Abstract
The (pro)renin receptor (PRR) is a transmembrane protein with multiple functions. However, its regulation and role in the pathogenesis of CKD remain poorly defined. Here, we report that PRR is a downstream target and an essential component of Wnt/β-catenin signaling. In mouse models, induction of CKD by ischemia-reperfusion injury (IRI), adriamycin, or angiotensin II infusion upregulated PRR expression in kidney tubular epithelium. Immunohistochemical staining of kidney biopsy specimens also revealed induction of renal PRR in human CKD. Overexpression of either Wnt1 or β-catenin induced PRR mRNA and protein expression in vitro. Notably, forced expression of PRR potentiated Wnt1-mediated β-catenin activation and augmented the expression of downstream targets such as fibronectin, plasminogen activator inhibitor 1, and α–smooth muscle actin (α-SMA). Conversely, knockdown of PRR by siRNA abolished β-catenin activation. PRR potentiation of Wnt/β-catenin signaling did not require renin, but required vacuolar H+ ATPase activity. In the mouse model of IRI, transfection with PRR or Wnt1 expression vectors promoted β-catenin activation, aggravated kidney dysfunction, and worsened renal inflammation and fibrotic lesions. Coexpression of PRR and Wnt1 had a synergistic effect. In contrast, knockdown of PRR expression ameliorated kidney injury and fibrosis after IRI. These results indicate that PRR is both a downstream target and a crucial element in Wnt signal transmission. We conclude that PRR can promote kidney injury and fibrosis by amplifying Wnt/β-catenin signaling.
Keywords: chronic kidney disease, renal fibrosis, (pro)renin receptor, Wnt, betacatenin, ischemia-reperfusion
The (pro)renin receptor (PRR) is thought to be a component of the renin-angiotensin system (RAS) that is expressed in a wide variety of organs including the kidney.1–3 As a single-pass transmembrane receptor, PRR specifically binds to renin or its inactive precursor prorenin.4 After binding to the PRR, renin increases its catalytic activity of converting angiotensinogen to angiotensin I,1,4 the rate-limiting step in the cascade of RAS activation. Prorenin also gains enzymatic activity by binding to the PRR. Apart from RAS activation, binding of renin or prorenin to the PRR also directly triggers intracellular signal transduction, leading to activation of the extracellular signal–regulated kinase 1 and 2 (ERK-1/2), by a mechanism independent of angiotensin II (Ang II) generation.4–6 This action of the PRR independently induces the expression of profibrotic genes such as TGF-β, fibronectin, collagen I, and plasminogen activator inhibitor–1 (PAI-1). Furthermore, PRR is also identified as an accessory subunit of the vacuolar–type H+ ATPase,7,8 which plays a pivotal role in acidifying intracellular compartments and transporting protons across cell membranes. Interestingly, recent studies from model organisms such as Xenopus and Drosophila reveal that PRR is also a component of the Wnt receptor complex and regulates embryonic development via this signal pathway.9,10 These studies illustrate that PRR may possess a wide array of biologic activities in different tissues at specific timings that extend far beyond RAS activation.11,12
In the kidney, PRR is predominantly expressed in renal tubular epithelium, particularly in the distal tubules and collecting duct, as well as in glomerular podocytes and mesangial cells.7,13 Studies have shown that PRR is critical for normal nephron formation, because targeted ablation of PRR in nephron progenitors caused a marked decrease in the number of developing nephrons, small cystic kidneys, and podocyte foot process effacement at birth.14 Similarly, PRR present in the ureteric bud epithelia is essential for their branching morphogenesis and collecting duct development during kidney development.15,16 PRR is also critical for podocyte function and survival, because podocyte-specific PRR-knockout mice developed nephrotic syndrome, characterized by albuminuria, podocyte foot-process effacement, and cytoskeletal changes, and animals died between 2 and 3 weeks after birth.17 Recent studies also demonstrate that PRR in renal tubular epithelia regulates BP and sodium transport.18,19 Taken together, these investigations establish a critical role for PRR in regulating normal kidney development, tissue hemostasis, and BP regulation.
The role of PRR in the pathogenesis of kidney diseases, however, is uncertain.20,21 Numerous studies have shown an upregulation of PRR in the kidneys of CKDs such as diabetic nephropathy.22–24 However, the mechanism underlying PRR induction remains to be elucidated. We have recently demonstrated that multiple components of the RAS, including angiotensinogen, renin, angiotensin converting enzyme, and Ang II type 1 receptor, are controlled by Wnt/β-catenin,25 a signal pathway that is activated in a variety of CKD.26–30 This finding prompted us to examine whether PRR is also regulated by Wnt/β-catenin in diseased kidneys. Furthermore, although PRR is shown to participate in Wnt signaling in model organisms including Xenopus and Drosophila, the functional interplay between PRR and Wnt/β-catenin remains to be interrogated in the mammalian system in the setting of CKD.
In this study, we examined the regulation of PRR in animal models of CKD and human kidney biopsy specimens, as well as renal tubular cells in vitro, and investigated the functional interplay between PRR and Wnt/β-catenin in kidney injury and fibrosis after ischemic insult. Our results indicate that PRR not only is a downstream target of Wnt/β-catenin but also plays a crucial role in transmitting this pathogenic signaling.
Results
Upregulation of PRR in Animal Models of CKD and Human Kidney Biopsy Specimens
To investigate PRR regulation after kidney injury, we studied three animal models of CKD induced by renal ischemia/reperfusion injury (IRI), adriamycin (ADR), or chronic Ang II infusion. These models are well established and widely used, and they represent different causes that lead to renal failure and fibrotic lesions.25,31 As shown in Figure 1, A and B, PRR protein was significantly induced at 11 days after unilateral IRI (UIRI) compared with sham controls, as shown by western blot analyses of whole-kidney lysates. Similarly, renal PRR protein levels were significantly upregulated at 4 weeks after chronic Ang II infusion (Figure 1, C and D). PRR protein was also induced at 5 weeks after ADR administration (Figure 1, E and F). These results suggest that upregulation of PRR is a common finding in a variety of animal models of CKD, regardless of the initial causes.
Figure 1.
PRR is upregulated in a variety of animal models of kidney disease and in human kidney biopsy specimens. (A and B) Western blot analyses and quantitative determination of PRR protein in the kidneys at 11 days after UIRI. Kidney homogenates from sham control (Ctrl) and ischemic mice (UIRI) were subjected to western blotting using PRR-specific antibody. *P<0.05 versus Ctrl. (C and D) Western blot analyses of renal expression of PRR in Ang II–induced hypertensive nephropathy. PRR expression was assessed in the kidneys at 4 weeks after chronic infusion of Ang II. *P<0.05 versus Ctrl. (E and F) Western blot analyses of renal expression of PRR in ADR nephropathy. PRR expression was assessed in the kidneys at 5 weeks after ADR injection. *P<0.05 versus Ctrl. Numbers (1 through 4) indicate each individual animal in a given group. (G) Representative micrographs showed PRR expression in various animal models of CKD. Kidney cryosections were immunofluorescence-stained with antibody against PRR. Arrows indicate positive staining. Scale bar, 50 μm. (H) Representative micrographs show PRR expression in kidney biopsy specimens from patients with various CKDs. Yellow arrows indicate positive staining. Control, adjacent nontumor kidney tissue from patients who had renal cell carcinoma; DN, diabetic nephropathy; MN, membranous nephropathy. Scale bar, 20 μm.
We further examined PRR protein expression and localization by immunostaining in diseased kidneys after various injuries. As shown in Figure 1G, immunofluorescence staining revealed a marked induction of PRR protein in the fibrotic kidneys induced by UIRI, Ang II, or ADR, compared with controls. It was predominantly localized in renal tubular epithelium (Figure 1G, arrows).
To investigate the potential clinical relevance of these observations, we studied PRR expression in human kidney biopsy specimens from patients with various CKDs by immunohistochemical staining. As shown in Figure 1H, PRR protein was barely detectable in two cases of control, nontumor kidney tissue specimens. However, this protein was readily visible in the vast majority of 23 biopsy specimens from the patients with different CKDs, albeit with varying staining intensity. Representative micrographs of PRR staining in kidney biopsy specimens from patients with diabetic nephropathy, membranous nephropathy, and SLE are presented in Figure 1H. The demographic and clinical data of the patients with CKD are presented in Supplemental Table 1. It is concluded, therefore, that PRR expression is induced in human CKD as well.
PRR Is a Downstream Target of Wnt/β-Catenin Signaling
We next investigated the potential mechanism underlying PRR upregulation in diseased kidneys. Earlier studies demonstrate that multiple components of RAS are targets of Wnt/β-catenin signaling,25,26 a developmental signal cascade that is also activated in virtually all CKD models tested.32–34 This finding prompted us to examine whether Wnt/β-catenin signaling is involved in regulating PRR gene expression as well. To test this hypothesis, we first analyzed the regulatory regions of the PRR gene promoter by a bioinformatics approach. As shown in Figure 2A, there were putative T cell factor (TCF)/lymphoid enhancer factor (LEF) binding sites (TBS) in the promoter region of human, mouse, and rat PRR genes which were perfectly matched with TBS consensus sequences (Figure 2A), suggesting that PRR might be controlled by Wnt/β-catenin signaling.
Figure 2.
PRR is a downstream target of Wnt/β-catenin signaling. (A) Bioinformatics analyses revealed the presence of putative TBS in the promoter region of human, mouse, and rat PRR genes. The sequences and positions of the putative TBS in the PRR genes are highlighted (red), whereas the TBS consensus sequence is given at the bottom of this panel. (B and C) Over-expression of Wnt1 (B) or constitutively active β-catenin (C) induced the mRNA expression of PRR in human kidney proximal tubular cells (HKC-8). HKC-8 cells were transiently transfected with Wnt1 expression vector (pHA-Wnt1), Flag-tagged N-terminally truncated β-catenin expression vector (pDel-β-cat), or empty vector (pcDNA3) for 24 hours. Cells were then analyzed for PRR mRNA expression by qRT-PCR. *P<0.05 versus pcDNA3 control. (D and E) Wnt1 induced PRR protein expression in a dose-dependent fashion. HKC-8 cells were transfected with different amounts of Wnt1 expression plasmid (microgram per well) or pcDNA3 empty vector (4 µg/well), as indicated. Cell lysates were analyzed for PRR protein expression by western blot. *P<0.05 versus pcDNA3 alone. (F and G) Constitutively active β-catenin induced PRR protein expression. HKC-8 cells were transfected with different amounts of Flag-tagged, truncated β-catenin expression plasmid, as indicated, or empty vector pcDNA3. Cell lysates were analyzed for PRR protein expression by western blot. *P<0.05 versus pcDNA3. (H and I) Wnt1 and β-catenin were colocalized with PRR in mouse kidney at 11 days after UIRI. Kidney cryosections were coimmunostained for Wnt1 (H) (green) or β-catenin (I) (green) and PRR (red). Arrows indicate Wnt1 or β-catenin and PRR colocalization in the same tubule. Scale bar, 50 μm.
To ascertain the regulation of PRR by Wnt/β-catenin signaling, we transiently transfected human kidney proximal tubular cells (HKC-8) with HA-tagged Wnt1 expression vector (pHA-Wnt1). As shown in Figure 2B, overexpression of Wnt1 substantially induced the mRNA expression of PRR, which was illustrated by quantitative, real-time RT-PCR (qRT-PCR) analysis. Similarly, transfection of HKC-8 cells with N-terminus–truncated, constitutively activated β-catenin expression vector (pDel-β-cat) also induced PRR mRNA expression (Figure 2C). Accordingly, forced expression of either exogenous Wnt1 or β-catenin induced PRR protein expression in HKC-8 cells in a dose-dependent fashion (Figure 2, D–G).
To establish the relationship between Wnt/β-catenin and PRR expression in vivo, we further examined the colocalization of Wnt/β-catenin and PRR by double immunofluorescence staining. Although little or no Wnt1, β-catenin, and PRR staining was observed in sham-operated kidney (data not shown), these proteins were markedly induced and colocalized in renal tubular epithelium at 11 days after UIRI (Figure 2, H and I, arrows). Taken together, these results indicate that PRR is a downstream target of Wnt/β-catenin signaling both in vitro and in vivo.
Over-Expression of PRR Augments Wnt1-Mediated Gene Transcription
To investigate the reciprocal regulation of PRR on Wnt/β-catenin, we examined the interplay between PRR and Wnt signaling. HKC-8 cells were transfected with the expression vectors encoding PRR (pCMV-PRR) and/or Wnt1 expression vector (pHA-Wnt1). As expected, expression of Wnt1 induced PRR (Figure 3A, lane 3 versus lane 1), and cotransfection of the Wnt1- and PRR-expression vectors further upregulated PRR protein (Figure 3, A and B). Of interest, although PRR alone did not affect Wnt1 expression (Figure 3A) or β-catenin–mediated gene transcription (Figure 3C), over-expression of PRR significantly augmented Wnt1-triggered gene transcription in the β-catenin–responsive TOP Flash luciferase reporter assay (Figure 3C), suggesting that PRR is able to potentiate Wnt/β-catenin signaling.
Figure 3.
Over-expression of PRR amplifies Wnt/β-catenin signaling in vitro. (A and B) Wnt1 and PRR expression was confirmed after transient transfection. HKC-8 cells were transfected with empty vector (pcDNA3), Wnt1 expression vector (pHA-Wnt1), or PRR expression vector (pCMV-PRR), as indicated, followed by incubation for 24 hours. Representative western blot (A) and quantitative data for PRR (B) are presented. *P<0.05 versus pcDNA3; †P<0.05 versus pHA-Wnt1 or pCMV-PRR alone. (C) PRR augmented the Wnt1-mediated gene expression. HKC-8 cells were cotransfected with TOP-Flash reporter plasmid, Wnt1 expression plasmid (pHA-Wnt1), PRR expression plasmid (pCMV-PRR), or combination, as indicated. Luciferase activity was assayed and reported after correction with transfection efficiency. *P<0.05 versus pcDNA3; †P<0.05 versus pHA-Wnt1 alone (n=5). (D–H) Western blot analyses and quantitative data demonstrated that PRR augmented Wnt1-mediated β-catenin activation and its target gene expression. HKC-8 cells were transfected with empty vector (pcDNA3), Wnt1 expression vector (pHA-Wnt1), or PRR expression vector (pCMV-PRR), as indicated, followed by incubation for 24 hours. Representative western blot (D) and quantitative data for active β-catenin (E), PAI-1 (F), fibronectin (G), and α-SMA (H) are present. *P<0.05 versus pcDNA3 control; †P<0.05 versus pHA-Wnt1. (I) Immunofluorescence staining showed that overexpression of PRR upregulated the Wnt1-mediated fibronectin expression and deposition. HKC-8 cells were transfected with empty vector (pcDNA3), Wnt1 expression plasmid (pHA-Wnt1), or PRR expression vector (pCMV-PRR), as indicated. Cells were then immunostained for fibronectin. Scale bar, 50 μm.
We further investigated the regulation of Wnt/β-catenin signaling by PRR by examining the expression of Wnt target genes. As shown in Figure 3, D–H, PRR did not induce β-catenin activation and its target gene expression under basal conditions. However, ectopic expression of PRR clearly promoted Wnt1-mediated β-catenin activation and the expression of PAI-1, fibronectin, and α-SMA proteins. Similar data were obtained when the cells were assessed for fibronectin expression by immunofluorescence staining (Figure 3I). These results indicate that PRR is able to promote the expression of the fibrosis-related genes by potentiating Wnt signaling.
PRR Is Obligatory for Transmitting Wnt/β-Catenin Signaling In Vitro
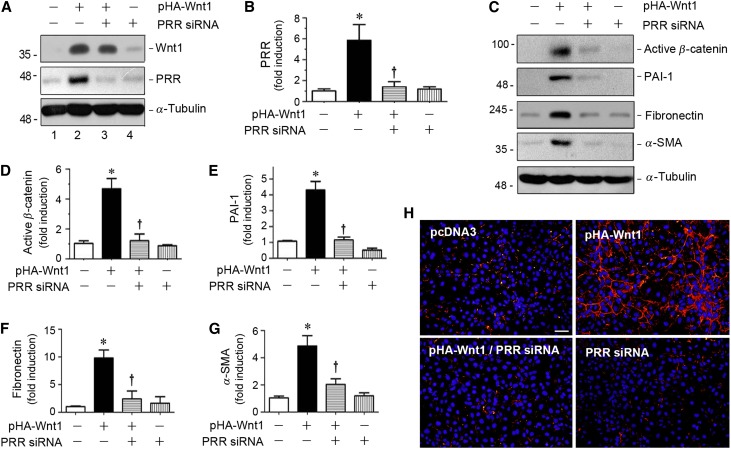
We next investigated the PRR regulation of Wnt/β-catenin signaling by knocking down PRR expression in kidney tubular cells. To this end, HKC-8 cells were transfected with control or PRR-specific small interfering RNA (siRNA) in the presence of pHA-Wnt1 plasmid. As shown in Figure 4, A and B, transfection of PRR-specific siRNA efficiently abolished the Wnt1-induced PRR protein expression in HKC-8 cells. Consistently, knockdown of PRR expression by siRNA also abrogated the Wnt1-triggered induction of active β-catenin, PAI-1, fibronectin, and α-SMA proteins in HKC-8 cells (Figure 4, C–G). Immunofluorescence staining also showed that knockdown of PRR reduced the Wnt1-mediated fibronectin protein expression and deposition. Therefore, PRR is required for Wnt1-mediated β-catenin activation and its downstream target gene expression.
Figure 4.
Knockdown of PRR inhibits Wnt/β-catenin signaling in vitro. (A and B) Western blot analyses showed the downregulation of PRR expression by siRNA-mediated inhibition. HKC-8 cells were transfected with empty vector (pcDNA3), Wnt1 expression vector (pHA-Wnt1) in the presence of control siRNA, or PRR-specific siRNA (PRR siRNA), as indicated, followed by incubation for 24 hours. Representative western blot (A) and quantitative data for PRR (B) are presented. *P<0.05 versus control; †P<0.05 versus pHA-Wnt1 plus control siRNA. (C–G) Western blot analyses and quantitative data demonstrated that knockdown of PRR expression by siRNA inhibited Wnt/β-catenin signaling. HKC-8 cells were transfected with empty vector (pcDNA3), Wnt1 expression vector (pHA-Wnt1) in the presence of control siRNA, or PRR-specific siRNA (PRR siRNA), as indicated, followed by incubation for 24 hours. Representative western blot (C) and quantitative data for active β-catenin (D), PAI-1 (E), fibronectin (F), and α-SMA (G) are presented. *P<0.05 versus control; †P<0.05 versus pWnt1 plus control siRNA. (H) Immunofluorescence staining showed that knockdown of PRR inhibited Wnt1-mediated fibronectin expression. HKC-8 cells were transfected with empty vector (pcDNA3), Wnt1 expression plasmid (pHA-Wnt1) in the presence of control siRNA, or PRR siRNA (PRR siRNA), as indicated. Cells were then immunostained for fibronectin. Scale bar, 50 μm.
PRR Potentiates Wnt/β-Catenin Signaling by a Renin-Independent Mechanism, but it Requires V-ATPase Activity
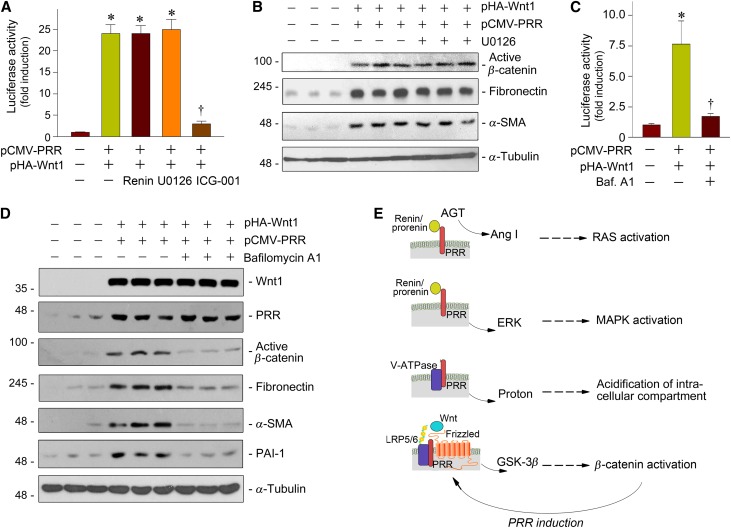
We further explored whether renin is required for PRR potentiation of Wnt/β-catenin signaling, because renin/prorenin binding to PRR is obligatory for PRR-mediated RAS activation and mitogen-activated protein (MAP) kinase activation.2,3 To this end, we employed the β-catenin–responsive TOP Flash luciferase reporter system to assess the functional consequence of the canonical Wnt signaling. As shown in Figure 5A, transfection of pHA-Wnt1 and pCMV-PRR plasmids in HKC-8 cells markedly induced luciferase reporter activity, as expected. However, incubation with renin did not affect luciferase activity, indicating that renin is not required for PRR-mediated reporter gene expression. Similarly, blockade of ERK activation by U0126 did not affect luciferase activity. ICG-001, a small molecule inhibitor that specifically blocks β-catenin–mediated gene transcription,35,36 was able to abolish luciferase activity induced by Wnt1 and PRR (Figure 5A). Similarly, U0126 also did not affect Wnt1/PRR-mediated β-catenin activation and its downstream fibronectin and α-SMA expression in HKC-8 cells (Figure 5B). These results suggest that, unlike PRR-mediated RAS and MAPK activation that requires renin/prorenin engagement, PRR augments Wnt/β-catenin signaling by a mechanism that is clearly independent of renin.
Figure 5.
PRR amplifies Wnt/β-catenin signaling by a mechanism independent of renin, but requires V-ATPase activity. (A) PRR augmented Wnt/β-catenin–targeted gene transcription in a renin-independent manner. HKC-8 cells were cotransfected with TOP-Flash reporter plasmid, Wnt1 expression plasmid (pHA-Wnt1), and PRR expression plasmid (pCMV-PRR), as indicated, in the absence or presence of renin (10−8 M), U0126 (10 μM), or ICG-001 (10 μM), respectively. *P<0.05 versus pcDNA3 control; †P<0.05 versus pHA-Wnt1 plus pCMV-PRR (n=5). (B) Inhibition of ERK1/2 did not affect Wnt1/PRR-induced β-catenin activation and its target gene expression. HKC-8 cells were cotransfected with pHA-Wnt1 and pCMV-PRR expression plasmids in the absence or presence of U0126 (10 μM), as indicated. Cell lysates were immunoblotted with antibodies against active β-catenin, fibronectin, α-SMA, and α-tubulin, respectively. (C) V-ATPase activity was required for Wnt-mediated gene transcription. HKC-8 cells were cotransfected with TOP-Flash reporter plasmid, pHA-Wnt1, and pCMV-PRR plasmids in the absence or presence of bafilomycin A1 (100 nM). *P<0.05 versus pcDNA3 control; †P<0.05 versus pHA-Wnt1 plus pCMV-PRR. (D) Inhibition of V-ATPase activity by bafilomycin A1 abolished Wnt1/PRR-mediated β-catenin activation and its target gene expression. Cell lysates were immunoblotted with antibodies against Wnt1, PRR, active β-catenin, fibronectin, α-SMA, PAI-1, and α-tubulin, respectively. (E) Diagram depicts the potential mechanism of PRR action. In addition to promoting RAS activation and MAPK activation, which requires prorenin/renin involvement, PRR stimulates V-ATPase activity and promotes Wnt/β-catenin signaling by a mechanism independent of renin. Because PRR is a downstream target of Wnt/β-catenin signaling, PRR upregulation and Wnt/β-catenin activation constitute a vicious cycle.
PRR is also known as an accessory protein of the vacuolar H+ ATPase (V-ATPase).7,8 Therefore, we further investigated whether V-ATPase function is required for PRR to promote Wnt/β-catenin signaling. As shown in Figure 5C, inhibition of V-ATPase activity by bafilomycin A1, a highly potent, selective inhibitor,37,38 abolished Wnt1/PRR-mediated gene transcription in the β-catenin–responsive TOP Flash luciferase reporter system. Similarly, bafilomycin A1 almost completely abolished the Wnt1/PRR-mediated β-catenin activation and fibronectin, α-SMA, and PAI-1 expression (Figure 5D). Therefore, it is conceivable that the ability of PRR to potentiate Wnt/β-catenin signaling depends on an intact V-ATPase activity (Figure 5E).
PRR Potentiates Wnt1/β-Catenin Signaling In Vivo
To establish the in vivo relevance of PRR on regulation of Wnt/β-catenin signaling, we next examined whether forced expression of PRR affects renal activation of β-catenin in a mouse model induced by UIRI, which is characterized by sustained Wnt/β-catenin activation and progressive fibrotic lesions after AKI.31 As shown in Figure 6A, at 4 days after UIRI, various expression plasmids including empty vector (pcDNA3), PRR expression vector (pCMV-PRR), Wnt1 expression vector (pHA-Wnt1), or in combination (pCMV-PRR + pHA-Wnt1), were injected via tail vein by a hydrodynamic-based gene transfer technique, an approach that results in significant renal expression of the transgenes.31,39 As shown in Figure 6, B–E, western blot analyses of whole-kidney homogenates revealed that delivery of exogenous PRR or Wnt1 genes upregulated renal expression of PRR and Wnt1 proteins at 11 days after UIRI, and combination of these expression plasmids resulted in more profound induction of PRR and Wnt1, compared with the groups receiving pCMV-PRR or pHA-Wnt1 alone. Consistently, expression of PRR also promoted renal β-catenin expression (Figure 6, B and E). Similar data were obtained when kidney sections were immunostained for Wnt1, PRR, and β-catenin, respectively (Figure 6F). These results indicate that over-expression of PRR promotes Wnt/β-catenin signaling in vivo as well.
Figure 6.
In vivo expression of exogenous PRR and Wnt1 synergistically promotes β-catenin activation after kidney injury. (A) Experimental design. Red arrows indicate plasmid injection of various expression vectors, including empty vector pcDNA3, pHA-Wnt1, pCMV-PRR, or in combination, at 4 days after UIRI (30 minutes). (B–E) Western blot analyses showed the expression levels of Wnt1, PRR, and β-catenin proteins in different groups as indicated. Representative western blot (A) and graphical presentation of Wnt1 (C), PRR (D), and β-catenin (E) are shown. Numbers (1, 2) indicate each individual animal in a given group (B). *P<0.05 versus sham; †P<0.05 versus UIRI injected with pcDNA3; ǂP<0.05 versus UIRI injected with pCMV-PRR; #P<0.05 versus UIRI injected with pHA-Wnt1 (n=6–7). (F) Representative micrographs showed Wnt1, PRR, and β-catenin protein expression in different groups as indicated. Kidney sections were stained immunohistochemically with antibodies against Wnt1 and β-catenin, whereas PRR was detected by immunofluorescence staining. Arrows indicate positive staining. Scale bar, 50 μm. UNx, unilateral nephrectomy.
Coexpression of PRR and Wnt1 Augments Kidney Dysfunction and Fibrosis In Vivo
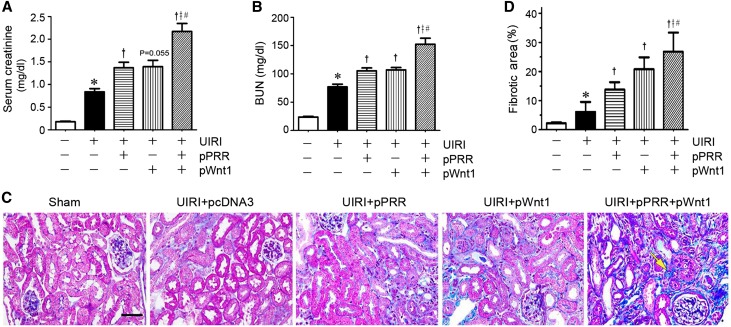
We found that coexpression of PRR and Wnt1 substantially aggravated kidney dysfunction after UIRI, because serum creatinine levels elevated significantly at 11 days after injury (Figure 7A). Ectopic expression of PRR or Wnt1 alone also increased serum creatinine, to a lesser extent (Figure 7A), but combination of both expression plasmids resulted in profound decline in renal function, suggesting a synergism between PRR and Wnt1 in driving kidney injury. Similar results were obtained when BUN was assessed in different groups (Figure 7B).
Figure 7.
PRR and Wnt1 synergistically aggravate kidney dysfunction and fibrosis after IRI. (A) Over-expression of PRR and Wnt1 expression vector starting at 4 days after IRI aggravated kidney dysfunction. Serum creatinine (A) and BUN (B) were assessed at 11 days after UIRI. *P<0.05 versus sham; †P<0.05 versus UIRI injected with pcDNA3; ǂP<0.05 versus UIRI injected with pCMV-PRR; #P<0.05 versus UIRI injected with pHA-Wnt1 (n=6). The P value (0.06) between UIRI group and UIRI plus pHA-Wnt1 was given. (C) Representative micrographs showed collagen deposition in different groups as indicated. Paraffin sections were subjected to MTS. Yellow arrow indicates positive staining. Scale bar, 50 μm. (D) Graphical presentation demonstrated kidney fibrotic lesions after quantitative determination. *P<0.05 versus sham; †P<0.05 versus UIRI injected with pcDNA3; ǂP<0.05 versus UIRI injected with pCMV-PRR; #P<0.05 versus UIRI injected with pHA-Wnt1.
We next examined renal fibrotic lesions in this model after various treatments. As shown in Figure 7, C and D, Masson trichrome staining (MTS) revealed that expression of either PRR or Wnt1 promoted renal fibrotic lesions, but coexpression of both plasmids in vivo resulted in substantial kidney lesions and fibrosis at 11 days after UIRI (7 days after plasmid injection), suggesting that PRR and Wnt act in concert to promote the development of kidney fibrosis.
PRR and Wnt1 Synergistically Promote Myofibroblast Activation and Matrix Expression In Vivo
We further examined whether PRR and Wnt affect myofibroblast activation, a key event in renal fibrogenesis.40 As shown in Figure 8, A and B, western blot analyses of whole-kidney lysates illustrated that both PRR and Wnt1 could upregulate renal α-SMA expression at 11 days after UIRI, and together, they synergistically promoted the expression of this protein. Immunohistochemical staining also demonstrated a profound induction of the α-SMA–positive myofibroblasts predominantly in renal interstitial space after coexpression of PRR and Wnt1 (Figure 8E, arrow), whereas expression of either PRR or Wnt1 alone only moderately increased myofibroblast activation. Similar results were observed when kidney sections were immunostained for fibroblast-specific protein 1 (Fsp1) (Figure 8E), suggesting that PRR and Wnt1 synergistically promoted the activation of the matrix-producing myofibroblasts.
Figure 8.
PRR and Wnt1 synergistically promote myofibroblast activation and fibrosis-related gene expression in vivo. (A–D) Western blot analyses showed the expression of α-SMA, fibronectin, and PAI-1 in different groups, as indicated. Representative western blot (A) and graphical presentation of α-SMA (B), fibronectin (C), and PAI-1 (D) are shown. Numbers (1, 2) indicate each individual animal in a given group. *P<0.05 versus sham; †P<0.05 versus UIRI injected with pcDNA3; ǂP<0.05 versus UIRI injected with pCMV-PRR; #P<0.05 versus UIRI injected with pHA-Wnt1 (n=6–7). (E) Representative micrographs showed α-SMA, Fsp1, and fibronectin in different groups, as indicated. Kidney sections were immunostained with different antibodies against α-SMA, Fsp1, and fibronectin, respectively. Arrows indicate positive staining. Scale bar, 50 μm.
Consistent with myofibroblast activation, western blot analyses showed that renal fibronectin protein was also upregulated after expression of PRR and Wnt1 (Figure 8, A and C). Comparing to the PRR- or Wnt1-expressing vector alone, simultaneous expression of both PRR and Wnt1 resulted in profound induction of fibronectin in the kidney after UIRI. A similar expression pattern of fibronectin protein was also observed by immunohistochemical staining (Figure 8E). In addition, expression of PAI-1, a direct downstream target of Wnt/β-catenin,41 was also induced by PRR, Wnt1, or in combination (Figure 8, A and D).
PRR and Wnt1 Promote Cytokine Expression and Macrophage Infiltration In Vivo
We next examined the effects of exogenous PRR and Wnt1 on renal inflammation after UIRI, as they are a major component of chronic kidney injury. As shown in Figure 9, A and B, qRT-PCR analyses demonstrated that in vivo expression of either PRR or Wnt1 upregulated proinflammatory RANTES (regulated on activation, normal T cell expressed and secreted), also known as C-C chemokine ligand 5 (CCL5), and TNF-α mRNA expression. Immunohistochemical staining also showed the induction of RANTES and TNF-α proteins predominantly in renal tubular epithelium, especially in the kidney after coexpression of both PRR and Wnt1 (Figure 9C). Consistently, renal F4/80-positive cells, a marker for the infiltrated macrophages, were increased after either PRR or Wnt1 expression, and coexpression of both genes resulted in more profound induction (Figure 9, C and D).
Figure 9.
PRR and Wnt1 induce chemokine expression and promote renal inflammation. (A and B) qRT-PCR showed renal expression of RANTES (A) and TNF-α (B) in different groups, as indicated. Fold-induction over the controls was reported. *P<0.05 versus sham; †P<0.05 versus UIRI injected with pcDNA3; ǂP<0.05 versus UIRI injected with pCMV-PRR; #P<0.05 versus UIRI injected with pHA-Wnt1 (n=6–7). (C) Representative micrographs showed renal expression of RANTES and TNF-α proteins, as well as renal F4/80+ cells in different groups, as indicated. Kidney sections were stained immunohistochemically with specific antibodies against RANTES and TNF-α, respectively. Immunofluorescence staining for F4/80 was performed on kidney cryosections. Arrows indicate positive staining. Scale bar, 50 μm. (D) Graphical presentation of F4/80+ macrophages in the kidney sections of different groups. Data are presented as the numbers of F4/80-positive cells per high-power field. *P<0.05 versus sham; †P<0.05 versus UIRI injected with pcDNA3; ǂP<0.05 versus UIRI injected with pCMV-PRR; #P<0.05 versus UIRI injected with pHA-Wnt1 (n=6–7).
Knockdown of PRR Expression Ameliorates Kidney Injury and Fibrosis In Vivo
To further establish the role of an increased PRR in kidney injury and fibrosis, we utilized an opposite strategy by downregulating endogenous PRR expression in vivo. To this end, mice were daily injected intravenously with control or PRR-specific siRNA for 7 days, starting at 4 days after UIRI (Figure 10A). We found that knockdown of PRR significantly improved kidney function. Both serum creatinine and BUN were substantially reduced at 11 days after UIRI (Figure 10, B and C). Western blot analyses of whole-kidney lysates confirmed the reduction of renal PRR protein after siRNA injection (Figure 10, D and E). Notably, knockdown of PRR inhibited renal β-catenin expression, and reduced fibronectin and α-SMA proteins (Figure 10, D, F, G, and H). Similar results were obtained when kidney sections were immunostained for PRR, β-catenin, fibronectin, and α-SMA proteins in different groups (Figure 10I). Not surprisingly, downregulation of PRR ameliorated kidney fibrotic lesions and collagen deposition, as assessed by MTS (Figure 10I).
Figure 10.
Knockdown of PRR by siRNA strategy ameliorates kidney injury and fibrosis in vivo. (A) Experimental design. Red arrows indicate intravenous injection of either control or PRR-specific siRNA. (B and C) Knockdown of PRR starting at 4 days after IRI improved kidney function. Serum creatinine (B) and BUN (C) were assessed at 11 days after severe UIRI (35 minutes). *P<0.05 versus sham; †P<0.05 versus UIRI injected with Ctrl-siR (control siRNA) (n=6–7). (D–H) Western blot analyses showed the expression of PRR, β-catenin, fibronectin, and α-SMA in different groups, as indicated. Representative western blot (D) and graphical presentation of PRR (E), β-catenin (F), fibronectin (G), and α-SMA (H) are shown. Numbers (1, 2, and 3) indicate each individual animal in a given group. *P<0.05 versus sham; †P<0.05 versus UIRI injected with Ctrl-siR (n=6–7). (I) Representative micrographs showed renal expression of PRR, β-catenin, fibronectin, and α-SMA proteins in different groups, as indicated. Kidney sections were immunostained with different antibodies against PRR, β-catenin, fibronectin, and α-SMA, respectively. MTS was used to assess collagen deposition (blue color) and fibrosis in different groups, as indicated. Arrows indicate positive staining. Scale bar, 50 μm. UNx, unilateral nephrectomy.
Discussion
PRR was discovered on the basis of its property of specific binding to renin or prorenin, which facilitates the conversion of angiotensinogen to angiotensin I, thereby promoting local RAS activation at the site where it expresses.1,4,42 Ligand/PRR engagement also triggers an intracellular signaling that leads to MAP kinase activation.4,5 In this study, we have described a novel ligand-independent pathway by which PRR promotes kidney injury and demonstrated a reciprocal, complex interaction between PRR and Wnt/β-catenin signaling (Figure 5E). We showed that Wnt/β-catenin controls PRR gene and induces its expression in kidney tubular cells (Figure 2). In return, PRR acts as an essential component of the Wnt receptor complex and is obligatory for its signal transduction (Figures 3 and 4). Therefore, PRR induction and Wnt/β-catenin activation in the kidney spontaneously instigate a vicious, self-perpetuating cycle (Figure 5E), which leads to the amplification of pathogenic Wnt/β-catenin signaling and ultimately drives the progressive loss of kidney structural integrity and function after injury. These studies not only identify Wnt/β-catenin as a critical upstream transcriptional regulator that controls PRR expression in the kidney, but also provide novel insights into the mechanism underlying the pathologic action of PRR in CKD progression.
PRR is ubiquitously expressed in a variety of organs such as the brain, heart, kidney, and liver,4,6 a pattern similar to Wnt/β-catenin.27,43,44 In the kidney, PRR is detected in renal tubular epithelium, particularly in the distal tubules and collecting duct, as well as in glomerular podocytes and mesangial cells.7,13 In this study, we found that renal PRR was induced, predominantly in renal tubular epithelium, in three CKD models tested, by UIRI, ADR, or chronic Ang II infusion, as well as in human kidney biopsy specimens from patients with various CKDs (Figure 1). Together with previous reports that renal tubular PRR is upregulated in diabetic nephropathy,22,23 these studies suggest that PRR induction perhaps is a common pathologic finding in the kidney after chronic injury, consistent with an increased intrarenal RAS activation and renal fibrosis found in these models.
One novel finding in this study is that PRR is a downstream target of Wnt/β-catenin signaling and subjected to its regulation. This conclusion is supported by several lines of in vitro and in vivo evidence (Figure 2). There are putative TCF–binding sites in the promoter region of human, mouse, and rat PRR genes, raising the possibility that β-catenin can control its expression. Indeed, forced expression of either Wnt1 ligand or constitutively active β-catenin induced PRR mRNA and protein expression in cultured kidney tubular cells. Furthermore, PRR was colocalized with Wnt ligand or β-catenin in renal tubular epithelium in the kidney after ischemic injury (Figure 2), and forced expression of exogenous Wnt1 induced PRR expression in vivo (Figure 6). It should be pointed out that multiple RAS genes have been identified as the downstream targets of Wnt/β-catenin in an earlier study.25 In that regard, the possibility also exists that Wnt/β-catenin could indirectly induce PRR expression in vivo by promoting Ang II generation, which in turn stimulates PRR expression as previously reported.45 Therefore, Wnt/β-catenin, as a master upstream regulator, is able to control virtually all of the RAS genes in a coordinated fashion. Such an ability of Wnt/β-catenin makes it a central and crucial player in driving kidney dysfunction and fibrosis under pathologic conditions.
The interplay between PRR and Wnt/β-catenin is clearly not unidirectional. In that regard, PRR also regulates Wnt/β-catenin signaling (Figures 3 and 4), presumably by acting as an adapter protein that recruits Wnt receptor Frizzled and coreceptors, the LDL-related protein 5 and 6 (LRP-5/6).9,10 In fact, PRR is absolutely required for proper Wnt signal transduction in kidney tubular cells, because knockdown of PRR expression completely abolished β-catenin activation and its target gene expression (Figure 4). Furthermore, the functional integrity of the V-ATPase is also indispensable, because inhibition of its activity by bafilomycin A1 blunted Wnt signaling (Figure 5, C and D), suggesting that local acidification of intracellular compartments plays a critical role in Wnt signal transduction. These results are consistent with earlier reports in model organisms such as Xenopus and Drosophila,9,10 suggesting that participation in Wnt signaling is an evolutionarily conserved, essential cellular function of PRR. It is worthwhile to note that our studies, together with a previous report,46 demonstrate that the intricate interplay between PRR and Wnt/β-catenin goes beyond Xenopus and Drosophila, and it occurs in human kidney cells in vitro (Figures 3 and 4) and in mouse models of CKD as well (Figures 6–8). Because PRR is commonly upregulated in diseased kidneys compared with normal controls (Figure 1), this suggests that Wnt/β-catenin signaling under pathologic conditions is primed to react to its ligand stimulation and produce an exaggerated response. In this regard, our studies have also delineated a new mechanism underlying the hyperactive Wnt/β-catenin signaling in diseased kidneys.
Since its initial discovery in 2002,4 the role of PRR in the pathogenesis of kidney diseases has been a subject of intense investigation. Because global ablation of PRR is embryonically lethal, evidence for PRR involvement in renal diseases almost exclusively relies on studies in transgenic animals overexpressing PRR, and so far the reported data are contradictory.20,21 Although earlier studies showing that transgenic rats overexpressing human PRR slowly developed proteinuria and nephropathy with normal renal level of Ang II and BP,20 a more recent study indicates that mice with constitutive overexpression of PRR had normal BP and failed to develop albuminuria and renal fibrosis at the age of 12 months, in spite of a 25- to 80-fold renal and up to 400-fold cardiac increase in PRR expression.21 The reason behind these discrepancies is unknown, and perhaps, to some extent, is related to the species and strains of animals used. We speculate that PRR by itself perhaps is not sufficient to induce a robust renal fibrosis in normal mice, because its action on Wnt/β-catenin completely relies on the preexisting Wnt ligands (Figure 2). Therefore, over-expression of PRR in normal mice, in which Wnt expression is relatively silenced,33,34 could have quite different outcomes in mice with CKD. We showed that expression of exogenous PRR at 4 days after IRI, a time point when Wnt ligands are highly expressed,31 promoted renal fibrosis and accelerated kidney function decline (Figures 6–9). Furthermore, coexpression of PRR and Wnt1 exhibits a synergistic effect, suggesting the importance of the preexisting Wnt ligands in conferring PRR fibrogenic actions. Therefore, it is reasonable to conclude that PRR is unlikely to initiate renal fibrosis by itself, but it can promote kidney destruction by amplifying Wnt/β-catenin signaling.
It should be stressed that the reciprocal interplay between PRR and Wnt/β-catenin does not require the participation of renin (Figure 5). Furthermore, blockade of the renin-triggered ERK-1/2 activation by U0126 did not affect PRR-mediated β-catenin activation and its downstream fibronectin and α-SMA expression (Figure 5), suggesting the absence of any crosstalk between (pro)renin/PRR-mediated MAP kinase signaling and Wnt/β-catenin (Figure 5E). In theory, an excessive PRR can promote kidney injury via (pro)renin-dependent RAS activation and ERK-1/2 stimulation, or (pro)renin-independent potentiation of Wnt/β-catenin (Figure 5E). However, it is highly likely that the interplay between PRR and Wnt/β-catenin, as identified in this study, plays a predominant role in mediating the pathologic actions of PRR. This notion is consistent with previous reports that over-expression of (pro)renin in transgenic rats or mice did not result in any kidney fibrosis over a period of 6 months.47,48 Nevertheless, we cannot exclude the possibility that in animal models when endogenous renin is present, ERK1/2 cascade may also play a role in mediating PRR-induced expression of the fibrosis-related proteins such as fibronectin and PAI-1 in vivo.
In summary, we herein demonstrate that PRR is commonly upregulated in kidney tubular cells in a variety of CKDs in animal models and in humans. PRR not only is a downstream target, but also acts as an integral component of the Wnt receptor complex and is required for its signal transduction. These results illustrate a novel, ligand-independent pathway of PRR in promoting kidney failure and fibrosis after injury.
Concise Methods
Mouse Models of CKD
Male BALB/c mice weighing 20–22 g were purchased from the Center of Experimental Animals in Southern Medical University (Guangzhou, China). UIRI was used to model CKD development after AKI. This model avoids animal loss after severe AKI induced by bilateral IRI, because one kidney is intact at the time of injury. The UIRI model is well established and kidney function can be assessed after removing the intact kidney by nephrectomy. Details of the UIRI protocol are described elsewhere.31 Briefly, 6-week-old male BALB/c mice were subjected to general anesthesia and a midline abdominal incision was made; the left renal pedicle was then clipped using microaneurysm clamps for 30 minutes. After removal of the clamps, reperfusion of the kidneys was visually confirmed, and the incision was then closed. During the ischemic period and recovery, body temperature was maintained between approximately 37°C and 38°C using a temperature-controlled heating system. Animals were then administered intraperitoneally with buprenorphine at 0.05 mg/kg body wt. At 10 days after UIRI, mice were subjected to unilateral nephrectomy to remove the intact contralateral kidney. Mice were euthanized at 11 days after UIRI. Serum and kidney tissues were collected for various analyses.
To investigate the renal expression of PRR in other CKD models, a chronic Ang II infusion model and ADR-induced nephropathy model were used as well. For the Ang II infusion model, an osmotic minipump (Model 2ML4; Alzet, Cupertino, CA) was implanted subcutaneously for the chronic administration of Ang II at the rate of 1 μg/kg per minute. At 4 weeks after Ang II infusion, mice were euthanized. Kidney tissues were collected for western blotting and immunostaining. For the ADR-induced nephropathy model, male BALB/c mice were administered with ADR (doxorubicin hydrochloride; Chembest Bioscience, Shanghai, China) via a single intravenous injection at 10 mg/kg body wt. At 5 weeks after ADR injection, all mice were euthanized. Kidney tissues were collected for western blotting and immunostaining. All animal studies were performed by the use of procedures approved by the Animal Ethics Committee at the Nanfang Hospital, Southern Medical University.
Human Kidney Biopsy Samples
Human kidney specimens were obtained from diagnostic renal biopsies performed at the Nanfang Hospital, Southern Medical University. Nontumor kidney tissues from the patients who had renal cell carcinoma and underwent nephrectomy were used as normal controls. Paraffin-embedded human kidney biopsy specimen sections (2.5 μm thickness) were prepared using a routine procedure. All studies involving human kidney sections were approved by the Institutional Ethics Committee at the Nanfang Hospital, Southern Medical University.
In Vivo Expression of PRR and Wnt1 Genes
In vivo expression of exogenous genes in mice was carried out by an established hydrodynamic-based gene transfer approach, as described previously.31,39 At 4 days after UIRI (30 minutes), BALB/c mice were subjected to a single intravenous injection of empty vector (pcDNA3), Wnt1 expression vector (pHA-Wnt1), PRR expression vector (pCMV-PRR), or in combination (Figure 5A). Briefly, 20 µg of plasmid DNA was diluted in 1.8 ml of saline and injected via the tail vein into the mouse circulation within 5–10 seconds. At 11 days after UIRI (7 days after plasmid injection), mice were euthanized, and serum and kidney tissues were collected for various analyses.
Knockdown of PRR Expression by siRNA Strategy In Vivo
Male BALB/c mice were subjected to severe UIRI (35 minutes). At 4 days after UIRI, mice were subjected to daily intravenous injections of control or PRR-specific siRNA (Ctrl-siR or PRR-siR) for 7 days (Figure 10A). Briefly, synthetic control or PRR-specific siRNA (50 µg dissolved in 1 ml of saline) was rapidly injected via the tail vein into mice within 5–10 seconds, once a day for 7 consecutive days (Figure 10A). Phosphoramidite-modified siRNA was synthesized by GenePharma (Shanghai, China). The target sequences used for knockdown of PRR in this study were as follows: sense 5′-CCCUUUGGAGAAUGCAGUUTT-3′ and antisense 5′AACUGCAUUCUCCAAAGGGTT-3′. At 11 days after UIRI (7 days after siRNA injection), mice were euthanized and serum and kidney tissues were collected for various analyses.
Cell Culture
Human kidney proximal tubular cells (HKC-8) were described previously.36 HKC-8 cells were transiently transfected with various expression vectors including pcDNA3, pHA-Wnt1, pDel-β-cat, pCMV-PRR, or in combination, as well as control and PRR-specific siRNA using Lipofectamine 2000 reagent according to the protocol specified by the manufacturer (Invitrogen, Grand Island, NY). In some experiments, HKC-8 cells were treated with ICG-001 (Chembest Bioscience, Shanghai, China) at 10 µM, or U0126 (#9903; Cell Signaling Technology, Danvers, MA) at 10 μM, recombinant human renin protein (REN-H5221; ACRO Biosystem, Newark, NJ) at 10−8 M, and bafilomycin A1 (Chembest, Shanghai, China) at 100 nM. At 24 hours after transfection, cells were collected and subjected to various analyses.
Serum Creatinine and BUN Assay
The level of serum creatinine and BUN levels were measured using an AU480 Automatic biochemical analyzer (Beckman Coulter, Brea, CA). Serum creatinine and BUN were expressed as milligrams per 100 ml.
Histology and Immunohistochemical Staining
Paraffin-embedded mouse kidney sections (3 μm thickness) were prepared by a routine procedure. Kidney sections were subjected to MTS for assessing collagen deposition and fibrotic lesions as described previously.33 Quantification of the fibrotic area was carried out by a computer-aided point-counting technique as described previously.39 Immunohistochemical staining was performed using routine protocol. Antibodies used were as follows: rabbit polyclonal anti-PRR (anti-ATP6IP2) (ab40790; Abcam, Cambridge, MA), rabbit polyclonal anti-Wnt1 (ab15251; Abcam), rabbit polyclonal anti–β-catenin (ab15180; Abcam), mouse monoclonal anti–α-SMA (A2547; Sigma-Aldrich), rabbit monoclonal anti-fibronectin (F3648; Sigma-Aldrich), rabbit polyclonal anti-Fsp1 (07–2274; EMD-Millipore, CA), goat polyclonal anti-RANTES (sc-1410, Santa), and rabbit polyclonal anti–TNF-α (ab9739; Abcam).
Immunofluorescence Staining
Kidney cryosections or cells cultured on coverslips were fixed with 3.7% paraformalin for 15 minutes at room temperature. After blocking with 10% donkey serum for 30 minutes, the slides were immunostained with primary antibodies against PRR (AF5716; R&D systems, Minneapolis, MN), fibronectin (F3648; Sigma-Aldrich), Wnt1 (ab15251; Abcam), β-catenin (610154; BD biosciences), and F4/80 (14–4801–82; eBioscience, San Diego, CA). To visualize the primary antibodies, slides were stained with cyanine dye–2– or cyanine dye–3–conjugated secondary antibodies (Jackson Immuno-Research Laboratories, West Grove, PA). Slides were viewed under an Olympus BX61 Microscope equipped with a digital camera.
Western Blot Analysis
Protein expression was analyzed by western blot analysis as described previously.31,39 The primary antibodies used were as follows: rabbit polyclonal anti-fibronectin (F3648; Sigma-Aldrich), rabbit monoclonal anti–β-catenin antibody (ab15180; Abcam), mouse monoclonal anti–α-SMA antibody (A2547; Sigma-Aldrich), goat anti–PAI-1 antibody (AF3828; R&D Systems), rabbit polyclonal anti–active–β-catenin antibody (4270s; Cell Signaling Technology), rabbit polyclonal anti-Wnt1 (ab15251; Abcam), rabbit polyclonal anti-PRR (ab40790; Abcam), mouse anti-Flag (KM8002, Sungene Biotech, Tianjin, China), mouse anti–glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (RM2002; Ray antibody Biotech, Beijing, China), and mouse anti–α-tubulin (RM2003; Ray antibody Biotech).
Real-Time RT-PCR
Total RNA isolation was carried out using the TRIzol RNA Isolation System (Life Technologies, Grand Island, NY) according to the manufacturer’s instruction. The first-strand cDNA synthesis was carried out by using a Reverse Transcription System Kit (Promega, Madison, WI). qRT-PCR was performed on an ABI PRISM 7000 Sequence Detection System (Applied Biosystems, Foster City, CA) as described previously.39 The PCR reaction mixture in a 25 μl volume contained 12.5 μl 2×SYBR Green PCR Master Mix (Applied Biosystems), 5 μl diluted reverse transcription product (1:10), and 0.5 μM sense and antisense primer sets. The sequences of the mouse primer pairs used in qRT-PCR are as follows. PRR (human): forward primer, 5′-CCATGGCTGTGTTTGTCGTG-3′, reverse primer, 5′-GGCCAAGAAAGGTCTTCTTTCAC-3′; RANTES (mouse): forward primer, 5′-TGCTGCTTTGCCTACCTCTC-3′, reverse primer, 5′-TTGAACCCACTTCTTCTCTGG-3′; TNF-α (mouse): forward primer, 5′-TCGTAGCAAACCACCAAGTG-3′, reverse primer, 5′-CCTTGAAGAGAACCTGGGAG-3′. The mRNA levels of various genes were calculated after normalizing with β-actin.
Transfection and Luciferase Assay
The β-catenin–mediated gene transcription was assessed by using the TOP Flash reporter plasmid containing two sets of three copies of the TCF–binding site upstream of the thymidine kinase minimal promoter and luciferase cDNA (GenePharma, Shanghai, China). HKC-8 cells were cotransfected using Lipofectamine 2000 reagent with TOP Flash plasmid and Wnt1 expression vector (pHA-Wnt1) or PRR expression vector (pCMV-PRR) in the absence or presence of renin (10−8 M), U0126 (10 μM), ICG-001 (10 μM), and bafilomycin A1 (100 nM), as indicated. An internal control reporter plasmid (0.1 mg) Renilla reniformis luciferase driven under thymidine kinase promoter (GenePharma, Shanghai, China) was also cotransfected for normalizing the transfection efficiency. Luciferase assay was performed using a dual luciferase assay system kit according to the manufacturer’s protocols (Promega). Relative luciferase activity (arbitrary units) was reported as fold induction over the controls after normalizing for transfection efficiency.
Statistical Analyses
All data examined are expressed as mean±SEM. Statistical analyses of the data were performed using Sigma Stat software (Jandel Scientific, San Rafael, CA). Comparisons between groups were made using one-way ANOVA followed by the Newman–Kuels test. P<0.05 was considered significant.
Disclosures
None.
Supplementary Material
Acknowledgments
This work was supported by the National Science Foundation of China Grants 81370839 and 81521003, Guangdong Science Foundation (2014A030312014), Guangzhou Projects Grant 201504010001, and the National Institutes of Health Grants DK064005 and DK106049.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2016070811/-/DCSupplemental.
References
- 1.Nguyen G, Muller DN: The biology of the (pro)renin receptor. J Am Soc Nephrol 21: 18–23, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Nguyen G: Renin, (pro)renin and receptor: An update. Clin Sci (Lond) 120: 169–178, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Oshima Y, Morimoto S, Ichihara A: Roles of the (pro)renin receptor in the kidney. World J Nephrol 3: 302–307, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nguyen G, Delarue F, Burcklé C, Bouzhir L, Giller T, Sraer JD: Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest 109: 1417–1427, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang Y, Noble NA, Zhang J, Xu C, Border WA: Renin-stimulated TGF-beta1 expression is regulated by a mitogen-activated protein kinase in mesangial cells. Kidney Int 72: 45–52, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Peng H, Li W, Seth DM, Nair AR, Francis J, Feng Y: (Pro)renin receptor mediates both angiotensin II-dependent and -independent oxidative stress in neuronal cells. PLoS One 8: e58339, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Advani A, Kelly DJ, Cox AJ, White KE, Advani SL, Thai K, Connelly KA, Yuen D, Trogadis J, Herzenberg AM, Kuliszewski MA, Leong-Poi H, Gilbert RE: The (pro)renin receptor: Site-specific and functional linkage to the vacuolar H+-ATPase in the kidney. Hypertension 54: 261–269, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Daryadel A, Bourgeois S, Figueiredo MF, Gomes Moreira A, Kampik NB, Oberli L, Mohebbi N, Lu X, Meima ME, Danser AH, Wagner CA: Colocalization of the (pro)renin receptor/Atp6ap2 with H+-ATPases in mouse kKidney but prorenin does not acutely regulate intercalated cell H+-ATPase activity. PLoS One 11: e0147831, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cruciat CM, Ohkawara B, Acebron SP, Karaulanov E, Reinhard C, Ingelfinger D, Boutros M, Niehrs C: Requirement of prorenin receptor and vacuolar H+-ATPase-mediated acidification for Wnt signaling. Science 327: 459–463, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Buechling T, Bartscherer K, Ohkawara B, Chaudhary V, Spirohn K, Niehrs C, Boutros M: Wnt/Frizzled signaling requires dPRR, the Drosophila homolog of the prorenin receptor. Curr Biol 20: 1263–1268, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Wu CH, Mohammadmoradi S, Thompson J, Su W, Gong M, Nguyen G, Yiannikouris F: Adipocyte (pro)renin-receptor deficiency induces lipodystrophy, liver steatosis and increases blood pressure in male mice. Hypertension 68: 213–219, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geisberger S, Maschke U, Gebhardt M, Kleinewietfeld M, Manzel A, Linker RA, Chidgey A, Dechend R, Nguyen G, Daumke O, Muller DN, Wright MD, Binger KJ: New role for the (pro)renin receptor in T-cell development. Blood 126: 504–507, 2015 [DOI] [PubMed] [Google Scholar]
- 13.Huang J, Siragy HM: Regulation of (pro)renin receptor expression by glucose-induced mitogen-activated protein kinase, nuclear factor-kappaB, and activator protein-1 signaling pathways. Endocrinology 151: 3317–3325, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song R, Preston G, Kidd L, Bushnell D, Sims-Lucas S, Bates CM, Yosypiv IV: Prorenin receptor is critical for nephron progenitors. Dev Biol 409: 382–391, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song R, Preston G, Ichihara A, Yosypiv IV: Deletion of the prorenin receptor from the ureteric bud causes renal hypodysplasia. PLoS One 8: e63835, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yosypiv IV: Prorenin receptor in kidney development. Pediatr Nephrol 32: 383–392, 2017 [DOI] [PubMed] [Google Scholar]
- 17.Riediger F, Quack I, Qadri F, Hartleben B, Park JK, Potthoff SA, Sohn D, Sihn G, Rousselle A, Fokuhl V, Maschke U, Purfürst B, Schneider W, Rump LC, Luft FC, Dechend R, Bader M, Huber TB, Nguyen G, Muller DN: Prorenin receptor is essential for podocyte autophagy and survival. J Am Soc Nephrol 22: 2193–2202, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramkumar N, Stuart D, Mironova E, Bugay V, Wang S, Abraham N, Ichihara A, Stockand JD, Kohan DE: Renal tubular epithelial cell prorenin receptor regulates blood pressure and sodium transport. Am J Physiol Renal Physiol 311: F186–F194, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quadri S, Siragy HM: (Pro)renin receptor contributes to regulation of renal epithelial sodium channel. J Hypertens 34: 486–494, discussion 494, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaneshiro Y, Ichihara A, Sakoda M, Takemitsu T, Nabi AH, Uddin MN, Nakagawa T, Nishiyama A, Suzuki F, Inagami T, Itoh H: Slowly progressive, angiotensin II-independent glomerulosclerosis in human (pro)renin receptor-transgenic rats. J Am Soc Nephrol 18: 1789–1795, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Rosendahl A, Niemann G, Lange S, Ahadzadeh E, Krebs C, Contrepas A, van Goor H, Wiech T, Bader M, Schwake M, Peters J, Stahl R, Nguyen G, Wenzel UO: Increased expression of (pro)renin receptor does not cause hypertension or cardiac and renal fibrosis in mice. Lab Invest 94: 863–872, 2014 [DOI] [PubMed] [Google Scholar]
- 22.Takahashi K, Yamamoto H, Hirose T, Hiraishi K, Shoji I, Shibasaki A, Kato I, Kaneko K, Sasano H, Satoh F, Totsune K: Expression of (pro)renin receptor in human kidneys with end-stage kidney disease due to diabetic nephropathy. Peptides 31: 1405–1408, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Siragy HM, Huang J: Renal (pro)renin receptor upregulation in diabetic rats through enhanced angiotensin AT1 receptor and NADPH oxidase activity. Exp Physiol 93: 709–714, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyazaki N, Murata I, Takemura G, Okada H, Kanamori H, Matsumoto-Miyazaki J, Yoshida G, Izumi K, Kashi H, Niimi K, Nishiwaki A, Miyazaki T, Ohno M, Ohashi H, Suzuki F, Minatoguchi S: Expression of prorenin receptor in renal biopsies from patients with IgA nephropathy. Int J Clin Exp Pathol 7: 7485–7496, 2014 [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou L, Li Y, Hao S, Zhou D, Tan RJ, Nie J, Hou FF, Kahn M, Liu Y: Multiple genes of the renin-angiotensin system are novel targets of Wnt/β-catenin signaling. J Am Soc Nephrol 26: 107–120, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou L, Liu Y: Wnt/β-catenin signaling and renin-angiotensin system in chronic kidney disease. Curr Opin Nephrol Hypertens 25: 100–106, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou D, Tan RJ, Fu H, Liu Y: Wnt/β-catenin signaling in kidney injury and repair: A double-edged sword. Lab Invest 96: 156–167, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tan RJ, Zhou D, Zhou L, Liu Y: Wnt/β-catenin signaling and kidney fibrosis. Kidney Int Suppl (2011) 4: 84–90, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DiRocco DP, Kobayashi A, Taketo MM, McMahon AP, Humphreys BD: Wnt4/β-catenin signaling in medullary kidney myofibroblasts. J Am Soc Nephrol 24: 1399–1412, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Surendran K, Schiavi S, Hruska KA: Wnt-dependent beta-catenin signaling is activated after unilateral ureteral obstruction, and recombinant secreted frizzled-related protein 4 alters the progression of renal fibrosis. J Am Soc Nephrol 16: 2373–2384, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Xiao L, Zhou D, Tan RJ, Fu H, Zhou L, Hou FF, Liu Y: Sustained activation of Wnt/β-catenin signaling drives AKI to CKD progression. J Am Soc Nephrol 27: 1727–1740, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He W, Kang YS, Dai C, Liu Y: Blockade of Wnt/β-catenin signaling by paricalcitol ameliorates proteinuria and kidney injury. J Am Soc Nephrol 22: 90–103, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He W, Dai C, Li Y, Zeng G, Monga SP, Liu Y: Wnt/beta-catenin signaling promotes renal interstitial fibrosis. J Am Soc Nephrol 20: 765–776, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dai C, Stolz DB, Kiss LP, Monga SP, Holzman LB, Liu Y: Wnt/beta-catenin signaling promotes podocyte dysfunction and albuminuria. J Am Soc Nephrol 20: 1997–2008, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Henderson WR Jr, Chi EY, Ye X, Nguyen C, Tien YT, Zhou B, Borok Z, Knight DA, Kahn M: Inhibition of Wnt/beta-catenin/CREB binding protein (CBP) signaling reverses pulmonary fibrosis. Proc Natl Acad Sci U S A 107: 14309–14314, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hao S, He W, Li Y, Ding H, Hou Y, Nie J, Hou FF, Kahn M, Liu Y: Targeted inhibition of β-catenin/CBP signaling ameliorates renal interstitial fibrosis. J Am Soc Nephrol 22: 1642–1653, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kinouchi K, Ichihara A, Sano M, Sun-Wada GH, Wada Y, Kurauchi-Mito A, Bokuda K, Narita T, Oshima Y, Sakoda M, Tamai Y, Sato H, Fukuda K, Itoh H: The (pro)renin receptor/ATP6AP2 is essential for vacuolar H+-ATPase assembly in murine cardiomyocytes. Circ Res 107: 30–34, 2010 [DOI] [PubMed] [Google Scholar]
- 38.Yoshimori T, Yamamoto A, Moriyama Y, Futai M, Tashiro Y: Bafilomycin A1, a specific inhibitor of vacuolar-type H(+)-ATPase, inhibits acidification and protein degradation in lysosomes of cultured cells. J Biol Chem 266: 17707–17712, 1991 [PubMed] [Google Scholar]
- 39.Zhou D, Li Y, Zhou L, Tan RJ, Xiao L, Liang M, Hou FF, Liu Y: Sonic hedgehog is a novel tubule-derived growth factor for interstitial fibroblasts after kidney injury. J Am Soc Nephrol 25: 2187–2200, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Y: Cellular and molecular mechanisms of renal fibrosis. Nat Rev Nephrol 7: 684–696, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He W, Tan R, Dai C, Li Y, Wang D, Hao S, Kahn M, Liu Y: Plasminogen activator inhibitor-1 is a transcriptional target of the canonical pathway of Wnt/beta-catenin signaling. J Biol Chem 285: 24665–24675, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang F, Lu X, Liu M, Feng Y, Zhou SF, Yang T: Renal medullary (pro)renin receptor contributes to angiotensin II-induced hypertension in rats via activation of the local renin-angiotensin system. BMC Med 13: 278, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clevers H, Nusse R: Wnt/β-catenin signaling and disease. Cell 149: 1192–1205, 2012 [DOI] [PubMed] [Google Scholar]
- 44.Angers S, Moon RT: Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol 10: 468–477, 2009 [DOI] [PubMed] [Google Scholar]
- 45.Li W, Liu J, Hammond SL, Tjalkens RB, Saifudeen Z, Feng Y: Angiotensin II regulates brain (pro)renin receptor expression through activation of cAMP response element-binding protein. Am J Physiol Regul Integr Comp Physiol 309: R138–R147, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li C, Siragy HM: High glucose induces podocyte injury via enhanced (pro)renin receptor-Wnt-β-catenin-snail signaling pathway. PLoS One 9: e89233, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peters B, Grisk O, Becher B, Wanka H, Kuttler B, Lüdemann J, Lorenz G, Rettig R, Mullins JJ, Peters J: Dose-dependent titration of prorenin and blood pressure in Cyp1a1ren-2 transgenic rats: Absence of prorenin-induced glomerulosclerosis. J Hypertens 26: 102–109, 2008 [DOI] [PubMed] [Google Scholar]
- 48.Mercure C, Prescott G, Lacombe MJ, Silversides DW, Reudelhuber TL: Chronic increases in circulating prorenin are not associated with renal or cardiac pathologies. Hypertension 53: 1062–1069, 2009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.