Abstract
Background
Abiraterone acetate plus prednisolone improves survival in men with relapsed prostate cancer. We assessed the effect of this combination in men starting long-term androgen-deprivation therapy (ADT), using a multigroup, multistage trial design.
Methods
We randomly assigned patients in a 1:1 ratio to receive ADT alone or ADT plus abiraterone acetate (1000 mg daily) and prednisolone (5 mg daily) (combination therapy). Local radiotherapy was mandated for patients with node-negative, nonmetastatic disease and encouraged for those with positive nodes. For patients with nonmetastatic disease with no radiotherapy planned and for patients with metastatic disease, treatment continued until radiologic, clinical, or prostate-specific antigen (PSA) progression; otherwise, treatment was to continue for 2 years or until any type of progression, whichever came first. The primary outcome measure was overall survival. The intermediate primary outcome was failure-free survival (treatment failure was defined as radiologic, clinical, or PSA progression or death from prostate cancer).
Results
A total of 1917 patients underwent randomization from November 2011 through January 2014. The median age was 67 years, and the median PSA level was 53 ng per milliliter. A total of 52% of the patients had metastatic disease, 20% had node-positive or node-indeterminate nonmetastatic disease, and 28% had node-negative, nonmetastatic disease; 95% had newly diagnosed disease. The median follow-up was 40 months. There were 184 deaths in the combination group as compared with 262 in the ADT-alone group (hazard ratio, 0.63; 95% confidence interval [CI], 0.52 to 0.76; P<0.001); the hazard ratio was 0.75 in patients with nonmetastatic disease and 0.61 in those with metastatic disease. There were 248 treatment-failure events in the combination group as compared with 535 in the ADT-alone group (hazard ratio, 0.29; 95% CI, 0.25 to 0.34; P<0.001); the hazard ratio was 0.21 in patients with nonmetastatic disease and 0.31 in those with metastatic disease. Grade 3 to 5 adverse events occurred in 47% of the patients in the combination group (with nine grade 5 events) and in 33% of the patients in the ADT-alone group (with three grade 5 events).
Conclusions
Among men with locally advanced or metastatic prostate cancer, ADT plus abiraterone and prednisolone was associated with significantly higher rates of overall and failure-free survival than ADT alone. (Funded by Cancer Research U.K. and others; STAMPEDE ClinicalTrials.gov number, NCT00268476, and Current Controlled Trials number, ISRCTN78818544.)
The Systemic Therapy in Advancing or Metastatic Prostate Cancer: Evaluation of Drug Efficacy (STAMPEDE) trial recruits patients commencing long-term androgen-deprivation therapy (ADT; orchiectomy or gonadotropin-releasing hormone [GnRH] agonists or antagonists) for the first time for locally advanced or metastatic prostate cancer. Several agents have improved overall survival (docetaxel, abiraterone acetate, enzalutamide, cabazitaxel, radium-223, and sipuleucel-T)1–8 or reduced morbidity (zoledronic acid and denosumab)9–11 among such patients. These agents were initially investigated in men with very advanced disease whose tumors had progressed during first-line ADT, a disease state now termed castration-resistant prostate cancer.
The STAMPEDE trial uses a novel multigroup, multistage (also called multiarm, multistage [MAMS]) platform design12–14 to test whether the addition of further treatments to ADT improves overall survival if used in the first-line setting. It uses intermediate activity analyses, based on failure-free survival, to cease randomization to research groups that are insufficiently active.12–14 We have previously reported that treatment with docetaxel at the inception of ADT increased median survival from 71 months to 81 months as well as overall survival (hazard ratio for death, 0.76).15,16 These results, along with those of a systematic review that included other trials17–19 and of a meta-analysis,16 led to docetaxel becoming a part of the standard of care for suitable patients with prostate cancer who had not received previous hormone therapy.
An important mechanism for escape from tumor control by androgen ablation includes the intracellular conversion of steroid precursors to androgenic steroids by prostate-cancer cells. Abiraterone acetate is a selective, irreversible inhibitor of CYP17, an enzyme that is critical in the production of androgens in the testes, adrenal glands, and prostate-tumor tissue. Inhibition of CYP17 in combination with ADT results in a more effective androgen depletion than can be induced by surgical castration or by GnRH analogues alone.20 Two large, phase 3 trials showed that the addition of abiraterone acetate with prednisolone to standard-of-care therapy prolonged survival among men with castration-resistant prostate cancer.4,5,21 The STAMPEDE trial is invesinvestigating whether the earlier use of abiraterone in men who are initiating long-term ADT could improve survival.
Methods
Trial Design and Patients
We used a multigroup, multistage platform design, incorporating a seamless phase 2–3 component. The primary outcome was overall survival, defined as the time from randomization to death from any cause. The intermediate primary outcome was failure-free survival, defined as the time to the first of the following forms of treatment failure: biochemical (prostate-specific antigen [PSA]) failure (see the protocol, available with the full text of this article at NEJM.org); progression of local, lymph-node, or distant metastases; or death from prostate cancer. Secondary outcome measures included adverse events, symptomatic skeletal events, progression-free survival (i.e., failure-free survival excluding biochemical failure), prostate cancer–specific survival, and (data not shown) quality of life.
The rationale and design were described previously.12–14,22 Full details are provided in the protocol. In summary, eligible patients had prostate cancer that was newly diagnosed and metastatic, node-positive, or high-risk locally advanced (with at least two of following: a tumor stage of T3 or T4, a Gleason score of 8 to 10, and a PSA level ≥40 ng per milliliter) or disease that was previously treated with radical surgery or radiotherapy and was now relapsing with high-risk features (in men no longer receiving therapy, a PSA level >4 ng per milliliter with a doubling time of <6 months, a PSA level >20 ng per milliliter, nodal or metastatic relapse, or <12 months of total ADT with an interval of >12 months without treatment). Patients were intended for treatment with long-term ADT that started no longer than 12 weeks before randomization. There were no age restrictions. Patients with clinically significant cardiovascular disease (e.g., severe angina, recent myocardial infarction, or a history of cardiac failure) were excluded.
Trial Oversight and Conduct
The trial was sponsored by the U.K. Medical Research Council (MRC) and conducted by the MRC Clinical Trials Unit at University College London (UCL). MRC provided funding for the trial through core funding to UCL. Cancer Research U.K. approved the trial design and subsequent amendments and provided funding support but had no further input. Janssen approved the design for this comparison (ADT plus abiraterone and prednisolone vs. ADT alone) and participated in discussions on the progress of the trial. Janssen also provided grant funding, abiraterone acetate, and funds for drug distribution. Representatives from Janssen were invited to comment on the manuscript. Further funding for the platform was provided by Astellas Pharma, Clovis Oncology, Novartis, Pfizer, and Sanofi Aventis. The trial was conducted in accordance with the principles of Good Clinical Practice guidelines and the Declaration of Helsinki, and the appropriate regulatory and ethics approvals were obtained. All the patients provided written informed consent.
The analyses were driven by prespecified criteria, and the decision to submit the manuscript for publication was made by the members of the Trial Management Group. Three authors at the MRC Clinical Trials Unit at UCL accessed the raw data; processed data that were released by the independent data monitoring committee and trial steering committee were available to all the coauthors. The authors vouch for the completeness and accuracy of the data and analyses and for the adherence of the trial to the protocol. No one who is not an author contributed to the writing of the manuscript.
Randomization and Masking
Randomization was performed centrally by telephone with the use of a computerized algorithm, which was developed and maintained by the MRC Clinical Trials Unit at UCL. Minimization with a random element of 80% was used, with stratification according to randomizing center, age at randomization (<70 vs. ≥70 years), the presence or absence of metastases, planned use of prostate radiotherapy (yes vs. no), nodal involvement (negative vs. indeterminate vs. positive), World Health Organization performance status (0 vs. 1 or 2 [on a scale of 0 to 4, with higher numbers indicating greater disability]), type of ADT, and regular, long-term use of aspirin or nonsteroidal antiinflammatory drugs (yes vs. no). The assignment ratio was 1:1 to standard of care alone or with abiraterone acetate and prednisolone (combination therapy). The comparison was open-label, because masking of the treatment assignment was deemed impracticable. Eligible patients could be assigned to any of the trial groups that were open; we focus here on patients assigned contemporaneously to ADT alone or with abiraterone and prednisolone.
Procedures
ADT was given for at least 2 years; radiotherapy at 6 to 9 months after randomization was mandatory for patients with node-negative, nonmetastatic disease and optional for patients with node-positive, nonmetastatic disease. Abiraterone (1000 mg) with prednisolone (5 mg) was given once daily. The treatment duration depended on disease stage and intent for radical radiotherapy: for patients with nonmetastatic disease with no radiotherapy planned and for patients with metastatic disease, treatment continued until PSA, radiologic, or clinical progression or until another treatment was started; for patients with nonmetastatic disease with radiotherapy planned, treatment was to continue for 2 years or until any type of progression, whichever came first. Dose modifications are described in the protocol.
Patients were assessed every 6 weeks during the first 6 months, then every 12 weeks until 2 years, then every 6 months until 5 years, and then annually; in addition, patients receiving combination therapy were assessed at least every 3 months. Assessments included PSA testing and ascertainment of adverse events; further tests were conducted at the discretion of the treating physician. The nadir PSA level (for the definition of PSA progression) was defined as the lowest level within 24 weeks after randomization. There was no protocol-mandated imaging except at baseline. Adverse events were assessed with the use of the National Cancer Institute Common Terminology Criteria for Adverse Events (initially, version 3.0; later, version 4.0). Serious adverse events and reactions were reported accordingly.
Statistical Analysis
The sample size was calculated with the use of Stata nstage and predecessor programs that allow for the design of multigroup, multistage trials.23 Assuming a median failure-free survival of 2 years and a median overall survival between 4 and 5 years for ADT, we targeted a 25% relative difference between the combination group and the ADT-alone group for both failure-free survival (hazard ratio for treatment failure, 0.75) and overall survival (hazard ratio for death, 0.75). The main analysis for the comparison of combination therapy against control for survival could be performed after the occurrence of approximately 267 deaths in the control group for 90% power and a one-sided alpha level of 2.5%, after we accounted for three intermediate lack-of-benefit analyses of failure-free survival. An independent data monitoring committee reviewed accumulating data, guided by lack-of-benefit stopping guidelines.
Data on patients without an event of interest were censored when they were last known to be event-free. The median follow-up was determined through reversing death and censoring indicators. Standard survival-analysis methods were used to analyze time-to-event data in Stata software, version 14, with Kaplan–Meier estimates for survival curves and Cox proportional-hazards models to estimate relative treatment effects. These estimates were adjusted for stratification factors (except randomizing center and ADT method) and were stratified according to time periods defined by corecruiting groups. A hazard ratio of less than 1.00 favored the combination group. The restricted mean survival time (restricted to 54 months) was calculated from flexible parametric models (5 degrees of freedom). The proportional-hazards assumption was tested; the restricted mean survival time was emphasized in the presence of nonproportionality. All the confidence intervals are at the 95% level. Prespecified subgroup analyses looked at the consistency of treatment effect according to stratification factors, time period, categorized Gleason score, and quintiles of PSA levels that were measured before the initiation of long-term ADT. Key subgroup analyses according to metastatic status were prespecified. Exploratory analyses considered prostate cancer–specific survival and progression-free survival according to the age at randomization.
All the patients were included in the efficacy analyses under their assigned treatment on an intention-to-treat basis. For safety analyses, patients were grouped according to the treatment that they started. In the analysis of the median duration of abiraterone treatment, data were censored at the date of last contact with the patient if the patient had not reported stopping the drug.
Results
Patients
Between November 15, 2011, and January 17, 2014, a total of 1917 consenting patients at 111 U.K. and 5 Swiss sites underwent randomization (957 to ADT alone and 960 to combination therapy). Data were frozen on February 10, 2017. The flow of patients through the trial is shown in Figure S1 in the Supplementary Appendix, available at NEJM.org; the baseline characteristics of the patients are shown in Table 1, and in Table S1 in the Supplementary Appendix. The median PSA level was 53 ng per milliliter. The median follow-up was 40 months.
Table 1. Characteristics of the Patients.*.
| Characteristic | ADT Alone (N = 957) | Combination Therapy (N = 960) |
|---|---|---|
| Age at randomization — yr | ||
| Median (IQR) | 67 (62 to 72) | 67 (63 to 72) |
| Range | 39 to 84 | 42 to 85 |
| PSA level before ADT — ng/ml | ||
| Median (IQR) | 56 (19 to 165) | 51 (19 to 158) |
| Range | 0 to 10,530 | 0 to 21,460 |
| WHO performance status — no. (%)† | ||
| 0 | 744 (78) | 745 (78) |
| 1 or 2 | 213 (22) | 215 (22) |
| Disease group — no. (%) | ||
| Newly diagnosed node-negative, nonmetastatic disease | 256 (27) | 253 (26) |
| Newly diagnosed node-positive, nonmetastatic disease | 187 (20) | 182 (19) |
| Newly diagnosed metastatic disease | 476 (50) | 465 (48) |
| Previously treated nonmetastatic disease | 12 (1) | 25 (3) |
| Previously treated metastatic disease | 26 (3) | 35 (4) |
| Gleason score — no. (%)‡ | ||
| ≤7 | 223 (23) | 221 (23) |
| 8 to 10 | 721 (75) | 715 (74) |
| Unknown | 13 (1) | 24 (2) |
| Planned or current long-term ADT — no. (%) | ||
| Orchiectomy | 5 (1) | 3 (<1) |
| Bicalutamide | 5 (1) | 5 (1) |
| Dual androgen blockade | 4 (<1) | 1 (<1) |
| LHRH-based§ | 943 (99) | 951 (99) |
| Time to initiation of ADT from randomization — days¶ | ||
| Median (IQR) | −45 (−67 to −23) | −44 (−63 to −24) |
| Range | −85 to 39 | −85 to 28 |
| Planned antiandrogen use — no. (%) | ||
| No | 50 (5) | 61 (6) |
| Short-term antiandrogen | 902 (94) | 895 (93) |
| Long-term antiandrogen | 5 (1) | 4 (<1) |
| Radiotherapy planned — no. (%)|| | ||
| No | 561 (59) | 564 (59) |
| Yes | 396 (41) | 396 (41) |
| Hypertension — no. (%) | ||
| No | 571 (60) | 557 (58) |
| Yes, but still fit for trial | 385 (40) | 401 (42) |
| Cardiovascular assessment not received | 1 (<1) | 2 (<1) |
Combination therapy was androgen-deprivation therapy (ADT) plus abiraterone acetate and prednisolone. Percentages may not sum to 100 because of rounding. IQR denotes interquartile range, and PSA prostate-specific antigen.
The World Health Organization (WHO) performance status was scored on a scale of 0 to 4, with higher numbers indicating greater disability.
Gleason scores range from 3 to 10, with higher scores indicating more aggressive disease, less differentiated tumor, and worse prognosis.
All patients with planned use of luteinizing hormone–releasing hormone (LHRH) analogues should have antiandrogens for disease flares, at least.
Data were missing for one patient in the ADT-alone group.
Radiotherapy was mandated for patients with newly diagnosed node-negative, nonmetastatic disease and strongly encouraged in patients with newly diagnosed node-positive, nonmetastatic disease.
Treatment
The median time from randomization to the initiation of abiraterone was 1.3 weeks, and the median time from the initiation of ADT to the initiation of abiraterone was 8.0 weeks (most patients started ADT before randomization) (Table S2 in the Supplementary Appendix). Of the 960 patients in the combination group, 10 (1%) did not receive abiraterone, mostly because they declined treatment. The median duration of abiraterone treatment was 23.7 months in the patients for whom the duration was capped at 2 years (67% reported stopping for treatment completion) and 33.2 months in the patients who could continue through progression (51% reported permanent stopping for progression and 20% for excessive toxic effects) (Table S2 and Fig. S2 in the Supplementary Appendix). A total of 265 patients (28%) had not yet permanently stopped treatment with abiraterone at the time of this report.
The planned rate of use of radiotherapy was 41% in the combination group and in the ADT-alone group. The reported rate of use of radiotherapy was 39% in the combination group and 40% in the ADT-alone group.
Overall Survival
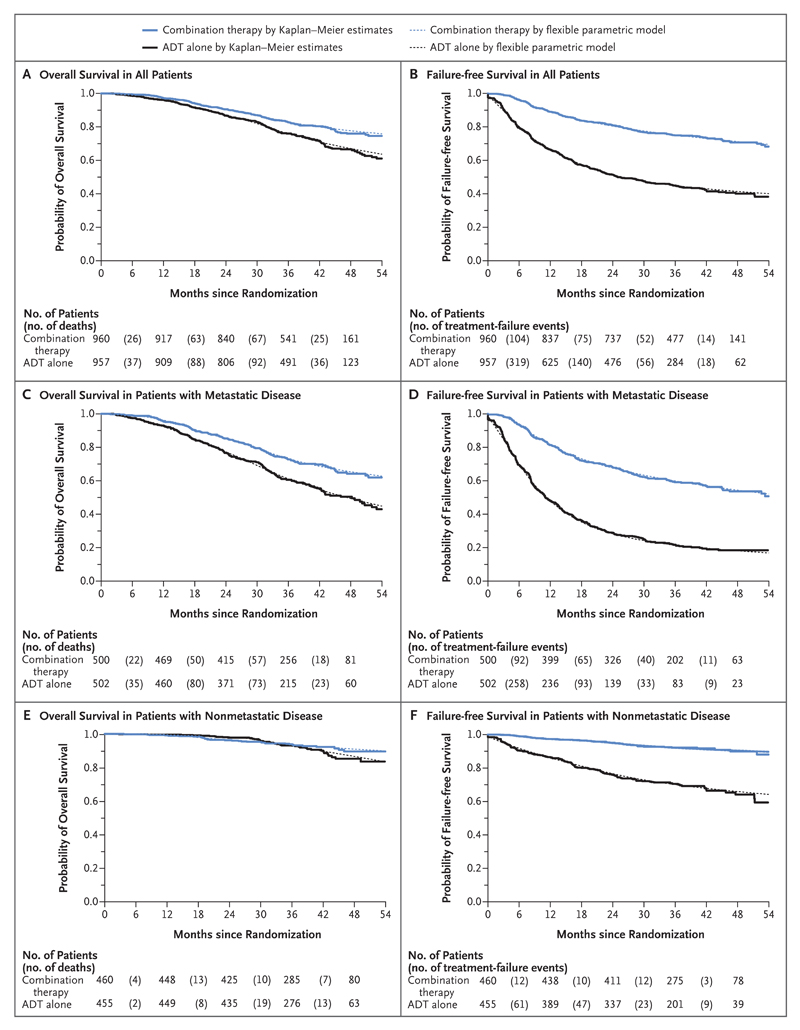
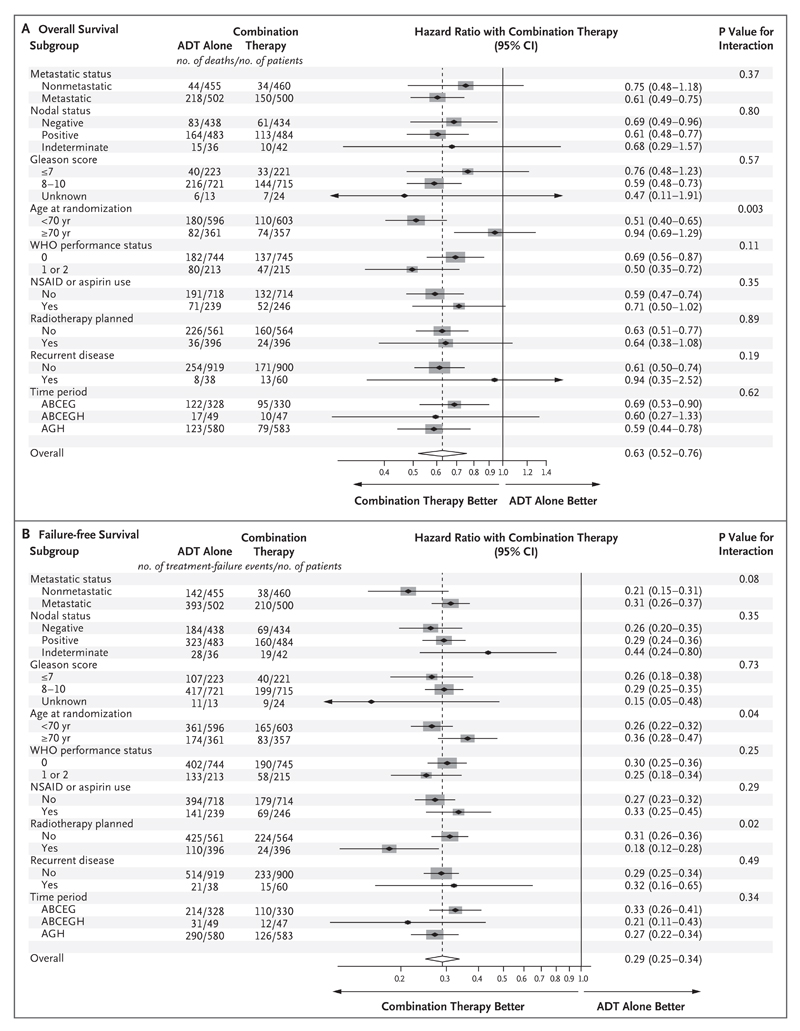
There were 184 deaths in the combination group and 262 in the ADT-alone group. There was strong evidence of a survival advantage in the combination group, with a 3-year survival of 83% as compared with 76% in the ADT-alone group (hazard ratio for death, 0.63; 95% confidence interval [CI], 0.52 to 0.76; P<0.001) (Fig. 1A). There was no evidence of nonproportional hazards (P = 0.31) or of heterogeneity of the treatment effect according to metastatic status at randomization. A preplanned subgroup analysis of 1002 patients with metastatic disease at entry included 368 deaths (Fig. 1C). The same comparisons in patients without metastatic disease at randomization are immature, currently with 78 deaths in total (Fig. 1E). There was no evidence of heterogeneity of effect in other subgroups (Fig. 2A).
Figure 1. Overall Survival and Failure-free Survival in All Patients and According to Metastatic Status at Randomization (Intention-to-Treat Population).
Combination therapy was androgen-deprivation therapy (ADT) plus abiraterone acetate and prednisolone. Treatment failure was defined as radiologic, clinical, or prostate-specific antigen (PSA) progression or death from prostate cancer.
Figure 2. Forest Plots of Treatment Effect on Overall and Failure-free Survival within Subgroups According to Stratification Factors at Randomization.
In each panel, the dashed vertical line is the point estimate of the hazard ratio for the analysis. Gleason scores range from 3 to 10, with higher scores indicating more aggressive disease, less differentiated tumor, and worse prognosis. The World Health Organization (WHO) performance status was scored on a scale of 0 to 4, with higher numbers indicating greater disability. Time period was defined by corecruiting group; ABCEG corresponds to November 2011 through January 2013, ABCEGH January 2013 through March 2013, and AGH April 2013 through January 2014. NSAID denotes nonsteroidal antiinflammatory drug.
Failure-free Survival
There were 248 events of treatment failure (radiologic, clinical, or PSA progression or death from prostate cancer) in the combination group and 535 in the ADT-alone group. The 3-year failure-free survival was 75% in the combination group and 45% in the ADT-alone group (hazard ratio for treatment failure, 0.29; 95% CI, 0.25 to 0.34; P<0.001) (Fig. 1B). There was evidence of nonproportional hazards (P = 0.001), so we present restricted mean failure-free survival time: 43.9 months in the combination group and 30.0 months in the ADT-alone group in the first 54 months after randomization, a difference of 13.9 months (95% CI, 12.3 to 15.4). The effect of abiraterone on failure-free survival was noted in all subgroups (Fig. 1D, Fig. 1F, and Fig. 2B).
Other Efficacy Outcome Measures
There were 198 events of radiologic or clinical progression or death from prostate cancer in the combination group and 379 in the ADT-alone group. The 3-year progression-free survival was 80% in the combination group and 62% in the ADT-alone group (hazard ratio for clinical or radiologic progression or death from prostate cancer, 0.40; 95% CI, 0.34 to 0.47; P<0.001) (Fig. S3 in the Supplementary Appendix). There were 113 symptomatic skeletal events reported so far in the combination group and 203 in the ADT-alone group. The 3-year rate without symptomatic skeletal events was 88% in the combination group and 78% in the ADT-alone group (hazard ratio for symptomatic skeletal events, 0.46; 95% CI, 0.37 to 0.58; P<0.001) (Fig. S4A in the Supplementary Appendix). This difference was most marked in the subgroup of patients with metastatic disease (Fig. S4B in the Supplementary Appendix). A total of 140 of the 184 deaths in the combination group (76%) and 216 of the 262 deaths in the ADT-alone group (82%) were attributed to prostate cancer on central review. The competing-risks subhazard ratio for death from prostate cancer was 0.58 (95% CI, 0.47 to 0.72).
Exploratory Analyses
Exploratory analyses that considered the outcomes of progression-free and prostate cancer–specific survival within subgroups of age at randomization suggested a favorable treatment effect regardless of age; proportionally fewer events were noted in older patients. In patients younger than 70 years of age, there were 113 events of radiologic or clinical progression or death from prostate cancer in the combination group and 263 in the ADT-alone group (hazard ratio, 0.36; 95% CI, 0.29 to 0.45); in patients 70 years of age or older, there were 65 events in the combination group and 116 in the ADT-alone group (hazard ratio, 0.50; 95% CI, 0.37 to 0.68; P = 0.10 for interaction). In patients younger than 70 years of age, there were 93 deaths from prostate cancer in the combination group and 154 in the ADT-alone group (subhazard ratio, 0.51; 95% CI, 0.39 to 0.66); in patients 70 years of age or older, there were 47 deaths from prostate cancer in the combination group and 62 in the ADT-alone group (subhazard ratio, 0.80; 95% CI, 0.54 to 1.17; P = 0.08 for interaction).
Adverse Events
Patients in the safety population who reported adverse events of grade 3 or higher during their entire time in the trial numbered 443 of 948 (47%) in the combination group and 315 of 960 (33%) in the ADT-alone group (Table 2). There were 12 grade 5 adverse events, including 9 in the combination group (2 events of pneumonia [1 including sepsis], 2 events of stroke, and 1 event each of dyspnea, lower respiratory tract infection, liver failure, pulmonary hemorrhage, and chest infection) and 3 in the ADT-alone group (2 events of myocardial infarction and 1 event of bronchopneumonia). Among the 1476 patients in the safety population in whom progression had not occurred within the first year, the prevalence of adverse events of grade 3 or higher was 15% in the combination group and 11% in the ADT-alone group. The main additional adverse events over and above the control therapy were hypertension, mild increases in aminotransferase levels, and respiratory disorders. Further details, including an overview according to age, are provided in Tables S4 through S32 in the Supplementary Appendix. The pattern and levels of adverse events were similar in the intention-to-treat population.
Table 2. Worst Adverse-Event Grade Reported during Entire Time in the Trial.*.
| Variable | ADT Alone | Combination Therapy |
|---|---|---|
| Safety population | ||
| No. of patients | 960 | 948 |
| Patients with an adverse event — no. (%) | ||
| Any grade | 950 (99) | 943 (99) |
| Grade 3–5 | 315 (33) | 443 (47) |
| Grade 5 only† | 3 (<1) | 9 (1) |
| Grade 3–5 adverse events — no. (%) | ||
| Endocrine disorders‡ | 133 (14) | 129 (14) |
| Cardiovascular disorders | 41 (4) | 92 (10) |
| Hypertension | 13 (1) | 44 (5) |
| Myocardial infarction | 9 (1) | 10 (1) |
| Cardiac dysrhythmia | 2 (<1) | 14 (1) |
| Musculoskeletal disorders | 46 (5) | 68 (7) |
| Gastrointestinal disorders | 40 (4) | 49 (5) |
| Hepatic disorders | 12 (1) | 70 (7) |
| Increased ALT level | 4 (<1) | 53 (6) |
| Increased AST level | 2 (<1) | 10 (1) |
| General disorders | 29 (3) | 45 (5) |
| Fatigue | 15 (2) | 21 (2) |
| Edema | 0 | 5 (1) |
| Respiratory disorders | 23 (2) | 44 (5) |
| Dyspnea | 7 (1) | 18 (2) |
| Laboratory abnormalities | 21 (2) | 34 (4) |
| Hypokalemia | 3 (<1) | 12 (1) |
| Intention-to-treat population | ||
| Total no. of patients | 957 | 960 |
| No. of patients in safety analysis | 953 | 955 |
| Patients with an adverse event — no. (%) | ||
| Any grade | 943 (99) | 950 (99) |
| Grade 3–5 | 312 (33) | 446 (47) |
| Grade 5 only† | 3 (<1) | 9 (1) |
ALT denotes alanine aminotransferase, and AST aspartate aminotransferase.
In the ADT-alone group, there were two events of myocardial infarction and one event of bronchopneumonia. In the combination group, there were two events of pneumonia (one including sepsis), two events of stroke, and one event each of dyspnea, lower respiratory tract infection, liver failure, pulmonary hemorrhage, and chest infection.
Endocrine disorders included hot flashes and impotence.
Treatment after Progression
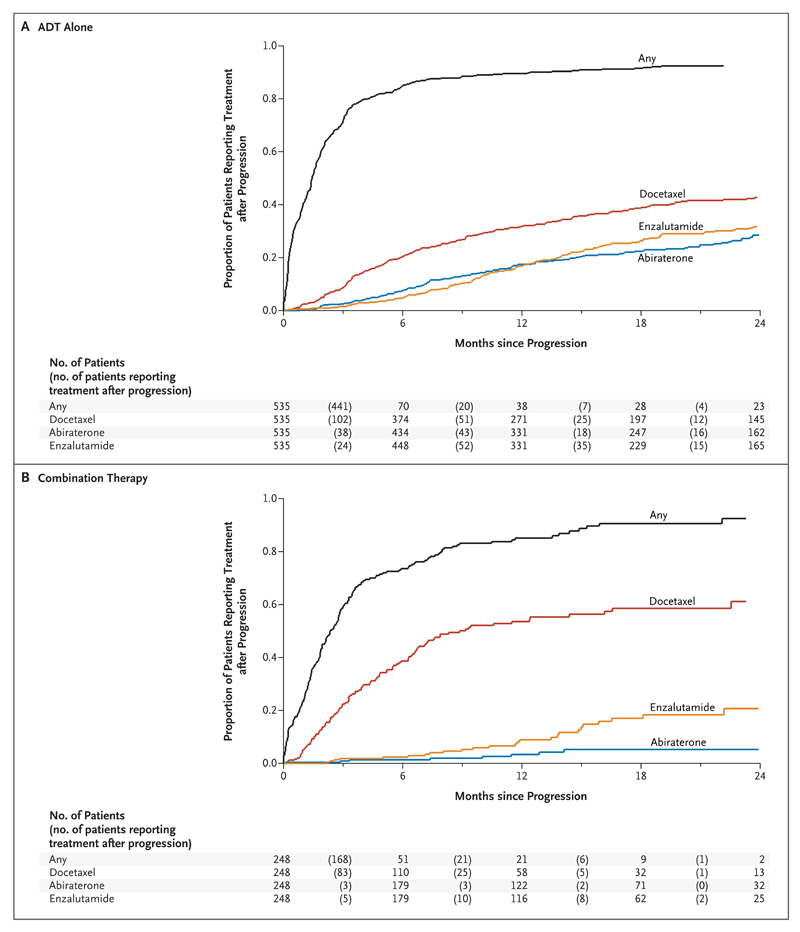
There are emerging differences in the therapies used at relapse (Fig. 3, and Table S3 in the Supplementary Appendix). The median time from relapse to life-prolonging therapy for castration-resistant prostate cancer (abiraterone, enzalutamide, docetaxel, cabazitaxel, or radium-223) or any subsequent therapy was similar in the two groups, but absolute numbers of patients reporting life-prolonging or any treatment after progression were higher in the ADT-alone group than in the combination group (ADT-alone group, 310 and 477, respectively, of 957 patients; combination group, 131 and 196, respectively, of 960 patients). The use of abiraterone at relapse in patients who received abiraterone as primary therapy was lower in patients in the combination group than in patients in the ADT-alone group; patients in the combination group could continue abiraterone beyond the first event of radiologic, clinical, or PSA progression until all three protocol-defined progression types were reached. The rate of docetaxel use was proportionately higher in the combination group than in the ADT-alone group. Because 1471 of the men (77%) in this comparison remain alive, these data will evolve with time as more patients have a relapse and receive further treatments.
Figure 3. Time until the Initiation of Second-Line Treatment after First Event of Radiologic, Clinical, or PSA Progression.
Discussion
STAMPEDE is a multigroup, multistage platform trial protocol investigating the efficacy of additional therapy at the time of inception of primary ADT in men with newly diagnosed, locally advanced, or metastatic disease or in those with relapsing disease and poor prognostic features. This is the sixth comparison in the trial for which we are reporting survival data15,16,24–27 and the first comparison incorporated after trial initiation.22
The use of ADT plus abiraterone and prednisolone as compared with ADT alone was associated with a 71% relative improvement in the time to treatment failure, which translated into a 37% difference in overall survival. These findings were consistent in patients with metastatic disease and those with nonmetastatic disease, although most deaths have occurred in the patients with metastatic disease. The between-group difference in survival among the patients with nonmetastatic disease, which is underpinned by a large difference in failure-free survival, occurred even though abiraterone was administered for 2 years or less in this group.
There was good adherence to abiraterone, which was associated with acceptable adverseevent rates, with most patients stopping therapy owing to the completion of planned therapy or protocol-defined progression rather than toxic effects. Adverse events were in line with previous experience in castration-resistant prostate cancer,4,5,21 despite the longer absolute duration of therapy, particularly in the patients with metastatic disease. Nearly 300 patients are still receiving treatment. Few patients stopped treatment because of side effects, but there were more grade 3 to 5 adverse events reported in the combination group than in the ADT-alone group. The lower dose of prednisolone that was used does not seem to have influenced the rate of pharmacologically relevant severe adverse events, such as hypertension or hypokalemia.20
The strengths of the comparison in this trial include broad recruitment through an academic network (116 centers, including most U.K. oncology centers, in collaboration with the Swiss Group for Clinical Cancer Research) encompassing a wide range of disease states. The size of the trial permits some examination of interactions, including those with other treatments known to be effective (e.g., radiotherapy) and prognostic factors used for stratification at randomization. Effects in all subgroups were similar, with no evidence of heterogeneity. The apparent inconsistency in survival-effect size in men 70 years of age or older is probably an artifact arising from a small number of events; the absolute difference is only 8 deaths (74 in the combination group and 82 in the ADT-alone group). The result is complicated by a higher risk of death from coexisting conditions and a relatively shorter follow-up among older men, because a higher proportion were recruited later, after recruitment to the docetaxel comparison in the protocol was completed. Results for failure-free, progression-free, and prostate cancer–specific survival showed a benefit of abiraterone in men regardless of age. Further exploration is possible in a preplanned meta-analysis with two other trials investigating the addition of abiraterone to standard ADT with overlapping patient inclusions: LATITUDE (to be published on June 4, 2017, at NEJM.org)28 and PEACE1 (ClinicalTrials.gov number, NCT01957436).
The effect size that we report with abiraterone is a little larger with respect to overall survival and substantially larger with respect to failure-free survival than the effect size that we reported with the addition of docetaxel in a similar patient group. The trial results raise the question of what should be regarded as the contemporary standard of care. Abiraterone has a better side-effect profile than docetaxel and is an easier treatment to administer logistically. Conversely, the treatment duration is longer, some patients have long-term effects of glucocorticoid use, and cost is a consideration. In the absence of comparative data, patient choice and the ability of health care systems to support the use of these drugs will determine the relative use of docetaxel and abiraterone.
Perhaps a more important question is whether the benefits of docetaxel and abiraterone can be combined. Although docetaxel may work in part by targeting the androgen-receptor pathway,29 it generally has a different mechanism of action than abiraterone, and abiraterone is active after docetaxel use.5 Thus, there may be an additive effect of giving abiraterone immediately after docetaxel in prostate cancer not previously treated with hormone therapy. Some future information will emerge from the STAMPEDE trial, which includes a further comparison involving abiraterone plus enzalutamide in which docetaxel was permitted as part of the standard of care in patients who had undergone randomization starting in 2016. The PEACE1 trial also now includes patients receiving docetaxel as part of the standard of care and will help address the question of whether the benefits seen with docetaxel15–17 are overlapping or additional to those seen with abiraterone.
Finally, the design used in the trial has allowed the STAMPEDE investigators to address multiple questions within a single trial platform, making efficient use of control-group patients.22 The current abiraterone comparison eventually recruited 1917 patients in 2 years 3 months, well ahead of target and underlining the power of this design to address multiple questions efficiently and rapidly. There are three further randomizations within the trial that will yield survival results in the coming years, which means that a single protocol will have answered at least 10 different primary questions in 15 years; two further comparisons are also in the late stages of planning. To address as many questions in separate trials would have taken considerably longer and required far more control-group patients.
In conclusion, men with locally advanced or metastatic prostate cancer who received ADT plus abiraterone and prednisolone had significantly higher rates of overall and failure-free survival than those who received ADT alone. In addition, combination therapy was associated with fewer symptomatic skeletal events.
Supplementary Material
Acknowledgments
Supported by Cancer Research U.K., Medical Research Council, Astellas Pharma, Clovis Oncology, Janssen, Novartis, Pfizer, and Sanofi Aventis.
Appendix
The authors’ full names and academic degrees are as follows: Nicholas D. James, Ph.D., Johann S. de Bono, Ph.D., Melissa R. Spears, M.Sc., Noel W. Clarke, Ch.M., Malcolm D. Mason, F.R.C.R., David P. Dearnaley, F.R.C.R., Alastair W.S. Ritchie, M.D., Claire L. Amos, Ph.D., Clare Gilson, M.R.C.P., Rob J. Jones, M.B., Ch.B., David Matheson, Ph.D., Robin Millman, Gerhardt Attard, M.D., Simon Chowdhury, Ph.D., William R. Cross, F.R.C.S., Silke Gillessen, M.D., Christopher C. Parker, M.D., J. Martin Russell, F.R.C.R., Dominik R. Berthold, M.D., Chris Brawley, M.Sc., Fawzi Adab, F.R.C.R., San Aung, M.R.C.P., Alison J. Birtle, F.R.C.R., Jo Bowen, F.R.C.R., Susannah Brock, F.R.C.R., Prabir Chakraborti, F.R.C.R., Catherine Ferguson, F.R.C.R., Joanna Gale, B.M., Emma Gray, F.R.C.R., Mohan Hingorani, Ph.D., Peter J. Hoskin, F.R.C.R., Jason F. Lester, F.R.C.R., Zafar I. Malik, F.R.C.R., Fiona McKinna, F.R.C.R., Neil McPhail, F.R.C.R., Julian Money-Kyrle, F.R.C.R., Joe O’Sullivan, Ph.D., Omi Parikh, F.R.C.R., Andrew Protheroe, F.R.C.P., Angus Robinson, F.R.C.R., Narayanan N. Srihari, F.R.C.R., Carys Thomas, M.R.C.P., John Wagstaff, Ch.B., James Wylie, F.R.C.R., Anjali Zarkar, F.R.C.R., Mahesh K.B. Parmar, D.Phil., and Matthew R. Sydes, M.Sc.
The authors’ affiliations are as follows: Institute of Cancer and Genomic Sciences, University of Birmingham (N.D.J.), and University Hospital Birmingham (A.Z.), Birmingham, the Institute of Cancer Research (J.S.B., D.P.D., G.A.), Medical Research Council Clinical Trials Unit at University College London (M.R. Spears, C.L.A., C.G., C.B., M.K.B.P., M.R. Sydes), King’s College London and Guy’s and St. Thomas’ NHS Foundation Trust (S.C.), and Royal Marsden NHS Foundation Trust (C.C.P.), London, Salford Royal NHS Foundation Trust, Salford (N.W.C.), Cardiff University School of Medicine (M.D.M.) and Velindre Cancer Centre (J.F.L.), Cardiff, Gloucestershire Royal Hospital (A.W.S.R.) and Gloucestershire Hospitals NHS Foundation Trust (J.B.), Gloucester, University of Glasgow, Glasgow (R.J.J., J.M.R.), St. James’s University Hospital, Leeds (W.R.C.), University Hospital of North-Midlands, Stoke-on-Trent (F.A.), Royal Devon and Exeter Hospital NHS Foundation Trust, Exeter (S.A.), Rosemere Cancer Centre, Royal Preston Hospital, Preston (A.J.B.), Dorset Cancer Centre, Poole Hospital, Poole (S.B.), Royal Derby Hospital, Derby (P.C.), Weston Park Hospital, Sheffield (C.F.), Portsmouth Oncology Centre, Queen Alexandra Hospital, Portsmouth (J.G.), Musgrove Park Hospital, Taunton (E.G.), Hull and East Yorkshire Hospitals NHS Trust, Hull (M.H.), Mount Vernon Cancer Centre, Northwood (P.J.H.), Clatterbridge Cancer Centre, Wirral (Z.I.M.), Brighton and Sussex University Hospitals NHS Trust (F.M.) and Sussex Cancer Centre, Royal Sussex County Hospital (A.R.), Brighton, NHS Highland, Inverness (N.M.), Royal Surrey County Hospital, Guildford (J.M.-K.), Northern Ireland Cancer and Queens University, Belfast (J.O.), Lancashire Teaching Hospitals NHS Trust, Preston (O.P.), Churchill Hospital, Oxford (A.P.), Shrewsbury and Telford Hospitals NHS Trust, Shrewsbury (N.N.S.), East Kent Hospitals NHS Trust, Canterbury (C.T.), Swansea University College of Medicine, Swansea (J. Wagstaff), and Christie NHS Foundation Trust, Manchester (J. Wylie) — all in the United Kingdom; and the Department of Medical Oncology, Kantonsspital St. Gallen, St. Gallen (S.G.), and University Hospital of Lausanne, Lausanne (D.R.B.) — both in Switzerland. Two of the authors (D.M., R.M.) were unaffiliated lay members of the STAMPEDE investigators.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–22. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 2.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–12. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 3.Petrylak DP, Tangen CM, Hussain MHA, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–20. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 4.Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138–48. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised openlabel trial. Lancet. 2010;376:1147–54. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 7.Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371:424–33. doi: 10.1056/NEJMoa1405095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–23. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 9.Lipton A, Fizazi K, Stopeck AT, et al. Superiority of denosumab to zoledronic acid for prevention of skeletal-related events: a combined analysis of 3 pivotal, randomised, phase 3 trials. Eur J Cancer. 2012;48:3082–92. doi: 10.1016/j.ejca.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Fizazi K, Carducci M, Smith M, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377:813–22. doi: 10.1016/S0140-6736(10)62344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saad F, Gleason DM, Murray R, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormonerefractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94:1458–68. doi: 10.1093/jnci/94.19.1458. [DOI] [PubMed] [Google Scholar]
- 12.Parmar MK, Barthel FM, Sydes M, et al. Speeding up the evaluation of new agents in cancer. J Natl Cancer Inst. 2008;100:1204–14. doi: 10.1093/jnci/djn267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.James ND, Sydes MR, Clarke NW, et al. Systemic therapy for advancing or metastatic prostate cancer (STAMPEDE): a multiarm multistage randomized controlled trial. BJU Int. 2009;103:464–9. doi: 10.1111/j.1464-410X.2008.08034.x. [DOI] [PubMed] [Google Scholar]
- 14.Sydes MR, James ND, Mason MD, et al. Flexible trial design in practice — dropping and adding arms in STAMPEDE: a multi-arm multi-stage randomised controlled trial. Trials. 2011;12(Suppl 1):A3. doi: 10.1186/1745-6215-13-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.James ND, Sydes MR, Clarke NW, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387:1163–77. doi: 10.1016/S0140-6736(15)01037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vale CL, Burdett S, Rydzewska LH, et al. Addition of docetaxel or bisphosphonates to standard of care in men with localised or metastatic, hormone-sensitive prostate cancer: a systematic review and meta-analyses of aggregate data. Lancet Oncol. 2016;17:243–56. doi: 10.1016/S1470-2045(15)00489-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sweeney CJ, Chen Y-H, Carducci M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373:737–46. doi: 10.1056/NEJMoa1503747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gravis G, Boher JM, Joly F, et al. Androgen deprivation therapy (ADT) plus docetaxel versus ADT alone in metastatic non castrate prostate cancer: impact of metastatic burden and long-term survival analysis of the randomized phase 3 GETUG-AFU15 trial. Eur Urol. 2016;70:256–62. doi: 10.1016/j.eururo.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 19.Gravis G, Fizazi K, Joly F, et al. Androgen-deprivation therapy alone or with docetaxel in non-castrate metastatic prostate cancer (GETUG-AFU 15): a randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14:149–58. doi: 10.1016/S1470-2045(12)70560-0. [DOI] [PubMed] [Google Scholar]
- 20.Attard G, Reid AH, Yap TA, et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol. 2008;26:4563–71. doi: 10.1200/JCO.2007.15.9749. [DOI] [PubMed] [Google Scholar]
- 21.Ryan CJ, Smith MR, Fizazi K, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015;16:152–60. doi: 10.1016/S1470-2045(14)71205-7. [DOI] [PubMed] [Google Scholar]
- 22.Sydes MR, Parmar MK, Mason MD, et al. Flexible trial design in practice — stopping arms for lack-of-benefit and adding research arms mid-trial in STAMPEDE: a multi-arm multi-stage randomized controlled trial. Trials. 2012;13:168. doi: 10.1186/1745-6215-13-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barthel FM-S, Royston P, Parmar MKB. A menu-driven facility for sample-size calculation in novel multiarm, multi-stage randomized controlled trials with a time-to-event outcome. Stata J. 2009;9:505–23. [Google Scholar]
- 24.Mason MD, Clarke NW, James ND, et al. Adding celecoxib with or without zoledronic acid for hormone-naïve prostate cancer: long-term survival results from an adaptive, multiarm, multistage, platform, randomized controlled trial. J Clin Oncol. 2017;35:1530–41. doi: 10.1200/JCO.2016.69.0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.James ND, Spears MR, Clarke NW, et al. Failure-free survival and radiotherapy in patients with newly diagnosed nonmetastatic prostate cancer: data from patients in the control arm of the STAMPEDE trial. JAMA Oncol. 2016;2:348–57. doi: 10.1001/jamaoncol.2015.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.James ND, Spears MR, Clarke NW, et al. Survival with newly diagnosed metastatic prostate cancer in the “docetaxel era”: data from 917 patients in the control arm of the STAMPEDE trial (MRC PR08, CRUK/06/019) Eur Urol. 2015;67:1028–38. doi: 10.1016/j.eururo.2014.09.032. [DOI] [PubMed] [Google Scholar]
- 27.James ND, Sydes MR, Mason MD, et al. Celecoxib plus hormone therapy versus hormone therapy alone for hormone-sensitive prostate cancer: first results from the STAMPEDE multiarm, multistage, randomised controlled trial. Lancet Oncol. 2012;13:549–58. doi: 10.1016/S1470-2045(12)70088-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fizazi K, Tran N, Fein L, et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. doi: 10.1056/NEJMoa1704174. [DOI] [PubMed] [Google Scholar]
- 29.Fitzpatrick JM, de Wit R. Taxane mechanisms of action: potential implications for treatment sequencing in metastatic castration-resistant prostate cancer. Eur Urol. 2014;65:1198–204. doi: 10.1016/j.eururo.2013.07.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.