Abstract
Cell-cell communication via Wnt ligands is necessary in regulating embryonic development and has been implicated in CKD. Because Wnt ligands are ubiquitously expressed, the exact cellular source of the Wnts involved in CKD remains undefined. To address this issue, we generated two conditional knockout mouse lines in which Wntless (Wls), a dedicated cargo receptor that is obligatory for Wnt secretion, was selectively ablated in tubular epithelial cells or interstitial fibroblasts. Blockade of Wnt secretion by genetic deletion of Wls in renal tubules markedly inhibited myofibroblast activation and reduced renal fibrosis after unilateral ureteral obstruction. This effect associated with decreased activation of β-catenin and downstream gene expression and preserved tubular epithelial integrity. In contrast, fibroblast-specific deletion of Wls exhibited little effect on the severity of renal fibrosis after obstructive or ischemia-reperfusion injury. In vitro, incubation of normal rat kidney fibroblasts with tubule-derived Wnts promoted fibroblast proliferation and activation. Furthermore, compared with kidney specimens from patients without CKD, biopsy specimens from patients with CKD also displayed increased expression of multiple Wnt proteins, predominantly in renal tubular epithelium. These results illustrate that tubule-derived Wnts have an essential role in promoting fibroblast activation and kidney fibrosis via epithelial-mesenchymal communication.
Keywords: renal fibrosis, fibroblast, Wnt signaling, chronic kidney disease, Wntless
Kidney fibrosis, characterized by excessive extracellular matrix deposition leading to scar formation in renal parenchyma, is generally considered as the final common consequence of a wide variety of CKDs.1–3 Although kidney injury in pathologic conditions often initiates from the tubular epithelium, an inevitable outcome is the subsequent activation of interstitial fibroblasts, leading to the over-production and deposition of extracellular matrix components. In this context, a fundamental question in the field is to understand how the injured tubules trigger interstitial fibroblast activation. In recent years, several hypotheses have been postulated to explain tubular responses after injury, such as partial epithelial-mesenchymal transition (EMT),4,5 cell cycle arrest, or metabolic alterations.6–9 Despite these distinct possibilities, one common and convergent finding is that tubular cells after injury are destined to switch to a secretory phenotype and produce numerous fibrogenic factors, which mediate an active epithelial-mesenchymal communication (EMC) and promote interstitial fibroblast activation.10,11 Therefore, identification and characterization of these tubule-derived factors will be essential for understanding the mechanisms of renal fibrosis and for designing effective therapeutic strategies.
Cell-to-cell communication is well known to play a crucial role in guiding the proper development of multicellular organisms during the embryonic stage and in the maintenance of tissue homeostasis in adults under normal physiologic conditions.12–15 Such a communication is often achieved through the engagement between secreted soluble cues from a particular cell and the neighboring responding cells via both autocrine and paracrine fashions. Earlier studies have shown that sonic hedgehog (Shh) and Wnt ligands are upregulated in diseased kidneys and could play a role in mediating fibroblast activation in the pathogenesis of CKD.16–20 Although the mode of action employed by Shh is well established,18,20,21 several key questions regarding Wnt signaling in renal fibrogenesis remain to be addressed.
Wnt ligands comprise a large family of secreted, hydrophobic glycoproteins that control a variety of cellular activities.22–25 Dysregulation of Wnt signaling is associated with various human diseases including developmental abnormalities, tissue fibrosis, and tumorigenesis.26–29 In various forms of CKD, many Wnt ligands are induced simultaneously in different types of kidney cells.26,27,30,31 However, the exact cellular source of the Wnt ligands that play a predominant role in mediating fibrogenic responses remains to be elucidated.
As secretory proteins, the intracellular trafficking and secretion of Wnt ligands in the producing cells are tightly controlled by Wntless (Wls), also known as G protein–coupled receptor 177 (GPR177) in mammals and Evenness Interrupted (Evi) in Drosophila.32,33 Wls is an evolutionarily conserved seven-pass transmembrane protein that functions as a Wnt cargo receptor and is therefore obligatory for the secretion of Wnt proteins.33 Because Wls controls the release of all Wnts from the producing cells, conditional deletion of Wls would be a powerful approach that allows spatial and temporal control of Wnt secretion in vivo.34 Conditional knockout of Wls in a specific cell type in other organs has shown that the lack of Wls blocks Wnt secretion from the producing cells and abolishes Wnt functions in the responding cells.35–39 However, a similar strategy to block Wnt secretion via conditional ablation of Wls in the kidney has not been reported.
In this study, we generated two lines of conditional knockout mice in which Wls was specifically deleted in either renal tubular epithelial cells or interstitial fibroblasts, respectively. Our results show that blockade of Wnt secretion in renal tubules, but not in fibroblasts, inhibited fibroblast activation and reduced renal fibrosis after injury. These studies suggest that tubule-derived Wnts play a predominant role in driving fibroblast activation and kidney fibrosis.
Results
Tubule-Specific Ablation of Wls Inhibits Renal β-Catenin Activation In Vivo
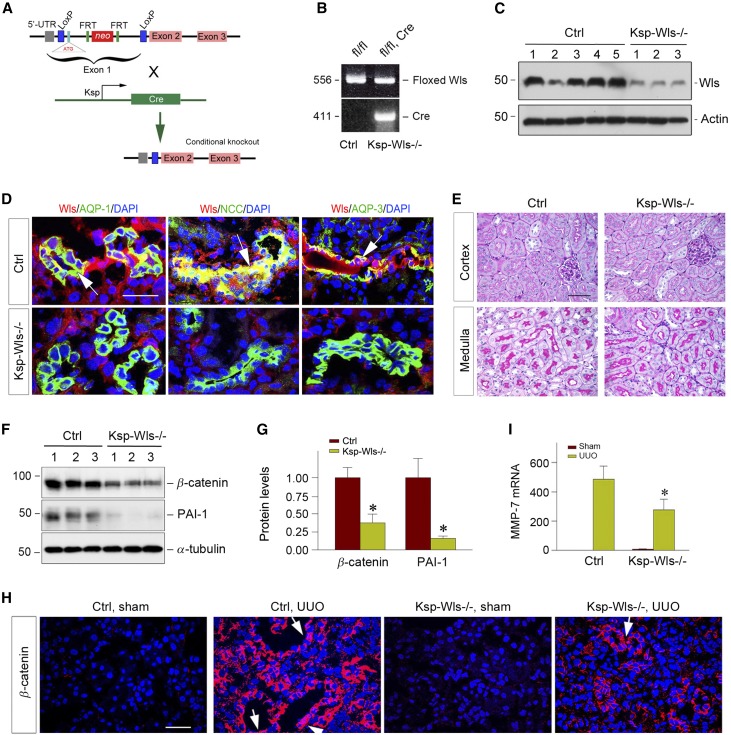
Wnt ligands are ubiquitously expressed in different types of cells in vivo.22,23 To delineate the cellular source of Wnts that play a major role in renal fibrogenesis, we generated conditional knockout mice with cell type–specific ablation of Wls, a dedicated seven-pass transmembrane cargo receptor that is essential for Wnt secretion.32,33 Homozygous Wls floxed mice were mated with transgenic mice expressing Cre recombinase under the control of Ksp-cadherin promoter (Ksp-Cre) (Figure 1A). As shown in Figure 1B, mice with tubule-specific ablation of Wls (designated as Ksp-Wls−/−) were generated (genotype: Wlsfl/fl, Cre) (Figure 1B, lane 2), and further confirmed by renal Cre mRNA expression (data not shown). Age- and sex- matched Wls floxed mice (genotype: Wlsfl/fl) from the same litters were used as controls (Figure 1B, lane 1).
Figure 1.
Tubule-specific ablation of endogenous Wls suppresses activation of Wnt/β-catenin signaling after UUO. (A) Schematic diagram shows the strategy of crossbreeding of Wls floxed mice (Wlsfl/fl) with Ksp-Cre transgenic mice. The Wls floxed mutant mice possess a loxP site before the ATG start site in the 5′ untranslated region and another in the upstream of exon 2 of Wls gene. (B) Mouse genotyping analyzed by PCR. Lane 1 shows the genotyping of the control mice used in this study (genotype: Wlsfl/fl), and lane 2 denotes the genotyping of the tubule-specific Wls knockout mice (genotype: Wlsfl/fl, Cre), designated as Ksp-Wls−/−. (C) Western blot analyses demonstrate a substantial reduction of renal Wls protein in Ksp-Wls−/− mice, compared to the controls. Kidney lysates were prepared from control and Ksp-Wls−/− mice at 7 days after UUO. Numbers (1–5) indicate each individual animal in a given group. (D) Costaining for Wls and tubule segment–specific markers in control and Ksp-Wls−/− kidneys. Immunofluorescence staining demonstrated the costaining of Wls (red) and various tubular markers (green) in the kidneys. Segment-specific tubular markers used are as follows: proximal tubule, aquaporin-1 (AQP1); distal tubule, thiazide-sensitive Na-Cl cotransporter (TSC)/NCC; and collecting duct, aquaporin-3 (AQP3). Arrows indicate Wls-positive tubules. Scale bar, 50 µm. (E) Tubule-specific deletion of Wls does not cause renal abnormality. Representative micrographs of PAS staining show kidney cortex and medulla in control and Ksp-Wls−/− mice. Scale bar, 50 µm. (F and G) Western blot analyses of renal expression of β-catenin and PAI-1 proteins in the obstructive kidneys of control and Ksp-Wls−/− mice at 7 days after UUO. Representative western blot (F) and quantitative data (G) are presented. Numbers (1–3) indicate each individual animal in a given group. *P<0.05 versus controls (n=6–9). (H) Representative immunofluorescence micrographs show β-catenin expression and localization in the control and Ksp-Wls−/− mice kidneys at 7 days after UUO. Arrows indicate positive nuclear β-catenin staining in kidney tubular epithelial cells. Scale bar, 50 µm. (I) qRT-PCR demonstrated renal MMP-7 mRNA levels at 7 days after UUO. *P<0.05 versus controls (n=6–9). Ctrl, control.
Western blot analyses confirmed a marked reduction of renal Wls protein in Ksp-Wls−/− mice, compared with the controls (Figure 1C). To further examine the site and efficiency of Ksp-Cre–mediated deletion of Wls, we performed double immunofluorescence staining for Wls and tubular segment–specific markers in control and Ksp-Wls−/− mice. As shown in Figure 1D, ablation of Wls protein was clearly evident in all tubular segments to varying extents, including proximal tubule, distal tubule, and collecting duct. Quantitative determination revealed that Wls was almost completely ablated in >95% of kidney distal tubule and collecting duct cells. However, deletion of Wls only took place in approximately 40%–50% of proximal tubular cells in Ksp-Wls−/− mice.
Because Wls is obligatory for the secretion of all Wnt proteins,33 ablation of Wls in a particular cell type would effectively block Wnt release from these cells. We found that blockade of Wnts secretion from renal tubular cells did not cause any kidney abnormality under basal conditions. There was no difference in kidney histology (Figure 1E), as well as body weight and renal function, between control and Ksp-Wls−/− mice.
We next examined whether blockade of Wnt secretion from renal tubules by genetic ablation of Wls affects renal activation of β-catenin, the principal intracellular mediator of canonic Wnt signaling. As shown in Figure 1F, β-catenin activation was clearly evident in the control mice at 7 days after unilateral ureteral obstruction (UUO), as previously reported.27,40 However, tubular ablation of Wls substantially inhibited renal β-catenin expression and activation in Ksp-Wls−/− mice (Figure 1, F and G). Similar results were obtained when kidney sections were subjected to immunofluorescence staining for β-catenin (Figure 1H). Accordingly, the downstream targets of Wnt/β-catenin signaling, such as plasminogen activator inhibitor–1 (PAI-1) and matrix metalloproteinase–7 (MMP-7),41,42 were repressed in the obstructed kidneys of Ksp-Wls−/− mice after UUO, compared with the controls (Figure 1, F, G and I). Taken together, these results indicate that tubule-derived Wnt ligands are mainly responsible for renal β-catenin activation after obstructive injury.
Tubule-Specific Ablation of Wls Ameliorates Kidney Fibrosis
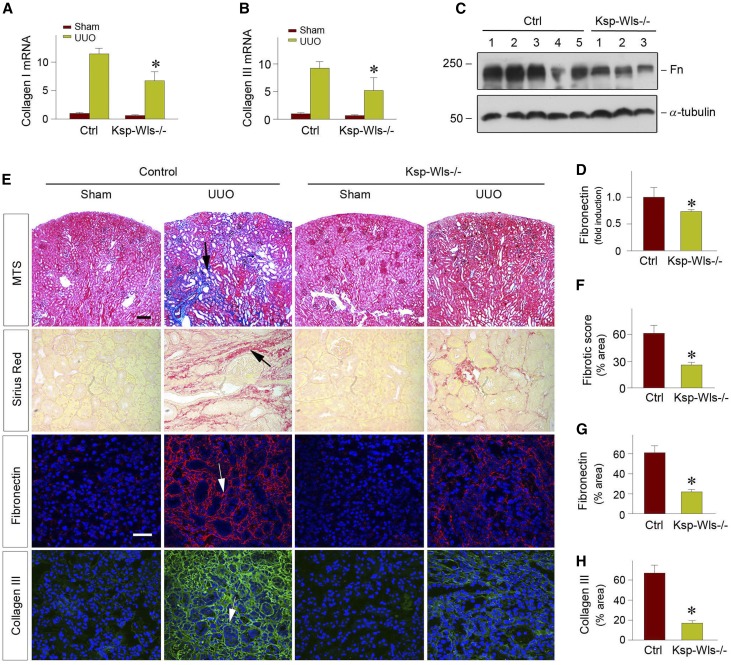
We next assessed the effects of blockade of Wnt secretion in renal tubules by conditional ablation of Wls on kidney fibrosis after obstructive injury. As shown in Figure 2, A and B, tubule-specific loss of Wls in the Ksp-Wls−/− mice significantly inhibited type I and type III collagen mRNA expression in the obstructive kidneys after UUO, compared with the control mice. Similar inhibition of fibronectin expression was evident, as shown by western blot analyses of whole kidney lysates (Figure 2, C and D). Consistently, Masson trichrome staining (MTS) and Picrosirius Red staining revealed a significant reduction of collagen deposition and fibrotic lesions after obstructive injury in the Ksp-Wls−/− mice, compared with the control mice (Figure 2, E and F). Immunofluorescence staining for fibronectin and type III collagen also demonstrated that loss of Wls in renal tubules attenuated renal expression and deposition of these interstitial matrix proteins after obstructive injury in vivo (Figure 2, E, G and H). Collectively, these results indicate that deletion of endogenous Wls in a tubule-specific fashion is sufficient to inhibit Wnt/β-catenin signaling and retard the progression of kidney fibrosis in vivo.
Figure 2.
Tubule-specific deletion of Wls ameliorates kidney fibrosis after UUO. (A and B) qRT-PCR analyses show significant downregulation of collagen type I and type III mRNA in the obstructed kidneys of Ksp-Wls−/− mice at 7 days after UUO, compared with the controls. *P<0.05 versus controls (n=3–5). (C and D) Western blot analyses demonstrate a decreased fibronectin protein in Ksp-Wls−/− kidney after UUO, compared with the controls. Representative western blot (C) and quantitative data (D) are presented. *P<0.05 versus controls (n=6–9). (E) Representative micrographs of MTS, Picrosirius Red staining, and immunofluorescence staining for fibronectin and collagen type III are presented. Arrows indicate positive staining. (F–H) Graphical presentation of quantitative data of fibrosis score (F), fibronectin (G), and collagen type III (H) in control and Ksp-Wls−/− mice at 7 days after UUO. *P<0.05 versus controls (n=3). Scale bar, 50 µm. Fn, fibronectin; Ctrl, control.
Deletion of Tubular Wls Preserves Tubular Epithelial Cell Integrity
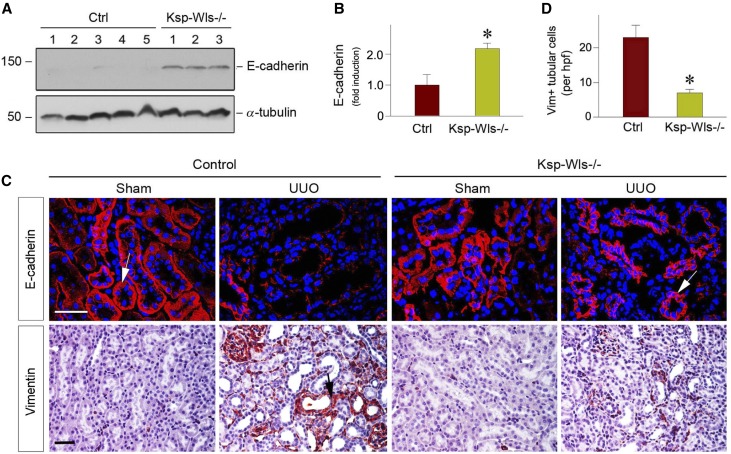
We further investigated the potential mechanisms by which blockade of tubular Wnt secretion ameliorates renal fibrosis. Because Wnt signaling is instrumental in mediating tubular injury,40 we then explored the effect of tubule-specific deletion of Wls on this process in obstructive nephropathy. Because loss of E-cadherin is known to associate with impairment of epithelial cell integrity, we examined renal E-cadherin protein expression by western blot analysis of whole kidney lysates. As shown in Figure 3, A and B, tubule-specific deletion of Wls largely preserved E-cadherin expression after UUO, compared with the controls. Immunofluorescence staining also revealed that E-cadherin protein was lost in the obstructed kidney at 7 days after UUO in the control mice, whereas it was largely preserved in Ksp-Wls−/− mice (Figure 3C). We also investigated the expression and localization of vimentin, an intermediate cytoskeleton protein and a marker for mesenchymal cells, in the Ksp-Wls−/− and control mice after UUO. As illustrated in Figure 3, C and D, immunohistochemical staining showed a decreased number of the tubular epithelial cells stained positively for vimentin in the fibrotic kidneys of Ksp-Wls−/− mice after UUO at 7 days, compared with the controls. Of note, Wnts-enriched conditioned medium (Wnts-CM), prepared from kidney proximal tubule cells overexpressing Wnt ligands, induced fibronectin and α-smooth muscle actin (α-SMA) expression in human proximal tubular cells (HKC-8) in vitro (Supplemental Figure 1), suggesting that tubule-derived Wnts can target themselves in an autocrine fashion. Therefore, blockade of Wnt secretion by deleting Wls in vivo leads to the preservation of tubular epithelial cell integrity in Ksp-Wls−/− mice.
Figure 3.
Tubule-specific deletion of Wls reduces partial EMT after UUO. (A and B) Western blot analyses show E-cadherin protein levels in the obstructive kidneys of the control and Ksp-Wls−/− mice at 7 days after UUO. Representative western blot (A) and quantitative data (B) are presented. *P<0.05 versus controls (n=6–9). Numbers (1–5) denote each individual animal in a given group. (C) Representative micrographs show E-cadherin and vimentin expression in control and Ksp-Wls−/− kidneys at 7 days after UUO. Arrows indicate positive staining in renal tubules. Scale bar, 50 μm. (D) Quantitative data show vimentin-positive tubular cells per high power field (hpf). *P<0.05 versus controls (n=3). Ctrl, control; Vim+, vimentin positive.
Deletion of Tubular Wls Inhibits Myofibroblast Activation and Inflammation
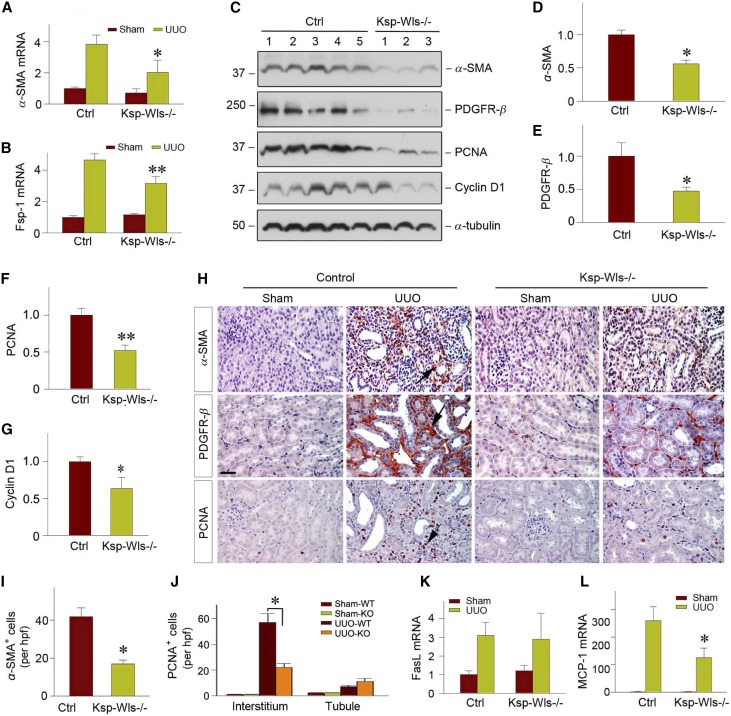
We next investigated whether tubule-specific deletion of Wls reduces activation of renal myofibroblasts. As shown in Figure 4A, quantitative, real-time RT-PCR (qRT-PCR) revealed that blockade of Wnt secretion from renal tubules in Ksp-Wls−/− mice substantially inhibited α-SMA mRNA expression. In addition, fibroblast-specific protein 1 (Fsp1), also known as S100A4, a direct target of canonic Wnt signaling,43 was also repressed in Ksp-Wls−/− kidneys at 7 days after UUO, compared with the controls (Figure 4B). However, qRT-PCR assessment revealed that blockade of Wnts secretion from kidney tubules did not significantly inhibit renal TGF-β1 mRNA expression.
Figure 4.
Tubule-specific deletion of Wls represses myofibroblast activation and renal inflammation after UUO. (A and B) Quantitative determination of renal α-SMA and Fsp-1 mRNA levels of the control and Ksp-Wls−/− mice at 7 days after UUO by qRT-PCR. *P<0.05, **P<0.01 versus controls (n=3–5). (C) Western blot analyses of renal α-SMA, PDGFR-β, PCNA, and Cyclin D1 in the control and Ksp-Wls−/− mice at 7 days after UUO. (D–G) Quantitative data on the protein expression of α-SMA (D), PDGFR-β (E), PCNA (F), and cyclin D1 (G) in the obstructed kidney after UUO. *P<0.05 versus controls (n=6–9). Numbers (1–5) indicate each individual animal in a given group. (H) Representative micrographs show α-SMA, PDGFR-β, and PCNA expression in the control and Ksp-Wls−/− kidneys at 7 days after UUO. Arrows indicate positive staining. Scale bar, 50 µm. (I) Quantitative data on renal α-SMA staining are shown. (J) Knockout of Wls in renal tubules decreases PCNA-positive cells in renal interstitium. PCNA-positive cells per high power field (hpf) are counted and shown. (K) qRT-PCR demonstrates renal mRNA levels of FasL in the control and Ksp-Wls−/− mice at 7 days after UUO. (L) qRT-PCR demonstrates renal mRNA levels of MCP-1 in the control and Ksp-Wls−/− mice at 7 days after UUO. *P<0.05 versus controls. Ctrl, control.
Western blot analyses confirmed a similar reduction of α-SMA protein in the Ksp-Wls−/− mice, compared with the controls (Figure 4, C and D). As shown in Figure 4, C and E, tubule-specific depletion of Wls also repressed platelet-derived growth factor receptor β (PDGFR-β) in Ksp-Wls−/− fibrotic kidneys, compared with the controls. Meanwhile, cell proliferation markers such as proliferating cell nuclear antigen (PCNA) and cyclin D1 were reduced in Ksp-Wls−/− fibrotic kidneys, compared with the controls (Figure 4, C, F and G). Immunohistochemical staining for α-SMA, PDGFR-β, and PCNA consistently showed a significant reduction in Ksp-Wls−/− mice at 7 days after UUO (Figure 4, H and I). The numbers of PCNA-positive cells in renal interstitium were markedly decreased in Ksp-Wls−/− kidneys, compared with the controls (Figure 4J). However, little difference was observed in tubular epithelial cell proliferation between Ksp-Wls−/− kidneys and the controls (Figure 4J). Of note, tubule-specific deletion of Wls did not affect Fas ligand (FasL) mRNA level (Figure 4K), suggesting that blockade of Wnts secretion in renal tubules has little effect on cell apoptosis and survival.
We also examined the effect of blockade of Wnt secretion in renal tubules on renal inflammation. Immunofluorescence staining showed a decreased infiltration of the CD45-positive cells in Ksp-Wls−/− mice, compared with the controls (Supplemental Figure 2). qRT-PCR analysis revealed that monocyte chemoattractant protein 1 (MCP-1) mRNA expression was repressed in Ksp-Wls−/− kidneys after UUO, compared with the controls (Figure 4L). Together, these data indicate that tubule-specific deletion of Wls alleviates renal infiltration of inflammatory cells.
Genetic Ablation of Wls in Fibroblasts Does Not Affect Renal Fibrosis after UUO and Ischemia/Reperfusion Injury
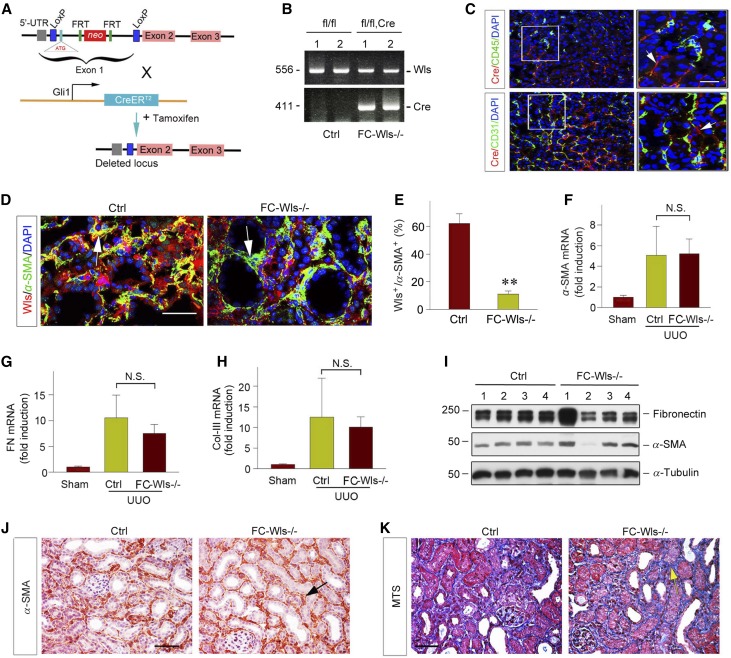
Because Wnts are expressed in interstitial cells as well, we then sought to determine the roles and relative contribution of the fibroblast-derived Wnts in renal fibrogenesis. To this end, we generated conditional knockout mice with fibroblast-specific ablation of Wls by crossing Wls floxed mice with an inducible Cre mouse line under the control of endogenous Gli1 promoter/enhancer elements (designate as FC-Wls−/−) (Figure 5, A and B). After injections of tamoxifen, Cre recombinase was induced in the Gli1-positive kidney interstitial fibroblasts, as reported previously,17,21 thereby resulting in specific Wls ablation in these cells. Notably, coimmunostaining showed no colocalization of Cre (red) and CD45 or CD31 antigens (green) (Figure 5C), indicating that there was an absence of Wls deletion in the CD45+ monocytes and CD31+ endothelial cells. Groups of sex-matched, 8-week-old FC-Wls−/− mice and their control littermates were given tamoxifen, and then subjected to UUO for 7 days. As shown in Figure 5D, ablation of Wls was evident in the α-SMA+, active fibroblasts in FC-Wls−/− mice, compared with controls. Quantitative determination revealed that in the α-SMA+ fibroblast population, approximately 60% were Wls+ in the controls, but only 10% in FC-Wls−/− mice (Figure 5E), suggesting an efficient and specific deletion of Wls in active fibroblasts. Interestingly, there was little difference in α-SMA, fibronectin, and type III collagen mRNA expression between FC-Wls−/− mice and control littermates at 7 days after UUO, as assessed by qRT-PCR (Figure 5, F–I). Similar results were obtained when renal α-SMA protein was detected by western blot analysis (Figure 5J). Consistently, immunohistochemical staining for α-SMA and MTS exhibited virtually identical levels of myofibroblast activation and collagen deposition in the obstructed kidneys in both FC-Wls−/− and control mice (Figure 5, K and L).
Figure 5.
Fibroblast-specific deletion of Wls exhibits little effect on kidney fibrosis after obstructive injury. (A) Schematic diagram depicting generation of fibroblast-specific deletion of Wls in mice by using Cre-LoxP system. Wls floxed mice (Wlsfl/fl) were crossbred with tamoxifen-inducible Cre transgenic mice under the control of endogenous Gli1 promoter/enhancer elements. Tamoxifen-inducible, Cre-mediated recombination resulted in deletion of the flanked sequences in Gli1-expressing fibroblasts. (B) Mouse genotyping analyzed by PCR. Lanes 1 and 2 show the genotyping of the control mice used in this study (genotype: Wlsfl/fl), lanes 3 and 4 denote the genotyping of fibroblast-specific Wls knockout mice (genotype: Wlsfl/fl Cre), designated as FC-Wls−/−. (C) Coimmunostaining with antibodies against Cre recombinase (red) and various cell type–specific markers (green). Arrows indicate Cre-positive cells. Scale bar, 20 µm. (D) Representative immunofluorescent micrographs show colocalization of Wls and α-SMA expression in control and FC-Wls−/− kidneys at 7 days after UUO. Arrows indicate α-SMA–positive, active fibroblasts in kidney interstitial space. Scale bar, 50 μm. (E) Quantitative data show the percentage of Wls+ cells in the α-SMA+ fibroblast population in control and FC-Wls−/− kidneys at 7 days after UUO. **P<0.01 versus controls (n=3). (F–H) qRT-PCR analyses show the relative mRNA levels of α-SMA, fibronectin, and collagen type III in the obstructive kidneys of control and FC-Wls−/− mice at 7 days after UUO (n=5). (I) Western blot analyses of renal fibronectin and α-SMA levels in control and FC-Wls−/− mice at 7 days after UUO. (J) Representative micrographs of immunohistochemical staining show α-SMA expression in control and FC-Wls−/− kidneys at 7 days after UUO. (K) Representative micrographs of MTS show collagen deposition in control and FC-Wls−/− kidneys at 7 days after UUO. Arrows indicate positive staining. Scale bar, 50 µm. Ctrl, control.
We further examined the effects of blockade of Wnt secretion from fibroblasts in FC-Wls−/− mice on renal fibrosis by using an ischemia/reperfusion injury (IRI) model. Groups of FS-Wls−/− mice and their control littermates were given tamoxifen, and then subjected to unilateral IRI for 10 days. As shown in Supplemental Figure 3A, fibroblast-specific deletion of Wls did not affect serum creatinine levels. Renal mRNA levels of α-SMA, a specific marker for myofibroblast activation, were similar after IRI in the FC-Wls−/− mice and their control littermates (Supplemental Figure 3). Consistently, western blot analyses for renal α-SMA protein confirmed this result. Therefore, genetic blockade of Wnt secretion from interstitial fibroblasts exhibits little effect on renal fibrosis after either obstructive or ischemic injury.
Exogenous Wnts Promote Renal Fibroblast Proliferation In Vitro
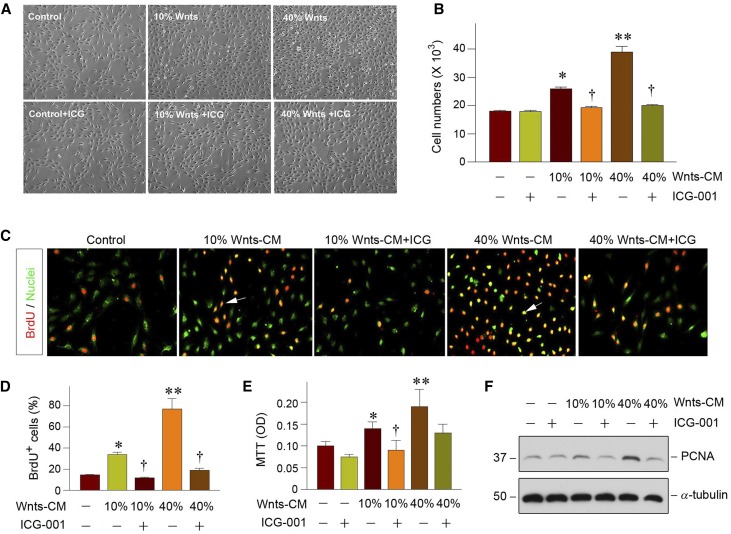
To ascertain the role of tubule-derived Wnts in renal fibrogenesis by promoting fibroblast expansion in a paracrine fashion, we mimicked the in vivo situation by incubating NRK-49F cells with Wnts-CM prepared from kidney proximal tubule cells (HKC-8) transfected with multiple Wnt expression vectors including Wnt1, Wnt2, Wnt3a, and Wnt4 in vitro, as described recently.44 As illustrated in Figure 6A, an appreciable increase in NRK-49F cell density was observed after incubation with different doses of Wnts-CM. Cell number counting revealed that Wnts-CM substantially induced fibroblast expansion in a dose-dependent manner (Figure 6B). However, treatment with ICG-001, a small molecule that specifically inhibits the canonic Wnt/β-catenin signaling,40,45 abolished the effect of Wnts-CM on fibroblast proliferation (Figure 6, A and B), suggesting a predominant role for Wnts in the conditioned medium accounting for its mitogenic effect. Furthermore, Wnts-CM was able to promote fibroblasts entering the cell cycle and undergoing DNA synthesis, which was reflected by bromodeoxyuridine (BrdU) incorporation. As shown in Figure 6, C and D, an increased BrdU incorporation in fibroblasts was observed after incubation with Wnts-CM, compared with the controls. Consistently, a quantitative colorimetric 3–(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay also exhibited that Wnts-CM stimulated NRK-49F cell proliferation in vitro (Figure 6E). Finally, we examined the protein expression of the PCNA by western blotting. As presented in Figure 6F, PCNA was significantly induced in NRK-49F cells after incubation with Wnts-CM, but was abolished by ICG-001 treatment. Therefore, tubule-derived Wnts are able to serve as a potent mitogen and promote fibroblast proliferation.
Figure 6.
Wnt ligands promote fibroblast proliferation and matrix production in vitro. (A) Representative micrographs show the phase-contrast images of fibroblasts after incubation with Wnts-CM in the absence or presence of ICG-001 for 48 hours. (B) Wnts promotes fibroblast proliferation. NRK-49F cells were incubated with Wnts-CM for 48 hours. Cell numbers were counted and presented. *P<0.05, **P<0.01 versus controls. †P<0.05, versus 10% or 40% Wnts-CM alone (n=3). (C) Representative micrographs show that Wnts-CM promoted fibroblasts DNA synthesis as demonstrated by BrdU incorporation. NRK-49F cells were incubated with 10% and 40% Wnts-CM for 48 hours, respectively. Cells were immunostained with mouse anti-BrdU antibody (red). SYTO-Green (green) was used to visualize the nuclei. Arrows indicate BrdU-positive cells. (D) Quantitative determination of the percentage of BrdU-positive cells after the treatment. *P<0.05, **P<0.01 versus controls. †P<0.05, versus 10% or 40% Wnt-CM (n=3). (E) Colorimetric MTT assay shows that Wnts-CM promoted fibroblast proliferation. *P<0.05, **P<0.01 versus controls. †P<0.05, versus 10% Wnts-CM (n=3). OD, optical density. (F) Western blot analyses show that Wnts-CM induced PCNA protein expression in fibroblasts. NRK-49F cells were incubated with varying concentrations of Wnts-CM for 48 hours as indicated.
To further confirm the role of Wnts in fibroblast activation, we stimulated NRK-49F cells with several purified, individual Wnt ligands. To this end, NRK-49 cells were incubated with human recombinant Wnt3a, Wnt4, and Wnt5a proteins for 48 hours, respectively. As shown in Supplemental Figure 4, recombinant Wnt proteins induced PDGFR-β and PCNA expression in vitro, and they also promoted fibronectin expression and deposition. Collectively, these results demonstrate that tubule-derived Wnts are sufficient to induce renal interstitial fibroblast proliferation and activation, thereby leading to kidney fibrosis.
Induction and Expression Pattern of Wnt Ligands in Human CKD
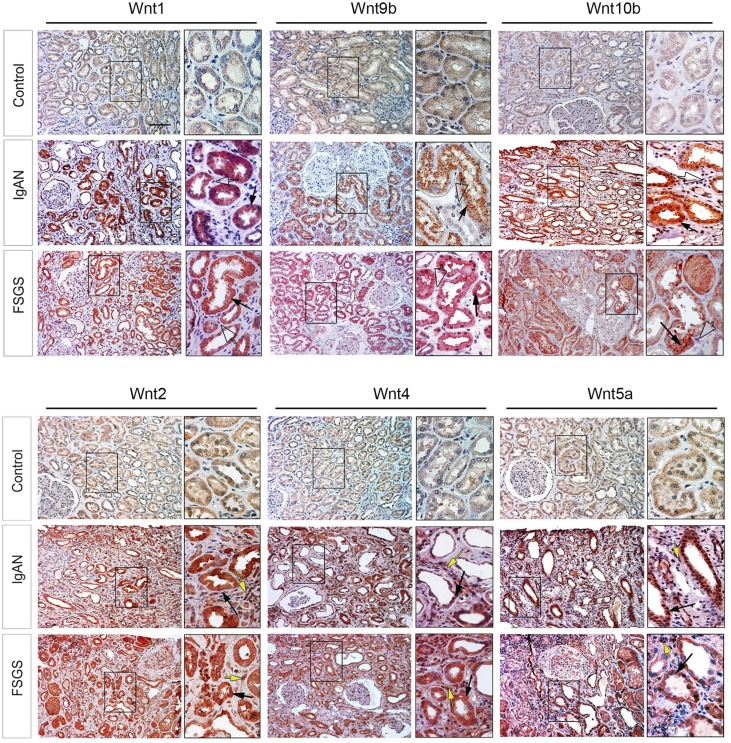
To study the clinical relevance of Wnts regulation to the pathogenesis of human CKD, we examined the protein expression of various Wnt ligands in kidney biopsy specimens from patients with a variety of CKDs. The demographic and clinical features of patients with CKD are presented in Supplemental Table 1. The majority of the biopsy specimens from these patients displayed a mild-to-moderate degree of renal interstitial fibrosis as assessed by MTS (Supplemental Figure 5). As shown in Figure 7, the staining for Wnt proteins, including Wnt1, Wnt2, Wnt4, Wnt5a, Wnt9b, and Wnt10b, was weak in human kidneys dissected from the nontumor region in patients with renal carcinoma (two patients tested). However, all of these Wnt ligands were induced to varying degrees in the kidney biopsy specimens from patients diagnosed with IgA nephropathy (five patients), FSGS (five patients), and membranous nephritis (five patients), as shown by immunohistochemical staining. Of interest, Wnt1, Wnt9b, and Wnt10b proteins were predominantly localized in renal tubular epithelium in human CKD (Figure 7, enlarged boxes, solid arrows), whereas they were largely absent in renal interstitial cells (Figure 7, enlarged boxes, open arrows). In contrast, Wnt2, Wnt4, and Wnt5a were widely expressed in both renal tubular and interstitial compartments (Figure 7, enlarged boxes, black and yellow arrows). These results indicate that multiple Wnt ligands are induced predominantly in renal tubular epithelium in human CKD as well.
Figure 7.
Multiple Wnt ligands are induced in human CKD. Human kidney biopsy samples were immunohistochemically stained with specific antibodies against Wnt1, Wnt2, Wnt4, Wnt5a, Wnt9b, and Wnt10b proteins. Representative micrographs show expression and localization of Wnts protein in a variety of human CKDs. Wnt1, Wnt9b, and Wnt10b were largely expressed in tubular epithelial cells (boxed area, solid arrows) but not in interstitial cells (open arrows). Wnt2, Wnt4, and Wnt 5a were detected in both tubular and interstitial cells in diseased kidneys (boxed area, black arrows and yellow arrows). Nontumor kidney tissue from the patients who had renal cell carcinoma and underwent nephrectomy was used as normal controls. IgAN, IgA nephropathy. Boxed areas are enlarged. Scale bar, 50 μm.
Discussion
Tubular epithelium comprises the major part of kidney parenchyma and is the epicenter of renal injury in response to various toxic, metabolic, and ischemic insults. Several different tubular responses after initial injury have been described in recent years, including partial EMT, cell cycle arrest at G2/M phase, and metabolic changes.4,5,8,9 However, all of these responses seem to converge onto a common outcome characterized by tubular cells switching to a secretory phenotype, with an increased release of a variety of soluble fibrogenic factors.10,11 In this study, we have demonstrated that multiple Wnt ligands are induced in tubular epithelium in human CKD (Figure 7), suggesting that Wnts are major constituents of the tubular secretome in pathologic conditions. More importantly, selective blockade of Wnt secretion by genetic ablation of Wls in renal tubules, but not in interstitial fibroblasts, markedly attenuates renal fibrosis. Our studies have established that tubule-derived Wnts play a predominant role in driving fibroblast activation and kidney fibrosis by mediating EMC in a paracrine fashion.
Because many Wnt ligands are concurrently induced in fibrotic kidneys, earlier studies have largely focused on β-catenin, the common intracellular mediator of canonic Wnt signaling.40,46,47 However, not all Wnt actions are mediated by β-catenin, and some of them are transmitted via the β-catenin–independent, noncanonic pathway. Furthermore, β-catenin has dual functions in both Wnt signaling and cell adhesion. Given these shortcomings, it is both necessary and important to interrogate the pathogenic role of Wnt ligands from different cellular sources and to assess their exact contribution in renal fibrogenesis. It is worthwhile to note that, among 19 Wnt ligands, 16 are upregulated in the fibrotic kidney after UUO, indicating an enormous activation of this signaling in CKD.27 In this context, assessment of the pathologic contribution of each individual Wnt might be difficult or unfruitful, because the function of a single Wnt which is absent might be compensated by other members of the Wnt family.26 Because Wls controls the release of all Wnts, cell type–specific ablation of Wls would be a powerful strategy to define the exact role and spatial contribution of Wnts from distinct sources.
The results of this study illustrate that blockade of Wnt secretion from renal tubules, but not fibroblasts, inhibits fibroblast activation and ameliorates kidney fibrosis. This finding is not completely surprising, because the majority of Wnts are induced in renal tubular epithelium under pathologic conditions. As the major resident cells in the kidney, injury to the tubular epithelium is a common finding in CKD with different causes. Although there is controversy regarding how tubular cells respond to injury and whether there is an EMT,4,5,48–50 one consensus exists in the field that tubular cells after injury switch to a secretory phenotype by releasing an assortment of fibrogenic factors.10,11 This study indicates that Wnt ligands are a major constituent of the tubular secretome in response to injury. Consistent with this notion, we show herein that at least six Wnt ligands, whose specific antibodies are currently available, are induced in renal tubular epithelium in human kidney biopsy specimens (Figure 7). It should be acknowledged that, due to the small sample size, our studies on Wnts expression in human CKD are preliminary in nature and only provide a proof-of-principle for its clinical relevance. Further studies, with large sample sizes and longitudinal design, are needed to address the relevance of Wnts expression to renal fibrosis in humans.
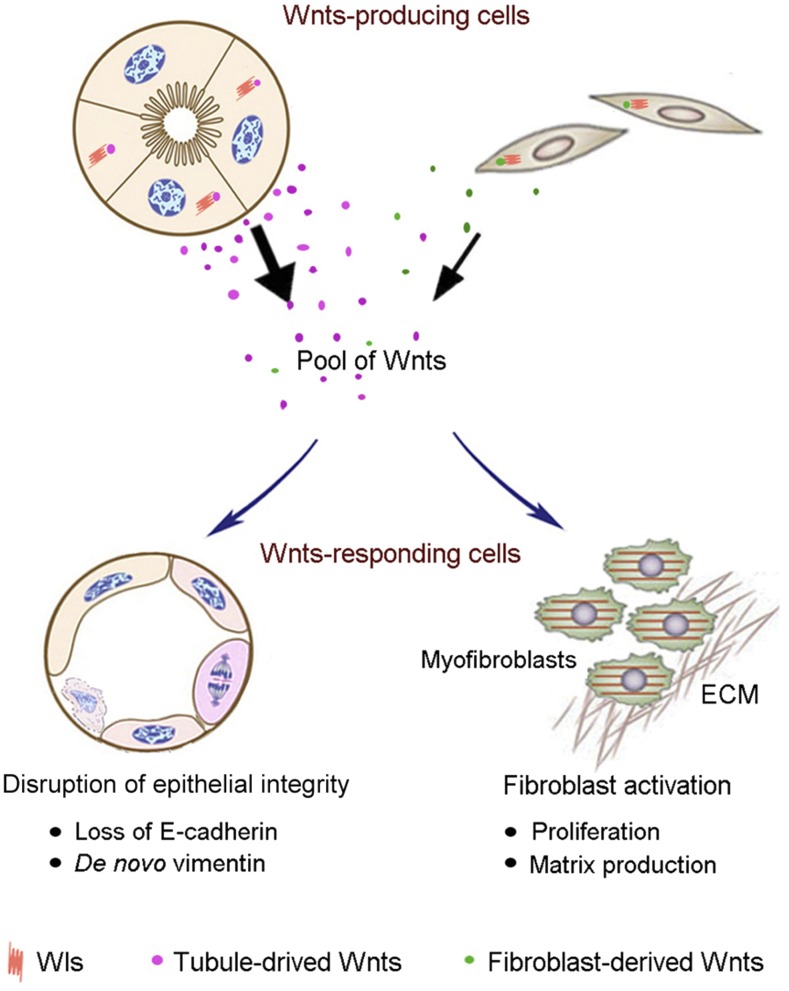
Selective blockade of Wnts secretion via tubule-specific ablation of Wls is sufficient to reduce fibroblast activation and renal fibrosis (Figures 2–4). Of note, the effect of tubule-derived Wnts on renal fibrosis is probably under-estimated in this study, because Wls ablation is incomplete and only occurs in approximately 40%–50% of proximal tubular cells in the Ksp-Wls−/− kidneys. There are several potential mechanisms that could account for the protective effect of tubular ablation of Wls after injury. First, blockade of Wnts secretion by renal tubules reduced renal β-catenin activation, particularly in tubular epithelium (Figure 1). This leads to dramatic preservation of tubular cell integrity, as demonstrated by retaining E-cadherin expression and inhibition of tubular expression of vimentin (Figure 3). In addition, blockade of Wnt secretion from renal tubules in Ksp-Wls−/− mice would reduce the Wnt concentration gradient in renal interstitium, which results in less activation of myofibroblasts, the principal matrix-producing cells. Consistent with this notion, tubule-derived Wnts are sufficient to cause fibroblast proliferation and expansion (Figure 6) and induce matrix over-production in vitro.19 We also observed a reduced renal inflammation in Ksp-Wls mice after UUO (Figure 4). It remains, however, unclear whether it is directly caused by reduced Wnt ligands or is due to an improved renal pathology after tubule-specific ablation of Wls. Nevertheless, our studies demonstrate that tubule-derived Wnts could impair tubular epithelial cell integrity by an autocrine manner, and promote fibroblast activation and matrix production in renal interstitium in a paracrine fashion (Figure 8).
Figure 8.
Tubule-derived Wnts play a major role in the pathogenesis of kidney fibrosis. Diagram shows the cellular sources of Wnt ligands and their actions in renal fibrogenesis. Multiple Wnts are induced in renal tubular epithelium as well as in interstitial fibroblasts after kidney injury and secreted by a Wls-dependent mechanism. Pool of Wnts targets tubular cells and impairs epithelial integrity characterized by loss of E-cadherin and de novo expression of vimentin, and promotes fibroblast activation characterized by an enhanced cell proliferation and matrix production. ECM, extracellular matrix.
Although fibroblast-specific activation of β-catenin is sufficient to cause renal fibrotic lesions,26 we found that genetic blockade of Wnt secretion from fibroblasts by ablating Wls does not significantly affect the severity of renal fibrosis in two models of nephropathies induced by UUO and IRI (Figure 5). This is in harmony with a previous report that conditional knockout of Wnt4 in renal fibroblasts has no effect on the severity of kidney fibrosis after injury.26 These findings, together with the results of tubule-specific ablation of Wls aforementioned, suggest that fibroblasts are major responding cells of Wnt ligands, but that their contribution to the pool of secreted Wnts in renal parenchyma is limited. Consistent with this, only selected Wnts are induced in renal interstitial cells in human CKD (Figure 7). It is unlikely that the lack of influence of fibroblast-derived Wnts on renal fibrosis is due to an insufficient ablation of Wls (Figure 5). However, the possibility exists that fibroblasts derived from non–Gli1-expressing cells could contribute to fibrosis via Wnt secretion. In addition, the Wnt-positive cells in the renal interstitium in human CKD could include endothelial cells and infiltrating inflammatory cells such as macrophages. Because the Cre is under the control of Gli1 promoter and only expressed in interstitial fibroblasts in the FC-Wls mice (Figure 5),21 we cannot exclude the likelihood that endothelial cells or macrophages may secrete Wnt ligands that play a critical role in renal fibrogenesis. Therefore, future studies are warranted to investigate the role of Wnts secreted by different kidney and infiltrating cells to completely understand this signaling in the development and progression of fibrotic CKD.
In summary, by creating two conditional knockout mouse lines with tubule- or fibroblast-specific deletion of Wls, we have demonstrated that tubule-derived, but not fibroblast-originated, Wnts are indispensable for fibroblasts activation and kidney fibrosis. This conclusion is consistent with that multiple Wnt ligands are induced predominantly in renal tubular epithelium in human kidney biopsy specimens from patients with CKD. These studies reinforce the notion that Wnt ligands, as a major constituent of the tubular secretome, promote kidney fibrotic lesions by mediating EMC in a paracrine fashion.
Concise Methods
Animal Models
Homozygous Wls floxed mice (C57BL/6J background) were obtained from the Jackson Laboratories (Stock #012888; Bar Harbor, ME). Transgenic mice that express Cre recombinase under the control of kidney-specific Ksp-Cre or endogenous Gli1 promoter/enhancer elements were reported elsewhere.47 By mating Wls floxed mice with Ksp-Cre or Gli1-Cre transgenic mice, conditional knockout mice (Ksp-Wls−/− or FS-Wls−/−) in which the Wls gene was specifically disrupted in renal tubular epithelial cells (genotype Wlsfl/fl, Ksp-Cre+/−) or fibroblasts (genotype Wlsfl/fl, FS-Cre+/−) were created. These mice were crossbred with homozygous Wls floxed mice (genotype Wlsfl/fl) to generate offspring with 50% Ksp-Wls−/− or FS-Wls−/− mice and 50% control mice (Wls floxed mice) within the same litters. A routine PCR protocol was used for genotyping of tail DNA samples with the following primer pairs: Cre transgene, 5′-AGG-TGT-AGA-GAA-GGC-ACT-TAGC-3′ and 5′-CTA-ATC-GCC-ATC-TTC-CAG-CAG-G-3′, which generated a 411-bp fragment; and Wls genotyping, 5′-AGG-CTT-CGA-ACG-TAA-CTG-ACC-3′ and 5′-CTC-AGA-ACT-CCC-TTC-TTG-AAG-C-3′, which yielded a 556-bp band for the floxed alleles. All animals were born normally at the expected Mendelian frequency, and they were normal in size and did not display any gross physical or behavioral abnormalities. In FC-Wls−/− mice, Cre is exclusively expressed in the Gli1-expressing cells. Mice were intraperitoneally injected with tamoxifen (T5648; Sigma-Aldrich, St. Louis, MO) at 25 mg/kg body wt for 5 days, and then subjected to UUO and unilateral renal IRI (UIRI). In this study, UUO and UIRI were performed by using established procedures, as described elsewhere.40 Mice were euthanized at day 7 after UUO. For the UIRI model, mice were subjected to unilateral nephrectomy to remove the intact, contralateral kidney at 10 days, and then euthanized at 11 days after UIRI. Blood and kidneys were collected for various analyses. Animal experiments were approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh.
Human Kidney Biopsy Samples
Human kidney specimens were obtained from diagnostic renal biopsies performed at the Presbyterian University Hospital, University of Pittsburgh Medical Center. Nontumor kidney tissues from patients who had renal cell carcinoma and underwent nephrectomy were used as normal controls. Paraffin-embedded human kidney biopsy specimen sections (2.5-μm thickness) were prepared using a routine procedure. All studies involving human kidney samples were approved by the Institutional Review Board at the University of Pittsburgh.
qRT-PCR
Total RNA was extracted using TRIzol RNA isolation system (Invitrogen). First-strand cDNA synthesis was carried out by using a reverse transcription system kit according to the instructions of the manufacturer (Promega, Madison, WI). qRT-PCR was performed on an ABI PRISM 7000 sequence detection system (Applied Biosystems, Foster City, CA) as described previously.17 The sequences of primer pairs for different genes are shown in Supplemental Table 2. The mRNA levels of various genes were calculated after normalizing with β-actin.
Western Blot Analysis
Kidney tissues were lysed with radioimmunoprecipitation assay buffer containing 1% NP-40, 0.1% SDS, 100 μg/ml PMSF, 1% protease inhibitor cocktail, and 1% phosphatase I and II inhibitor cocktail (Sigma-Aldrich) in PBS on ice. The supernatants were collected after centrifugation at 13,000 × g at 4°C for 15 minutes. Protein expression was analyzed by western blot analysis. The primary antibodies used were as follows: anti-Wls (MABS87; EMD Millipore, Billerica, MA), anti–β-catenin (#610154; BD Transduction Laboratories, San Jose, CA), anti–E-cadherin (#3195; Cell Signaling Technology, Danvers, MA), anti–PAI-1 (sc-5297), anti–PDGFR-β (sc-432), anti-PCNA (sc-56) (Santa Cruz Biotechnology, Santa Cruz, CA), anti–cyclin D1 (RB904-P0; NeoMarkers, Fremont, CA), anti-fibronectin (F3648), anti–α-SMA (A2547), anti–α-tubulin (T9026) (Sigma-Aldrich), and anti-actin (MAB1501; EMD Millipore).
Histology and Immunohistochemical Staining
Paraffin-embedded mouse kidney sections (3-μm thickness) or human kidney biopsy sections were prepared by a routine procedure. The sections were subjected to MTS and Picrosirius Red staining by standard protocol. Immunohistochemical staining was performed according to the established protocol as described previously.44 The antibodies against Wnt1 (ab15251), Wnt2 (ab150608), Wnt4 (ab15699), Wnt5a (ab72583), Wnt9b (ab151220), β-catenin (ab15180), α-SMA (ab5694; Abcam, Inc., Cambridge, MA), Wnt10b (sc6546) (Santa Cruz Biotechnology), vimentin (#5741s), PDGFR-β (#3169), and PCNA (#2586; Cell Signaling Technology) were used.
Coimmunofluorescence Staining and Confocal Microscopy
Kidney cryosections were fixed with 3.7% paraformaldehyde for 15 minutes at room temperature and immersed in 0.2% Triton X-100 for 10 minutes. After blocking with 10% donkey serum in PBS for 1 hour, slides were coimmunostained with the following antibodies: anti-Cre (MAB3120; EMD Millipore), anti-fibronectin (F3648; Sigma-Aldrich), anti–collagen type III (#234189; EMD Millipore), anti-CD45 (ab60291; Abcam, Inc.), and anti-CD31 (#550274; BD Pharmingen, San Jose, CA). To visualize the primary antibodies, slides were stained with cyanine Cy2- or Cy3-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA). For double immunostaining, cryosections from control and Ksp-Wls−/− mice were stained with anti-Wls (MABS87; EMD Millipore) and one of the following antibodies: anti–aquaporin 1 (AQP1, sc-9878; Santa Cruz Biotechnology), anti–Thiazide-sensitive Na-Cl cotransporter (NCC, AB3553; EMD Millipore), or anti–aquaporin 3 (AQP3, ab125045; Abcam, Inc.). Double immunostaining was also performed using antibodies against Wls (EMD Millipore) and α-SMA (ab5694; Abcam, Inc.) in control and FC-Wls−/− kidneys. Stained slides were viewed under a Leica TCS-SL confocal microscope equipped with a digital camera (Buffalo Grove, IL).
Serum Creatinine Assay
Serum was collected from control and FC-Wls−/− mice at 11 days after UIRI. Serum creatinine level was determined by use of a QuantiChrom creatinine assay kit, according to the protocols specified by the manufacturer (BioAssay Systems, Hayward, CA). The level of serum creatinine was expressed as milligrams per 100 ml (deciliter).
Cell Culture
Normal rat kidney interstitial fibroblast (NRK-49F) cells were obtained from the American Type Culture Collection (Manassas, VA). Cells were maintained as described previously.17 Serum-starved NRK-49F was treated with recombinant human Wnts protein (StemRD Inc., Burlingame, CA) and Wnts-CM prepared from human kidney tubular cells (HKC-8) cells transfected with different Wnt-expression vectors, as described previously.44 After incubation for various periods of time as indicated, NRK-49F cells were then collected and subjected to various analyses.
Cell Proliferation Assay
Cell proliferation was assessed by two approaches: cell counting and MTT assay. Cell numbers were counted by using a hemocytometer. Cell proliferation was also determined quantitatively by an MTT assay. Briefly, NRK-49F was seeded into a 96-well plate at a density of 2 × 103/well. After adherence of cells to the plate, the culture was changed to the serum-free medium and incubated for 24 hours, followed by treatment with or without Wnts-CM at different concentrations for various periods of time as indicated. MTT (5 mg/ml) was added to the medium at 10 μl/well, followed by incubation at 37°C for 4 hours. After the medium was removed, cells were lysed with 100 μl dimethyl sulfoxide. Absorbance of each well was measured by a microplate reader at 490 nm wavelength.
BrdU Incorporation Assay
The effect of Wnts on fibroblast DNA synthesis was evaluated by BrdU incorporation. Briefly, cells were seeded onto 24-well plates and treated with various concentrations of Wnts-CM for 48 hours, and then pulsed with BrdU (10 μM) for 24 hours. Cells were fixed with ice-cold 70% ethanol for 20 minutes, and DNA was denatured by incubation with 2.5 M HCL for 20 minutes, followed by neutralization with 0.1 M boric acid. Endogenous peroxidase activity was quenched by incubating the cells with 3% H2O2 in PBS for 20 minutes, and nonspecific binding was blocked by incubating the cells with 10% donkey serum for 10 minutes at room temperature, as described previously.21 Incorporated BrdU was detected with a mouse monoclonal anti-BrdU antibody (B2531; Sigma-Aldrich), followed by incubation with cyanine Cy3–conjugated, affinity-purified secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA). Stained cells were mounted with Vectashield antifade mounting medium by using SYTO-Green to visualize the nuclei. Stained samples were viewed under an Eclipse E600 epifluorescence microscope equipped with a digital camera (Nikon, Melville, NY).
Statistical Analyses
All data were expressed as mean±SEM. Statistical analyses of the data were performed using SigmaStat software (Jandel Scientific Software, San Rafael, CA). Comparisons between groups were made using one-way ANOVA, followed by the Student–Newman–Keuls test. P<0.05 was considered significant.
Disclosures
None.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health Grants DK064005, DK091239, and DK106049; National Natural Science Foundation of China Grants 81370839 and 81521003; Guangdong Science Foundation Innovative Group Grant 2014A030312014; and Guangzhou Research Fund 201504010001.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2016080902/-/DCSupplemental.
References
- 1.Liu Y: Cellular and molecular mechanisms of renal fibrosis. Nat Rev Nephrol 7: 684–696, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eddy AA: Overview of the cellular and molecular basis of kidney fibrosis. Kidney Int Suppl (2011) 4: 2–8, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duffield JS: Cellular and molecular mechanisms in kidney fibrosis. J Clin Invest 124: 2299–2306, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lovisa S, LeBleu VS, Tampe B, Sugimoto H, Vadnagara K, Carstens JL, Wu CC, Hagos Y, Burckhardt BC, Pentcheva-Hoang T, Nischal H, Allison JP, Zeisberg M, Kalluri R: Epithelial-to-mesenchymal transition induces cell cycle arrest and parenchymal damage in renal fibrosis. Nat Med 21: 998–1009, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grande MT, Sánchez-Laorden B, López-Blau C, De Frutos CA, Boutet A, Arévalo M, Rowe RG, Weiss SJ, López-Novoa JM, Nieto MA: Snail1-induced partial epithelial-to-mesenchymal transition drives renal fibrosis in mice and can be targeted to reverse established disease. Nat Med 21: 989–997, 2015 [DOI] [PubMed] [Google Scholar]
- 6.Zhu F, Liu W, Li T, Wan J, Tian J, Zhou Z, Li H, Liu Y, Hou FF, Nie J: Numb contributes to renal fibrosis by promoting tubular epithelial cell cycle arrest at G2/M. Oncotarget 7: 25604–25619, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonventre JV: Primary proximal tubule injury leads to epithelial cell cycle arrest, fibrosis, vascular rarefaction, and glomerulosclerosis. Kidney Int Suppl (2011) 4: 39–44, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang L, Besschetnova TY, Brooks CR, Shah JV, Bonventre JV: Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med 16: 535–543, 2010 [DOI] [PMC free article] [PubMed]
- 9.Kang HM, Ahn SH, Choi P, Ko YA, Han SH, Chinga F, Park AS, Tao J, Sharma K, Pullman J, Bottinger EP, Goldberg IJ, Susztak K: Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat Med 21: 37–46, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou D, Liu Y: Renal fibrosis in 2015: Understanding the mechanisms of kidney fibrosis. Nat Rev Nephrol 12: 68–70, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ovadya Y, Krizhanovsky V: A new Twist in kidney fibrosis. Nat Med 21: 975–977, 2015 [DOI] [PubMed] [Google Scholar]
- 12.Gill PS, Rosenblum ND: Control of murine kidney development by sonic hedgehog and its GLI effectors. Cell Cycle 5: 1426–1430, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Zhou D, Tan RJ, Liu Y: Sonic hedgehog signaling in kidney fibrosis: A master communicator. Sci China Life Sci 59: 920–929, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bang C, Antoniades C, Antonopoulos AS, Eriksson U, Franssen C, Hamdani N, Lehmann L, Moessinger C, Mongillo M, Muhl L, Speer T, Thum T: Intercellular communication lessons in heart failure. Eur J Heart Fail 17: 1091–1103, 2015 [DOI] [PubMed] [Google Scholar]
- 15.Combes AN, Davies JA, Little MH: Cell-cell interactions driving kidney morphogenesis. Curr Top Dev Biol 112: 467–508, 2015 [DOI] [PubMed] [Google Scholar]
- 16.Surendran K, Schiavi S, Hruska KA: Wnt-dependent beta-catenin signaling is activated after unilateral ureteral obstruction, and recombinant secreted frizzled-related protein 4 alters the progression of renal fibrosis. J Am Soc Nephrol 16: 2373–2384, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Ding H, Zhou D, Hao S, Zhou L, He W, Nie J, Hou FF, Liu Y: Sonic hedgehog signaling mediates epithelial-mesenchymal communication and promotes renal fibrosis. J Am Soc Nephrol 23: 801–813, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kramann R, Schneider RK, DiRocco DP, Machado F, Fleig S, Bondzie PA, Henderson JM, Ebert BL, Humphreys BD: Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell 16: 51–66, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maarouf OH, Aravamudhan A, Rangarajan D, Kusaba T, Zhang V, Welborn J, Gauvin D, Hou X, Kramann R, Humphreys BD: Paracrine Wnt1 drives interstitial fibrosis without inflammation by tubulointerstitial cross-talk. J Am Soc Nephrol 27: 781–790, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rauhauser AA, Ren C, Lu D, Li B, Zhu J, McEnery K, Vadnagara K, Zepeda-Orozco D, Zhou XJ, Lin F, Jetten AM, Attanasio M: Hedgehog signaling indirectly affects tubular cell survival after obstructive kidney injury. Am J Physiol Renal Physiol 309: F770–F778, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou D, Li Y, Zhou L, Tan RJ, Xiao L, Liang M, Hou FF, Liu Y: Sonic hedgehog is a novel tubule-derived growth factor for interstitial fibroblasts after kidney injury. J Am Soc Nephrol 25: 2187–2200, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Angers S, Moon RT: Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol 10: 468–477, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Clevers H, Nusse R: Wnt/β-catenin signaling and disease. Cell 149: 1192–1205, 2012 [DOI] [PubMed] [Google Scholar]
- 24.Zhou D, Tan RJ, Fu H, Liu Y: Wnt/β-catenin signaling in kidney injury and repair: A double-edged sword. Lab Invest 96: 156–167, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawakami T, Ren S, Duffield JS: Wnt signalling in kidney diseases: Dual roles in renal injury and repair. J Pathol 229: 221–231, 2013 [DOI] [PubMed] [Google Scholar]
- 26.DiRocco DP, Kobayashi A, Taketo MM, McMahon AP, Humphreys BD: Wnt4/β-catenin signaling in medullary kidney myofibroblasts. J Am Soc Nephrol 24: 1399–1412, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He W, Dai C, Li Y, Zeng G, Monga SP, Liu Y: Wnt/beta-catenin signaling promotes renal interstitial fibrosis. J Am Soc Nephrol 20: 765–776, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klaus A, Birchmeier W: Wnt signalling and its impact on development and cancer. Nat Rev Cancer 8: 387–398, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Yang J, Mowry LE, Nejak-Bowen KN, Okabe H, Diegel CR, Lang RA, Williams BO, Monga SP: β-catenin signaling in murine liver zonation and regeneration: A Wnt-Wnt situation! Hepatology 60: 964–976, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou L, Liu Y: Wnt/β-catenin signalling and podocyte dysfunction in proteinuric kidney disease. Nat Rev Nephrol 11: 535–545, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Surendran K, Simon TC, Liapis H, McGuire JK: Matrilysin (MMP-7) expression in renal tubular damage: Association with Wnt4. Kidney Int 65: 2212–2222, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Bänziger C, Soldini D, Schütt C, Zipperlen P, Hausmann G, Basler K: Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell 125: 509–522, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Das S, Yu S, Sakamori R, Stypulkowski E, Gao N: Wntless in Wnt secretion: Molecular, cellular and genetic aspects. Front Biol (Beijing) 7: 587–593, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carpenter AC, Rao S, Wells JM, Campbell K, Lang RA: Generation of mice with a conditional null allele for Wntless. Genesis 48: 554–558, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen SR, Tang JX, Cheng JM, Hao XX, Wang YQ, Wang XX, Liu YX: Does murine spermatogenesis require WNT signalling? A lesson from Gpr177 conditional knockout mouse models. Cell Death Dis 7: e2281, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cornett B, Snowball J, Varisco BM, Lang R, Whitsett J, Sinner D: Wntless is required for peripheral lung differentiation and pulmonary vascular development. Dev Biol 379: 38–52, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Augustin I, Gross J, Baumann D, Korn C, Kerr G, Grigoryan T, Mauch C, Birchmeier W, Boutros M: Loss of epidermal Evi/Wls results in a phenotype resembling psoriasiform dermatitis. J Exp Med 210: 1761–1777, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhong Z, Zylstra-Diegel CR, Schumacher CA, Baker JJ, Carpenter AC, Rao S, Yao W, Guan M, Helms JA, Lane NE, Lang RA, Williams BO: Wntless functions in mature osteoblasts to regulate bone mass. Proc Natl Acad Sci USA 109: E2197–E2204, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fu J, Ivy Yu HM, Maruyama T, Mirando AJ, Hsu W: Gpr177/mouse Wntless is essential for Wnt-mediated craniofacial and brain development. Dev Dyn 240: 365–371, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hao S, He W, Li Y, Ding H, Hou Y, Nie J, Hou FF, Kahn M, Liu Y: Targeted inhibition of β-catenin/CBP signaling ameliorates renal interstitial fibrosis. J Am Soc Nephrol 22: 1642–1653, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He W, Tan R, Dai C, Li Y, Wang D, Hao S, Kahn M, Liu Y: Plasminogen activator inhibitor-1 is a transcriptional target of the canonical pathway of Wnt/beta-catenin signaling. J Biol Chem 285: 24665–24675, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.He W, Tan RJ, Li Y, Wang D, Nie J, Hou FF, Liu Y: Matrix metalloproteinase-7 as a surrogate marker predicts renal Wnt/β-catenin activity in CKD. J Am Soc Nephrol 23: 294–304, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stein U, Arlt F, Walther W, Smith J, Waldman T, Harris ED, Mertins SD, Heizmann CW, Allard D, Birchmeier W, Schlag PM, Shoemaker RH: The metastasis-associated gene S100A4 is a novel target of beta-catenin/T-cell factor signaling in colon cancer. Gastroenterology 131: 1486–1500, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Xiao L, Zhou D, Tan RJ, Fu H, Zhou L, Hou FF, Liu Y: Sustained activation of Wnt/β-catenin signaling drives AKI to CKD progression. J Am Soc Nephrol 27: 1727–1740, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Henderson WR Jr, Chi EY, Ye X, Nguyen C, Tien YT, Zhou B, Borok Z, Knight DA, Kahn M: Inhibition of Wnt/beta-catenin/CREB binding protein (CBP) signaling reverses pulmonary fibrosis. Proc Natl Acad Sci USA 107: 14309–14314, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dai C, Stolz DB, Kiss LP, Monga SP, Holzman LB, Liu Y: Wnt/beta-catenin signaling promotes podocyte dysfunction and albuminuria. J Am Soc Nephrol 20: 1997–2008, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou D, Li Y, Lin L, Zhou L, Igarashi P, Liu Y: Tubule-specific ablation of endogenous β-catenin aggravates acute kidney injury in mice. Kidney Int 82: 537–547, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Quaggin SE, Kapus A: Scar wars: Mapping the fate of epithelial-mesenchymal-myofibroblast transition. Kidney Int 80: 41–50, 2011 [DOI] [PubMed] [Google Scholar]
- 49.Humphreys BD, Lin SL, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, Valerius MT, McMahon AP, Duffield JS: Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol 176: 85–97, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG: Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest 110: 341–350, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.