Abstract
By meta-analyzing rare coding variants in whole-exome sequences of 4,133 schizophrenia cases and 9,274 controls, de novo mutations in 1,077 trios, and copy number variants from 6,882 cases and 11,255 controls, we show that individuals with schizophrenia carry a significant burden of rare damaging variants in 3,488 genes previously identified as having a near-complete depletion of loss-of-function variants. In schizophrenia patients who also have intellectual disability, this burden is concentrated in risk genes associated with neurodevelopmental disorders. After excluding known neurodevelopmental disorder risk genes, a significant rare variant burden persists in other loss-of-function intolerant genes, and while this effect is notably stronger in schizophrenia patients with intellectual disability, it is also seen in patients who do not have intellectual disability. Together, our results show that rare damaging variants contribute to the risk of schizophrenia both with and without intellectual disability, and support an overlap of genetic risk between schizophrenia and other neurodevelopmental disorders.
Introduction
Schizophrenia is a common and debilitating psychiatric illness characterized by positive symptoms (hallucinations, delusions, disorganized speech and behaviour), negative symptoms (social withdrawal and diminished emotional expression), and cognitive impairment that result in social and occupational dysfunction1,2. Operational diagnostic criteria for the disorder as described in the DSM-V require the presence of at least two of the core symptoms over a period of six months with at least one month of active symptoms3. It is increasingly recognized that current categorical psychiatric classifications have a number of shortcomings, in particular that they overlook the increasing evidence for etiological and mechanistic overlap between psychiatric disorders4.
A diverse range of pathophysiological processes may contribute to the clinical features of schizophrenia5. Indeed, previous studies have suggested a number of hypotheses about schizophrenia pathogenesis, including abnormal pre-synaptic dopaminergic activity6, postsynaptic mechanisms involved in synaptic plasticity7, dysregulation of synaptic pruning8, and disruption to early brain development9,10. This complexity is underpinned by the varied nature of genetic contributions to risk of schizophrenia. Genome-wide association studies have identified over 100 independent loci defined by common (minor allele frequency [MAF] > 1%) single nucleotide variants (SNVs)11, and a recent analysis determined that more than 71% of all one-megabase regions in the genome contain at least one common risk allele12. The modest effects of these variants (median odds ratio [OR] = 1.08) combine to produce a polygenic contribution that explains only a fraction of the overall liability12. In addition, a number of rare variants have been identified that have far larger effects on individual risk. These are best exemplified by eleven large, rare recurrent copy number variants (CNVs) but evidence from whole-exome sequencing studies implies that many other rare coding SNVs and de novo mutations also confer substantial individual risk13–17. There is growing evidence that some of the same genes and pathways are affected by both common and rare variants7,18. Pathway analyses of common variants and hypothesis-driven gene set analyses of rare variants have begun to enumerate some of these specific biological processes, including histone methylation, transmission at glutamatergic synapses, calcium channel signaling, synaptic plasticity, and translational regulation by the fragile X mental retardation protein (FMRP)11,13,14,19,20.
In addition to exploring the biological mechanisms underlying schizophrenia, genetic analyses can also be used to understand its relationship to other neuropsychiatric and neurodevelopmental disorders. For instance, schizophrenia, bipolar disorder, and autism (ASD) show substantial sharing of common risk variants21,22. Sequencing studies of neurodevelopmental disorders suggest that this sharing of genetic risk may extend to rare variants of large effect. In the largest sequencing study of ASD to date, 20 of the 46 genes and all six CNVs implicated (false discovery rate [FDR] < 5%) had been previously described as dominant causes of developmental disorders23. Furthermore, an analysis of 60,706 whole exomes led by the ExAC consortium identified 3,230 genes with near-complete depletion of protein-truncating variants, and de novo loss-of-function (LoF) mutations identified in individuals with ASD or developmental disorders were concentrated in this set of “LoF intolerant” genes23–25. Similarly, evidence from rare variants for a broader shared genetic etiology between schizophrenia and neurodevelopmental disorders has begun to emerge. Analyses of whole-exome data provided support for an enrichment of schizophrenia rare variants in intellectual disability genes, and schizophrenia cases were also found to have a higher concentration of ultra-rare disruptive SNVs in the ExAC LoF intolerant genes compared to controls13,17,26.
However, the contribution of these rare variants to risk in the wider population of individuals diagnosed with schizophrenia, including those without intellectual disability, remains unclear. Intriguingly, the 11 rare CNVs found to be highly penetrant for schizophrenia also increased risk for intellectual disability and other congenital defects16,27, and more recently, a meta-analysis of whole-exome sequence data showed that LoF variants in SETD1A conferred substantial risk for both schizophrenia and neurodevelopmental disorders18. Concurrent analyses of autism whole-exome data found that de novo loss-of-function (LoF) mutations identified in ASD probands, particularly those that disrupt genes associated with neurodevelopmental disorders, were disproportionately found in individuals with intellectual disability23,28. These emerging results raise the possibility that rare schizophrenia risk variants may be concentrated in a subset of schizophrenia patients with co-morbid intellectual disability. Here, we present the one of the largest accumulation of schizophrenia rare variant data to date, which we jointly analyze with phenotype data on cognitive function. Using this data set, we attempt to identify groups of genes disrupted by schizophrenia rare risk variants, and determine if a subset of patients disproportionately carry these damaging alleles.
Results
Study design
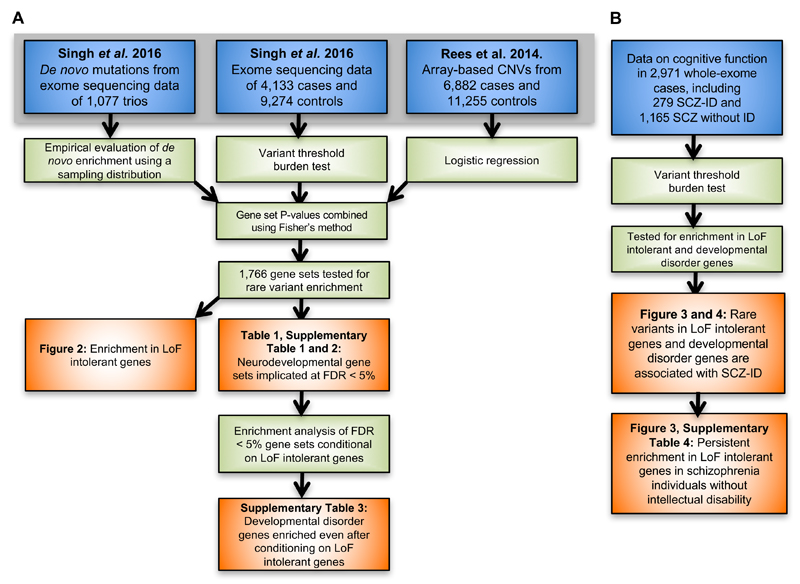
To maximize our power to detect enrichment of damaging variants in schizophrenia cases in groups of genes, we performed a meta-analysis of three different types of rare coding variant studies: (1) high-quality SNV calls from whole-exome sequences of 4,133 schizophrenia cases and 9,274 matched controls, (2) de novo mutations identified in 1,077 schizophrenia parent-proband trios (Figure 1), and (3) CNV calls from genotyping array data of 6,882 cases and 11,255 controls. The ascertainment of these samples, data production, and quality control were described previously18,29. All de novo mutations included in our analysis had been validated through Sanger sequencing, and stringent quality control steps were performed on the case-control data to ensure that sample ancestry and batch were closely matched between cases and controls (Online Methods).
Figure 1.
Analysis workflow. Data sets are shown in blue, statistical methods and analysis steps are shown in green, and results (figures and tables) from the analysis are shown in orange. A: Enrichment analyses in 1,766 gene sets using the entire rare variant data set. B: Enrichment analyses in LoF intolerant and developmental disorder genes in the subset of cases with information on cognitive function. ID: intellectual disability; SCZ: schizophrenia; SCZ-ID: schizophrenia patients with intellectual disability.
For each data type, we used appropriate methods to test for an excess of rare variants (Figure 1, Online Methods). In analyses of case-control SNV data, we applied an extension of the variant threshold burden test that corrected for exome-wide differences between cases and controls30. We tested all allele frequency thresholds below 0.1% observed in our data, and assessed statistical significance by permutation testing. In analyses of de novo SNV data, we compared the observed number of de novo mutations to random samples from an expected distribution based on a gene-specific mutation rate model to calculate an empirical P-value. For both types of whole-exome sequencing data, we restricted our analyses to loss-of-function variants. Finally, in analyses of case-control CNV data, we used a logistic regression framework that compares the rate of CNVs overlapping a specific gene set while correcting for differences in CNV size and number of genes disrupted7,19,31. To ensure our model was well calibrated, we restricted our analyses to small deletions and duplications overlapping fewer than seven genes with MAF < 0.1% (Supplementary Figure 1, Online Methods).
We tested for an excess of rare damaging variants in schizophrenia patients in 1,766 gene sets (Online Methods, Supplementary Table 1, and detailed results below). Gene set P-values were computed using the three methods and variant definitions described above, and then meta-analyzed using Fisher’s Method to provide a single P-value for each gene set. Because we gave each data type equal weight, gene sets achieving significance typically show at least some signal in all three types of data. We observed a marked inflation in the quantile-quantile (Q-Q) plot of gene set P-values (Supplementary Figure 2), so we conducted two analyses to ensure our results were robust and not biased due to methodological or technical artifacts. First, we observed no inflation of P-values when testing for enrichment of synonymous variants in our case-control and de novo analyses (Supplementary Figure 2). Second, we created random gene sets by sampling uniformly across the genome, and observed null distributions in Q-Q plots regardless of variant class and analytical method (Supplementary Figure 3). These findings suggested that our methods sufficiently corrected for known genome-wide differences in LoF and CNV burden between cases and controls, and other technical confounders like batch and ancestry.
Rare, damaging schizophrenia variants are concentrated in LoF intolerant genes
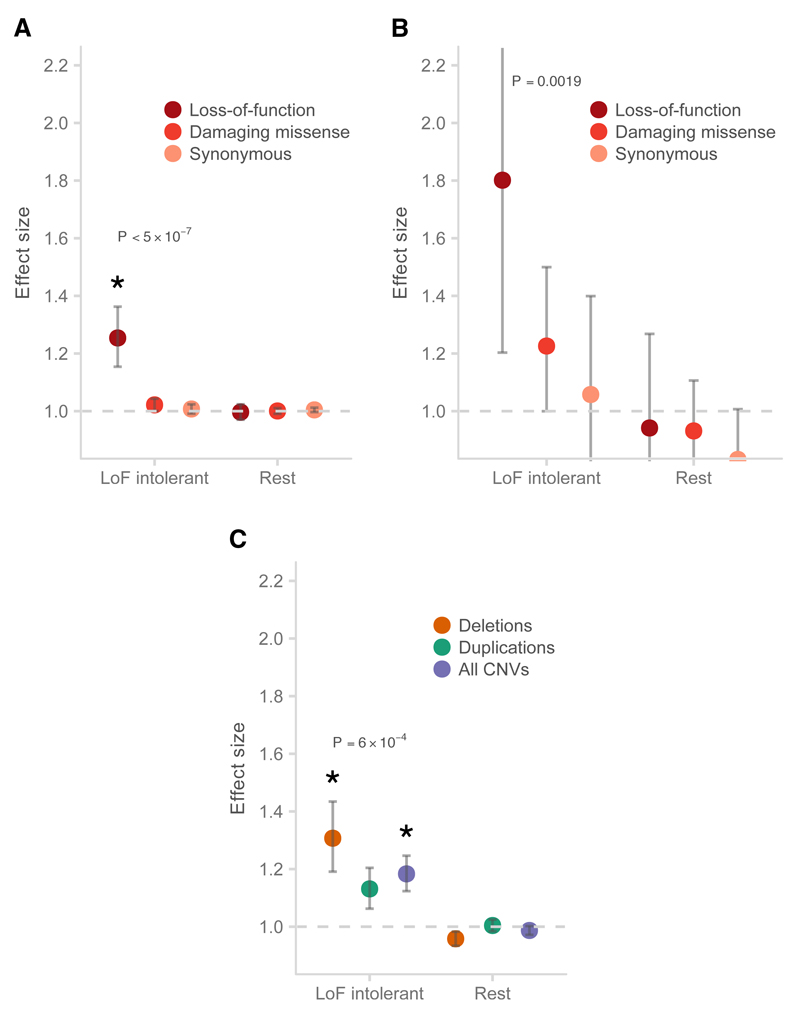
We first tested whether rare schizophrenia risk variants were consistently concentrated in genes defined loss-of-function intolerant across study design and variant type. Because some of our schizophrenia exome data was included in the ExAC database, we focused on the subset of 45,376 ExAC exomes without a known psychiatric diagnosis and that were not present in our study. From this subset, 3,488 genes were found to have near-complete depletion of such variants, which we defined as the LoF intolerant gene set. We found that rare damaging variants in schizophrenia cases were enriched in LoF intolerant genes (P < 3.6×10−10, Table 1, Figure 2), with support in case-control SNVs (P < 5×10−7; OR 1.24, 1.16-1.31, 95% CI), case-control CNVs (P = 2.6×10−4; OR 1.21, 1.15 – 1.28, 95% CI), and de novo mutations (P = 6.7×10−3; OR 1.36, 1.1 – 1.68, 95% CI). While this result was consistent with observations in intellectual disability and ASD24,32 the absolute effect size is smaller (e.g. de novos, Supplementary Figure 4 and 5). We observed no excess burden of rare damaging variants in the remaining 14,753 genes (Figure 2, Supplementary Figure 5). Furthermore, this signal was spread among many different LoF intolerant genes: if we rank genes by decreasing significance, the enrichment disappears in the case-control SNV analysis (P > 0.05) only after the exclusion of the top 50 genes. This suggests that the contribution of damaging rare variants in schizophrenia is not concentrated in just a handful of genes, but instead spread across many genes.
Table 1.
Gene sets enriched for rare coding variants conferring risk for schizophrenia at FDR < 1%. The effect sizes and corresponding P-values from enrichment tests of each variant type (case-control SNVs, DNM, and case-control CNVs) are shown for each gene set, along with the Fisher’s combined P-value (Pmeta) and the FDR-corrected Q-value (Qmeta). We only show the most significant gene set if there are multiple ones from the same data set or biological process (see Supplementary Table 1 for all 1,766 gene sets). Ngenes: number of genes in the gene set; Est: effect size estimate and its lower and upper bound assuming a 95% CI; DNM: de novo mutation.
| Name | Ngenes | EstSNV | 95% CI of EstSNV | PSNV | EstDNM | 95% CI of EstDNM | PDNM | EstCNV | 95% CI of EstCNV | PCNV | Pmeta | Qmeta |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ExAC LoF intolerant genes (pLI > 0.9) |
3488 | 1.24 | 1.16-1.31 | < 5.0 x 10-7 | 1.36 | 1.1-1.68 | 0.0067 | 1.21 | 1.15-1.28 | 0.00026 | < 3.60 x 10-10 | 4.30 x 10-7 |
| Dominant, diagnostic DDG2P genes, in which LoF variants result in developmental disorders with brain abnormalities | 156 | 1.42 | 1.07-1.88 | 0.011 | 4.18 | 2.21-8.03 | 0.00073 | 1.92 | 1.54-2.39 | 0.0016 | 2.30 x 10-6 | 0.00067 |
| Sanders et al. autism risk genes (FDR < 10%) | 66 | 1.28 | 0.97-1.69 | 0.0095 | 3.96 | 1.65-9.94 | 0.019 | 2.21 | 1.75-2.79 | 0.00033 | 9.50 x 10-6 | 0.0017 |
| Darnell et al. targets of FMRP | 790 | 1.24 | 1.13-1.36 | 8.5 x 10-6 | 1.31 | 0.83-2.09 | 0.17 | 1.32 | 1.2-1.47 | 0.0032 | 9.30 x 10-7 | 0.00038 |
| Cotney et al. CHD8-targeted promoters (hNSC and human brain tissue) | 2920 | 1.09 | 1.02-1.16 | 0.0008 | 1.77 | 1.36-2.31 | 0.00025 | 1.11 | 1.05-1.18 | 0.027 | 1.10 x 10-6 | 0.00038 |
| G2CDB: mouse cortex post-synaptic density consensus | 1527 | 1.20 | 1.11-1.3 | 2.5 x 10-6 | 1.57 | 1.06-2.33 | 0.028 | 1.04 | 0.96-1.11 | 0.32 | 3.90 x 10-6 | 0.00097 |
| Weynvanhentenryck et al. CLIP targets of RBFOX | 967 | 1.21 | 1.11-1.33 | 4.8 x 10-5 | 1.84 | 1.21-2.8 | 0.0085 | 1.07 | 0.98-1.17 | 0.2 | 1.30 x 10-5 | 0.002 |
| NMDAR network (defined in Purcell et al.) | 61 | 1.66 | 1.09-2.54 | 0.0061 | 5.60 | 2.06-16.09 | 0.017 | 2.46 | 1.78-3.4 | 0.0028 | 3.70 x 10-5 | 0.0044 |
| GOBP: chromatin modification (GO:0016568) | 519 | 1.29 | 1.13-1.49 | 0.00018 | 2.26 | 1.32-3.94 | 0.0099 | 1.12 | 0.99-1.28 | 0.18 | 4.20 x 10-5 | 0.0046 |
Figure 2.
Enrichment of schizophrenia rare variants in genes intolerant of loss-of-function variants. A: Schizophrenia cases compared to controls for rare SNVs and indels; B: Rates of de novo mutations in schizophrenia probands compared to control probands; C: Case-control CNVs. P-values shown were from the test of LoF enrichment in A, LoF enrichment in B, and all CNVs enrichment in C. Error bars represent the 95% CI of the point estimate. LoF intolerant: 3,448 genes with near-complete depletion of truncating variants in the ExAC database; Rest: the remaining genes in the genome with pLI < 0.9; Damaging missense: missense variants with CADD phred > 15. Asterisk: P < 1 x 10-3.
Schizophrenia risk genes are shared with other neurodevelopmental disorders
Given the significant enrichment of rare damaging variants in LoF intolerant genes in developmental disorders, autism and schizophrenia, we next asked whether these variants affected the same genes. We found that autism risk genes identified from exome sequencing meta-analyses23 and genes in which LoF variants are known causes of severe developmental disorders as defined by the DDD study33,34 were significantly enriched for rare variants in individuals with schizophrenia (PASD = 9.5 ×10−6; PDD = 2.3 ×10−6; Table 1, Online Methods). Previous analyses have shown an enrichment of rare damaging variants in genes whose mRNA are bound by FMRP in both schizophrenia and autism35,13,32, so we sought to identify further shared biology by testing targets of neural regulatory genes previously implicated in autism32,36. We observed enrichment of both such sets: promoter targets of CHD8 (P = 1.1×10−6) and splice targets of RBFOX (P = 1.3×10−5) (Table 1). We noted that some published gene lists attributed to same biological process differed due to choices of assay, cell type, method of sample extraction, and threshold of statistical significance, leading to distinct results in our gene set analyses. For example, we observed a significant enrichment in the published FMRP binding gene set based on mouse brain data37, but with no signal in one based on a human kidney cell line38.
We also tested an additional 1,759 gene sets from databases of biological pathways with at least 100 genes, as we lacked power to detect weak enrichments in smaller sets (Online Methods). We observed enrichment of damaging rare variants in schizophrenia cases at FDR q < 0.05 in 35 of these gene sets (Supplementary Table 1, 2). These included previously implicated gene sets, like the NMDA receptor and ARC complexes13,14,35,37, as well as novel gene sets, such as genes involved in cytoskeleton (GO: 0007010), chromatin modification (GO:0016568), and chromatin organization (GO: 0006325). Furthermore, the gene sets most significantly enriched (FDR q < 0.01) for schizophrenia rare variants (Table 1) had all been previously linked to autism, intellectual disability, and severe developmental disorders23,32,33. Our enrichment results matched some of the findings from a pathway analysis of common risk variants in psychiatric disorders, which also implicated neuronal and chromatin gene sets20. However, unlike that study, we found no enrichment of rare variants in immune-related gene sets.
We noticed that the 1,759 gene sets we tested were collectively enriched with LoF intolerant genes when compared to a random sampling of genes from the genome (Supplementary Figure 6 and 7). For some of the gene sets associated with schizophrenia, this over-representation was quite substantial: 67% of the gene targets of FMRP and 74% of the genes associated with severe neurodevelopmental disorders are LoF intolerant. To better understand the consequences of this overlap on our results, we extended the gene set enrichment methods (Online Methods) to condition on LoF intolerance and brain-expression for the 35 gene sets with FDR q < 0.05 in the previous analysis (Supplementary Table 2). We first observed that 22 of the 35 gene sets remained significant even after conditioning on brain expression (Supplementary Tables 3, Online Methods), suggesting they represent more specific biological processes involved in schizophrenia. However, only known autism risk genes (P = 4.4×10−4) and neurodevelopmental disorder genes (P = 3×10−5) had an excess of rare coding variants above the enrichment already observed in LoF intolerant genes (Supplementary Table 3). Thus, in addition to biological pathways implicated specifically in schizophrenia, at least a portion of the schizophrenia risk conferred by rare variants of large effect is shared with childhood onset disorders of neurodevelopment.
Schizophrenia patients with intellectual disability have a greater burden of rare damaging variants
In autism spectrum disorders, the observed excess of rare damaging variants has been shown to be greater in individuals with intellectual disability than those with normal levels of cognitive function28. We observed a similar phenomenon in schizophrenia cases carrying SETD1A LoF variants18, so next sought to explore whether this pattern is consistent in gene sets implicated in schizophrenia. We acquired relevant cognitive phenotype data for 2,971 of the 4,131 schizophrenia patients with whole-exome sequencing data (Supplementary Figure 8). Of these individuals, 279 were clinically diagnosed with intellectual disability in addition to fulfilling the full diagnostic criteria for schizophrenia (SCZ-ID subgroup, Online Methods). We also identified 1,165 individuals for whom we could rule out cognitive impairment (by excluding pre-morbid IQ < 85, fewer than 12 years of schooling or lowest decile of composite cognitive measures, depending on available data, Online Methods). Finally, we identified 1,527 individuals who were not diagnosed with intellectual disability, but in whom some cognitive impairment could not be excluded.
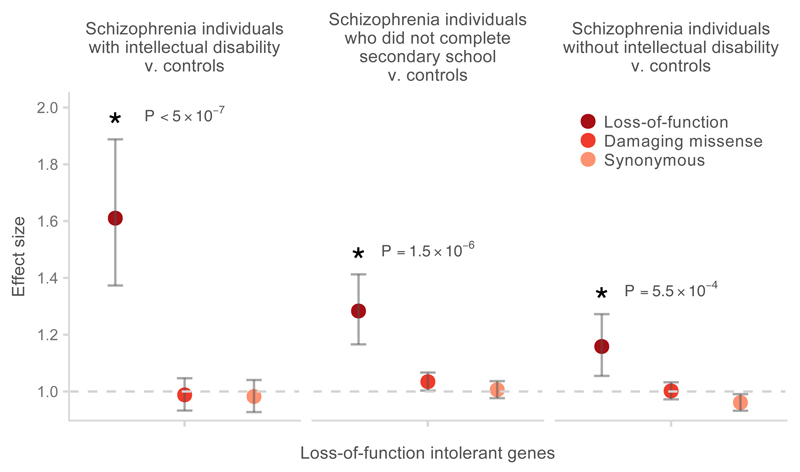
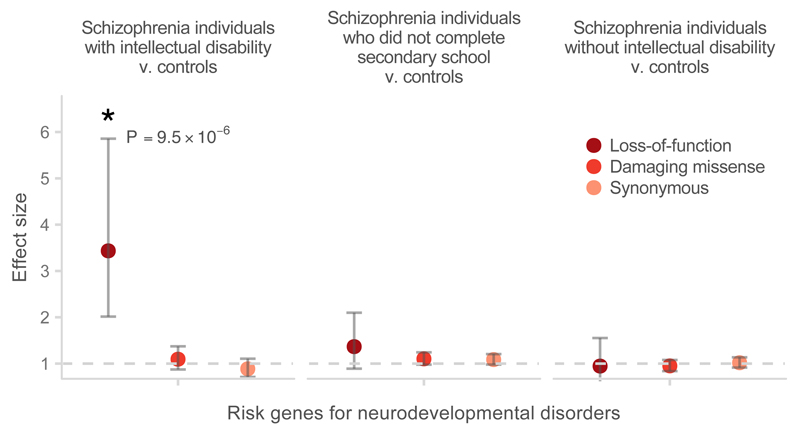
When stratifying into these three groups (intellectual disability, no intellectual disability but cognitive impairment not excluded, no cognitive impairment), we observed that the burden of rare damaging variants in LoF intolerant genes was significantly greater in the SCZ-ID subgroup than in the remaining schizophrenia cases (P = 2.6×10−4; OR 1.3, 1.12– 1.51, 95% CI) or controls (P < 5×10−7; OR 1.61, 1.37 – 1.89, 95% CI; Figure 3). In the LoF intolerant gene set, 0.27 (0.2 – 0.35, 95% CI) extra singleton (defined as having an allele count of one in our data set) LoF variants were observed per exome in SCZ-ID cases compared to controls, while 0.10 (0.065 – 0.13, 95% CI) extra singleton LoF variants per exome were observed in the remaining schizophrenia cases compared to controls (Online Methods). Furthermore, SCZ-ID individuals had significant enrichment of rare LoF variants in developmental disorder genes compared to the other cases (P = 9×10−4; OR 2.36, 1.41– 3.92, 95% CI) or to controls (P = 9.5×10−6; OR 3.43, 2.01– 5.86, 95% CI; Figure 4). Compared to controls, the SCZ-ID individuals carried 0.045 (0.03 – 0.06, 95% CI) extra singleton LoF variants in developmental disorder genes per exome, suggesting that around 4% of these cases had a LoF variant that is relevant to their clinical presentation. No enrichment in neurodevelopmental disorder genes was observed in schizophrenia patients without intellectual disability, suggesting that these genes were relevant only for that subset of schizophrenia patients (Figure 4, Supplementary Table 4). Notably, even after excluding known developmental disorder genes from the set of LoF intolerant genes, we still observed an enrichment of rare variants in SCZ-ID patients compared to the remaining cases (P = 1×10−3; 1.26, 1.08 – 1.47, 95% CI) or to controls (P < 5×10−7; OR 1.54, 1.31– 1.81, 95% CI; Supplementary Figure 9). Rare variation in these genes contributes more to disease risk in the subset of patients with both schizophrenia and intellectual disability.
Figure 3.
Enrichment of rare loss-of-function variants in LoF intolerant genes in schizophrenia cases stratified by information on cognitive function compared to controls. The P-values shown were calculated using the variant threshold method comparing LoF burden between the corresponding cases and controls. Error bars represent the 95% CI of the point estimate. Damaging missense: missense variants with CADD phred > 15.
Figure 4.
Enrichment of rare loss-of-function variants in known severe developmental disorder genes in schizophrenia cases stratified by information on cognitive function compared to controls. The P-values shown were calculated using the variant threshold method comparing LoF burden between the corresponding cases and controls. Error bars represent the 95% CI of the point estimate. Damaging missense: missense variants with CADD phred > 15.
Rare variants confer risk for schizophrenia in individuals without intellectual disability
While rare damaging variants in LoF intolerant genes were most enriched in the subset of schizophrenia patients with intellectual disability, we still observed a weaker but significant enrichment in individuals with schizophrenia for whom we could confirm do not have intellectual disability (P = 5.5 × 10−4; 1.16, 1.05 – 1.27, 95% CI; Figure 3). Therefore, rare risk variants for schizophrenia follow the pattern previously described in autism: concentrated in individuals with intellectual disability, but not exclusive to that group. To produce a more accurate estimate of the effect of damaging rare variants on schizophrenia conditional on their effects on overall cognition, we recalculated the enrichment of rare variants in LoF intolerant genes in a subset of 2,161 schizophrenia cases and 2,398 controls for which data on years of education was available and for whom intellectual disability could be excluded (Supplementary Figure 8). After controlling for differences in educational attainment (Online Methods), individuals with schizophrenia have a 1.26-fold excess of rare variants in LoF intolerant genes (P = 2 × 10−6; 1.14 – 1.38, 95% CI). This increase in our observed odds ratio is consistent with previous accounts that rare damaging variants also affect educational attainment in controls39, thus biasing our unconditional estimate.
Discussion
Our integrated analysis of thousands of whole-exome sequences demonstrates that rare damaging variants increase risk of schizophrenia both with and without co-morbid intellectual disability. While the identification of individual genes remains difficult at current samples sizes, we show that the burden of damaging de novo mutations, rare SNVs and CNVs in schizophrenia is not scattered across the genome but is primarily concentrated in 3,488 genes intolerant of loss-of-function variants. This observation is shared with autism, intellectual disability, and severe neurodevelopmental disorders32,40. We recapitulate enrichment in previously published gene sets, including transmission at glutamatergic synapses and translational regulation by FMRP, and implicate other gene sets previously linked to autism, intellectual disability, and severe developmental disorders. However, we find that all of these gene sets share a large number of underlying genes, and are especially enriched with the 3,488 genes intolerant of LoF variants. These overlaps among gene sets originating from very different analyses, as well as the subtleties of how they are defined, suggest caution in interpreting biological explanations from observed enrichments.
We jointly analyzed the case-control SNV data with information on cognitive function for 2,971 patients, and find that LoF variants disrupting genes associated with severe developmental disorders are disproportionately found in individuals with schizophrenia with co-morbid intellectual disability, with 4% of these cases having a single LoF variant that is relevant to their clinical presentation. Even after excluding variants in known developmental disorder genes, rare variants contribute a greater degree to schizophrenia risk in the SCZ-ID subgroup of patients than the remaining schizophrenia population. These results show that some of these genetic perturbations have clear manifestations in childhood, and that rare risk variants in schizophrenia are particularly associated with co-morbid intellectual disability. Our observations are consistent with results in autism in which rare risk variants are associated with intellectual disability22,23,28. Notably, a weaker but still significant rare variant burden was observed in schizophrenia patients without cognitive impairment, and this signal persists even after controlling for educational attainment. Together, these results demonstrate that rare variants have different contributions to schizophrenia risk depending on the degree of cognitive impairment. Importantly, they do not simply confer risk for a small subset of patients but contribute to disease pathogenesis more broadly.
Our study supports the observation that genetic risk factors for psychiatric and neurodevelopmental disorders do not follow clear diagnostic boundaries. Coding variants disrupting the same genes, and quite possibly, the same biological processes, increase risk for a range of phenotypic manifestation. This clinically variable presentation is reminiscent of LoF variants in SETD1A and 11 large copy number variant syndromes, previously shown to confer risk for schizophrenia in addition to other prominent developmental defects16,18. It is possible that these genes contain an allelic series of variants conferring gradations of risk. A recent schizophrenia GWAS meta-analysis demonstrated that the common variant association signal was similarly enriched in LoF intolerant genes41, suggesting that schizophrenia risk genes may be perturbed by common variants of subtle effects and disrupted by rare variants of high penetrance in the population. This possibility is also supported by the overlap in at least some of the pathways affected by both rare and common variation, such as chromatin remodeling. However, the most common deletion in the 22q11.2 locus and a recurrent two base deletion in SETD1A are associated with both schizophrenia and more severe neurodevelopmental disorders, suggesting the same variants can also confer risk for a range of clinical features18,42,43. Ultimately, it may prove difficult to clearly partition patients genetically into subtypes with similar clinical features, especially if genes and variants previously thought to cause well-characterized Mendelian disorders can have such varied outcomes. This pattern is consistent with the hypothesis that LoF variants in genes under genic constraint result in a spectrum of neurodevelopmental outcomes with the burden of mutations highest in intellectual disability and least in schizophrenia, corresponding to a gradient of neurodevelopmental pathology indexed by the degree of cognitive impairment, age of onset, and severity4.
Despite the complex nature of genetic contributions to risk of schizophrenia, it is notable that across study design (trio or case-control) and variant class (SNVs or CNVs), risk loci of large effect are concentrated in a small subset of genes. Previous rare variant analyses in other neurodevelopmental disorders, such as autism, have successfully integrated information across de novo SNVs and CNVs to identify novel risk loci23. As sample sizes increase, meta-analyses leveraging the shared genetic risk across study designs and variant types, including those we did not consider here, such as classical recessive inheritance, will be similarly well powered to identify additional risk genes in schizophrenia.
Online Methods
Sample collections
The ascertainment, data production, and quality control of the schizophrenia case-control whole-exome sequencing data set had been described in detail in an earlier publication18. Briefly, the data set was composed of schizophrenia cases recruited as part of eight collections in the UK10K sequencing project, and matched population controls from non-psychiatric arms of the UK10K project, healthy blood donors from the INTERVAL project, and five Finnish population studies. The UK10K data set was combined and analyzed with published data from a Swedish schizophrenia case-control study35. The data production, quality control, and analysis of the case-control CNV data set was described in an earlier publication29. The schizophrenia cases were recruited as part of the CLOZUK and CardiffCOGS studies, which consisted of both schizophrenia individuals taking the antipsychotic clozapine and a general sample of cases from the UK. Matched controls were selected from four publicly available non-psychiatric data sets. All samples were genotyped using Illumina arrays, and processed and called under the same protocol. Sanger-validated de novo mutations identified through whole exome-sequencing in seven published studies of schizophrenia parent-proband trios were aggregated and re-annotated for enrichment analyses13,44–49. A full description of each trio study, including sequencing and capture technology and sample recruitment was previously described18.
Sample and variant quality control
We jointly called each case data set with its nationality-matched controls, and excluded samples based on contamination, coverage, non-European ancestry, and excess relatedness18. A number of empirically derived filters were applied at the variant and genotype level, including filters on GATK VQSR, genotype quality, read depth, allele balance, missingness, and Hardy-Weinberg disequilibrium18. After variant filtering, the per-sample transition-to-transversion ratio was ~3.2 across the entire data set, as expected for populations of European ancestry50. For the case-control CNV analysis, we similarly excluded samples based on excess relatedness, and only CNVs supported by more than 10 probes and greater than 10 kilobases in size were retained to ensure high quality calls. All de novo mutations in our study had been validated using Sanger sequencing.
We used the Ensembl Variant Effect Predictor (VEP) version 75 to annotate all variants (SNVs and CNVs) according to Gencode v.19 coding transcripts. We defined frameshift, stop gained, splice acceptor, and donor variants as loss-of-function (LoF), and missense or initiator codon variants with the recommended CADD Phred score cut-off of greater than 15 as damaging missense51. A gene was annotated as disrupted by a deletion if part of its coding sequence overlapped the copy number event. We more conservatively defined genes as duplicated only if the entire canonical transcript of the gene overlapped with the duplication event.
Statistical tests of the case-control exome data used case-control permutations within each population (UK, Finnish, Swedish) to generate empirical P-values to test hypotheses. No genome-wide inflation was observed in burden tests of individual genes18. In the curated set of de novo mutations, we observed the expected exome-wide number of synonymous mutations given gene mutation rates from previously validated models24, suggesting variant calling was generally unbiased across Gencode v.19 coding genes. Lastly, the case-control CNV data set had been previously analyzed for burden of CNVs affecting individual genes, and enrichment analyses in targeted gene sets7,29.
Rare variant gene set enrichment analyses
Case-control enrichment burden tests
For the case-control SNV data set, we performed permutation-based gene set enrichment tests using an extension of the variant threshold method30. This method assumed that variants with a MAF below an unknown threshold T were more likely to be damaging than variants with a MAF above T, and this threshold was allowed to differ for every gene or pathway tested. To consider different possible values for threshold T, a gene or gene set test statistic t(T) was calculated for every allowable T, and the maximum test-statistic, or tmax, was selected. The statistical significance of tmax was evaluated by permuting phenotypic labels, and calculating tmax from the permuted data such that different values of T could be selected following each permutation. In Price et al., t(T) was defined as the z-score calculated from regressing the phenotype on the sum of the allele counts of variants in a gene with MAF < T. We extended this method to test for enrichment in gene sets by regressing schizophrenia status on the total number of damaging alleles in the gene set of interest with MAF < T (Xin,T) while correcting for the total number of damaging alleles genome-wide with MAF < T (Xall,T). Xin,T controlled for exome-wide differences between schizophrenia cases and controls, ensuring any significant gene set result was significant beyond baseline differences. t(T) was defined as the t-statistic testing if the regression coefficient of Xin,T deviated from 0. We then calculated t(T) for all observed thresholds below a minor allele frequency of 0.1%, and selected the maximum value for the tmax based on the observed data. To calculate a null distribution for tmax, we performed two million case-control permutations within each population (UK, Finnish, and Swedish) to control for batch and ancestry, and calculated tmax for each permuted sample while allowing T to vary. The P-value for each gene set was calculated as the fraction of the two million permuted samples that had a greater tmax than what was observed in the unpermuted data. The odds ratio and 95% confidence interval of each gene set was calculated using a logistic regression model, regressing schizophrenia status on Xin while controlling for total number of variants genome-wide (Xall) and population (UK, Finnish, and Swedish). Unlike gene set P-values which were calculated using permutation across multiple frequency thresholds, the odds ratios an d 95% CI were calculated using only variants observed once in our data set (allele count of 1) to ensure they were comparable between tested gene sets.
CNV logistic regression
We adapted a logistic regression framework described in Raychaudhuri et al. and implemented in PLINK to compare the case-control differences in the rate of CNVs overlapping a specific gene set while correcting for differences in CNV size and total genes disrupted7,19,31. We first restricted our analyses to coding deletions and duplications, and tested for enrichment using the following model:
where for individual i, pi is the probability they have schizophrenia i, si is the total length of CNVs, gall is the total number of genes overlapping CNVs, and gin is the number of genes within the gene set of interest overlapping CNVs. It has been shown that β1 and β2 sufficiently controlled for the genome-wide differences in the rate and size of CNVs between cases and control, while β3 captured the true gene set enrichment above this background rate7,19,31. For each gene set, we reported the one-sided P-value, odds ratio, and 95% confidence interval of β3.
Weighted permutation-based sampling of de novo mutations
For each variant class of interest, we first determined the total number of de novo mutations observed in the 1,077 schizophrenia trios. We then generated 2 million random samples with the same number of de novo mutations, weighting the probability of observing a mutation in a gene by its estimated mutation rate. The baseline gene-specific mutation rates were obtained using the method described in Samocha et al. and adapted to produce LoF and damaging missense rates for each Gencode v.19 gene. These mutation rates adjusted for both sequence context and gene length, and were successfully applied in the primary analyses of large-scale exome sequencing of autism and severe developmental disorders with replicable results23,32,40. For each gene set, one-sided enrichment P-values were calculated as the fraction of two million random samples that had a greater or equal number of de novo mutations in the gene set of interest than what is observed in the 1,077 trios. The effect size of the enrichment was calculated as the ratio between the number of observed mutations in the gene set of interest and the average number of mutations in the gene set across the two million random samples. We adapted a method in Fromer et al. to calculate 95% credible intervals for the enrichment statistic13. We first generated a list of one thousand evenly spaced values between 0 and ten times the point estimate of the enrichment. For each value, the mutation rates of genes in the gene set of interest were multiplied by that amount, and 50,000 random samples of de novo mutations were generated using these weighted rates. The probability of observing the number of mutations in the gene set of interest given each effect size multiplier was calculated as the fraction of samples in which the number of mutations in the gene set is the same as the observed number in the 1,077 trios. We normalized the probabilities across the 1,000 values to generate a posterior distribution of the effect size, and calculated the 95% credible interval using this empirical distribution.
Combined joint analysis
Gene set P-values calculated using the case-control SNV, case-control CNV, and de novo data were meta-analyzed using Fisher’s combined probability method with df = 6 to provide a single test statistic for each gene set. We corrected for the number of gene sets tested in the discovery analysis (n = 1,776) by controlling the false discovery rate (FDR) using the Benjamini-Hochberg approach, and reported only results with a q-value of less than 5%.
Description of gene sets
The full list of tested gene sets is found in Supplementary Table 1, and a detailed description is provided in the Supplementary Note. Briefly, we tested all gene sets with more than 100 genes from five public pathway databases. We additionally tested additional gene sets selected based on biological hypotheses about schizophrenia risk, and genome-wide screens investigating rare variants in intellectual disability, autism spectrum disorders, and other neurodevelopmental disorders. All gene identifiers were mapped to the GENCODE v.19 release, and all non-coding genes were excluded. A total of 1,766 gene sets were included in our analysis.
Selection of allele frequency thresholds and consequence severity
For the case-control whole-exome data, we applied an extension of the variant threshold model (described above). With this method, we tested damaging variants at a number of frequency thresholds without specifying an a priori MAF cut-off. All thresholds below a MAF of 0.1% observed in our data were tested, and we assessed statistical significance by permutation testing. For all the whole-exome data (case-control and trio data), we restricted our analyses to loss-of-function variants. These variants have a clear and severe predicted functional consequence in that they putatively cause a single-copy loss of a gene. Furthermore, this class of variants had been demonstrated to have the strongest genome-wide enrichment between cases and controls across neurodevelopmental and psychiatric disorders18,32,40. When selecting MAF cut-offs for case-control CNVs, we found that while the bulk of the test statistics were not inflated, the tail of gene set P-values were dramatically inflated even when testing for enrichment in the random gene sets (Supplementary Figure 1). This inflation in the tail of the Q-Q plot was driven in part by very large (overlapping more than 10 genes), more common (MAF between 0.1% and 1%) CNVs observed mainly in cases or controls. Some of these, such as the known syndromic CNVs, likely harbored true risk genes. However, because these CNVs were highly recurrent in cases and depleted in controls, and disrupted a large number of genes, any gene set that included even a single gene within these CNVs would appear to be significant, even after controlling for total CNV length and genes overlapped. To ensure our model was well calibrated and its P-values followed a null distribution for random gene sets, we explored different frequency and size thresholds, and conservatively restricted our analysis to copy number events overlapping less than seven genes (excluding the largest 10% of CNVs) with MAF < 0.1% (Supplementary Figure 1). Our main conclusions remained unchanged even if we selected a more stringent (excluding the largest 15% of CNVs) or less stringent (excluding the largest 5% of CNVs) size threshold.
Robustness of enrichment analyses
We uniformly sampled genes from the genome (as defined by Gencode v.19) to generate random gene sets with the same size distribution as the 1,776 gene sets in our discovery analysis. For each random set, we calculated gene set P-values for the case-control SNV data, case-control CNV data, and de novo data using the appropriate method and frequency cut-offs across all variant classes. A Q-Q plot was generated using P-values from enrichment tests of each data set and variant type. Reassuringly, we observed null distributions in all such Q-Q plots (Supplementary Figure 3).
Comparison of de novo enrichment with broader neurodevelopmental disorders
We aggregated and re-annotated de novo mutations from four studies: 1,113 severe DD probands40, 4,038 ASD probands23,32, and 2,134 control probands28,32. We used the Poisson exact test to calculate differences in de novo rates in constrained genes between schizophrenia, ASD, and DD and controls. Counts in each functional class (synonymous, missense, damaging missense, and LoF) were tested separately, and the one-sided P-value, rate ratio, and 95% CI of each comparison were reported and plotted in Figure 2, Supplementary Figure 4 and 5.
Conditional analyses
In each of the three methods we used for gene set enrichment, we restricted all variants analyzed to those that reside in the background gene list, and tested for an excess of rare variants in genes shared between the gene set of interest (K) and the background list (B). Brain-enriched genes from GTEx, and the ExAC LoF intolerant genes (pLI > 0.9) were used as backgrounds (see above). For the case-control SNV data, we modified the variant threshold method to regress schizophrenia status on the total number of damaging alleles in genes present in both the gene set of interest and the background gene set (K ∩ B), while correcting for the total number of damaging alleles in the set of all background genes (B). The logistic regression model for the case-control CNV data was modified to:
where gB is the total number of background genes overlapping a CNV, and gK ∩ B is the number of genes in the intersection of the gene set of interest and the background list overlapping a CNV. Finally, we determined the total number of de novo mutations within the background gene list observed in the 1,077 schizophrenia trios, and generated 2 million random samples with the same number of de novo mutations. For each gene set, one-sided enrichment P-values were calculated as the fraction of two million random samples that had a greater or equal number of de novo mutations in genes in K ∩ B than what is observed in the 1,077 trios. Gene set P-values were combined using Fisher’s method. We restricted our conditional enrichment analysis to gene sets with q-value < 5% in the discovery analysis, and adjusted for multiple testing using Bonferroni correction (P = 0.00071, or 0.05/67 tests; see Supplementary Table 3).
Rare variants and cognition in schizophrenia
Within the UK10K study, 97 individuals from the MUIR collection were given discharge diagnoses of mild learning disability and schizophrenia (ICD-8 and -9). The recruitment guidelines of the MUIR collection were described in detail in a previous publication52. In brief, evidence of remedial education was a prerequisite to inclusion, and individuals with pre-morbid IQs below 50 or above 70, severe learning disabilities, or were unable to give consent were excluded. The Schizophrenia and Affective Disorders Schedule-Lifetime version (SADS-L) in people with mild learning disability, PANSS, RDC, and DSM-III-R, and St. Louis Criterion were applied to all individuals to ensure that any diagnosis of schizophrenia was robust. Using the clinical information provided alongside the Swedish and Finnish case-control data sets, we identified additional 182 schizophrenia individuals who were similarly diagnosed with intellectual disability, for a total of 279 individuals.
Cognitive testing and educational attainment data available for a subset of samples were used identify schizophrenia individuals without cognitive impairment. For 502 individuals from the Cardiff collection in the UK10K study, we acquired their pre-morbid IQ as extrapolated from National Adult Reading Test (NART), and identified 412 individuals for analysis after excluding all individuals with predicted pre-morbid IQ of less than 85 (or below one standard deviation of the population distribution for IQ). We additionally acquired information on educational attainment in 54 schizophrenia individuals in the UK10K London collection, and retained 27 individuals without intellectual disability and who completed at least 12 years of schooling. Lastly, the California Verbal Learning Test was conducted on 124 Finnish schizophrenia individuals sequenced as part of UK10K, and a composite score was generated from measures of verbal and visual working memory, verbal abilities, visuoconstructive abilities, and processing speed. All individuals with intellectual disability had been excluded from cognitive testing. Within this set of samples, we additionally excluded any individuals who ranked in the lowest decile in CVLT composite score, and retained 92 individuals for analysis. According to these criteria, we identified 531 of 697 schizophrenia individuals from the UK and Finnish data sets with cognitive data as not having intellectual disability. We additionally acquired data on educational attainment for the Swedish schizophrenia cases and controls from the Swedish National Registry. After excluding individuals with intellectual disability, we identified 1,527 schizophrenia individuals who did not complete secondary school (less than 12 years of schooling), and 634 schizophrenia individuals who completed at least compulsory and upper secondary schooling (at least 12 years of schooling). The last group with the greatest educational attainment and without intellectual disability was defined as cases without cognitive impairment. In the Swedish sample, 49.4% of control samples had lower educational attainment than the 634 individuals with schizophrenia defined as having no cognitive impairment, suggesting that our definition was sufficiently strict. In total, combining the UK, Finnish, and Swedish data, we identified 1,165 schizophrenia individuals without cognitive impairment.
Using the variant threshold method, we tested for differences in rare LoF burden between the three case groups (intellectual disability, did not complete secondary school, no cognitive impairment) against controls. We restricted these analyses to three gene sets (LoF intolerant genes, genes in which LoF variants are diagnostic for severe developmental disorders, and LoF intolerant genes after excluding severe developmental disorders genes), and adjusted for multiple testing using Bonferroni correction (P = 0.0038, or 0.05/13 tests). Supplementary Table 4 enumerated all the statistical tests performed. To estimate the per-exome excess of rare singleton (defined as having an allele count of one in our data set) LoF variants in cases compared to controls, we regressed Xin (the number of LoF variants in the gene set of interest) on case status (0 or 1) while controlling for Xall (the total number of LoF variants genome-wide) and population (UK, Finnish, and Swedish). The effect size and 95% CI of the regression coefficient of case status predictor were reported.
Data Availability
Sequence data and processed VCFs for the UK10K project were deposited into the European Genome-phenome Archive (EGA) under study accession code EGAO00000000079. The processed VCFs from the Swedish case-control study were deposited in dbGAP under accession code (phs000473.v1.p1). Rare variant counts, and gene-level association results from combining the whole-exome sequencing data sets were described in a previous publication18 and were made available on the PGC results and download page (https://www.med.unc.edu/pgc/results-and-downloads).
Supplementary Material
Acknowledgements
We gratefully thank all participants in these studies. We thank Timi Touloupoulou, Marco Picchioni, Chiara Nosarti, Fiona Gaughran, and Oliver Howes for contributing clinical data used in this study. The UK10K project was funded by Wellcome Trust grant WT091310. The INTERVAL sequencing studies are funded by Wellcome Trust grant WT098051. T.S. is supported by the Williams College Dr. Herchel Smith Fellowship. A.P. is supported by Academy of Finland grants 251704 and 286500, NIMH U01MH105666 and the Sigrid Juselius Foundation. The work at Cardiff University was funded by Medical Research Council (MRC) Centre (G0801418) and Program Grants (G0800509). P.F.S. gratefully acknowledges support from the Swedish Research Council (Vetenskapsrådet, award D0886501). Creation of the Sweden schizophrenia study data was supported by NIMH R01 MH077139 and the Stanley Center of the Broad Institute. Participants in INTERVAL were recruited with the active collaboration of NHS Blood and Transplant England, which has supported fieldwork and other elements of the trial. DNA extraction and genotyping was funded by the National Institute of Health Research (NIHR), the NIHR BioResource and the NIHR Cambridge Biomedical Research Centre. The academic coordinating centre for INTERVAL was supported by core funding from: NIHR Blood and Transplant Research Unit in Donor Health and Genomics, UK Medical Research Council (G0800270), and British Heart Foundation (SP/09/002). We would like to acknowledge the contribution of data from outside sources: (i) Genetic Architecture of Smoking and Smoking Cessation accessed through dbGAP: Study Accession: phs000404.v1.p1. Funding support for genotyping, which was performed at the Center for Inherited Disease Research (CIDR), was provided by 1 X01 HG005274-01. CIDR is fully funded through a federal contract from the National Institutes of Health to The Johns Hopkins University, contract number HHSN268200782096C. Assistance with genotype cleaning, as well as with general study coordination, was provided by the Gene Environment Association Studies (GENEVA) Coordinating Center (U01 HG004446). Funding support for collection of datasets and samples was provided by the Collaborative Genetic Study of Nicotine Dependence (COGEND; P01 CA089392) and the University of Wisconsin Transdisciplinary Tobacco Use Research Center (P50 DA019706, P50 CA084724). (ii). High-Density SNP Association Analysis of Melanoma: Case–Control and Outcomes Investigation, dbGaP Study Accession: phs000187.v1.p1. Research support to collect data and develop an application to support this project was provided by 3P50CA093459, 5P50CA097007, 5R01ES011740 and 5R01CA133996. (iii) Genetic Epidemiology of Refractive Error in the KORA Study, dbGaP Study Accession: phs000303.v1.p1. Principal investigators: Dwight Stambolian, University of Pennsylvania, Philadelphia, PA, USA; H. Erich Wichmann, Institut für Humangenetik, Helmholtz-Zentrum München, Germany, National Eye Institute, National Institutes of Health, Bethesda, MD, USA. Funded by R01 EY020483, National Institutes of Health, Bethesda, MD, USA. (iv) WTCCC2 study: Samples were downloaded from https://www.ebi.ac.uk/ega/ and include samples from the National Blood Donors Cohort, EGAD00000000024 and samples from the 1958 British Birth Cohort, EGAD00000000022. Funding for these projects was provided by the Wellcome Trust Case Control Consortium 2 project (085475/B/08/Z and 085475/Z/08/Z), the Wellcome Trust (072894/Z/03/Z, 090532/Z/09/Z and 075491/Z/04/B) and NIMH grants (MH 41953 and MH083094).
Footnotes
Author contributions
T.S., J.C.B conceived and designed the experiments.
T.S performed the statistical analysis.
T.S., J.T.R.W., M.J., D.C., J.S., M.T., E.R., P.F.S analysed the data.
T.S., J.T.R.W., M.J., J.S., M.T., E.R., C.I., D.B., A.M.M., G.K., D.G., R.M.M., M.D.F., E.B., M.G., C.M.H., P.S., A.P., M.C.O., M.J.O., J.C.B contributed reagents/materials/analysis tools.
T.S., D.C., M.J.O., J.C.B wrote the paper
Competing financial interests statement
We have no competing financial interests to declare.
References
- 1.van Os J, Kapur S. Schizophrenia. Lancet. 2009;374:635–45. doi: 10.1016/S0140-6736(09)60995-8. [DOI] [PubMed] [Google Scholar]
- 2.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5{®}) American Psychiatric Publishing; 2013. [Google Scholar]
- 3.Tandon R, et al. Definition and description of schizophrenia in the DSM-5. Schizophr Res. 2013;150:3–10. doi: 10.1016/j.schres.2013.05.028. [DOI] [PubMed] [Google Scholar]
- 4.Owen MJ. New approaches to psychiatric diagnostic classification. Neuron. 2014;84:564–571. doi: 10.1016/j.neuron.2014.10.028. [DOI] [PubMed] [Google Scholar]
- 5.Owen MJ, Sawa A, Mortensen PB. Schizophrenia. Lancet. 2016;6736:1–12. doi: 10.1016/S0140-6736(15)01121-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III--the final common pathway. Schizophr Bull. 2009;35:549–62. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pocklington AJ, et al. Novel Findings from CNVs Implicate Inhibitory and Excitatory Signaling Complexes in Schizophrenia. Neuron. 2015;86:1203–1214. doi: 10.1016/j.neuron.2015.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sekar A, et al. Schizophrenia risk from complex variation of complement component 4. Nature. 2016;530:177–183. doi: 10.1038/nature16549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Owen MJ, O’Donovan MC, Thapar A, Craddock N. Neurodevelopmental hypothesis of schizophrenia. Br J Psychiatry. 2011;198:173–5. doi: 10.1192/bjp.bp.110.084384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rapoport JL, Giedd JN, Gogtay N. Neurodevelopmental model of schizophrenia: update 2012. Mol Psychiatry. 2012;17:1228–38. doi: 10.1038/mp.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–7. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loh P-R, et al. Contrasting genetic architectures of schizophrenia and other complex diseases using fast variance-components analysis. Nat Genet. 2015;47:1385–1392. doi: 10.1038/ng.3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fromer M, et al. De novo mutations in schizophrenia implicate synaptic networks. Nature. 2014;506:179–184. doi: 10.1038/nature12929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirov G, et al. De novo CNV analysis implicates specific abnormalities of postsynaptic signalling complexes in the pathogenesis of schizophrenia. Mol Psychiatry. 2012;17:142–53. doi: 10.1038/mp.2011.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The International Schizophrenia Consortium. Rare chromosomal deletions and duplications increase risk of schizophrenia. Nature. 2008;455:237–41. doi: 10.1038/nature07239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rees E, et al. Analysis of copy number variations at 15 schizophrenia-associated loci. Br J Psychiatry. 2014;204:108–14. doi: 10.1192/bjp.bp.113.131052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu X, Need AC, Petrovski S, Goldstein DB. One gene, many neuropsychiatric disorders: lessons from Mendelian diseases. Nat Neurosci. 2014;17:773–781. doi: 10.1038/nn.3713. [DOI] [PubMed] [Google Scholar]
- 18.Singh T, et al. Rare loss-of-function variants in SETD1A are associated with schizophrenia and developmental disorders. Nat Neurosci. 2016;19:571–577. doi: 10.1038/nn.4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szatkiewicz JP, et al. Copy number variation in schizophrenia in Sweden. Mol Psychiatry. 2014;19:762–773. doi: 10.1038/mp.2014.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Psychiatric Genetics Consortium. Psychiatric genome-wide association study analyses implicate neuronal, immune and histone pathways. Nat Neurosci. 2015 doi: 10.1038/nn.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee SH, et al. Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat Genet. 2013;45:984–94. doi: 10.1038/ng.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robinson EB, et al. Genetic risk for autism spectrum disorders and neuropsychiatric variation in the general population. Nat Genet. 2016;48:552–555. doi: 10.1038/ng.3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanders SJ, et al. Insights into Autism Spectrum Disorder Genomic Architecture and Biology from 71 Risk Loci. Neuron. 2015;87:1215–1233. doi: 10.1016/j.neuron.2015.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Samocha KE, et al. A framework for the interpretation of de novo mutation in human disease. Nat Genet. 2014;46:944–950. doi: 10.1038/ng.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lek M, et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–291. doi: 10.1038/nature19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Genovese G, et al. Increased burden of ultra-rare protein-altering variants among 4,877 individuals with schizophrenia. Nat Neurosci. 2016 doi: 10.1038/nn.4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirov G, et al. The penetrance of copy number variations for schizophrenia and developmental delay. Biol Psychiatry. 2014;75:378–85. doi: 10.1016/j.biopsych.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iossifov I, et al. The contribution of de novo coding mutations to autism spectrum disorder. Nature. 2014;515:216–21. doi: 10.1038/nature13908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rees E, et al. CNV analysis in a large schizophrenia sample implicates deletions at 16p12.1 and SLC1A1 and duplications at 1p36.33 and CGNL1. Hum Mol Genet. 2014;23:1669–76. doi: 10.1093/hmg/ddt540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Price AL, et al. Pooled Association Tests for Rare Variants in Exon-Resequencing Studies. Am J Hum Genet. 2010;86:832–838. doi: 10.1016/j.ajhg.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raychaudhuri S, et al. Accurately assessing the risk of schizophrenia conferred by rare copy-number variation affecting genes with brain function. PLoS Genet. 2010;6 doi: 10.1371/journal.pgen.1001097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Rubeis S, et al. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature. 2014;515:209–15. doi: 10.1038/nature13772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Firth HV, et al. DECIPHER: Database of Chromosomal Imbalance and Phenotype in Humans Using Ensembl Resources. Am J Hum Genet. 2009;84:524–33. doi: 10.1016/j.ajhg.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deciphering Developmental Disorders Study. Prevalence and architecture of de novo mutations in developmental disorders. Nature. 2017;542:433–438. doi: 10.1038/nature21062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Purcell SM, et al. A polygenic burden of rare disruptive mutations in schizophrenia. Nature. 2014;506:185–90. doi: 10.1038/nature12975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cotney J, et al. The autism-associated chromatin modifier CHD8 regulates other autism risk genes during human neurodevelopment. Nat Commun. 2015;6:6404. doi: 10.1038/ncomms7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Darnell JC, et al. FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell. 2011;146:247–61. doi: 10.1016/j.cell.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ascano M, et al. FMRP targets distinct mRNA sequence elements to regulate protein expression. Nature. 2012;492:382–386. doi: 10.1038/nature11737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ganna A, et al. Ultra-rare disruptive and damaging mutations influence educational attainment in the general population. Nat Neurosci. 2016;19:1563–1565. doi: 10.1038/nn.4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.The Deciphering Developmental Disorders Study. Large-scale discovery of novel genetic causes of developmental disorders. Nature. 2015;519:223–8. doi: 10.1038/nature14135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pardiñas AF, et al. Common schizophrenia alleles are enriched in mutation-intolerant genes and maintained by background selection. bioRxiv. 2016:68593. doi: 10.1101/068593. [DOI] [Google Scholar]
- 42.Ben-Shachar S, et al. 22q11.2 Distal Deletion: A Recurrent Genomic Disorder Distinct from DiGeorge Syndrome and Velocardiofacial Syndrome. Am J Hum Genet. 2008;82:214–221. doi: 10.1016/j.ajhg.2007.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Michaelovsky E, et al. Genotype-phenotype correlation in 22q11.2 deletion syndrome. BMC Med Genet. 2012;13:122. doi: 10.1186/1471-2350-13-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guipponi M, et al. Exome sequencing in 53 sporadic cases of schizophrenia identifies 18 putative candidate genes. PLoS One. 2014;9:e112745. doi: 10.1371/journal.pone.0112745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Girard SL, et al. Increased exonic de novo mutation rate in individuals with schizophrenia. Nat Genet. 2011;43:860–3. doi: 10.1038/ng.886. [DOI] [PubMed] [Google Scholar]
- 46.McCarthy SE, et al. De novo mutations in schizophrenia implicate chromatin remodeling and support a genetic overlap with autism and intellectual disability. Mol Psychiatry. 2014;19:652–8. doi: 10.1038/mp.2014.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takata A, et al. Loss-of-function variants in schizophrenia risk and SETD1A as a candidate susceptibility gene. Neuron. 2014;82:773–80. doi: 10.1016/j.neuron.2014.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu B, et al. Exome sequencing supports a de novo mutational paradigm for schizophrenia. Nat Genet. 2011;43:864–8. doi: 10.1038/ng.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu B, et al. De novo gene mutations highlight patterns of genetic and neural complexity in schizophrenia. Nat Genet. 2012;44:1365–9. doi: 10.1038/ng.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Do R, et al. Exome sequencing identifies rare LDLR and APOA5 alleles conferring risk for myocardial infarction. Nature. 2014;518:102–106. doi: 10.1038/nature13917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kircher M, et al. A general framework for estimating the relative pathogenicity of human genetic variants. Nat Genet. 2014;46:310–5. doi: 10.1038/ng.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Doody GA, Johnstone EC, Sanderson TL, Owens DG, Muir WJ. ‘Pfropfschizophrenie’ revisited. Schizophrenia in people with mild learning disability. Br J Psychiatry. 1998;173:145–153. doi: 10.1192/bjp.173.2.145. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sequence data and processed VCFs for the UK10K project were deposited into the European Genome-phenome Archive (EGA) under study accession code EGAO00000000079. The processed VCFs from the Swedish case-control study were deposited in dbGAP under accession code (phs000473.v1.p1). Rare variant counts, and gene-level association results from combining the whole-exome sequencing data sets were described in a previous publication18 and were made available on the PGC results and download page (https://www.med.unc.edu/pgc/results-and-downloads).