Abstract
Adaptation of the organism to potassium (K+) deficiency requires precise coordination among organs involved in K+ homeostasis, including muscle, liver, and kidney. How the latter performs functional and molecular changes to ensure K+ retention is not well understood. Here, we investigated the role of ubiquitin-protein ligase NEDD4-2, which negatively regulates the epithelial sodium channel (ENaC), Na+/Cl− cotransporter (NCC), and with no-lysine-kinase 1 (WNK1). After dietary K+ restriction for 2 weeks, compared with control littermates, inducible renal tubular NEDD4-2 knockout (Nedd4LPax8/LC1) mice exhibited severe hypokalemia and urinary K+ wasting. Notably, expression of the ROMK K+ channel did not change in the distal convoluted tubule and decreased slightly in the cortical/medullary collecting duct, whereas BK channel abundance increased in principal cells of the connecting tubule/collecting ducts. However, K+ restriction also enhanced ENaC expression in Nedd4LPax8/LC1 mice, and treatment with the ENaC inhibitor, benzamil, reversed excessive K+ wasting. Moreover, K+ restriction increased WNK1 and WNK4 expression and enhanced SPAK-mediated NCC phosphorylation in Nedd4LPax8/LC1 mice, with no change in total NCC. We propose a mechanism in which NEDD4-2 deficiency exacerbates hypokalemia during dietary K+ restriction primarily through direct upregulation of ENaC, whereas increased BK channel expression has a less significant role. These changes outweigh the compensatory antikaliuretic effects of diminished ROMK expression, increased NCC phosphorylation, and enhanced WNK pathway activity in the distal convoluted tubule. Thus, NEDD4-2 has a crucial role in K+ conservation through direct and indirect effects on ENaC, distal nephron K+ channels, and WNK signaling.
Keywords: ENaC, ion transport, K channels, signaling
Organisms handle potassium (K+) deficiency by activating mechanisms aimed at conserving K+ and establishing normokalemia.1,2 Hypokalemia may result from renal and extrarenal disorders and is a side effect of many drug therapies.3–5 The renal response to K+ deficiency occurs in the distal nephron, where K+ secretion via principal cells is diminished and K+ reabsorption in intercalated cells is activated, allowing the decrease of K+ excretion to near zero.1,2,6 Genetic, physiologic, and pathophysiologic evidences indicate that net urinary K+ excretion results from an interplay between different ion transport processes in the ASDN. Indeed, the Na+/Cl− cotransporter (NCC) in the distal convoluted tubule (DCT) controls how much Na+ is delivered to the CNT/CD, which promotes electrogenic Na+ reabsorption via epithelial sodium channel (ENaC) and K+ excretion via ROMK and BK channels.7–9 The regulation of these proteins involves both aldosterone-dependent and -independent mechanisms.8,10–14 Aldosterone-dependent K+ secretion predominates during hyperkalemia, when aldosterone is elevated. This is supported by observations that deletion of aldosterone synthase, the mineralocorticoid receptor (MR), the aldosterone-induced kinase SGK1, or αENaC all result in hyperkalemia.10,12,15–18 It is well accepted that under such hyperkalemic conditions, SGK1 is strongly expressed and phosphorylates the ubiquitin-protein ligase NEDD4-2, thereby interfering with downregulation of ENaC and the WNK/SPAK/NCC pathway.17,19–21 On the other hand, it is less well understood what mechanisms are active during K+ restriction, when the body has to preserve K+. Under such low aldosterone conditions, the aldosterone/MR/SGK1 regulatory axis is switched off. Nevertheless, NCC is stimulated because of the aldosterone-independent effect of hypokalemia.11,22,23 One can reasonably assume that mechanisms that interfere with K+ secretion are important as well, and that such inhibitory mechanisms might involve NEDD4-2.
Although NEDD4-2 is best characterized as an ENaC inhibitor,24–32 it was recently shown that its role is more complex. Both in vitro, as well as in NEDD4-2 knockout (Nedd4LPax8/LC1) mice given a high Na+ diet (HSD), NEDD4-2 acts as a NCC inhibitor,20,21 whereas it affects ENaC to a lesser degree. These effects were observed under HSD, a maneuver that suppresses endogenous aldosterone production, thereby maximizing NEDD4-2 activity. However, it is not known how regulation of these proteins by NEDD4-2 is coordinated during K+ restriction, a qualitatively different stress that also suppresses aldosterone. If the effect of NEDD4-2 on NCC predominates under K+ restriction, then the increased NCC activity seen with NEDD4-2 deletion would result in diminished Na+ delivery to the CNT/CCD, minimizing the hypokalemic effect of K+ deficiency. In contrast, if NEDD4-2–dependent inhibition of ENaC outweighs its effect on NCC, NEDD4-2 deletion would be expected to increase voltage-dependent distal K+ excretion, exacerbating the hypokalemia. Our findings in nephron-specific Nedd4LPax8/LC1 mice20 clearly demonstrate that the latter is the case, particularly when dietary K+ is restricted over the long term. Thus, taken together with prior observations, our data indicate that the primary regulatory target of NEDD4-2 action may change depending on the type of physiologic stimulus that suppresses aldosterone secretion; under conditions of volume expansion caused by dietary Na+ loading, NEDD4-2 primarily regulates NCC,20 whereas under conditions where hypokalemia is induced by K+ restriction, NEDD4-2 primarily regulates ENaC. These observations reveal a previously unrecognized role for NEDD4-2 as an essential suppressor of tubular ENaC activity when dietary K+ is scarce.
Results
The Early Adaptation to K+ Deficiency Is Independent of Renal Tubular NEDD4-2
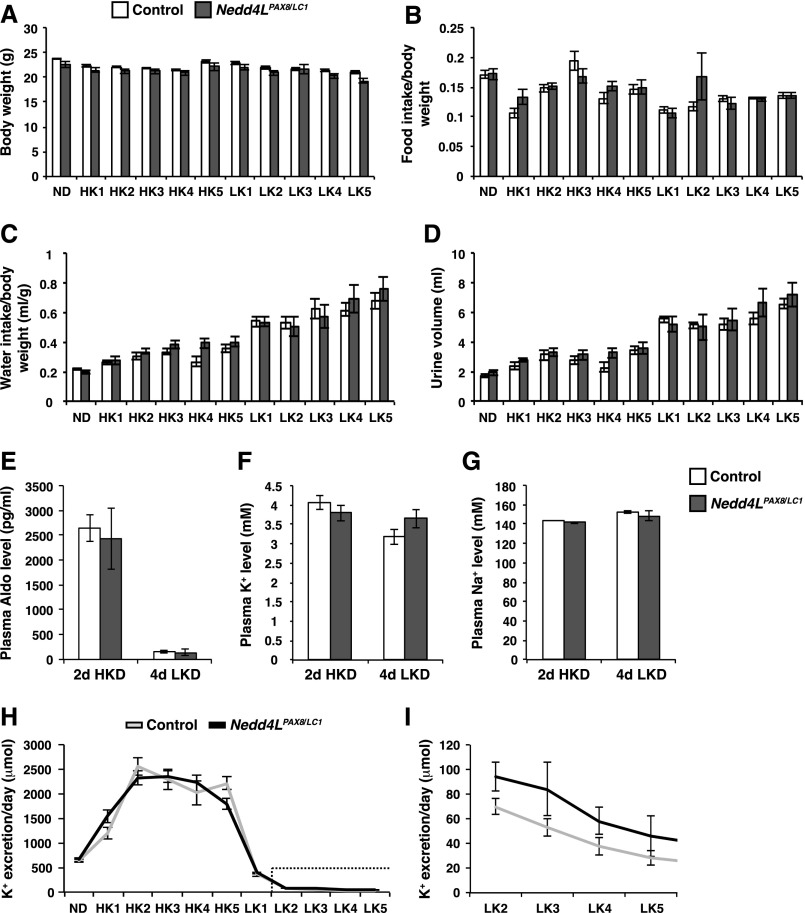
To evaluate how NEDD4-2 regulates renal tubular transport processes under varying K+ diets, control and Nedd4LPax8/LC1 mice were initially kept under high K+ diet (HKD) for 5 days, followed by another 5 days of low K+ diet (LKD). During this maneuver, no difference in body weight, food/water consumption, and urine volume was observed between genotypes (Figure 1, A–D). As expected, HKD increased and LKD decreased plasma aldosterone levels in all mice (Figure 1E). HKD induced kaliuresis, which reached a maximum after 2 days (Figure 1H). Upon switching to LKD, K+ excretion declined dramatically (Figure 1H), and Nedd4LPax8/LC1 mice tended to preserve less K+ (Figure 1I). Plasma K+ and Na+ at 4 days of LKD were similar in both genotypes (Figure 1, F and G). Similar to recent observations by Walter et al.,23 urinary Na+ excretion transiently increased under LKD in both groups (Supplemental Figure 1A) but was recovered after 2 weeks (Figure 2D). This transient LKD-induced natriuresis is most likely because of diminished aldosterone and reduction of ENaC activity,33,34 and diminution of NKCC2 expression/activity attributed to a reduction in filtered K+.35–38 These observations suggest that the kidney is able to conserve K+ independently of NEDD4-2 action in the short term.
Figure 1.
Metabolic parameters of Nedd4LPax8/LC1 mice challenged by HKD and LKD. (A–D) Body weight (A), food intake/body weight (B), water intake/body weight (C), and urine volume (D). No significant difference was observed between control and mutant mice in the measured parameters (eight controls and seven Nedd4LPax8/LC1 mice). (E–G) Plasma aldosterone (E), plasma K+ (F), and plasma Na+ (G) levels after 2 days of HKD (ten controls and seven Nedd4LPax8/LC1 mice) and after 4 days of LKD (five controls and four Nedd4LPax8/LC1 mice). Note the sharp reduction in aldosterone levels in response to LKD. No difference was observed between control and mutant mice in plasma aldosterone, Na+, and K+ levels. (H and I) Urinary K+ excretion per day under normal diet and during 5 days of HKD and 5 days of LKD; (I) is a magnification of the dotted box in (H), indicating K+ excretion under LKD starting from day 2 until day 5. Mutant mice seem to be less able to reduce their urinary K+ excretion than control littermates. The difference was not statistically significant (eight controls and seven Nedd4LPax8/LC1 mice). HK, day of high K+ diet; LK, day of low K+ diet; ND, normal diet.
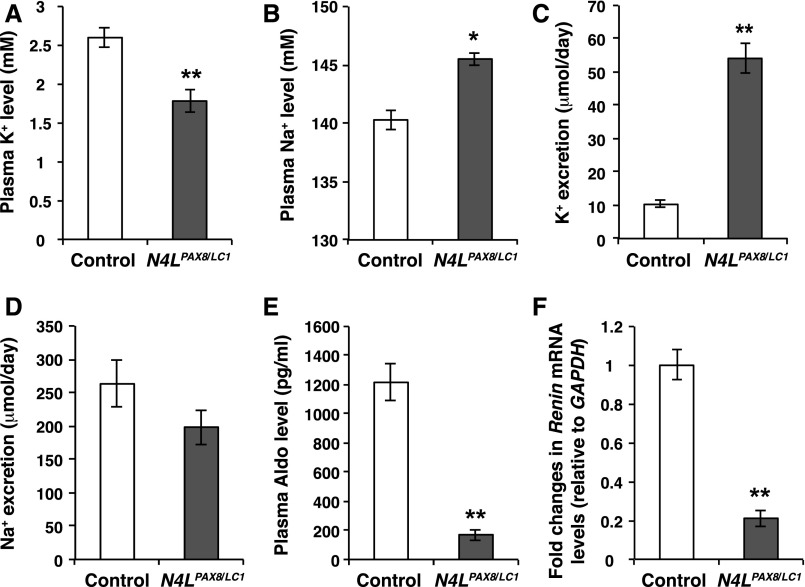
Figure 2.
Nedd4LPax8/LC1 mice develop a K+ wasting phenotype after 2 weeks of LKD. (A and B) Plasma K+ and Na+ in control (white) and Nedd4LPax8/LC1 (gray) mice after 2 weeks of LKD. The renal suppression of NEDD4-2 results in severe hypokalemia and a slight increase in plasma [Na+] (n= 12 controls and five Nedd4LPax8/LC1 mice). (C and D) Analysis of 24-hour urinary K+ and Na+ excretion reveal elevated K+ excretion in mutant mice and concomitant but not significant Na+ retention (six mice in each group). (E and F) Plasma aldosterone (n=12 controls and five Nedd4LPax8/LC1 mice) and renin expression (n= five control and five Nedd4LPax8/LC1 mice) are decreased in Nedd4LPax8/LC1 mice as compared with controls. *P<0.05; **P<0.01.
Nedd4LPax8/LC1 Mice Display a Defect in K+ Retention under Prolonged K+ Deficiency
As mutant mice showed a tendency to K+ loss, we extended the LKD treatment to 2 weeks, expecting total body K+ to be depleted, inducing frank hypokalemia. Indeed, under these conditions both groups developed hypokalemia. However, in the Nedd4LPax8/LC1 mice, the hypokalemia was more severe (Figure 2A) and was accompanied by increased urinary K+ excretion (Figure 2C). Mutant mice exhibited slight hypernatremia and a tendency toward decreased Na+ excretion (Figure 2, B and D). Because both aldosterone and renin expression were decreased in Nedd4LPax8/LC1 mice compared with controls (Figure 2, E and F), hypervolemia is likely the primary cause for the relative discrepancy in aldosterone. We note the surprisingly high aldosterone levels measured in control mice as already previously reported for standard diet (1350 pg/ml),20 which appears to be inherent to the model used. No differences in body weight, food and water intake, urine volume (Supplemental Table 1), or urine osmolality (Supplemental Figure 1B) were observed.
These findings suggest that NEDD4-2 is regulated by alterations in dietary K+. To evaluate this further, we subjected control mice either to LKD for 2 weeks or HKD for 2 days and analyzed NEDD4-2 expression and phosphorylation levels in total kidney lysates. After 2 weeks of LKD, NEDD4-2 was less phosphorylated at S222 and S328 by approximately 75% and 65%, respectively, compared with HKD, with no significant change in total NEDD4-2 (Supplemental Figure 2, A and B). However, immunofluorescence further showed that total NEDD4-2 is upregulated in the CNT/CD under LKD (Supplemental Figure 2, C and D). This effect was masked in Western blots, likely because of expression of NEDD4-2 elsewhere in the kidney.39
Decreased ROMK Membrane Localization in Nedd4LPax8/LC1 Mice
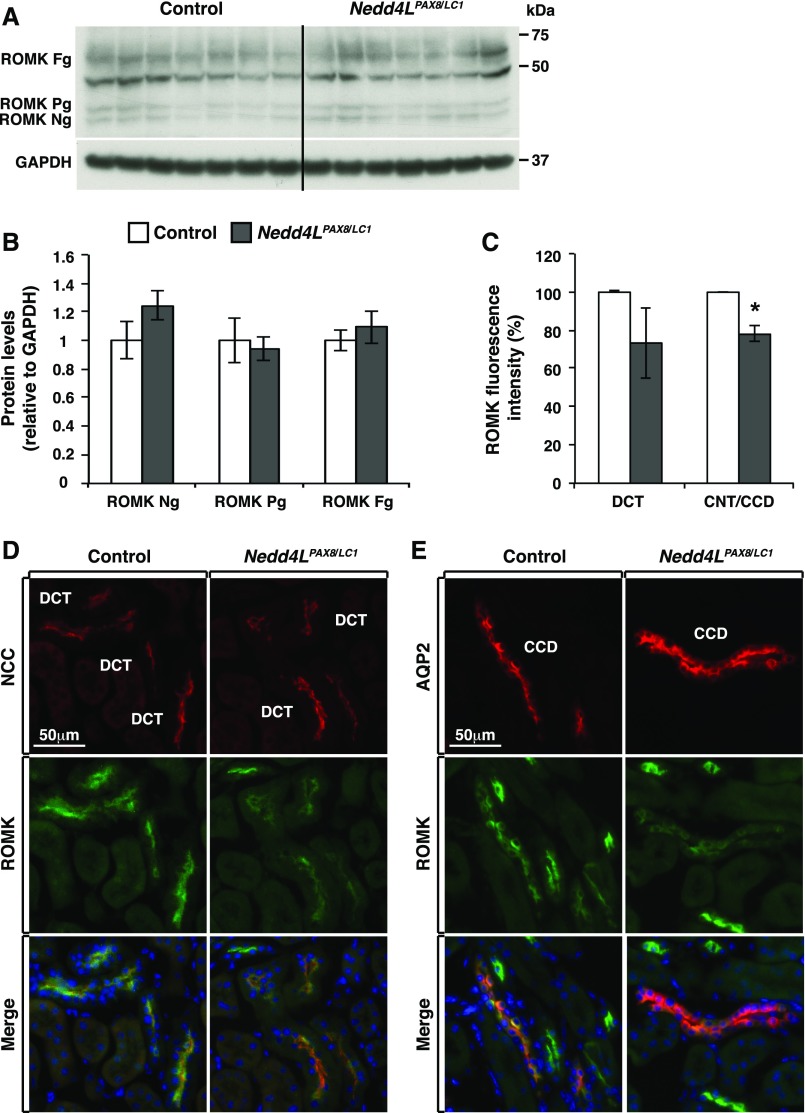
Previously, Nedd4LPax8/LC1 mice challenged by HSD for 10 days exhibited enhanced abundance and apical localization of ROMK in the distal nephron.20 We therefore analyzed ROMK expression and membrane abundance in control and mutant mice after 2 weeks of LKD. Western blot analyses showed no difference in ROMK expression (Figure 3, A and B). In contrast, immunofluorescence staining indicated that ROMK membrane localization was less abundant in the CNT/CD by about 20% (Figure 3, C and E), whereas no difference was observed in the DCT (Figure 3, C and D). These data suggest that CNT/CD ROMK expression is altered in mutant mice; however, this alteration does not explain the observed K+ wasting.
Figure 3.
ROMK apical localization is decreased in the distal nephron of Nedd4LPax8/LC1 mice under LKD. (A) Western blot analysis of ROMK from control and Nedd4LPax8/LC1 mice after 2 weeks of LKD; the band at 50 kDa is nonspecific.17 (B) Protein quantification of (A) showing similar expression levels of ROMK in both genotypes (seven mice in each group). (C) ROMK fluorescence intensity in DCT and CNT/CCD segments (from D and E) from control and Nedd4LPax8/LC1 mice. Shown are the ratios of cortical ROMK labeling over the surface area of NCC or AQP2-expressing cells, respectively. *P<0.05. (D and E) Costaining of ROMK (green) with NCC (red) (D) and AQP2 (red) (E) from control and Nedd4LPax8/LC1 mice. ROMK level is significantly decreased in the CNT/CCD (C and E) segments of Nedd4LPax8/LC1 mice, the decrease of ROMK in the DCT is not statistically significant (C and D) (four mice in each group). Fg, fully glycosylated; Ng, nonglycosylated ROMK; Pg, partially glycosylated.
The Hypokalemia in Nedd4LPax8/LC1 Mice Is Caused by Increased ENaC Activity
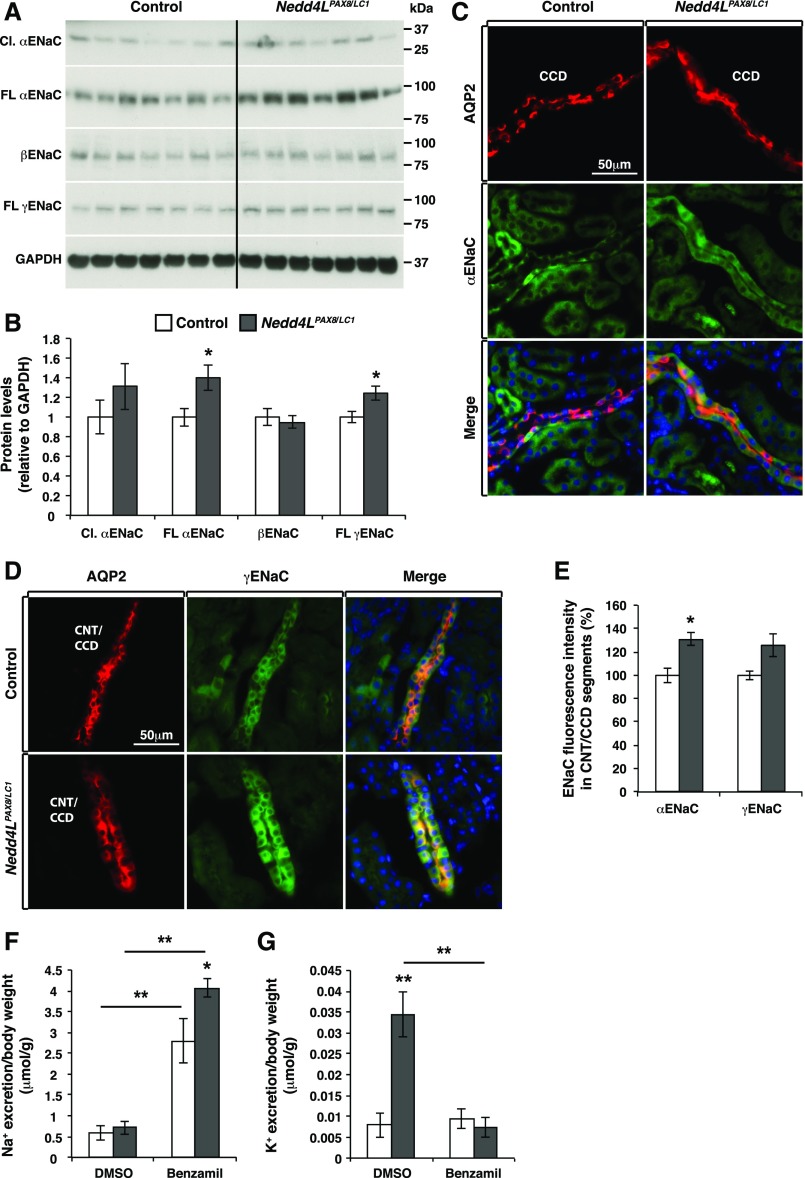
We assessed ENaC expression by Western blot and observed increased full-length (but not cleaved) αENaC, and full-length γENaC in mutant mice (Figure 4, A and B). The cleaved form of γENaC was not detectable, likely because ENaC was not sufficiently expressed under LKD conditions. Immunofluorescence analyses suggested a 30% increase in the intracellular staining of αENaC (Figure 4, C and E) and a tendency toward elevated γENaC (Figure 4, D and E). No obvious differences were observed for βENaC staining, consistent with the immunoblotting data (Supplemental Figure 3B). ENaC mRNAs were similar in both genotypes (Supplemental Figure 3A). To analyze ENaC activity, we treated mice with a single dose (1 mg/kg body wt) of benzamil, a specific ENaC inhibitor.40 Benzamil treatment increased urine volume to the same extent in control and mutant mice (Supplemental Figure 3C) but induced a significantly stronger natriuresis (145%) in Nedd4LPax8/LC1 mice (Figure 4F). Interestingly, intraperitoneal benzamil injection completely reversed excessive K+ wasting in mutant mice (Figure 4G). In contrast, we did not detect an antikaliuretic effect of benzamil treatment in control mice, indicating that ENaC-mediated Na+ reabsorption was not linked to K+ excretion under these hypokalemic conditions. Taken together, these data suggest that the exacerbated hypokalemia observed in Nedd4LPax8/LC1 versus control mice is caused by ENaC overactivation.
Figure 4.
ENaC expression and activity are increased in Nedd4LPax8/LC1 mice after 2 weeks of LKD. (A and B) Western blot analysis of ENaC in control and Nedd4LPax8/LC1 mice after 2 weeks of LKD and protein quantification showing a significant increase in the full-length αENaC and γENaC subunits (seven mice in each group). (C and D) Costaining of αENaC (C) and γENaC (D) (both in green) and AQP2 (in red) in control and Nedd4LPax8/LC1 mice. (E) Mean of αENaC and γENaC fluorescence intensity in CNT/CCD segments showing a significant increase in αENaC (but not γENaC) abundance in Nedd4LPax8/LC1 mice compared with controls (four mice in each group). (F) Benzamil significantly increased Na+ excretion compared with vehicle (DMSO) in both genotypes, and the loss of Na+ in the benzamil-treated Nedd4LPax8/LC1 mice is significantly higher than that of control mice. (G) Benzamil treatment efficiently prevents the K+ wasting observed in Nedd4LPax8/LC1 mice (seven mice in each group). *P<0.05; **P<0.01
The WNK/SPAK/NCC Pathway Is Enhanced in Nedd4LPax8/LC1 Mice
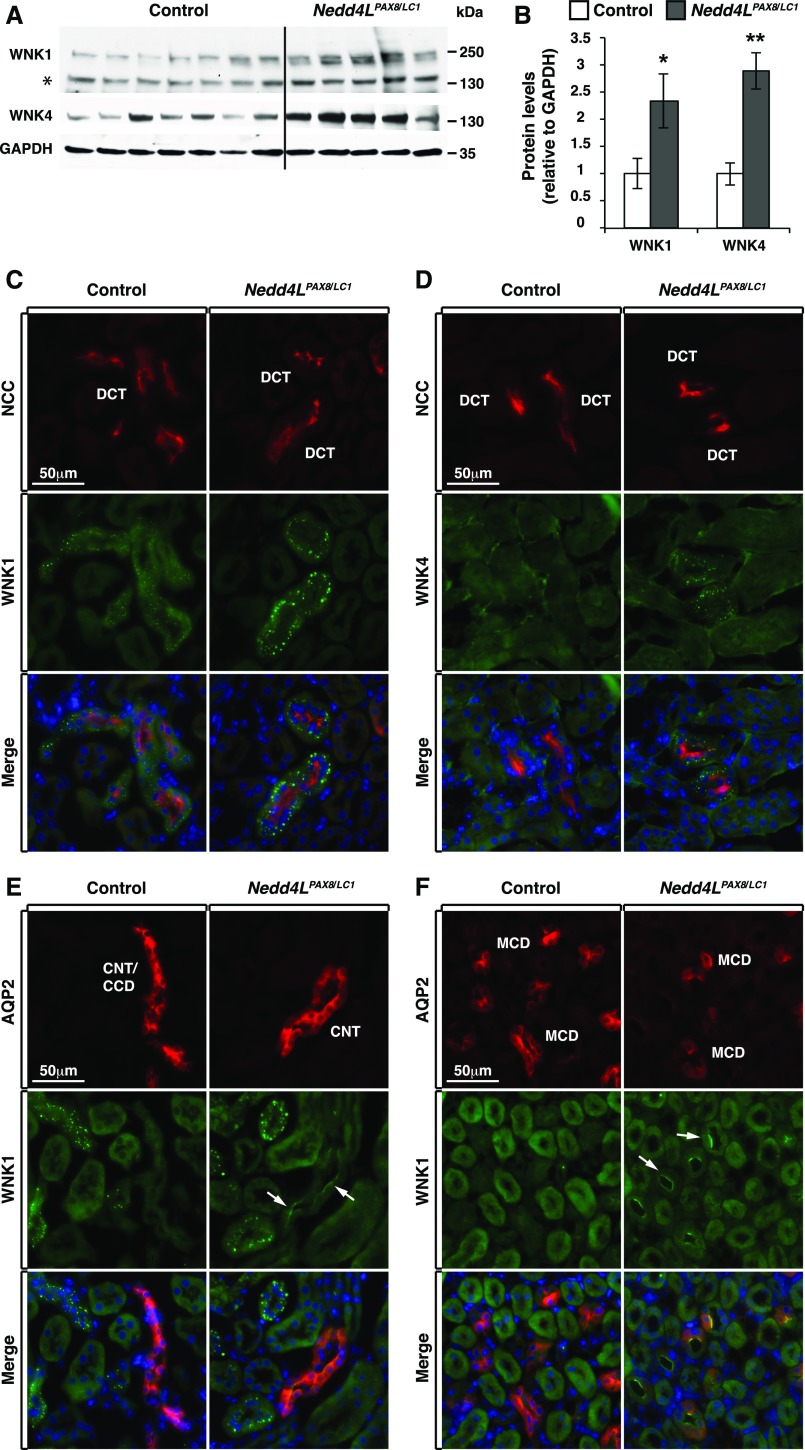
Previous work showed that Nedd4LPax8/LC1 mice fed a HSD/normal K+ diet exhibited an increase in WNK1 protein abundance, resulting in enhanced SPAK-mediated NCC phosphorylation.19 We therefore wondered if NEDD4-2 deletion affects WNK1 regulation under hypokalemic conditions. Accordingly, we analyzed WNK1 abundance and cellular localization using a C-terminal antibody specific for NEDD4-2–sensitive isoforms19 and found increased WNK1 protein levels in mutant mice (Figure 5, A and B). Furthermore, immunofluorescence analyses showed that WNK1 exhibited a differential segment-specific pattern in the distal nephron. Specifically, WNK1 accumulated in intracellular puncta in the DCT (Figure 5C). In the DCT of Nedd4LPax8/LC1 mice, WNK1 puncta were larger and more intense than in control littermates (Figure 5C). In addition, WNK1 protein expression was enhanced at the apical membrane of both cortical (Figure 5E) and medullary CNT/CD in Nedd4LPax8/LC1 mice (Figure 5F). Interestingly, the enhanced WNK1 signal in the CNT/CD was primarily localized in principal cells, as shown by AQP2 costaining (Figure 5, E and F). In addition, total WNK4 expression was enhanced in mutant mice relative to controls during long-term K+ restriction (Figure 5, A and B), and similarly to WNK1, WNK4 puncta were more prominent in the DCT of mutant mice (Figure 5D). These data indicate that the protein abundance of WNK1 and WNK4 is increased in Nedd4LPax8/LC1 versus control mice under severe hypokalemic conditions, and that there is a difference in subcellular localization in different nephron segments.
Figure 5.
WNK1 and WNK4 are upregulated in Nedd4LPax8/LC1-deficient mice. (A and B) Western blot analysis of WNK1 and WNK4 in control and Nedd4LPax8/LC1 mice after 2 weeks of LKD, and protein quantification showing a significant increase in the two WNK proteins in Nedd4LPax8/LC1 mice (* indicates a nonspecific band; seven controls and five Nedd4LPax8/LC1 mice). (C and D) Costaining of NCC (red) with WNK1 (C) and WNK4 (D) (both in green) in control and Nedd4LPax8/LC1 mice. WNK1 and WNK4 foci are more prominent in the DCT of mutant versus control mice. (E and F) Costaining of WNK1 (green) and AQP2 (red) showing an increase in WNK1 apical localization in the CNT/CCD (E) (arrows) and MCD (F) (arrows) of mutant mice. *P<0.05; *P<0.01
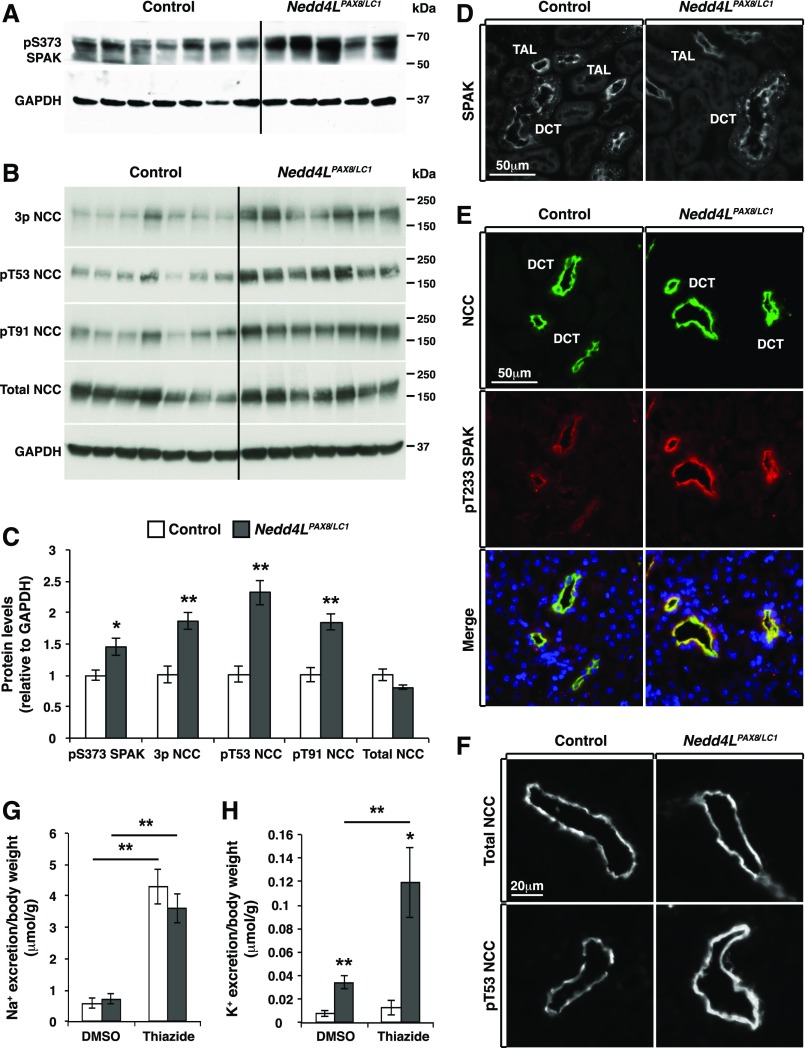
Because WNK1 and WNK4 are well known to regulate NCC via phosphorylation by Ste20- and SPS1-related proline alanine-rich kinase (SPAK), we investigated how LKD affects SPAK-mediated regulation of NCC in control and Nedd4LPax8/LC1 mice. Western blot analysis revealed increased phosphorylation of SPAK at S373 in mutant mice (Figure 6, A and C). SPAK phosphorylation at this site has previously been reported to be a signature of WNK-mediated activation.19,41 Consistent with this observation, the SPAK T-loop activation site (T233) exhibited stronger phosphorylation in the DCT, with no apparent change in total SPAK expression or localization (Figure 6, D and E). This correlated with increased NCC phosphorylation, whereas total NCC expression and membrane localization were not different (Figure 6, B, C, E, and F). A single injection of thiazide resulted in similar natriuresis in both genotypes (Figure 6G), whereas thiazide-sensitive kaliuresis was greater in mutant versus control mice (Figure 6H), indicating that NCC cannot be the cause of K+ wasting and hypokalemia observed in mutant mice. Altogether, our data suggest that the WNK/SPAK/NCC pathway is activated in mutant mice, functioning as a compensatory mechanism that attenuates the excessive urinary K+ wasting associated with NEDD4-2 deletion.
Figure 6.
NCC and SPAK phosphorylation is increased in Nedd4LPax8/LC1 mice after 2 weeks of LKD. (A and B) Western blot analysis of SPAK (A) and NCC (B) phosphorylation in control and Nedd4LPax8/LC1 mice. (C) Protein quantification from (A) and (B) showing a significant increase in S373 phosphorylated SPAK and NCC phosphorylation at several residues including T53, T91, or the combination of T43, T53, and T58 (3P-NCC), with no modification of total NCC (seven controls and five to seven Nedd4LPax8/LC1 mice). (D) Immunostaining of total SPAK in control and Nedd4LPax8/LC1 mice showing comparable level and localization of the protein in the cortex of both genotypes. (E) Costaining of NCC (green) and T233 phosphorylated SPAK (red) in control and Nedd4LPax8/LC1 mice. An increase in SPAK phosphorylation was observed in the DCT of mutant mice. (F) Immunostaining of total (upper panel) and T53 phosphorylated NCC (lower panel) in control and Nedd4LPax8/LC1 mice after 2 weeks of LKD. Increased NCC phosphorylation is observed in Nedd4LPax8/LC1 mice, with similar apical localization of the transporter in both genotypes. (G and H) Thiazide treatment induced an equivalent increase in Na+ excretion in control and Nedd4LPax8/LC1 mice compared with vehicle (DMSO) (G), whereas K+ excretion was exacerbated in mutant mice by thiazide treatment (H) (seven mice in each group). *P<0.05; **P<0.01.
BK Channels Are Increased in Principal Cells of the CNT/CD in Nedd4LPax8/LC1 Mice
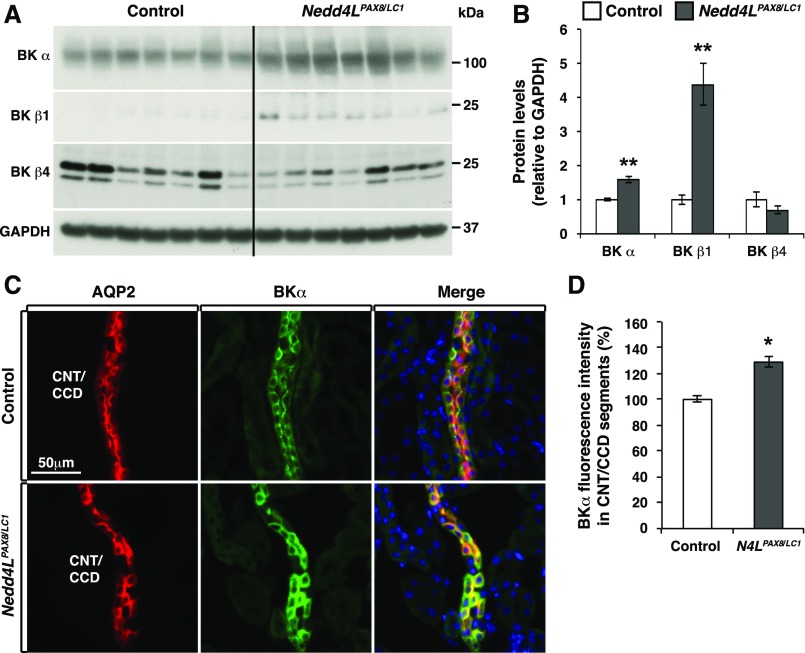
Because WNK1 is a positive regulator of BK channels,42,43 we also assessed BK expression by Western blot, and found that the channel-forming BKα and BKβ1 (but not BKβ4) subunits were increased in total kidney extracts (Figure 7, A and B). Immunostaining showed that BKα subunits were detectable in the CNT/CD despite active hypokalemia, and their expression was enhanced by about 30% in the CNT/CCD of Nedd4LPax8/LC1 mice (Figure 7, C and D). Interestingly, the increased BKα abundance in Nedd4LPax8/LC1 mice appeared to be restricted to principal cells, as demonstrated by AQP2 costaining (Figure 7C). These findings indicate that under hypokalemic conditions, the K+ loss in Nedd4LPax8/LC1 mice correlates with enhanced expression of BK channels in principal cells, suggesting that enhanced BK-mediated K+ secretion may contribute to the profound hypokalemia observed in these mice.
Figure 7.
BK channels are upregulated in CNT/CCD principal cells of Nedd4LPax8/LC1 mice. (A and B) Western blot analysis of BK in control and Nedd4LPax8/LC1 mice after 2 weeks of LKD, and protein quantification revealing a significant increase in BKα and BKβ1 expression (seven mice of each genotype). (C) Costaining of BKα (green) and AQP2 (red) in control and Nedd4LPax8/LC1 mice. (D) Quantification of BKα fluorescence intensity in the CNT/CCD segments. BKα labeling was significantly increased in the principal cells of mutant CNT/CCD segments. *P<0.05; **P<0.01.
Evaluation of Epithelial Hypertrophy in the CNT/CD of Nedd4LPax8/LC1 Mice under LKD
Rats fed with LKD for 2 weeks had been previously shown to exhibit distal nephron hypertrophy and diabetes insipidus, evidenced by decreased AQP2 expression.44–46 We therefore assessed AQP2 expression and found no difference between genotypes (Supplemental Figure 4, A and B). Moreover, to evaluate distal hypertrophy, we quantified the size of at least 150 CNT/CD cells per genotype and detected a tendency toward epithelial hypertrophy in mutant versus control mice, although the difference was not statistically significant (Supplemental Figure 4C). These observations suggest that distal tubular morphology and concentrating functions are not different under LKD between control and Nedd4LPax8/LC1 mice.
Discussion
NEDD4-2 is a well appreciated master regulator of distal nephron Na+ reabsorption and BP,47–49 but its role in regulating these processes during changes in K+ balance is not well understood. Here, we show that Nedd4LPax8/LC1 mice exhibit severe urinary K+ wasting, exacerbating the hypokalemia caused by chronic dietary K+ deficiency. This severe hypokalemia was associated with increased ENaC expression and activity, increased BK channel abundance, enhanced NCC phosphorylation, and decreased ROMK apical localization in the CNT/CD. Thus, the hypokalemic phenotype observed in these mice under chronic LKD seems to be driven by increased ENaC and BK channel expression.
Not surprisingly, our data show that HKD-induced kaliuresis is preserved in Nedd4LPax8/LC1 mice. Under such conditions, NEDD4-2 activity is low because of inhibition by aldosterone and SGK1.17,50 Hence, the knockout of NEDD4-2 in K+-loaded mice would have minimal effects on K+ balance relative to K+-loaded controls. This contrasts with the inactivation of stimulatory actors in the pathway, such as aldosterone synthase, MR, SGK1, and αENaC, all of which cause hyperkalemia after genetic deletion.10,12,15–18 Taking this into consideration, we investigated what would happen to Nedd4LPax8/LC1 mice when restricting K+ supply, a condition that should be associated with high NEDD4-2 activity. Would overactivation of NCC (as shown in Ronzaud et al.20) lead to a reduction of Na+ delivery to downstream segments, and consequently balance the K+ excretion? Or would NEDD4-2 deletion lead to increased ENaC activity, enhanced K+ excretion, and hypokalemia, as is the case in Liddle syndrome51,52? Our findings suggest that the second scenario predominates, namely that NEDD4-2 suppression of ENaC is most active under K+-restricted conditions.
Numerous prior reports have shown that NEDD4-2 interacts with and ubiquitylates ENaC, reducing channel cell surface expression.24–26,28,32,53,54 The physiologic relevance of ENaC/NEDD4-2 interactions has largely been explored within the context of Liddle syndrome or signaling pathways that block NEDD4-2 activity. However, a rationale explaining why NEDD4-2 was selected during evolution to tonically inhibit ENaC activity when aldosterone levels are low has been lacking. This study suggests that the inhibition of ENaC by NEDD4-2 is crucial under conditions where dietary K+ is scarce, to limit excessive K+ wasting and prevent life-threatening hypokalemia.
Our biochemical analyses show an upregulation of primarily intracellular αENaC. Accumulation of intracellular βENaC and γENaC was observed in the same model under dietary Na+ loading,20 and it was suggested by an accompanying editorial by Ellison that NEDD4-2 may not control directly the cell surface expression, but intracellular degradation.55 Our data support such a model. Additionally, they suggest that under chronic K+ depletion, NEDD4-2–dependent regulation of ENaC may act independently of factors that regulate its proteolytic cleavage. Intriguingly, ENaC regulation seems to be diet-dependent, although both HSD and LKD result in reduced circulating aldosterone. When mice were kept under HSD, intracellular βENaC and γENaC were increased, but αENaC (including αENaC cleavage) was decreased because of low aldosterone,20 and no increase in amiloride sensitivity was observed. In contrast, LKD results in accumulation of intracellular αENaC, a slight increase in γENaC, and no alteration of βENaC, together with increased benzamil sensitivity. It is well known that ENaC has a high conductance, and only a low number of channels per cell are expected to be active at the plasma membrane; consequently, the biochemical or histochemical detection of ENaC may not be sensitive enough to detect subtle changes at the membrane. This may explain that alterations related to ENaC activation (cleavage of either αENaC or γENaC) are not observable under the LKD conditions.56,57 Similar data regarding ENaC abundance have been reported in other germline models of Nedd4L inactivation.24–26
Recently, it was shown that SGK1/NEDD4-2 regulates WNK1.19 Similarly, we found that WNK1 is increased in the DCT and the CNT/CD of Nedd4LPax8/LC1 mice under LKD. In addition, we observed a net augmentation in WNK signaling, as both WNK1 and WNK4 were increased, and phosphorylation of the WNK effectors SPAK/OSR1 was enhanced. Moreover, both WNK1 and WNK4 exhibited a punctate pattern in the DCT, a finding that was previously reported in instances where WNK kinases are active.11,58 By contrast, in the CNT/CD, WNK1 localized to the apical membrane, which was particularly evident in mutant mice. This segment-specific subcellular distribution may reflect a difference in WNK1 interactors or regulatory networks, and supports extensive literature implicating WNK1 as a regulator of ion transport processes in the CNT/CD that influence K+ balance.59
We also show that NCC phosphorylation is enhanced in Nedd4LPax8/LC1. This correlates with plasma K+ and the observed increase in DCT WNK/SPAK pathway activity in mutant mice. Consistent with increased NCC activity in the mutant model, thiazide-mediated inhibition of NCC exacerbated urinary K+ losses. This suggests that the high NCC activity may, in part, be a compensatory response during active hypokalemia to limit distal Na+ delivery. In this regard, recent studies have suggested that NCC phosphorylation status is tightly linked to the concentration of extracellular K+.11,60 Specifically, hypokalemia has been shown to be a potent NCC activator. The effect is believed to be because of changes in the basolateral membrane voltage of the DCT, which becomes hyperpolarized during hypokalemia, resulting in enhanced chloride efflux and activation of WNK kinases.11 The experiments presented here did not dissociate the effect of NEDD4-2 from the role of hypokalemia in WNK regulation. However, it is notable that under high NaCl/normal K+ dietary conditions, Nedd4LPax8/LC1 mice exhibit increased WNK1 protein abundance relative to controls, despite no difference in plasma K+ levels.19,20 On the basis of these results, we propose that the stimulatory effect of NEDD4-2 ablation on WNK activity under LKD conditions is because of a combined effect of reduced NEDD4-2–mediated WNK degradation and hypokalemia.
In addition, our data revealed that Nedd4LPax8/LC1 mice exhibit an increase in BK protein, and a decrease in ROMK apical localization in the CNT/CD. Recently, Liu et al. and Webb et al. both found that WNK1 activates BK channels in vitro, through the regulation of protein expression and trafficking.42,43 Because WNK1 protein abundance was increased in the CNT and CD in Nedd4LPax/LC1 mice, it seems plausible that the increase in BK expression is WNK1-dependent. However, the increased expression of BK subunits did not appear to be the predominant cause of K+ wasting in Nedd4LPax8/LC1 mice, as the defective kaliuresis was fully corrected by benzamil administration, suggesting that the increased K+ losses are primarily ENaC-dependent in etiology. The decreased ROMK expression might be explained by enhanced WNK signaling because L-WNK1 and WNK4 are well known inhibitors of ROMK, as evidenced in various in vitro and in vivo systems.61–64 On the other hand, ROMK was more abundant in the normokalemic Nedd4LPax8/LC1 mice under HSD conditions,20 a condition also associated with enhanced WNK1/4 expression.19 Thus, the observed changes in ROMK cannot be fully explained by a regulatory effect of the WNK signaling pathway. Other molecular components of the ROMK regulatory network, or perhaps a WNK-independent effect of plasma [K+] itself, may predominate under the conditions studied here.
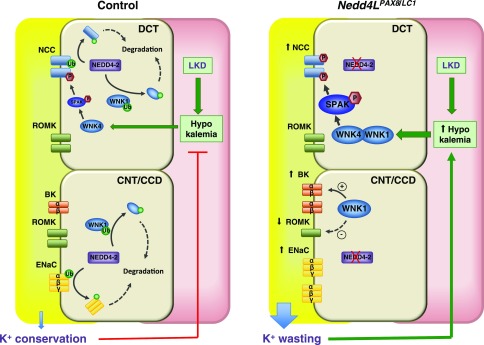
In summary, this study demonstrates that K+-restricted mice lacking renal tubular NEDD4-2 exhibit severe urinary K+ wasting and hypokalemia, and describes how NEDD4-2 orchestrates the regulation of its different targets in order to ensure adequate K+ handling (Figure 8). The primary defect caused by NEDD4-2 deletion is the impaired downregulation of ENaC, which subsequently increases the driving force for K+ secretion. Our findings suggest that the tonic inhibition of ENaC by NEDD4-2 may be an evolutionarily conserved mechanism that allows the kidney to conserve K+ and prevent life-threatening hypokalemia when access to dietary K+ is limited.
Figure 8.
Model of NEDD4-2 action in K+ conservation in the CNT/CCD. Under K+ restriction, the aldosterone/MR/SGK1 regulatory axis is switched off, resulting in maximal NEDD4-2 activity. In controls, the active NEDD4-2 primarily downregulates ENaC. WNK1 is also downregulated, resulting in limited BK channel activity, Moreover, hypokalemia causes WNK4 activation, and stimulation of SPAK and NCC to limit distal Na+ delivery to ENaC and voltage-dependent K+ excretion via ROMK. Collectively, these transport processes act synergistically to promote K+ conservation. The deregulation of this process in Nedd4LPax8/LC1 mice results in K+ wasting, primarily because of the release of ENaC from tonic NEDD4-2 inhibition. WNK1 is also increased, causing elevated BK expression. The severe hypokalemia caused by enhanced K+ wasting further stimulates WNK1/WNK4, SPAK and NCC, which, together with reduced ROMK expression, partially compensates for the K+ wasting induced by NEDD4-2 deletion.
Concise Methods
Handling and Induction of Renal Tubule–Specific Nedd4LPax8/LC1 Mice
Inducible renal tubule–specific Nedd4Lflox/flox/Pax8-rTA/LC1 knockout (Nedd4LPax8/LC1) mice were generated as described previously.20 Mice were housed in a temperature-controlled room (19°C–22°C) with a 12:12-hour light/dark cycle. To induce the gene deletion, mice aged 21–24 days were treated with doxycycline (2 mg/ml in 2% sucrose in drinking water) for 12 days. Genotype was identified by PCR performed on ear biopsies, Nedd4L gene deletion was analyzed by PCR performed on genomic DNA preparation from kidneys.
Dietary Manipulation
Control (Nedd4LPax8 or Nedd4LLC1) and mutant (Nedd4LPax8/LC1) doxycycline-treated mice were fed a standard or control diet (0.3% K+; 0.2% Na+; Ssniff Spezialitäten GmbH), LKD (<0.03% K+; 0.2% Na+; Ssniff Spezialitäten GmbH), or HKD (5% K+; 0.2% Na+; Ssniff Spezialitäten GmbH) for the periods indicated in the related results. Mice were given free access to food and water during the period of experiments.
Plasma and Organs Collection
Mice were anesthetized by ketamine/xylazine intraperitoneal injection; blood was collected by exsanguination from the retro-orbital plexus in SARSTEDT heparin-containing microtubes and plasma was separated according to the manufacturer’s instructions. Mice were then humanely euthanized by cervical dislocation. Experimental protocols were designed with respect to the Swiss Animal Welfare Act and approved by the veterinary administration of the Canton of Vaud, Switzerland.
Metabolic Cages, Diuretic Treatment, and Urine and Plasma Analyses
After 2 days of adaptation in metabolic cages, data related to body weight and food and water intake were registered, and 24-hour urine samples were collected. Urine analysis (Na+, K+, creatinine, and urea) was performed by the Laboratory of Clinical Chemistry at the Lausanne Hospital (CHUV) using a Modular Analytics System (Roche Diagnostics). For diuretics treatment, control and mutant mice were intraperitoneally injected with vehicle containing benzamil (1 mg/kg body wt) and thiazide (20 mg/kg body wt), and urine samples were collected 3 hours after injection. Diuretic treatment was applied during the light cycle (when the aldosterone level was minimal) to avoid any overlap between aldosterone effect and the effect induced by drug treatment. Plasma Na+ and K+ levels were measured with a flame photometer (Cole-Palmer Instrument). Plasma aldosterone measurements was performed at the Service of Nephrology of the CHUV using the radioimmunoassay kit ALDO-RIACT, according to the manufacturer’s instructions.
Kidney Lysates Preparation and Western Blot Analyses
Frozen tissues were homogenized using buffer containing Tris/HCl 50 mM at pH 7.5, EDTA 1mM, EGTA 1 mM, sucrose 0.27 M, NaF 50 mM, and Na-pyrophosphate 5 mM in addition to protease inhibitors purchased from Roche (Complete, no. 11836145001). Protein homogenates were then centrifuged at 10,000×g for 10 minutes at 4°C, supernatants were collected, and protein concentration was measured using the BradFord method (no. UPF86420; Uptima). For ROMK detection, an additional ultracentrifugation step at 100,000×g for 1 hour was performed for membrane enrichment. Proteins were separated by SDS-PAGE and transferred to nitrocellulose membranes using a wet transfer apparatus (Bio-Rad). Membranes were blocked with 5% nonfat milk in TBS and 0.1% Tween 20 for 40 minutes and incubated with primary antibodies overnight at 4°C. Secondary antibodies were applied for 2 hours at room temperature. A list of the antibodies used in the study is available in Supplemental Table 2. Western blot data were quantified using ImageJ software.
Immunofluorescence
Mice were anesthetized by ketamine/xylazine intraperitoneal injection. Cardiac perfusions of PBS followed by 4% PFA were performed to fix the kidneys. Before freezing, fixed kidneys were kept in 30% sucrose in PBS solution at 4°C overnight. Immunostaining was performed on 5 μm cryosections using primary antibodies applied overnight at 4°C. Secondary antibodies were applied for 2 hours at room temperature. The antibodies and dilutions used are listed in Supplemental Table 2. DNA was counterstained with 0.1 μg/ml DAPI. Samples were mounted in Dako immunofluorescence medium, and imaged by fluorescence microscopy using either a Zeiss Axiovision (v4.8) or Zeiss Axioscan Z1 microscope. Entire kidney sections containing at least 200 segments of interest were quantified using ImageJ software. Integrated density and corrected total cell fluorescence were assessed for all analyzed signals.65 To evaluate tubular hypertrophy in the distal nephron, CNT/CD were determined as AQP2-expressing segments. Kidney sections were imaged with the ×40 objective of a Zeiss Axiovision microscope. The surface area of each individual cell of at least ten CNT/CD segments in each condition were analyzed using ImageJ ROI manager and measurement tools, as described in the ImageJ user guide.66,67 The mean size of 150 cells from control and 200 cells from mutant mice was calculated.
RNA Extraction and Quantitative RT-PCR
Total RNA was purified from total kidney using TRIzol reagent (Ambion) and precipitated by isopropyl alcohol. cDNA was synthetized from 2 to 5 μg total RNA using Superscript II reverse transcription (Invitrogen) and random hexamers. TaqMan Gene Expression Assays (Applied Biosystems) were used to analyze gene expression. Primers/probes were Scann1a (αENaC, Mm00803386_m1; Applied Biosystems), Scann1b (βENaC; Mm00441215_m1), Scann1c (γENaC; Mm00441228_m1), Ren1 (Renin1; Mm02342889_g1), Gapdh (GAPDH; Mm99999915_g1).
Statistical Analyses
Data were statistically analyzed using an unpaired paired t test. Values were considered significant when P≤0.05. Data are represented as means±SEM. The significance of the metabolic parameters of the experimental groups under different diets were analyzed using two-way ANOVA followed by Bonferroni multiple comparisons tests. Values were considered significant when α<0.05.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Matteo Stifanelli, Andrée Tedjani, and Marianne Sidhom for technical help.
This work was supported by the Swiss National Science Foundation grant no. 310030_159735 (to O.S.), the National Centre of Competence in Research “Swiss Kidney.ch” (to O.S.), networking support by the European Cooperation in Science and Technology (COST) Action Aldosterone and Mineralocorticoid Receptor: Pathophysiology, clinical implication, and therapeutic innovations (ADMIRE) BM1301 (to O.S.), Novartis Foundation for medical biological research (to O.S.), and National Institutes of Health grant R01DK098145 (to A.R.S.). L.A.-Q. was supported by a fellowship of the Marie Curie cofunding International Fellowship Program on Integrative Kidney Physiology and Pathophysiology.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2016070732/-/DCSupplemental.
References
- 1.Greenlee M, Wingo CS, McDonough AA, Youn JH, Kone BC: Narrative review: Evolving concepts in potassium homeostasis and hypokalemia. Ann Intern Med 150: 619–625, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Youn JH, McDonough AA: Recent advances in understanding integrative control of potassium homeostasis. Annu Rev Physiol 71: 381–401, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gennari FJ: Hypokalemia. N Engl J Med 339: 451–458, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Kjeldsen K: Hypokalemia and sudden cardiac death. Exp Clin Cardiol 15: e96–e99, 2010 [PMC free article] [PubMed] [Google Scholar]
- 5.Unwin RJ, Luft FC, Shirley DG: Pathophysiology and management of hypokalemia: A clinical perspective. Nat Rev Nephrol 7: 75–84, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Crambert G: H-K-ATPase type 2: Relevance for renal physiology and beyond. Am J Physiol Renal Physiol 306: F693–F700, 2014 [DOI] [PubMed] [Google Scholar]
- 7.Staub O, Loffing J: Mineralocorticoid action in the aldosterone sensitive distal nephron. In: The Kidney: Physiology and Pathophysiology, edited by Alpern RJ, Caplan MJ, Moe OW, San Diego, Elsevier Inc., 2013, pp 1181–1211 [Google Scholar]
- 8.Penton D, Czogalla J, Loffing J: Dietary potassium and the renal control of salt balance and blood pressure. Pflugers Arch 467: 513–530, 2015 [DOI] [PubMed] [Google Scholar]
- 9.Cornelius RJ, Wen D, Li H, Yuan Y, Wang-France J, Warner PC, Sansom SC: Low Na, high K diet and the role of aldosterone in BK-mediated K excretion. PLoS One 10: e0115515, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Todkar A, Picard N, Loffing-Cueni D, Sorensen MV, Mihailova M, Nesterov V, Makhanova N, Korbmacher C, Wagner CA, Loffing J: Mechanisms of renal control of potassium homeostasis in complete aldosterone deficiency. J Am Soc Nephrol 26: 425–438, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Terker AS, Zhang C, McCormick JA, Lazelle RA, Zhang C, Meermeier NP, Siler DA, Park HJ, Fu Y, Cohen DM, Weinstein AM, Wang WH, Yang CL, Ellison DH: Potassium modulates electrolyte balance and blood pressure through effects on distal cell voltage and chloride. Cell Metab 21: 39–50, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Terker AS, Yarbrough B, Ferdaus MZ, Lazelle RA, Erspamer KJ, Meermeier NP, Park HJ, McCormick JA, Yang CL, Ellison DH: Direct and indirect mineralocorticoid effects determine distal salt transport. J Am Soc Nephrol 27: 2436–2445, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sorensen MV, Grossmann S, Roesinger M, Gresko N, Todkar AP, Barmettler G, Ziegler U, Odermatt A, Loffing-Cueni D, Loffing J: Rapid dephosphorylation of the renal sodium chloride cotransporter in response to oral potassium intake in mice. Kidney Int 83: 811–824, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Rengarajan S, Lee DH, Oh YT, Delpire E, Youn JH, McDonough AA: Increasing plasma [K+] by intravenous potassium infusion reduces NCC phosphorylation and drives kaliuresis and natriuresis. Am J Physiol Renal Physiol 306: F1059–F1068, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Canonica J, Sergi C, Maillard M, Klusonova P, Odermatt A, Koesters R, Loffing-Cueni D, Loffing J, Rossier B, Frateschi S, Hummler E: Adult nephron-specific MR-deficient mice develop a severe renal PHA-1 phenotype. Pflugers Arch 468: 895–908, 2016 [DOI] [PubMed] [Google Scholar]
- 16.Huang DY, Wulff P, Völkl H, Loffing J, Richter K, Kuhl D, Lang F, Vallon V: Impaired regulation of renal K+ elimination in the sgk1-knockout mouse. J Am Soc Nephrol 15: 885–891, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Al-Qusairi L, Basquin D, Roy A, Stifanelli M, Rajaram RD, Debonneville A, Nita I, Maillard M, Loffing J, Subramanya AR, Staub O: Renal tubular SGK1 deficiency causes impaired K+ excretion via loss of regulation of NEDD4-2/WNK1 and ENaC. Am J Physiol Renal Physiol 311: F330–F342, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perrier R, Boscardin E, Malsure S, Sergi C, Maillard MP, Loffing J, Loffing-Cueni D, Sørensen MV, Koesters R, Rossier BC, Frateschi S, Hummler E: Severe salt-losing syndrome and hyperkalemia induced by adult nephron-specific knockout of the epithelial sodium channel α-subunit. J Am Soc Nephrol 27: 2309–2318, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roy A, Al-Qusairi L, Donnelly BF, Ronzaud C, Marciszyn AL, Gong F, Chang YP, Butterworth MB, Pastor-Soler NM, Hallows KR, Staub O, Subramanya AR: Alternatively spliced proline-rich cassettes link WNK1 to aldosterone action. J Clin Invest 125: 3433–3448, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ronzaud C, Loffing-Cueni D, Hausel P, Debonneville A, Malsure SR, Fowler-Jaeger N, Boase NA, Perrier R, Maillard M, Yang B, Stokes JB, Koesters R, Kumar S, Hummler E, Loffing J, Staub O: Renal tubular NEDD4-2 deficiency causes NCC-mediated salt-dependent hypertension. J Clin Invest 123: 657–665, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arroyo JP, Lagnaz D, Ronzaud C, Vázquez N, Ko BS, Moddes L, Ruffieux-Daidié D, Hausel P, Koesters R, Yang B, Stokes JB, Hoover RS, Gamba G, Staub O: Nedd4-2 modulates renal Na+-Cl- cotransporter via the aldosterone-SGK1-Nedd4-2 pathway. J Am Soc Nephrol 22: 1707–1719, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castañeda-Bueno M, Cervantes-Perez LG, Rojas-Vega L, Arroyo-Garza I, Vázquez N, Moreno E, Gamba G: Modulation of NCC activity by low and high K(+) intake: Insights into the signaling pathways involved. Am J Physiol Renal Physiol 306: F1507–F1519, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walter C, Tanfous MB, Igoudjil K, Salhi A, Escher G, Crambert G: H,K-ATPase type 2 contributes to salt-sensitive hypertension induced by K(+) restriction. Pflugers Arch 468: 1673–1683, 2016 [DOI] [PubMed] [Google Scholar]
- 24.Kimura T, Kawabe H, Jiang C, Zhang W, Xiang YY, Lu C, Salter MW, Brose N, Lu WY, Rotin D: Deletion of the ubiquitin ligase Nedd4L in lung epithelia causes cystic fibrosis-like disease. Proc Natl Acad Sci USA 108: 3216–3221, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boase NA, Rychkov GY, Townley SL, Dinudom A, Candi E, Voss AK, Tsoutsman T, Semsarian C, Melino G, Koentgen F, Cook DI, Kumar S: Respiratory distress and perinatal lethality in Nedd4-2–deficient mice. Nat Commun 2: 287, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi PP, Cao XR, Sweezer EM, Kinney TS, Williams NR, Husted RF, Nair R, Weiss RM, Williamson RA, Sigmund CD, Snyder PM, Staub O, Stokes JB, Yang B: Salt-sensitive hypertension and cardiac hypertrophy in mice deficient in the ubiquitin ligase Nedd4-2. Am J Physiol Renal Physiol 295: F462–F470, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou R, Patel SV, Snyder PM: Nedd4-2 catalyzes ubiquitination and degradation of cell surface ENaC. J Biol Chem 282: 20207–20212, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Lu C, Pribanic S, Debonneville A, Jiang C, Rotin D: The PY motif of ENaC, mutated in Liddle syndrome, regulates channel internalization, sorting and mobilization from subapical pool. Traffic 8: 1246–1264, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Snyder PM, Olson DR, Thomas BC: Serum and glucocorticoid-regulated kinase modulates Nedd4-2–mediated inhibition of the epithelial Na+ channel. J Biol Chem 277: 5–8, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Harvey KF, Dinudom A, Cook DI, Kumar S: The Nedd4-like protein KIAA0439 is a potential regulator of the epithelial sodium channel. J Biol Chem 276: 8597–8601, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Kamynina E, Debonneville C, Hirt RP, Staub O: Liddle’s syndrome: A novel mouse Nedd4 isoform regulates the activity of the epithelial Na(+) channel. Kidney Int 60: 466–471, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Minegishi S, Ishigami T, Kino T, Chen L, Nakashima-Sasaki R, Araki N, Yatsu K, Fujita M, Umemura S: An isoform of Nedd4-2 is critically involved in the renal adaptation to high salt intake in mice. Sci Rep 6: 27137, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frindt G, Palmer LG: Effects of dietary K on cell-surface expression of renal ion channels and transporters. Am J Physiol Renal Physiol 299: F890–F897, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nguyen MT, Yang LE, Fletcher NK, Lee DH, Kocinsky H, Bachmann S, Delpire E, McDonough AA: Effects of K+-deficient diets with and without NaCl supplementation on Na+, K+, and H2O transporters’ abundance along the nephron. Am J Physiol Renal Physiol 303: F92–F104, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gutsche HU, Peterson LN, Levine DZ: In vivo evidence of impaired solute transport by the thick ascending limb in potassium-depleted rats. J Clin Invest 73: 908–916, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Greger R, Schlatter E: Presence of luminal K+, a prerequisite for active NaCl transport in the cortical thick ascending limb of Henle’s loop of rabbit kidney. Pflugers Arch 392: 92–94, 1981 [DOI] [PubMed] [Google Scholar]
- 37.Hebert SC, Andreoli TE: Control of NaCl transport in the thick ascending limb. Am J Physiol 246: F745–F756, 1984 [DOI] [PubMed] [Google Scholar]
- 38.Elkjaer ML, Kwon TH, Wang W, Nielsen J, Knepper MA, Frøkiaer J, Nielsen S: Altered expression of renal NHE3, TSC, BSC-1, and ENaC subunits in potassium-depleted rats. Am J Physiol Renal Physiol 283: F1376–F1388, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Loffing-Cueni D, Flores SY, Sauter D, Daidié D, Siegrist N, Meneton P, Staub O, Loffing J: Dietary sodium intake regulates the ubiquitin-protein ligase nedd4-2 in the renal collecting system. J Am Soc Nephrol 17: 1264–1274, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Grimm PR, Lazo-Fernandez Y, Delpire E, Wall SM, Dorsey SG, Weinman EJ, Coleman R, Wade JB, Welling PA: Integrated compensatory network is activated in the absence of NCC phosphorylation. J Clin Invest 125: 2136–2150, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saritas T, Borschewski A, McCormick JA, Paliege A, Dathe C, Uchida S, Terker A, Himmerkus N, Bleich M, Demaretz S, Laghmani K, Delpire E, Ellison DH, Bachmann S, Mutig K: SPAK differentially mediates vasopressin effects on sodium cotransporters. J Am Soc Nephrol 24: 407–418, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Webb TN, Carrisoza-Gaytan R, Montalbetti N, Rued A, Roy A, Socovich AM, Subramanya AR, Satlin LM, Kleyman TR, Carattino MD: Cell-specific regulation of L-WNK1 by dietary K. Am J Physiol Renal Physiol 310: F15–F26, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu Y, Song X, Shi Y, Shi Z, Niu W, Feng X, Gu D, Bao HF, Ma HP, Eaton DC, Zhuang J, Cai H: WNK1 activates large-conductance Ca2+-activated K+ channels through modulation of ERK1/2 signaling. J Am Soc Nephrol 26: 844–854, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Amlal H, Krane CM, Chen Q, Soleimani M: Early polyuria and urinary concentrating defect in potassium deprivation. Am J Physiol Renal Physiol 279: F655–F663, 2000 [DOI] [PubMed] [Google Scholar]
- 45.Marples D, Frøkiaer J, Dørup J, Knepper MA, Nielsen S: Hypokalemia-induced downregulation of aquaporin-2 water channel expression in rat kidney medulla and cortex. J Clin Invest 97: 1960–1968, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khositseth S, Uawithya P, Somparn P, Charngkaew K, Thippamom N, Hoffert JD, Saeed F, Michael Payne D, Chen SH, Fenton RA, Pisitkun T: Autophagic degradation of aquaporin-2 is an early event in hypokalemia-induced nephrogenic diabetes insipidus. Sci Rep 5: 18311, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rizzo F, Staub O: NEDD4-2 and salt-sensitive hypertension. Curr Opin Nephrol Hypertens 24: 111–116, 2015 [DOI] [PubMed] [Google Scholar]
- 48.Goel P, Manning JA, Kumar S: NEDD4-2 (NEDD4L): The ubiquitin ligase for multiple membrane proteins. Gene 557: 1–10, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ronzaud C, Staub O: Ubiquitylation and control of renal Na+ balance and blood pressure. Physiology (Bethesda) 29: 16–26, 2014 [DOI] [PubMed] [Google Scholar]
- 50.van der Lubbe N, Moes AD, Rosenbaek LL, Schoep S, Meima ME, Danser AH, Fenton RA, Zietse R, Hoorn EJ: K+-induced natriuresis is preserved during Na+ depletion and accompanied by inhibition of the Na+-Cl- cotransporter. Am J Physiol Renal Physiol 305: F1177–F1188, 2013 [DOI] [PubMed] [Google Scholar]
- 51.Liddle GW, Bledsoe T, Coppage WS Jr: A familial renal disorder simulating primary aldosteronism but with negligible aldosterone secretion. Trans Assoc Am Physicians 76: 199–213, 1963 [Google Scholar]
- 52.Shimkets RA, Warnock DG, Bositis CM, Nelson-Williams C, Hansson JH, Schambelan M, Gill JR Jr, Ulick S, Milora RV, Findling JW, Canessa CM, Rossier BC, Lifton RP: Liddle’s syndrome: Heritable human hypertension caused by mutations in the beta subunit of the epithelial sodium channel. Cell 79: 407–414, 1994 [DOI] [PubMed] [Google Scholar]
- 53.Abriel H, Loffing J, Rebhun JF, Pratt JH, Schild L, Horisberger JD, Rotin D, Staub O: Defective regulation of the epithelial Na+ channel by Nedd4 in Liddle’s syndrome. J Clin Invest 103: 667–673, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kamynina E, Debonneville C, Bens M, Vandewalle A, Staub O: A novel mouse Nedd4 protein suppresses the activity of the epithelial Na+ channel. FASEB J 15: 204–214, 2001 [DOI] [PubMed] [Google Scholar]
- 55.Ellison DH: Ubiquitylation and the pathogenesis of hypertension. J Clin Invest 123: 546–548, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kellenberger S, Schild L: Epithelial sodium channel/degenerin family of ion channels: A variety of functions for a shared structure. Physiol Rev 82: 735–767, 2002 [DOI] [PubMed] [Google Scholar]
- 57.Frindt G, Palmer LG: Na channels in the rat connecting tubule. Am J Physiol Renal Physiol 286: F669–F674, 2004 [DOI] [PubMed] [Google Scholar]
- 58.Schumacher FR, Siew K, Zhang J, Johnson C, Wood N, Cleary SE, Al Maskari RS, Ferryman JT, Hardege I, Yasmin, Figg NL, Enchev R, Knebel A, O’Shaughnessy KM, Kurz T: Characterisation of the Cullin-3 mutation that causes a severe form of familial hypertension and hyperkalaemia. EMBO Mol Med 7: 1285–1306, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hadchouel J, Ellison DH, Gamba G: Regulation of renal electrolyte transport by WNK and SPAK-OSR1 kinases. Annu Rev Physiol 78: 367–389, 2016 [DOI] [PubMed] [Google Scholar]
- 60.Terker AS, Zhang C, Erspamer KJ, Gamba G, Yang CL, Ellison DH: Unique chloride-sensing properties of WNK4 permit the distal nephron to modulate potassium homeostasis. Kidney Int 89: 127–134, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cope G, Murthy M, Golbang AP, Hamad A, Liu CH, Cuthbert AW, O’Shaughnessy KM: WNK1 affects surface expression of the ROMK potassium channel independent of WNK4. J Am Soc Nephrol 17: 1867–1874, 2006 [DOI] [PubMed] [Google Scholar]
- 62.Cheng CJ, Huang CL: Activation of PI3-kinase stimulates endocytosis of ROMK via Akt1/SGK1-dependent phosphorylation of WNK1. J Am Soc Nephrol 22: 460–471, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vidal-Petiot E, Elvira-Matelot E, Mutig K, Soukaseum C, Baudrie V, Wu S, Cheval L, Huc E, Cambillau M, Bachmann S, Doucet A, Jeunemaitre X, Hadchouel J: WNK1-related familial hyperkalemic hypertension results from an increased expression of L-WNK1 specifically in the distal nephron. Proc Natl Acad Sci USA 110: 14366–14371, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fang L, Garuti R, Kim BY, Wade JB, Welling PA: The ARH adaptor protein regulates endocytosis of the ROMK potassium secretory channel in mouse kidney. J Clin Invest 119: 3278–3289, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Parry WL, Hemstreet GP 3rd: Cancer detection by quantitative fluorescence image analysis. J Urol 139: 270–274, 1988 [DOI] [PubMed] [Google Scholar]
- 66.Collins TJ: ImageJ for microscopy. Biotechniques 43[Suppl]: 25–30, 2007 [DOI] [PubMed] [Google Scholar]
- 67.Jensen EC: Quantitative analysis of histological staining and fluorescence using ImageJ. Anat Rec (Hoboken) 296: 378–381, 2013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.