Abstract
Lower eGFR 1 year after kidney transplant is associated with shorter allograft and patient survival. We examined how practice changes in the past decade correlated with time trends in average eGFR at 1 year after kidney transplant in the United States in a cohort of 189,944 patients who received a kidney transplant between 2001 and 2013. We calculated the average eGFR at 1 year after transplant for the recipient cohort of each year using the appropriate Modification of Diet in Renal Disease equation depending on the prevailing methodology of creatinine measurement, and used linear regression to model the effects of practice changes on the national post-transplant eGFR trend. Between the 2001–2005 period and the 2011–2013 period, average 1-year post-transplant eGFR remained essentially unchanged, with differences of 1.34 (95% confidence interval, 1.03 to 1.65) ml/min per 1.73 m2 and 0.66 (95% confidence interval, 0.32 to 1.01) ml/min per 1.73 m2 among deceased and living donor kidney transplant recipients, respectively. Over time, the mean age of recipients increased and more marginal organs were used; adjusting for these trends unmasked a larger temporal improvement in post-transplant eGFR. However, changes in immunosuppression practice had a positive effect on average post-transplant eGFR and balanced out the negative effect of recipient/donor characteristics. In conclusion, average 1-year post-transplant eGFR remained stable, despite increasingly unfavorable attributes in recipients and donors. With an aging ESRD population and continued organ shortage, preservation of average post-transplant eGFR will require sustained improvement in immunosuppression and other aspects of post-transplant care.
Keywords: chronic kidney disease, transplantation, glomerular filtration rate
Many kidney transplant (KT) recipients have an eGFR<60 ml/min per 1.73 m2 on the basis of the Modification of Diet in Renal Disease (MDRD) equation at their 1-year anniversary, placing them in stage 3T CKD or worse. The degree of GFR impairment at 1 year post-KT has prognostic value and is associated with lower GFR at 5 years, higher risk of eventual graft failure, and cardiovascular death.1–5 Increasingly, GFR at 1 year post-KT is used as a surrogate endpoint for long-term graft outcome in clinical trials.6
Given its prognostic value, trends in 1-year post-KT GFR in a national cohort can serve as an indicator of success in transplantation over time. The improvement in transplant care and reduction of acute rejection from better immunosuppression over time could have resulted in a rise in the average post-transplant GFR.7,8 On the other hand, increased acceptance of older donors and donors with cardiac death (DCD) in the past decade may have had an opposite effect and instead led to a decline in the average post-KT GFR.
Using data from the Scientific Registry of Transplant Recipients (SRTR), we studied how competing trends in KT practices affected the national trend of average post-KT eGFR from 2001 to 2013. We grouped transplant practices into four categories to study their effects on post-KT GFR separately and collectively: recipient characteristics, donor and organ characteristics, immunosuppression choices, and post-transplant course.
In our study, post-KT GFR was estimated from serum creatinine reported to SRTR using the MDRD equation, so any systematic drift in creatinine measurement could affect the secular trend in eGFR. Our study period coincided with efforts to standardize creatinine assays using reference materials traceable to isotope dilution mass spectrometry (IDMS). IDMS-calibrated creatinine values are generally 0.1–0.2 mg/dl lower, and this difference necessitated a separate IDMS-calibrated MDRD equation.9,10 Our analysis accounted for this adoption by applying the appropriate MDRD equation to different subperiods of the study: the non–IDMS-calibrated equation for the early period of 2001–2005, and the IDMS-calibrated equation for the recent period of 2011–2013. Both equations were used to estimate the upper and lower bounds of average eGFR for the transition period of 2006–2010.
Results
Table 1 shows the descriptive statistics of the variables by the three subperiods and by donor types. Over the study period, significant trends were observed in each of the four categories of variables, as discussed further below. Briefly, the proportion of elderly and obese recipients increased, more marginal deceased donor kidneys were used, the use of tacrolimus and mycophenolate increased, and incidence of acute rejection decreased.
Table 1.
Descriptive characteristics at the time of transplant by era and donor type
| Characteristic | DDKT | LDKT | ||||
|---|---|---|---|---|---|---|
| 2001–2005 (n=35,486) | 2006–2010 (n=42,124) | 2011–2013 (n=25,079) | 2001–2005 (n=26,085) | 2006–2010 (n=26,364) | 2011–2013 (n=13,549) | |
| Recipient characteristics | ||||||
| Recipient age (mean±SD), yr | 50.7±12.6 | 53.1±12.6 | 54.3±12.7 | 46.9±13.2 | 48.8±13.6 | 49.6±13.8 |
| Recipient age >65 yr, % | 13.4 | 18.4 | 21.1 | 8.8 | 11.8 | 14.2 |
| Male recipient, % | 60.2 | 60.8 | 60.3 | 58.9 | 61.5 | 62.4 |
| Race, % | ||||||
| White | 50.6 | 46.1 | 43.7 | 68.0 | 67.0 | 66.4 |
| Black | 29.3 | 31.9 | 31.8 | 14.9 | 14.0 | 13.5 |
| Other | 7.0 | 7.9 | 8.5 | 5.1 | 5.6 | 5.9 |
| Recipient disease burden | ||||||
| Peak panel-reactive antibodies (mean±SD) | 18.3±30.6 | 21.4±32.3 | 22.2±32.3 | 9.3±21.6 | 12.3±24.4 | 12.6±24.3 |
| Peak PRA information missing, % | 0.9 | 1.0 | 5.1 | 2.3 | 2.1 | 8.8 |
| Peak PRA=0, % | 44.8 | 44.3 | 43.9 | 60.5 | 57.0 | 54.1 |
| 0<peak PRA≤20, % | 30.0 | 25.3 | 20.1 | 24.3 | 23.0 | 19.0 |
| 20<peak PRA≤80, % | 14.3 | 17.8 | 20.3 | 9.6 | 13.5 | 14.4 |
| Peak PRA >80, % | 10.0 | 11.6 | 10.6 | 3.4 | 4.4 | 3.7 |
| Pretransplant dialysis (mean±SD), yr | 3.4±3.1 | 3.7±3.3 | 3.9±3.4 | 1.3±2.2 | 1.2±2.0 | 1.3±2.0 |
| With diabetes, % | 29.8 | 29.1 | 30.8 | 27.4 | 24.5 | 24.1 |
| BMI (mean±SD), kg/m2 | 27.1±5.2 | 27.9±5.4 | 28.4±5.4 | 26.8±5.2 | 27.5±5.3 | 27.8±5.4 |
| BMI information missing, % | 14.5 | 6.1 | 2.7 | 13.5 | 5.5 | 2.3 |
| 18.5<BMI (underweight), % | 2.1 | 1.9 | 1.7 | 2.6 | 2.2 | 2.4 |
| 18.5≤BMI<25 (normal), % | 30.5 | 28.8 | 26.7 | 33.3 | 31.6 | 30.0 |
| 25≤BMI<30 (overweight), % | 29.6 | 32.3 | 33.1 | 28.5 | 32.0 | 32.8 |
| BMI≥30 (obese), % | 23.3 | 30.9 | 35.8 | 22.0 | 28.7 | 32.5 |
| Donor and organ quality factors | ||||||
| Donor age (mean±SD) | 37.4±16.8 | 39.2±16.5 | 38.8±16.2 | 40.6±10.9 | 41.8±11.5 | 42.9±11.8 |
| Donor age >50 yr, % | 26.3 | 29.9 | 29.0 | 20.9 | 26.2 | 30.3 |
| Male donor, % | 59.6 | 60.4 | 60.2 | 40.8 | 39.8 | 37.8 |
| Donor race, % | ||||||
| White | 72.5 | 69.1 | 68.2 | 69.9 | 69.8 | 70.0 |
| Black | 11.6 | 13.7 | 14.7 | 13.6 | 12.1 | 11.4 |
| Hispanic | 12.8 | 14.1 | 13.7 | 12.0 | 13.4 | 13.7 |
| Other | 3.0 | 3.1 | 3.5 | 4.6 | 4.7 | 4.9 |
| ECD, % | 15.8 | 17.7 | 15.6 | |||
| DCD, % | 5.0 | 12.0 | 16.1 | |||
| Pulsatile perfusion after harvest, % | 17.3 | 32.6 | 40.2 | |||
| Donors with hypertension, % | 21.1 | 27.4 | 27.7 | 0.5 | 2.2 | 3.3 |
| Donors with diabetes, % | 4.5 | 7.0 | 7.3 | – | – | – |
| Donor serum creatinine >1.5 mg/dl, % | 13.0 | 16.4 | 17.2 | – | – | – |
| Cold ischemia time (mean±SD), h | 18.5±8.3 | 17.9±9.8 | 17.0±8.7 | 2.3±5.2 | 2.1±5.3 | 2.1±4.7 |
| Cold ischemia time information missing, % | 12.90 | 4.50 | 1.50 | – | – | – |
| Cold ischemia time <10 h, % | 14.10 | 19.20 | 22.20 | – | – | – |
| 10≤cold ischemia time<20 h, % | 39.90 | 44.00 | 46.30 | – | – | – |
| 20<cold ischemia time≤30 h, % | 26.60 | 24.60 | 22.00 | – | – | – |
| Cold ischemia time >30 h, % | 6.50 | 7.70 | 8.00 | – | – | – |
| Number of HLA mismatches (A, B, DR) | 3.6±1.9 | 4.0±1.7 | 4.1±1.6 | 3.1±1.7 | 3.3±1.7 | 3.5±1.7 |
| Immunosuppression practice | ||||||
| Induction agent | ||||||
| Polyclonals, % | 37.0 | 50.6 | 54.2 | 29.1 | 41.0 | 44.0 |
| Anti-IL2R, % | 33.9 | 25.6 | 22.6 | 34.3 | 30.4 | 30.4 |
| Alemtuzumab, % | 4.0 | 10.8 | 13.0 | 4.5 | 13.7 | 17.1 |
| Calcineurin inhibitor choice for maintenance | ||||||
| Tacrolimus, % | 68.3 | 87.9 | 93.0 | 67.1 | 86.0 | 91.5 |
| Cyclosporine, % | 25.3 | 7.8 | 3.0 | 27.2 | 8.9 | 3.7 |
| Antiproliferative agent for maintenance | ||||||
| Mycophenolate, % | 83.4 | 92.3 | 94.6 | 81.4 | 90.9 | 93.7 |
| mTora, % | 13.8 | 4.4 | 2.2 | 14.1 | 5.8 | 2.9 |
| Azathioprine, % | 1.9 | 0.6 | 0.3 | 2.2 | 0.6 | 0.3 |
| Post-transplant course | ||||||
| DGF, % | 21.3 | 22.5 | 23.8 | 3.2 | 2.9 | 2.5 |
| Acute rejection by 1 yr, % | 13.3 | 10.4 | 9.5 | 12.0 | 10.1 | 9.7 |
PRA, panel reactive antibodies; –, not applicable.
mTOR inhibitor group included those on both mTOR inhibitor and mycophenolate.
In Tables 2 and 3, linear regressions were used to model the change in 1-year post-KT eGFR between early and recent periods. The transition period (2006–2010) was not included in the regression analysis given the uncertainty in GFR estimation.
Table 2.
Estimates from linear regression predicting 1-yr post-KT eGFR among recipients of DDKTs
| Variable | Unadjusted (Model 1D), β (95% CI) | Recipient Characteristics (Model 2D), β (95% CI) | Donor and Organ Characteristics (Model 3D), β (95% CI) | Immunosuppression Practice (Model 4D), β (95% CI) | Post-Transplant Course (Model 5D), β (95% CI) | Fully Adjusted (Model 6D), β (95% CI) |
|---|---|---|---|---|---|---|
| Transplant period (2011–2013 versus 2001–2005) | 1.34 (1.03 to 1.65) | 1.88 (1.56 to 2.21) | 2.63 (2.32 to 2.93) | 0.31 (−0.04 to 0.65) | 1.08 (0.77 to 1.39) | 1.05 (0.72 to 1.39) |
| Recipient characteristics | ||||||
| Age (per 10 yr) | ||||||
| Age ≤40 | −3.62 (−4.14 to −3.10) | −2.96 (−3.43 to −2.50) | ||||
| 40<age≤55 | −0.09 (−0.46 to 0.29) | 0.77 (0.44 to 1.10) | ||||
| Age >55 | −1.19 (−1.52 to −0.86) | 0.90 (0.61 to 1.20) | ||||
| Race (ref=White) | ||||||
| Black | 3.28 (2.91 to 3.64) | 4.48 (4.14 to 4.81) | ||||
| Hispanic | 4.01 (3.55 to 4.48) | 4.02 (3.59 to 4.45) | ||||
| Other | 3.91 (3.31 to 4.50) | 4.94 (4.40 to 5.48) | ||||
| Male (ref=female) | 1.26 (0.94 to 1.58) | 1.69 (1.40 to 1.97) | ||||
| Peak PRA (ref=0) | ||||||
| Missing PRA | 0.09 (−0.89 to 1.06) | −0.43 (−1.38 to 0.52) | ||||
| 0<peak PRA≤20 | −0.28 (−0.66 to 0.09) | −0.18 (−0.52 to 0.15) | ||||
| 20<peak PRA≤80 | 0 (−0.44 to 0.44) | −0.28 (−0.68 to 0.12) | ||||
| Peak PRA ≥80 | 0.84 (0.30 to 1.39) | −0.24 (−0.73 to 0.25) | ||||
| Length of pretransplant dialysis (per yr) | −0.12 (−0.17 to −0.08) | −0.03 (−0.07 to 0.01) | ||||
| With diabetes | 0.86 (0.51 to 1.20) | 1.44 (1.13 to 1.75) | ||||
| Recipient weight (ref=normal: 18.5≤BMI<25) | ||||||
| Recipient BMI missing | −3.09 (−3.66 to −2.52) | −2.48 (−2.99 to −1.97) | ||||
| Underweight (18.5<BMI) | 3.67 (2.54 to 4.81) | 3.11 (2.10 to 4.11) | ||||
| Overweight (25≤BMI<30) | −2.68 (−3.07 to −2.28) | −2.18 (−2.53 to −1.83) | ||||
| Obese (BMI≥30) | −5.18 (−5.59 to −4.77) | −4.29 (−4.66 to −3.92) | ||||
| Donor and organ factors | ||||||
| Age (per 10 yr) | ||||||
| Age ≤25 | −1.25 (−1.59 to −0.90) | −0.85 (−1.19 to −0.52) | ||||
| Age >25 | −4.79 (−4.95 to −4.63) | −4.67 (−4.83 to −4.51) | ||||
| Male (ref=female) | 3.39 (3.10 to 3.68) | 3.43 (3.14 to 3.71) | ||||
| Race (ref=White) | ||||||
| Black | −1.15 (−1.58 to −0.71) | −1.72 (−2.14 to −1.30) | ||||
| Hispanic | 1.24 (0.81 to 1.66) | 0.16 (−0.26 to 0.58) | ||||
| Other | −1.82 (−2.62 to −1.02) | −2.69 (−3.46 to −1.91) | ||||
| ECD | −0.09 (−0.62 to 0.44) | 0.01 (−0.07 to 0.09) | ||||
| DCD | −3.51 (−4.02 to −2.99) | −1.77 (−2.37 to −1.17) | ||||
| Pulsatile perfusion after harvest | −0.40 (−0.75 to −0.05) | −1.86 (−2.26 to −1.47) | ||||
| Donors with hypertension | −2.13 (−2.52 to −1.74) | −0.47 (−0.98 to 0.05) | ||||
| Donor with diabetes | −1.74 (−2.37 to −1.12) | −2.97 (−3.47 to −2.47) | ||||
| Donor with serum creatinine >1.5 mg/dL | −2.25 (−2.66 to −1.84) | −0.43 (−0.77 to −0.09) | ||||
| Cold ischemia time (ref:<10 h) | ||||||
| Cold ischemia time: missing | −0.32 (−0.93 to 0.29) | −1.94 (−2.31 to −1.57) | ||||
| 10≤cold ischemia time<20 h | −0.82 (−1.22 to −0.41) | −0.34 (−0.93 to 0.26) | ||||
| 20≤cold ischemia time<30 h | −1.45 (−1.90 to −1.00) | −0.55 (−0.94 to −0.17) | ||||
| Cold ischemia time >30 h | −2.88 (−3.51 to −2.25) | −1.05 (−1.48, −0.61) | ||||
| Number of HLA mismatches (A, B, DR) | 0.13 (0.05 to 0.21) | −2.51 (−3.12 to −1.90) | ||||
| Immunosuppression practice | ||||||
| Induction (ref=anti-IL2R) | ||||||
| Polyclonals | −1.62 (−2.01 to −1.23) | −0.61 (−0.96 to −0.26) | ||||
| Alemtuzumab | −2.65 (−3.29 to −2.00) | −0.95 (−1.53 to −0.38) | ||||
| No induction recorded | −0.51 (−0.95 to −0.07) | −0.87 (−1.27 to −0.48) | ||||
| Choice of CNI for maintenance (ref=cyclosporine) | ||||||
| Tacrolimus | 4.46 (4.01 to 4.91) | 4.23 (3.83 to 4.62) | ||||
| No CNI recorded | 2.10 (1.31 to 2.89) | 4.31 (3.61 to 5.01) | ||||
| Choice of antiproliferative for maintenance (ref=mTOR inhibitora) | ||||||
| Mycophenolate | 3.19 (2.62 to 3.76) | 3.23 (2.72 to 3.73) | ||||
| Azathioprine | 4.90 (3.17 to 6.64) | 4.28 (2.76 to 5.81) | ||||
| Other agents or none | 1.84 (0.99 to 2.69) | 2.25 (1.50 to 3.00) | ||||
| Post-transplant course | ||||||
| DGF | −5.16 (−5.52 to −4.79) | −3.26 (−3.60 to −2.92) | ||||
| Acute rejection by 1 yr | −10.2 (−10.7 to −9.76) | −9.18 (−9.60 to −8.76) |
These models included 59,480 recipients with no missing variables. 95% CI, 95% confidence interval; ref, reference group; PRA, panel-reactive antibodies; CNI, calcineurin inhibitor.
mTOR inhibitor group included those on both mTOR inhibitor and mycophenolate.
Table 3.
Estimates from linear regression predicting 1-yr post-KT eGFR among recipients of LDKTs
| Variable | Unadjusted (Model 1L), β (95% CI) | Recipient Characteristics (Model 2L), β (95% CI) | Donor and Organ Characteristics (Model 3L), β (95% CI) | Immunosuppression Practice (Model 4L), β (95% CI) | Post-Transplant Course (Model 5L), β (95% CI) | Fully Adjusted (Model 6L), β (95% CI) |
|---|---|---|---|---|---|---|
| Transplant period (2011–2013 versus 2001–2005) | 0.66 (0.32 to 1.01) | 1.03 (0.68 to 1.39) | 1.82 (1.49 to 2.16) | −0.46 (−0.84 to −0.08) | 0.43 (0.09 to 0.77) | 0.67 (0.31 to 1.04) |
| Recipient characteristics | ||||||
| Age (per 10 yr) | ||||||
| Age ≤40 | −2.71 (−3.13 to −2.29) | −2.24 (−2.64 to −1.85) | ||||
| 40<age≤55 | −0.52 (−0.91 to −0.14) | 0.12 (−0.23 to 0.48) | ||||
| Age >55 | 0.60 (0.18 to 1.01) | 1.02 (0.63 to 1.41) | ||||
| Race (ref=White) | ||||||
| Black | 4.93 (4.45 to 5.40) | 4.76 (3.87 to 5.65) | ||||
| Hispanic | 4.61 (3.89 to 5.34) | 3.02 (2.23 to 3.81) | ||||
| Other | 6.22 (5.73 to 6.72) | 3.86 (2.87 to 4.84) | ||||
| Male (ref=female) | 0.89 (0.55 to 1.22) | 1.17 (0.85 to 1.49) | ||||
| Peak PRA (ref=0) | ||||||
| Missing PRA | −0.35 (−1.14 to 0.45) | −0.64 (−1.41 to 0.12) | ||||
| 0<peak PRA≤20 | −0.21 (−0.61 to 0.19) | −0.14 (−0.52 to 0.23) | ||||
| 20<peak PRA≤80 | −0.13 (−0.66 to 0.40) | −0.15 (−0.65 to 0.35) | ||||
| Peak PRA ≥80 | −0.38 (−1.29 to 0.52) | −0.35 (−1.21 to 0.50) | ||||
| Length of pretransplant dialysis (per yr) | 0.12 (0.04 to 0.20) | 0.14 (0.07 to 0.21) | ||||
| With diabetes yes (ref=no) | 1.73 (1.36 to 2.11) | 1.65 (1.30 to 2.01) | ||||
| Recipient weight (ref=normal: 18.5≤BMI<25) | ||||||
| Recipient BMI missing | −2.05 (−2.64 to −1.45) | −2.13 (−2.69 to −1.57) | ||||
| Underweight (18.5<BMI) | 3.64 (2.58 to 4.71) | 3.72 (2.73 to 4.72) | ||||
| Overweight (25≤BMI<30) | −2.39 (−2.80 to −1.97) | −2.42 (−2.80 to −2.03) | ||||
| Obese (BMI≥30) | −4.11 (−4.55 to −3.67) | −4.10 (−4.51 to −3.69) | ||||
| Donor and organ factors | ||||||
| Age (per 10 yr) | ||||||
| Age ≤25 | −1.83 (−3.73 to 0.06) | −1.12 (−2.96 to 0.71) | ||||
| Age >25 | −4.10 (−4.26 to −3.94) | −3.98 (−4.13 to −3.82) | ||||
| Male (ref=Female) | 3.48 (3.16 to 3.80) | 3.53 (3.22 to 3.84) | ||||
| Race (ref=White) | ||||||
| Black | 2.26 (1.78 to 2.74) | −1.73 (−2.66 to −0.80) | ||||
| Hispanic | 4.21 (3.73 to 4.69) | 1.25 (0.46 to 2.04) | ||||
| Other | 3.38 (2.63 to 4.12) | −0.51 (−1.56 to 0.54) | ||||
| Number of HLA mismatch (A, B, DR) | −0.29 (−0.38 to −0.19) | −0.13 (−0.22 to −0.04) | ||||
| Donors with hypertension | −0.83 (−2.16 to 0.51) | −0.49 (−1.78 to 0.80) | ||||
| Immunosuppression practice | ||||||
| Induction (ref=anti-IL2R) | ||||||
| Polyclonals | −1.33 (−1.74 to −0.92) | −0.76 (−1.15 to −0.38) | ||||
| Alemtuzumab | −0.54 (−1.18 to 0.11) | −0.55 (−1.15 to 0.05) | ||||
| No induction recorded | −0.16 (−0.60 to 0.28) | −0.14 (−0.54 to 0.26) | ||||
| Choice of CNI for maintenance (ref=cyclosporine) | ||||||
| Tacrolimus | 4.33 (3.89 to 4.77) | 3.99 (3.59 to 4.40) | ||||
| No CNI recorded | 3.67 (2.85 to 4.50) | 4.37 (3.61 to 5.13) | ||||
| Choice of antiproliferative for maintenance (ref=mTOR inhibitora) | ||||||
| Mycophenolate | 2.74 (2.17 to 3.30) | 2.84 (2.32 to 3.36) | ||||
| Azathioprine | 3.50 (1.92 to 5.07) | 4.03 (2.58 to 5.48) | ||||
| Other agents or none | 1.77 (0.89 to 2.65) | 1.48 (0.67 to 2.29) | ||||
| Post-transplant course | ||||||
| DGF | −4.76 (−5.71 to −3.80) | −4.53 (−5.42 to −3.63) | ||||
| Acute rejection by 1 yr | −8.98 (−9.49 to −8.46) | −8.24 (−8.72 to −7.76) |
These models included 38,822 recipients with no missing variables. 95% CI, 95% confidence interval; ref, reference group; PRA, panel-reactive antibodies; CNI, calcineurin inhibitor.
mTOR inhibitor group included those on both mTOR inhibitor and mycophenolate.
Time Trends in eGFR at 1 Year post-Transplant (without Adjustment)
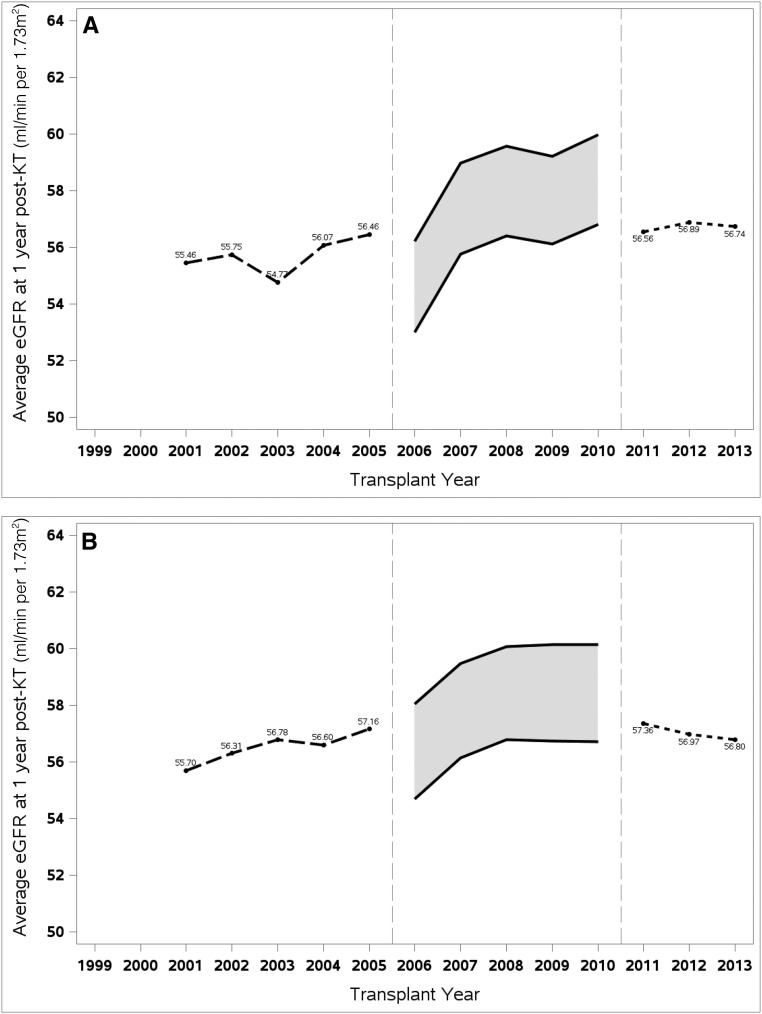
Figure 1 shows the generally stable trend of average post-KT eGFR between the early and the recent periods. Among deceased-donor KT (DDKT) recipients (Figure 1A), average 1-year post-KT eGFR ranged from 54.8 to 56.5 ml/min per 1.73 m2 in 2001–2005, and 56.6 to 56.9 in 2011–2013. Among living-donor KT (LDKT) recipients (Figure 1B), the difference in 1-year post-KT eGFR between the two periods was similarly small. The shaded boxes in both panels between 2006 and 2010 represent the uncertainty in eGFR estimation during this transition period. Assuming that all laboratories adopted the IDMS-calibrated methodology in 2006 would result in the lower bound estimate, whereas the upper bound estimate would result from a universal delay in adoption until 2011.
Figure 1.
Average eGFR at 1 year post-kidney transplant has remained essentially unchanged for both deceased donor kidney transplant and living donor kidney transplant recipients between 2001 and 2013. Average eGFR at 1 year post-KT was calculated by donor type and year transplanted: (A) for DDKT recipients and (B) for LDKT recipients. Non–IDMS-calibrated MDRD equation was applied for recipients between 2001 and 2005, and IDMS-calibrated version was applied for recipients between 2011 and 2013. For patients transplanted between 2006 and 2010, both versions of the MDRD equation were used to generate the upper bound and lower bound average eGFR.
In Tables 2 and 3, regression results of the unadjusted models (models 1D and 1L) summarize numerically what Figure 1 presents graphically. Among DDKT recipients, the coefficient βperiod 1.34 indicates that the average 1-year post-KT eGFR increased by 1.34 ml/min per 1.73 m2 (95% confidence interval, 1.03 to 1.65) between early and recent periods (Table 2). Among LDKT recipients, the change in average 1-year eGFR between the two periods was 0.66 ml/min per 1.73 m2 (95% confidence interval, 0.32 to 1.01) (Table 3).
Trends in Recipient Characteristics and Associated Change in 1-Year eGFR
Recipients steadily became older, with the proportion of recipients over age 65 years nearly doubling between early and recent periods (Table 1). Average dialysis vintage increased, as would be expected from the longer waiting time nationwide.11 Although the average recipient body mass index (BMI) increased only slightly, the proportion of obese recipients (BMI≥30 kg/m2) grew by about 50% for both DDKT and LDKT categories.
As shown in models 2D and 2L, recipient obesity was associated with lower post-KT eGFR. Compared with those with normal BMI, recipient BMI≥30 kg/m2 was associated with post-KT eGFR reduction of 5.18 and 4.11 ml/min per 1.73 m2 in DDKT and LDKT recipients, respectively (Tables 2 and 3). On the other hand, the effects of other recipient characteristics on post-KT eGFR are less clear. With inclusion of recipient characteristics in models 2D and 2L, βperiod estimates became higher at 1.88 and 1.03, respectively, for DDKT and LDKT. Compared with the unadjusted models (models 1D and 1L), these results suggest that the average 1-year post-KT eGFR would have improved slightly more between the two periods without the changes in recipient characteristics.
Trends in Donor and Organ Characteristics and Associated Change in 1-Year eGFR
In Table 1, both the deceased and living donor pools became older over time, with growth seen particularly with living donors over age 50 years. Use of deceased donors with DCD, hypertension, diabetes, and terminal creatinine >1.5 mg/dl all increased over time as well, and each was associated with lower expected 1-year post-KT eGFR in models 3D and 3L (Tables 2 and 3). With the inclusion of donor and organ characteristics, βperiod estimates in models 3D and 3L became higher than in the unadjusted models (1D and 1L), at 2.63 and 1.82, respectively, for DDKT and LDKT recipients. Stated differently, if donor and organ characteristics had remained unchanged, average 1-year eGFR would have been expected to increase more between the two periods.
Trends in Immunosuppression Practice and Associated Change in 1-Year eGFR
Practices of both induction and maintenance immunosuppression changed over the study period (Table 1). Induction with polyclonals and alemtuzumab became more common, and both are associated with slightly lower post-KT eGFR (models 4D and 4L in Tables 2 and 3). For maintenance regimen, both tacrolimus and mycophenolate became more prevalent, and both are associated with higher 1-year eGFR in regression models compared with cyclosporine or another antiproliferative. Compared with the unadjusted models (1D and 1L), incorporating immunosuppression practice changes in models 4D and 4L lowered βperiod estimates to 0.31 and −0.46 for DDKT and LDKT, respectively. In other words, little or no improvement in average 1-year eGFR would be expected between the two periods after adjusting for changes in immunosuppression practice.
Trends in post-Transplant Course and Associated Change in 1-Year eGFR
Incidence of delayed graft function (DGF) remained steady for both DDKT and LDKT cohorts over the study period, although the incidence of acute rejection decreased slightly (Table 1). In models 5D and 5L, both acute rejection and DGF were associated with lower 1-year post-KT eGFR (Tables 2 and 3). However, when compared with the unadjusted models (1D and 1L), adjusting these two factors had very little effect on the βperiod estimates.
Fully Adjusted Models
In models 2 through 5, categories of explanatory variables were considered separately to highlight the effect of adjusting for each. Models 6D and 6L adjusted for all variables, and estimates of selected variables are shown in Figure 2, A and B. Estimates of βperiod were 0.99 for DDKT and 0.67 for LDKT, nearly identical to those in the unadjusted models (1D and 1L) Tables 2 and 3).
Figure 2.
Acute rejection, delayed graft function, and several donor characteristics are associated with lower 1-year post-kidney transplant eGFR, whereas use of mycophenolate and tacrolimus are associated with higher 1-year post-KT eGFR. (A) Selected estimates from fully adjusted linear regression (model 6D from Table 2) predicting 1-year post-KT eGFR among recipients of DDKTs. Model adjusted for recipient characteristics (age, race, sex, panel reactive antibody [PRA], length of pretransplant dialysis, diabetes, BMI), donor and organ factors (age, sex, race, ECD, DCD, pulsatile perfusion after harvest, number of HLA mismatches [A, B, DR], hypertension, diabetes, cold ischemia time), immunosuppression practice (induction agents: polyclonals, anti-IL2R, or alemtuzumab; calcineurin inhibitor [CNI] choice for maintenance: tacrolimus, cyclosporine, or none; antiproliferative choice for maintenance: mycophenolate, azathioprine, or mTOR inhibitor), and post-transplant course (DGF, acute rejection by 1 year). (B) Selected estimates from fully adjusted linear regression (model 6L from Table 3) predicting 1-year post-KT eGFR among recipients of LDKTs. Model adjusted for recipient characteristics (age, race, sex, PRA, pretransplant dialysis, diabetes, BMI), donor and organ factors (age, sex, race, number of HLA mismatches [A, B, DR], hypertension), immunosuppression practice (induction agents: polyclonals, anti-IL2R, or alemtuzumab; CNI choice for maintenance: tacrolimus, cyclosporine, or none; antiproliferative choice for maintenance:mycophenolate, azathioprine, or mTOR inhibitor), and post-transplant course (DGF, acute rejection by 1 year). Post-Tx, post-transplant.
Sensitivity Analyses
Complete results for the sensitivity analyses are provided in (Supplemental Figure 1, Supplemental Table 1, A and B). Briefly, similar to the main analyses, there was only a minimal improvement in 1-year post-KT eGFR, increasing by 0.28 ml/min per 1.73 m2 per year and 0.19 ml/min per 1.73 m2 per year over the study period among DDKT and LDKT recipients, respectively, in the fully adjusted models.
Discussion
Long-term allograft and patient survival are the best measures of success after KT, but they require prolonged follow-up to manifest. An intermediate endpoint such as 1-year post-KT eGFR is associated with eventual patient and graft survival and has some prognostic value, as shown in multiple observational studies in both adult and pediatric populations.1,3–5,12–15 In our study, the average 1-year post-KT eGFR of United States DDKT and LDKT recipients transplanted in 2001 was 55.5 and 55.7 ml/min per 1.73 m2, respectively—higher than previously reported estimates around 50 ml/min per 1.73 m2 in the 1990s.16,17 However, between 2001 and 2013, the average 1-year post-KT eGFR remained essentially unchanged, despite substantial changes in immunosuppression and other management of the transplant population.
Our analysis of contemporaneous trends in kidney transplantation helps explain this apparent lack of further progress in 1-year post-KT eGFR. In models that highlighted each category of explanatory variables separately, we showed that some categories were associated with enhanced 1-year post-KT eGFR whereas others had the opposite effect.
Many of the donor and organ factors modeled, including older donor age, DCD status, donor hypertension, donor terminal creatinine >1.5 mg/dl, and prolonged cold ischemia time, were associated with lower post-KT eGFR, as has been shown in previous observational studies. The adjustment of its component criteria likely rendered the expanded criteria donor (ECD) status insignificant in our model, and the selective use of pulsatile perfusion in organs at risk of DGF likely explains its association with lower eGFR. In response to the organ shortage, use of higher-risk donors increased over time and may have slowed the improvement in aggregate post-KT renal function. Some changes in recipient characteristics, in particular the increase in BMI, also were associated with lower eGFR, although to a smaller extent compared with the donor factors.
In contrast, changes in immunosuppression practice overall were associated with better transplant outcome in both the DDKT and LDKT cohorts. The use of tacrolimus increased and its positive effect on 1-year post-KT eGFR has also been shown in a randomized controlled trial comparing tacrolimus to cyclosporine.18 The association between induction with polyclonals or alemtuzumab and lower post-KT renal function may reflect their selective use in recipients with higher rejection risk, because full adjustment of organ and recipient characteristics attenuated the negative association. Overall, adjusting for immunosuppression changes attenuated the increase in 1-year post-KT eGFR between the two periods, suggesting a possible role of immunosuppression practice in the modest improvement of post-transplant renal function over time.
On the other hand, despite the detrimental association between DGF and early acute rejection with eGFR in a given recipient, their incidences changed only slightly over time. The stability of these two factors likely limited their effect on the temporal trend of post-KT renal function, and their inclusion in the models only minimally altered the expected difference in average eGFR between the two periods.
When all four groups of variables were considered, the negative effect of donor/recipient factors and the positive effect of immunosuppression advancement essentially balanced each other. In both cohorts, the expected differences in 1-year post-KT eGFR between the two periods were nearly identical in unadjusted and fully adjusted models.
Assuming an average GFR loss between 1 and 2 ml/min per 1.73 m2 per year post-transplant as reported in the literature,16,19–22 the small increase in 1-year post-KT eGFR observed between the two periods is unlikely to be clinically significant or translate into noticeable improvement in graft survival. However, with the changes in recipient and donor characteristics, one might have expected the aggregate post-transplant renal function to decline over time, and its stability is reassuring.
Although the stability is reassuring, further advancements in transplant care may allow additional improvements in post-transplant eGFR. With regard to immunosuppression practice, the use of tacrolimus and mycophenolate has plateaued at >90% in the most recent cohort of KT recipients, and they are therefore unlikely to have further effect on the temporal trend of post-KT eGFR in the future. On the other hand, everolimus and belatacept are two newer, less employed agents, and their de novo use has been associated with higher post-KT eGFR compared with more traditional maintenance regimens.23,24 During the study period, the proportion of KT recipients initiated on everolimus increased slightly but steadily from 0.2% to 0.5%. It remains to be seen whether these newer agents will gain wider acceptance and affect the average post-KT eGFR. With regard to acute rejection, rates are low and have changed very little over the last decade, suggesting no substantial room for improvement without significantly intensifying immunosuppression and the attendant risks of infection. There may be opportunities to improve the incidence of DGF, however. The incidence of DGF in DDKT increased between the 1980s and 1990s in the United States,25 but the upward trend has been arrested since the early 2000s in our results despite continued increases in recipient obesity, ECD status, and DCD status, all risk factors for DGF.25,26 The stability of DGF incidence may be attributable to the popularization of pulsatile perfusion, which has been shown in randomized controlled trials to decrease incidences of DGF and improve 1-year eGFR in recipients of ECD and DCD kidneys.27,28 The success of pulsatile perfusion suggests DGF as a modifiable outcome, and research is underway to find additional methods, including pharmacologic agents, to ameliorate ischemic reperfusion injury that leads to DGF.25,29 Improvement of DGF incidence over time may translate into higher average post-KT eGFR in the future.
There are some limitations to this study. We relied on GFR estimation using the MDRD equation rather than actual measurement to assess transplant kidney function. Although GFR measurement by iothalamate or similar methods is considered the gold standard, this is impractical on a large scale.19 Because the MDRD equation was originally derived from a transplant-naïve CKD population, it is less accurate in KT recipients.30,31 However, the MDRD equation outperforms serum creatinine alone or the Cockcroft–Gault equation,32,33 and it remains a useful tool in KT recipients for both clinical and research applications.
Also, because the creatinine measurement techniques at different laboratories were not uniform and changed over time, we made assumptions about the technique used for each subperiod. Although the adoption of IDMS-calibrated methods started in earnest in 2006, some laboratories might have already been using calibrated creatinine assays before 2006. The use of the non–IDMS-traceable MDRD equation would overestimate eGFR on results from these laboratories and may mask a temporal improvement in average post-KT eGFR.
It should also be pointed out that association between any of the explanatory variables and post-KT eGFR in our regression models does not prove causality. For example, our models suggested a beneficial effect on aggregate post-KT eGFR from the adoption of tacrolimus. However, if another contemporaneous innovation had a similarly rising implementation over time, our analysis would not be able to separate the effects of the two practices. Although we controlled for the use of pulsatile perfusion and other immunosuppression medications, other practice changes were not modeled due to lack of data. One example would be the routine screening for BK polyoma virus, which became popular over the past decade and has reduced the incidence of severe BK nephropathy.34 Nonetheless, the positive effect on 1-year eGFR of tacrolimus versus cyclosporine has also been observed in a randomized controlled trial,18 which corroborates our conclusion that change in immunosuppression practice could have had a positive effect on the aggregate 1-year eGFR at the national level.
In conclusion, aggregate eGFR among KT recipients at 1 year post-transplant remained stable between 2001 and 2013, despite increase in recipient and donor characteristics that are associated with lower post-KT renal function. Our results demonstrate that changes in immunosuppression practice helped raise the post-KT GFR and countered the unfavorable changes in recipient and donor characteristics. It is reassuring that the transplant community has been able to respond to the increased demand for kidney transplantation and ongoing organ shortage without compromising post-transplant renal function. Efforts to sustain preservation of post-KT GFR should focus on optimizing immunosuppressive therapy, reducing DGF, and formulating other innovations in post-transplant care.
Concise Methods
Study Population
A total of 189,944 adult (age>20 years) primary KT recipients engrafted in the United States between 2001 and 2013 were identified in the SRTR (http://www.srtr.org/who.aspx). The SRTR database is updated annually with data collected by the Organ Procurement and Transplantation Network from hospitals and organ procurement organizations. Recipients with simultaneous extrarenal transplants, death before 1-year anniversary, or missing creatinine at 1 year (n=21,257) were excluded. The breakdown of excluded recipients is shown in Supplemental Table 2.
eGFR Estimation, Changes in Creatinine Measurement, and the Three Study Subperiods
The main outcome variable of interest, eGFR at 1 year post-KT, was derived from reported serum creatinine values using the MDRD equation. Over the past decade, United States laboratories gradually adopted the gold standard of IDMS-traceable creatinine calibration. Following the recommendation from the National Institutes of Health, the adoption accelerated after 2006 and was nearly complete in 2011.35
To account for changes in creatinine assays, we applied different versions of the MDRD equation during different periods. One year post-KT creatinine values for recipients transplanted between 2001 and 2005 (early period) were assumed to be non–IDMS-traceable, and the corresponding MDRD equation was used. In recipients transplanted between 2011 and 2013 (recent period), the IDMS-calibrated MDRD equation was used instead. However, accurate GFR estimation was infeasible during the transition period between 2006 and 2010 without knowing how each individual creatinine was measured. Both MDRD equations were therefore used to calculate upper and lower bounds of average eGFR during the transition period. Furthermore, to better understand the 2006–2010 period, we implemented a sensitivity analysis on a selection of transplant facilities with sufficient data to estimate the transition date more precisely.
Variables Affecting 1-Year post-KT eGFR and Their Temporal Trend
Four categories of explanatory variables were considered in the study: (1) Recipient demographics and disease burden: age (in years, as a continuous variable with splines at 40 and 55 years), sex, race/ethnicity, BMI, peak panel reactive antibody, dialysis vintage (in years), and history of diabetes. (2) Donor and organ quality characteristics: age (in years, as a continuous variable with a spline at 25 years), sex, race/ethnicity, ECD status, DCD status, history of diabetes and hypertension, serum creatinine >1.5 mg/dl, use of pulsatile perfusion, number of HLA mismatches between recipient and donor, and cold ischemia time (in hours). (3) Immunosuppression practice: induction with polyclonal antibodies (equine or rabbit antithymocyte antibodies), induction with monoclonal anti–IL-2 receptor antibodies (basiliximab or daclizumab), induction with alemtuzumab, initial choice of calcineurin inhibitor in maintenance regimen (tacrolimus, cyclosporine, or no calcineurin inhibitor), initial choice of antiproliferative in maintenance regimen (mycophenolate, azathioprine, or a mammalian target of rapamycin [mTOR] inhibitor). Recipients on both mycophenolate and an mTOR inhibitor were included in the mTOR inhibitor group. (4) Post-transplant events: acute rejection within the first post-transplant year and DGF, defined as dialysis requirement within the first week.
To demonstrate changes in transplant practices over time, descriptive statistics of each explanatory variable were trended over three subperiods as described above: 2001–2005 (early), 2006–2010, and 2011–2013 (recent).
Regression Analysis
Twelve linear regression models were used to study the effect of various changes on 1-year post-KT eGFR, six each for DDKT and LDKT cohorts (models 1D–6D and 1L–6L, respectively). Some variables pertained only to deceased donors, necessitating separate DDKT and LDKT models. Recipients transplanted in the 2006–2010 period were excluded in this analysis given the uncertainty in their GFR estimation. To study the temporal trend, the transplant period (early, 2001–2005, versus recent, 2011–2013) was also included in the models as the main explanatory variable of interest.
The four categories of explanatory variables were introduced in a staged fashion to isolate the effect of each. Models 1D and 1L adjusted for only the transplant period. From models 2D and 2L through 5D and 5L, each of the four categories of variables was added separately. In models 6D and 6L, all four categories of variables were adjusted.
Sensitivity Analyses for the Timing of IDMS-Calibration Implementation
We identified 44 (out of 305) transplant facilities with at least 5000 serum creatinine values for KT recipients at any time pre- or post-transplant during January of 2003 through December of 2011, which encompassed the IDMS transition period. For each selected facility, we used linear regression with eGFR as the outcome to estimate when the facility transitioned to the IDMS-traceable assay. The analyses described above estimating the temporal trend of 1-year post-KT eGFR were repeated for the 44 selected facilities, using transplant year as a continuous variable in the models.
All analyses were performed using SAS 9.3 (SAS, Cary, NC).
Disclosures
None.
Supplementary Material
Acknowledgments
The authors would like to acknowledge editorial support by Ruth Shamraj.
This work was supported by the US Centers for Disease Control and Prevention (CDC) award 5U58DP003836-04 and U58DP003836.
This work has been presented as an oral presentation at the annual meeting of American Society of Nephrology, Philadelphia, November 10, 2011 (J Am Soc Nephrol 22: 15A, 2011).
Publication and report contents are solely the responsibility of the authors and do not necessarily represent the official views of the CDC or the United States government. The data reported here have been supplied by the Minneapolis Medical Research Foundation (MMRF) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government. This study used data from the Scientific Registry of Transplant Recipients (SRTR). The SRTR data system includes data on all donor, wait-listed candidates, and transplant recipients in the US, submitted by the members of the Organ Procurement and Transplantation Network (OPTN). The Health Resources and Services Administration (HRSA), U.S. Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors.
Research idea and study design: Y.H., R.S., V.S., and A.T.; data acquisition: A.T., R.S.; data analysis/interpretation: A.T., V.S., B.G., Y.H, and R.S.; statistical analysis: A.T., B.G.; overall supervision and mentorship: B.G., V.S., and R.S. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. Y.H. and R.S. take responsibility that this study has been reported honestly, accurately, and transparently, that no important aspects of the study have been omitted, and that any discrepancies from the study as planned (and, if relevant, registered) have been explained. T.B., V.G., N.P., N.R.B., and M.P. have reviewed the manuscript for its intellectual content.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2016050543/-/DCSupplemental.
References
- 1.Salvadori M, Rosati A, Bock A, Chapman J, Dussol B, Fritsche L, Kliem V, Lebranchu Y, Oppenheimer F, Pohanka E, Tufveson G, Bertoni E: Estimated one-year glomerular filtration rate is the best predictor of long-term graft function following renal transplant. Transplantation 81: 202–206, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Meier-Kriesche HU, Baliga R, Kaplan B: Decreased renal function is a strong risk factor for cardiovascular death after renal transplantation. Transplantation 75: 1291–1295, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Kasiske BL, Israni AK, Snyder JJ, Skeans MA; Patient Outcomes in Renal Transplantation (PORT) Investigators : The relationship between kidney function and long-term graft survival after kidney transplant. Am J Kidney Dis 57: 466–475, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Kasiske BL, Israni AK, Snyder JJ, Skeans MA, Peng Y, Weinhandl ED: A simple tool to predict outcomes after kidney transplant. Am J Kidney Dis 56: 947–960, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Hariharan S, McBride MA, Cherikh WS, Tolleris CB, Bresnahan BA, Johnson CP: Post-transplant renal function in the first year predicts long-term kidney transplant survival. Kidney Int 62: 311–318, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Ibrahim A, Garg AX, Knoll GA, Akbari A, White CA: Kidney function endpoints in kidney transplant trials: A struggle for power. Am J Transplant 13: 707–713, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Meier-Kriesche HU, Li S, Gruessner RW, Fung JJ, Bustami RT, Barr ML, Leichtman AB: Immunosuppression: Evolution in practice and trends, 1994-2004. Am J Transplant 6: 1111–1131, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Webster AC, Woodroffe RC, Taylor RS, Chapman JR, Craig JC: Tacrolimus versus ciclosporin as primary immunosuppression for kidney transplant recipients: Meta-analysis and meta-regression of randomised trial data. BMJ 331: 810, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levey AS, Coresh J, Greene T, Marsh J, Stevens LA, Kusek JW, Van Lente F; Chronic Kidney Disease Epidemiology Collaboration : Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem 53: 766–772, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Stevens LA, Manzi J, Levey AS, Chen J, Deysher AE, Greene T, Poggio ED, Schmid CH, Steffes MW, Zhang YL, Van Lente F, Coresh J: Impact of creatinine calibration on performance of GFR estimating equations in a pooled individual patient database. Am J Kidney Dis 50: 21–35, 2007 [DOI] [PubMed] [Google Scholar]
- 11.US Renal Data System : USRDS 2013 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2013 [Google Scholar]
- 12.Muscheites J, Wigger M, Drueckler E, Klaassen I, John U, Wygoda S, Fischer DC, Kundt G, Misselwitz J, Müller-Wiefel DE, Haffner D: Estimated one-yr glomerular filtration rate is an excellent predictor of long-term graft survival in pediatric first kidney transplants. Pediatr Transplant 13: 365–370, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Salvadori M, Rosati A, Bock A, Chapman J, Dussol B, Fritsche L, Jeffery J, Kliem V, Lebranchu Y, Oppenheimer F, Pohanka E, Tufveson G: One-year posttransplant renal function is a strong predictor of long-term kidney function: Results from the Neoral-MOST observational study. Transplant Proc 35: 2863–2867, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Lenihan CR, O’Kelly P, Mohan P, Little D, Walshe JJ, Kieran NE, Conlon PJ: MDRD-estimated GFR at one year post-renal transplant is a predictor of long-term graft function. Ren Fail 30: 345–352, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Resende L, Guerra J, Santana A, Mil-Homens C, Abreu F, da Costa AG: First year renal function as a predictor of kidney allograft outcome. Transplant Proc 41: 846–848, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Gill JS, Tonelli M, Mix CH, Johnson N, Pereira BJ: The effect of maintenance immunosuppression medication on the change in kidney allograft function. Kidney Int 65: 692–699, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Gourishankar S, Hunsicker LG, Jhangri GS, Cockfield SM, Halloran PF: The stability of the glomerular filtration rate after renal transplantation is improving. J Am Soc Nephrol 14: 2387–2394, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Ekberg H, Tedesco-Silva H, Demirbas A, Vítko S, Nashan B, Gürkan A, Margreiter R, Hugo C, Grinyó JM, Frei U, Vanrenterghem Y, Daloze P, Halloran PF; ELITE-Symphony Study : Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med 357: 2562–2575, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Gera M, Slezak JM, Rule AD, Larson TS, Stegall MD, Cosio FG: Assessment of changes in kidney allograft function using creatinine-based estimates of glomerular filtration rate. Am J Transplant 7: 880–887, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Marcen R, Morales JM, Fernández-Rodriguez A, Capdevila L, Pallardó L, Plaza JJ, Cubero JJ, Puig JM, Sanchez-Fructuoso A, Arias M, Alperovich G, Serón D: Long-term graft function changes in kidney transplant recipients. NDT Plus 3[Suppl_2]: ii2–ii8, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Udayaraj UP, Casula A, Ansell D, Dudley CR, Ravanan R: Chronic kidney disease in kidney transplant recipients-is it different from chronic native kidney disease? Transplantation 90: 765–770, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Saran R, Li Y, Robinson B, Abbott KC, Agodoa LY, Ayanian J, Bragg-Gresham J, Balkrishnan R, Chen JL, Cope E, Eggers PW, Gillen D, Gipson D, Hailpern SM, Hall YN, He K, Herman W, Heung M, Hirth RA, Hutton D, Jacobsen SJ, Kalantar-Zadeh K, Kovesdy CP, Lu Y, Molnar MZ, Morgenstern H, Nallamothu B, Nguyen DV, O’Hare AM, Plattner B, Pisoni R, Port FK, Rao P, Rhee CM, Sakhuja A, Schaubel DE, Selewski DT, Shahinian V, Sim JJ, Song P, Streja E, Kurella Tamura M, Tentori F, White S, Woodside K, Hirth RA: US renal data system 2015 annual data report: Epidemiology of kidney disease in the United States. Am J Kidney Dis 67[Suppl 1]: Svii–S305, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loriga G, Ciccarese M, Pala PG, Satta RP, Fanelli V, Manca ML, Serra G, Dessole P, Cossu M: De novo everolimus-based therapy in renal transplant recipients: Effect on proteinuria and renal prognosis. Transplant Proc 42: 1297–1302, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Vincenti F, Rostaing L, Grinyo J, Rice K, Steinberg S, Gaite L, Moal MC, Mondragon-Ramirez GA, Kothari J, Polinsky MS, Meier-Kriesche HU, Munier S, Larsen CP: Belatacept and long-term outcomes in kidney transplantation. N Engl J Med 374: 333–343, 2016 [DOI] [PubMed] [Google Scholar]
- 25.Siedlecki A, Irish W, Brennan DC: Delayed graft function in the kidney transplant. Am J Transplant 11: 2279–2296, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moreira TR, Bassani T, de Souza G, Manfro RC, Gonçalves LF: Obesity in kidney transplant recipients: Association with decline in glomerular filtration rate. Ren Fail 35: 1199–1203, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Jochmans I, Moers C, Smits JM, Leuvenink HG, Treckmann J, Paul A, Rahmel A, Squifflet JP, van Heurn E, Monbaliu D, Ploeg RJ, Pirenne J: Machine perfusion versus cold storage for the preservation of kidneys donated after cardiac death: A multicenter, randomized, controlled trial. Ann Surg 252: 756–764, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Treckmann J, Moers C, Smits JM, Gallinat A, Maathuis MH, van Kasterop-Kutz M, Jochmans I, Homan van der Heide JJ, Squifflet JP, van Heurn E, Kirste GR, Rahmel A, Leuvenink HG, Pirenne J, Ploeg RJ, Paul A: Machine perfusion versus cold storage for preservation of kidneys from expanded criteria donors after brain death. Transpl Int 24: 548–554, 2011 [DOI] [PubMed] [Google Scholar]
- 29.Legendre C, Sberro-Soussan R, Zuber J, Rabant M, Loupy A, Timsit MO, Anglicheau D: Eculizumab in renal transplantation. Transplant Rev (Orlando) 27: 90–92, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Mariat C, Alamartine E, Afiani A, Thibaudin L, Laurent B, Berthoux P, De Filippis JP, Thibaudin D, Mayor B, Elessawy AB, Berthoux F: Predicting glomerular filtration rate in kidney transplantation: Are the K/DOQI guidelines applicable? Am J Transplant 5: 2698–2703, 2005 [DOI] [PubMed] [Google Scholar]
- 31.White CA, Huang D, Akbari A, Garland J, Knoll GA: Performance of creatinine-based estimates of GFR in kidney transplant recipients: A systematic review. Am J Kidney Dis 51: 1005–1015, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Pöge U, Gerhardt T, Palmedo H, Klehr HU, Sauerbruch T, Woitas RP: MDRD equations for estimation of GFR in renal transplant recipients. Am J Transplant 5: 1306–1311, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Poggio ED, Wang X, Weinstein DM, Issa N, Dennis VW, Braun WE, Hall PM: Assessing glomerular filtration rate by estimation equations in kidney transplant recipients. Am J Transplant 6: 100–108, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Sawinski D, Goral S: BK virus infection: An update on diagnosis and treatment. Nephrol Dial Transplant 30: 209–217, 2015 [DOI] [PubMed] [Google Scholar]
- 35.Myers GL, Miller WG, Coresh J, Fleming J, Greenberg N, Greene T, Hostetter T, Levey AS, Panteghini M, Welch M, Eckfeldt JH; National Kidney Disease Education Program Laboratory Working Group : Recommendations for improving serum creatinine measurement: A report from the laboratory working group of the national kidney disease education program. Clin Chem 52: 5–18, 2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.