating ART ≥3 years of age has continuing programmatic relevance.
In adults, a 2010 Cochrane review concluded that nevirapine and efavirenz had equivalent efficacy based on 7 randomized controlled trials(5). A separate examination of 5 observational studies in low- and middle-income countries generally favored efavirenz; 6 studies in high-income countries were more heterogeneous. The 2012 systematic review(2), on which the WHO guidelines were based, included 26 trials and 7 observational studies with tenofovir+lamivudine or tenofovir+emtricitabine backbones(6). This review, and a 2013 meta-analysis(7) of 10 trials and 28 studies with no 2NRTI backbone restriction, concluded efavirenz had superior efficacy. However, a 2012 systematic review(8) restricted to 7 trials in resource-limited settings concluded that nevirapine and efavirenz showed similar efficacy.
In children, observational studies have also generally concluded that efvairenz had superior efficacy. In 804 Batswana children 3-16 years of age initiating ART with nevirapine (median age 7 years) or efavirenz (8 years) (NNRTI chosen by clinician), 101/383 (26%) receiving nevirapine and 57/421 (14%) efavirenz experienced virologic failure (lack of suppression to <400 copies/ml by 6 months or confirmed ≥400 copies/ml post-suppression) (unadjusted hazard ratio (HR)=2.0 [95% CI 1.4-2.7] p<0.001, adjusted HR (aHR) reported as similar)(9). Thai studies have generally reported similar results(10, 11), most recently in 2015(11) where nevirapine was a predictor for virologic failure (≥1000 copies/ml after ≥24 weeks ART) (aHR=1.63 [1.14-2.32] p=0.004), although a 2011 study(12) reported no significant difference (unadjusted HR=1.46 [0.66-3.22]). In 675 children <18 years (84% ≥3 years) in the UK/Ireland Collaborative HIV Paediatric Study (CHIPS) initiating 2NRTI plus nevirapine (median age 4 years) or efavirenz (10 years), suppression <400 copies/ml within 12 months did not significantly differ (adjusted rate ratio (aRR)(efavirenz:nevirapine)=1.16 [0.95-1.41]) but over all follow-up, risk of subsequent virologic failure (confirmed >400 copies/ml) was lower with efavirenz (aRR=0.54 [0.40-0.72])(13). Differences were most pronounced in the first 2 years (interaction p=0.03). Two cross-sectional Tanzanian studies reported that risk of virologic failure was lower with efavirenz(14, 15). A study of 250 Ugandan children/adolescents (median age 9 years, range 0-18) compared efavirenz predominantly with zidovudine+lamivudine vs nevirapine predominantly with stavudine+lamivudine. Twelve-month virologic failure was higher in nevirapine (adjusted odds ratio(aOR)(nevirapine:efavirenz)=2.46 [1.23-4.90] p=0.01)(16).
Considering toxicity, a meta-analysis found nevirapine had more adverse events (AEs) resulting in drug substitution or treatment discontinuation (OR=2.2 [1.9-2.6] in adults ≥15 years; limited data in infants/children with each study reporting different outcomes)(3). However, with efavirenz, one concern is central nervous system (CNS) events, with a risk ratio (vs nevirapine) of 1.4 [0.75-2.59] in children/adolescents ≥5 years in one Ugandan study(17). However, in the recent CHIPS study, discontinuation due to toxicity was 27/370 (7.3%) with 2 or 3 NRTIs plus nevirapine vs 32/424 (7.5%) with efavirenz(13).
We therefore compared VL response and treatment discontinuation on first-line nevirapine- and efavirenz-based ART initiated in children ≥3 years of age in the ARROW trial(18).
Materials and Methods
Observational analyses included 836 previously untreated Ugandan/Zimbabwean children initiating efavirenz- or nevirapine-based ART, 3-17 years of age in the ARROW trial (ISCRTN24791884)(18). Children were randomized 1:1:1 to open-label lamivudine+abacavir+NNRTI continuously (Arm-A, control, no zidovudine); induction-maintenance with 4-drug lamivudine+abacavir+NNRTI+zidovudine for 36 weeks, then lamivudine+abacavir+NNRTI (Arm-B; short-term zidovudine) or lamivudine+abacavir+zidovudine (Arm-C; long-term zidovudine). The NNRTI (nevirapine/efavirenz) was chosen by clinicians; both were available in all centers throughout the trial for initial ART and substitutions. Simultaneously, children were randomized 1:1 in a factorial design to clinically driven monitoring vs laboratory plus clinical monitoring for toxicity (hematology/biochemistry) and efficacy (CD4). After ≥36 weeks, eligible children taking lamivudine+abacavir twice-daily were randomized to continue twice-daily or move to once-daily. Children were recruited from one Zimbabwean (University of Zimbabwe, Harare) and three Ugandan centers (Joint Clinical Research Centre, Kampala; Baylor-Uganda, Mulago; MRC/UVRI Uganda Research Unit on AIDS, Entebbe). ARROW was approved by Research Ethics Committees in Uganda, Zimbabwe and the UK. Caregivers gave written consent.
Post-baseline VL was assayed retrospectively on stored plasma at 4, 24, 36, 48 and 144 weeks in all children <5 years at ART initiation. VL was also assayed at these time points and 24-weekly post-week 48 in a subset of children enrolled post-June 2008 (immunology sub study); and at, and 48 and 96 weeks after, randomization to once- vs twice-daily lamivudine+abacavir (shown to be virologically equivalent)(19). Assays used Abbott RealTime and Roche Amplicor 1.5: because many samples had low volumes and had to be diluted 1:2, the lower limit of detection was 80 copies/ml. Analysis used closest measurements to nominal time points in equally spaced windows (results available for 37%(n=309), 35%(n=282), 42%(n=341), 44%(n=241), 49%(n=265), respectively, of those alive and in follow-up, broadly similar in nevirapine vs efavirenz). In 145 Arm-A/B immunology sub study children alive and in follow-up at 24 weeks (with complete VLs), virologic failure was defined as ≥400 copies/ml at week 24 or subsequent confirmed ≥400 copies/ml through 3 years (9, 13, 16).
The primary (non-randomized) exposure was NNRTI received at ART initiation (efavirenz vs nevirapine) using intention-to-treat. Child characteristics were compared across these groups using chi-squared (categorical factors) and Wilcoxon (continuous factors) tests. Suppression <80, <400 and <1000 copies/ml with efavirenz and nevirapine was compared using generalized estimating equations for global tests over time (binomial distribution, independent working correlation). 36, 48 and 144 weeks post-ART initiation, predictors of suppression <80, <400 and <1000 copies/ml were identified using logistic regression, forcing efavirenz vs nevirapine and age at ART initiation into models. Models included children with VL at baseline and the relevant time point (92/89/83% of those with VLs at 36/48/144 weeks). Other factors considered as potential confounders were pre-ART WHO stage, CD4%, weight/height-for-age(20), VL, gender, center, ART-strategy randomization, monitoring randomization, whether the caregiver/child reported missed doses in the last 4 weeks, and percentage of scheduled visits to date with missed doses in the last 4 weeks. For each time point, independent predictors of suppression <80 copies/ml were identified using backward elimination (exit p≥0.1 to develop an explanatory models; interactions between variables in final models retained where p<0.1). Additional predictors of suppression <400 and <1000 copies/ml were then identified using forward selection (entry p=0.1), forcing in factors included in the <80 copies/ml model. Factors in any of the models were then included in final time point-specific models for each threshold, allowing the impact of the same factor to be assessed over the different thresholds. Nonlinearity was explored using natural cubic splines(21) (knots at 10th, 50th, 90th centiles), then represented by categorization. Potential confounders of the association between efavirenz/nevirapine and early death (before week 36) and permanent discontinuation of initial NNRTI were identified using logistic (adjusting for CD4% only (low number of events)) and Cox regression (backwards elimination) respectively. Analyses used Stata 14.1 (StataCorp). p-values are two-sided.
Results
836 previously untreated children 3-17 years of age initiated ART between March 2007-October 2008. 445(53%) received efavirenz and 391(47%) nevirapine. Children on efavirenz were more likely to be male and WHO stage 1/2, were older and less underweight/stunted at ART initiation (p<0.01), but had similar VL (p=0.17) (Table 1). Reflecting local availability, center strongly predicted receiving efavirenz vs nevirapine (p<0.001).
Table 1. Characteristics of children receiving efavirenz and nevirapine at ART initiation.
| Efavirenz (n=445) | Nevirapine (n=391) | P* | |
|---|---|---|---|
| Male | 242 (54%) | 176 (45%) | 0.007 |
| Age (years): median (IQR) | 8.6 (6.4-11.0) | 7.5 (4.9-10.1) | <0.001 |
| CD4 (cells/µl): median (IQR) | 277 (115-436) | 261 (102-476) | 0.94 |
| CD4% | 12 (6-18) | 10 (5-17) | 0.05 |
| Weight-for-age Z-score: median (IQR) | -1.7 (-2.6 to -1.0) | -2.4 (-3.6 to -1.5) | <0.001 |
| Height-for-age Z-score: median (IQR) | -1.9 (-2.8 to -1.1) | -2.6 (-3.5 to -1.6) | <0.001 |
| Viral load (copies/ml): median (IQR) | 160000 (52000-422000)** | 199000 (67000-453000)*** | 0.17 |
| WHO stage 3/4 | 269 (60%) | 322 (82%) | <0.001 |
| On TB treatment | 43 (10%) | 0 (0%) | <0.001 |
| Randomized treatment strategy | 0.61 | ||
| Arm-A (3TC/ABC/NNRTI throughout) | 141 (32%) | 135 (35%) | |
| Arm-B
(3TC/ABC/NNRTI
throughout, ZDV until week 36) |
153 (34%) | 134 (34%) | |
| Arm-C
(3TC/ABC/ZDV
throughout, NNRTI until week 36) |
151 (34%) | 122 (31%) | |
| Allocated monitoring strategy | 0.75 | ||
| Routine CD4 monitoring | 225 (51%) | 202 (52%) | |
| No CD4 monitoring | 220 (49%) | 189 (48%) | |
| Country/centre*** | <0.001 | ||
| Uganda/Entebbe | 52 (12%) | 94 (24%) | |
| Uganda/JCRC | 205 (46%) | 25 (6%) | |
| Uganda/PIDC | 98 (22%) | 45 (12%) | |
| Zimbabwe/Harare | 90 (20%) | 227 (58%) |
Chi-squared tests for categorical measures and Wilcoxon rank-sum tests for continuous measures unless otherwise indicated
n=260 (185 no baseline viral loads)
n=235 (156 no baseline viral load)
36% received efavirenz in Entebbe, 89% in JCRC, 69% in PIDC, 28% in Harare
Four of four hundred forty-five (1%) initiating efavirenz and 18/391 (5%) initiating nevirapine died before week 36. Whilst the difference persisted after adjusting for CD4% (p=0.01), causes of death were primarily infection-related and similar between efavirenz/nevirapine (septicemia/meningitis 0/7, pneumonia 1/4, chronic diarrhea/wasting/hypokalemia 1/3, stroke/cerebrovascular 1/2, uncertain 1/2), with similarly low CD4% (median(IQR) 8(5-11) vs 3(1-14) respectively). The initial NNRTI was permanently discontinued before week 36 in 8(2%) initiating efavirenz [2 AE; 4 voluntary decision; 2 pregnancy-related] and 22(6%) initiating nevirapine [9 AE; 13 tuberculosis treatment] (p=0.001 Cox regression adjusting for age at ART initiation). At week 36, those randomized to Arm-C (3NRTI maintenance) discontinued NNRTI (excepting five/two previously discontinuing zidovudine (anemia)/abacavir (hypersensitivity) respectively). Amongst children randomized to 2NRTI+NNRTI maintenance (Arm-A/B), 13 died after week 36 [6/294 (2%) efavirenz; 7/269 (3%) nevirapine]. After week 36, the initial NNRTI was permanently discontinued in 37/294 (13%) initiating efavirenz [5 AE; 17 switch to second-line; 4 pregnancy-related; 1 voluntary decision; 10 other] and 41/269 (15%) nevirapine [23 switch to second-line; 13 tuberculosis treatment; 1 voluntary decision; 4 other] (Cox p=0.31 unadjusted, 0.009 adjusted for age and CD4% at ART initiation and center). Overall, Arm-A/B children initiating efavirenz or nevirapine spent 94.4% and 88.9% follow-up-time through to their last clinic visit (median 4 years follow-up) on efavirenz or nevirapine-containing ART, respectively.
Over total follow-up, the initial NNRTI was permanently discontinued due to an AE in 7/445 (2%) initiating efavirenz (3 lipodystrophy (2 Arm-B previously receiving zidovudine); 2 gynaecomastia; 2 hypersensitivity reaction) and 9/391 (2%) nevirapine (4 hypersensitivity reaction; 1 Stevens-Johnson Syndrome; 1 rash; 1 acute febrile episode; 1 lactic acidosis; 1 raised liver enzymes) (p=0.46 Fisher’s exact test). In nevirapine, all 9 AEs were <10 weeks post-ART initiation (median 23 days (IQR 15-30)) and 8/9 substituted with efavirenz; in efavirenz, 2 were <10 weeks, the remaining 5 were a median 3.3 years (3.2-3.8) after ART initiation.
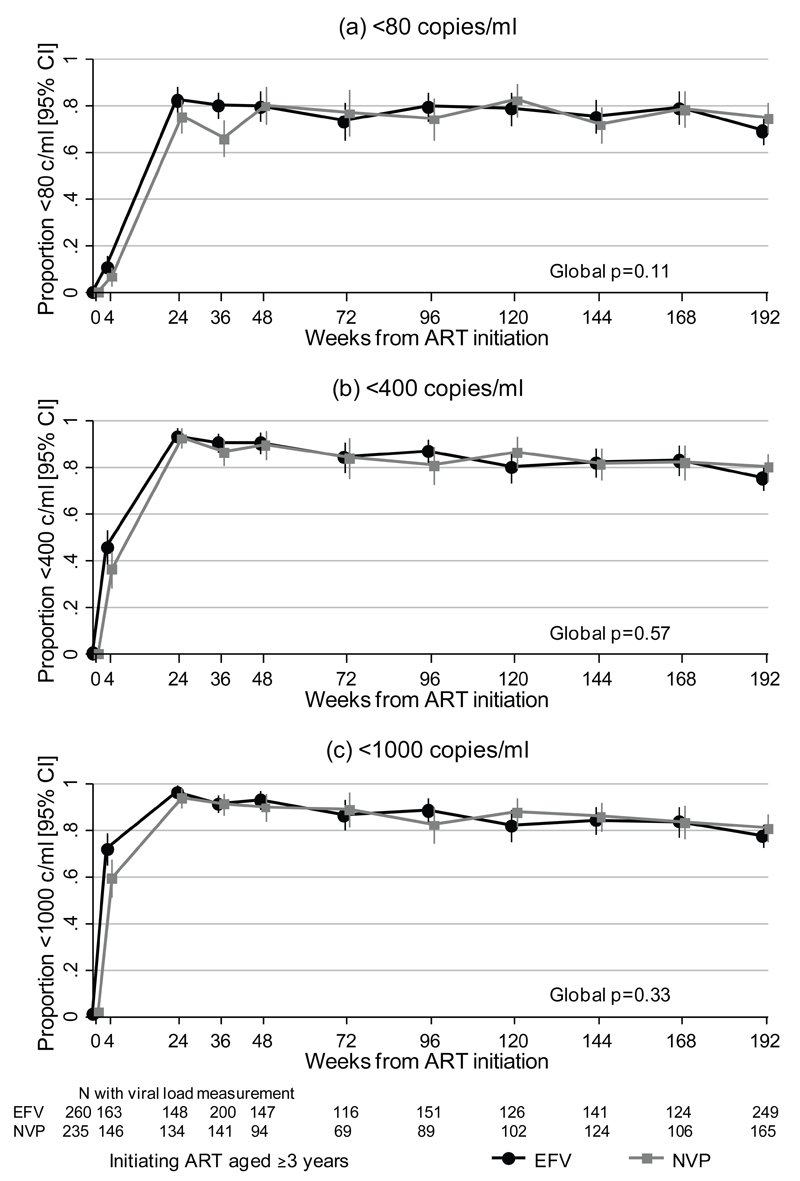
459/563 (81.5%) initiating long-term NNRTI (Arm-A/B) had ≥1 post-baseline VL. Over all follow-up there was a trend to better suppression <80 copies/ml with efavirenz (global unadjusted p=0.11), driven by effects before 36 weeks (p=0.007 ≤36 weeks, p=0.83 ≥48 weeks). There was no evidence of difference for <400 copies/ml (p=0.57) or <1000 copies/ml (p=0.33), with a difference in <1000 copies/ml at week 4 only (p=0.02, ≥24 weeks p=0.77) (Figure 1). Mean VL reduction from weeks 0–4 was 2.4log10 with efavirenz and 2.3log10 with nevirapine (p=0.18, n=302). At week 36, 160/200 (80.0%) efavirenz vs 93/141 (66.0%) nevirapine were <80 copies/ml (+14.0% [95% CI 4.5,23.6] p=0.004), compared to 181(90.5%) vs 122(86.5%) respectively <400 copies/ml (+4.0% [-3.0,10.9] p=0.25). By week 48, suppression <80, <400 and <1000 copies/ml was similar for efavirenz and nevirapine (p=0.97, p=0.78, p=0.40 respectively). Despite little switching to second-line ART (similar with both NNRTIs), suppression remained similar at week 144 (106/141 (75.2%) vs 89/124 (71.8%) <80 copies/ml, 116(82.3%) vs 101(81.5%) <400 copies/ml, 118(83.7%) vs 106(85.5%) <1000 copies/ml; p>0.5).
Figure 1.
Suppression (a) <80 copies/ml over time, (b) <400 copies/ml over time and (c) <1000 copies/ml over time
Footnote 1: Only including long-term NNRTI (Arm-A/B) from week 48 onwards
However, in adjusted analyses (Table 2), differences in suppression between efavirenz vs nevirapine differed by both baseline VL and age at ART initiation; and these relationships varied over follow-up. At week 36, suppression <80 copies/ml did not depend on pre-ART VL in efavirenz, but declined with increasing pre-ART VL in nevirapine; the net effect was greater suppression with efavirenz in those with pre-ART VL >35,000 copies/ml (overall effect of efavirenz vs nevirapine p=0.0004; heterogeneity/interaction p=0.007) (Table 2; Supplementary Figure 1a). Suppression <400 and <1000 copies/ml was broadly similar (Supplementary Table 1). This effect had weakened by week 48 (heterogeneity/interaction p=0.15; Table 2; Supplementary Figure 1b), and by week 144 suppression <80 copies/ml was lower in those with higher pre-ART VL (p=0.05) in both groups (heterogeneity/interaction p=0.8).
Table 2. Independent predictors of viral load suppression <80 copies/ml at 36, 48 and 144 weeks after ART initiation.
| 36 weeks (n=314, Arms A/B/C) | 48 weeks* (n=213, Arms A/B) | 144 weeks* (n=221, Arms A/B) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| aOR [95% CI] | P | aOR [95% CI] | P | aOR [95% CI] | P | |
| VL at ART initiation | ||||||
| Per log10 higher if taking efavirenz | 1.01 [0.58-1.76] | 0.96 | 0.76 [0.39-1.49] | 0.42 | 0.59 [0.35-1.00] | 0.05 |
| Per log10 higher if taking nevirapine | 0.29 [0.15-0.56] | <0.001 | 0.33 [0.13-0.86] | 0.02 | ||
| Heterogeneity | 0.003 | 0.15 | ||||
| Efavirenz, vs. nevirapine if VL 200,000 copies/ml at ART initiation | 2.55 [1.29-5.03] | 0.007 | 0.62 [0.27-1.43] | 0.27 | ||
| Efavirenz, vs. nevirapine if aged 10 years at ART initiation | 1.14 [0.43-3.08] | 0.79 | ||||
| Age at ART initiation | ||||||
| Per year older if taking efavirenz | 0.79 [0.69-0.90] | <0.001 | ||||
| Per year older if taking nevirapine | 0.94 [0.79-1.11] | 0.46 | ||||
| Heterogeneity | 0.09 | |||||
| Age at ART initiation, vs 5-9years | 0.02 | 0.09 | ||||
| 3-4 | 0.73 [0.36-1.46] | 0.37 | 0.47 [0.19-1.15] | 0.10 | ||
| 10+ | 0.34 [0.16-0.73] | 0.006 | 0.38 [0.14-1.00] | 0.05 | ||
| 3TC/ABC/NNRTI throughout, vs additional 36 weeks ZDV induction* | 0.52** [0.27-1.00] | 0.05 | ||||
| Missed any doses in last 4 weeks, vs not missed any doses | 0.51 [0.17-1.52] | 0.23 | ||||
| % visits to date with missed doses in last 4 weeks (per 10% higher) | 0.76 [0.57-1.00] | 0.05 | 0.84 [0.59-1.21] | 0.36 | ||
| Centre, vs. A | 0.47*** | |||||
| B | 1.00 [0.45, 2.25] | 0.99 | ||||
| C | 0.58 [0.28-1.23] | 0.16 | ||||
| D | 0.69 [0.31-1.49] | 0.34 | ||||
| Overall efavirenz vs nevirapine (incorporating interaction effects above) | 0.0004 | 0.26 | 0.05 | |||
aOR=adjusted odds ratio, n=n complete cases
Only including children receiving long-term NNRTIs (Arm A/B) in analyses from week 48 onwards
There was no evidence of interaction between NNRTI and allocated ART strategy (p>0.1).
Included because significant variation in suppression <400 copies/ml and <1000 copies/ml by centre at week 36, Supplementary Table 1.
Considering age at ART initiation, at week 36 and 48, suppression was poorer in the youngest and older children, irrespective of NNRTI (global p=0.02, p=0.003, p=0.0006 for <80, <400, <1000 respectively at week 36; p=0.09, p=0.009, p=0.03 respectively at week 48). However, at week 144, older children/adolescents had poorer suppression <80 copies/ml on efavirenz (aOR per year older=0.79 [95%CI 0.69-0.90] p<0.001) but suppression was independent of age on nevirapine (aOR=0.94 [0.79-1.11] p=0.46) (overall effect of efavirenz vs nevirapine p=0.05; heterogeneity/interaction p=0.09) (Table 2; Supplementary Figure 1c). Effect sizes were similar for <400 copies/ml (efavirenz: aOR per year older=0.74 [0.64-0.87] p<0.001; nevirapine: aOR=0.88 [0.72-1.08] p=0.23) and <1000 copies/ml (efavirenz: aOR=0.72 [0.61-0.85] p<0.001; nevirapine: aOR=0.85 [0.69-1.06] p=0.14) but evidence for heterogeneity was weaker (p=0.15, 0.19 respectively) (Supplementary Table 1). The relationship in those on efavirenz increased year-by-year across the age range with no evidence of non-linearity (noting those 3-4 years of age at ART initiation were 5-9 by week 144).
Effects of other factors did not vary by NNRTI (p>0.1 for all thresholds). Suppression at weeks 48 and 144 was generally poorer in those reporting missing ART doses at a greater percentage of scheduled visits, with larger effects on the higher VL thresholds (<400, <1000 copies/ml) than <80 copies/ml (Supplementary Table 1). Interestingly, at week 144 suppression was lower in lamivudine+abacavir+NNRTI (Arm-A) vs lamivudine+abacavir+NNRTI+36 weeks zidovudine (Arm-B) (p=0.05).
In the subset with regular VLs, there was no evidence of a difference in virologic failure (≥400 copies/ml at week 24 or subsequent confirmed ≥400 copies/ml through 3 years) (18/93 (19.4%) efavirenz vs 8/52 (15.4%) nevirapine, p=0.56 Cox regression adjusting for age at ART initiation and center).
Discussion
WHO guidelines recommend children/adolescents ≥3 years initiate ART with 2NRTI+NNRTI, where the NNRTI efavirenz is preferred over nevirapine. Overall, in this non-randomized comparison in the ARROW trial, short-term suppression favored efavirenz, particularly for <80 copies/ml, but there was little difference from week 24 at <400 and <1000 copies/ml despite the vast majority remaining on the same NNRTI. Longer-term (≥48 weeks) differences were small at all thresholds, although analysis at 144 weeks favored efavirenz (p=0.05). This is broadly consistent with other pediatric studies, which generally found efavirenz associated with better virologic outcome(9–11, 13–16). The UK/Irish CHIPS study also found the most pronounced differences were shorter-term (<2 years).
However, short-term performance of efavirenz vs nevirapine also varied by pre-ART VL, with efavirenz similar at 36 and 48 weeks regardless of pre-ART VL but nevirapine better in those with lower VL (Supplementary Figure 1a). Longer-term (144 weeks), this was not apparent: suppression was independently lower in those with higher pre-ART VL regardless of NNRTI received and independently of age, potentially reflecting greater pre-ART reservoir with higher VL, but regardless of cause, highlights the importance of prompt ART initiation. The fact that the initial superiority of efavirenz at high pre-ART VL waned over time raises questions regarding the relevance of short-term suppression as an outcome, since long-term suppression is the goal of treatment.
The other consistent predictor of suppression at every time point was age. Contrasting pre-ART VL, effects of age on early suppression occurred independently of NNRTI, with younger and older children having lower suppression at all thresholds, independently of pre-ART VL and self-reported adherence. Children >10 years were predominantly responsible for their own medication intake, highlighting challenges in pre-adolescence and adolescent adherence. Most children >3 years were already taking divided tablets, so poorer adherence with syrups is not the cause of lower suppression in the younger children(22). However, after 3 years ART, in contrast to early suppression, the relative performance of NNRTIs depended on age. Overall there was at most a modest decline in suppression with age in children on nevirapine, contrasting a marked decline on efavirenz (Supplementary Figure 1c), the net result being children 10+ years of age at ART initiation (13+ years at VL measurement) having better long-term suppression with nevirapine, even after adjusting for self-reported adherence. One plausible explanation is the well-documented CNS side-effects of efavirenz; pre-adolescents/adolescents may have taken more unreported treatment interruptions. Certainly, sub-clinical CNS side-effects were commonly reported in adolescents in the weekends-off BREATHER trial(23): at enrolment, adolescents reported CNS side-effects and occasional missed doses which they found difficult to report to clinic staff (S Bernays, personal communication). Children/adolescents with CNS-related signs/symptoms at ART initiation may have been less likely to receive efavirenz, and simultaneously to have lower adherence. If anything, this would tend to favor efavirenz, particularly given necessarily incomplete adjustment for self-reported adherence. It is also unclear whether previous studies investigated varying differences between efavirenz and nevirapine by age (interactions), so these effects may have been missed.
Interestingly, at 144 weeks, children/adolescents who had received an additional NRTI (zidovudine) until week 36 (Arm-B) had marginally better suppression than those who had not (Arm-A) (p=0.05). In contrast in all children(18), including those <3 years of age, there was no evidence of difference (191/259 (73.7%) vs 169/232 (72.8%); p=0.82) (heterogeneity by age <3 vs ≥3 years at ART initiation p=0.08).Long-term CD4 recovery was also greater in the induction-maintenance Arm-B(24). Long-term 3NRTI+NNRTI has been quite widely used (from infancy) in Europe(13), as this is more palatable than 3-drug lopinavir/ritonavir regimens and potentially more forgiving where persuading children to take medication is problematic.
Our results confirm the generally favorable toxicity profile of both nevirapine and efavirenz, with only eight nevirapine and two efavirenz hypersensitivity reactions, and ~2% permanently discontinuing each NNRTI due to any AE, in a clinical setting where clinicians may not discontinue a drug despite toxicity if the toxicity is not life-threatening. Gynaecomastia resulted in permanent discontinuation of efavirenz in two children, 1% of those receiving it long-term. Permanent discontinuations for non-AE reasons were more frequent with nevirapine, particularly tuberculosis treatment which is an advantage of efavirenz in children at high risk of tuberculosis shortly after ART initiation(25). No switches to second-line treatment occurred before 48 weeks with either regimen.
Although children were not randomized to nevirapine or efavirenz, potential confounders were considered for inclusion in models; nonetheless, there is always the possibility of residual confounding. A major limitation of our study is incomplete VL sampling: however assays were performed to answer questions not depending on receipt of nevirapine or efavirenz, so will not bias this comparison. Although sampling was incomplete, restricting power for interactions particularly, our study is of similar size to others (with 4-year follow-up); the suggestion that older children had better long-term suppression with nevirapine requires further study. All children received the WHO-recommended backbone NRTIs lamivudine+abacavir(6). Although approximately one third also received zidovudine until week 36, this did not affect relative performance of efavirenz vs. nevirapine; other regimens are unlikely to have altered relative impact of different NNRTIs. We considered three thresholds for suppression (<80, <400, <1000 copies/ml); while <80 copies/ml provides a sensitive investigation of impact of low-level resistant variants, <400 and <1000 copies/ml may be more relevant in clinical practice, particularly resource-limited settings. Results were generally similar over thresholds. We pre-specified an exit p-value of 0.1 for backwards selection to develop an explanatory model; however this increases the chance of Type I error (false-positives).
In summary, we confirm results from other pediatric studies that efavirenz is associated with better initial virologic outcome than nevirapine, particularly at higher pre-ART VLs, but longer-term we found they are broadly similar, with nevirapine even having some advantage in older children. This supports the 2013 WHO recommendation that nevirapine should continue as a reasonable alternative to efavirenz, particularly in older children/adolescents. It also suggests there is no particular reason to substitute nevirapine in children doing well on first-line ART. Both nevirapine and efavirenz have generally favorable toxicity profiles, although clinicians need to remain alert to the possibility of hypersensitivity reactions with either.
Supplementary Material
Supplementary Digital Content
Acknowledgements
We thank the children, caregivers and staff from all the centers participating in the ARROW trial, and the ARROW Trial Steering Committee for access to data.
Source of Funding: The main ARROW trial was funded by the UK Medical Research Council and the UK Department for International Development (DFID). ViiV Healthcare/GlaxoSmithKline donated first-line drugs for ARROW and provided funding for VL assays.
Footnotes
Conflicts of Interest: No conflicts of interest.
MRC/UVRI Uganda Research Unit on AIDS, Entebbe, Uganda: P Munderi, P Nahirya-Ntege, R Katuramu, J Lutaakome, F Nankya, G Nabulime, I Sekamatte, J Kyarimpa, A Ruberantwari, R Sebukyu, G Tushabe, D Wangi, M Musinguzi, M Aber, L Matama, D Nakitto-Kesi, P Kaleebu, S Nassimbwa Joint Clinical Research Centre, Kampala, Uganda: P Mugyenyi, V Musiime, R Keishanyu, VD Afayo, J Bwomezi, J Byaruhanga, P Erimu, C Karungi, H Kizito, WS Namala, J Namusanje, R Nandugwa, TK Najjuko, E Natukunda, M Ndigendawani, SO Nsiyona, R Kibenge, B Bainomuhwezi, D Sseremba, J Tezikyabbiri, CS Tumusiime, A Balaba, A Mugumya, F Nghania, D Mwebesa, M Mutumba, E Bagurukira, F Odongo, S Mubokyi, M Ssenyonga, M Kasango, E Lutalo, P Oronon, ED Williams, O Senfuma, L Mugarura, J Nkalubo, S Abunyang, O Denis, R Lwalanda, I Nankya, E Ndashimye, E Nabulime, D Mulima University of Zimbabwe, Harare, Zimbabwe: KJ Nathoo, MF Bwakura-Dangarembizi, F Mapinge, E Chidziva, T Mhute, T Vhembo, R Mandidewa, M Chipiti, R Dzapasi, C Katanda D Nyoni, GC Tinago, J Bhiri, S Mudzingwa, D Muchabaiwa, M Phiri, V Masore, CC Marozva, SJ Maturure, S Tsikirayi, L Munetsi, KM Rashirai, J Steamer, R Nhema, W Bikwa, B Tambawoga, E Mufuka, M Munjoma, K Mataruka, Y Zviuya Zvitambo, Harare: P Kurira, K Mutasa Baylor College of Medicine Children’s Foundation Uganda, Mulago Hospital Uganda: A Kekitiinwa, P Musoke, S Bakeera-Kitaka, R Namuddu, P Kasirye, A Babirye, J Asello, S Nakalanzi, NC Ssemambo, J Nakafeero, J Tikabibamu, G Musoba, J Ssanyu, M Kisekka MRC Clinical Trials Unit at UCL, London, UK: DM Gibb, MJ Thomason, AS Walker, AD Cook, AJ Szubert, B Naidoo-James, MJ Spyer, C Male, AJ Glabay, LK Kendall, J Crawley, AJ Prendergast
Independent ARROW Trial Monitors: I Machingura, S Ssenyonjo. Trial Steering Committee: I Weller (Chair), E Luyirika, H Lyall, E Malianga, C Mwansambo, M Nyathi, F Miiro, DM Gibb, A Kekitiinwa, P Mugyenyi, P Munderi, KJ Nathoo, AS Walker; Observers S Kinn, M McNeil, M Roberts, W Snowden. Data and Safety Monitoring Committee: A Breckenridge (Chair), A Pozniak, C Hill, J Matenga, J Tumwine. Endpoint Review Committee (independent members): G Tudor-Williams (Chair), H Barigye, HA Mujuru, G Ndeezi; Observers: S Bakeera-Kitaka, MF Bwakura-Dangarembizi, J Crawley, V Musiime, P Nahirya-Ntege, A Prendergast, M Spyer.
Economics Group: P Revill, T Mabugu, F Mirimo, S Walker, MJ Sculpher.
Presented in part at CROI 2014, Boston.
References
- 1.World Health Organisation. Geneva: 2013. Global Update on HIV Treatment 2013. [Google Scholar]
- 2.Tang MW, Kanki PJ, Shafer RW. A Review of the Virological Efficacy of the 4 World Health Organization–Recommended Tenofovir-Containing Regimens for Initial HIV Therapy. Clinical Infectious Diseases. 2012;54:862–75. doi: 10.1093/cid/cir1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shubber Z, Calmy A, Andrieux-Meyer I, Vitoria M, Renaud-Théry F, Shaffer N, et al. Adverse events associated with nevirapine and efavirenz-based first-line antiretroviral therapy: a systematic review and meta-analysis. AIDS. 2013;27(9):1403–12. doi: 10.1097/QAD.0b013e32835f1db0. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organisation. Geneva: 2015. Antiretroviral medicines in low-and middle-income countries: forecasts of global and regional demand for 2014-2018. [Google Scholar]
- 5.Mbuagbaw L, Irlam J, Spaulding A, Rutherford G, Siegfried N. Efavirenz or nevirapine in three-drug combination therapy with two nucleoside-reverse transcriptase inhibitors for initial treatment of HIV infection in antiretroviral-naïve individuals (Review) Cochrane Database of Systematic Reviews. 2010;12 doi: 10.1002/14651858.CD004246.pub3. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organisation. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: Recommendations for a public health approach2013. Available from: http://www.who.int/hiv/pub/guidelines/paediatric020907.pdf.
- 7.Pillay P, Ford N, Shubber Z, Ferrand RA. Outcomes for Efavirenz versus Nevirapine-Containing Regimens for Treatment of HIV-1 Infection: A Systematic Review and Meta-Analysis. PLUS ONE. 2013;8(7):e68995. doi: 10.1371/journal.pone.0068995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castillo-Mancilla JR, Campbell TB. Comparative effectiveness of efavirenz-based antiretroviral regimens in resource-limited settings. Journal of Comparative Effectiveness Research. 2012;1(2):157–70. doi: 10.2217/cer.12.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lowenthal ED, Ellenberg JH, Machine E, Sagdeo A, Boiditswe S, Steenhoff AP, et al. Association Between Efavirenz-Based Compared With Nevirapine-Based Antiretroviral Regimens and Virological Failure in HIV-Infected Children. The Journal of the American Medical Association. 2013;309(17):1803–9. doi: 10.1001/jama.2013.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jittamala P, Puthanakit T, Chaiinseeard S, Sirisanthana V. Predictors of Virologic Failure and Genotypic Resistance Mutation Patterns in Thai Children Receiving Non-Nucleoside Reverse Transcriptase Inhibitor–Based Antiretroviral Therapy. The Pediatric Infectious Disease Journal. 2009;28(9):826–30. doi: 10.1097/INF.0b013e3181a458f9. [DOI] [PubMed] [Google Scholar]
- 11.Bunupuradah T, Sricharoenchai S, Hansudewechakul R, Virat K, Teeraananchai S, Orasri W, et al. Risk of First-line Antiretroviral Therapy Failure in HIV-infected Thai Children and Adolescents. The Pediatric Infectious Disease Journal. 2015;34(3):58–62. doi: 10.1097/INF.0000000000000584. [DOI] [PubMed] [Google Scholar]
- 12.Bunupuradah T, Puthanakit T, Kosalaraksa P, Kerr S, Boonrak P, Prasitsuebsai W, et al. Immunologic and virologic failure after first-line NNRTI-based antiretroviral therapy in Thai HIVinfected children. AIDS Research and Therapy. 2011;8(40) doi: 10.1186/1742-6405-8-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duong T, Judd A, Collins IJ, Doerholt K, Lyall H, Foster C, et al. Long-term virological outcome in children on antiretroviral therapy in the UK and Ireland. AIDS. 2014;28(16):2395–405. doi: 10.1097/QAD.0000000000000438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Emmett SD, Cunningham CK, Mmbaga BT, Kinabo GD, Schimana W, Swai ME, et al. Predicting Virologic Failure Among HIV-1-Infected Children Receiving Antiretroviral Therapy in Tanzania: a Cross-Sectional Study. Journal of Acquired Immune Deficiency Syndromes. 2010;54(4):368–75. doi: 10.1097/QAI.0b013e3181cf4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mgelea EM, Kisenge R, Aboud S. Detecting virological failure in HIV-infected Tanzanian children. South African Medical Journal. 2014;103(10):696–9. doi: 10.7196/samj.7807. [DOI] [PubMed] [Google Scholar]
- 16.Kamya MR, Mayanja-Kizza H, Kambugu A, Bakeera-Kitaka S, Semitala F, Mwebaze-Songa P, et al. Predictors of Long-Term Viral Failure Among Ugandan Children and Adults Treated With Antiretroviral Therapy. Journal of Acquired Immune Deficiency Syndromes. 2007;46(2):187–93. doi: 10.1097/QAI.0b013e31814278c0. [DOI] [PubMed] [Google Scholar]
- 17.Tukei VJ, Asiimwe A, Maganda A, Atugonza R, Sebuliba I, Bakeera-Kitaka S, et al. Safety and Tolerability of Antiretroviral Therapy Among HIV-Infected Children And Adolescents In Uganda. Journal of Acquired Immune Deficiency Syndromes. 2012;59(3):274–80. doi: 10.1097/QAI.0b013e3182423668. [DOI] [PubMed] [Google Scholar]
- 18.ARROW Trial team. Routine vs clinically driven laboratory monitoring and first-line antiretroviral therapy strategies in African children with HIV (ARROW): a 5-year open-label randomised factorial trial. The Lancet. 2013;381(9875):1391–403. doi: 10.1016/S0140-6736(12)62198-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Musiime V, Kasirye P, Naidoo–James B, Nahirya-Ntege P, Mhute T, Cook A, et al. Once- versus twice-daily abacavir and lamivudine in African children: the randomised controlled ARROW Trial. AIDS. doi: 10.1097/QAD.0000000000001116. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wade A, Ades A. Age-related reference ranges: significance tests for models and confidence intervals for centiles. Statistics in Medicine. 1994;13:2359–67. doi: 10.1002/sim.4780132207. [DOI] [PubMed] [Google Scholar]
- 21.Hess K. Assessing time-by-covariate interactions in proportional hazards regression models using cubic spline functions. Statistics in Medicine. 1994;13:1045–62. doi: 10.1002/sim.4780131007. [DOI] [PubMed] [Google Scholar]
- 22.Musoke P, Szubert AJ, Musiime V, Nathoo K, Nahirya-Ntege P, Mutasa K, et al. Single Dose Nevirapine Exposure does not affect Response to Anti-retroviral Therapy in HIV-infected African Children aged <3 Years. AIDS. 2015;29:1623–32. doi: 10.1097/QAD.0000000000000749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Butler K. ART With Weekends Off Is Noninferior to Continuous ART in Young People on EFV+2NRTI. CROI 2015; Seattle, WA: 2015. CROI. [Google Scholar]
- 24.Picat M-Q, Lewis J, Musiime V, Prendergast A, Nathoo K, Kekitiinwa A, et al. Predicting Patterns of Long-Term CD4 Reconstitution in HIV-Infected Children Starting Antiretroviral Therapy in Sub-Saharan Africa: A Cohort-Based Modelling Study. PLOS Medicine. 2013;10(10) doi: 10.1371/journal.pmed.1001542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crook A, Turkova A, Musiime V, Bwakura-Dangarembizi M, Bakeera-Kitaka S, Nahirya-Ntege P, et al. Tuberculosis incidence is high in HIV-infected African children but is reduced by co-trimoxazole and time on antiretroviral therapy. BMC Medicine. 2016;14(1):50. doi: 10.1186/s12916-016-0593-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.