Abstract
Secondary hyperparathyroidism commonly complicates CKD and associates with morbidity and mortality. We profiled microRNA (miRNA) in parathyroid glands from experimental hyperparathyroidism models and patients receiving dialysis and studied the function of specific miRNAs. miRNA deep-sequencing showed that human and rodent parathyroids share similar profiles. Parathyroids from uremic and normal rats segregated on the basis of their miRNA expression profiles, and a similar finding was observed in humans. We identified parathyroid miRNAs that were dysregulated in experimental hyperparathyroidism, including miR-29, miR-21, miR-148, miR-30, and miR-141 (upregulated); and miR-10, miR-125, and miR-25 (downregulated). Inhibition of the abundant let-7 family increased parathyroid hormone (PTH) secretion in normal and uremic rats, as well as in mouse parathyroid organ cultures. Conversely, inhibition of the upregulated miR-148 family prevented the increase in serum PTH level in uremic rats and decreased levels of secreted PTH in parathyroid cultures. The evolutionary conservation of abundant miRNAs in normal parathyroid glands and the regulation of these miRNAs in secondary hyperparathyroidism indicates their importance for parathyroid function and the development of hyperparathyroidism. Specifically, let-7 and miR-148 antagonism modified PTH secretion in vivo and in vitro, implying roles for these specific miRNAs. These findings may be utilized for therapeutic interventions aimed at altering PTH expression in diseases such as osteoporosis and secondary hyperparathyroidism.
Keywords: hyperparathyroidism, chronic kidney disease, mineral metabolism, gene expression, microRNA
MicroRNAs (miRNAs) are conserved RNAs that regulate gene expression by directing RNA-induced silencing complex to the 3′-UTRs of mRNAs, thereby inhibiting translation or inducing degradation.1 Up to 60% of human protein-coding genes are regulated by miRNAs.2 miRNAs are categorized into groups on the basis of cistronic expression and the mature miRNA sequence.3 miRNAs are essential for normal development and are involved in fine-tuning of many biologic processes. Their role as key regulators boosts their value as therapeutic targets and biomarkers.4,5 Small-RNA sequencing provides both the sequence and frequency of RNA molecules, and is thus an attractive approach for quantitative, global miRNA profiling.6
Binding of miRNA to target mRNA is essential for regulatory function. One inhibition strategy is on the basis of oligonucleotides that complement the specific miRNA sequence (anti-miRs). These oligonucleotides compete with cellular mRNAs.7 Chemical modifications have been employed to prevent oligonucleotide degradation, improve affinity for target miRNA, and promote tissue uptake in the course of in vivo delivery,8–10 providing effective knockdown reagents for determining the function of specific miRNAs.11–14
Parathyroid hormone (PTH) determines extracellular calcium homeostasis and bone strength. It is secreted by parathyroid glands in response to decrease in serum calcium. Secondary hyperparathyroidism (SHP) is a complication of CKD characterized by increased PTH secretion, PTH gene expression, and parathyroid cell proliferation.15,16 In experimental SHP increased PTH gene expression is mediated predominantly by post-transcriptional mechanisms determined by the regulated binding of trans-acting proteins to the PTH mRNA.17–19 We showed that in addition to protein-PTH mRNA interactions, miRNAs are also necessary for the stimulation of the parathyroid by hypocalcemia and uremia. Dicer mediates the final step of miRNA maturation and its deletion results in disruption of miRNA activity in the cell. We have recently shown that parathyroid-specific deletion of Dicer1 in PT-Dicer−/− mice resulted in impaired response to both acute and chronic hypocalcemia and uremia.20 Thus, miRNAs are essential for the increase in PTH mRNA and serum levels during both acute and chronic hypocalcemia and uremia.
Although miRNA profiles have been studied in parathyroid tumors,21–23 there is no information about miRNA expression and function in normal parathyroid physiology and in SHP. Furthermore, parathyroid miRNA profiles of patients with SHP and experimental models have not been studied so far. We profiled the mouse, rat, and human parathyroid miRNome by small-RNA sequencing. We characterized the abundant parathyroid miRNA families in these species, identified several miRNAs that are dysregulated in SHP, and targeted abundant/upregulated miRNA using previously validated anti-miRs in vivo and in vitro. Our findings suggest roles for specific miRNAs in parathyroid physiology and disease.
Results
Conserved miRNA Patterns in Human and Murine Parathyroid
The most abundant miRNA sequence families in normal human, mouse, and rat parathyroid tissues, established by deep-sequencing of small-RNA libraries, are shown in Figure 1A (complete profiles of all study specimens are depicted in Supplemental Tables 1–10). Among the 50 most abundant sequence families in human parathyroid, 37 were also top 50 in mouse and 39 in rat (Figure 1B). Abundance correlation of top miRNAs between species was 0.60–0.78 (Figure 1C). let-7 members were the most highly expressed in human (25%), mouse (32%), and rat (23%) parathyroids, followed by miR-30 members (8.9%–14%) and miR-141/200 members (4.5%–8.5%). miR-375 was high in mouse (8.3%) and rat (7.6%), but lower in human tissues (0.5%). miR-148a/b composed 2.6% in human and mouse and 7.0% in rat (Figure 1B). These findings show expression conservation of miRNA among species, suggesting regulatory significance.
Figure 1.
miRNA abundance is similar in normal human, mouse, and rat parathyroid glands. (A) Heat map representation of the most abundant miRNA sequence families in pooled normal specimens. The top 50 sequence families (not individual miRNAs) in humans are shown, with corresponding values from rats and mice. miRNA sequence families (rows) and species (columns) were hierarchically clustered applying Manhattan distance metric and Ward clustering method. The color scale represents abundance, where brighter shades correspond to higher values. (B) Venn diagram showing vast overlaps of top 50 miRNA sequence families between species. (C) Three-dimensional scatter plot showing interspecies expression correlations of top 25 miRNA sequence families (as determined in humans). Input data for these analyses (raw counts arranged in sequence families) are presented in Supplemental Tables 2, 5, and 8.
SHP Is Accompanied by Major miRNome Alterations
miRNA sequencing was performed upon ESRD-related hyperplastic human tissues and several models of hyperplastic glands in rats: short-term uremia (induced by 1-week adenine diet with high-phosphorus content), intermediate/long-term uremia (6–8 weeks of the diet), and hypocalcemia (induced by feeding weaned rats a diet with low-calcium content). Principal component analysis of samples by miRNA profiles, plotted in Figure 2, is suggestive of major miRNA transcriptome alterations, which are apparent in both human and experimental SHP models (Supplemental Figure 1). Dose-response is also evident in the experimental SHP, wherein more severe disease is accompanied by outlying profiles.
Figure 2.
Principal component analysis (PCA) of miRNA profiles discriminates normal from hyperplastic glands. Plots depicting the main variables (PC1 and PC2) derived from PCAs which capture miRNA abundance levels into new variables. These new variables do not correlate with each other, however, each principal component correlates (or anticorrelates) with all miRNA, to different degrees. PCA plots of miRNA profiles (A) or differentially expressed miRNA (B) from normal rats and rats with SHP induced by either uremia or low-calcium diet. (C) PCA plot of miRNA profiles from normal deparaffinized human parathyroid glands and glands from ESRD patients with SHP. Ca, calcium.
SHP-Driven miRNA Alterations Display Common Patterns of Dysregulation
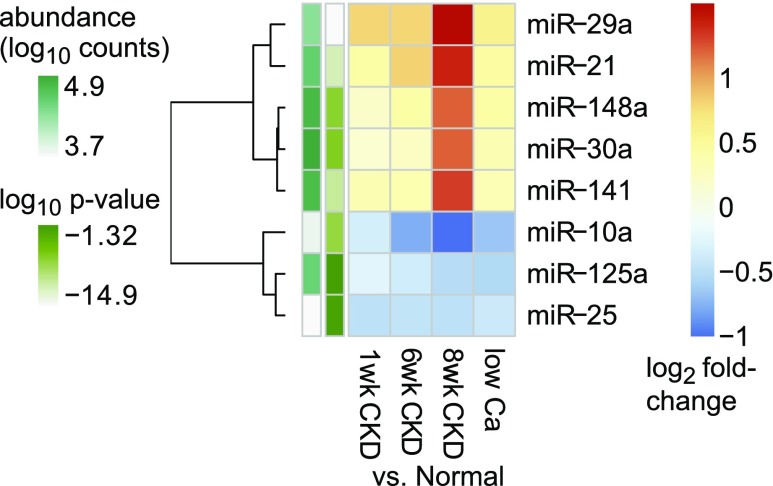
To identify specific miRNAs that are dysregulated in SHP, we evaluated miRNA differential expression between normal and SHP rats (Supplemental Tables 11–13). Figure 3 displays the most abundant differentially expressed miRNA sequence families, exemplifying a common pattern in which an miRNA family is either upregulated or downregulated at early CKD, and these trends progress gradually through late CKD, whereas the changes in the 3-week hypocalcemia model resemble intermediate-duration CKD. For example, miR-148 and miR-141 members both increase, gradually peaking at 8-week CKD, whereas hypocalcemia resembles 6-week CKD. miR-10 members display inverse trends. However, among lower-expressed miRNAs significant changes are seen that follow different patterns, such as a rise at 1-week CKD followed by partial normalization (e.g., miR-499 sequence family; Supplemental Figure 2, Supplemental Table 13).
Figure 3.
Top differentially expressed miRNAs in experimental SHP show common patterns. Heat map showing the most abundant differentially expressed miRNA sequence families (derived from Supplemental Table 13, columns C–F). Color shades represent the fold change of the indicated miRNA in the indicated SHP model compared with control rats. Rows (miRNA families) were clustered applying Euclidean distance and complete clustering method. The side (row) annotations represent the ANOVA-like P values and the average abundance levels of the indicated miRNA families. Ca, calcium.
Predicted targets of the abundant upregulated miRNA sequence families (see Figure 3) are overrepresented in KEGG “pathways in cancer,” “focal adhesion,” “axon guidance,” “small-cell lung cancer,” “MAPK signaling,” “regulation of actin cytoskeleton,” “olfactory transduction,” “glioma,” “TGF-b signaling,” “neurotrophin signaling,” and other pathways (Supplemental Table 14A). Predicted targets of abundant downregulated miRNA families are overrepresented in KEGG pathways “MAPK signaling,” “neurotrophin signaling,” “regulation of actin cytoskeleton,” “focal adhesion,” “WNT signaling,” “amyotrophic lateral sclerosis,” “pathways in cancer,” “ECM-receptor interaction,” “glioma,” “calcium signaling,” and others (Supplemental Table 14B).
Differential expression analyses of human miRNA in ESRD-hyperplastic parathyroid glands compared with normal tissues are shown in Supplemental Figure 3 and Supplemental Tables 15–17. These substantial miRNA expression alterations suggest that miRNAs are involved in SHP pathogenesis and highlight specific miRNAs that can be manipulated to potentially manage the disease.
Functional Delivery of miRNA-Antagonizing Oligonucleotides to the Rat Parathyroid
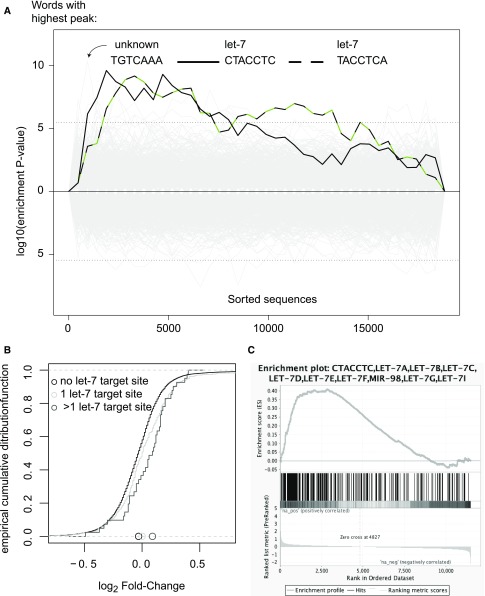
To study the effect of inhibiting specific miRNAs by antagonizing oligonucleotides, we first demonstrated the functional delivery of systemically injected anti-miRs to the rat parathyroid. We chose to antagonize the let-7 family, because let-7s are the most abundant miRNAs in parathyroids. Interestingly, individual let-7 members displayed significant alterations in rat SHP (Supplemental Figure 4), but the family at its entirety did not change (Supplemental Table 13, sf-rno-miR-98(8)). Thus, anti–let-7 or scrambled control oligonucleotides were injected to normal rats, twice weekly for 4 weeks. Gene expression changes were examined in pooled microdissected parathyroids at the end of the study period (Supplemental Table 18). Sylamer analysis (Figure 4A) and cumulative distribution function plots of mRNA fold-changes (Figure 4B) demonstrated distinct regulatory consequences of anti–let-7 oligonucleotides in parathyroids, confirming functional delivery. Ranked gene set enrichment analysis24 also confirmed that the let-7 anti-miRs engaged with their targets and functionally deactivated them (Figure 4C). Interestingly, the leading derepressed let-7 target in this analysis was Pbx2, an oncogenic transcription factor25 that has been shown to be targeted by let-7 in leukemia.26 Likewise, gene set enrichment analysis disclosed that let-7 antagonists brought upon a RIG-I–like receptor response, likely resulting from nonself RNA (despite the fact that the control group was treated with scrambled oligonucleotides of similar backbone chemistry—possibly due to concomitant inhibition of xenobiotic metabolism, see below). Rat mRNAs that were upregulated by let-7 antagonism were enriched with genes included in PPAR singling pathway (KEGG), ABC transporters (KEGG), cation homeostasis (GO-bp), G-protein signaling-adenylate cyclase activating pathway (GO-bp), actin filament organization (GO-bp), IFN response (hallmark gene sets), and TNF signaling via NF-kB (hallmark). mRNAs downregulated by let-7 antagonism were enriched with ribosome and spliceosome genes (KEGG), drug/xenobiotic metabolism by cytochrome P450 genes (KEGG), base excision repair genes (KEGG), myc targets (hallmark), unfolded protein response genes (hallmark), early and late estrogen response genes (hallmark), and mTORC1-upregulated genes (hallmark). mTORC1 activation has been shown to contribute to parathyroid cell proliferation.27 Estrogen has been shown to alter PTH production directly through parathyroid estrogen receptors,28 and possibly also indirectly.29
Figure 4.
Evidence for delivery and functional target engagement of anti–let-7 oligonucleotides in parathyroid. Pooled total RNA from normal rats injected with anti–let-7 or control oligonucleotides underwent mRNA array analysis (Affymetrix GeneChip Rat Genome 230 2.0 Arrays). The fold-change values of protein coding genes included in the array were used to detect derepression of let-7 targets. (A) Sylamer analysis shows that two out of three words with highest peak enrichment in 3′utr of upregulated genes are reverse-complementary to the let-7 seed. (B) Empirical cumulative distribution function plot of mRNA log2 fold-changes showing a shift to the right (upregulation) among miranda-predicted let-7 targets compared with nontarget mRNAs: 11,191 nontarget mRNAs; 339 single-targeted mRNAs (P value 7.8e−05); 41 multitargeted mRNAs (P value 0.003). (C) Gene set enrichment analysis shows striking enrichment of predicted let-7 targets among genes upregulated by let-7 antagonizing oligonucleotides. Putative target genes according to this analysis are labeled as such in Supplemental Table 18.
let-7 Antagonism Increases Serum PTH Levels in Control and CKD Rats
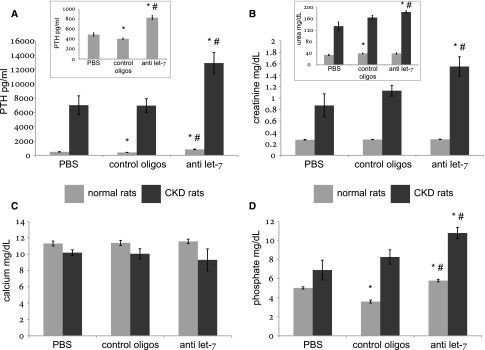
As seen above, let-7 is very abundant in parathyroids (Figure 1), and some of its members were deregulated in rat and human SHP (Supplemental Tables 11 and 14). To test let-7 involvement in SHP, we antagonized them in normal and CKD rats. Compared with scramble oligonucleotides, injection of anti–let-7 to both normal and CKD rats resulted after 4 weeks in nearly doubled serum PTH levels (Figure 5A); namely, it induced hyperparathyroidism in normal rats and aggravated hyperparathyroidism in uremic rats. Serum calcium levels were not affected by the experimental interventions (Figure 5C), whereas creatinine was increased by anti–let-7 oligonucleotides in CKD but not in normal rats (Figure 5B). Serum phosphate levels were moderately increased by the intervention (Figure 5D), denoting possible extraparathyroid effects of the antagonizing oligonucleotides. The higher levels of serum PTH were independent of phosphate, calcium, and creatinine according to multiple linear regression in anti–let-7–treated normal rats (P<0.05) but not in CKD rats (P=0.18). Nonetheless, an interim analysis after 2 weeks of CKD-inducing diet and anti-miR administration was consistent with a direct, mineral-independent effect of the anti–let-7 oligonucleotides on serum PTH levels in CKD (Supplemental Figure 5, Supplemental Table 19). In normal rats, increased PTH serum levels provoked by let-7 antagonists were not grossly associated with parathyroid cell proliferation (Supplemental Figure 6).
Figure 5.
Serum chemistry and PTH levels are altered in rats treated for 4 weeks with let-7 anti-miRs. Serum PTH (A), creatinine (B), urea (B; inset), calcium (C), and phosphate (D) levels in normal and CKD rats treated with let-7 anti-miRs, scrambled controls (“control oligos”), or PBS. *P<0.05 for the comparison with PBS-treated rats (within the same disease study group—normal/CKD); #P<0.05 for the comparison with control oligonucleotides-treated rats. Numbers of animals were six in each subgroup of normal rats and five in each subgroup of uremic rats.
let-7 Antagonism Increases PTH Secretion In Vitro
To confirm a direct (primary) effect of anti–let-7 oligonucleotides upon the parathyroid glands we performed an in vitro organ culture experiment. Normal mouse thyro-parathyroid organs and surrounding tissues were incubated in culture media containing scrambled oligonucleotides or anti–let-7 oligonucleotides. Anti–let-7s caused increased PTH accumulation at 1–5 hours (Figure 6), confirming a mineral-independent direct effect. Thus, let-7 antagonism increases PTH secretion in vivo and in vitro.
Figure 6.
PTH accumulation in vitro is augmented by let-7 anti-miRs. Mouse thyro-parathyroid blocks from normal mice were incubated in cell culture media in the presence of anti–let-7 oligonucleotides or scrambled controls (0.5 mg/ml), and medium was sampled for PTH ELISA at the indicated time points. *P<0.05 for the comparison with control oligonucleotides at the same time point.
Anti–miR-148, but Not Anti–miR-141, Oligonucleotides Decrease Serum PTH
Members of both miR-141 and miR-148 sequence families are abundant in human, rat, and mouse parathyroids and increase in SHP (Figure 3). To test whether manipulation of miR-141/148 activity influences the course of SHP, we antagonized these miRNAs in rats with CKD (6 weeks). Administration of anti–miR-141 did not consistently affect serum PTH or serum biochemistry compared with controls (Figure 7A). However, anti–miR-148 significantly decreased serum PTH in the uremic rats (Figure 7B) without altering serum creatinine or phosphate levels. Serum calcium levels were mildly increased by anti–miR-148, suggesting possible extraparathyroid effects of the antagonizing oligonucleotides.
Figure 7.
Serum chemistry and PTH levels are altered in rats treated with miR-141/miR-148 antagonists. Serum PTH (top left panel), creatinine (top right panel), calcium (bottom right panel), and phosphate (bottom left panel) levels in CKD rats treated for 4 weeks with the indicated miRNA antagonists or scrambled control oligonucleotides. *P<0.05 for the comparison with control oligonucleotides. An interim analysis at 2 weeks is presented in Supplemental Figure 5.
Anti–miR-148 Oligonucleotides Decrease PTH Secretion In Vitro
To determine if the repressing effect of anti–miR-148 oligonucleotides upon PTH is a direct effect on parathyroid glands, anti–miR-148s were studied in parathyroid organ cultures. Mice were fed 0.2% adenine high-phosphorus diet for 8 days to induce SHP.30 Subsequently, thyro-parathyroid organs were removed and cultured as above in media containing anti–miR-148 or control oligonucleotides for 21 hours. miR-148–antagonizing media led to a significant decrease in accumulated PTH compared with control throughout the incubation period (Figure 8), confirming a primary, mineral-independent effect on parathyroid tissue. Hence, antagonism of miR-148 decreases the high PTH levels accompanying CKD in vivo and in vitro.
Figure 8.
PTH accumulation in vitro is diminished by miR-148 antagonism. Mouse thyro-parathyroid blocks from CKD mice were incubated in cell culture media in the presence of anti–miR-148 oligonucleotides or scrambled controls (0.5 mg/ml), and medium was sampled for PTH ELISA at the indicated time points. Overall accumulation was significantly lower in the presence of miR-148 anti-miRs (P<0.05 by repeated measures ANOVA).
Discussion
Transcriptome-wide miRNA profiling in parathyroid glands has not been previously reported in SHP. To identify specific miRNA involvement, we first characterized the normal human, rat, and mouse parathyroid miRNome. We found that humans, rats, and mice share similar profiles of abundant miRNA, suggesting evolutionarily conserved regulation or functions in parathyroid physiology. We next studied miRNA expression in hyperplastic glands. Principal component analysis revealed that miRNA profiles from SHP rats are distinguishable from miRNA profiles of normal glands, and that each condition leading to SHP forms a distinct group, suggesting common patterns of SHP together with specific patterns dependent on the cause of SHP. Experimental SHP due to prolonged CKD displayed the most divergent profile. Differences observed in parathyroids from human patients with ESRD compared with normal parathyroid tissues, although reflecting a very advanced and chronic process, were in part similar to the alterations in the experimental rat models, e.g., increase in miR-21, miR-25, and miR-34 family members and decrease in miR-10, miR-134, and miR-138 members. However, we also noted opposing alteration (Supplemental Tables 13 and 17).
Analysis of differential expression revealed that several abundant miRNAs are consistently deregulated in SHP. Depletion of all miRNAs in PT-Dicer−/− mice led to failure of these mice to increase serum PTH and develop SHP.20 The abundant upregulated miRNA families (miR-21, miR-29, miR-30, miR-141, and miR-148) may thus contribute to the SHP phenotype. Predicted targets of these upregulated miRNAs are overrepresented in KEGG “pathways in cancer,” MAPK signaling, TGFβ signaling, and neurotrophin signaling. Predicted targets of abundant downregulated miRNA families (miR-10, miR-25, and miR-125) are also overrepresented in KEGG pathways MAPK signaling, neurotrophin signaling, and “pathways in cancer,” reinforcing possible connections between miRNA deregulation and the hyperplastic/hypersecreting parathyroid phenotype.
It is intriguing that SHP leads to dysregulation of a subset of miRNAs, regardless of whether it was provoked by different stages of uremia or hypocalcemia. This suggests shared miRNA-dependent pathways that induce SHP. Additionally, mice with Dicer1 deletion in the parathyroid had impaired increase in serum PTH and aberrant development of SHP induced by both hypocalcemia and uremia. These findings indicate that miRNAs are important for the development of SHP by both triggers and that an overlapping set of miRNAs contributes to over-activity of the parathyroid in SHP. SHP induced by both chronic hypocalcemia and uremia is characterized by increased serum PTH, PTH mRNA levels, and parathyroid cell proliferation.15,31 The increase in PTH mRNA levels was shown to be mediated by post-transcriptional mechanisms resulting in increased PTH mRNA stability.17,31,32 This increase in PTH mRNA stability is determined by the regulated binding of stabilizing AUF1 (AU rich binding factor 1) and destabilizing KSRP (K homology splicing regulatory protein), which is orchestrated by the peptidyl-prolyl cis-trans isomerase Pin1.19,33,34 miRNAs provide an additional post-transcriptional mechanism of regulation of PTH expression in hypocalcemia and uremia. Possible links between miRNAs and protein-RNA interactions in the parathyroid remain to be determined.
Of the six most abundant miRNA families in rat parathyroid, four families (miR-30, miR-148, miR-141, and miR-21) were significantly upregulated in SHP, whereas two (let-7 and miR-375) were nonsignificantly downregulated (the latter is upregulated in human ESRD; Supplemental Tables 13 and 16). The role of specific miRNAs in parathyroid physiology and in SHP has not been studied. To address this question, we used oligonucleotides that inhibit specific miRNA.9 Chemically modified anti-miR oligonucleotides injected subcutaneously accumulate in tissues, including liver and kidney.35 In our study, we showed that anti-miRs were functionally delivered to the parathyroid glands. Treatment of normal rats with anti–let-7 oligonucleotides increased serum PTH. The increase in serum PTH was not related to renal function, although there was a moderate increase in serum phosphate. The direct effect of let-7 inhibition on the parathyroid was shown in parathyroid organ cultures that avoid any systemic effects of the anti-miRs.36 let-7 anti-miRs added to the growth medium of mouse thyro-parathyroid organ cultures increased PTH secretion by the parathyroids as they did in vivo. These results demonstrate the direct effect of anti–let-7 oligonucleotides to increase PTH secretion.
Let-7 antagonism also increased serum PTH in CKD rats. Individual members of the let-7 family were disparately altered in SHP (Supplemental Figure 4, Supplemental Table 11). However, overall let-7 family levels did not change in SHP (Supplemental Table 13). Thus, maintenance of stable let-7 expression is obtained through opposing regulation of let-7 clusters, made possible by its complex genomic organization and post-transcriptional regulation.37 Our results, demonstrating increased serum PTH levels after inhibition of all let-7 family members, suggest that let-7s maintain serum PTH at normal physiologic concentrations by restraining PTH production or secretion. Proliferation was not grossly affected by let-7 antagonism, but increase in Pbx2 transcription factor levels may harbinger subsequent hyperplasia.
We have recently shown that the mTOR pathway is activated in the parathyroid glands of rats with SHP.27 In this study, let-7 antagonism brought about SHP concomitantly with downregulation of mTORC1-activated mRNA, pointing to effects downstream of mTORC1 activation. Estrogen may affect PTH indirectly29 or directly through estrogen receptors.28,38,39 We have shown that estrogen increases PTH gene expression in ovariectomized rats.28 In this study, mRNAs downregulated by let-7 antagonism were enriched with early and late estrogen response genes, providing another link to the mechanism of SHP exacerbation by let-7–antagonizing oligonucleotides.
We also inhibited miRNA sequence families that were increased in 8-week CKD rats: miR-141 and miR-148. Administration of anti–miR-141 led to an insignificant decrease in serum PTH compared with controls. However, anti–miR-148 led to significant decrease in serum PTH in the CKD rats. There was no change in serum creatinine or phosphate levels, but a mild increase in serum calcium in the anti–miR-148 group. The increase in serum calcium may contribute to the decreased PTH in the anti–miR-148–injected CKD rats. However, the decrease in serum PTH by anti–miR-148 also occurred in vitro in mouse parathyroid organ cultures. The organ culture experiments were performed with parathyroids from CKD mice to increase basal PTH secretion. Anti–miR-148 oligonucleotides decreased the levels of PTH in the medium compared with controls. Therefore, anti–miR-148s act on the parathyroid directly to decrease PTH levels. Interestingly, the miR-148 family has been linked with various neoplastic diseases,40 and inhibition of miR-148 was reported to downregulate insulin mRNA in pancreatic islet cells.41
Limitations of our study include the partial selection of miRNAs for investigation and partial mechanistic analysis of their implications in parathyroid function. Also, specific genes targeted by the identified key miRNA have not been explored, and these should be pursued for the betterment of our understanding of parathyroid molecular physiology in uremic and hypocalcemic disorders.
In summary, parathyroid miRNAs are crucial to the activation of parathyroid gland function at the levels of PTH secretion, gene expression, and parathyroid cell proliferation.20 Our studies demonstrate that SHP leads to dysregulation of many miRNAs supporting a key role for miRNAs in SHP of uremia. We have identified several specific miRNA families that are increased or decreased in SHP. Downregulation of two specific miRNAs affects PTH secretion in vivo in and in vitro. Our studies suggest that let-7 members restrict PTH secretion, whereas miR-148 members promote secretion. In CKD, the expression of parathyroid let-7 and the increase in miR-148 members may contribute to the development of SHP. Future studies may identify miRNAs as new therapeutic targets for the management of SHP, a common complication of CKD and a source of morbidity and mortality in these patients.
Concise Methods
Animal studies were performed on male Sprague Dawley rats and C57BL/6 mice. Rodents were fed a control or an adenine high-phosphorus diet for 1, 6, or 8 weeks to induce CKD-SHP.30,42,43 To induce chronic hypocalcemia, weanling rats were fed a calcium-deficient diet for 2 weeks.15,44 Human parathyroid paraffin sections from archival parathyroids removed at surgery from patients with ESRD and from patients with normal renal function removed incidentally during total thyroidectomy were received from the pathology department. Human parathyroid cells from parathyroids of patients with CKD and normal human glands, as well as mouse parathyroid glands, were captured from formalin-fixed deparaffinized sections. Small-RNA sequencing libraries were generated as previously published.6 All cited sequencing data has been deposited at the NCBI gene expression omnibus and can be accessed at: http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE79727. Specific 2'-O-MOE–modified anti-miR oligonucleotides were provided by Regulus Therapeutics.45 Anti-miRs were injected subcutaneously at 5 mg/kg twice weekly for 4 weeks. At the end of the experiments, serum was analyzed for biochemistry and PTH. Parathyroids were microdissected for RNA extraction. RNA pooled from parathyroids of five rats from each experimental group was used for mRNA profiling (GeneChip Rat Genome 230 2.0 arrays; Affymetrix). Examination of functional delivery of systemically injected anti–let-7 oligonucleotides to the parathyroid glands was established by Sylamer analysis of mRNA expression data46 and by empirical cumulative distribution function of the log fold-changes. For in vitro studies, mouse thyro-parathyroid and surrounding tissue blocks were maintained in culture as previously described,20,36 in the presence of anti-miR or control oligonucleotides (0.5 mg/ml). Serum and growth medium PTH levels were measured using mouse or rat intact PTH ELISA kits.
Statistical Analyses
Normalization of the profile data was done by calculating relative read frequency to derive actual abundance in each subsample. Analyses of RNA sequencing data were performed after batch correction using ComBat.47 Data reduction, differential expression, and statistical analyses were conducted with DESeq248 and additional packages in R software environment for statistical computing and graphics (https://www.r-project.org/). Analysis scripts can be provided to readers upon request.
Study Approval
All animal experiments were approved by the Hadassah Hebrew University Animal Care and Use Committee. Analysis of archived human paraffin-embedded parathyroid tissue was done in compliance with an exemption from the requirement for informed consent, given by the Hadassah Medical Center Helsinki Committee.
Disclosures
None.
Supplementary Material
Acknowledgments
Vivek Kaimal (Regulus Therapeutics) assisted with mRNA expression array analysis. miRNA antagonizing oligonucleotides were provided by Regulus Therapeutics.
The work was supported by the Israel Academy of Science (grant 358/12, T.N.-M.) and the Harold and Ethel Pupkewitz Research Fund (T.N.-M.). I.Z.B.-D. is supported in part by the Israeli Centers of Research Excellence (I-CORE) Program of the Planning and Budgeting Committee and the Israel Science Foundation (grant no. 41/11).
Parts of this work were presented as a poster abstract at the American Society of Nephrology meeting, Chicago, November 17, 2016.
V.S. conducted the animal and organ culture experiments, participated in data analysis, and drafted the manuscript; I.M.-Y.L. acquired human samples; R.A. and A.M. prepared small-RNA cDNA libraries for miRNA deep-sequencing; G.W. assisted with animal experiments; T.N.-M. and I.Z.B.-D. designed the research, supervised the experiments, analyzed the data, and revised the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2016050585/-/DCSupplemental.
References
- 1.Bartel DP: MicroRNAs: Target recognition and regulatory functions. Cell 136: 215–233, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedman RC, Farh KK, Burge CB, Bartel DP: Most mammalian mRNAs are conserved targets of microRNAs. Genome Res 19: 92–105, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farazi TA, Horlings HM, Ten Hoeve JJ, Mihailovic A, Halfwerk H, Morozov P, Brown M, Hafner M, Reyal F, van Kouwenhove M, Kreike B, Sie D, Hovestadt V, Wessels LFA, van de Vijver MJ, Tuschl T: MicroRNA sequence and expression analysis in breast tumors by deep sequencing. Cancer Res 71: 4443–4453, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Etheridge A, Lee I, Hood L, Galas D, Wang K: Extracellular microRNA: A new source of biomarkers. Mutat Res 717: 85–90, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng CJ, Saltzman WM, Slack FJ: Canonical and non-canonical barriers facing antimiR cancer therapeutics. Curr Med Chem 20: 3582–3593, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hafner M, Renwick N, Farazi TA, Mihailović A, Pena JTG, Tuschl T: Barcoded cDNA library preparation for small RNA profiling by next-generation sequencing. Methods 58: 164–170, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krützfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, Manoharan M, Stoffel M: Silencing of microRNAs in vivo with ‘antagomirs’. Nature 438: 685–689, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Davis S, Lollo B, Freier S, Esau C: Improved targeting of miRNA with antisense oligonucleotides. Nucleic Acids Res 34: 2294–2304, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esau CC: Inhibition of microRNA with antisense oligonucleotides. Methods 44: 55–60, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Davis S, Propp S, Freier SM, Jones LE, Serra MJ, Kinberger G, Bhat B, Swayze EE, Bennett CF, Esau C: Potent inhibition of microRNA in vivo without degradation. Nucleic Acids Res 37: 70–77, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Esau C, Davis S, Murray SF, Yu XX, Pandey SK, Pear M, Watts L, Booten SL, Graham M, McKay R, Subramaniam A, Propp S, Lollo BA, Freier S, Bennett CF, Bhanot S, Monia BP: miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab 3: 87–98, 2006 [DOI] [PubMed] [Google Scholar]
- 12.McGlinn E, Yekta S, Mansfield JH, Soutschek J, Bartel DP, Tabin CJ: In ovo application of antagomiRs indicates a role for miR-196 in patterning the chick axial skeleton through Hox gene regulation. Proc Natl Acad Sci USA 106: 18610–18615, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park JK, Lee EJ, Esau C, Schmittgen TD: Antisense inhibition of microRNA-21 or -221 arrests cell cycle, induces apoptosis, and sensitizes the effects of gemcitabine in pancreatic adenocarcinoma. Pancreas 38: e190–e199, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Chau BN, Xin C, Hartner J, Ren S, Castano AP, Linn G, Li J, Tran PT, Kaimal V, Huang X, Chang AN, Li S, Kalra A, Grafals M, Portilla D, MacKenna DA, Orkin SH, Duffield JS: MicroRNA-21 promotes fibrosis of the kidney by silencing metabolic pathways. Sci Transl Med 4: 121ra18, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naveh-Many T, Rahamimov R, Livni N, Silver J: Parathyroid cell proliferation in normal and chronic renal failure rats. The effects of calcium, phosphate, and vitamin D. J Clin Invest 96: 1786–1793, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silver J, Naveh-Many T, Kronenberg HM: Parathyroid hormone: molecular biology. In: Principles of Bone Biology, edited by Bilezikian JB, Raisz LG, Rodan GA, San Diego, Academic Press, 2002, pp 407–422 [Google Scholar]
- 17.Kilav R, Silver J, Naveh-Many T: A conserved cis-acting element in the parathyroid hormone 3′-untranslated region is sufficient for regulation of RNA stability by calcium and phosphate. J Biol Chem 276: 8727–8733, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Naveh-Many T, Nechama M: Regulation of parathyroid hormone mRNA stability by calcium, phosphate and uremia. Curr Opin Nephrol Hypertens 16: 305–310, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Nechama M, Uchida T, Mor Yosef-Levi I, Silver J, Naveh-Many T: The peptidyl-prolyl isomerase Pin1 determines parathyroid hormone mRNA levels and stability in rat models of secondary hyperparathyroidism. J Clin Invest 119: 3102–3114, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shilo V, Ben-Dov IZ, Nechama M, Silver J, Naveh-Many T: Parathyroid-specific deletion of dicer-dependent microRNAs abrogates the response of the parathyroid to acute and chronic hypocalcemia and uremia. FASEB J 29: 3964–3976, 2015 [DOI] [PubMed] [Google Scholar]
- 21.Rahbari R, Holloway AK, He M, Khanafshar E, Clark OH, Kebebew E: Identification of differentially expressed microRNA in parathyroid tumors. Ann Surg Oncol 18: 1158–1165, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corbetta S, Vaira V, Guarnieri V, Scillitani A, Eller-Vainicher C, Ferrero S, Vicentini L, Chiodini I, Bisceglia M, Beck-Peccoz P, Bosari S, Spada A: Differential expression of microRNAs in human parathyroid carcinomas compared with normal parathyroid tissue. Endocr Relat Cancer 17: 135–146, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Verdelli C, Forno I, Vaira V, Corbetta S: Epigenetic alterations in human parathyroid tumors. Endocrine 49: 324–332, 2015 [DOI] [PubMed] [Google Scholar]
- 24.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP: Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 102: 15545–15550, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allen TD, Zhu YX, Hawley TS, Hawley RG: TALE homeoproteins as HOX11-interacting partners in T-cell leukemia. Leuk Lymphoma 39: 241–256, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Pelosi A, Careccia S, Lulli V, Romania P, Marziali G, Testa U, Lavorgna S, Lo-Coco F, Petti MC, Calabretta B, Levrero M, Piaggio G, Rizzo MG: miRNA let-7c promotes granulocytic differentiation in acute myeloid leukemia. Oncogene 32: 3648–3654, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Volovelsky O, Cohen G, Kenig A, Wasserman G, Dreazen A, Meyuhas O, Silver J, Naveh-Many T: Phosphorylation of ribosomal protein S6 mediates mammalian target of rapamycin complex 1-induced parathyroid cell proliferation in secondary hyperparathyroidism. J Am Soc Nephrol 27: 1091–1101, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naveh-Many T, Almogi G, Livni N, Silver J: Estrogen receptors and biologic response in rat parathyroid tissue and C cells. J Clin Invest 90: 2434–2438, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carrillo-López N, Román-García P, Rodríguez-Rebollar A, Fernández-Martín JL, Naves-Díaz M, Cannata-Andía JB: Indirect regulation of PTH by estrogens may require FGF23. J Am Soc Nephrol 20: 2009–2017, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jia T, Olauson H, Lindberg K, Amin R, Edvardsson K, Lindholm B, Andersson G, Wernerson A, Sabbagh Y, Schiavi S, Larsson TE: A novel model of adenine-induced tubulointerstitial nephropathy in mice. BMC Nephrol 14: 116, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moallem E, Kilav R, Silver J, Naveh-Many T: RNA-Protein binding and post-transcriptional regulation of parathyroid hormone gene expression by calcium and phosphate. J Biol Chem 273: 5253–5259, 1998 [DOI] [PubMed] [Google Scholar]
- 32.Nechama M, Ben-Dov IZ, Briata P, Gherzi R, Naveh-Many T: The mRNA decay promoting factor K-homology splicing regulator protein post-transcriptionally determines parathyroid hormone mRNA levels. FASEB J 22: 3458–3468, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Sela-Brown A, Silver J, Brewer G, Naveh-Many T: Identification of AUF1 as a parathyroid hormone mRNA 3′-untranslated region-binding protein that determines parathyroid hormone mRNA stability. J Biol Chem 275: 7424–7429, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Nechama M, Peng Y, Bell O, Briata P, Gherzi R, Schoenberg DR, Naveh-Many T: KSRP-PMR1-exosome association determines parathyroid hormone mRNA levels and stability in transfected cells. BMC Cell Biol 10: 70–81, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seth PP, Siwkowski A, Allerson CR, Vasquez G, Lee S, Prakash TP, Wancewicz EV, Witchell D, Swayze EE: Short antisense oligonucleotides with novel 2′-4′ conformationaly restricted nucleoside analogues show improved potency without increased toxicity in animals. J Med Chem 52: 10–13, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Galitzer H, Ben-Dov IZ, Silver J, Naveh-Many T: Parathyroid cell resistance to fibroblast growth factor 23 in secondary hyperparathyroidism of chronic kidney disease. Kidney Int 77: 211–218, 2010 [DOI] [PubMed] [Google Scholar]
- 37.Roush S, Slack FJ: The let-7 family of microRNAs. Trends Cell Biol 18: 505–516, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Epstein E, Silver J, Almogi G, Livni N, Naveh-Many T: Parathyroid hormone mRNA levels are increased by progestins and vary during rat estrous cycle. Am J Physiol 270: E158–E163, 1996 [DOI] [PubMed] [Google Scholar]
- 39.Haglund F, Rosin G, Nilsson IL, Juhlin CC, Pernow Y, Norenstedt S, Dinets A, Larsson C, Hartman J, Höög A: Tumour nuclear oestrogen receptor beta 1 correlates inversely with parathyroid tumour weight. Endocr Connect 4: 76–85, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen Y, Song YX, Wang ZN: The microRNA-148/152 family: Multi-faceted players. Mol Cancer 12: 43, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Melkman-Zehavi T, Oren R, Kredo-Russo S, Shapira T, Mandelbaum AD, Rivkin N, Nir T, Lennox KA, Behlke MA, Dor Y, Hornstein E: miRNAs control insulin content in pancreatic β-cells via downregulation of transcriptional repressors. EMBO J 30: 835–845, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yokozawa T, Oura H, Okada T: Metabolic effects of dietary purine in rats. J Nutr Sci Vitaminol (Tokyo) 28: 519–526, 1982 [DOI] [PubMed] [Google Scholar]
- 43.Levi R, Ben-Dov IZ, Lavi-Moshayoff V, Dinur M, Martin D, Naveh-Many T, Silver J: Increased parathyroid hormone gene expression in secondary hyperparathyroidism of experimental uremia is reversed by calcimimetics: Correlation with posttranslational modification of the trans acting factor AUF1. J Am Soc Nephrol 17: 107–112, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Naveh-Many T, Silver J: Regulation of parathyroid hormone gene expression by hypocalcemia, hypercalcemia, and vitamin D in the rat. J Clin Invest 86: 1313–1319, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hogan DJ, Vincent TM, Fish S, Marcusson EG, Bhat B, Chau BN, Zisoulis DG: Anti-miRs competitively inhibit microRNAs in Argonaute complexes. PLoS One 9: e100951, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Dongen S, Abreu-Goodger C, Enright AJ: Detecting microRNA binding and siRNA off-target effects from expression data. Nat Methods 5: 1023–1025, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leek JT, Johnson WE, Parker HS, Jaffe AE, Storey JD: The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics 28: 882–883, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Love MI, Huber W, Anders S: Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15: 550, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.