Temporal organization of biological processes is a critical feature of life. In addition to orderly, carefully timed developmental processes, precise temporal organization is also observed on a daily time scale. On Earth, organisms ranging from bacteria to humans have evolved internal timekeeping mechanisms called circadian clocks that control biochemistry, physiology, and behavior to coordinate with the 24-hour day (1–3). Circadian clocks have long been known to drive rhythms in such things as activity, sleep, and hormone secretion, but in recent years it has become clear that these clocks influence the normal homeostasis of organisms on a profound scale, controlling processes as diverse as cell cycle regulation, cell signaling, metabolic events, and cognitive function. Disruption of clock function in mammals results in abnormalities in many physiological functions, resulting in increases in risks for cardiovascular and gastrointestinal diseases, sleep abnormalities, and cancer (reviewed in refs. 1 and 4). In a recent issue of PNAS, Gorbacheva et al. (5) describe a mechanistic link between the circadian clock and sensitivity to the chemotherapeutic agent cyclophosphamide (CY). Although several prior studies have implicated time of day as an important indicator of the morbidity of some cancer treatments, these authors go beyond the vague idea that clocks somehow affect susceptibility to these drugs. Of particular significance is their observation that sensitivity is not dictated by rhythmicity or arrhythmicity per se but instead depends on the specific molecular state of the circadian clock. This study provides a clear mechanistic link between the clock and tolerance of an anticancer drug.
Connections between circadian clocks and disease are numerous (reviewed in ref. 1). Many studies have shown increased incidence of disease with disruption of normal clock function by tissue lesions (removal of the central clock in the suprachiasmatic nucleus or removal of the pineal gland, which is the tissue responsible for rhythmic melatonin synthesis) or through altered light/dark cycles, such as those experienced by shift workers. More recently, genetic lesions, such as the targeted disruption of the Period 2 gene, which is a critical component of the circadian clock, have also shown a propensity for increased risk of tumors in mice (6). Perhaps these effects on a wide range of processes should not be surprising, because microarray analyses have revealed that 3–10% of all transcripts are under circadian control, although the specific rhythmic mRNAs vary in a tissue-specific manner (reviewed in ref. 2). The rhythmic mRNA classes suggest that the circadian clock impinges on nearly all major cellular regulatory pathways, including many cell-signaling pathways, apoptotic signaling, metabolic processes, detoxification mechanisms, and cell cycle control. Many of these rhythmic genes are directly related to regulatory mechanisms that are important in the control or treatment of cancer.
Sensitivity to chemotherapeutic agents depends on the specific molecular state of the circadian clock.
Not only is it known that altered rhythmicity can exacerbate cancer, but it is also known that many chemotherapeutic agents have drastically different levels of efficacy when given at different phases in the circadian cycle. This disparity in efficacy is due to differences in the susceptibility of the tumor to the drug and to the level of tolerance of the host. The goal of circadian-modulated chemotherapy or “chronotherapy” is to find times for drug delivery that result in low toxicity to the host and high toxicity to the cancer cell. Circadian clocks are present in many tissues, and cells of the body and tumors often maintain robust circadian rhythms. The chronotherapy approach capitalizes on asynchronies in cell division and drug metabolic rhythms between the normal and cancerous cells. This approach has been used with good effect to treat several animal cancer models and has recently been extended to patients with advanced stage cancers, including gastrointestinal cancer and colorectal cancer with unresectable liver metastases (reviewed in refs. 4 and 7).
Despite these advances, the mechanism involved in circadian sensitivity to chemotherapeutic agents is still obscure. Because of the clock's involvement in so many important regulatory processes, it is difficult to know whether sensitivity to the drug is a specific function of the clock mechanism or is instead some more general “downstream” feature of the rhythmic physiology of the organism. The paper by Gorbacheva et al. (5) begins to investigate these mechanisms through the use of genetically altered mice with well characterized defects in their clocks' molecular machinery.
The mammalian circadian oscillator is an intracellular mechanism composed of a set of interlocking transcription/translation feedback loops that complete one cycle each day (reviewed in refs. 2 and 3). The Period genes (Per1 and Per2) and Cryptochrome genes (Cry1 and Cry2) are at the center of the core negative feedback loop, which is required for a functional clock. These genes are transcriptionally activated by the basic helix–loop–helix PAS transcription factors CLOCK and BMAL1, which heterodimerize and bind to E-box enhancer elements in the promoters of these genes. The Per and Cry mRNAs then give rise to PER and CRY proteins that, along with Casein Kinase Iε (CKIε), interact to form a repression complex that translocates back into the nucleus, interact directly with CLOCK and BMAL1, and result in loss of their activation activity (Fig. 1B).
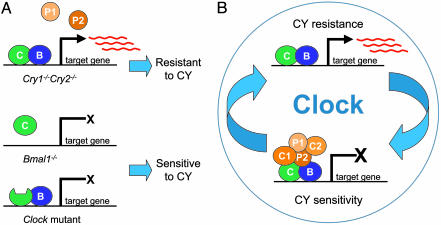
Fig. 1.
The sensitivity to CY depends on the molecular state of the circadian oscillator mechanism. (A) Mice lacking both Cry genes cannot form a functional repressive complex, and the CLOCK/BMAL1 heterodimer remains in a constitutively active state, with high levels of expression of their target mRNAs. In contrast, mice with disruption of the Bmal1 gene or with a mutation in the Clock gene are not capable of activating the CLOCK/BMAL1 target genes, therefore locking the system in an inactivated state. Both classes of mutations show arrhythmic behavioral phenotypes but drastically different sensitivities to CY. (B) These results suggest that the normal rhythmicity in sensitivity to CY is driven by the rhythmic activity of the CLOCK/BMAL1 heterodimer and that the mechanism for CY resistance may be controlled by the expression of the CLOCK/BMAL1 target genes. Red lines represent mRNA. C1, CRY1; C2, CRY2; C, CLOCK; B, BMAL1; P1, PER1; P2, PER2.
Although mutations in any of these core clock components in mice result in disruption of normal circadian rhythmicity, the “state” of the molecular oscillator varies between the different mutations. The mutant mouse strains used in the Gorbacheva et al. (5) paper are an excellent example of how the clock can be locked into different molecular states (Fig. 1 A). The Cry1-/-Cry2-/- double knockout genotype results in lack of a functional repressive complex; therefore, CLOCK and BMAL1 are in a constitutively active state, transcribing high levels of target genes at all times of the circadian cycle (8–10). In contrast, mice with a targeted disruption of the Bmal1 gene (Bmal1-/-) (11) or mice with the Clock/Clock mutant (12–14), both of which are also behaviorally arrhythmic, express constitutively low levels of the CLOCK/BMAL1 target genes because the heterodimeric activator cannot form (Bmal1-/-) or is not active (Clock/Clock).
Gorbacheva et al. (5) report that normal mice show a robust rhythm in sensitivity to CY, with mortality and morbidity rates peaking when the drug is given during the late night/early morning. The animals showed increased resistance to the drug when it was given in the late afternoon/early evening, a time when the level of CLOCK/BMAL1 transcriptional activation is high in most peripheral tissues. Interestingly, the arrhythmic mutants all showed loss of rhythms in CY sensitivity but with very different profiles. The Bmal1-/- and Clock/Clock mutants showed high levels of sensitivity to CY at all circadian times, whereas the Cry1-/-Cry2-/- mice showed constitutively high resistance to the drug. Another surprise from this work is the finding that these rhythms of sensitivity are not caused by rhythmic metabolism of CY but instead result from CLOCK/BMAL1-mediated changes in B lymphocyte survival or recovery after the CY challenge.
These findings are important for several reasons. First, the observation that circadian control of sensitivity to chemotherapeutics depends not just on rhythmicity but on the activation state of CLOCK/BMAL1 provides an inroad to future studies on the mechanisms involved. Perhaps the B cell survival factors are direct targets of CLOCK/BMAL1. Many direct targets are already known, and this question can easily be explored. In addition, drug exposure to patients could be timed to coincide with times when CLOCK and BMAL1 are maximally active, as has been done with other chemotherapeutic agents. Second, as the authors point out, if the sensitivity of these animals to CY is dictated by B cell survival, then perhaps the tolerance of patients to such drugs can be improved by treatments that enhance the survival of this cell class. Third, why is B cell survival after CY administration under circadian control? Understanding the influence of the circadian clock on the regulation of these important immune cells could provide insight into yet another important level of circadian control.
Perhaps the most important aspect of the work by Gorbacheva et al. (5) is that the delineation of mechanisms yield confidence and better-tailored treatments. The strategies and tools used in this paper can be applied to other drugs used in chronomedicine and other circadian manipulations that seem to influence cancer and other diseases. One hopes that exploitation of such daily temporal changes in physiology may soon become a common weapon in the clinician's arsenal.
See companion article on page 3407 in issue 9 of volume 102.
References
- 1.Hastings, M. H., Reddy, A. B. & Maywood, E. S. (2003) Nat. Rev. Neurosci. 4, 649-661. [DOI] [PubMed] [Google Scholar]
- 2.Lowrey, P. L. & Takahashi, J. S. (2004) Annu. Rev. Genomics Hum. Genet. 5, 407-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reppert, S. M. & Weaver, D. R. (2002) Nature 418, 935-941. [DOI] [PubMed] [Google Scholar]
- 4.Fu, L. & Lee, C. C. (2003) Nat. Rev. Cancer 3, 350-361. [DOI] [PubMed] [Google Scholar]
- 5.Gorbacheva, V. Y., Kondratov, R. V., Zhang, R., Cherukuri, S., Gudkov, A. V., Takahashi, J. S. & Antoch, M. P. (2005) Proc. Natl. Acad. Sci. USA 102, 3407-3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fu, L., Pelicano, H., Liu, J., Huang, P. & Lee, C. (2002) Cell 111, 41-50. [DOI] [PubMed] [Google Scholar]
- 7.Mormont, M. C. & Levi, F. (2003) Cancer 97, 155-169. [DOI] [PubMed] [Google Scholar]
- 8.Vitaterna, M. H., Selby, C. P., Todo, T., Niwa, H., Thompson, C., Fruechte, E. M., Hitomi, K., Thresher, R. J., Ishikawa, T., Miyazaki, J., et al. (1999) Proc. Natl. Acad. Sci. USA 96, 12114-12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okamura, H., Miyake, S., Sumi, Y., Yamaguchi, S., Yasui, A., Muijtjens, M., Hoeijmakers, J. H. & van der Horst, G. T. (1999) Science 286, 2531-2534. [DOI] [PubMed] [Google Scholar]
- 10.van der Horst, G. T., Muijtjens, M., Kobayashi, K., Takano, R., Kanno, S., Takao, M., de Wit, J., Verkerk, A., Eker, A. P., van Leenen, D., et al. (1999) Nature 398, 627-630. [DOI] [PubMed] [Google Scholar]
- 11.Bunger, M. K., Wilsbacher, L. D., Moran, S. M., Clendenin, C., Radcliffe, L. A., Hogenesch, J. B., Simon, M. C., Takahashi, J. S. & Bradfield, C. A. (2000) Cell 103, 1009-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vitaterna, M. H., King, D. P., Chang, A.-M., Kornhauser, J. M., Lowrey, P. L., McDonald, J. D., Dove, W. F., Pinto, L. H., Turek, F. W. & Takahashi, J. S. (1994) Science 264, 719-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gekakis, N., Staknis, D., Nguyen, H. B., Davis, F. C., Wilsbacher, L. D., King, D. P., Takahashi, J. S. & Weitz, C. J. (1998) Science 280, 1564-1569. [DOI] [PubMed] [Google Scholar]
- 14.King, D. P., Zhao, Y., Sangoram, A. M., Wilsbacher, L. D., Tanaka, M., Antoch, M. P., Steeves, T. D., Vitaterna, M. H., Kornhauser, J. M., Lowrey, P. L., et al. (1997) Cell 89, 641-653. [DOI] [PMC free article] [PubMed] [Google Scholar]