Abstract
Metabolic acidosis is associated with poor outcomes in CKD. Because impaired renal ammonium excretion is important in the pathogenesis of acidosis, urine ammonium excretion might be a better and perhaps earlier acid–base indicator of risk than serum bicarbonate, particularly in patients without acidosis. We evaluated the association between baseline ammonium excretion and clinical outcomes in African American Study of Kidney Disease and Hypertension participants (n=1044). Median daily ammonium excretion was 19.5 (95% confidence interval [95% CI], 6.5 to 43.2) mEq. In Cox regression models (adjusted for demographics, measured GFR, proteinuria, body mass index, net endogenous acid production, and serum potassium and bicarbonate), hazard ratios of the composite outcome of death or dialysis were 1.46 (95% CI, 1.13 to 1.87) in the low tertile and 1.14 (95% CI, 0.89 to 1.46) in the middle tertile of daily ammonium excretion compared with the high tertile. Among participants without acidosis at baseline, the adjusted hazard ratio for those with ammonium excretion <20 mEq/d was 1.36 (95% CI, 1.09 to 1.71) compared with those with ammonium excretion ≥20 mEq/d. Additionally, compared with participants in the high ammonium tertile, those in the low ammonium tertile had higher adjusted odds of incident acidosis at 1 year (adjusted odds ratio, 2.56; 95% CI, 1.04 to 6.27). In conclusion, low ammonium excretion is associated with death and renal failure in hypertensive kidney disease, even among those without acidosis. Low ammonium excretion could identify patients with CKD and normal bicarbonate levels who might benefit from alkali before acidosis develops.
Keywords: acidosis., chronic kidney disease, dialysis, mortality, AASK (African American Study of Kidney Disease and Hypertension)
In nondialysis-requiring CKD, the prevalence of metabolic acidosis, typically defined as serum total carbon dioxide (tCO2) <22 mEq/L, is approximately 15%.1,2 The prevalence increases with advancing CKD, from 7% in stage 2 to 37% in stage 4 CKD.2 Some, but not all, observational studies identified low tCO2 as a risk factor for GFR decline and mortality.3–7 Differences in population characteristics and methodologic approaches may explain these discrepancies. Another possibility is that tCO2, although convenient and commonly measured in clinical practice and research settings, may not be the best acid–base indicator of risk.
Because urine ammonium (uNH4+) excretion is critical for the maintenance of normal tCO2 and reduced uNH4+ excretion plays an important role in the development of metabolic acidosis in CKD, it may be an alternative and perhaps earlier indicator of risk than tCO2. Indeed, Vallet et al. found that lower uNH4+ excretion was a risk factor for ESRD in >1000 patients with CKD in the NephroTest cohort.8 However, they did not adjust for serum tCO2 or nutritional indicators of acid-alkali content, such as protein intake or net endogenous acid production (NEAP), in their main analyses. Hence, it is uncertain whether uNH4+ excretion has prognostic value above and beyond tCO2 or if the excess risk of ESRD associated with low uNH4+ is because of poor nutritional status. Nevertheless, this finding highlights the potential to use uNH4+ measurements to identify at-risk individuals. This may be especially important for those with normal tCO2, who comprise the vast majority of patients with CKD.1,2 For instance, if low uNH4+ were shown to be a risk factor for poor outcomes in patients with CKD and normal tCO2, it is plausible that base administration before acidosis ensues might prevent acidosis-related complications in CKD. Thus, the main objective of this study was to determine if uNH4+ excretion is associated with long-term clinical end points in CKD independent of serum tCO2, NEAP, GFR, and other potential confounders. Secondary objectives were to determine, in the subgroup of patients with CKD without acidosis, whether daily uNH4+ excretion is associated with subsequent acidosis as well as clinical events.
Results
We evaluated data from participants in the African American Study of Kidney Disease and Hypertension (AASK) Study. Table 1 presents baseline characteristics of the 1044 participants included in the main analyses. Daily uNH4+ excretion was higher in men and those with higher body mass index (BMI), dietary protein intake, and NEAP. Measured GFR (mGFR) and tCO2 were higher and serum potassium was lower in the highest uNH4+ tertile.
Table 1.
Baseline characteristics
| Variable | Total Population, n=1044 | Daily uNH4+ Tertile 1, n=348 | Daily uNH4+ Tertile 2, n=348 | Daily uNH4+ Tertile 3, n=348 | P Value |
|---|---|---|---|---|---|
| uNH4+, mEq/da | 19.5 (6.5 to 43.2) | 10.5 (4.2 to 14.8) | 19.4 (15.6 to 23.5) | 31.4 (24.9 to 53.1) | — |
| uNH4+, mEq/kg per day | 0.24 (0.13) | 0.13 (0.05) | 0.23 (0.06) | 0.37 (0.11) | <0.001 |
| Age, yr | 54 (11) | 53 (11) | 54 (11) | 54 (10) | 0.52 |
| Men, % | 62 | 48 | 63 | 75 | <0.001 |
| Heart disease, % | 52 | 49 | 51 | 55 | 0.27 |
| Current smoker, % | 29 | 29 | 27 | 31 | 0.38 |
| Past smoker, % | 29 | 26 | 31 | 29 | |
| Never smoker, % | 42 | 45 | 42 | 39 | |
| SBP, mmHg | 150 (24) | 151 (25) | 149 (23) | 151 (24) | 0.28 |
| BMI, kg/m2 | 30.6 (6.6) | 29.3 (6.5) | 30.5 (6.2) | 31.9 (7.0) | <0.001 |
| Protein intake, g/d | 69 (25) | 54 (18) | 68 (18) | 86 (27) | <0.001 |
| NEAP, mEq/d | 83 (37) | 78 (43) | 81 (30) | 89 (35) | <0.001 |
| ACE-I/ARB use, % | 39 | 38 | 38 | 42 | 0.44 |
| Diuretic use, % | 64 | 63 | 61 | 67 | 0.24 |
| mGFR, ml/min per 1.73 m2 | 47 (14) | 41 (13) | 47 (13) | 52 (13) | <0.001 |
| mGFR<30 ml/min per 1.73 m2, % | 16 | 24 | 14 | 9 | <0.001 |
| Urine protein-to-creatinine, mg/gb | 80 (29–342) | 96 (38–349) | 73 (28–470) | 67 (24–275) | 0.58 |
| Urine protein-to-creatinine ≥220 mg/g, % | 32 | 33 | 34 | 30 | 0.47 |
| Serum tCO2, mEq/L | 25.1 (3.0) | 24.7 (3.2) | 25.2 (2.8) | 25.4 (2.8) | 0.01 |
| Serum tCO2 <22 mEq/L, % | 12 | 16 | 11 | 9 | 0.01 |
| Serum potassium, mEq/L | 4.2 (0.6) | 4.4 (0.7) | 4.2 (0.6) | 4.1 (0.5) | <0.001 |
Continuous variables presented as mean (SD) unless noted otherwise. —, not applicable; SBP, systolic BP; ACE-I, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Presented as median with 95% CIs.
Presented as median with interquartile range.
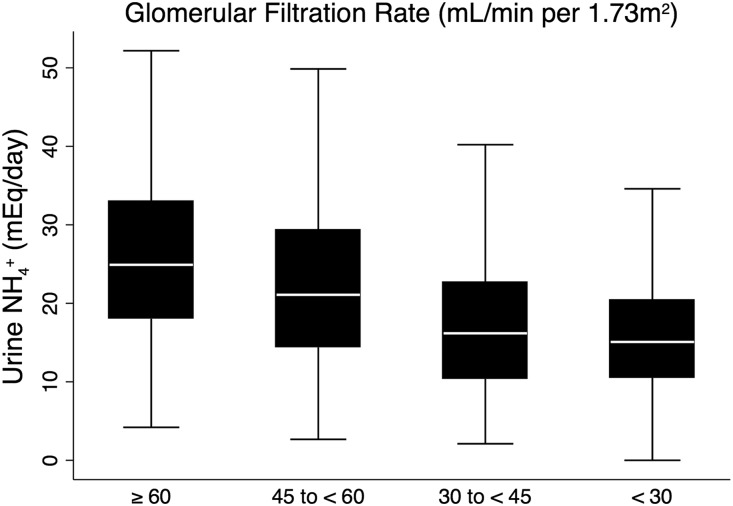
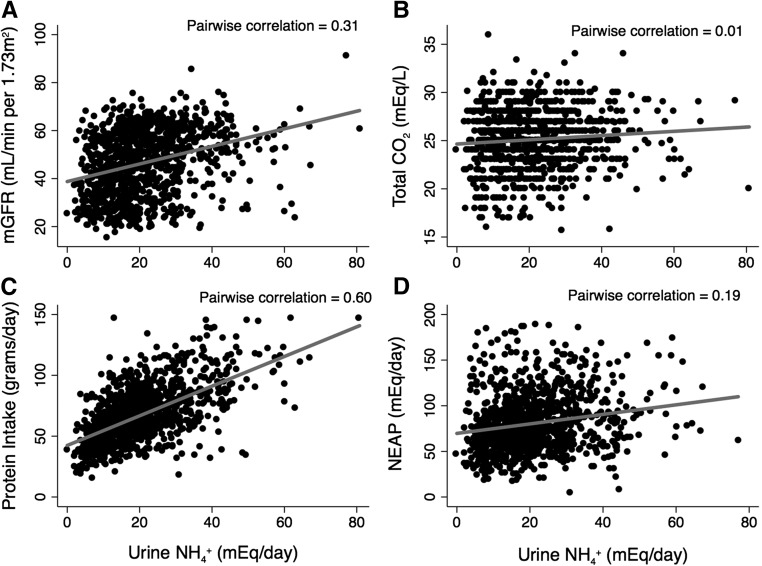
As expected, uNH4+ excretion was lower with lower mGFR (Figure 1). Figure 2 presents unadjusted scatterplots between uNH4+ and mGFR, tCO2, NEAP, and protein intake. Daily protein intake had a moderate correlation with uNH4+, mGFR and NEAP had low correlation with uNH4+, and tCO2 did not correlate with uNH4+ in the unadjusted analyses. Table 2 presents the adjusted multivariate linear regression results of the association between daily uNH4+ excretion and key clinical factors. Sex (men), obesity, higher NEAP, higher mGFR, higher urine protein-to-creatinine ratio, and lower serum potassium were associated with higher uNH4+ excretion. There was no association between serum tCO2 and uNH4+ after multivariate adjustment.
Figure 1.
Daily uNH4+ excretion decreases with lower mGFR. P<0.001 for trend.
Figure 2.
Daily uNH4+ excretion is modestly correlated with protein intake, and there is little to no correlation with mGFR, total CO2, and NEAP. Correlations of uNH4+ with (A) mGFR, (B) serum total CO2, (C) protein intake, and (D) NEAP at baseline.
Table 2.
Multivariate regression model results of the associations between daily uNH4+ excretion and key clinical factors
| Variable | Δ uNH4+, mEq/d | 95% CI |
|---|---|---|
| Age <50 yr | ||
| versus 50–60 yr | 0.5 | −1.2 to 2.1 |
| versus >60 yr | 1.4 | −0.3 to 3.0 |
| Men versus women | 4.4 | 3.0 to 5.7 |
| Heart disease, yes versus no | 0.9 | −0.5 to 2.2 |
| Current smoker | ||
| versus past | −1.0 | −2.8 to 0.8 |
| versus never | −1.3 | −2.9 to 0.3 |
| SBP<140 mmHg | ||
| versus 140 to <160 mmHg | 0.1 | −1.5 to 1.7 |
| versus ≥160 mmHg | −0.6 | −2.3 to 1.0 |
| BMI<25 kg/m2 | ||
| versus 25–30 kg/m2 | 0.9 | −1.0 to 2.8 |
| versus >30 kg/m2 | 4.1 | 2.2 to 6.0 |
| NEAP tertile 1, mEq/d | ||
| versus tertile 2 | 2.3 | 0.7 to 3.9 |
| versus tertile 3 | 4.2 | 2.6 to 5.8 |
| ACE-I/ARB use, yes versus no | 1.2 | −0.2 to 2.6 |
| Diuretic use, yes versus no | 0.7 | −0.7 to 2.1 |
| GFR≥60 ml/min per m2 | ||
| versus 30 to <59 ml/min per m2 | −3.7 | −5.5 to −1.8 |
| versus 15 to <30 ml/min per m2 | −7.9 | −10.0 to −5.9 |
| versus <15 ml/min per m2 | −8.9 | −11.4 to −6.4 |
| PCR ≥220 versus <220 mg/g | 2.2 | 0.6 to 3.7 |
| Serum tCO2 22–28 mEq/L | ||
| versus <22 mEq/L | −0.4 | −2.5 to 1.8 |
| versus >28 mEq/L | −0.3 | −2.9 to 2.3 |
| Serum potassium, mEq/L | −2.5 | −3.7 to −1.4 |
SBP, systolic BP; ACE-I, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; PCR, urine protein-to-creatinine ratio.
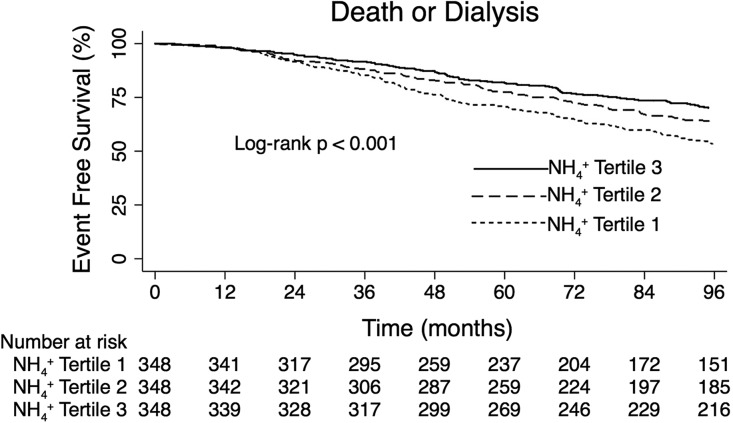
Table 3 presents the number and incidence rates of death or ESRD events during follow-up in the entire cohort and according to uNH4+ tertile. The number and incidence of events were higher with lower daily uNH4+ excretion. Figure 3 presents the unadjusted composite outcome of death or ESRD by tertiles of baseline daily uNH4+ excretion. Event-free survival was highest in the highest tertile of uNH4+ excretion and lowest in the lowest tertile of uNH4+ excretion.
Table 3.
Number and incidence rates of death and ESRD events during follow-up
| Daily uNH4+ Excretion | No. of ESRD Events | No. of Deaths before ESRD Events | Total No. of Events | Follow-Up Time (patient-yr) | Incidence Rate (95% CI) (per 1000 patient-yr) |
|---|---|---|---|---|---|
| Tertile 1 | 128 | 60 | 188 | 2388 | 79 (68 to 91) |
| Tertile 2 | 95 | 51 | 146 | 2648 | 55 (47 to 65) |
| Tertile 3 | 73 | 57 | 130 | 2825 | 46 (39 to 55) |
| Total | 296 | 168 | 464 | 7862 | 59 (54 to 65) |
Incidence rate shows the total number of events per 1000 patient-yr.
Figure 3.
Participants with lower daily uNH4+ excretion had higher unadjusted likelihood of death or ESRD during follow-up.
Table 4 presents unadjusted and adjusted hazards of the death or ESRD composite outcome by baseline daily uNH4+ excretion. Compared with the highest tertile of uNH4+ excretion, those in the lowest tertile had 46% higher risk of the composite outcome after adjusting for the main potential confounders (demographics, mGFR, proteinuria, NEAP, BMI, serum potassium, and serum tCO2.) in model 1. The results were similar after including other potential confounders (smoking status, systolic BP, heart disease, use of renin-angiotensin system inhibitors, and use of diuretics) in model 2. There was no statistically significantly different risk of the composite outcome for those in the middle tertile compared with the highest tertile of uNH4+ excretion in model 1 or model 2, although there was a suggestion of a dose–response relationship. There was no evidence of effect modification by serum bicarbonate categories (P=0.31) or tertiles of mGFR (P=0.15), NEAP (P=0.87), or protein intake (P=0.24).
Table 4.
Unadjusted and adjusted HRs of the death or ESRD composite outcome
| uNH4+, mEq/d | Unadjusted | Model 1 | Model 2 | |||
|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Tertile 1 | 1.76 | 1.41 to 2.21 | 1.46 | 1.13 to 1.87 | 1.50 | 1.16 to 1.95 |
| Tertile 2 | 1.21 | 0.96 to 1.54 | 1.14 | 0.89 to 1.46 | 1.13 | 0.88 to 1.45 |
| Tertile 3 | Ref | Ref | Ref | |||
P=0.15 for interaction by baseline mGFR tertiles. P=0.87 for interaction by baseline NEAP tertiles. P=0.24 for interaction by baseline protein intake tertiles. P=0.31 for interaction by baseline bicarbonate category (<22, 22–28, and >28 mEq/L). Model 1: adjusted for age, sex, randomized group, mGFR, proteinuria, NEAP, serum potassium, and serum tCO2. Stratified by BMI. Model 2: adjusted for model 1 variables, smoking status, systolic BP, heart disease, and use of angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and diuretics. Stratified by BMI. Ref, reference group.
Table 5 presents the associations between daily uNH4+ excretion and the individual outcomes of ESRD and death before ESRD, adjusted for model 1 variables. Daily uNH4+ excretion had a statistically significant relationship with ESRD but not with death before ESRD. The results were not significantly different for either outcome after adjusting for model 2 variables (data not shown).
Table 5.
Association between uNH4+ excretion and the individual ESRD and death before ESRD outcomes
| uNH4+, mEq/d | ESRD | Death before ESRD | ||
|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |
| Tertile 1 | 1.56 | 1.12 to 2.17 | 1.22 | 0.82 to 1.81 |
| Tertile 2 | 1.27 | 0.92 to 1.75 | 0.91 | 0.62 to 1.34 |
| Tertile 3 | Ref | Ref | ||
Model 1: adjusted for age, sex, randomized group, mGFR, proteinuria, NEAP, serum potassium, and serum tCO2. Stratified by BMI. Ref, reference group.
A sensitivity analysis that included 13 outliers who were excluded from the main analysis (because of high uNH4+ excretion per kilogram of body weight) showed similar hazard ratios (HRs) for the composite outcome of death or ESRD (HR, 1.36; 95% confidence interval [95% CI], 1.06 to 1.75 for the lowest uNH4+ tertile and HR, 1.08; 95% CI, 0.85 to 1.38 for the middle uNH4+ tertile compared with the highest uNH4+ tertile, adjusted for model 1 variables). An additional sensitivity analysis using uNH4+ indexed to body weight in mEq/kg per day as the independent variable showed similar results. Those in the lowest tertile of uNH4+ in mEq/kg per day had higher risk of the composite outcome (HR, 1.51; 95% CI, 1.17 to 1.94) than those in the highest tertile. Compared with those in the upper tertile of uNH4+ in mEq/kg per day, the HR for death or ESRD was 1.15 (95% CI, 0.90 to 1.47) for those in the middle tertile.
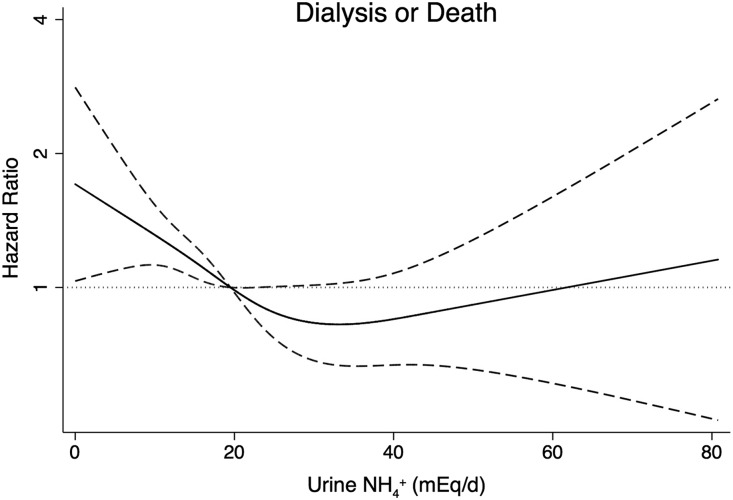
Figure 4 presents the cubic spline regression model results using median daily uNH4+ excretion (19.5 mEq/d) as the reference point. The lowest risk of the composite outcome was approximately 30 mEq/d uNH4+ excretion. Below 30 mEq/d, there was a linear and inverse association with the composite outcome. The cubic spline regression plot was similar after adjusting for model 2 variables (not shown).
Figure 4.
The risks of death or ESRD increase below uNH4+ 30 mEq/d. Shown is the cubic spline regression plot of the association between baseline uNH4+ excretion and the composite outcome of death or ESRD. The median value of uNH4+ excretion (19.5 mEq/d) served as the reference point. The solid line represents the mean HR and the dashed lines represent the 95% CIs. Adjusted for age, sex, randomized group, mGFR, proteinuria, NEAP, serum potassium, and serum tCO2. Stratified by BMI.
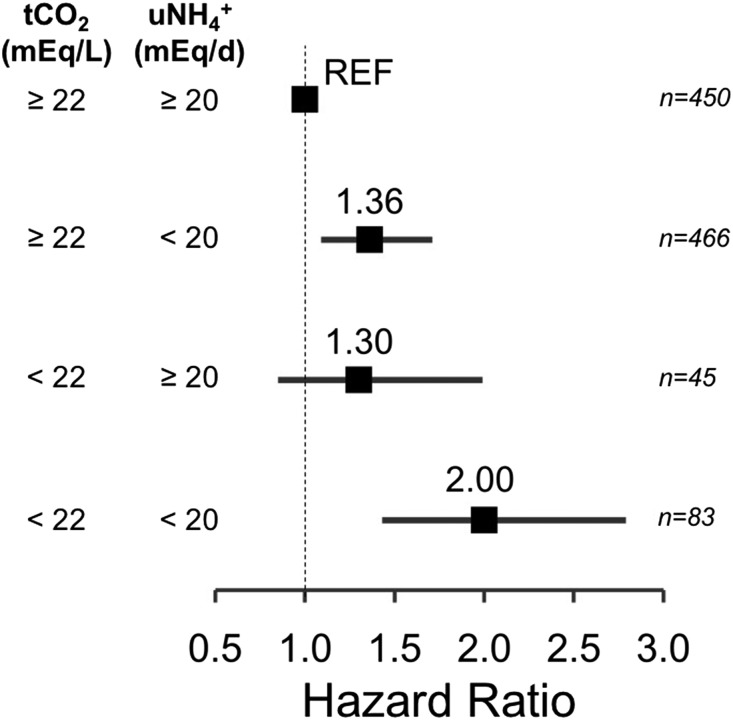
Figure 5 shows the adjusted Cox model results in which participants were categorized into one of four groups according to serum tCO2 and daily uNH4+ excretion: (1) tCO2<22 mEq/L and uNH4+ excretion <20 mEq/d, (2) tCO2<22 mEq/L and uNH4+ excretion ≥20 mEq/d, (3) tCO2≥22 mEq/L and uNH4+ excretion <20 mEq/d, or (4) tCO2≥22 mEq/L and uNH4+ excretion ≥20 mEq/d. Compared with the reference group (tCO2≥22 mEq/L and uNH4+ excretion ≥20 mEq/d), those with uNH4+ excretion <20 mEq/d and tCO2≥22 mEq/L had 36% higher risk of the composite outcome. Those with uNH4+ excretion ≥20 mEq/d and tCO2<22 mEq/L had 30% higher risk of the composite outcome, although statistical significance was not observed. Finally, those with uNH4+ excretion <20 mEq/d and tCO2<22 mEq/L had two-fold higher risk of the composite outcome. The results were similar after adjusting for model 2 variables (data not shown). There was no additive (relative excess risk from interaction = 0.34; 95% CI, −0.41 to 1.09; P=0.38) or multiplicative interaction (ratio of HRs =1.13; 95% CI, 0.68 to 1.87; P=0.64) between tCO2 and uNH4+ with the death or ESRD composite outcome.
Figure 5.
Low uNH4+ predicts death or ESRD in participants without acidosis. Shown are HRs with 95% CIs of the composite outcome of death or ESRD according to uNH4+ excretion and serum tCO2. Adjusted for age, sex, randomized group, mGFR, proteinuria, NEAP, and serum potassium. Stratified by BMI. REF, reference group.
To evaluate the relationship between uNH4+ and later development of acidosis, we determined the odds of acidosis at the 1-year follow-up visit by uNH4+ tertile among participants without acidosis at baseline. Of the 1044 participants in the main analysis, 916 did not have acidosis at baseline. Of these, 866 participants had serum tCO2 measured at 1 year; 287, 282, and 297 in the lowest, middle, and highest tertiles of uNH4+ at baseline, respectively. At 1 year, 87 (10%) of these participants developed acidosis; 50 in the lowest, 22 in the middle, and 15 in the highest tertile of baseline uNH4+. After adjusting for randomized treatment assignment and baseline characteristics, those in the lowest tertile of uNH4+ had significantly higher odds of acidosis than those in the highest tertile of uNH4+ at 1 year (Table 6).
Table 6.
Odds of acidosis at 1 year by baseline uNH4+ tertiles among participants without acidosis at baseline
| uNH4+, mEq/d | Odds Ratio of Acidosis | 95% CI |
|---|---|---|
| Tertile 1 | 2.56 | 1.04 to 6.27 |
| Tertile 2 | 1.21 | 0.47 to 3.09 |
| Tertile 3 | Ref | Ref |
Adjusted for age, sex, randomized group, mGFR, proteinuria, NEAP, serum potassium, and BMI. Ref, reference group.
Discussion
Renal ammonia (NH3) production and consequent uNH4+ excretion are critically important processes that help maintain acid–base homeostasis. The decline in uNH4+ excretion that accompanies reduced GFR is a key contributing factor to the development of metabolic acidosis in CKD, which is associated with poor outcomes in this population. Since reduced uNH4+ excretion precedes the development of metabolic acidosis in this setting, measurements of uNH4+ excretion could be an indicator of risk, and perhaps a better indicator of risk, than tCO2. In this study of black patients with hypertensive CKD, we identified an association between lower daily uNH4+ excretion and higher risk of death or ESRD independent of demographic factors, CKD severity factors, tCO2, and nutritional indicators of acid and alkali intake. The findings were similar when uNH4+ excretion was indexed to body weight. Analyses of the individual outcomes found a relationship between uNH4+ excretion and ESRD but not mortality, perhaps because of the greater number of ESRD events than death before ESRD events in the AASK cohort. Our results are consistent with those of a previous study that observed a higher risk of ESRD with lower uNH4+ excretion.8 Here, we show that this relationship is independent of serum tCO2 and nutritional determinants of acid–base status. In addition, we show that among individuals without acidosis, uNH4+ excretion <20 mEq/d is associated with higher risk of death or ESRD (36%) than daily uNH4+ excretion ≥20 mEq/d. Finally, we have quantitated the strength of the relationship between low uNH4+ excretion and the risk of subsequent acidosis for the first time. Cumulatively, these data suggest that low daily uNH4+ excretion has a prognostic value in CKD in terms of acidosis risk, as well as long-term clinical outcomes, irrespective of systemic tCO2 and other factors.
As expected, uNH4+ directly correlated with mGFR and inversely correlated with serum potassium after multivariate adjustment. Obese participants had higher uNH4+ excretion than nonobese participants, perhaps because of dietary factors or higher NEAP, as did men and those with proteinuria. Although renal acid excretion is thought to decline with aging, there was a trend toward higher uNH4+ excretion in older AASK participants. Daily uNH4+ also directly correlated with protein intake and NEAP after multivariate adjustment. Hence, it is plausible that the reason why low uNH4+ is associated with poor outcomes in CKD is because of poor nutritional status. However, we adjusted for nutritional factors including NEAP and BMI in our analyses. Furthermore, if low uNH4+ were the result of low acid intake from poor nutritional status, then it would be expected that uNH4+ excretion would match dietary acid intake and acid–base balance would be relatively stable over time. However, those in the lowest uNH4+ tertile had >2.5-fold higher odds of subsequent acidosis at 1 year compared with those in the highest tertile of uNH4+ excretion. Hence, it is likely that participants with low uNH4+ had impaired renal acid excretion rather than poor nutritional status, with consequent acid retention, higher risk of subsequent acidosis, and poor clinical outcomes. Nevertheless, the associations between uNH4+ excretion and the clinical outcomes in this cohort of black patients with hypertensive CKD were independent of these important confounders.
The results of this study may have important implications for research and perhaps clinical practice. Clinical practice guidelines recommend correcting acidosis with chronic oral alkali to prevent bone demineralization and protein catabolism, and results from single-center studies also suggest that correcting acidosis may slow CKD progression.9–11 Hence, patients with CKD and acidosis are commonly prescribed oral alkali. However, several lines of evidence from animal models of CKD and observational studies in humans suggest that alkaline therapy may be beneficial in patients with CKD without acidosis.3,5,12 This notion is reinforced by findings from a single-center interventional study in patients with hypertensive stage 2 CKD and mean tCO2 in the normal range (approximately 26 mEq/L).13 In this study, individuals who received oral sodium bicarbonate had better renal function than a placebo group after 5 years, despite having similar GFR at baseline. Thus, oral alkaline therapy is a promising intervention in patients with CKD and normal tCO2. However, the vast majority of patients with CKD have normal tCO2, and administering alkali to these individuals may lead to overtreatment on a population level.1,2 The results from this and the NephroTest cohort suggest that uNH4+ could be used to risk-stratify individuals with CKD without acidosis who might be more likely to benefit from alkali. Our results suggest that an uNH4+ excretion threshold <20 mEq/d could be used to risk-stratify individuals.
One of the mechanisms by which acidosis and subclinical acidosis is thought to contribute to GFR decline in CKD is via NH3-mediated activation of the alternative pathway of complement, ultimately leading to tubulointerstitial fibrosis.14,15 Thus, it might be expected that higher uNH4+ excretion would predict CKD progression. However, we found an association between lower uNH4+ excretion and higher risk of poor outcomes, particularly as it relates to renal function. Although these findings are seemingly contradictory to the hypothesis that NH3 is a direct cause of renal injury, it is important to note that uNH4+ excretion may not necessarily reflect tissue levels of NH3. For instance, low uNH4+ excretion because of impaired NH3/NH4+ transport could lead to high interstitial concentrations of NH3/NH4+ and consequent intrarenal complement activation. It is more likely that low uNH4+ excretion is simply an indicator of poor acid excretory capacity and kidney function. Thus, the results of this study do not necessarily contradict the notion that intrarenal NH3 contributes to renal injury in CKD.
Our study has several strengths. The AASK Study has a well characterized cohort with long-term follow-up and careful data collection. Furthermore, >95% of randomized participants were included in the main analyses. Second, GFR was measured by 125iodine-iothalamate clearance in the AASK Study, which is important considering the strong relationship between renal function and uNH4+ excretion. Third, carefully collected 24-hour urine samples were required before randomization. Notably, the median daily uNH4+ excretion in the AASK cohort was 19.5 mEq, which is similar to the median daily value in the NephroTest cohort (18.5 mEq).8 Like all observational studies, residual confounding remains possible. uNH4+ was measured once at baseline, hence we were not able to assess variability of uNH4+ excretion over time in this study. We evaluated black patients with hypertensive CKD, hence our findings may not be generalizable to other populations.
In summary, lower uNH4+ excretion is a risk factor for acidosis and poor renal and survival outcomes among blacks with hypertensive CKD irrespective of important confounders, tCO2, and nutritional determinants of acid–base status. Similar findings were observed in another CKD cohort.8 Hence, uNH4+ excretion has prognostic value above and beyond tCO2. With accumulating interest in the potential use of alkaline therapy to slow CKD progression in patients without overt acidosis, the observation that lower daily uNH4+ excretion in this setting signals a higher risk of death or ESRD suggests that uNH4+ may better risk-stratify these individuals.
Concise Methods
Study Participants
The details of the AASK Study have been published.16–18 Briefly, black patients aged 18–70 years with hypertensive CKD (defined by an mGFR between 20 and 65 ml/min per 1.73 m2 by renal clearance of 125iodine-iothalamate and diastolic BP >95 mmHg) were eligible for the study. Key exclusion criteria included elevated fasting or random blood glucose, treatment for diabetes, and urine protein-to-creatinine ratio >2.5 g/g. Between April of 1995 and September of 1998, 1094 participants were randomized to ramipril, metoprolol, or amlodipine, and to one of two BP goals (usual mean arterial pressure goal of 102–107 mmHg or a low mean arterial pressure goal of ≤92 mmHg). At the end of the randomized trial phase, participants who did not reach ESRD were eligible to enroll in the nonrandomized AASK Cohort Study, during which BP was targeted to <130/80 mmHg using ramipril or an angiotensin receptor blocker as the first-line therapy in all participants. Baseline (prerandomization) urine samples were available for 1057 participants. We excluded 13 participants with daily uNH4+ excretion normalized by body wt≥0.7 mEq/kg in the main analyses out of concern for falsely elevated uNH4+ excretion because of bacterial overgrowth (these individuals were included in a sensitivity analysis). Hence, 1044 participants were evaluated, corresponding to 99% of participants with available samples and 95% of randomized participants. The AASK Study was overseen by institutional review boards of the participating sites and was performed under the principles embodied in the Declaration of Helsinki.
Measurements
Using standardized forms, trained personnel obtained data on baseline demographic, clinical, and laboratory data. uNH4+ was measured by the glutamate dehydrogenase method from aliquots of the baseline 24-hour urine collection; daily uNH4+ excretion was determined from 24-hour urine volumes. The 24-hour urine samples were confirmed to have been collected according to the AASK Study protocol and were necessary before randomization. Serum tCO2 was measured using either the kinetic ultraviolet method (Roche Hitachi 747 autoanalyzer; Roche, Indianapolis, IN) or a carbon dioxide electrode (Beckman CX3 Delta autoanalyzer; Beckman, Brea, CA). Urine protein excretion was expressed as protein-to-creatinine ratio from the 24-hour urine collection. Daily dietary protein intake (grams per day) was calculated from 24-hour urine urea nitrogen excretion using the following equation: 6.25×[urine urea nitrogen+(weight×0.031)].19 NEAP was calculated using the following formula: –10.2+(54.5×protein intake in grams per day)/urine potassium in mEq/d.20
Statistical Analyses
Participants were categorized by tertiles of daily uNH4+ excretion. Continuous variables are presented as means with SDs, unless otherwise specified. Categorical variables are presented as percentages. Significance tests were performed using analysis of variance for continuous variables and chi-squared tests for dichotomous variables.
Multivariate linear regression models examined the cross-sectional relationship between daily uNH4+ excretion and key clinical features at baseline. The longitudinal outcome of interest was the composite of death or ESRD, which were adjudicated by the outcomes committee. A series of Cox regression models were fit to relate the composite outcome of death or ESRD to daily uNH4+ excretion using the highest tertile of uNH4+ as the reference. Follow-up time was censored at the administrative end date, permanent loss-to-follow-up, or if the participant did not enroll in the cohort phase. The initial model was unadjusted, followed by adjustment for age, sex, randomized group, mGFR, proteinuria, NEAP, serum potassium, and serum tCO2 (model 1). This model was considered the main model because it included key demographic characteristics and biologically important potential confounders of the relationship between uNH4+ and the outcomes. A subsequent exploratory model additionally adjusted for smoking status, systolic BP, heart disease, and use of angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and diuretics (model 2). Similar Cox models evaluated the association between daily uNH4+ with death and ESRD separately. Proportional hazards assumptions were evaluated using a formal significance test on the basis of the unscaled and scaled Schoenfeld residuals and a graphical assessment of log-log survival curves. BMI violated the proportional hazards assumption and was included as a stratification variable (<25, 25–<30, and ≥30 kg/m2) in the adjusted models. Interaction of the relationship between the composite of death or ESRD and daily uNH4+ excretion by (1) mGFR (tertiles), (2) NEAP (tertiles), (3) dietary protein intake (tertiles), and (4) serum tCO2 (<22, 22–28, and >28 mEq/L) was investigated by including a multiplicative interaction term in the main model. A sensitivity analysis included the 13 individuals who were excluded from the main analyses. Another sensitivity analysis evaluated the hazards of the composite ESRD or death outcome using daily uNH4+ excretion indexed to body weight (uNH4+/kg per day) as the predictor variable; the highest tertile of uNH4+/kg per day was the reference. Both sensitivity analyses were adjusted for variables in the main model (model 1). A cubic spline regression analysis adjusted for the main model variables was performed using daily uNH4+ excretion as the predictor variable. Knots were placed at quartiles of daily uNH4+ excretion and the median value (19.5 mEq) was the reference point.
An additional Cox model adjusted for main model variables was performed by grouping participants into one of four categories, according to whether daily uNH4+ excretion was <20 mEq or ≥20 mEq and whether serum tCO2 was <22 or ≥22 mEq/L. Those with daily uNH4+ excretion ≥20 mEq and serum tCO2 ≥22 mEq/L served as the reference. Additive interaction was evaluated by determining the relative excess risk from interaction with 95% CIs, and multiplicative interaction was evaluated by determining the ratio of HRs with 95% CIs.21,22 Logistic regression analyses (adjusted for demographic factors, randomized treatment assignment, and baseline mGFR, proteinuria, NEAP, serum potassium, and BMI) determined the odds of acidosis at 1 year among participants without acidosis at baseline by baseline uNH4+, using the highest tertile of uNH4+ as the reference group.
The analyses were performed using Stata 14 (StataCorp, College Station, TX).
Disclosures
None.
Acknowledgments
K.L.R. receives support from the US Department of Veterans Affairs Clinical Sciences Research and Development Service Career Development Award (IK2 CX000537), Robert Wood Johnson Foundation (Harold Amos Medical Faculty Development Award), and National Institutes of Diabetes, Digestive, and Kidney Disease (grant no. 1U01DK099933).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Urine Ammonium and Preclinical Acidosis in CKD,” on pages 2258–2260.
References
- 1.Moranne O, Froissart M, Rossert J, Gauci C, Boffa JJ, Haymann JP, M’rad MB, Jacquot C, Houillier P, Stengel B, Fouqueray B; NephroTest Study Group : Timing of onset of CKD-related metabolic complications. J Am Soc Nephrol 20: 164–171, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raphael KL, Zhang Y, Ying J, Greene T: Prevalence of and risk factors for reduced serum bicarbonate in chronic kidney disease. Nephrology (Carlton) 19: 648–654, 2014 [DOI] [PubMed] [Google Scholar]
- 3.Kovesdy CP, Anderson JE, Kalantar-Zadeh K: Association of serum bicarbonate levels with mortality in patients with non-dialysis-dependent CKD. Nephrol Dial Transplant 24: 1232–1237, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Menon V, Tighiouart H, Vaughn NS, Beck GJ, Kusek JW, Collins AJ, Greene T, Sarnak MJ: Serum bicarbonate and long-term outcomes in CKD. Am J Kidney Dis 56: 907–914, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Raphael KL, Wei G, Baird BC, Greene T, Beddhu S: Higher serum bicarbonate levels within the normal range are associated with better survival and renal outcomes in African Americans. Kidney Int 79: 356–362, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raphael KL, Zhang Y, Wei G, Greene T, Cheung AK, Beddhu S: Serum bicarbonate and mortality in adults in NHANES III. Nephrol Dial Transplant 28: 1207–1213, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah SN, Abramowitz M, Hostetter TH, Melamed ML: Serum bicarbonate levels and the progression of kidney disease: A cohort study. Am J Kidney Dis 54: 270–277, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vallet M, Metzger M, Haymann JP, Flamant M, Gauci C, Thervet E, Boffa JJ, Vrtovsnik F, Froissart M, Stengel B, Houillier P; NephroTest Cohort Study group : Urinary ammonia and long-term outcomes in chronic kidney disease. Kidney Int 88: 137–145, 2015 [DOI] [PubMed] [Google Scholar]
- 9.National Kidney Foundation : K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 42[Suppl 3]: S1–S201, 2003 [PubMed] [Google Scholar]
- 10.de Brito-Ashurst I, Varagunam M, Raftery MJ, Yaqoob MM: Bicarbonate supplementation slows progression of CKD and improves nutritional status. J Am Soc Nephrol 20: 2075–2084, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phisitkul S, Khanna A, Simoni J, Broglio K, Sheather S, Rajab MH, Wesson DE: Amelioration of metabolic acidosis in patients with low GFR reduced kidney endothelin production and kidney injury, and better preserved GFR. Kidney Int 77: 617–623, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Kanda E, Ai M, Yoshida M, Kuriyama R, Shiigai T: High serum bicarbonate level within the normal range prevents the progression of chronic kidney disease in elderly chronic kidney disease patients. BMC Nephrol 14: 4, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahajan A, Simoni J, Sheather SJ, Broglio KR, Rajab MH, Wesson DE: Daily oral sodium bicarbonate preserves glomerular filtration rate by slowing its decline in early hypertensive nephropathy. Kidney Int 78: 303–309, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Nath KA, Hostetter MK, Hostetter TH: Pathophysiology of chronic tubulo-interstitial disease in rats. Interactions of dietary acid load, ammonia, and complement component C3. J Clin Invest 76: 667–675, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tolins JP, Hostetter MK, Hostetter TH: Hypokalemic nephropathy in the rat. Role of ammonia in chronic tubular injury. J Clin Invest 79: 1447–1458, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Appel LJ, Middleton J, Miller ER 3rd , Lipkowitz M, Norris K, Agodoa LY, Bakris G, Douglas JG, Charleston J, Gassman J, Greene T, Jamerson K, Kusek JW, Lewis JA, Phillips RA, Rostand SG, Wright JT: The rationale and design of the AASK cohort study. J Am Soc Nephrol 14[Suppl 2]: S166–S172, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Appel LJ, Wright JT Jr, Greene T, Kusek JW, Lewis JB, Wang X, Lipkowitz MS, Norris KC, Bakris GL, Rahman M, Contreras G, Rostand SG, Kopple JD, Gabbai FB, Schulman GI, Gassman JJ, Charleston J, Agodoa LY; African American Study of Kidney Disease and Hypertension Collaborative Research Group : Long-term effects of renin-angiotensin system-blocking therapy and a low blood pressure goal on progression of hypertensive chronic kidney disease in African Americans. Arch Intern Med 168: 832–839, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wright JT Jr, Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, Cheek D, Douglas-Baltimore JG, Gassman J, Glassock R, Hebert L, Jamerson K, Lewis J, Phillips RA, Toto RD, Middleton JP, Rostand SG; African American Study of Kidney Disease and Hypertension Study Group : Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: Results from the AASK trial. JAMA 288: 2421–2431, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Maroni BJ, Steinman TI, Mitch WE: A method for estimating nitrogen intake of patients with chronic renal failure. Kidney Int 27: 58–65, 1985 [DOI] [PubMed] [Google Scholar]
- 20.Frassetto LA, Todd KM, Morris RC Jr, Sebastian A: Estimation of net endogenous noncarbonic acid production in humans from diet potassium and protein contents. Am J Clin Nutr 68: 576–583, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Knol MJ, VanderWeele TJ: Recommendations for presenting analyses of effect modification and interaction. Int J Epidemiol 41: 514–520, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li R, Chambless L: Test for additive interaction in proportional hazards models. Ann Epidemiol 17: 227–236, 2007 [DOI] [PubMed] [Google Scholar]