Abstract
Sickle cell disease (SCD)–associated nephropathy is a major source of morbidity and mortality in patients because of the lack of efficacious treatments targeting renal manifestations of the disease. Here, we describe a long-term treatment strategy with the selective endothelin-A receptor (ETA) antagonist, ambrisentan, designed to interfere with the development of nephropathy in a humanized mouse model of SCD. Ambrisentan preserved GFR at the level of nondisease controls and prevented the development of proteinuria, albuminuria, and nephrinuria. Microscopy studies demonstrated prevention of podocyte loss and structural alterations, the absence of vascular congestion, and attenuation of glomerulosclerosis in treated mice. Studies in isolated glomeruli showed that treatment reduced inflammation and oxidative stress. At the level of renal tubules, ambrisentan treatment prevented the increased excretion of urinary tubular injury biomarkers. Additionally, the treatment strategy prevented tubular brush border loss, diminished tubular iron deposition, blocked the development of interstitial fibrosis, and prevented immune cell infiltration. Furthermore, the prevention of albuminuria in treated mice was associated with preservation of cortical megalin expression. In a separate series of identical experiments, combined ETA and ETB receptor antagonism provided only some of the protection observed with ambrisentan, highlighting the importance of exclusively targeting the ETA receptor in SCD. Our results demonstrate that ambrisentan treatment provides robust protection from diverse renal pathologies in SCD mice, and suggest that long-term ETA receptor antagonism may provide a strategy for the prevention of renal complications of SCD.
Keywords: sickle nephropathy, endothelin, proteinuria, renal injury, glomerular filtration barrier
Sickle cell disease (SCD) is an autosomal recessive monogenetic blood disorder that affects approximately 100,000 people in the United States and millions globally.1,2 The central pathology of the disease is the polymerization of mutant hemoglobin S (HbS). This process sets in motion a cascade of events, beginning with erythrocyte sickling, endothelial activation, and vaso-occlusion, that ultimately result in chronic tissue hypoxia, inflammation, and organ damage.3 Advances in the management of SCD throughout the past half-century have led to a progressive increase in life expectancy with a concomitant increase in the prevalence of chronic disease manifestations.4,5 Sickle cell nephropathy now represents one of the most serious organ-specific complications facing patients with SCD. Renal pathologies in SCD occur at both the structural and functional level and include deterioration of the glomerular filtration barrier, progressive loss of functional glomeruli, tubulointerstitial injury, defective urine-concentrating ability, and dysfunction of the distal nephron.6 Evidence of nephropathy, as manifested by albuminuria or proteinuria, occurs in nearly two-third of adults with SCD, and presently, almost one in five patients with SCD die of renal disease.7,8 This level of disease burden occurs due to lack of availability of robustly renal-protective therapies targeted to the mechanisms that underlie sickle nephropathy.
Endothelin-1 (ET-1) is a signaling peptide produced by diverse cell types that exerts its physiologic and pathophysiologic actions by binding to two receptor subtypes, ETA and ETB.9,10 ETA receptor activation induces vasoconstriction, inflammation, mitogenesis, and nociception.11–15 These effects can be counteracted by ETB activation in some tissues, highlighting the importance of the balance between the two receptor subtypes.16 ET-1 expression is induced by established hallmarks of the SCD milieu, including hypoxia, oxidative stress, and thrombosis.17 This theoretic connection between SCD and ET-1 overproduction was validated by multiple studies that demonstrated elevated ET-1 levels in both plasma and urine of patients with SCD.17 In the kidney, ET-1 has been implicated as a mechanistic contributor to development of proteinuria in various forms of CKD.18–22 Moreover, the elevated urinary ET-1 excretion reported in patients with SCD correlates with microalbuminuria,23 suggesting a pathophysiologic link between ET-1 induction and renal injury in SCD. Direct evidence to demonstrate that ET-1 contributes to renal dysfunction in SCD comes from a study by Sabaa and colleagues who reported that SCD mice treated with the dual ET receptor antagonist, bosentan, were protected from hypoxia-induced decreases in renal blood flow and renal inflammation.24 These studies have provided both clinical and preclinical evidence to support the hypothesis that ET-1 signaling is a mechanistic mediator of organ-specific pathology in SCD. However, no study has provided rigorous evidence to characterize ET receptor antagonism as a treatment strategy for renal complications of SCD.
This study aimed to investigate the renal protective potential of ET receptor antagonism in the treatment of sickle nephropathy and determine the importance of receptor subtype targeting and duration of treatment. We demonstrate that long-term ETA receptor antagonism can prevent the development of many of the glomerular and tubular pathologies that characterize the nephropathy associated with SCD. Additionally, short-term ETA receptor antagonism demonstrated the ability to reduce, but not fully reverse, the severity of established nephropathy. Dual ETA/B receptor antagonism was examined under the same duration treatment protocols and was found to provide less renal protection than ETA receptor antagonism alone. These results establish ET receptor antagonism as a highly efficacious treatment strategy for sickle nephropathy and highlight that maximal benefit is provided by long-term antagonism of the ETA receptor.
Results
Characteristics of Experimental Animals
Vehicle-treated 14-week-old HbSS mice had significantly higher spleen-to–body weight ratio and daily urine volume, and decreased urine osmolality, when compared with age-matched vehicle-treated HbAA mice. Two-week treatment with selective ETA receptor antagonist, ambrisentan, significantly reduced spleen-to–body weight ratio of HbSS mice. No parameters were changed with nonselective ETA/B receptor antagonist, A-182086, treatment in HbSS mice (Tables 1 and 2). Neither antagonist had any effect on the characteristics of control mice (Tables 1 and 2). HbSS mice had elevated plasma ET-1 concentration compared with controls, and both 2-week and 10-week ambrisentan treatment protocols significantly reduced plasma ET-1 level (Figure 1A, Table 3). This result suggests that in SCD the ETA receptor signals through a positive feedback loop to increase ET-1 expression.
Table 1.
Characteristics of experimental animals after 2 weeks of treatment with ET receptor antagonists
| Characteristics | HbAA | HbSS | ||||
|---|---|---|---|---|---|---|
| Vehicle | Ambrisentan | A-182086 | Vehicle | Ambrisentan | A-182086 | |
| Body weight, g | M: 25.7±1.2 | M: 28.8±0.8 | M: 26.8±0.4 | M: 27.5±1.1 | M: 27.5±0.8 | M: 28.2±0.5 |
| F: 22.3±1.0 | F: 21.7±0.5 | F: 21.7±0.7 | F: 21.9±1.0 | F: 21.1±0.9 | F: 23.0±0.4 | |
| Right kidney/body weight, mg/g | 6.6±0.3 | 6.5±0.4 | 6.3±0.4 | 7.4±0.4 | 7.6±0.5 | 7.8±0.5 |
| Left kidney/body weight, mg/g | 6.2±0.3 | 5.8±0.3 | 5.9±0.3 | 7.2±0.4 | 7.7±0.5 | 7.0±0.4 |
| Spleen/body weight ratio, % | 0.9±0.1 | 0.8±0.1 | 0.9±0.1 | 6.9±0.3a | 5.7±0.3a,b | 7.0±0.5a |
| Water intake, ml/24 h | 3.2±0.3 | 4.7±0.5 | 4.6±0.6 | 5.2±0.5 | 6.0±0.5 | 5.8±0.5 |
| Urine excretion, ml/24 h | 1.0±0.2 | 1.3±0.1 | 1.6±0.3 | 2.1±0.3a | 2.3±0.4a | 2.7±0.3a |
| Urine osmolality, mOsm/kg | 2482±147 | 2299±121 | 2601±121 | 1177±135a | 1551±174a | 1624±80a |
| Systolic BP, mmHg | 120±1 | 119±2 | 121±1 | 123±1 | 119±3 | 122±2 |
| Serum creatinine, mg/dl | 0.09±0.01 | 0.10±0.01 | 0.12±0.01 | 0.11±0.01 | 0.11±0.01 | 0.09±0.01 |
Data are means±SEM (n=12–14 in HbSS groups and n=6–9 in HbAA groups). M, male; F, female.
P<0.05 versus HbAA vehicle.
P<0.05 versus HbSS vehicle.
Table 2.
Characteristics of experimental animals after 10 weeks of treatment with ET receptor antagonists
| Characteristics | HbAA | HbSS | ||||
|---|---|---|---|---|---|---|
| Vehicle | Ambrisentan | A-182086 | Vehicle | Ambrisentan | A-182086 | |
| Body weight, g | M: 32.2±0.9 | M: 30.5±0.9 | M: 32.1±1.3 | M: 29.3±0.4 | M: 28.6±0.5 | M: 27.5±1.1 |
| F: 24.9±1.5 | F: 24.3±0.7 | F: 22.2±0.7 | F: 24.2±0.7 | F: 25.7±0.8 | F: 24.2±0.7 | |
| Right kidney/body weight, mg/g | 6.6±0.3 | 6.4±0.3 | 6.3±0.2 | 6.8±0.3 | 6.5±0.3 | 6.4±0.3 |
| Left kidney/body weight, mg/g | 5.6±0.5 | 5.8±0.2 | 6.0±0.1 | 6.4±0.2 | 6.0±0.2 | 6.0±0.2 |
| Spleen/body weight ratio, % | 0.8±0.1 | 1.0±0.1 | 0.8±0.1 | 7.0±0.3a | 5.9±0.4a | 6.7±0.4a |
| Water intake, ml/24 h | 3.8±0.6 | 4.0±0.3 | 3.5±0.4 | 4.6±0.4 | 6.3±1.2 | 6.0±0.3 |
| Urine excretion, ml/24 h | 1.0±0.3 | 0.9±0.1 | 1.0±0.2 | 2.0±0.3a | 2.1±0.4a | 1.8±0.1a |
| Urine osmolality, mOsm/kg | 2574±415 | 2257±185 | 2336±247 | 1519±42a | 1519±136a | 1370±149a |
| Systolic BP, mmHg | 121±2 | 115±2 | 121±4 | 125±4 | 118±4 | 126±3 |
| Serum creatinine, mg/dl | 0.10±0.01 | 0.11±0.01 | 0.10±0.01 | 0.09±0.01 | 0.10±0.01 | 0.10±0.01 |
Data are means±SEM (n=9–10 in HbSS groups and n=6–9 in HbAA groups). M, male; F, female.
P<0.05 versus HbAA vehicle.
P<0.05 versus HbSS vehicle.
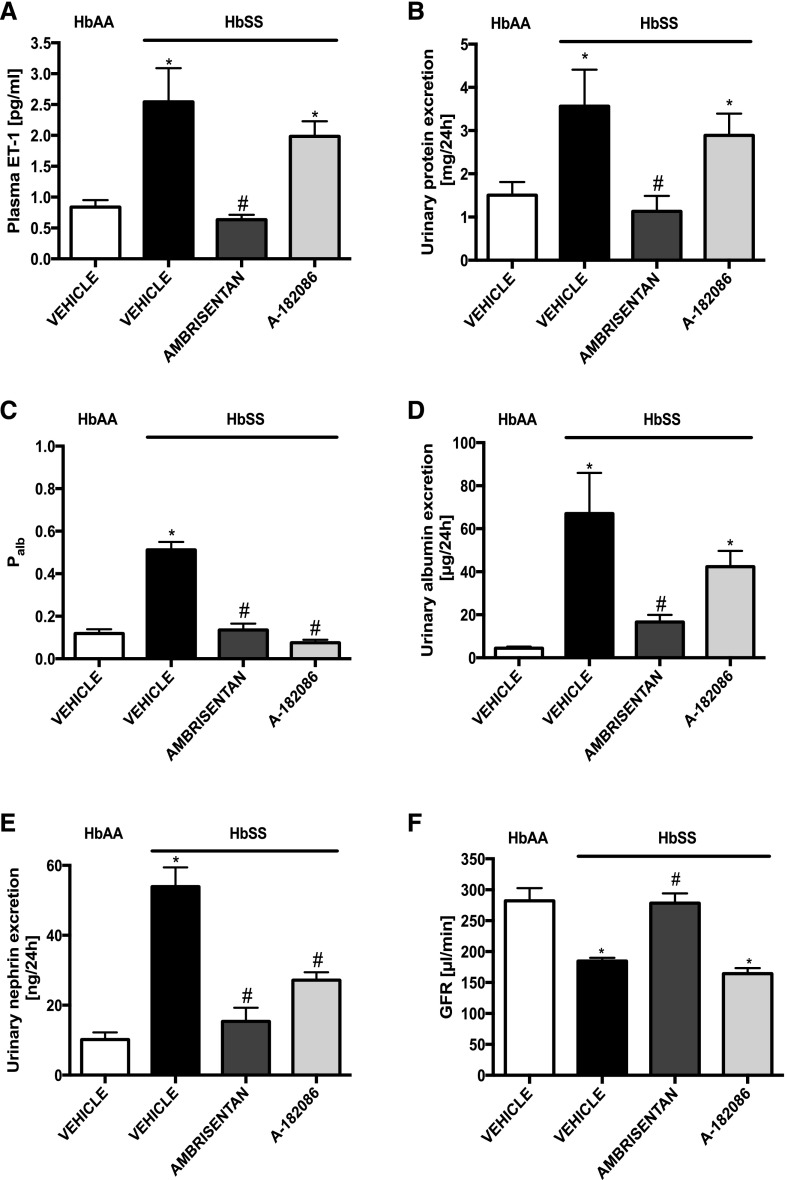
Figure 1.
Long-term selective ETA receptor antagonism preserves glomerular function by preventing glomerular injury in humanized sickle cell mice. Plasma ET-1 and markers of renal function from genetic control (HbAA) and humanized sickle mice (HbSS) treated with vehicle, the selective ETA antagonist, ambrisentan, or the combined ETA/B antagonist, A-182086, for 10 weeks beginning at 4 weeks of age. (A) Depicts the average ET-1 in plasma after 10-week treatment protocol. (B) Depicts the average proteinuria after 10-week treatment protocol. (C) Depicts the average Palb after 10-week treatment protocol. (D) Depicts the average urinary albumin excretion after 10-week treatment protocol. (E) Depicts the average urinary nephrin excretion after 10-week treatment protocol. (F) Depicts the average GFR after 10-week treatment protocol. Data are mean±SEM; *P<0.05 versus vehicle-treated HbAA; #P<0.05 versus vehicle-treated HbSS; n=9–10 in HbSS and n=9 in vehicle-treated HbAA group.
Table 3.
Short-term ET receptor antagonism attenuates glomerular and tubular injury in HbSS mice
| Variables | HbAA | HbSS | ||||
|---|---|---|---|---|---|---|
| Vehicle | Ambrisentan | A-182086 | Vehicle | Ambrisentan | A-182086 | |
| Plasma ET-1, pg/ml | 0.6±0.1 | 0.8±0.2 | 0.7±0.1 | 1.8±0.2a | 1.0±0.1b | 1.6±0.2a |
| Urinary protein excretion, mg/24 h | 1.8±0.3 | 1.7±0.5 | 2.1±0.6 | 3.2±0.4a | 2.4±0.3b | 3.5±0.4a |
| Palb | 0.12±0.02 | 0.13±0.02 | 0.12±0.02 | 0.48±0.05a | 0.24±0.03b | 0.27±0.04a,b |
| Urinary albumin excretion, μg/24 h | 30.1±7.9 | 25.6±5.7 | 26.0±7.7 | 81.7±18.0a | 35.2±6.2b | 108.3±27.1a |
| Urinary nephrin excretion, ng/24 h | 7.5±1.9 | 5.5±1.8 | 10.8±4.1 | 52.8±7.6a | 31.6±2.1a,b | 37.8±3.1a |
| Urinary KIM-1 excretion, pg/24 h | 37±8 | 112±26 | 113±49 | 272±46a | 122±22b | 323±34a |
| Urinary NAG excretion, mU/24 h | 9.4±1.6 | 5.4±2.7 | 6.4±2.8 | 40.2±8.3a | 15.7±2.9b | 27.5±7.1a |
Data are means±SEM (n=12–14 in HbSS groups and n=6–9 in HbAA groups).
P<0.05 versus HbAA vehicle.
P<0.05 versus HbSS vehicle.
Short-Term ET Receptor Antagonism Attenuates Glomerular and Tubular Injury
To investigate the contribution of ET signaling to the maintenance and progression of established nephropathy in SCD, we first examined the effect of 2-week treatment with ET receptor antagonists on glomerular and tubular dysfunction in HbSS and HbAA mice. Vehicle-treated HbSS mice had significantly higher proteinuria, albuminuria, nephrinuria, and glomerular permeability to albumin (Palb) when compared with HbAA controls (Table 3). Ambrisentan significantly reduced albuminuria, nephrinuria, and Palb in HbSS mice, whereas A-182086 significantly attenuated only Palb, and the effect of dual antagonism did not reach statistical significance in the reduction of albuminuria and nephrinuria (Table 3). These results suggest that ETA activation contributes to the maintenance of dysfunction of the glomerular filtration barrier in established sickle nephropathy, a finding that is consistent with previous results from our laboratory.22
We also determined if ET signaling contributes to the maintenance of tubular injury in SCD, because final urinary albumin excretion is the result of both glomerular filtration and proximal tubular uptake. Thus, we measured urinary excretion of kidney injury marker 1 (KIM-1) and N-acetyl-β-D-glucosaminidase (NAG), markers of proximal tubule injury. Both markers were significantly increased in vehicle-treated HbSS mice compared with controls. Ambrisentan significantly attenuated KIM-1 and NAG, whereas A-182086 had no significant effect on these markers in HbSS mice (Table 3). Neither antagonist had any effect on markers of glomerular or tubular injury in control HbAA mice (Table 3).
Long-Term Ambrisentan Treatment Prevents Onset of Glomerular Injury
To investigate the pathophysiologic relevance of ET signaling in the development of sickle nephropathy, we examined the effect of 10-week treatment (from weaning into adulthood) with ET receptor antagonists on proteinuria in HbSS mice. Selective ETA receptor antagonist prevented proteinuria in HbSS mice when compared with vehicle-treated mice (1.1±0.3 versus 3.57±0.8 mg/24 h, respectively), whereas the nonselective ETA/B receptor antagonist had no effect on urinary protein excretion (Figure 1B).
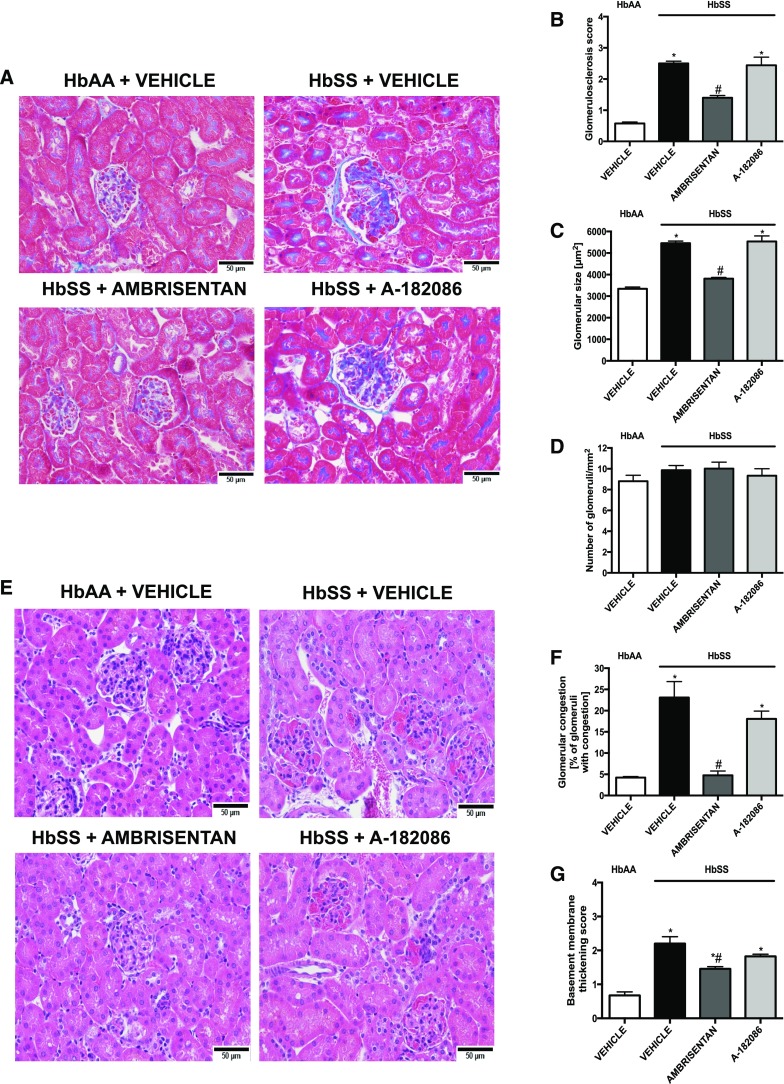
Next, we examined the role of ET signaling in the development of sickle cell glomerulopathy by measuring structural and functional markers of glomerular injury in HbSS mice. Both ambrisentan and A-182086 prevented the increase in Palb in glomeruli isolated from HbSS mice (Figure 1C). Moreover, both antagonists prevented increases in nephrinuria (Figure 1E). However, only the selective ETA receptor antagonist prevented albuminuria in HbSS mice (Figure 1D). Importantly, ambrisentan treatment preserved GFR to the level of nondisease controls (Figure 1F), whereas A-182086 treatment had no effect on the decline in GFR observed in HbSS mice. (Figure 1F). Histologic analysis of glomerular structure showed glomerular hypertrophy, basement membrane of Bowman’s capsule thickening, glomerulosclerosis, and vascular congestion in vehicle-treated HbSS mice. Only ambrisentan significantly prevented or attenuated all of these changes in glomerular morphology in HbSS mice (Figure 2). Neither antagonist had any effect on markers of glomerular structure or function in control HbAA mice (Supplemental Figures 1 and 2). These results suggest that ETA activation contributes to the development and progression of glomerulopathy in SCD.
Figure 2.
Histological analysis reveals that selective ETA receptor antagonism preserves glomerular morphology and prevents vascular congestion in humanized sickle cell mice. Histologic examination of the renal cortex of genetic control (HbAA) and humanized sickle mice (HbSS) treated with vehicle, the selective ETA antagonist, ambrisentan, or the combined ETA/B antagonist, A-182086, for 10 weeks beginning at 4 weeks of age. (A) Depicts representative Masson trichrome–stained sections of glomeruli. Original magnification, ×40 (scale bar=50 μm). (B) Depicts quantification of (A) represented as sclerosis index score. (C) Depicts glomerulomegaly represented as mean area of glomeruli (square micrometer). (D) Depicts number of glomeruli per square millimeter. (E) Depicts representative hematoxylin and eosin–stained sections of glomeruli. Original magnification, ×40 (scale bar=50 μm). (F) Depicts glomerular congestion represented as percentage of glomeruli with congestion. (G) Depicts basement membrane of Bowman’s capsule thickening score. Data are mean±SEM; n=5 in HbAA and HbSS groups; *P<0.05 versus vehicle-treated HbAA; #P<0.05 versus vehicle-treated HbSS. All of the glomerular characteristics were counted in ten sections per slide (minimum 20 glomeruli).
Long-Term Ambrisentan Treatment Prevents Onset of Podocyte Injury
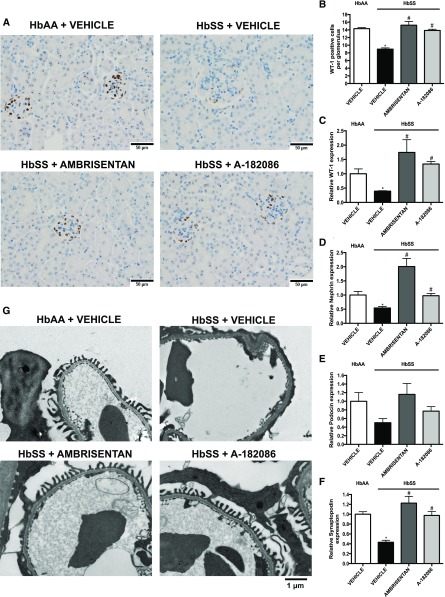
Podocyte injury is a key feature of alteration in the integrity of the glomerular filtration barrier and, ultimately, proteinuric nephropathy. Thus, we examined the effect of the long-term ET receptor antagonism on podocyte phenotypic changes. Podocyte number per glomerulus, as determined by Wilms tumor 1 (WT-1) immunohistochemistry, was significantly decreased in vehicle-treated HbSS mice, and was concomitant with reduced mRNA levels of the podocyte markers nephrin, podocin, and synaptopodin. Both antagonists prevented podocyte loss, demonstrated by WT-1–positive glomerular cells and mRNA expression of podocyte markers (Figure 3, A–F). Transmission electron microscopy studies in glomeruli of vehicle-treated HbSS mice showed evidence of more podocyte foot process fusion and effacement, and glomerular basement membrane thickening, whereas prevalence of these structural defects was similar to vehicle-treated nondisease controls in mice treated with both antagonists (Figure 3G). Neither antagonist had any effect on markers of podocyte injury in control HbAA mice (Supplemental Figure 3). These results suggest that ETA activation contributes to the development and progression of podocyte injury in SCD.
Figure 3.
ETA receptor antagonism prevents podocyte loss and preserves podocyte structure in humanized sickle cell mice. Immunohistochemical examination and mRNA expression of markers of podocyte injury of kidneys from genetic control (HbAA) and humanized sickle mice (HbSS) treated with vehicle, the selective ETA antagonist, ambrisentan, or the combined ETA/B antagonist, A-182086, for 10 weeks beginning at 4 weeks of age. (A) Depicts representative WT-1–positive stained sections of glomeruli. Original magnification, ×40 (scale bar=50 μm). (B) Depicts the quantification of (A) represented as the average number of WT-1–positive cells per glomerulus. Podocytes were counted in minimum 20 glomeruli in ten sections per slide. Data are mean±SEM; n=5 in HbAA and HbSS groups. (C) Depicts the relative WT-1 mRNA expression in glomeruli after 10-week treatment protocol. (D) Depicts the relative nephrin mRNA expression in glomeruli after 10-week treatment protocol. (E) Depicts the relative podocin mRNA expression in glomeruli after 10-week treatment protocol. (F) Depicts the relative synaptopodin mRNA expression in glomeruli after 10-week treatment protocol. (G) Depicts representative photomicrographs of transmission electron microscopy sections of glomeruli. Data are mean±SEM; *P<0.05 versus vehicle-treated HbAA; #P<0.05 versus vehicle-treated HbSS; n=5–6 in HbSS and n=6 in vehicle-treated HbAA group.
Long-Term Ambrisentan Treatment Prevents Glomerular Dysfunction
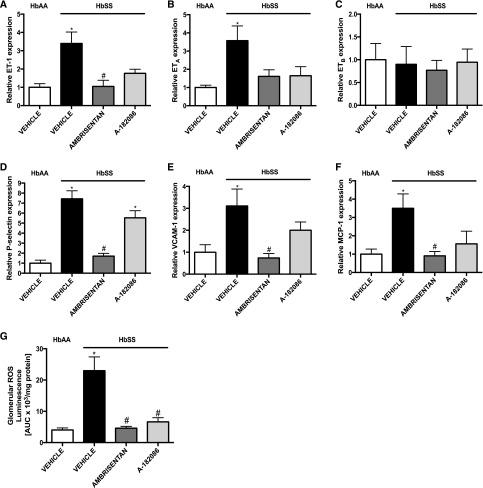
To elucidate the mechanism underlying the nephroprotective effect of ET receptor antagonism on glomeruli of HbSS mice, we examined endothelial activation, inflammation, and oxidative stress, hallmarks of glomerular dysfunction. Isolated glomeruli from HbSS mice treated with both antagonists demonstrated nondisease control–level sensitivity to phorbol 12-myristate 13-acetate–stimulated reactive oxygen species (ROS) production (Figure 4G). By contrast, only the selective ETA receptor antagonist prevented the induction of vascular adhesion molecules and proinflammatory marker expression in glomeruli from HbSS mice (Figure 4, D–F). Neither antagonist had any effect on glomerular dysfunction in control HbAA mice (Supplemental Figure 4). These results suggest that ETA receptor antagonism successfully protects from ET-mediated glomerular alterations in sickle nephropathy.
Figure 4.
Evidence from isolated glomeruli suggests that ETA receptor antagonism prevents the onset of glomerular inflammation and oxidative stress in humanized sickle cell mice. Expression of components of the ET system, inflammation, and oxidative stress in glomeruli from genetic control (HbAA) and humanized sickle mice (HbSS) treated with vehicle, the selective ETA antagonist, ambrisentan, or the combined ETA/B antagonist, A-182086, for 10 weeks beginning at 4 weeks of age. (A) Depicts the relative ET-1 mRNA expression in glomeruli after 10-week treatment protocol. (B) Depicts the relative ETA receptor mRNA expression in glomeruli after 10-week treatment protocol. (C) Depicts the relative ETB receptor mRNA expression in glomeruli after 10-week treatment protocol. (D) Depicts the relative P-selectin mRNA expression in glomeruli after 10-week treatment protocol. (E) Depicts the relative vascular cell adhesion molecule 1 (VCAM-1) mRNA expression in glomeruli after 10-week treatment protocol. (F) Depicts the relative monocyte chemoattractant protein-1 (MCP-1) mRNA expression in glomeruli after 10-week treatment protocol. (G) Depicts the glomerular ROS production after 10-week treatment protocol. Data are mean±SEM; *P<0.05 versus vehicle-treated HbAA; #P<0.05 versus vehicle-treated HbSS; n=5–6 in HbSS and n=6 in vehicle-treated HbAA group. AUC, area under the curve.
Long-Term Ambrisentan Treatment Prevented Tubular Injury
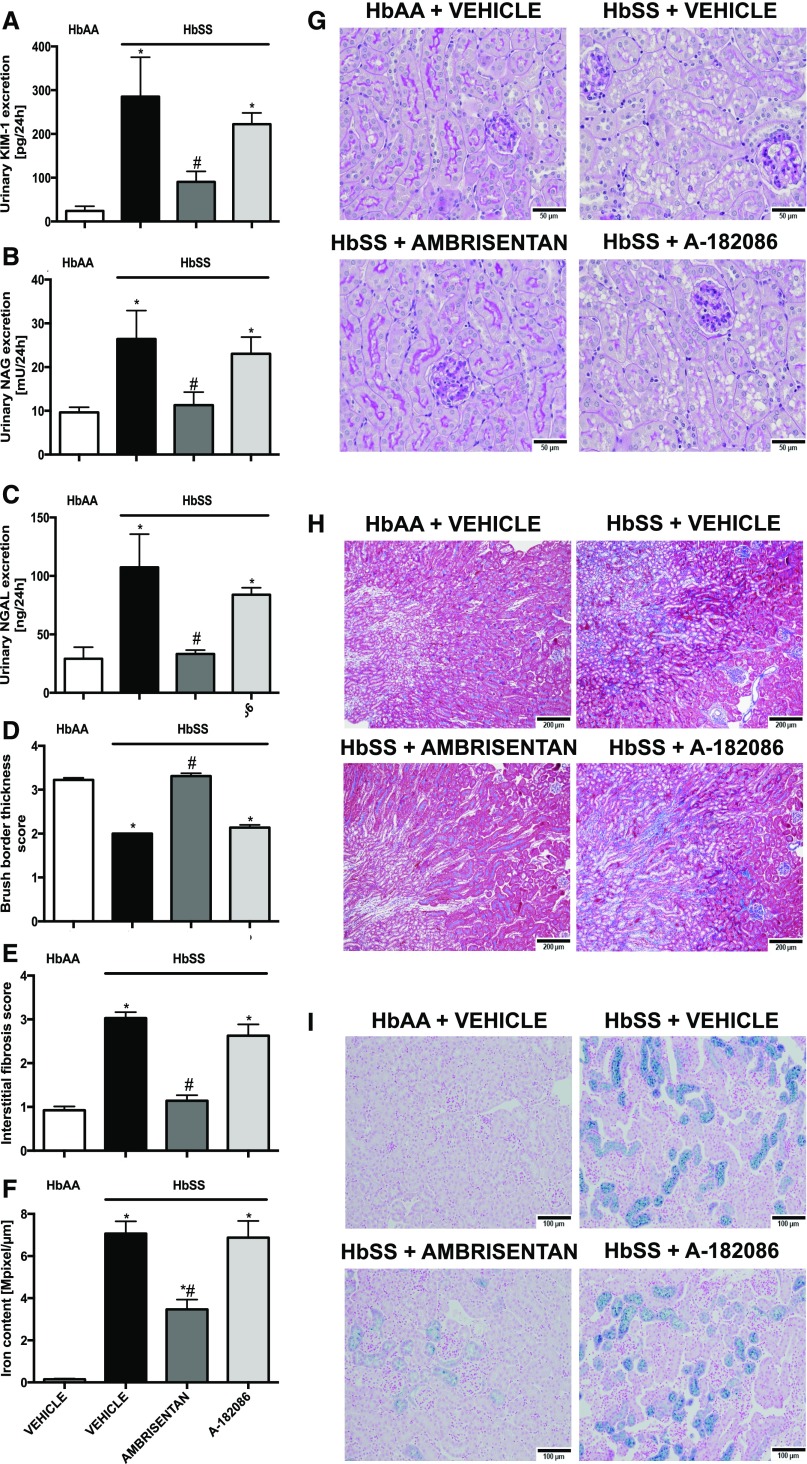
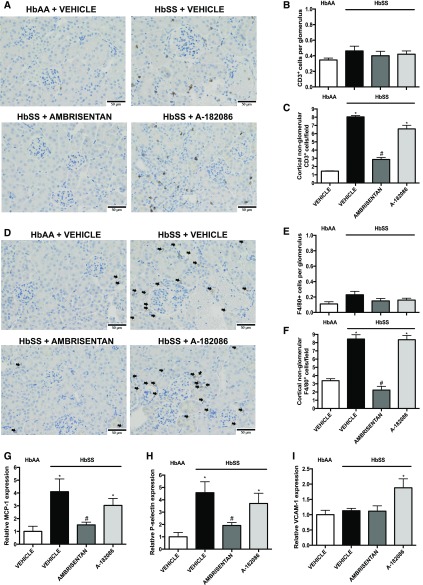
On the basis of data from the short-term treatment protocol, we analyzed markers of injury and histologic structure of the proximal tubules in HbSS mice. Increased urinary KIM-1 and NAG excretion were prevented by ambrisentan compared with vehicle-treated HbSS mice (Figure 5, A and B). Moreover, ambrisentan prevented renal inflammation and fibrosis, possibly through blocking T cell and macrophage infiltration (Figure 6, A–F) as well as the induction of proinflammatory mediators (Figure 6, G–I). In contrast, antagonism of both ET receptors had no significant effect on tubular injury in HbSS mice when compared with the selective ETA receptor antagonist (Figures 5 and 6). These results were confirmed by histologic analysis where ambrisentan preserved brush border thickness (Figure 5, D and G), prevented interstitial fibrosis (Figure 5, E and H), and reduced tubular iron deposition associated with nondisease levels of urinary neutrophil gelatinase-associated lipocalin (NGAL), iron binding protein, excretion in HbSS mice (Figure 5, C, F and I). Neither antagonist had any effect on tubular structure and function in control HbAA mice (Supplemental Figures 5 and 6).
Figure 5.
Long-term ETA receptor antagonism prevents renal tubular injury in humanized sickle cell mice. Histologic examination of the renal cortex and urinary excretion of markers of tubular injury of genetic control (HbAA) and humanized sickle mice (HbSS) treated with vehicle, the selective ETA antagonist, ambrisentan, or the combined ETA/B antagonist, A-182086, for 10 weeks beginning at 4 weeks of age. (A) Depicts the average urinary KIM-1 excretion after 10-week treatment protocol. (B) Depicts the average urinary NAG excretion after 10-week treatment protocol. (C) Depicts the average urinary NGAL excretion, iron-binding protein, after 10-week treatment protocol. Data are mean±SEM; *P<0.05 versus vehicle-treated HbAA; #P<0.05 versus vehicle-treated HbSS; n=9–10 in HbSS and n=9 in untreated HbAA group. (D) Depicts brush border thickness index score. (E) Depicts interstitial fibrosis index score. (F) Depicts quantification of iron deposition in the whole kidney sections (megapixel per micrometer). (G) Depicts representative periodic acid Schiff–hematoxylin–stained sections of renal cortex. Original magnification, ×40 (scale bar=50 μm). (H) Representative Masson trichrome–stained sections of renal cortex and medulla. Original magnification, ×10 (scale bar=200 μm, respectively). (I) Depicts Prussian blue iron–stained sections of renal cortex. Original magnification, ×20 (scale bar=100 µm). Fibrosis and brush border thickness were assessed in ten sections per slide. Data are mean±SEM; *P<0.05 versus vehicle-treated HbAA; #P<0.05 versus vehicle-treated HbSS; n=5 in HbAA and HbSS groups.
Figure 6.
Selective ETA receptor antagonism prevents renal cortical inflammation and immune cell infiltration in humanized sickle cell mice. Immunohistochemical examination and mRNA expression of proinflammatory and profibrotic markers in renal cortex of genetic control (HbAA) and humanized sickle mice (HbSS) treated with vehicle, the selective ETA antagonist, ambrisentan, or the combined ETA/B antagonist, A-182086, for 10 weeks beginning at 4 weeks of age. (A) Depicts representative CD3+–stained cortical sections. Original magnification, ×40 (scale bar=50 μm). (B) Depicts the quantification of glomerular CD3+ cells represented as the average number of CD3+ cells per glomerulus (minimum 20 glomeruli counted). (C) Depicts the quantification of cortical nonglomerular CD3+ cells represented as the average number of nonglomerular CD3+ cells per field. (D) Depicts representative F4/80+-stained cortical sections. Original magnification, ×40 (scale bar=50 μm). Arrows indicate F4/80+ cells. (E) Depicts the quantification of glomerular F4/80+ cells represented as the average number of F4/80+ cells per glomerulus (minimum 20 glomeruli counted). (F) Depicts the quantification of cortical nonglomerular F4/80+ cells represented as the average number of nonglomerular F4/80+ cells per field. CD3+ and F4/80+ cells were assessed in ten sections per slide and calculated. Data are mean±SEM; n=5 in HbAA and HbSS groups. (G) Depicts the relative MCP-1 mRNA expression in cortex after 10-week treatment protocol. (H) Depicts the relative P-selectin mRNA expression in cortex after 10-week treatment protocol. (I) Depicts the relative VCAM-1 mRNA expression in cortex after 10-week treatment protocol. Data are mean±SEM *P<0.05 versus vehicle-treated HbAA; #P<0.05 versus vehicle-treated HbSS; n=5–6 in HbSS and n=6 in vehicle-treated HbAA group.
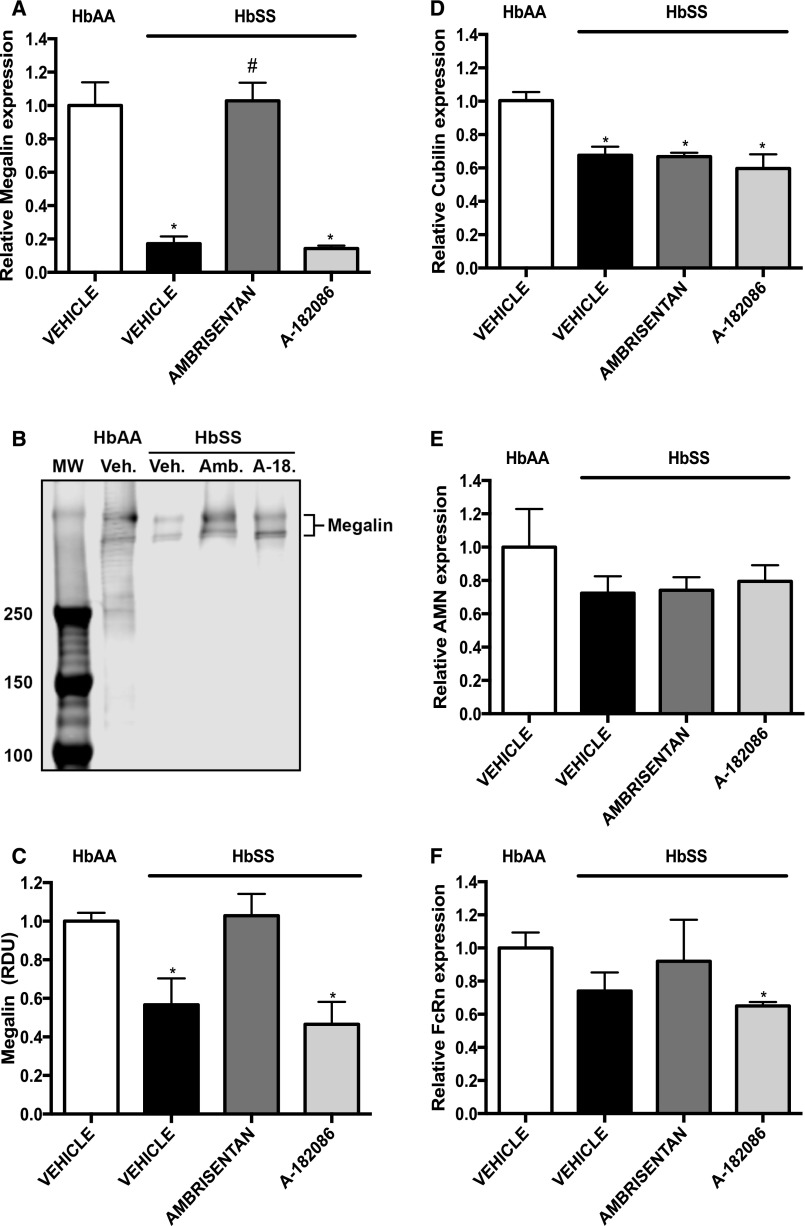
Long-Term ET Receptor Antagonism Treatment Preserves Tubular Megalin Expression
The contradictory observations that treatment of HbSS mice with either selective or nonselective ET antagonists prevented the increase in Palb, whereas only the selective ETA antagonist prevented albuminuria, led us to investigate if the two antagonists had differing effects on proximal tubular protein handling. We measured expression of the receptors involved in proximal tubular endocytosis of filtered albumin. HbSS mice had significantly lower megalin mRNA and protein expression when compared with HbAA mice, and ambrisentan, but not A-182086, restored expression to nondisease levels (Figure 7, A–C). In addition, suppressed neonatal Fc receptor (FcRn) mRNA expression was observed only in ETA/B antagonist–treated HbSS mice (Figure 7F). Neither antagonist had any effect on proximal tubule protein handling receptors in control HbAA mice (Supplemental Figure 7). These results suggest that proximal tubular ETB receptor signaling may play a role in albumin uptake regulation and contribute to the degree of albuminuria in HbSS mice.
Figure 7.
Analysis of tubular albumin transporters reveals that ETA receptor antagonism preserves megalin expression in the renal cortex of humanized sickle cell mice. Expression of receptors that participate in albumin handling from renal cortex of genetic control (HbAA) and humanized sickle mice (HbSS) treated with vehicle, the selective ETA antagonist, ambrisentan, or the combined ETA/B antagonist, A-182086, for 10 weeks beginning at 4 weeks of age. (A) Depicts the relative megalin mRNA expression in cortex after 10-week treatment protocol. (B) Depicts Western blot analysis of megalin expression in cortical extracts. (C) Depicts quantification of Western blot bands for megalin. (D) Depicts the relative cubilin mRNA expression in cortex after 10-week treatment protocol. (E) Depicts the relative amnionless (AMN) receptor mRNA expression in cortex after 10-week treatment protocol. (F) Depicts the relative FcRn mRNA expression in cortex after 10-week treatment protocol. Data are mean±SEM; *P<0.05 versus vehicle-treated HbAA; #P<0.05 versus vehicle-treated HbSS; n=5–6 in HbSS and n=6 in vehicle-treated HbAA group. a-18, A-182086; Amb., Ambrisentan; MW, molecular weight; RDU, relative densitometry units; Veh., vehicle.
Discussion
Renal complications of SCD include a variety of glomerular and tubular abnormalities, however, the pathogenesis of these complications remains unclear. This study demonstrated that long-term ETA receptor antagonism prevented the development and progression of sickle nephropathy, thus demonstrating that ETA receptor signaling is a critical mechanistic mediator in renal complications associated with SCD. These studies provide the basis for the use of ETA receptor antagonism as a potential therapeutic approach for the prevention of renal involvement in SCD.
Abundant evidence implicates endothelial dysfunction in many proteinuric renal diseases,19,20,22,25–27 suggesting that activation of ET receptors may lead to glomerular endothelial and tubular dysfunction, thereby contributing to proteinuria. Our group has previously demonstrated that hypoxia increases glomerular ET-1 expression in C57BL/6J mice, but not in endothelial-specific ET-1 knockout mice.28 Also, ET-1 mRNA expression in glomeruli isolated from HbSS mice was shown to be significantly elevated.22 These data may suggest a mechanism for ET-1–induced nephropathy in SCD. In this study, we observed significantly elevated plasma levels of ET-1 and proteinuria in HbSS mice, consistent with prior reports of elevated ET-1 levels and proteinuria in patients with SCD.23,29–31 Albuminuria is linked with progression of nephropathy in SCD32,33 and has been associated with a decline in GFR and glomerular permselectivity in SCD.34 This is consistent with other proteinuric renal diseases where proteinuria correlates with decline in GFR.35 In line with human disease, our results in humanized SCD mice provided evidence that prevention of proteinuria, with selective ETA receptor antagonism, was associated with preserved GFR.
Our observations suggest a pathophysiologic link between increased ET signaling and the impairment of glomerular function in SCD. Moreover, given the often opposing actions of ETA and ETB receptors, we asked the important question of whether selective ETA or nonselective ETA/B receptor antagonism provides superior or equivalent renal protection in SCD. This is particularly relevant given that both the combined ETA/B receptor antagonists, bosentan and macitentan, and the selective ETA receptor antagonist, ambrisentan, are currently Food and Drug Administration–approved for treatment of pulmonary arterial hypertension. The clinical experience that now exists with both selective and nonselective ET receptor antagonists minimizes barriers in the path to clinical translation of our findings.
The contribution of ETB receptor signaling to proteinuria and Palb in SCD could not be directly assessed using a selective ETB receptor antagonist in vivo because of hypertension, reduced ET-1 clearance, and increased ETA receptor activation that result from ETB receptor antagonism.36 Our results demonstrated that treatment with a selective ETA receptor antagonist significantly attenuated Palb in glomeruli from HbSS mice. Treatment with the combined ETA/B receptor antagonist had a similar effect, indicating that the effect of ET-1 on Palb is most likely exclusively mediated by ETA receptors. On the basis of the lower expression of ETB receptors compared with ETA receptors in glomeruli,22,37 our results support a specific role of ETA receptors in controlling Palb and are consistent with previous studies that have shown that ETB receptor antagonism does not alter ET-1–induced elevation of Palb in vitro.18 However, because Palb measurements are performed in isolated decapsulated glomeruli independent of hemodynamic influences, a potential role of ETB receptors on glomerular filtration through hemodynamic mechanisms in SCD cannot be excluded. The mechanism contributing to glomerular injury in mice treated with the combined ETA/B receptor antagonist may be an inability to prevent congestion in glomerular capillaries, ultimately leading to impairment of renal hemodynamics evidenced by reduced GFR. This idea is in accordance with other studies showing a relationship between early alterations in renal hemodynamics and heightened histologic injury after renal ischemia in SCD.38 Moreover, rats lacking ETB receptors develop more severe renal injury and proteinuria in response to hyperglycemia39 or nephrectomy.40 In contrast, we observed significant decreases in urinary albumin and protein excretion only in HbSS mice treated with the selective ETA receptor antagonist, again suggesting a crucial role of ETA receptors in mediating glomerular injury in SCD.
Intact podocytes are essential for the integrity of the glomerular filtration barrier and podocyte injury leads to albuminuria.41,42 Importantly, podocytes express ETA and ETB receptors suggesting a target for elevated ET-1. In addition, several studies have shown that disruption of the podocyte actin cytoskeleton occurs after exposure to ET-1.20,27 Moreover, previous studies have shown a direct effect of chronic ET-1 infusion on urinary excretion of nephrin, a marker of podocyte injury.18 Interestingly, 1-week administration of a selective ETA receptor antagonist attenuated this effect in SCD mice, suggesting that antagonism of ETA receptors may prevent podocyte injury in SCD.22 Here, we provide evidence that ET-1, via ETA receptor activation, mediates podocyte injury in SCD, and this effect can be prevented by chronic ETA receptor antagonism. Other investigators provided similar evidence in diabetic and nondiabetic glomerular injury18,43–45 and preeclampsia.46 When considering our findings in the context of these prior studies, the data suggest that disruption of glomerular structure by ET-1, via ETA receptor activation, leads to alterations in podocyte morphology and function, resulting in increased Palb and albuminuria/proteinuria in SCD mice. However, a recently published study demonstrated beneficial effects of podocyte-specific deletion of both ETA and ETB receptors, resulting in protection from podocyte loss and mesangial matrix expansion in diabetic nephropathy.25 Further studies with the use of podocyte-specific ETA or ETB receptor knockout mice are required to resolve this important question in SCD.
In this study, a protective effect on glomerular congestion and size was observed in HbSS mice treated with the selective ETA, but not the dual ETA/B, receptor antagonist. In contrast, Lenoir et al. reported significant benefit on glomerular structure in hypoxia-exposed SCD mice treated with the dual ET receptor antagonist, bosentan.47 However, bosentan is 30 times more selective for ETA versus ETB receptors,48 and when considered in the context of this study, this suggests that renal protection in previous studies may have been mediated primarily by blockade of ETA receptors in bosentan-treated SCD mice.24 Thus, our data highlight the importance of selectively targeting the ETA receptor to achieve maximal renal protection in SCD.
Recent studies have re-emphasized the role of proximal tubular uptake of albumin in protection against albuminuria.49,50 Renal tubules are known to be highly susceptible to hypoxic conditions51 and it has been reported that urinary KIM-1 and NAG levels, biomarkers of tubular injury, strongly correlated with albuminuria in patients with SCD.52 Therefore, we investigated the contribution of proximal tubular injury to albuminuria in SCD. We found that selective ETA receptor antagonism protects against tubular injury in HbSS mice, which may contribute to the antiproteinuric effect of the ETA receptor antagonist. These observations are also in agreement with those reported by Hocher et al.53 and may suggest an additional benefit of the use of ETA receptor antagonists in the treatment of sickle nephropathy. Our results suggest that renal tubular protein uptake may involve ETB receptor activation. Although proteinuria is often presumed to be a result of glomerular damage in SCD,54 the question of whether tubular injury contributes to impaired albuminuria or whether tubular injury is the consequence of a continual protein load remains unanswered. One explanation of the lack of reduction in albuminuria and proteinuria after administration of the dual ETA/B receptor antagonist in HbSS mice may involve increased albumin endocytosis in the proximal tubule followed by cell proliferation and injury. A second hypothesis is that blocking of ETB receptors in renal proximal tubules may cause a decrease in tubular albumin uptake, an increase in urinary albumin excretion, and ultimately tubular apoptosis and injury. This hypothesis is supported by several studies implicating ET-1 as proapoptotic in the kidney.55–58 Our results demonstrate that, although the nonselective ETA/B receptor antagonist improved glomerular structure and function to some extent in HbSS mice, increased urinary albumin excretion was still present, thus excluding glomerular injury as the only cause of albuminuria. Also, the observed downregulation of megalin and FcRn may be related to the oxidative stress–mediated transcription factor, NFκB, activation and facilitated release of proinflammatory and profibrotic mediators, including MCP-1 or P-selectin.59 These results are also consistent with the protective role of ETB receptor in proximal tubules. Moreover, data from our laboratory has shown a protective role of ETB receptor activation in tunicamycin-induced renal tubular apoptosis.60 Thus, our results suggest that the albuminuria observed in HbSS mice treated with the dual ETA/B receptor antagonist is a combination of glomerular and tubular injury or dysfunction.
In conclusion, this study implicates the ETA receptor as an important mediator in the development and progression of renal injury in SCD, and suggests that selective ETA receptor antagonism is sufficient to prevent development and progression of renal injury in SCD. These findings provide rigorous proof-of-concept evidence to support the use of ETA receptor antagonism in the treatment of sickle nephropathy, and by utilizing a well tolerated and clinically approved drug in our treatment strategy, the barriers to the next steps of clinical investigation are minimal.
Concise Methods
Animals
Studies used humanized SCD knock-in mice, with notation: B6;129-Hbatm1(HBA)Tow Hbbtm2(HBG1,HBB*)Tow/Hbbtm3(HBG1,HBB)Tow/J, developed by Townes et al.61 Experimental animals (HbSS) were homozygous for mutant β globin (E to V at position 6) and expressed human HbS. Control animals (HbAA), derived from the same colony, were homozygous for wild-type β globin and expressed human HbA. All studies were performed in 14-week-old HbSS mice with similar numbers of males and females in each group and age-matched genetic controls. Group numbers varied depending on availability of mice from the breeding colony. All mice were housed under conditions of constant temperature, humidity, and 12-hour light/dark cycle, and were provided with food (Harlan Teklad) and water ad libitum. Separate groups of mice were placed in standard metabolic cages for 48 hours before the experiments. Mice were allowed to adapt to metabolic cages for 1 day before collection of 24-hour urine samples. All mice were maintained and studied in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals following a protocol reviewed and approved by the University of Alabama at Birmingham Institutional Animal Care and Use Committees.
Drug Treatment Protocols
The selective ETA receptor antagonist ambrisentan (10mg/kg per day; AbbVie, Inc., Abbott Park, IL), nonselective ETA/ETB receptor antagonist A-182086 (10mg/kg per day; AbbVie, Inc., Abbott Park, IL), or vehicle were administrated in drinking water and the concentrations were adjusted daily according to the water intake, as previously described.62 To assess the treatment of established nephropathy, antagonists were administrated for 2 weeks beginning at 12 weeks of age, and this strategy was referred to as the 2-week treatment protocol. To assess ability of treatment to prevent the onset of nephropathy, antagonists were administrated for 10 weeks beginning at 4 weeks of age (time of weaning), and this strategy was referred to as the 10-week treatment protocol.
BP Measurements
Standard tail-cuff BP measurements, using a pneumatic transducer, were obtained on five consecutive days from 13-week-old HbSS and HbAA mice that had undergone 5 days of prior training as previously described.63 Data are presented as the average of five measurements for each animal.
GFR Measurements
GFR was measured in 14-week-old HbSS and HbAA mice (1 day before metabolic cage studies) using transcutaneous measurement of fluorescein isothiocyanate–labeled sinestrin technique as previously described.64
Measurements of Palb
Glomeruli were isolated on ice by consecutive sieving technique as previously described with minor modifications.65,66 Briefly, renal cortical tissue was passed through a series of filters (with pore size 180, 100, and 50 μm) resulting in suspension of decapsulated, intact glomeruli in ice-cold Hanks Balanced Salt Solution, pH 7.4 (Corning, NY). Freshly isolated glomeruli were used for measurements of Palb as previously described.66–68 In brief, change in glomerular volume was measured in response to an oncotic gradient induced by defined concentrations of albumin in the buffer solution.
Glomerular ROS Assay
Glomerular ROS production was measured using a luminescence assay as previously described.22,28
Plasma and Urine Analyses
All urine analyses were performed on 24-hour urine samples collected in metabolic cages. Urinary protein concentration was measured using the Bradford assay (BioRad Laboratories, CA). Urinary albumin concentration was determined using an immunoperoxidase assay according to the manufacturer’s instructions (GenWay Biotech Inc., CA). Urinary nephrin concentration was measured using a mouse NPHN ELISA kit (Uscn Life Science Inc., TX). Urine levels of tubular injury markers were measured using ELISA for mouse KIM-1 (ab119596-KIM-1 [TIM-1] Mouse ELISA kit; Abcam, UK), NAG (Mouse NAG kit; Crystal Chem, IL), and NGAL (Lipocalin-2 [NGAL] Mouse ELISA kit; Abcam, UK). Plasma levels of ET-1 were determined by ELISA according to manufacturer’s instructions (QuantiGlo ET-1 Kit; R&D Systems, MN). University of Alabama at Birmingham–University of California San Diego O’Brien Center for Acute Kidney Injury Research bioanalytic core facility measured plasma creatinine levels by liquid chromatography–tandem mass spectrometry.
Histologic Analysis
Kidneys isolated from 10-week treatment protocol mice were immersed in 10% formalin and embedded in paraffin. Kidney sections (4-μm thick) were processed for histopathology studies and immunohistochemistry assay. Sections were stained with hematoxylin and eosin, Masson trichrome, periodic acid Schiff–hematoxylin, or Prussian blue using standard protocols. Tissues were evaluated blindly according to the criteria used for quantification of the changes in renal structures, as previously described.69–72 Briefly, the extent of glomerular sclerosis was assessed on Masson trichrome–stained sections using a grading score from 0 to 4: 0 for no sclerosis; 1 for sclerosis in up to 25% of the glomerulus; 2 for sclerosis in up to 50% of the glomerulus; 3 for sclerosis in up to 75% of glomerulus; and 4 for sclerosis in up to 100% of the glomerulus. Glomerular basement membrane thickening score was assessed using the following 0–4 scale: 0 for no basement membrane thickening; 1 for mild thickening; 2 for moderate thickening; 3 for severe thickening; and 4 for massive thickening. Glomerular vascular congestion was assessed on hematoxylin and eosin–stained sections and calculated as the percentage of total glomeruli with congestion present in at least 25% of the glomerulus. A minimum of 20 glomeruli were evaluated under 400× magnification from ten different bright-field regions of renal cortex and results were averaged for each kidney. Tubular brush border thickness was assessed on periodic acid Schiff–hematoxylin–stained sections using a 0–4 grading scale: 0 for no changes; 1 for lesions involving <25% of the area; 2 for lesions involving 25%–50% of the area; 3 for lesions involving >50% of the area; and 4 for lesions involving nearly 100% of the area. Results were averaged for each animal. Tubulointerstitial fibrosis was assessed in ten fields per kidney section and graded as follows: 0 for no fibrosis; 1 for mild fibrosis; 2 for moderate fibrosis; 3 for severe fibrosis; and 4 for fibrosis involving nearly the entire area, and results averaged for each animal. Renal iron deposition was assessed on scanned images of whole kidney by MetaMorph software (MetaMorph; Molecular Devices LLC, CA).
For immunohistochemistry, sections were stained for WT-1 using anti–Wilms tumor protein antibody (CAN-R9[IHC]-56–2; ab89901, Abcam, UK), anti-CD3 (T cells) antibody (ab16669,1:600; Abcam, UK), and anti-F/4/80 (macrophages) antibody (MCA497GA, 1:200; BioRad). WT-1–positive cells were counted under 400× magnification from a minimum of 20 glomeruli from ten different bright-field regions of the cortex and results were averaged for each kidney. Renal cortical CD3+ and F4/80+ cells were blindly quantified in ten microscopic fields (200 × 200 μm, 400× magnification) and results were averaged for each kidney.
Electron Microscopy
Small cubes of renal cortex (1.5 × 1.5 × 1.5 mm) were drop-fixed in 2.5% glutaraldehyde/2.5% formalin, postfixed in 1% osmium tetroxide, and processed into resin blocks. Ultrathin sections were cut using a diamond knife, placed in a copper grid, stained with uranyl acetate and lead citrate, and examined using an FEI Tecnai T12 Spirit 20–120 kv transmission electron microscope.
Total RNA Extraction and Quantitative Real Time PCR
Total RNA extraction of renal cortex or glomeruli was performed using Ambion PureLink RNA Mini Kit or RNAqueous-Micro Total RNA Isolation Kit (Ambion, Austin, TX), and reverse transcription with the iScript cDNA synthesis kit (BioRad, Hercules, CA). Real-time amplification was performed with ABI 7300 Real-Time PCR System using iTaq Universal Probes Supermix (BioRad, Hercules, CA) and TaqMan primer gene expression assay (Applied Biosystems, Foster City, CA) of ET-1 (Mn00438656_m1), ETA (Mn01243722_m1), ETB (Mn00432989_m1), WT-1 (Mn01337048), nephrin (Mn00497828), podocin (Mn01292252_m1), synaptopodin (Mn03413333_m1), MCP-1 (Mn00441242_m1), P-selectin (Mn01295931_m1), V-CAM1 (Mn01320970_m1), megalin (Mn01328171_m1), cubilin (Mn01325077_m1), amnionless (AMN; Mn00473870_m1), and FcRn (Mn01205451_m1) according to the manufacturer’s instructions. The comparative method of relative quantification (2-ΔΔCT) was used to calculate the expression level of each target gene, normalized to β-actin. Data are presented as the relative mRNA expression of the specific gene of interest.
Western Blotting
Thirty micrograms of protein was electrophoresed on 4%–15% gels (#4561086; Bio-Rad) as previously described.73 Blots were probed with a rabbit polyclonal antibody against the megalin c-terminus (ab76969; Abcam, UK). Equivalent protein loading was confirmed by Coomassie staining.
Statistical Analyses
Analyses were performed using GraphPad Prism 6.0 software (GraphPad Software Inc., CA). Data were compared using paired t test or two-way ANOVA with Bonferroni post hoc correction (variables: genotype, treatment). One-way ANOVA with Bonferroni's post hoc correction for multiple comparisons was utilized for Western blot data. Results are expressed as mean±SEM with α=0.05.
Disclosures
None.
Supplementary Material
Acknowledgments
The technical assistance of Binli Tao and Kaquanta Barlow is greatly appreciated. The authors also thank Xiaofen Liu and Ping Hua for assistance with histologic and Western blot studies. We acknowledge the University of Alabama at Birmingham High Resolution Shared Imaging Facility for assistance with electron microscopy, as well as the Center for Metabolic Bone Disease Core Laboratory for assistance in preparing tissues for histologic evaluation.
This research was supported by grants from the National Heart, Lung, and Blood Institute, U01 HL117684 to A.K., J.S.P., and D.M.P.; K99 1K99HL127178 and T32DK079339 to J.S.S.; National Institutes of Health T32 DK007545 to C.D.M.; the National Institute of Diabetes and Digestive and Kidney Disease, F30 DK107194 to B.M.F.; the American Heart Association, 15POST25090329 to E.Y.G.; the American Society of Nephrology, Joseph A. Carlucci Research Fellowship to M.K. and P30 DK079337 (the core resource of the University of Alabama at Birmingham–University of California San Diego O’Brien Center).
Parts of this work were presented during Kidney week, November 7, 2015, San Diego, CA, and Kidney Week, November 18, 2016, Chicago, IL.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Endothelin-A Receptor Antagonism Retards the Progression of Murine Sickle Cell Nephropathy,” on pages 2253–2255.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2016070711/-/DCSupplemental.
References
- 1.Hassell KL: Population estimates of sickle cell disease in the U.S. Am J Prev Med 38[Suppl]: S512–S521, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Weatherall DJ, Clegg JB: Inherited haemoglobin disorders: An increasing global health problem. Bull World Health Organ 79: 704–712, 2001 [PMC free article] [PubMed] [Google Scholar]
- 3.Nath KA, Hebbel RP: Sickle cell disease: Renal manifestations and mechanisms. Nat Rev Nephrol 11: 161–171, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lanzkron S, Carroll CP, Haywood C Jr: Mortality rates and age at death from sickle cell disease: U.S., 1979-2005. Public Health Rep 128: 110–116, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yawn BP, Buchanan GR, Afenyi-Annan AN, Ballas SK, Hassell KL, James AH, Jordan L, Lanzkron SM, Lottenberg R, Savage WJ, Tanabe PJ, Ware RE, Murad MH, Goldsmith JC, Ortiz E, Fulwood R, Horton A, John-Sowah J: Management of sickle cell disease: Summary of the 2014 evidence-based report by expert panel members. JAMA 312: 1033–1048, 2014 [DOI] [PubMed] [Google Scholar]
- 6.Ataga KI, Orringer EP: Renal abnormalities in sickle cell disease. Am J Hematol 63: 205–211, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Elmariah H, Garrett ME, De Castro LM, Jonassaint JC, Ataga KI, Eckman JR, Ashley-Koch AE, Telen MJ: Factors associated with survival in a contemporary adult sickle cell disease cohort. Am J Hematol 89: 530–535, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamideh D, Alvarez O: Sickle cell disease related mortality in the United States (1999-2009). Pediatr Blood Cancer 60: 1482–1486, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Davenport AP, Hyndman KA, Dhaun N, Southan C, Kohan DE, Pollock JS, Pollock DM, Webb DJ, Maguire JJ: Endothelin. Pharmacol Rev 68: 357–418, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yanagisawa M, Masaki T: Molecular biology and biochemistry of the endothelins. Trends Pharmacol Sci 10: 374–378, 1989 [DOI] [PubMed] [Google Scholar]
- 11.Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T: A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 332: 411–415, 1988 [DOI] [PubMed] [Google Scholar]
- 12.Kowalczyk A, Kleniewska P, Kolodziejczyk M, Skibska B, Goraca A: The role of endothelin-1 and endothelin receptor antagonists in inflammatory response and sepsis. Arch Immunol Ther Exp (Warsz) 63: 41–52, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosanò L, Spinella F, Bagnato A: Endothelin 1 in cancer: Biological implications and therapeutic opportunities. Nat Rev Cancer 13: 637–651, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Khodorova A, Montmayeur JP, Strichartz G: Endothelin receptors and pain. J Pain 10: 4–28, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Speed JS, Fox BM, Johnston JG, Pollock DM: Endothelin and renal ion and water transport. Semin Nephrol 35: 137–144, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maguire JJ, Davenport AP: Endothelin receptors and their antagonists. Semin Nephrol 35: 125–136, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fox BM, Kasztan M: Endothelin receptor antagonists in sickle cell disease: A promising new therapeutic approach. Life Sci 159: 15–19, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saleh MA, Boesen EI, Pollock JS, Savin VJ, Pollock DM: Endothelin-1 increases glomerular permeability and inflammation independent of blood pressure in the rat. Hypertension 56: 942–949, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ortmann J, Amann K, Brandes RP, Kretzler M, Münter K, Parekh N, Traupe T, Lange M, Lattmann T, Barton M: Role of podocytes for reversal of glomerulosclerosis and proteinuria in the aging kidney after endothelin inhibition. Hypertension 44: 974–981, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Morigi M, Buelli S, Angioletti S, Zanchi C, Longaretti L, Zoja C, Galbusera M, Gastoldi S, Mundel P, Remuzzi G, Benigni A: In response to protein load podocytes reorganize cytoskeleton and modulate endothelin-1 gene: Implication for permselective dysfunction of chronic nephropathies. Am J Pathol 166: 1309–1320, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peppa-Patrikiou M, Dracopoulou M, Dacou-Voutetakis C: Urinary endothelin in adolescents and young adults with insulin-dependent diabetes mellitus: Relation to urinary albumin, blood pressure, and other factors. Metabolism 47: 1408–1412, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Heimlich JB, Speed JS, O’Connor PM, Pollock JS, Townes TM, Meiler SE, Kutlar A, Pollock DM: Endothelin-1 contributes to the progression of renal injury in sickle cell disease via reactive oxygen species. Br J Pharmacol 173: 386–395, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tharaux PL, Hagège I, Placier S, Vayssairat M, Kanfer A, Girot R, Dussaule JC: Urinary endothelin-1 as a marker of renal damage in sickle cell disease. Nephrol Dial Transplant 20: 2408–2413, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Sabaa N, de Franceschi L, Bonnin P, Castier Y, Malpeli G, Debbabi H, Galaup A, Maier-Redelsperger M, Vandermeersch S, Scarpa A, Janin A, Levy B, Girot R, Beuzard Y, Leboeuf C, Henri A, Germain S, Dussaule JC, Tharaux PL: Endothelin receptor antagonism prevents hypoxia-induced mortality and morbidity in a mouse model of sickle-cell disease. J Clin Invest 118: 1924–1933, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lenoir O, Milon M, Virsolvy A, Hénique C, Schmitt A, Massé JM, Kotelevtsev Y, Yanagisawa M, Webb DJ, Richard S, Tharaux PL: Direct action of endothelin-1 on podocytes promotes diabetic glomerulosclerosis. J Am Soc Nephrol 25: 1050–1062, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benigni A, Zoja C, Corna D, Orisio S, Longaretti L, Bertani T, Remuzzi G: A specific endothelin subtype A receptor antagonist protects against injury in renal disease progression. Kidney Int 44: 440–444, 1993 [DOI] [PubMed] [Google Scholar]
- 27.Morigi M, Buelli S, Zanchi C, Longaretti L, Macconi D, Benigni A, Moioli D, Remuzzi G, Zoja C: Shigatoxin-induced endothelin-1 expression in cultured podocytes autocrinally mediates actin remodeling. Am J Pathol 169: 1965–1975, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heimlich JB, Speed JS, Bloom CJ, O’Connor PM, Pollock JS, Pollock DM: ET-1 increases reactive oxygen species following hypoxia and high-salt diet in the mouse glomerulus. Acta Physiol (Oxf) 213: 722–730, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rybicki AC, Benjamin LJ: Increased levels of endothelin-1 in plasma of sickle cell anemia patients. Blood 92: 2594–2596, 1998 [PubMed] [Google Scholar]
- 30.Hammerman SI, Kourembanas S, Conca TJ, Tucci M, Brauer M, Farber HW: Endothelin-1 production during the acute chest syndrome in sickle cell disease. Am J Respir Crit Care Med 156: 280–285, 1997 [DOI] [PubMed] [Google Scholar]
- 31.Graido-Gonzalez E, Doherty JC, Bergreen EW, Organ G, Telfer M, McMillen MA: Plasma endothelin-1, cytokine, and prostaglandin E2 levels in sickle cell disease and acute vaso-occlusive sickle crisis. Blood 92: 2551–2555, 1998 [PubMed] [Google Scholar]
- 32.Gosmanova EO, Zaidi S, Wan JY, Adams-Graves PE: Prevalence and progression of chronic kidney disease in adult patients with sickle cell disease. J Investig Med 62: 804–807, 2014 [DOI] [PubMed] [Google Scholar]
- 33.Powars DR, Elliott-Mills DD, Chan L, Niland J, Hiti AL, Opas LM, Johnson C: Chronic renal failure in sickle cell disease: Risk factors, clinical course, and mortality. Ann Intern Med 115: 614–620, 1991 [DOI] [PubMed] [Google Scholar]
- 34.Guasch A, Cua M, Mitch WE: Early detection and the course of glomerular injury in patients with sickle cell anemia. Kidney Int 49: 786–791, 1996 [DOI] [PubMed] [Google Scholar]
- 35.Remuzzi G, Bertani T: Pathophysiology of progressive nephropathies. N Engl J Med 339: 1448–1456, 1998 [DOI] [PubMed] [Google Scholar]
- 36.Pollock DM, Pollock JS: Evidence for endothelin involvement in the response to high salt. Am J Physiol Renal Physiol 281: F144–F150, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Karet FE, Kuc RE, Davenport AP: Novel ligands BQ123 and BQ3020 characterize endothelin receptor subtypes ETA and ETB in human kidney. Kidney Int 44: 36–42, 1993 [DOI] [PubMed] [Google Scholar]
- 38.Juncos JP, Grande JP, Croatt AJ, Hebbel RP, Vercellotti GM, Katusic ZS, Nath KA: Early and prominent alterations in hemodynamics, signaling, and gene expression following renal ischemia in sickle cell disease. Am J Physiol Renal Physiol 298: F892–F899, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pfab T, Thöne-Reineke C, Theilig F, Lange I, Witt H, Maser-Gluth C, Bader M, Stasch JP, Ruiz P, Bachmann S, Yanagisawa M, Hocher B: Diabetic endothelin B receptor-deficient rats develop severe hypertension and progressive renal failure. J Am Soc Nephrol 17: 1082–1089, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Tazawa N, Okada Y, Nakata M, Izumoto H, Takasu M, Takaoka M, Gariepy CE, Yanagisawa M, Matsumura Y: Exaggerated vascular and renal pathology in endothelin-B-receptor-deficient rats with subtotal nephrectomy. J Cardiovasc Pharmacol 44[Suppl 1]: S467–S470, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Kerjaschki D: Caught flat-footed: Podocyte damage and the molecular bases of focal glomerulosclerosis. J Clin Invest 108: 1583–1587, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johnstone DB, Holzman LB: Clinical impact of research on the podocyte slit diaphragm. Nat Clin Pract Nephrol 2: 271–282, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Kohan DE, Rossi NF, Inscho EW, Pollock DM: Regulation of blood pressure and salt homeostasis by endothelin. Physiol Rev 91: 1–77, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wenzel RR, Littke T, Kuranoff S, Jürgens C, Bruck H, Ritz E, Philipp T, Mitchell A; SPP301 (Avosentan) Endothelin Antagonist Evaluation in Diabetic Nephropathy Study Investigators : Avosentan reduces albumin excretion in diabetics with macroalbuminuria. J Am Soc Nephrol 20: 655–664, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dhaun N, MacIntyre IM, Kerr D, Melville V, Johnston NR, Haughie S, Goddard J, Webb DJ: Selective endothelin-A receptor antagonism reduces proteinuria, blood pressure, and arterial stiffness in chronic proteinuric kidney disease. Hypertension 57: 772–779, 2011 [DOI] [PubMed] [Google Scholar]
- 46.Collino F, Bussolati B, Gerbaudo E, Marozio L, Pelissetto S, Benedetto C, Camussi G: Preeclamptic sera induce nephrin shedding from podocytes through endothelin-1 release by endothelial glomerular cells. Am J Physiol Renal Physiol 294: F1185–F1194, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Lenoir O, Sabaa N, Henrique C, Guyonnet L, Fligny C, Audard V, Tharaux PL: Endothelin receptor antagonism in sickle cell nephropathy. Presented at the 14th International Conference on Endothelin: Physiology, Pathophysiology and Therapeutics, Savannah, GA, Physiologist, 2015 [Google Scholar]
- 48.Opitz CF, Ewert R, Kirch W, Pittrow D: Inhibition of endothelin receptors in the treatment of pulmonary arterial hypertension: Does selectivity matter? Eur Heart J 29: 1936–1948, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brunskill NJ: Molecular interactions between albumin and proximal tubular cells. Exp Nephrol 6: 491–495, 1998 [DOI] [PubMed] [Google Scholar]
- 50.Dickson LE, Wagner MC, Sandoval RM, Molitoris BA: The proximal tubule and albuminuria: Really! J Am Soc Nephrol 25: 443–453, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bonventre JV, Yang L: Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest 121: 4210–4221, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sundaram N, Bennett M, Wilhelm J, Kim MO, Atweh G, Devarajan P, Malik P: Biomarkers for early detection of sickle nephropathy. Am J Hematol 86: 559–566, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hocher B, Kalk P, Slowinski T, Godes M, Mach A, Herzfeld S, Wiesner D, Arck PC, Neumayer HH, Nafz B: ETA receptor blockade induces tubular cell proliferation and cyst growth in rats with polycystic kidney disease. J Am Soc Nephrol 14: 367–376, 2003 [DOI] [PubMed] [Google Scholar]
- 54.Marsenic O, Couloures KG, Wiley JM: Proteinuria in children with sickle cell disease. Nephrol Dial Transplant 23: 715–720, 2008 [DOI] [PubMed] [Google Scholar]
- 55.Hocher B, Rohmeiss P, Thöne-Reineke C, Schwarz A, Burst V, van der Woude F, Bauer C, Theuring F: Apoptosis in kidneys of endothelin-1 transgenic mice. J Cardiovasc Pharmacol 31[Suppl 1]: S554–S556, 1998 [DOI] [PubMed] [Google Scholar]
- 56.Dong F, Zhang X, Wold LE, Ren Q, Zhang Z, Ren J: Endothelin-1 enhances oxidative stress, cell proliferation and reduces apoptosis in human umbilical vein endothelial cells: Role of ETB receptor, NADPH oxidase and caveolin-1. Br J Pharmacol 145: 323–333, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meyer TW: Tubular injury in glomerular disease. Kidney Int 63: 774–787, 2003 [DOI] [PubMed] [Google Scholar]
- 58.Zoja C, Morigi M, Figliuzzi M, Bruzzi I, Oldroyd S, Benigni A, Ronco P, Remuzzi G: Proximal tubular cell synthesis and secretion of endothelin-1 on challenge with albumin and other proteins. Am J Kidney Dis 26: 934–941, 1995 [DOI] [PubMed] [Google Scholar]
- 59.Baines RJ, Brunskill NJ: The molecular interactions between filtered proteins and proximal tubular cells in proteinuria. Nephron Exp Nephrol 110: e67–e71, 2008 [DOI] [PubMed] [Google Scholar]
- 60.De Miguel C, Pollock DM, Pollock JS: Endothelium-derived ET-1 and the development of renal injury. Am J Physiol Regul Integr Comp Physiol 309: R1071–R1073, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu LC, Sun CW, Ryan TM, Pawlik KM, Ren J, Townes TM: Correction of sickle cell disease by homologous recombination in embryonic stem cells. Blood 108: 1183–1188, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saleh MA, Pollock JS, Pollock DM: Distinct actions of endothelin A-selective versus combined endothelin A/B receptor antagonists in early diabetic kidney disease. J Pharmacol Exp Ther 338: 263–270, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pollock DM, Rekito A: Hypertensive response to chronic NO synthase inhibition is different in Sprague-Dawley rats from two suppliers. Am J Physiol 275: R1719–R1723, 1998 [DOI] [PubMed] [Google Scholar]
- 64.Ellery SJ, Cai X, Walker DD, Dickinson H, Kett MM: Transcutaneous measurement of glomerular filtration rate in small rodents: Through the skin for the win? Nephrology (Carlton) 20: 117–123, 2015 [DOI] [PubMed] [Google Scholar]
- 65.Misra RP: Isolation of glomeruli from mammalian kidneys by graded sieving. Am J Clin Pathol 58: 135–139, 1972 [DOI] [PubMed] [Google Scholar]
- 66.Piwkowska A, Rogacka D, Kasztan M, Angielski S, Jankowski M: Insulin increases glomerular filtration barrier permeability through dimerization of protein kinase G type Iα subunits. Biochim Biophys Acta 1832: 791–804, 2013 [DOI] [PubMed] [Google Scholar]
- 67.Savin VJ, Sharma R, Lovell HB, Welling DJ: Measurement of albumin reflection coefficient with isolated rat glomeruli. J Am Soc Nephrol 3: 1260–1269, 1992 [DOI] [PubMed] [Google Scholar]
- 68.Kasztan M, Piwkowska A, Kreft E, Rogacka D, Audzeyenka I, Szczepanska-Konkel M, Jankowski M: Extracellular purines’ action on glomerular albumin permeability in isolated rat glomeruli: Insights into the pathogenesis of albuminuria. Am J Physiol Renal Physiol 311: F103–F111, 2016 [DOI] [PubMed] [Google Scholar]
- 69.Hocher B, Thöne-Reineke C, Rohmeiss P, Schmager F, Slowinski T, Burst V, Siegmund F, Quertermous T, Bauer C, Neumayer HH, Schleuning WD, Theuring F: Endothelin-1 transgenic mice develop glomerulosclerosis, interstitial fibrosis, and renal cysts but not hypertension. J Clin Invest 99: 1380–1389, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Amann K, Koch A, Hofstetter J, Gross ML, Haas C, Orth SR, Ehmke H, Rump LC, Ritz E: Glomerulosclerosis and progression: Effect of subantihypertensive doses of alpha and beta blockers. Kidney Int 60: 1309–1323, 2001 [DOI] [PubMed] [Google Scholar]
- 71.Raij L, Azar S, Keane WF: Role of hypertension in progressive glomerular immune injury. Hypertension 7: 398–404, 1985 [PubMed] [Google Scholar]
- 72.Wang Y, Doshi M, Khan S, Li W, Zhang PL: Utility of iron staining in identifying the cause of renal allograft dysfunction in patients with sickle cell disease. Case Rep Transplant 2015: 528792, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hyndman KA, Musall JB, Xue J, Pollock JS: Dynamin activates NO production in rat renal inner medullary collecting ducts via protein-protein interaction with NOS1. Am J Physiol Renal Physiol 301: F118–F124, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.