Abstract
The kinin system is activated during vasculitis and may contribute to chronic inflammation. C1-inhibitor is the main inhibitor of the kinin system. In this study, we investigated the presence of the kinin B1 receptor on endothelial microvesicles and its contribution to the inflammatory process. Compared with controls (n=15), patients with acute vasculitis (n=12) had markedly higher levels of circulating endothelial microvesicles, identified by flow cytometry analysis, and significantly more microvesicles that were positive for the kinin B1 receptor (P<0.001). Compared with microvesicles from wild-type cells, B1 receptor-positive microvesicles derived from transfected human embryonic kidney cells induced a significant neutrophil chemotactic effect, and a B1 receptor antagonist blocked this effect. Likewise, patient plasma induced neutrophil chemotaxis, an effect decreased by reduction of microvesicle levels and by blocking the B1 receptor. We used a perfusion system to study the effect of patient plasma (n=6) and control plasma (n=6) on the release of microvesicles from glomerular endothelial cells. Patient samples induced the release of significantly more B1 receptor-positive endothelial microvesicles than control samples, an effect abrogated by reduction of the microvesicles in the perfused samples. Perfusion of C1-inhibitor–depleted plasma over glomerular endothelial cells promoted excessive release of B1 receptor-positive endothelial microvesicles compared with normal plasma, an effect significantly decreased by addition of C1-inhibitor or B1 receptor-antagonist. Thus, B1 receptor-positive endothelial microvesicles may contribute to chronic inflammation by inducing neutrophil chemotaxis, and the reduction of these microvesicles by C1-inhibitor should be explored as a potential treatment for neutrophil-induced inflammation.
Keywords: vasculitis, complement, Chronic inflammation, glomerular endothelial cells, neutrophils, kinin
Activation of the kinin/contact system is proinflammatory, inducing pain and capillary leakage symptomatic of severe vascular inflammation.1 The kinin system consists of a cascade of proteins ultimately leading to the release of bradykinin from high molecular weight kininogen.1 In addition to bradykinin, other kinins are released during inflammation, including a 13-amino acid peptide termed proteinase 3-kinin (PR3-kinin) cleaved by neutrophil-derived proteinase 3 (PR3) and thus presumably active during conditions associated with neutrophil influx, such as the chronic vascular inflammatory condition vasculitis.2 Bradykinin binds to the constitutively expressed B2-receptor, whereas des-arg9-bradykinin and PR3-kinin bind to the B1-receptor (B1R), expressed primarily during chronic inflammation.2,3 The major inhibitor of the kinin system is C1-inhibitor.1
The kinin system may be assembled and activated on endothelial cells and neutrophils,4 as well as on blood cell-derived microvesicles (MVs)5 with a proinflammatory effect. MVs are vesicular membrane blebs (0.1–1.0 μm in diameter) shed by cells during activation, injury and/or apoptosis that contain proteins, RNA, histones, and other cellular components from the parent cell.6–8 MVs can be found in the plasma of healthy individuals but are increased during inflammatory conditions.9 MVs can promote inflammation10 and thrombosis11 and thus prolong the inflammatory process. This may be achieved by membrane exposure of, or release of, proinflammatory cytokines, chemokines, selectins, adhesion molecules, and complement factors7,12 as well as phosphatidylserine, which may activate coagulation factors, and tissue factor (predominantly on monocytic MVs),13 thus promoting thrombosis.11 MVs may also transfer the kinin B1R.14 In the circulation, MVs may interact with cells by receptor binding, membrane fusion or endocytosis/engulfment of the entire MV, as reviewed by Meckes and Raab-Traub.15 Thus, MVs may facilitate intercellular communication.
Endothelial cells also react to stress by shedding MVs (termed endothelial cell-derived microvesicles; EMVs).16 EMVs are increased in various conditions associated with vascular damage, such as diabetes and hypertension.17 Circulating EMVs as well as platelets and leukocytes have been detected systemically in patients with vasculitis.9,14,18–21 Vasculitis is a disease characterized by inflammation in and around vessel walls affecting multiple organs, including the kidneys, skin, joints, intestines, and lungs. The most common vasculitis in childhood is Henoch–Schönlein purpura (HSP), whereas adults are more frequently affected by ANCA-associated vasculitis including granulomatosis with polyangiitis, microscopic polyangiitis, and eosinophilic granulomatosis with polyangiitis.22 The kinin system has been shown to be activated during vasculitis.2,23 Levels of MVs have correlated to disease activity as assessed by the Birmingham vasculitis activity score (BVAS),18,24 thus serving as disease biomarkers.
The presence of B1R on EMVs would reflect a phenomenon occurring on the endothelial cell during the inflammatory process and may also exacerbate the condition. The aim of this study was to investigate if B1R is present on EMVs shed during vasculitis and to study the contribution of B1R on EMVs to the inflammatory process, specifically neutrophil chemotaxis. Furthermore, the study aimed to explore if C1-inhibitor could regulate the release of B1R-positive EMVs from glomerular endothelial cells.
Results
EMVs in Vasculitis Plasma Are B1R-Positive
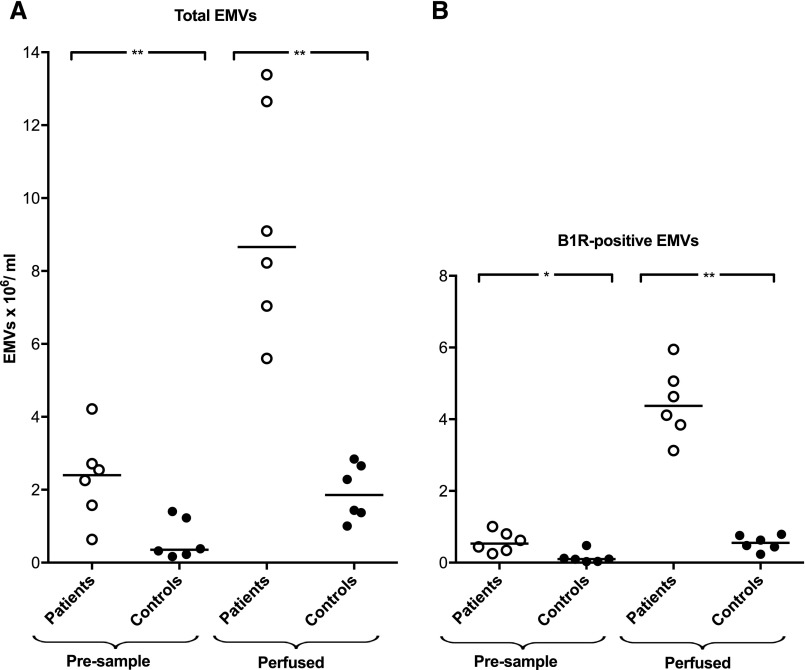
Plasma from patients with vasculitis (n=12) and healthy controls (n=15) were analyzed by flow cytometry for the presence of EMVs, defined as positive for CD105 and/or CD144. Patient plasma exhibited significantly more EMVs than controls. Furthermore, a significantly higher amount of EMVs were B1R-positive compared with controls (Figure 1). Of the total number of patient EMVs, a median of 41% (range, 8%–69%) were B1R-positive compared with 13% (range, 0%–49%) in controls. No correlation was found between B1R-positive EMV levels and variables such as specific diagnosis, presence/absence of immunotherapy, creatinine levels, or BVAS (Table 1). Patient EMVs were also found to be IL-8–positive, with a median of 86% (range, 51%–97%; n=5). Most patient EMVs that were B1R-positive were also IL-8–positive, with a median of 92% (range, 86%–96%). Fewer control EMVs were IL-8–positive, with a median of 46% (range, 6%–65%; n=5, data not shown).
Figure 1.
EMVs in vasculitis plasma express B1R. Plasma samples from patients with vasculitis (n=12, patients 1–7 and 9–13 in Table 1) and healthy controls (n=15) exhibited significantly higher levels of EMVs, positive for CD105 and/or CD144. Circulating EMVs were B1R-positive and samples from patients with vasculitis had significantly higher levels of B1R compared with controls. Samples were run using a BD FACSCanto Cytometer. ***P<0.001.
Table 1.
Description of patients included in this study
| Patient No. | Sex | Age at Sampling | Diagnosis | ANCA | Clinical Findings | Creatinine,a μmol/L | BP at Admission, mmHg | BVAS Score | Blood Sample Taken Before/After Start of Immunosuppressive Therapy |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 43 | HSP | — | Purpura, nephritis, arthritis | 89 | 170/100 | 15 | B |
| 2 | M | 16 | HSP | — | Purpura, arthritis | 69 | 122/70 | 3 | B |
| 3b | M | 80 | MPA | MPO | Rash, nephritis | 259 | 168/72 | 23 | B |
| 4 | M | 62 | GPA | PR3 | URT symptoms, pulmonary granuloma, temporal arteritis | 61 | 130/90 | 6 | B |
| 5b | F | 19 | SLE | — | Rash, myalgia, scleritis, nephritis | 62 | 110/60 | 17 | A |
| 6b | F | 76 | GPA | PR3 | Gingivitis, rhinitis, hematuria | 74 | 130/70 | 14 | B |
| 7b | F | 12 | GPA | PR3 MPO | Fever, arthralgia, nephritis | 312 | 120/70 | 15 | A |
| 8b | F | 61 | GPA | MPO | Polychondritis, cough, rhinitis, nephritis | 54 | 150/80 | 13 | B |
| 9 | M | 10 | HSP | — | Purpura, arthritis, nephritis | 47 | 102/60 | 7 | B |
| 10 | F | 51 | GPA | PR3 | Hemoptysis, cough, nephritis | 132 | 164/96 | 8 | A |
| 11 | M | 73 | MPA | MPO | Hearing loss, nephritis | 173 | 155/93 | 15 | A |
| 12 | F | 68 | GPA | PR3 | Myalgia, arthralgia, nephritis | 86 | 136/79 | 13 | A |
| 13 | F | 67 | MPA | MPO | Myalgia, confusion, nephritis | 148 | 130/60 | 22 | B |
| 14 | F | 9 | HSP | — | Purpura, myalgia, nephritis, arthritis | 51 | 110/62 | 18 | B |
| 15 | M | 13 | GPA | PR3 | Dyspnea, pulmonary infiltrates, arthralgia, nephritis | 84 | 120/70 | 24 | B |
| 16 | F | 17 | GPA | MPO | Dyspnea, myalgia, arthralgia, gastrointestinal symptoms, nephritis | 86 | 115/75 | 26 | B |
| 17 | F | 13 | GPA | PR3 | Myalgia, pansinusitis, exophthalmos, pulmonary infiltrates | 48 | 110/75 | 17 | B |
M, male; —, no ANCA detected; B, before; MPA, microscopic polyangiitis; MPO, myeloperoxidase; GPA, granulomatosis with polyangiitis; URT, upper respiratory tract; F, female; A, after.
Normal creatinine values of 45–90 μmol/L for women and 60–105 μmol/L for men.
MVs with B1R Induce Neutrophil Migration
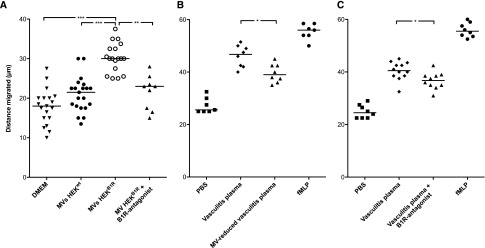
Experiments were designed to investigate if the presence of B1R on MVs induced neutrophil chemotaxis. Neutrophils migrated toward MVs derived from human embryonic kidney (HEK) cells transfected with B1R (HEKB1R cells) or MVs derived from wild-type cells (HEKwt cells; 70 μg/ml MVs). Enhanced migration was demonstrated when MVs derived from HEKB1R cells were placed in the lower compartment compared with MVs derived from HEKwt cells (Figure 2A). Neutrophil migration toward MVs derived from HEKB1R cells was significantly reduced by preincubation with the B1R-antagonist R715. Neutrophil migration toward MVs derived from HEKwt cells was comparable with MVs derived from HEKB1R cells preincubated with the R715 B1R-antagonist.
Figure 2.
Neutrophils migrate toward B1R-expressing MVs. Isolated neutrophils from healthy donors migrated through a membrane filter in a Boyden chamber assay. The y-axis represents the distance of neutrophil migration. (A) HEKwt and HEKB1R cell-derived MVs (70 µg/ml) and DMEM (negative control) were placed in the lower compartment. More neutrophil migration was induced by MVs from HEKB1R compared with HEKwt and HEKB1R preincubated with the B1R-antagonist R715. (B) Purified neutrophils migrated toward plasma from patients with vasculitis as well as toward the same samples with reduced MV content (median 7.5% of the original EMVs). Neutrophil migration was decreased in MV-reduced vasculitis plasma. (C) Purified neutrophils migrated toward plasma from patients with vasculitis preincubated with or without the B1R-antagonist R715. Neutrophil migration was decreased in samples preincubated with the B1R-antagonist. Experiments in (B and C) were carried out at separate occasions. DMEM and PBS were used as the negative controls. N-formyl-met-leu-phe (fMLP) was used as the positive control. ***P<0.001; **P<0.01; *P<0.05.
Patient-Derived B1R-Positive MVs Are Chemotactic for Neutrophils
Plasma from patients with vasculitis induced neutrophil migration (Figure 2B). The chemotactic effect was lowered significantly by reduction of the amount of MVs (Figure 2B). Likewise, addition of the B1R-antagonist decreased neutrophil migration (Figure 2C). Addition of anti-human CXCL8/IL-8 further reduced chemotaxis (35.7 μm; range, 31–42.5, data not shown). This implies that plasma from patients with vasculitis contains MVs with chemotactic properties, indicated by the presence of B1Rs, as well as other chemotactic substances, such as IL-8.
Release of B1R-Positive EMVs from Primary Glomerular Endothelial Cells
The release of B1R-positive EMVs was analyzed after perfusion of plasma from patients with vasculitis (n=6; ANCA-positive vasculitis n=4, patients 3, 4, 7, and 15; HSP n=2, patients 2 and 14; Table 1) and controls (n=6) over primary glomerular endothelial cells (PGECs) using a microfluidic perfusion system. Patient preperfusion samples exhibited higher amounts of circulating EMVs positive for B1R compared with controls. Perfusion of plasma over PGECs led to release of B1R-positive EMVs. Significantly more EMVs were released from PGECs when perfused with patient plasma compared with controls, and these EMVs were B1R-positive (Figure 3). No marked difference in PGEC response was noted when using samples from patients with ANCA-positive vasculitis or patients with HSP. Samples from two patients with ANCA-positive vasculitis (patients 7 and 15) were compared with the same samples after IgG depletion by protein-G to remove ANCA. Removal of IgG did affect the release of EMVs in the perfusion system (data not shown), suggesting that ANCA did not contribute to EMV release.
Figure 3.
B1R is found on EMVs released after perfusion of PGECs. (A) Plasma samples from patients with vasculitis (n=6) and healthy controls (n=6) perfused over PGECs at a shear stress of 2 dynes/cm2 for 15 minutes induced the release of EMVs (CD144-positive and CD105-positive MVs). Patient plasma samples contained more EMVs than control samples before perfusion (presample). Perfused plasma samples from patients with vasculitis exhibited significantly higher levels of EMVs than controls. (B) Patient samples contained more B1R-positive EMVs before perfusion (presample). Perfused samples from patients with vasculitis had significantly higher levels of B1R-positive EMVs compared with controls (the lowest value of B1R-positive EMVs in control plasma was 0.03×106/ml). Statistical comparisons were carried out between patients and controls, but not between presamples and perfused samples. Samples were analyzed using a BD FACSCanto Cytometer. **P<0.01; *P<0.05.
Plasma from patients with vasculitis in which MV levels were reduced (7.5% of the original quantity of EMVs) did not exhibit an increase in the number of EMVs after perfusion over PGECs (Supplemental Figure 1A). Likewise, an increase of B1R-positive EMVs was not registered when the vasculitis plasma with reduced MVs was perfused over the cells (Supplemental Figure 1B).
C1-Inhibitor and B1R-Antagonist Reduced Release of B1R-Positive EMVs from PGECs
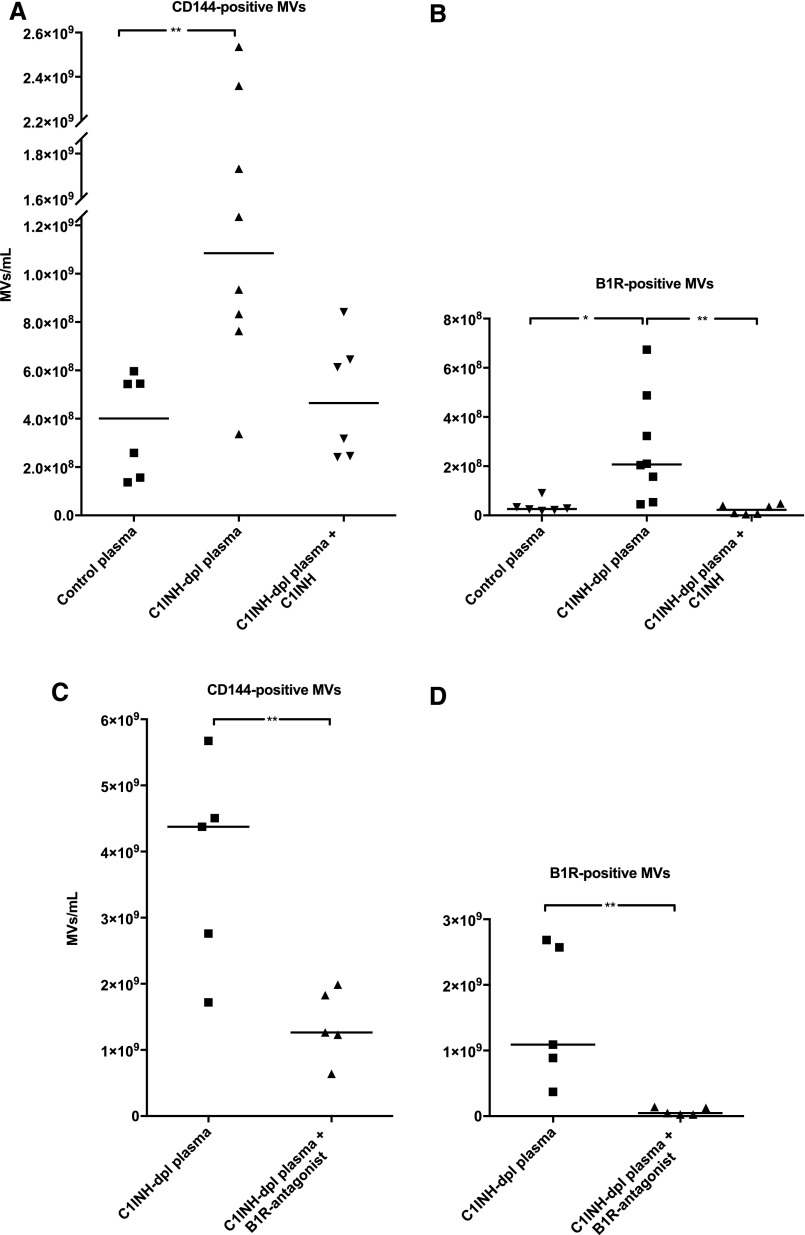
C1-inhibitor–depleted plasma exhibited lower total EMVs (CD144-positive MVs) and B1R-positive EMVs before perfusion (median EMVs, 90; range, 38–250×106/ml; B1R-positive EMVs median, 8; range, 1–24×106/ml) in comparison to normal plasma (median EMVs, 330; range, 80–536×106/ml; B1R-positive EMVs median, 53; range, 8–76×106/ml). After perfusion, EMV release was higher in the C1-inhibitor–depleted plasma compared with normal plasma (Figure 4A). C1-inhibitor–depleted plasma with added C1-inhibitor (Berinert) exhibited lower EMV release that did not reach statistical significance when compared with C1-inhibitor–depleted plasma and control plasma (Figure 4A). Perfused C1-inhibitor–depleted plasma also exhibited significantly elevated B1R-positive EMVs that were reduced in the presence of C1-inhibitor (Figure 4B), suggesting that the lack of C1-inhibitor triggered kinin system activation on PGECs and EMVs. Kinin system activation was confirmed by measuring kinin levels in C1-inhibitor–depleted plasma perfused over PGECs showing a higher level in the absence of C1-inhibitor (227.2 pg/ml) compared with a sample with added C1-inhibitor (20.6 pg/ml). Likewise, addition of the B1R-antagonist (R715) to C1-inhibitor–depleted plasma reduced total EMV release and B1R-positive EMVs (Figure 4, C and D, respectively). Taken together, these results suggest that C1-inhibitor and B1R-antagonist reduced the total number of EMVs as well as levels of B1R-positive EMVs.
Figure 4.
C1-inhibitor and B1R-antagonist reduce release of B1R-positive EMVs after perfusion of PGECs. (A) Perfusion of normal plasma, C1-inhibitor–depleted plasma, and C1-inhibitor–depleted plasma complemented with C1-inhibitor, over PGECs at a shear stress of 5 dynes/cm2 for 5 minutes induced the release of EMVs (CD144-positive). C1-inhibitor–depleted plasma showed higher levels of total EMVs compared with control plasma. Complementing the C1-inhibitor–depleted plasma with C1-inhibitor reduced the total number of EMVs. (B) Cells perfused with C1-inhibitor–depleted plasma exhibited significantly higher levels of B1R-positive EMVs than control plasma. Addition of C1-inhibitor significantly reduced the number of B1R-positive EMVs. (C) The amounts of total EMVs released from PGECs perfused with C1-inhibitor–depleted plasma to which the B1R-antagonist R715 was added were significantly reduced compared with cells perfused with C1-inhibitor–depleted plasma. (D) PGECs exposed to C1-inhibitor–depleted plasma in the presence of the B1R-antagonist exhibited significantly lower levels of B1R-positive EMVs. Samples were run using a CyFlow Cube 8 flow cytometer. **P<0.01; *P<0.05. C1INH, C1-inhibitor; C1INH-dpl plasma, C1-inhibitor–depleted plasma.
B1R-Positive EMVs Are Chemotactic for Neutrophils
C1-inhibitor–depleted plasma perfused over PGECs and containing B1R-positive EMVs (Figure 4) exhibited chemotactic activity toward neutrophils. This activity was specific for B1R because it was significantly reduced in the presence of the B1R-antagonist R715 (Figure 5).
Figure 5.
Neutrophils migrate toward B1R-positive endothelial MVs. Purified neutrophils, from healthy donors, migrated through a filter in a Boyden chamber toward a chemoattractant. The y-axis represents the distance of neutrophil migration. PBS was used as the negative control. C1-inhibitor–depleted plasma (preincubated with or without the B1R-antagonist R715) derived from perfusion experiments over PGECs was used as the chemoattractant. Migration of neutrophils was decreased in plasma incubated with the B1R-antagonist R715. *P<0.05. C1INH-dpl plasma, C1-inhibitor–depleted plasma.
Discussion
MVs have been shown to be important mediators of cell-to-cell communication and they also reflect the state of activation of the parent cell from which they were shed. Here, we show that EMVs carry kinin receptors during chronic vascular inflammation, and that the presence of B1R on MVs induced neutrophil chemotaxis. Thus, MVs actively promote and sustain the inflammatory state. B1R-positive EMVs were observed in patients with vasculitis and reproduced in an in vitro perfusion system using patient plasma as well as C1-inhibitor–depleted plasma. Importantly, both C1-inhibitor and a B1R-antagonist inhibited release of B1R-positive EMVs by blocking activation of the kinin system on the endothelium. These results suggest that C1-inhibitor can block the release of chemotactic EMVs during vascular inflammation.
C1-inhibitor inhibits plasma kallikrein, thereby preventing release of kinins.25,26 Patients with vasculitis have elevated levels of kinins, both systemically and locally in the kidneys.2,23 High levels of bradykinin are also found in patients with hereditary angioedema because of mutations in C1-inhibitor or neutralizing autoantibodies.27,28 We have previously shown that patients with vasculitis have normal C1-inhibitor levels.23 Moreover, genetic sequencing of the C1-inhibitor reactive site did not show alterations.23 Thus patients with vasculitis most probably have circulating C1-inhibitor capable of reducing the release of B1R-positive EMVs. Kinin system activation may, however, overwhelm the inhibitor and thus levels of C1-inhibitor, although normal, may not suffice to inhibit excessive release of chemotactic B1R-positive EMVs. The results presented here suggest that treatment with C1-inhibitor may, to a certain extent, prevent neutrophil influx at inflammatory sites.
The kinin system is activated in both children and adults with vasculitis.2,23,29 The components of the kinin system play a role in the intrinsic coagulation pathway as well as in the induction of inflammation.1 During this process there is a local reduction of BP, promotion of inflammation and pain, inhibition of platelet aggregation, induction of fibrinolysis, and capillary leakage.3 B1R, expressed during chronic inflammation, binds des-arg9-bradykinin and des-arg10-kallidin, as well as PR3-kinin2,3 and the receptor remains on the plasma membrane after ligand binding. Thus, the presence of the B1R on the surface of cells suggests that the ligand is bound, as the receptor is internalized under resting conditions, in the absence of signaling.3 Ligand binding activates endothelial formation of nitric oxide30 and partakes in the induction of pain.31,32 EMVs most probably do not possess the cellular machinery required for internalization of the B1R, and would thus be expected to continuously express the receptor.
B1R stimulation leads to neutrophil migration, as shown in both in vivo and in vitro models.32–34 Our results strengthen these findings, showing that even circulating B1R-positive MVs induce chemotaxis, and reduction of MV levels in plasma of patients with vasculitis or blockade of the B1R in the plasma reduced chemotaxis. Importantly, the B1R was demonstrated in kidneys from patients with GN, including vasculitis, and a B1R-antagonist ameliorated the inflammatory response in a mouse model of GN.35 Thus, the presence of B1R on EMVs suggests that it is expressed on these cells during vasculitis. Perfusion experiments demonstrated that patient plasma induced release of B1R-positive EMVs from PGECs, which would be expected to be a major source of EMVs in vasculitis as glomerular injury is predominant. The results suggest that B1R-positive EMVs could induce neutrophil chemotaxis at sites of inflammation.
Endothelial cell injury is a major manifestation of vasculitis in general, and circulating endothelial cells have been detected, indicating vascular wall injury.20,36 EMVs are elevated during vasculitis18,19 and decrease upon treatment,20 suggesting that they are adequate biomarkers of endothelial injury during vasculitis. Circulating EMVs in patients with vasculitis were shown to be E-selectin positive.9 Furthermore, levels of EMVs were higher in pediatric patients with vasculitis that developed thromboembolic events.21 In this study, high levels of EMVs could not be correlated to patient diagnosis, BVAS, renal function, or immunosuppressive treatment, probably because of the limited number of patients and their heterogeneity. Nevertheless, reducing MV levels in vasculitis plasma decreased EMV release from PGECs, and a novel function of EMVs contributing to the local inflammation was demonstrated. Both B1R and IL-8, demonstrated on EMVs, were found to be chemotactic. Blockade of B1R reduced the chemotactic potential of EMVs, an effect further enhanced, albeit minimally, by additional blockade of IL-8 on the EMVs, showing that EMVs have potent inflammatory properties. The chemotactic potential of B1R-positive MVs was not specific for EMVs, as even MVs derived from HEKB1R cells induced neutrophil migration. Although not addressed here, we speculate that even other MVs bearing B1R, such as neutrophil-derived MVs,14 could induce a similar chemotactic and proinflammatory effect.
The factor/s inducing kinin system activation and EMV release during vasculitis are most probably numerous. EMVs have been associated with vascular injury and metabolic derangements16,18–20 and may in themselves be injurious to endothelial cells.37 MV release may be beneficial for the parent cell as a means to rid the cell of unwanted components,38 and general abrogation of MV release may thus not be favorable. C1-inhibitor reduced the total release of EMVs and, more specifically, those that were B1R-positive. As B1R-positive MVs induced neutrophil chemotaxis, a major feature of inflammation, the effect of C1-inhibitor on B1R-positive EMV release could be beneficial. We therefore suggest that C1-inhibitor should be explored as a treatment for neutrophil-associated vascular inflammation.
Concise Methods
Patients and Controls
Blood samples were available from 17 patients (ten females and seven males, from children and adults; median age, 43 years) with vasculitis treated at the Department of Nephrology and the Department of Pediatrics, Section of Pediatric Nephrology, Skåne University Hospital, Lund and Malmö, Sweden. Details regarding patients’ age, sex, clinical and laboratory findings, diagnosis, and BP are given in Table 1. None of the patients were on hemodialysis at, or before, the time of sampling. Samples were obtained at onset or during the acute phase of disease and used for flow cytometry analysis and perfusion assays. Disease activity was assessed using the BVAS (Table 1).24 Blood was also available from 27 healthy adult controls (15 women, 12 men; median age, 42 years) not using any medications at the time of sampling. These samples were used for flow cytometry analysis, perfusion and neutrophil chemotaxis assays. Samples from the patients and controls were obtained after written consent of the subjects or their parents. The study was carried out with the approval of the Regional Ethics Review Board of Lund University.
Blood Samples
Whole blood was drawn into citrated tubes and centrifuged to obtain platelet-poor plasma and platelet-free plasma. These samples were analyzed for the presence of EMVs and used in perfusion experiments. In certain plasma samples the EMV content was reduced by centrifugation to 7.5% (range, 5.7%–11.3%) of the original EMV content. For details see Supplemental Material.
Culture of HEK Cells and Isolation of MVs
HEKwt and HEK cells stably expressing human B1R (HEKB1R)39 were cultured as detailed in the Supplemental Material. Medium from cells was collected and centrifuged at 2500×g for 5 minutes and further at 100,000×g for 3 hours to collect the pellets containing MVs. The concentration of MVs derived from HEKwt and HEKB1R was 70 µg/ml (corresponding to 36.7×108 MVs/ml). Kinin levels were measured in the supernatants of HEKwt and HEKB1R cells using an ELISA kit described below, and were found to be below the detection limit.
Culture of PGECs
PGECs (Cell Systems, Kirkland, WA) were cultured as described in the Supplemental Material and used in perfusion experiments.
Perfusion Experiments over PGECs
A semiautomated microfluidic perfusion system (VenaFlux; Cellix, Dublin, Ireland) was used to mimic shear in the microvasculature. Microcapillary channels (Vena8 Endothelial+ biochips; Cellix) were coated with fibronectin (Sigma-Aldrich, St. Louis, MO) and the PGEC suspension as described.40 PGECs were prestimulated with 200 µM histamine (Sigma-Aldrich) in Dulbecco phosphate buffered saline (DPBS; PAA Laboratories) for 10 minutes at a shear stress of 5 dynes/cm2 obtained with a Mirus Evo nanopump (Cellix). Histamine prestimulated samples mimic the activated endothelium present during inflammation.41 Histamine-stimulated endothelial cells were found to release more EMVs, confirming previous results,40 and were therefore used in all described experiments.
Plasma samples from patients and controls were perfused over the PGECs at 2–5 dynes/cm2 for 5–15 minutes, as described in the Supplemental Material. In certain experiments, IgG was depleted from plasma by adsorption to a Protein G Sepharose column (Amersham Biosciences, Uppsala, Sweden). After perfusion, the presample (plasma before perfusion over PGECs) and the perfused sample were collected and centrifuged for 5 minutes at 10,000×g. Dilution of the perfused samples was adjusted for by protein concentrations. These samples were later assayed by flow cytometry for detection EMVs.
C1-inhibitor–depleted plasma (Milan Analytica AG, Rheinfelden, Switzerland) as well as vasculitis plasma were diluted 1:1 in filtered DPBS and similarly perfused over the cells at a shear stress of 5 dynes/cm2 for 5 minutes. In certain experiments C1-inhibitor (Berinert, final concentration of 1 IU in suspension; CSL Behring, Marburg Germany) or B1R-antagonist R715 (1 μM; a kind gift from D. Regoli, Universite de Sherbrooke, Quebec, Canada) was added to the plasma just before perfusion.
Kinin ELISA
Kinin levels in HEK supernatants and perfused C1-inhibitor–depleted plasma samples were measured using the MARKIT-M bradykinin-ELISA kit (Dainippon Pharmaceutical, Osaka, Japan) as per the manufacturer’s instructions.
Detection of MVs Derived from Endothelial Cells and B1R on the EMVs
Plasma samples and samples collected from perfusion experiments were incubated with mouse anti-human CD144 (conjugated with phycoerythrin, 1:200; peridinin chlorophyll protein-cyanin5.5, 1:600; or FITC, 1:200) and mouse anti-human CD105:peridinin chlorophyll protein-cyanin5.5 at 1:800. MVs labeled with either or both antibodies were considered to derive from endothelial cells.
To detect B1R, rabbit anti-human B1R from two sources (in-house developed antibody42 at 10 μg/ml final concentration or BDKRB1 at 1:50; Abgent, San Diego, CA) was incubated with the plasma or perfusion samples. To detect IL-8, mouse anti-human IL-8:phycoerythrin at 1:800 was used. Flow cytometry was performed using a BD FACSCanto Cytometer and FACSDiva Software version 6.0 (Becton Dickinson Immunocytometry Systems, San Jose, CA) or a CyFlow Cube 8 flow cytometer (Sysmex Partec, Norderstedt, Germany) with FCS Express 4 Flow Research Edition software version 4.07.0003 (De Novo Software, Glendale, CA). The latter flow cytometer detects vesicles of submicron size and therefore identifies more events corresponding to MVs. Details are given in the Supplemental Material.
Neutrophil Migration Assay
Neutrophils were purified from citrated whole blood using Lymphoprep (Fresenius Kabi, Oslo, Norway), as previously described43, and adjusted to a concentration of 2×106 cells/ml. A neutrophil migration assay was performed with a Boyden chamber micropore filter assay (Neuro Probe, Gaithersburg, MD).44 Purified neutrophils from healthy donors (n=10) were placed in the upper compartment of the chamber. MVs derived from HEKwt cells, HEKB1R cells, and PGECs in perfusion experiments were placed in the lower compartment with or without preincubation with 1 μM B1R-antagonist R715, for 30 minutes at room temperature. Alternatively, plasma (diluted at 1:4) was placed in the lower compartment, in the presence or absence of the B1R-antagonist R715. In certain experiments, anti–hCXCL8/IL-8 (1 μg/ml; R&D Systems, Minneapolis, MN) was added to the plasma 30 minutes before running the assay. Migration in the membrane (measured in micrometers) was assayed by light microscopy (Zeiss Axiostar Plus; Zeiss, Göttingen, Germany). For details, see the Supplemental Material.
Statistical Analyses
To compare EMV levels between patient and control samples, in plasma as well as in the perfusion experiments, the Mann–Whitney U test was used. Correlations between B1R-positive EMVs and variables such as diagnosis, treatment, creatinine levels, and the BVAS were carried out using Spearman correlation coefficient. Neutrophil migration and perfusion experiments were compared using Kruskal–Wallis and Dunn multiple comparisons test. The Wilcoxon test was used to compare neutrophil migration in paired samples. A P value ≤0.05 was considered significant. Statistical analysis was performed using GraphPad prism software (version 7; GraphPad Software, La Jolla, CA).
Disclosures
None.
Supplementary Material
Acknowledgments
This study was supported by grants from The Swedish Research Council (K2013-64X-14008-13-5 and K2015-99X-22877-01-6), The Knut and Alice Wallenberg Foundation (Wallenberg Clinical Scholar 2015.0320), The Torsten Söderberg Foundation, Skåne Centre of Excellence in Health, IngaBritt och Arne Lundberg’s Research Foundation, Crown Princess Lovisa’s Society for Child Care Region Skåne, and The Konung Gustaf V:s 80-årsfond (all to D.K.); The Alfred Österlund Foundation (to L.M.F.L.-L. and R.K.); and The Swedish Rheumatism Association, The Medical Faculty of the University of Lund, The Anna-Greta Crafoord Foundation, Greta and Johan Kock’s Foundation, The Samariten Foundation, Fanny Ekdahl Foundation, The Jerring Foundation, and The Thelma Zoegas Foundation (to R.K.).
Preliminary results were presented by D.K. during a talk given at the 17th Congress of the International Pediatric Nephrology Association at Iguacu Brazil, September 20–24, 2016.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Endothelium-Neutrophil Communication via B1-Kinin Receptor–Bearing Microvesicles in Vasculitis,” on pages 2255–2258.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2016060637/-/DCSupplemental.
References
- 1.Colman RW, Schmaier AH: Contact system: A vascular biology modulator with anticoagulant, profibrinolytic, antiadhesive, and proinflammatory attributes. Blood 90: 3819–3843, 1997 [PubMed] [Google Scholar]
- 2.Kahn R, Hellmark T, Leeb-Lundberg LM, Akbari N, Todiras M, Olofsson T, Wieslander J, Christensson A, Westman K, Bader M, Müller-Esterl W, Karpman D: Neutrophil-derived proteinase 3 induces kallikrein-independent release of a novel vasoactive kinin. J Immunol 182: 7906–7915, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Leeb-Lundberg LM, Marceau F, Müller-Esterl W, Pettibone DJ, Zuraw BL: International union of pharmacology. XLV. Classification of the kinin receptor family: From molecular mechanisms to pathophysiological consequences. Pharmacol Rev 57: 27–77, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Schmaier AH: The contact activation and kallikrein/kinin systems: Pathophysiologic and physiologic activities. J Thromb Haemost 14: 28–39, 2016 [DOI] [PubMed] [Google Scholar]
- 5.Oehmcke S, Mörgelin M, Malmström J, Linder A, Chew M, Thorlacius H, Herwald H: Stimulation of blood mononuclear cells with bacterial virulence factors leads to the release of pro-coagulant and pro-inflammatory microparticles. Cell Microbiol 14: 107–119, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Lacroix R, Dignat-George F: Microparticles: New protagonists in pericellular and intravascular proteolysis. Semin Thromb Hemost 39: 33–39, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Mause SF, Weber C: Microparticles: Protagonists of a novel communication network for intercellular information exchange. Circ Res 107: 1047–1057, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Amabile N, Rautou PE, Tedgui A, Boulanger CM: Microparticles: Key protagonists in cardiovascular disorders. Semin Thromb Hemost 36: 907–916, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Brogan PA, Shah V, Brachet C, Harnden A, Mant D, Klein N, Dillon MJ: Endothelial and platelet microparticles in vasculitis of the young. Arthritis Rheum 50: 927–936, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Distler JH, Huber LC, Gay S, Distler O, Pisetsky DS: Microparticles as mediators of cellular cross-talk in inflammatory disease. Autoimmunity 39: 683–690, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Zwicker JI, Trenor CC 3rd, Furie BC, Furie B: Tissue factor-bearing microparticles and thrombus formation. Arterioscler Thromb Vasc Biol 31: 728–733, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burnier L, Fontana P, Kwak BR, Angelillo-Scherrer A: Cell-derived microparticles in haemostasis and vascular medicine. Thromb Haemost 101: 439–451, 2009 [PubMed] [Google Scholar]
- 13.Osterud B, Bjorklid E: Tissue factor in blood cells and endothelial cells. Front Biosci (Elite Ed) 4: 289–299, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Kahn R, Mossberg M, Ståhl AL, Johansson K, Lopatko Lindman I, Heijl C, Segelmark M, Mörgelin M, Leeb-Lundberg LM, Karpman D: Microvesicle transfer of kinin B1-receptors is a novel inflammatory mechanism in vasculitis. Kidney Int 91: 96–105, 2017 [DOI] [PubMed] [Google Scholar]
- 15.Meckes DG Jr, Raab-Traub N: Microvesicles and viral infection. J Virol 85: 12844–12854, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leroyer AS, Anfosso F, Lacroix R, Sabatier F, Simoncini S, Njock SM, Jourde N, Brunet P, Camoin-Jau L, Sampol J, Dignat-George F: Endothelial-derived microparticles: Biological conveyors at the crossroad of inflammation, thrombosis and angiogenesis. Thromb Haemost 104: 456–463, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Diamant M, Tushuizen ME, Sturk A, Nieuwland R: Cellular microparticles: New players in the field of vascular disease? Eur J Clin Invest 34: 392–401, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Brogan PA, Dillon MJ: Endothelial microparticles and the diagnosis of the vasculitides. Intern Med 43: 1115–1119, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Kümpers P, Erdbrügger U, Grossheim M, Meyer GP, Hiss M, Gwinner W, Haller H, Haubitz M: Endothelial microparticles as a diagnostic aid in Churg-Strauss vasculitis-induced cardiomyopathy. Clin Exp Rheumatol 26[Suppl 49]: S86–S89, 2008 [PubMed] [Google Scholar]
- 20.Clarke LA, Hong Y, Eleftheriou D, Shah V, Arrigoni F, Klein NJ, Brogan PA: Endothelial injury and repair in systemic vasculitis of the young. Arthritis Rheum 62: 1770–1780, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Eleftheriou D, Hong Y, Klein NJ, Brogan PA: Thromboembolic disease in systemic vasculitis is associated with enhanced microparticle-mediated thrombin generation. J Thromb Haemost 9: 1864–1867, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Eleftheriou D, Dillon MJ, Brogan PA: Advances in childhood vasculitis. Curr Opin Rheumatol 21: 411–418, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Kahn R, Herwald H, Müller-Esterl W, Schmitt R, Sjögren AC, Truedsson L, Karpman D: Contact-system activation in children with vasculitis. Lancet 360: 535–541, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Mukhtyar C, Lee R, Brown D, Carruthers D, Dasgupta B, Dubey S, Flossmann O, Hall C, Hollywood J, Jayne D, Jones R, Lanyon P, Muir A, Scott D, Young L, Luqmani RA: Modification and validation of the Birmingham vasculitis activity score (version 3). Ann Rheum Dis 68: 1827–1832, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Joseph K, Kaplan AP: Formation of bradykinin: A major contributor to the innate inflammatory response. Adv Immunol 86: 159–208, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Kaplan AP, Joseph K: Pathogenic mechanisms of bradykinin mediated diseases: Dysregulation of an innate inflammatory pathway. Adv Immunol 121: 41–89, 2014 [DOI] [PubMed] [Google Scholar]
- 27.Davis AE, 3rd: C1 inhibitor and hereditary angioneurotic edema. Annu Rev Immunol 6: 595–628, 1988 [DOI] [PubMed] [Google Scholar]
- 28.Jackson J, Sim RB, Whelan A, Feighery C: An IgG autoantibody which inactivates C1-inhibitor. Nature 323: 722–724, 1986 [DOI] [PubMed] [Google Scholar]
- 29.Karpman D, Kahn R: The contact/kinin and complement systems in vasculitis. APMIS 117[Suppl 127]: 48–54, 2009 [DOI] [PubMed]
- 30.Kuhr F, Lowry J, Zhang Y, Brovkovych V, Skidgel RA: Differential regulation of inducible and endothelial nitric oxide synthase by kinin B1 and B2 receptors. Neuropeptides 44: 145–154, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seabrook GR, Bowery BJ, Heavens R, Brown N, Ford H, Sirinathsinghi DJ, Borkowski JA, Hess JF, Strader CD, Hill RG: Expression of B1 and B2 bradykinin receptor mRNA and their functional roles in sympathetic ganglia and sensory dorsal root ganglia neurones from wild-type and B2 receptor knockout mice. Neuropharmacology 36: 1009–1017, 1997 [DOI] [PubMed] [Google Scholar]
- 32.Pesquero JB, Araujo RC, Heppenstall PA, Stucky CL, Silva JA Jr, Walther T, Oliveira SM, Pesquero JL, Paiva AC, Calixto JB, Lewin GR, Bader M: Hypoalgesia and altered inflammatory responses in mice lacking kinin B1 receptors. Proc Natl Acad Sci U S A 97: 8140–8145, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahluwalia A, Perretti M: Involvement of bradykinin B1 receptors in the polymorphonuclear leukocyte accumulation induced by IL-1 beta in vivo in the mouse. J Immunol 156: 269–274, 1996 [PubMed] [Google Scholar]
- 34.Ehrenfeld P, Millan C, Matus CE, Figueroa JE, Burgos RA, Nualart F, Bhoola KD, Figueroa CD: Activation of kinin B1 receptors induces chemotaxis of human neutrophils. J Leukoc Biol 80: 117–124, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Klein J, Gonzalez J, Decramer S, Bandin F, Neau E, Salant DJ, Heeringa P, Pesquero JB, Schanstra JP, Bascands JL: Blockade of the kinin B1 receptor ameloriates glomerulonephritis. J Am Soc Nephrol 21: 1157–1164, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clarke LA, Shah V, Arrigoni F, Eleftheriou D, Hong Y, Halcox J, Klein N, Brogan PA: Quantitative detection of circulating endothelial cells in vasculitis: Comparison of flow cytometry and immunomagnetic bead extraction. J Thromb Haemost 6: 1025–1032, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Dignat-George F, Boulanger CM: The many faces of endothelial microparticles. Arterioscler Thromb Vasc Biol 31: 27–33, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Tissot JD, Canellini G, Rubin O, Angelillo-Scherrer A, Delobel J, Prudent M, Lion N: Blood microvesicles: From proteomics to physiology. Transl Proteomics 1: 38–52, 2013 [Google Scholar]
- 39.Enquist J, Skröder C, Whistler JL, Leeb-Lundberg LM: Kinins promote B2 receptor endocytosis and delay constitutive B1 receptor endocytosis. Mol Pharmacol 71: 494–507, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Tati R, Kristoffersson AC, Ståhl AL, Rebetz J, Wang L, Licht C, Motto D, Karpman D: Complement activation associated with ADAMTS13 deficiency in human and murine thrombotic microangiopathy. J Immunol 191: 2184–2193, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nolasco LH, Turner NA, Bernardo A, Tao Z, Cleary TG, Dong JF, Moake JL: Hemolytic uremic syndrome-associated Shiga toxins promote endothelial-cell secretion and impair ADAMTS13 cleavage of unusually large von Willebrand factor multimers. Blood 106: 4199–4209, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sandén C, Leeb-Lundberg LM: Kinin B1 receptor homo-oligomerization is required for receptor trafficking to the cell surface. Int Immunopharmacol 15: 121–128, 2013 [DOI] [PubMed] [Google Scholar]
- 43.Sørensen OE, Follin P, Johnsen AH, Calafat J, Tjabringa GS, Hiemstra PS, Borregaard N: Human cathelicidin, hCAP-18, is processed to the antimicrobial peptide LL-37 by extracellular cleavage with proteinase 3. Blood 97: 3951–3959, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Falk W, Goodwin RH Jr, Leonard EJ: A 48-well micro chemotaxis assembly for rapid and accurate measurement of leukocyte migration. J Immunol Methods 33: 239–247, 1980 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.