Abstract
Significant advances in understanding the pathogenesis of GN have occurred in recent decades. Among those advances is the finding that both innate and adaptive immune cells contribute to the development of GN. Neutrophils were recognized as key contributors in early animal models of GN, at a time when the prevailing view considered neutrophils to function as nonspecific effector cells that die quickly after performing antimicrobial functions. However, advances over the past two decades have shown that neutrophil functions are more complex and sophisticated. Specifically, research has revealed that neutrophil survival is regulated by the inflammatory milieu and that neutrophils demonstrate plasticity, mediate microbial killing through previously unrecognized mechanisms, demonstrate transcriptional activity leading to the release of cytokines and chemokines, interact with and regulate cells of the innate and adaptive immune systems, and contribute to the resolution of inflammation. Therefore, neutrophil participation in glomerular diseases deserves re-evaluation. In this review, we describe advances in understanding classic neutrophil functions, review the expanded roles of neutrophils in innate and adaptive immune responses, and summarize current knowledge of neutrophil contributions to GN.
Keywords: glomerulonephritis, immunology, glomerular disease, neutrophil
Despite advances in understanding the pathogenesis of various forms of GN, therapy has not advanced past nonspecific inhibition of immune and inflammatory responses. Those therapies induce complete or partial remissions in 50% or fewer patients, and they are associated with significant side effects. A more complete understanding of the effectors of glomerular injury will identify targets for new therapies. Early studies using animal models of immune complex and anti–glomerular basement membrane (GBM) GN recognized neutrophils as a necessary cellular component of glomerular injury.1 At the time those studies were performed, the prevailing view was that neutrophils were short-lived, terminally differentiated cells serving as nonspecific effectors; were present briefly during the acute phase of inflammation; and possessed little or no synthetic capability. Consequently, the majority of the nephrology research community shifted focus to the contribution of B cells, monocytes/macrophages, T cells, dendritic cells, and intrinsic glomerular cells.2,3 The past two decades have witnessed the discovery of exciting new information about neutrophils, indicating that survival is regulated by the external milieu, expanded mechanisms for microbial killing exist, a number of cytokines and chemokines are expressed and released, cells of both innate and adaptive immune systems interact with and are activated by neutrophils, and lipids that promote resolution of inflammation are produced.4–6 In light of those expanded capabilities, we believe the participation of neutrophils in the development of various forms of GN deserves re-evaluation. In this review, we summarize new findings related to classic neutrophil functions, describe expanded functional activities in innate and adaptive immune responses, and review current knowledge of neutrophil participation in GN. Those expanded functional capabilities suggest that neutrophils may be more important contributors to various forms of GN, and identifying those contributions may provide new therapeutic targets.
New Understanding of Classic Neutrophil Functions and Their Role in GN
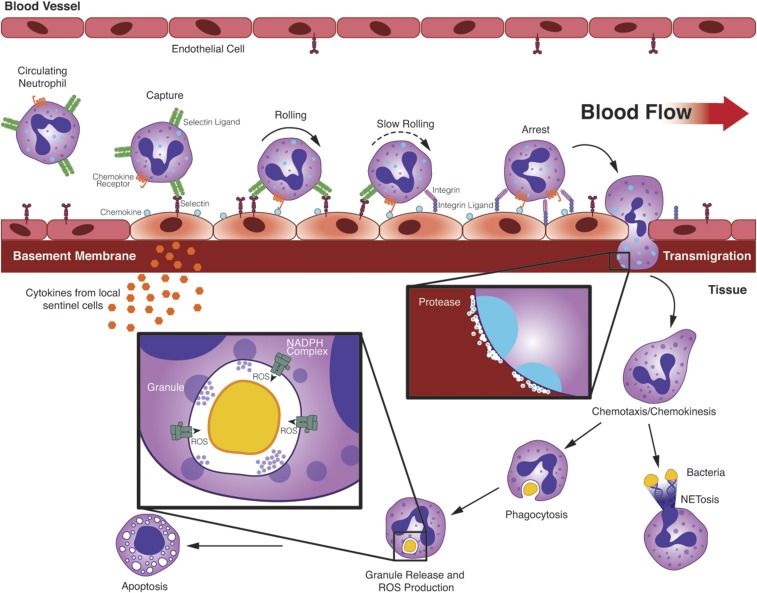
Figure 1 illustrates the series of coordinated events leading to neutrophil elimination of bacteria at sites of infection, including adhesion to activated vascular endothelial cells, transmigration across the vascular wall, chemotaxis to the site of infection, phagocytosis of bacteria, and generation and release of chemicals that kill those organisms.7 To accomplish those tasks, neutrophils express >100 cell surface receptors to sense their microenvironment and regulate their cellular responses (Table 1). This section summarizes recent advances in understanding those coordinated events relevant to neutrophil participation in glomerular inflammation.
Figure 1.
The sequential steps of classic neutrophil recruitment from the vasculature to tissue in response to infection are shown. Sentinel cells are stimulated by PAMPs or DAMPs to release chemokines, cytokines, and lipid mediators that activate vascular endothelial cells to express selectins and chemokines. Neutrophils undergo capture and fast rolling through the interaction of selectins on endothelial cells with their ligands on neutrophils. Selectins and chemokines induce integrin-mediated slow rolling and neutrophil arrest. Neutrophils undergo integrin-mediated crawling to a site for transmigration. Transmigration at endothelial cell junctions is mediated by a number of additional adhesion molecules. Release of proteolytic enzymes allows neutrophils to enter the extravascular space. Extravascular neutrophils follow a chemoattractant gradient to the site of infection. Pathogens are attacked by phagocytosis and by release of NETs. Microbes within phagosomes are killed by a combination of granule contents and generation of ROS. Infiltrating neutrophils are eliminated by apoptosis.
Table 1.
Neutrophil cell surface receptors
| Neutrophil Cell Surface Receptors |
|---|
| G-protein coupled receptors |
| Chemoattractant receptors: FPR1 (FPR), FPR2 (FPRL1), FPR3 (FPRL2), BLT1 (LTB4R), BLT2 (LTB4R), PAFR, C5aR |
| Chemokine receptors: CXCR1 (human), CXCR2, CXCR4, CCR1, CCR2, CX3CR1 |
| Fc receptors |
| FcγRs: FcγRI, FcγRIIA (human), FcγRIIB (inhibitory), FcγRIII (mouse), FcγRIIIB (human), FcγRIV (mouse) |
| Fcα-receptors: FcαRI (human) |
| Fcε-receptors: FcεRI, FcεRII |
| Adhesion receptors |
| Selectins and selectin ligands: L-selectin, PSGL-1, ESL-1, CD44 |
| Integrins: LFA-1 (αLβ2), Mac-1 (αMβ2), VLA-4 (α4β1) |
| Cytokine receptors |
| Type I cytokine receptors: IL-4R, IL-6R, IL-12R, IL-15R, G-CSFR, GM-CSFR |
| Type II cytokine receptors: IFNAR, IFNGR, IL-10R |
| IL-1R family: IL-1RI, IL1RII, IL-18R |
| TNFR family: TNFR1 (p55), TNFR2 (p75), Fas, LTβR, RANK, TRAIL-R2, TRAIL-R3 |
| Innate immune receptors |
| TLRs: TLR1, TLR2, TLR4, TLR5, TLR6, TLR7 (?), TLR8, TLR9 |
| C-type lectins: Dectin-1, Mincle, MDL-1, Mcl, CLEC-2 |
| NOD-like receptors: NOD2, NLRP3 |
| RIG-like receptors: RIG-I, MDA5 |
| Adenosine receptors |
| A1, A2A, A2B, A3 |
| ITIM-bearing inhibitory receptors |
| CD300a, CD300f, CD200R, CEACAM1, CLEC12a, CLEC4a, LILRB2, LILRB3, PILRa, SIGLEC-5, SIGLEC-9, SIRL-1, SIRP-a |
Neutrophil Recruitment
Recruitment of circulating neutrophils is a multistep cascade triggered by the release of pathogen-associated molecular pattern molecules (PAMPs) during infection or damage-associated molecular pattern molecules (DAMPs) during tissue injury (Figure 1). PAMPs and DAMPs interact with pattern recognition receptors on the surface (Toll-like receptors [TLRs] and C-type lectin receptors) and in the cytoplasm (Nod-like receptors, RIG-I–like receptors, and TLR-7, -9, and -10) of sentinel cells, including macrophages and dendritic cells, causing them to release chemokines, cytokines (e.g., IL-1β and TNFα), and lipid mediators that initiate neutrophil recruitment.8,9 Those mediators induce vascular endothelial cells to increase surface expression of selectins and enhance access to selectins through changes in the thickness and composition of the glucosaminoglycan endothelial surface layer.10–12
Contact between neutrophils and activated endothelial cells in postcapillary venules leads to neutrophil capture and fast rolling through the interaction of E- and P-selectins on endothelial cells with P-selectin glycoprotein ligand–1 (PSGL-1), E-selectin ligand–1 (ESL-1), and CD44 on neutrophils.11,13,14 Rolling exposes neutrophils to chemokines immobilized on the endothelial cell surface.12 The combination of intracellular signals generated by selectins and chemokine receptors leads to a conformation change of the integrin, LFA-1 (CD11a/CD18), that results in an intermediate affinity for ICAM-1.14 The combination of interaction of LFA-1 with ICAM-1 and selectins with PSGL-1 results in slow rolling. Continued exposure to chemokine- and selectin-mediated signaling induces a high affinity conformation of LFA-1, resulting in neutrophil arrest. Additionally, those signals stimulate increased neutrophil surface expression of the integrin Mac-1 (CD11b/CD18) through granule exocytosis.15,16 After arrest, neutrophils undergo intraluminal crawling toward a site favorable for transmigration through the interaction of Mac-1 with ICAM-1.12,17 Transmigration through the endothelial cell layer occurs primarily at endothelial cell junctions and is mediated by a number of adhesion molecules, including MAC-1; platelet/endothelial cell adhesion molecule–1; junctional adhesion molecules –A, –B, and–C; and endothelial cell selective adhesion molecules, CD99 and CD99L2.12,14 Once through the endothelial cell layer, neutrophils use proteolytic enzymes, such as gelatinase, released from intracellular granules to traverse the vascular basement membrane and enter the extravascular space.18 Extravascular neutrophils are exposed to a sequential cascade of chemoattractants that stimulate chemotaxis and chemokinesis to the location of infection or inflammation.19 Long-distance recruitment is usually mediated by “intermediate target” chemoattractants, leukotriene B4 and IL-8, that abruptly switch to “end-target” chemoattractants, formylated peptides and C5a, at 100–150 µm from the source of inflammation.19,20 Neutrophils arrive at the site of inflammation in two waves. The first wave is initiated by recruitment, described above. In the second wave, commonly referred to as swarming, long-distance neutrophil recruitment is driven by LTB4 produced by neutrophils recruited in the first wave.21 This is one of many examples of amplification loops initiated by neutrophils.22
This description of neutrophil recruitment was derived from studies of the systemic vasculature and recruitment was typically induced by exposure to soluble inflammatory mediators. Alternative methods of neutrophil recruitment are used in the specialized vascular beds of glomerular capillaries (Figure 2).23–26 P-selectin on platelets adherent to endothelial cells can serve as a bridge between endothelial cells and neutrophils, a process termed “secondary capture.”11 Capture of circulating neutrophils by platelets has been described in murine anti-GBM GN.23,27 Immune complex deposition can lead to neutrophil capture through Fcγ receptor (FcγR) recognition of IgG whereas Mac-1 integrins maintain adhesion under flow conditions.24 The requirement of FcγR and MAC-1 expression for glomerular neutrophil recruitment and development of proteinuria was described in a murine model of anti-GBM GN.24,28,29 Passive transfer of sera from patients with SLE to CD11b/CD18-deficient mice expressing human FcγRIIA and FcγRIIIB on neutrophils showed that FcγRIIA mediates neutrophil accumulation and glomerular injury.30 The role of endothelial cell selectins in glomerular neutrophil recruitment remains unclear. Evidence for and against selectin-mediated neutrophil recruitment in anti-GBM GN has been published, and an inhibitory role has even been described.23,25,27,31,32
Figure 2.
Three alternative methods of glomerular neutrophil recruitment are shown. “Secondary capture” involves platelets serving as a bridge between endothelial cells and neutrophils. A second method of neutrophil capture occurs by direct interaction with immune complexes through FcγRs or complement receptors. A third method involves neutrophil retention by slowing their crawling along glomerular capillaries. Increased neutrophil retention requires direct interaction between monocytes and neutrophils, production of TNFα by patrolling monocytes, and integrin expression by neutrophils.
Multiphoton microscopy of hydronephrotic mouse kidneys showed that neutrophils and monocytes constantly patrol normal glomeruli by crawling along capillaries (Figure 2).26 Induction of anti-GBM GN increased neutrophil and monocyte retention within the glomerulus by slowing their travel along the capillary bed, but did not induce transmigration. Prolonged glomerular dwell time of both neutrophils and monocytes was also induced by administration of antibodies against myeloperoxidase (MPO) and by activated glomerular T cells, but not by noninflammatory podocyte injury.26 Increased neutrophil retention required direct interaction between monocytes and neutrophils, as well as production of TNFα by patrolling monocytes.33 Prolonged neutrophil transit time through inflamed glomeruli required Mac-1 expression, whereas prolonged monocyte transit time was dependent on LFA-1, Mac-1, and CX3CR1.26,33
The effects of these alternative methods of glomerular neutrophil recruitment on neutrophil responses, such as enhanced respiratory burst activity and granule exocytosis, transcriptional activation, and the rate of apoptosis, have not been determined. The discovery that neutrophils undergoing prolonged glomerular transit time demonstrated enhanced reactive oxygen species (ROS) generation and CXCL1 release, but did not produce TNFα, suggests that differences in some of those responses will be found.33
Complement Activation
Activation of the C cascade by immune complexes has long been identified as a mechanism for neutrophil recruitment and glomerular cell injury.28,34–37 An additional role of C activation in ANCA-associated vasculitis (AAV) has been described. C depletion or C5a receptor blockage was reported to protect anti-MPO–treated mice from developing necrotizing crescentic GN.38,39 ANCA-stimulated neutrophils were found to activate the alternative C pathway, leading to generation of C5a.40 C5a recruited additional neutrophils and primed those neutrophils for an enhanced respiratory burst and degranulation in response to ANCA.41,42 Patients with active AAV have high levels of C5a, and other C factors.43 A clinical trial to evaluate the safety and efficacy of an oral inhibitor of the C5a receptor (CCX168) in AAV was completed in 2016 (NCT01363388), but study results have yet to be posted (clinicaltrials.gov).
Mechanisms of Microbial Killing
ROS and antimicrobial granule components cooperate to kill invading microbes engulfed by neutrophils into phagosomes (Figure 1).44 Extracellular release of those microbial toxins leads to tissue injury. The molecular events leading to ROS generation by the neutrophil NADPH oxidase have been extensively studied.4 Signals generated by microbial invasion or tissue injury, including TLR agonists, chemokines, proinflammatory cytokines, and proinflammatory lipids enhance neutrophil production of ROS in response to activation signals, a process termed “priming.”45–48 In addition to enhanced ROS generation, primed neutrophils demonstrate enhanced chemotaxis, exocytosis, adhesion, and transcriptional activity in addition to delayed apoptosis. Neutrophil priming is postulated to play a critical role in development of GN in AAV.39,40,49 Priming by TNFα also enhances neutrophil recruitment by immune complexes.47,50
Evidence for induction of, and protection against, glomerular injury by ROS has been reported. Suzuki et al. reported that neutrophil interaction with glomerular immune complexes through FcγRs led to ROS-dependent NF-κB activation, TNFα overexpression, and further neutrophil recruitment.51 Feith et al. reported that albuminuria after 72 hours of the heterologous phase of anti-GBM nephritis was dependent on neutrophil ROS generation.52 Hypohalous acid, but not H2O2, was shown to increase albumin permeability of isolated rat glomeruli, and intrarenal generation of hypohalous acid by renal artery injection of MPO and H2O2, but not H2O2 alone, induced proteinuria in rats.53,54 Odobasic et al. reported that MPO contributes to ROS-mediated glomerular injury in the heterologous phase of anti-GBM GN, but attenuates injury during the autologous phase by suppressing T cell proliferation and cytokine production.55 Mice genetically deficient in neutrophil NADPH oxidase failed to develop proteinuria after administration of anti-GBM antibodies.26 On the other hand, Schreiber et al. reported that mice genetically deficient in NADPH oxidase components (gp91phox, p47phox) demonstrated accelerated ANCA-mediated crescentic GN, due to diminished ROS-mediated downregulation of IL-1β.56
The role of neutrophil granule components in glomerular disease is most completely understood in AAV-mediated GN. Target antigens for ANCA (MPO, PR3, and possibly LAMP-2) are stored in neutrophil granules and secretory vesicles.57–59 Target antigens in granules are not accessible to circulating ANCA; however, neutrophil priming increases their plasma membrane expression, allowing ANCA to stimulate neutrophil degranulation and ROS production through neutrophil FcγRs.60–63 Depletion of circulating neutrophils also protects mice from anti-MPO–induced GN.64 The extent of neutrophil ROS production and degranulation depends on the level of ANCA expression on plasma membranes.65 Using a mouse model of AAV, Schreiber et al. showed that glomerular cytokine generation, neutrophil and monocyte infiltration into glomeruli, and crescent formation were dependent on neutrophil release of granule proteases.66 Lu et al. reported that in vitro neutrophil-mediated endothelial cell injury by ANCA was induced by serine proteases, not ROS.42
Neutrophil serine proteases (neutrophil elastase, cathepsin G, and proteinase 3) have been studied in other models of GN. Feith et al. showed that neutrophil release of serine proteases was required for albuminuria at 24 hours in a model of anti-GBM nephritis.52 Schrijver et al. used C57BL/6J,bg/bg (beige) mice, in which neutrophils are genetically deficient in neutrophil elastase and cathepsin G but demonstrate normal ROS generation, to examine their role in anti-GBM GN.67 Beige mice failed to develop proteinuria despite equivalent glomerular neutrophil accumulation and normal neutrophil respiratory burst activity.67 Suzuki et al. reported that administration of an inhibitor of neutrophil elastase prevented development of proteinuria, hematuria, and crescent formation in a rat model of anti-GBM nephritis.68 Both neutrophil elastase and cathepsin G degrade GBM in vitro.69 Neutrophil elastase has also been detected in glomeruli and urine from patients with crescentic GN, MPGN, and membranous nephropathy.70,71 Another neutrophil granule component, gelatinase (MMP9), was shown by immunohistochemistry and zymography to be present in glomeruli of patients with several types of GN, including AAV, IgA nephropathy, acute postinfectious GN, and lupus nephritis (LN).72 Infusion of elastase and other MMPs into isolated rat glomeruli increased albumin permeability.73
The studies summarized above suggest that generation of ROS and release of granule enzymes participate in neutrophil-mediated glomerular injury, although their relative contribution depends on factors yet to be identified. The understanding of the mechanisms of injury and the granule components responsible is incomplete. Proteomic analysis of the four subsets of neutrophil granules (secretory vesicles; gelatinase or tertiary granules; specific or secondary granules; and azurophilic or primary granules) identified >800 protein constituents.57,58,74 A number of those newly discovered granule proteins have been shown to contribute to induction and resolution of the inflammatory response.75–79 The large number of neutrophil granule constituents identified suggests that a focus on granule proteases is too narrow.
Expanded Neutrophil Functions and Potential Role in GN
Neutrophil Heterogeneity and Plasticity
The life span of neutrophils in the circulation was thought to be limited to a few hours by constitutive apoptosis. Pillay et al. challenged that concept and suggested the normal life span may be up to several days.80 This observed increase in life span, however, was subsequently challenged on methodologic grounds.81 Neutrophil apoptosis does not occur at a fixed rate, but can be increased or decreased at sites of inflammation by various cytokines, growth factors, and bacterial products.82 Thus, the neutrophil contribution to severity and resolution of inflammatory responses is influenced by the rate at which they die. It is unclear if regulation of neutrophil apoptosis during glomerular inflammation resembles that found in other inflammatory conditions. Less than 20% of neutrophils recruited to the glomerulus in a model of immune complex GN were reported to undergo apoptosis.83
Subpopulations of neutrophils with different surface molecules and densities demonstrate unique functional characteristics.84,85 For example, a low-density subpopulation of neutrophils that express programmed death receptor 1 ligand (PDL-1) shows immunosuppressive properties in HIV-infected patients.86,87 Immunosuppressive neutrophils also have been identified in patients with autoimmune diseases, cancer, and pregnancy.88–93 Neutrophil plasticity is also demonstrated by their ability to acquire properties of dendritic cells or macrophages, including antigen presentation, upon exposure to specific cytokine combinations.84 Thus, subpopulations of resting and stimulated neutrophils may perform important immune regulatory functions in GN, although a role of neutrophil subpopulations in pathophysiology remains to be determined.
Neutrophil Extracellular Traps
In 2004, a new antimicrobial mechanism was described in which neutrophils form an extracellular network of chromatin fibers containing globular domains of antimicrobial proteins derived from intracellular granules, termed neutrophil extracellular traps, or NETs.94 NET formation is stimulated by activation of Fc receptors, TLRs, and receptors for C components, IL-8, TNFα, IFNs, and by exposure to gram-positive and gram-negative bacteria and fungi.94–97 NET formation occurs by a unique form of cell death, termed NETosis, in which nuclear and granule membranes dissolve, the nuclear content decondenses into the cytoplasm, and the plasma membrane ruptures releasing strands of chromatin decorated with granular proteins.98 Bacteria, fungi, and parasites bind to NETs through a charge interaction, leading to their exposure to high concentrations of antimicrobial proteins, including histones, defensins, lysozyme, and serine proteases.99 Although NETs possess antibacterial activity in vitro, their ability to kill bacteria in vivo has proven difficult to demonstrate.100
A number of reports suggest that NETs are involved in autoimmune and glomerular diseases, including AAV and LN.101 Kessenbrock et al. showed that neutrophils from normal subjects primed by TNFα in vitro demonstrated robust NET formation upon exposure to ANCA containing IgG fractions and mouse monoclonal anti-PR3.102 NETs were shown to be present in glomeruli in renal biopsy samples from patients with rapidly deteriorating renal function due to AAV.102,103 Recently, multiphoton microscopy identified NETs in about 20% of glomeruli in murine anti-GBM GN.104 However, those NETs were present only transiently, and their disruption with DNase did not alter the development of proteinuria.104
Neutrophils from patients with SLE are also more likely to form NETs which contain antigens (dsDNA, histones) recognized by lupus autoantibodies, particularly when they are stimulated with immune complexes.105–108 In addition to providing antigens recognized by autoantibodies, components of NETs stimulate dendritic cells to release IFN-α through TLR-9 and enhance T cell activation by antigen through an unknown mechanism.106–109 Hakkim et al. showed that a subset of patients with SLE had impaired degradation of NETs, and those patients were significantly more likely to have LN.110 Leffler et al. showed that patients with SLE with impaired degradation of NETs had significantly lower serum levels of C3 and C4, and those NETs were able to directly activate either the classic or lectin C pathways.111
Microparticles
Another new mechanism by which neutrophils may control bacterial invasion is through release of microparticles.112 Microparticles are a member of a group of vesicles (that includes exosomes, ectosomes, microvesicles, and shedding microvesicles) released from all activated or dying cells that contribute to cell-cell communication. Timar and colleagues reported that incubation of isolated human neutrophils with opsonized bacteria stimulated the rapid release of microparticles able to inhibit growth of both opsonized and nonopsonized bacteria through bacterial aggregation.112 Neutrophil-derived microparticles with the same surface markers were present with bacterial aggregates in the blood of patients with bacteremia. Those microparticles showed higher expression of granule antibacterial proteins. Neutrophil-derived microparticles have also been reported to induce pro- and anti-inflammatory macrophage phenotypes, and they participate in venous thrombogenesis.113–115
Evidence for participation of neutrophil-derived microparticles in glomerular diseases is sparse, but suggestive. Microparticles derived from neutrophils and platelets are present in the plasma in patients with active vasculitis.116 Anti-neutrophil cytoplasmic antibodies were reported to stimulate the release of microparticles from neutrophils.117 Those microparticles attached to endothelial cells in an integrin-dependent manner, where they stimulated increased adhesion molecule expression, ROS generation, cytokine release, and thrombin generation.
A number of studies described an increased concentration of microparticles derived from endothelial cells, platelets, and leukocytes in patients with SLE and antiphospholipid syndrome.118–120 Nielsen et al. reported that the concentration of cell-derived microparticles was decreased in SLE, although the ability of those microparticles to bind annexin V differed from normal individuals.121 Because some microparticles contain nucleic acids, the ability of microparticles to contribute to immune complex formation has been examined.122 Nielsen et al. showed that circulating microparticles from patients with SLE contained increased IgG, IgM, and C1q, compared with patients with rheumatoid arthritis and systemic sclerosis or healthy controls.123 Ullal et al. reported that anti-DNA and anti-nucleosomal antibodies bound to microparticles derived from cultured monocytes, T cells, and neutrophilic cells.124 Thus, cell-derived microparticles may participate in the generation of autoantibodies and in the formation and size of immune complexes that induce LN.
Neutrophils Interact with Innate and Adaptive Immune Cells
Neutrophils are now known to contribute to both innate and adaptive immune responses through direct and indirect interactions with monocytes, endothelial cells, T- and B-lymphocytes, natural killer (NK) cells, and dendritic cells. Neutrophils influence the immune response through synthesis and secretion of cytokines and chemokines (Table 2) and release of granule contents.75,125,126 Serine proteases, azurocidin, α-defensins, and LL-37 are granule components that induce monocyte adhesion and chemotaxis and activate endothelial cells to increase expression of adhesion molecules.127–129 Those proteins have also been reported to induce release of monocyte-attracting chemokines by endothelial cells and tissue macrophages, including MCP-1, MIP-1, and IL-8.130,131 Serine proteases proteolytically modify cytokines and chemokines and their receptors, resulting in modified activity.79 Azurocidin and PR3 disrupt endothelial cell integrity leading to increased vascular permeability and edema.128,132,133 LL-37 has both proinflammatory and anti-inflammatory activities through regulation of TLRs on neutrophils and monocytes.134
Table 2.
Cytokines and chemokines produced by neutrophils that contribute to experimental and human GN
| Cytokine/Chemokine | Animal Model of GN | Human GN |
|---|---|---|
| CC chemokines | ||
| CCL2 (MCP1)126 | LN154 | AAV,155 Crescentic GN,156 LN157 |
| CCL3(MIP1α)126 | LN158 | Crescentic GN,156 SLE159 |
| CCL4(MIP1β)126 | Crescentic GN156 | |
| CCL18126,160 | AAV161 | |
| CCL20126 | Anti-GBM,162 IgAN163 | |
| CXC chemokines | ||
| CXCL1126 | AAV164 | IgAN,165 LN166 |
| CXCL5126 | Anti-GBM167 | AAV167 |
| CXCL8 (IL-8)126 | Immune Complex GN168 | AAV164,169 |
| CXCL9126 | Anti-GBM162 | |
| CXCL10126 | LN157 | |
| CXCL 12126 | LN154 | |
| CXCL 13126 | LN170 | AAV,171 LN170,172 |
| Colony-stimulating factors | ||
| G-CSF125 | AAV173 | AAV174 |
| GM-CSF125 | AAV173 | |
| Proinflammatory cytokines | ||
| IL-1α125,175 | AAV,176 IgAN,177 MPGN,178 LN178 | |
| IL-1β125,175,179 | AAV,66,180 LN181 | AAV,182,183 IgAN,177 LN,178,184 MPGN178 |
| IL-6125,185,186 | Anti-GBM,187 LN188–190 | AAV,183 IgAN,191 LN,178 MPGN178 |
| IL-17125,192,193 | AAV,194 Anti-GBM,195,196 LN195 | LN197 |
| IL-18125,198,199 | LN200 | AAV,201 LN,197,202,203 |
| MIF125 | LN204 | Crescentic GN,205 FSGS,205 LN,205 MPGN205 |
| Anti-inflammatory cytokines | ||
| IL-1ra125,206 | Anti-GBM207 | IgAN,177 LN208 |
| IL-10a125,209–212 | LN/SLE202,213 | |
| IL-4125,214 | Anti-GBM215 | MN216 |
| TGFβ1125,217 | Anti-GBM218 | IgAN219 |
| Immunoregulatory cytokines | ||
| IL-12125,209,220,221 | LN222,223 | LN/SLE197,202,224 |
| IL-21125 | LN225 | |
| IL-27125,226 | Anti-GBM227 | |
| IFN-α125,228,229 | LN230,231 | FSGS,232LN/SLE229,233–236 |
| IFN-β125 | FSGS,232,237 MPGN238 | |
| IFN-γ125,229,239 | LN222,240–242 | AAV,216 FSGS,232 MPGN,216 LN/SLE229,243 |
| TNF superfamily members | ||
| TNF-α244,245 | Anti-GBM180 | AAV,176,182 IgAN,191 LN/SLE,178,246 MPGN178 |
| APRIL247,248 | LN/SLE249 | |
| BAFF247,250 | LN251,252 | LN/SLE249,253 |
| FasL126 | Anti-GBM254 | LN255 |
| TRAIL126 | LN256 |
Neutrophils are capable of expressing and producing a number of cytokines, although in smaller quantities than other immune cells. Several cytokines/chemokines have been implicated in experimental and human GN. However, the contribution from neutrophils is not well described and requires further inquiry. IgAN, IgA nephropathy; MPGN, membranoproliferative GN; MN, membranous nephropathy.
Evidence for and against production by neutrophils.
Several antimicrobial peptides released from neutrophil granules play an active role in recruiting and activating dendritic cells. Those peptides, termed ‘alarmins’, include LL-37, α-defensins, and lactoferrin.76,135–137 There is now evidence that neutrophils interact in a network with dendritic cells, T-cells, and NK cells to modulate the immune response in both infection and chronic inflammation.84,138,139 That modulation includes recruitment of T helper 1 and T helper 17 cells to infected sites by neutrophil-derived chemokines.140 In concert with dendritic cells, neutrophils potentiate the release of GM-CSF and IFN-γ by NK cells, which, in turn, promotes neutrophil survival and priming of ROS production.139,141,142 Upon migration into tissues, neutrophils undergo transcriptional activation resulting in production of proinflammatory cytokines, a number of which have been shown to participate in GN (Table 2).75,77,143 Although the ability of neutrophils to produce those cytokines and chemokines is a fraction of monocyte capacity, the large number of migrating neutrophils may provide physiologically relevant quantities. The contribution of glomerular neutrophils to the production of cytokines and other agents that regulate innate and adaptive immunity requires further investigation.
Neutrophils and Resolution of Inflammation
Emerging evidence suggests that neutrophils play a role in the resolution of inflammation.144 In the late stages of an inflammatory response, neutrophils switch their eicosanoid biosynthesis from proinflammatory leukotriene B4 to anti-inflammatory lipoxin A4, which inhibits neutrophil recruitment.145 Wu et al. demonstrated increased levels of lipoxin A4 in the glomeruli and leukocytes of patients with poststreptococcal GN.146 Poststreptococcal GN is usually a self-limited illness, and high levels of lipoxin A4 may contribute to disease resolution. Lipoxin A4 attenuates PDGF-mediated mesangial proliferation in cell culture and decreases neutrophil recruitment in an animal model of anti-GBM nephritis.147,148 Neutrophils also contribute to the resolution of inflammation through the production of resolvins, which can inhibit neutrophil transendothelial migration and tissue infiltration.145 The role of resolvins in GN has not been examined.
The expanded functional capabilities of neutrophils outlined in this review suggest that neutrophils may contribute to GN through initiation of disease, causing direct injury to glomerular cells or the filtration barrier, regulating activity of other cells of the innate and adaptive immune systems, or initiating resolution programs. Defining the role neutrophils play in development and resolution of various forms of GN will enhance our understanding of the systems biology of immune glomerular injury. The complex interactions between neutrophils and other immune cells and intrinsic glomerular cells suggests that the time for examining the role of individual cell types in GN may be coming to an end. Examining the complex cellular interactions within the glomerulus is likely to expand our understanding of the pathophysiology of GN and to identify new therapeutic targets. Examples of that potential were recently provided by two studies examining the effect of selective inhibition of neutrophil granule exocytosis in animal models of disease. Uriarte and colleagues inhibited neutrophil granule exocytosis in vivo by intravenous infusion of a cell-permeable peptide that blocked SNARE protein interactions.149 Treatment with that peptide significantly reduced acute lung injury induced by immune complex deposition. Recently, high through-put screening identified small molecules that inhibited neutrophil exocytosis in vivo by interfering in the interaction between the small GTPase Rab27a and its effector JFC1.150 Administration of one of those molecules to mice with endotoxin-induced systemic inflammation resulted in reduced neutrophil extravasation into tissues. Those examples illustrate the potential for new therapeutic avenues that will be identified by further understanding of the complex cellular interactions leading to glomerular inflammation.
Disclosures
D.J.C. receives research support from Mallinckrodt Pharmaceuticals.
Acknowledgments
The authors were supported by grants from the National Institutes of Health (1K08DK102542-01A1 to D.J.C., AI103980 to D.W.P. and K.R.M.) and a US Department of Veterans Affairs Merit Review (BX001838 to K.R.M.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Cochrane CG, Unanue ER, Dixon FJ: A role of polymorphonuclear leukocytes and complement in nephrotoxic nephritis. J Exp Med 122: 99–116, 1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sung SS, Bolton WK: T cells and dendritic cells in glomerular disease: The new glomerulotubular feedback loop. Kidney Int 77: 393–399, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rood IM, Hofstra JM, Deegens JK, Wetzels JF: B cell suppression in primary glomerular disease. Adv Chronic Kidney Dis 21: 166–181, 2014 [DOI] [PubMed] [Google Scholar]
- 4.Nauseef WM, Borregaard N: Neutrophils at work. Nat Immunol 15: 602–611, 2014 [DOI] [PubMed] [Google Scholar]
- 5.Jaillon S, Galdiero MR, Del Prete D, Cassatella MA, Garlanda C, Mantovani A: Neutrophils in innate and adaptive immunity. Semin Immunopathol 35: 377–394, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Radic M, Marion TN: Neutrophil extracellular chromatin traps connect innate immune response to autoimmunity. Semin Immunopathol 35: 465–480, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Witko-Sarsat V, Rieu P, Descamps-Latscha B, Lesavre P, Halbwachs-Mecarelli L: Neutrophils: Molecules, functions and pathophysiological aspects. Lab Invest 80: 617–653, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Takeuchi O, Akira S: Pattern recognition receptors and inflammation. Cell 140: 805–820, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Sadik CD, Kim ND, Luster AD: Neutrophils cascading their way to inflammation. Trends Immunol 32: 452–460, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marki A, Esko JD, Pries AR, Ley K: Role of the endothelial surface layer in neutrophil recruitment. J Leukoc Biol 98: 503–515, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zarbock A, Ley K, McEver RP, Hidalgo A: Leukocyte ligands for endothelial selectins: Specialized glycoconjugates that mediate rolling and signaling under flow. Blood 118: 6743–6751, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kolaczkowska E, Kubes P: Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol 13: 159–175, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Sundd P, Pospieszalska MK, Ley K: Neutrophil rolling at high shear: Flattening, catch bond behavior, tethers and slings. Mol Immunol 55: 59–69, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmidt S, Moser M, Sperandio M: The molecular basis of leukocyte recruitment and its deficiencies. Mol Immunol 55: 49–58, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Jones DH, Anderson DC, Burr BL, Rudloff HE, Smith CW, Krater SS, Schmalstieg FC: Quantitation of intracellular Mac-1 (CD11b/CD18) pools in human neutrophils. J Leukoc Biol 44: 535–544, 1988 [DOI] [PubMed] [Google Scholar]
- 16.Molad Y, Haines KA, Anderson DC, Buyon JP, Cronstein BN: Immunocomplexes stimulate different signalling events to chemoattractants in the neutrophil and regulate L-selectin and beta 2-integrin expression differently. Biochem J 299: 881–887, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phillipson M, Heit B, Colarusso P, Liu L, Ballantyne CM, Kubes P: Intraluminal crawling of neutrophils to emigration sites: A molecularly distinct process from adhesion in the recruitment cascade. J Exp Med 203: 2569–2575, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reichel CA, Rehberg M, Bihari P, Moser CM, Linder S, Khandoga A, Krombach F: Gelatinases mediate neutrophil recruitment in vivo: Evidence for stimulus specificity and a critical role in collagen IV remodeling. J Leukoc Biol 83: 864–874, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Foxman EF, Campbell JJ, Butcher EC: Multistep navigation and the combinatorial control of leukocyte chemotaxis. J Cell Biol 139: 1349–1360, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foxman EF, Kunkel EJ, Butcher EC: Integrating conflicting chemotactic signals. The role of memory in leukocyte navigation. J Cell Biol 147: 577–588, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lämmermann T, Afonso PV, Angermann BR, Wang JM, Kastenmüller W, Parent CA, Germain RN: Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature 498: 371–375, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Németh T, Mócsai A: Feedback Amplification of Neutrophil Function. Trends Immunol 37: 412–424, 2016 [DOI] [PubMed] [Google Scholar]
- 23.Kuligowski MP, Kitching AR, Hickey MJ: Leukocyte recruitment to the inflamed glomerulus: a critical role for platelet-derived P-selectin in the absence of rolling. J Immunol 176: 6991–6999, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Coxon A, Cullere X, Knight S, Sethi S, Wakelin MW, Stavrakis G, Luscinskas FW, Mayadas TN: Fc gamma RIII mediates neutrophil recruitment to immune complexes. a mechanism for neutrophil accumulation in immune-mediated inflammation. Immunity 14: 693–704, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Devi S, Kuligowski MP, Kwan RY, Westein E, Jackson SP, Kitching AR, Hickey MJ: Platelet recruitment to the inflamed glomerulus occurs via an alphaIIbbeta3/GPVI-dependent pathway. Am J Pathol 177: 1131–1142, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Devi S, Li A, Westhorpe CL, Lo CY, Abeynaike LD, Snelgrove SL, Hall P, Ooi JD, Sobey CG, Kitching AR, Hickey MJ: Multiphoton imaging reveals a new leukocyte recruitment paradigm in the glomerulus. Nat Med 19: 107–112, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Tipping PG, Huang XR, Berndt MC, Holdsworth SR: A role for P selectin in complement-independent neutrophil-mediated glomerular injury. Kidney Int 46: 79–88, 1994 [DOI] [PubMed] [Google Scholar]
- 28.Tang T, Rosenkranz A, Assmann KJ, Goodman MJ, Gutierrez-Ramos JC, Carroll MC, Cotran RS, Mayadas TN: A role for Mac-1 (CDIIb/CD18) in immune complex-stimulated neutrophil function in vivo: Mac-1 deficiency abrogates sustained Fcgamma receptor-dependent neutrophil adhesion and complement-dependent proteinuria in acute glomerulonephritis. J Exp Med 186: 1853–1863, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wakayama H, Hasegawa Y, Kawabe T, Hara T, Matsuo S, Mizuno M, Takai T, Kikutani H, Shimokata K: Abolition of anti-glomerular basement membrane antibody-mediated glomerulonephritis in FcRgamma-deficient mice. Eur J Immunol 30: 1182–1190, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Rosetti F, Tsuboi N, Chen K, Nishi H, Ernandez T, Sethi S, Croce K, Stavrakis G, Alcocer-Varela J, Gómez-Martin D, van Rooijen N, Kyttaris VC, Lichtman AH, Tsokos GC, Mayadas TN: Human lupus serum induces neutrophil-mediated organ damage in mice that is enabled by Mac-1 deficiency. J Immunol 189: 3714–3723, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Vriese AS, Endlich K, Elger M, Lameire NH, Atkins RC, Lan HY, Rupin A, Kriz W, Steinhausen MW: The role of selectins in glomerular leukocyte recruitment in rat anti-glomerular basement membrane glomerulonephritis. J Am Soc Nephrol 10: 2510–2517, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Rosenkranz AR, Mendrick DL, Cotran RS, Mayadas TN: P-selectin deficiency exacerbates experimental glomerulonephritis: A protective role for endothelial P-selectin in inflammation. J Clin Invest 103: 649–659, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Finsterbusch M, Hall P, Li A, Devi S, Westhorpe CL, Kitching AR, Hickey MJ: Patrolling monocytes promote intravascular neutrophil activation and glomerular injury in the acutely inflamed glomerulus. Proc Natl Acad Sci USA 113: E5172–E5181, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tipping PG, Boyce NW, Holdsworth SR: Relative contributions of chemo-attractant and terminal components of complement to anti-glomerular basement membrane (GBM) glomerulonephritis. Clin Exp Immunol 78: 444–448, 1989 [PMC free article] [PubMed] [Google Scholar]
- 35.Huang XR, Holdsworth SR, Tipping PG: Th2 responses induce humorally mediated injury in experimental anti-glomerular basement membrane glomerulonephritis. J Am Soc Nephrol 8: 1101–1108, 1997 [DOI] [PubMed] [Google Scholar]
- 36.Hébert MJ, Takano T, Papayianni A, Rennke HG, Minto A, Salant DJ, Carroll MC, Brady HR: Acute nephrotoxic serum nephritis in complement knockout mice: relative roles of the classical and alternate pathways in neutrophil recruitment and proteinuria. Nephrol Dial Transplant 13: 2799–2803, 1998 [DOI] [PubMed] [Google Scholar]
- 37.Trendelenburg M, Fossati-Jimack L, Cortes-Hernandez J, Turnberg D, Lewis M, Izui S, Cook HT, Botto M: The role of complement in cryoglobulin-induced immune complex glomerulonephritis. J Immunol 175: 6909–6914, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Xiao H, Schreiber A, Heeringa P, Falk RJ, Jennette JC: Alternative complement pathway in the pathogenesis of disease mediated by anti-neutrophil cytoplasmic autoantibodies. Am J Pathol 170: 52–64, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiao H, Dairaghi DJ, Powers JP, Ertl LS, Baumgart T, Wang Y, Seitz LC, Penfold ME, Gan L, Hu P, Lu B, Gerard NP, Gerard C, Schall TJ, Jaen JC, Falk RJ, Jennette JC: C5a receptor (CD88) blockade protects against MPO-ANCA GN. J Am Soc Nephrol 25: 225–231, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schreiber A, Xiao H, Jennette JC, Schneider W, Luft FC, Kettritz R: C5a receptor mediates neutrophil activation and ANCA-induced glomerulonephritis. J Am Soc Nephrol 20: 289–298, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ewert BH, Becker ME, Jennette JC, Falk RJ: Antimyeloperoxidase antibodies induce neutrophil adherence to cultured human endothelial cells. Ren Fail 17: 125–133, 1995 [DOI] [PubMed] [Google Scholar]
- 42.Lu X, Garfield A, Rainger GE, Savage CO, Nash GB: Mediation of endothelial cell damage by serine proteases, but not superoxide, released from antineutrophil cytoplasmic antibody-stimulated neutrophils. Arthritis Rheum 54: 1619–1628, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Gou SJ, Yuan J, Chen M, Yu F, Zhao MH: Circulating complement activation in patients with anti-neutrophil cytoplasmic antibody-associated vasculitis. Kidney Int 83: 129–137, 2013 [DOI] [PubMed] [Google Scholar]
- 44.Nauseef WM: How human neutrophils kill and degrade microbes: An integrated view. Immunol Rev 219: 88–102, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Murray J, Barbara JA, Dunkley SA, Lopez AF, Van Ostade X, Condliffe AM, Dransfield I, Haslett C, Chilvers ER: Regulation of neutrophil apoptosis by tumor necrosis factor-alpha: requirement for TNFR55 and TNFR75 for induction of apoptosis in vitro. Blood 90: 2772–2783, 1997 [PubMed] [Google Scholar]
- 46.Ward RA, Nakamura M, McLeish KR: Priming of the neutrophil respiratory burst involves p38 mitogen-activated protein kinase-dependent exocytosis of flavocytochrome b558-containing granules. J Biol Chem 275: 36713–36719, 2000 [DOI] [PubMed] [Google Scholar]
- 47.Lauterbach M, O’Donnell P, Asano K, Mayadas TN: Role of TNF priming and adhesion molecules in neutrophil recruitment to intravascular immune complexes. J Leukoc Biol 83: 1423–1430, 2008 [DOI] [PubMed] [Google Scholar]
- 48.Volk AP, Barber BM, Goss KL, Ruff JG, Heise CK, Hook JS, Moreland JG: Priming of neutrophils and differentiated PLB-985 cells by pathophysiological concentrations of TNF-α is partially oxygen dependent. J Innate Immun 3: 298–314, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kallenberg CG, Heeringa P, Stegeman CA: Mechanisms of Disease: Pathogenesis and treatment of ANCA-associated vasculitides. Nat Clin Pract Rheumatol 2: 661–670, 2006 [DOI] [PubMed] [Google Scholar]
- 50.Donovan KL, Coles GA, Williams JD: Tumor necrosis factor-alpha augments the pro-inflammatory interaction between PMN and GBM via a CD18 dependent mechanism. Kidney Int 48: 698–704, 1995 [DOI] [PubMed] [Google Scholar]
- 51.Suzuki Y, Gómez-Guerrero C, Shirato I, López-Franco O, Gallego-Delgado J, Sanjuán G, Lázaro A, Hernández-Vargas P, Okumura K, Tomino Y, Ra C, Egido J: Pre-existing glomerular immune complexes induce polymorphonuclear cell recruitment through an Fc receptor-dependent respiratory burst: Potential role in the perpetuation of immune nephritis. J Immunol 170: 3243–3253, 2003 [DOI] [PubMed] [Google Scholar]
- 52.Feith GW, Assmann KJ, Bogman MJ, van Gompel AP, Schalkwijk J, Koene RA: Different mediator systems in biphasic heterologous phase of anti-GBM nephritis in mice. Nephrol Dial Transplant 11: 599–607, 1996 [DOI] [PubMed] [Google Scholar]
- 53.Li JZ, Sharma R, Dileepan KN, Savin VJ: Polymorphonuclear leukocytes increase glomerular albumin permeability via hypohalous acid. Kidney Int 46: 1025–1030, 1994 [DOI] [PubMed] [Google Scholar]
- 54.Johnson RJ, Couser WG, Chi EY, Adler S, Klebanoff SJ: New mechanism for glomerular injury. Myeloperoxidase-hydrogen peroxide-halide system. J Clin Invest 79: 1379–1387, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Odobasic D, Kitching AR, Semple TJ, Holdsworth SR: Endogenous myeloperoxidase promotes neutrophil-mediated renal injury, but attenuates T cell immunity inducing crescentic glomerulonephritis. J Am Soc Nephrol 18: 760–770, 2007 [DOI] [PubMed] [Google Scholar]
- 56.Schreiber A, Luft FC, Kettritz R: Phagocyte NADPH oxidase restrains the inflammasome in ANCA-induced GN. J Am Soc Nephrol 26: 411–424, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lominadze G, Powell DW, Luerman GC, Link AJ, Ward RA, McLeish KR: Proteomic analysis of human neutrophil granules. Mol Cell Proteomics 4: 1503–1521, 2005 [DOI] [PubMed] [Google Scholar]
- 58.Uriarte SM, Powell DW, Luerman GC, Merchant ML, Cummins TD, Jog NR, Ward RA, McLeish KR: Comparison of proteins expressed on secretory vesicle membranes and plasma membranes of human neutrophils. J Immunol 180: 5575–5581, 2008 [DOI] [PubMed] [Google Scholar]
- 59.Kain R, Matsui K, Exner M, Binder S, Schaffner G, Sommer EM, Kerjaschki D: A novel class of autoantigens of anti-neutrophil cytoplasmic antibodies in necrotizing and crescentic glomerulonephritis: The lysosomal membrane glycoprotein h-lamp-2 in neutrophil granulocytes and a related membrane protein in glomerular endothelial cells. J Exp Med 181: 585–597, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Falk RJ, Terrell RS, Charles LA, Jennette JC: Anti-neutrophil cytoplasmic autoantibodies induce neutrophils to degranulate and produce oxygen radicals in vitro. Proc Natl Acad Sci USA 87: 4115–4119, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kettritz R: How anti-neutrophil cytoplasmic autoantibodies activate neutrophils. Clin Exp Immunol 169: 220–228, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mulder AH, Heeringa P, Brouwer E, Limburg PC, Kallenberg CG: Activation of granulocytes by anti-neutrophil cytoplasmic antibodies (ANCA): A Fc gamma RII-dependent process. Clin Exp Immunol 98: 270–278, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kettritz R, Jennette JC, Falk RJ: Crosslinking of ANCA-antigens stimulates superoxide release by human neutrophils. J Am Soc Nephrol 8: 386–394, 1997 [DOI] [PubMed] [Google Scholar]
- 64.Xiao H, Heeringa P, Liu Z, Huugen D, Hu P, Maeda N, Falk RJ, Jennette JC: The role of neutrophils in the induction of glomerulonephritis by anti-myeloperoxidase antibodies. Am J Pathol 167: 39–45, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schreiber A, Luft FC, Kettritz R: Membrane proteinase 3 expression and ANCA-induced neutrophil activation. Kidney Int 65: 2172–2183, 2004 [DOI] [PubMed] [Google Scholar]
- 66.Schreiber A, Pham CT, Hu Y, Schneider W, Luft FC, Kettritz R: Neutrophil serine proteases promote IL-1β generation and injury in necrotizing crescentic glomerulonephritis. J Am Soc Nephrol 23: 470–482, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schrijver G, Schalkwijk J, Robben JC, Assmann KJ, Koene RA: Antiglomerular basement membrane nephritis in beige mice. Deficiency of leukocytic neutral proteinases prevents the induction of albuminuria in the heterologous phase. J Exp Med 169: 1435–1448, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Suzuki S, Gejyo F, Kuroda T, Kazama JJ, Imai N, Kimura H, Arakawa M: Effects of a novel elastase inhibitor, ONO-5046, on nephrotoxic serum nephritis in rats. Kidney Int 53: 1201–1208, 1998 [DOI] [PubMed] [Google Scholar]
- 69.Davies M, Barrett AJ, Travis J, Sanders E, Coles GA: The degradation of human glomerular basement membrane with purified lysosomal proteinases: Evidence for the pathogenic role of the polymorphonuclear leucocyte in glomerulonephritis. Clin Sci Mol Med 54: 233–240, 1978 [DOI] [PubMed] [Google Scholar]
- 70.Kuźniar J, Kuźniar TJ, Marchewka Z, Lembas-Bogaczyk J, Rabczyński J, Kopeć W, Klinger M: Elastase deposits in the kidney and urinary elastase excretion in patients with glomerulonephritis--Evidence for neutrophil involvement in renal injury. Scand J Urol Nephrol 41: 527–534, 2007 [DOI] [PubMed] [Google Scholar]
- 71.Oda T, Hotta O, Taguma Y, Kitamura H, Sudo K, Horigome I, Chiba S, Yoshizawa N, Nagura H: Involvement of neutrophil elastase in crescentic glomerulonephritis. Hum Pathol 28: 720–728, 1997 [DOI] [PubMed] [Google Scholar]
- 72.Sanders JS, van Goor H, Hanemaaijer R, Kallenberg CG, Stegeman CA: Renal expression of matrix metalloproteinases in human ANCA-associated glomerulonephritis. Nephrol Dial Transplant 19: 1412–1419, 2004 [DOI] [PubMed] [Google Scholar]
- 73.Sharma R, Suzuki K, Nagase H, Savin VJ: Matrix metalloproteinase (stromelysin-1) increases the albumin permeability of isolated rat glomeruli. J Lab Clin Med 128: 297–303, 1996 [DOI] [PubMed] [Google Scholar]
- 74.Rørvig S, Østergaard O, Heegaard NH, Borregaard N: Proteome profiling of human neutrophil granule subsets, secretory vesicles, and cell membrane: Correlation with transcriptome profiling of neutrophil precursors. J Leukoc Biol 94: 711–721, 2013 [DOI] [PubMed] [Google Scholar]
- 75.Soehnlein O, Weber C, Lindbom L: Neutrophil granule proteins tune monocytic cell function. Trends Immunol 30: 538–546, 2009 [DOI] [PubMed] [Google Scholar]
- 76.Yang D, de la Rosa G, Tewary P, Oppenheim JJ: Alarmins link neutrophils and dendritic cells. Trends Immunol 30: 531–537, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Borregaard N, Sørensen OE, Theilgaard-Mönch K: Neutrophil granules: A library of innate immunity proteins. Trends Immunol 28: 340–345, 2007 [DOI] [PubMed] [Google Scholar]
- 78.DiStasi MR, Ley K: Opening the flood-gates: How neutrophil-endothelial interactions regulate permeability. Trends Immunol 30: 547–556, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pham CT: Neutrophil serine proteases: Specific regulators of inflammation. Nat Rev Immunol 6: 541–550, 2006 [DOI] [PubMed] [Google Scholar]
- 80.Pillay J, den Braber I, Vrisekoop N, Kwast LM, de Boer RJ, Borghans JA, Tesselaar K, Koenderman L: In vivo labeling with 2H2O reveals a human neutrophil lifespan of 5.4 days. Blood 116: 625–627, 2010 [DOI] [PubMed] [Google Scholar]
- 81.Lahoz-Beneytez J, Elemans M, Zhang Y, Ahmed R, Salam A, Block M, Niederalt C, Asquith B, Macallan D: Human neutrophil kinetics: Modeling of stable isotope labeling data supports short blood neutrophil half-lives. Blood 127: 3431–3438, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.McCracken JM, Allen LA: Regulation of human neutrophil apoptosis and lifespan in health and disease. J Cell Death 7: 15–23, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hughes J, Johnson RJ, Mooney A, Hugo C, Gordon K, Savill J: Neutrophil fate in experimental glomerular capillary injury in the rat. Emigration exceeds in situ clearance by apoptosis. Am J Pathol 150: 223–234, 1997 [PMC free article] [PubMed] [Google Scholar]
- 84.Scapini P, Cassatella MA: Social networking of human neutrophils within the immune system. Blood 124: 710–719, 2014 [DOI] [PubMed] [Google Scholar]
- 85.Silvestre-Roig C, Hidalgo A, Soehnlein O: Neutrophil heterogeneity: implications for homeostasis and pathogenesis. Blood 127: 2173–2181, 2016 [DOI] [PubMed] [Google Scholar]
- 86.Bowers NL, Helton ES, Huijbregts RP, Goepfert PA, Heath SL, Hel Z: Immune suppression by neutrophils in HIV-1 infection: Role of PD-L1/PD-1 pathway. PLoS Pathog 10: e1003993, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cloke T, Munder M, Bergin P, Herath S, Modolell M, Taylor G, Müller I, Kropf P: Phenotypic alteration of neutrophils in the blood of HIV seropositive patients. PLoS One 8: e72034, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sagiv JY, Michaeli J, Assi S, Mishalian I, Kisos H, Levy L, Damti P, Lumbroso D, Polyansky L, Sionov RV, Ariel A, Hovav AH, Henke E, Fridlender ZG, Granot Z: Phenotypic diversity and plasticity in circulating neutrophil subpopulations in cancer. Cell Reports 10: 562–573, 2015 [DOI] [PubMed] [Google Scholar]
- 89.Amirbeagi F, Thulin P, Pullerits R, Pedersen B, Andersson BA, Dahlgren C, Welin A, Bylund J: Olfactomedin-4 autoantibodies give unusual c-ANCA staining patterns with reactivity to a subpopulation of neutrophils. J Leukoc Biol 97: 181–189, 2015 [DOI] [PubMed] [Google Scholar]
- 90.Hu N, Mora-Jensen H, Theilgaard-Mönch K, Doornbos-van der Meer B, Huitema MG, Stegeman CA, Heeringa P, Kallenberg CG, Westra J: Differential expression of granulopoiesis related genes in neutrophil subsets distinguished by membrane expression of CD177. PLoS One 9: e99671, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dumitru CA, Moses K, Trellakis S, Lang S, Brandau S: Neutrophils and granulocytic myeloid-derived suppressor cells: Immunophenotyping, cell biology and clinical relevance in human oncology. Cancer Immunol Immunother 61: 1155–1167, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Carmona-Rivera C, Kaplan MJ: Low-density granulocytes: A distinct class of neutrophils in systemic autoimmunity. Semin Immunopathol 35: 455–463, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ssemaganda A, Kindinger L, Bergin P, Nielsen L, Mpendo J, Ssetaala A, Kiwanuka N, Munder M, Teoh TG, Kropf P, Müller I: Characterization of neutrophil subsets in healthy human pregnancies. PLoS One 9: e85696, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A: Neutrophil extracellular traps kill bacteria. Science 303: 1532–1535, 2004 [DOI] [PubMed] [Google Scholar]
- 95.Neeli I, Khan SN, Radic M: Histone deimination as a response to inflammatory stimuli in neutrophils. J Immunol 180: 1895–1902, 2008 [DOI] [PubMed] [Google Scholar]
- 96.Martinelli S, Urosevic M, Daryadel A, Oberholzer PA, Baumann C, Fey MF, Dummer R, Simon HU, Yousefi S: Induction of genes mediating interferon-dependent extracellular trap formation during neutrophil differentiation. J Biol Chem 279: 44123–44132, 2004 [DOI] [PubMed] [Google Scholar]
- 97.Munks MW, McKee AS, Macleod MK, Powell RL, Degen JL, Reisdorph NA, Kappler JW, Marrack P: Aluminum adjuvants elicit fibrin-dependent extracellular traps in vivo. Blood 116: 5191–5199, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Papayannopoulos V, Zychlinsky A: NETs: A new strategy for using old weapons. Trends Immunol 30: 513–521, 2009 [DOI] [PubMed] [Google Scholar]
- 99.Brinkmann V, Zychlinsky A: Neutrophil extracellular traps: Is immunity the second function of chromatin? J Cell Biol 198: 773–783, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sørensen OE, Borregaard N: Neutrophil extracellular traps - The dark side of neutrophils. J Clin Invest 126: 1612–1620, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gupta S, Kaplan MJ: The role of neutrophils and NETosis in autoimmune and renal diseases. Nat Rev Nephrol 12: 402–413, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kessenbrock K, Krumbholz M, Schönermarck U, Back W, Gross WL, Werb Z, Gröne HJ, Brinkmann V, Jenne DE: Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med 15: 623–625, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yoshida M, Sasaki M, Sugisaki K, Yamaguchi Y, Yamada M: Neutrophil extracellular trap components in fibrinoid necrosis of the kidney with myeloperoxidase-ANCA-associated vasculitis. Clin Kidney J 6: 308–312, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Westhorpe CL, Bayard JE, O’Sullivan KM, Hall P, Cheng Q, Kitching AR, Hickey MJ: In Vivo imaging of inflamed glomeruli reveals dynamics of neutrophil extracellular trap formation in glomerular capillaries. Am J Pathol 187: 318–331, 2017 [DOI] [PubMed] [Google Scholar]
- 105.Craft JE: Dissecting the immune cell mayhem that drives lupus pathogenesis. Sci Transl Med 3: 73ps9, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Garcia-Romo GS, Caielli S, Vega B, Connolly J, Allantaz F, Xu Z, Punaro M, Baisch J, Guiducci C, Coffman RL, Barrat FJ, Banchereau J, Pascual V: Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci Transl Med 3: 73ra20, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lande R, Ganguly D, Facchinetti V, Frasca L, Conrad C, Gregorio J, Meller S, Chamilos G, Sebasigari R, Riccieri V, Bassett R, Amuro H, Fukuhara S, Ito T, Liu YJ, Gilliet M: Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci Transl Med 3: 73ra19, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Villanueva E, Yalavarthi S, Berthier CC, Hodgin JB, Khandpur R, Lin AM, Rubin CJ, Zhao W, Olsen SH, Klinker M, Shealy D, Denny MF, Plumas J, Chaperot L, Kretzler M, Bruce AT, Kaplan MJ: Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J Immunol 187: 538–552, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tillack K, Breiden P, Martin R, Sospedra M: T lymphocyte priming by neutrophil extracellular traps links innate and adaptive immune responses. J Immunol 188: 3150–3159, 2012 [DOI] [PubMed] [Google Scholar]
- 110.Hakkim A, Fürnrohr BG, Amann K, Laube B, Abed UA, Brinkmann V, Herrmann M, Voll RE, Zychlinsky A: Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci USA 107: 9813–9818, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Leffler J, Martin M, Gullstrand B, Tydén H, Lood C, Truedsson L, Bengtsson AA, Blom AM: Neutrophil extracellular traps that are not degraded in systemic lupus erythematosus activate complement exacerbating the disease. J Immunol 188: 3522–3531, 2012 [DOI] [PubMed] [Google Scholar]
- 112.Timár CI, Lorincz AM, Csépányi-Kömi R, Vályi-Nagy A, Nagy G, Buzás EI, Iványi Z, Kittel A, Powell DW, McLeish KR, Ligeti E: Antibacterial effect of microvesicles released from human neutrophilic granulocytes. Blood 121: 510–518, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gasser O, Schifferli JA: Activated polymorphonuclear neutrophils disseminate anti-inflammatory microparticles by ectocytosis. Blood 104: 2543–2548, 2004 [DOI] [PubMed] [Google Scholar]
- 114.Prakash PS, Caldwell CC, Lentsch AB, Pritts TA, Robinson BR: Human microparticles generated during sepsis in patients with critical illness are neutrophil-derived and modulate the immune response. J Trauma Acute Care Surg 73: 401–406, discussion 406–407, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Myers DD, Hawley AE, Farris DM, Wrobleski SK, Thanaporn P, Schaub RG, Wagner DD, Kumar A, Wakefield TW: P-selectin and leukocyte microparticles are associated with venous thrombogenesis. J Vasc Surg 38: 1075–1089, 2003 [DOI] [PubMed] [Google Scholar]
- 116.Daniel L, Fakhouri F, Joly D, Mouthon L, Nusbaum P, Grunfeld JP, Schifferli J, Guillevin L, Lesavre P, Halbwachs-Mecarelli L: Increase of circulating neutrophil and platelet microparticles during acute vasculitis and hemodialysis. Kidney Int 69: 1416–1423, 2006 [DOI] [PubMed] [Google Scholar]
- 117.Hong Y, Eleftheriou D, Hussain AA, Price-Kuehne FE, Savage CO, Jayne D, Little MA, Salama AD, Klein NJ, Brogan PA: Anti-neutrophil cytoplasmic antibodies stimulate release of neutrophil microparticles. J Am Soc Nephrol 23: 49–62, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vikerfors A, Mobarrez F, Bremme K, Holmström M, Ågren A, Eelde A, Bruzelius M, Antovic A, Wallén H, Svenungsson E: Studies of microparticles in patients with the antiphospholipid syndrome (APS). Lupus 21: 802–805, 2012 [DOI] [PubMed] [Google Scholar]
- 119.Dignat-George F, Camoin-Jau L, Sabatier F, Arnoux D, Anfosso F, Bardin N, Veit V, Combes V, Gentile S, Moal V, Sanmarco M, Sampol J: Endothelial microparticles: A potential contribution to the thrombotic complications of the antiphospholipid syndrome. Thromb Haemost 91: 667–673, 2004 [DOI] [PubMed] [Google Scholar]
- 120.Sellam J, Proulle V, Jüngel A, Ittah M, Miceli Richard C, Gottenberg JE, Toti F, Benessiano J, Gay S, Freyssinet JM, Mariette X: Increased levels of circulating microparticles in primary Sjögren’s syndrome, systemic lupus erythematosus and rheumatoid arthritis and relation with disease activity. Arthritis Res Ther 11: R156, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nielsen CT, Østergaard O, Johnsen C, Jacobsen S, Heegaard NH: Distinct features of circulating microparticles and their relationship to clinical manifestations in systemic lupus erythematosus. Arthritis Rheum 63: 3067–3077, 2011 [DOI] [PubMed] [Google Scholar]
- 122.Pisetsky DS, Gauley J, Ullal AJ: Microparticles as a source of extracellular DNA. Immunol Res 49: 227–234, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nielsen CT, Østergaard O, Stener L, Iversen LV, Truedsson L, Gullstrand B, Jacobsen S, Heegaard NH: Increased IgG on cell-derived plasma microparticles in systemic lupus erythematosus is associated with autoantibodies and complement activation. Arthritis Rheum 64: 1227–1236, 2012 [DOI] [PubMed] [Google Scholar]
- 124.Ullal AJ, Reich CF 3rd, Clowse M, Criscione-Schreiber LG, Tochacek M, Monestier M, Pisetsky DS: Microparticles as antigenic targets of antibodies to DNA and nucleosomes in systemic lupus erythematosus. J Autoimmun 36: 173–180, 2011 [DOI] [PubMed] [Google Scholar]
- 125.Tecchio C, Micheletti A, Cassatella MA: Neutrophil-derived cytokines: Facts beyond expression. Front Immunol 5: 508, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tecchio C, Cassatella MA: Neutrophil-derived chemokines on the road to immunity. Semin Immunol 28: 119–128, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.David A, Kacher Y, Specks U, Aviram I: Interaction of proteinase 3 with CD11b/CD18 (beta2 integrin) on the cell membrane of human neutrophils. J Leukoc Biol 74: 551–557, 2003 [DOI] [PubMed] [Google Scholar]
- 128.Soehnlein O, Oehmcke S, Ma X, Rothfuchs AG, Frithiof R, van Rooijen N, Mörgelin M, Herwald H, Lindbom L: Neutrophil degranulation mediates severe lung damage triggered by streptococcal M1 protein. Eur Respir J 32: 405–412, 2008 [DOI] [PubMed] [Google Scholar]
- 129.Soehnlein O, Xie X, Ulbrich H, Kenne E, Rotzius P, Flodgaard H, Eriksson EE, Lindbom L: Neutrophil-derived heparin-binding protein (HBP/CAP37) deposited on endothelium enhances monocyte arrest under flow conditions. J Immunol 174: 6399–6405, 2005 [DOI] [PubMed] [Google Scholar]
- 130.Taekema-Roelvink ME, Kooten C, Kooij SV, Heemskerk E, Daha MR: Proteinase 3 enhances endothelial monocyte chemoattractant protein-1 production and induces increased adhesion of neutrophils to endothelial cells by upregulating intercellular cell adhesion molecule-1. J Am Soc Nephrol 12: 932–940, 2001 [DOI] [PubMed] [Google Scholar]
- 131.Tjabringa GS, Aarbiou J, Ninaber DK, Drijfhout JW, Sørensen OE, Borregaard N, Rabe KF, Hiemstra PS: The antimicrobial peptide LL-37 activates innate immunity at the airway epithelial surface by transactivation of the epidermal growth factor receptor. J Immunol 171: 6690–6696, 2003 [DOI] [PubMed] [Google Scholar]
- 132.Di Gennaro A, Kenne E, Wan M, Soehnlein O, Lindbom L, Haeggström JZ: Leukotriene B4-induced changes in vascular permeability are mediated by neutrophil release of heparin-binding protein (HBP/CAP37/azurocidin). FASEB J 23: 1750–1757, 2009 [DOI] [PubMed] [Google Scholar]
- 133.Gautam N, Olofsson AM, Herwald H, Iversen LF, Lundgren-Akerlund E, Hedqvist P, Arfors KE, Flodgaard H, Lindbom L: Heparin-binding protein (HBP/CAP37): A missing link in neutrophil-evoked alteration of vascular permeability. Nat Med 7: 1123–1127, 2001 [DOI] [PubMed] [Google Scholar]
- 134.Akashi-Takamura S, Miyake K: TLR accessory molecules. Curr Opin Immunol 20: 420–425, 2008 [DOI] [PubMed] [Google Scholar]
- 135.Davidson DJ, Currie AJ, Reid GS, Bowdish DM, MacDonald KL, Ma RC, Hancock RE, Speert DP: The cationic antimicrobial peptide LL-37 modulates dendritic cell differentiation and dendritic cell-induced T cell polarization. J Immunol 172: 1146–1156, 2004 [DOI] [PubMed] [Google Scholar]
- 136.Yang D, Chen Q, Chertov O, Oppenheim JJ: Human neutrophil defensins selectively chemoattract naive T and immature dendritic cells. J Leukoc Biol 68: 9–14, 2000 [PubMed] [Google Scholar]
- 137.de la Rosa G, Yang D, Tewary P, Varadhachary A, Oppenheim JJ: Lactoferrin acts as an alarmin to promote the recruitment and activation of APCs and antigen-specific immune responses. J Immunol 180: 6868–6876, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Müller I, Munder M, Kropf P, Hänsch GM: Polymorphonuclear neutrophils and T lymphocytes: Strange bedfellows or brothers in arms? Trends Immunol 30: 522–530, 2009 [DOI] [PubMed] [Google Scholar]
- 139.Costantini C, Cassatella MA: The defensive alliance between neutrophils and NK cells as a novel arm of innate immunity. J Leukoc Biol 89: 221–233, 2011 [DOI] [PubMed] [Google Scholar]
- 140.Pelletier M, Maggi L, Micheletti A, Lazzeri E, Tamassia N, Costantini C, Cosmi L, Lunardi C, Annunziato F, Romagnani S, Cassatella MA: Evidence for a cross-talk between human neutrophils and Th17 cells. Blood 115: 335–343, 2010 [DOI] [PubMed] [Google Scholar]
- 141.Bhatnagar N, Hong HS, Krishnaswamy JK, Haghikia A, Behrens GM, Schmidt RE, Jacobs R: Cytokine-activated NK cells inhibit PMN apoptosis and preserve their functional capacity. Blood 116: 1308–1316, 2010 [DOI] [PubMed] [Google Scholar]
- 142.Costantini C, Micheletti A, Calzetti F, Perbellini O, Pizzolo G, Cassatella MA: Neutrophil activation and survival are modulated by interaction with NK cells. Int Immunol 22: 827–838, 2010 [DOI] [PubMed] [Google Scholar]
- 143.Scapini P, Lapinet-Vera JA, Gasperini S, Calzetti F, Bazzoni F, Cassatella MA: The neutrophil as a cellular source of chemokines. Immunol Rev 177: 195–203, 2000 [DOI] [PubMed] [Google Scholar]
- 144.Jones HR, Robb CT, Perretti M, Rossi AG: The role of neutrophils in inflammation resolution. Semin Immunol 28: 137–145, 2016 [DOI] [PubMed] [Google Scholar]
- 145.Serhan CN, Chiang N, Van Dyke TE: Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol 8: 349–361, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wu SH, Liao PY, Yin PL, Zhang YM, Dong L: Elevated expressions of 15-lipoxygenase and lipoxin A4 in children with acute poststreptococcal glomerulonephritis. Am J Pathol 174: 115–122, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.McMahon B, Mitchell D, Shattock R, Martin F, Brady HR, Godson C: Lipoxin, leukotriene, and PDGF receptors cross-talk to regulate mesangial cell proliferation. FASEB J 16: 1817–1819, 2002 [DOI] [PubMed] [Google Scholar]
- 148.Ohse T, Ota T, Kieran N, Godson C, Yamada K, Tanaka T, Fujita T, Nangaku M: Modulation of interferon-induced genes by lipoxin analogue in anti-glomerular basement membrane nephritis. J Am Soc Nephrol 15: 919–927, 2004 [DOI] [PubMed] [Google Scholar]
- 149.Uriarte SM, Rane MJ, Merchant ML, Jin S, Lentsch AB, Ward RA, McLeish KR: Inhibition of neutrophil exocytosis ameliorates acute lung injury in rats. Shock 39: 286–292, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Johnson JL, Ramadass M, He J, Brown SJ, Zhang J, Abgaryan L, Biris N, Gavathiotis E, Rosen H, Catz SD: Identification of neutrophil exocytosis inhibitors (Nexinhibs), small molecule inhibitors of neutrophil exocytosis and inflammation: DRUGGABILITY OF THE SMALL GTPase Rab27a. J Biol Chem 291: 25965–25982, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Barletta KE, Ley K, Mehrad B: Regulation of neutrophil function by adenosine. Arterioscler Thromb Vasc Biol 32: 856–864, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Futosi K, Fodor S, Mócsai A: Neutrophil cell surface receptors and their intracellular signal transduction pathways. Int Immunopharmacol 17: 638–650, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Favier B: Regulation of neutrophil functions through inhibitory receptors: An emerging paradigm in health and disease. Immunol Rev 273: 140–155, 2016 [DOI] [PubMed] [Google Scholar]
- 154.Devarapu SK, Kumar Vr S, Rupanagudi KV, Kulkarni OP, Eulberg D, Klussmann S, Anders HJ: Dual blockade of the pro-inflammatory chemokine CCL2 and the homeostatic chemokine CXCL12 is as effective as high dose cyclophosphamide in murine proliferative lupus nephritis. Clin Immunol 169: 139–147, 2016 [DOI] [PubMed] [Google Scholar]
- 155.Ohlsson S, Bakoush O, Tencer J, Torffvit O, Segelmark M: Monocyte chemoattractant protein 1 is a prognostic marker in ANCA-associated small vessel vasculitis. Mediators Inflamm 2009: 584916, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Liu ZH, Chen SF, Zhou H, Chen HP, Li LS: Glomerular expression of C-C chemokines in different types of human crescentic glomerulonephritis. Nephrol Dial Transplant 18: 1526–1534, 2003 [DOI] [PubMed] [Google Scholar]
- 157.Abujam B, Cheekatla S, Aggarwal A: Urinary CXCL-10/IP-10 and MCP-1 as markers to assess activity of lupus nephritis. Lupus 22: 614–623, 2013 [DOI] [PubMed] [Google Scholar]
- 158.Turner JE, Paust HJ, Bennstein SB, Bramke P, Krebs C, Steinmetz OM, Velden J, Haag F, Stahl RA, Panzer U: Protective role for CCR5 in murine lupus nephritis. Am J Physiol Renal Physiol 302: F1503–F1515, 2012 [DOI] [PubMed] [Google Scholar]
- 159.Mamtani M, Rovin B, Brey R, Camargo JF, Kulkarni H, Herrera M, Correa P, Holliday S, Anaya JM, Ahuja SK: CCL3L1 gene-containing segmental duplications and polymorphisms in CCR5 affect risk of systemic lupus erythaematosus. Ann Rheum Dis 67: 1076–1083, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Auer J, Bläss M, Schulze-Koops H, Russwurm S, Nagel T, Kalden JR, Röllinghoff M, Beuscher HU: Expression and regulation of CCL18 in synovial fluid neutrophils of patients with rheumatoid arthritis. Arthritis Res Ther 9: R94, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Brix SR, Stege G, Disteldorf E, Hoxha E, Krebs C, Krohn S, Otto B, Klätschke K, Herden E, Heymann F, Lira SA, Tacke F, Wolf G, Busch M, Jabs WJ, Özcan F, Keller F, Beige J, Wagner K, Helmchen U, Noriega M, Wiech T, Panzer U, Stahl RA: CC chemokine ligand 18 in ANCA-associated crescentic GN. J Am Soc Nephrol 26: 2105–2117, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Paust HJ, Turner JE, Riedel JH, Disteldorf E, Peters A, Schmidt T, Krebs C, Velden J, Mittrücker HW, Steinmetz OM, Stahl RA, Panzer U: Chemokines play a critical role in the cross-regulation of Th1 and Th17 immune responses in murine crescentic glomerulonephritis. Kidney Int 82: 72–83, 2012 [DOI] [PubMed] [Google Scholar]
- 163.Meng T, Li X, Ao X, Zhong Y, Tang R, Peng W, Yang J, Zou M, Zhou Q: Hemolytic Streptococcus may exacerbate kidney damage in IgA nephropathy through CCL20 response to the effect of Th17 cells. PLoS One 9: e108723, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Summers SA, van der Veen BS, O’Sullivan KM, Gan PY, Ooi JD, Heeringa P, Satchell SC, Mathieson PW, Saleem MA, Visvanathan K, Holdsworth SR, Kitching AR: Intrinsic renal cell and leukocyte-derived TLR4 aggravate experimental anti-MPO glomerulonephritis. Kidney Int 78: 1263–1274, 2010 [DOI] [PubMed] [Google Scholar]
- 165.Zhao Y, Zhu L, Zhou T, Zhang Q, Shi S, Liu L, Lv J, Zhang H: Urinary CXCL1: A novel predictor of IgA nephropathy progression. PLoS One 10: e0119033, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Imaizumi T, Aizawa T, Segawa C, Shimada M, Tsuruga K, Kawaguchi S, Matsumiya T, Yoshida H, Joh K, Tanaka H: Toll-like receptor 3 signaling contributes to the expression of a neutrophil chemoattractant, CXCL1 in human mesangial cells. Clin Exp Nephrol 19: 761–770, 2015 [DOI] [PubMed] [Google Scholar]
- 167.Disteldorf EM, Krebs CF, Paust HJ, Turner JE, Nouailles G, Tittel A, Meyer-Schwesinger C, Stege G, Brix S, Velden J, Wiech T, Helmchen U, Steinmetz OM, Peters A, Bennstein SB, Kaffke A, Llanto C, Lira SA, Mittrücker HW, Stahl RA, Kurts C, Kaufmann SH, Panzer U: CXCL5 drives neutrophil recruitment in TH17-mediated GN. J Am Soc Nephrol 26: 55–66, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wada T, Tomosugi N, Naito T, Yokoyama H, Kobayashi K, Harada A, Mukaida N, Matsushima K: Prevention of proteinuria by the administration of anti-interleukin 8 antibody in experimental acute immune complex-induced glomerulonephritis. J Exp Med 180: 1135–1140, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Cockwell P, Brooks CJ, Adu D, Savage CO: Interleukin-8: A pathogenetic role in antineutrophil cytoplasmic autoantibody-associated glomerulonephritis. Kidney Int 55: 852–863, 1999 [DOI] [PubMed] [Google Scholar]
- 170.Worthmann K, Gueler F, von Vietinghoff S, Davalos-Mißlitz A, Wiehler F, Davidson A, Witte T, Haller H, Schiffer M, Falk CS, Schiffer L: Pathogenetic role of glomerular CXCL13 expression in lupus nephritis. Clin Exp Immunol 178: 20–27, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Monach PA, Warner RL, Tomasson G, Specks U, Stone JH, Ding L, Fervenza FC, Fessler BJ, Hoffman GS, Iklé D, Kallenberg CG, Krischer J, Langford CA, Mueller M, Seo P, St Clair EW, Spiera R, Tchao N, Ytterberg SR, Johnson KJ, Merkel PA: Serum proteins reflecting inflammation, injury and repair as biomarkers of disease activity in ANCA-associated vasculitis. Ann Rheum Dis 72: 1342–1350, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lee HT, Shiao YM, Wu TH, Chen WS, Hsu YH, Tsai SF, Tsai CY: Serum BLC/CXCL13 concentrations and renal expression of CXCL13/CXCR5 in patients with systemic lupus erythematosus and lupus nephritis. J Rheumatol 37: 45–52, 2010 [DOI] [PubMed] [Google Scholar]
- 173.Kitching AR, Ru Huang X, Turner AL, Tipping PG, Dunn AR, Holdsworth SR: The requirement for granulocyte-macrophage colony-stimulating factor and granulocyte colony-stimulating factor in leukocyte-mediated immune glomerular injury. J Am Soc Nephrol 13: 350–358, 2002 [DOI] [PubMed] [Google Scholar]
- 174.Freeley SJ, Coughlan AM, Popat RJ, Dunn-Walters DK, Robson MG: Granulocyte colony stimulating factor exacerbates antineutrophil cytoplasmic antibody vasculitis. Ann Rheum Dis 72: 1053–1058, 2013 [DOI] [PubMed] [Google Scholar]
- 175.Lord PC, Wilmoth LM, Mizel SB, McCall CE: Expression of interleukin-1 alpha and beta genes by human blood polymorphonuclear leukocytes. J Clin Invest 87: 1312–1321, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Rastaldi MP, Ferrario F, Crippa A, Dell’Antonio G, Casartelli D, Grillo C, D’Amico G: Glomerular monocyte-macrophage features in ANCA-positive renal vasculitis and cryoglobulinemic nephritis. J Am Soc Nephrol 11: 2036–2043, 2000 [DOI] [PubMed] [Google Scholar]
- 177.Hahn WH, Cho BS, Kim SD, Kim SK, Kang S: Interleukin-1 cluster gene polymorphisms in childhood IgA nephropathy. Pediatr Nephrol 24: 1329–1336, 2009 [DOI] [PubMed] [Google Scholar]
- 178.Takemura T, Yoshioka K, Murakami K, Akano N, Okada M, Aya N, Maki S: Cellular localization of inflammatory cytokines in human glomerulonephritis. Virchows Arch 424: 459–464, 1994 [DOI] [PubMed] [Google Scholar]
- 179.Marucha PT, Zeff RA, Kreutzer DL: Cytokine regulation of IL-1 beta gene expression in the human polymorphonuclear leukocyte. J Immunol 145: 2932–2937, 1990 [PubMed] [Google Scholar]
- 180.Tomosugi NI, Cashman SJ, Hay H, Pusey CD, Evans DJ, Shaw A, Rees AJ: Modulation of antibody-mediated glomerular injury in vivo by bacterial lipopolysaccharide, tumor necrosis factor, and IL-1. J Immunol 142: 3083–3090, 1989 [PubMed] [Google Scholar]
- 181.Lebedeva TV, Singh AK: Increased responsiveness of B. cells in the murine MRL/lpr model of lupus nephritis to interleukin-1 beta. J Am Soc Nephrol 5: 1530–1534, 1995 [DOI] [PubMed] [Google Scholar]
- 182.Noronha IL, Krüger C, Andrassy K, Ritz E, Waldherr R: In situ production of TNF-alpha, IL-1 beta and IL-2R in ANCA-positive glomerulonephritis. Kidney Int 43: 682–692, 1993 [DOI] [PubMed] [Google Scholar]
- 183.O’Brien EC, Abdulahad WH, Rutgers A, Huitema MG, O’Reilly VP, Coughlan AM, Harrington M, Heeringa P, Little MA, Hickey FB: Intermediate monocytes in ANCA vasculitis: Increased surface expression of ANCA autoantigens and IL-1β secretion in response to anti-MPO antibodies. Sci Rep 5: 11888, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Shin MS, Kang Y, Lee N, Wahl ER, Kim SH, Kang KS, Lazova R, Kang I: Self double-stranded (ds)DNA induces IL-1β production from human monocytes by activating NLRP3 inflammasome in the presence of anti-dsDNA antibodies. J Immunol 190: 1407–1415, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ericson SG, Zhao Y, Gao H, Miller KL, Gibson LF, Lynch JP, Landreth KS: Interleukin-6 production by human neutrophils after Fc-receptor cross-linking or exposure to granulocyte colony-stimulating factor. Blood 91: 2099–2107, 1998 [PubMed] [Google Scholar]
- 186.Faldt J, Dahlgren C, Ridell M: Difference in neutrophil cytokine production induced by pathogenic and non-pathogenic mycobacteria. APMIS, 110: 593–600, 2002 [DOI] [PubMed] [Google Scholar]
- 187.Karkar AM, Smith J, Tam FW, Pusey CD, Rees AJ: Abrogation of glomerular injury in nephrotoxic nephritis by continuous infusion of interleukin-6. Kidney Int 52: 1313–1320, 1997 [DOI] [PubMed] [Google Scholar]
- 188.Cash H, Relle M, Menke J, Brochhausen C, Jones SA, Topley N, Galle PR, Schwarting A: Interleukin 6 (IL-6) deficiency delays lupus nephritis in MRL-Faslpr mice: the IL-6 pathway as a new therapeutic target in treatment of autoimmune kidney disease in systemic lupus erythematosus. J Rheumatol 37: 60–70, 2010 [DOI] [PubMed] [Google Scholar]
- 189.Suzuki H, Yasukawa K, Saito T, Narazaki M, Hasegawa A, Taga T, Kishimoto T: Serum soluble interleukin-6 receptor in MRL/lpr mice is elevated with age and mediates the interleukin-6 signal. Eur J Immunol 23: 1078–1082, 1993 [DOI] [PubMed] [Google Scholar]
- 190.Tang B, Matsuda T, Akira S, Nagata N, Ikehara S, Hirano T, Kishimoto T: Age-associated increase in interleukin 6 in MRL/lpr mice. Int Immunol 3: 273–278, 1991 [DOI] [PubMed] [Google Scholar]
- 191.Yoshioka K, Takemura T, Murakami K, Okada M, Yagi K, Miyazato H, Matsushima K, Maki S: In situ expression of cytokines in IgA nephritis. Kidney Int 44: 825–833, 1993 [DOI] [PubMed] [Google Scholar]
- 192.Li L, Huang L, Vergis AL, Ye H, Bajwa A, Narayan V, Strieter RM, Rosin DL, Okusa MD: IL-17 produced by neutrophils regulates IFN-gamma-mediated neutrophil migration in mouse kidney ischemia-reperfusion injury. J Clin Invest 120: 331–342, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Moran EM, Heydrich R, Ng CT, Saber TP, McCormick J, Sieper J, Appel H, Fearon U, Veale DJ: IL-17A expression is localised to both mononuclear and polymorphonuclear synovial cell infiltrates. PLoS One 6: e24048, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Gan PY, Steinmetz OM, Tan DS, O’Sullivan KM, Ooi JD, Iwakura Y, Kitching AR, Holdsworth SR: Th17 cells promote autoimmune anti-myeloperoxidase glomerulonephritis. J Am Soc Nephrol 21: 925–931, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Riedel JH, Paust HJ, Krohn S, Turner JE, Kluger MA, Steinmetz OM, Krebs CF, Stahl RA, Panzer U: IL-17F promotes tissue injury in autoimmune kidney diseases. J Am Soc Nephrol 27: 3666–3677, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ramani K, Pawaria S, Maers K, Huppler AR, Gaffen SL, Biswas PS: An essential role of interleukin-17 receptor signaling in the development of autoimmune glomerulonephritis. J Leukoc Biol 96: 463–472, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Wong CK, Ho CY, Li EK, Lam CW: Elevation of proinflammatory cytokine (IL-18, IL-17, IL-12) and Th2 cytokine (IL-4) concentrations in patients with systemic lupus erythematosus. Lupus 9: 589–593, 2000 [DOI] [PubMed] [Google Scholar]
- 198.Fortin CF, Ear T, McDonald PP: Autocrine role of endogenous interleukin-18 on inflammatory cytokine generation by human neutrophils. FASEB J 23: 194–203, 2009 [DOI] [PubMed] [Google Scholar]
- 199.Verri WA Jr, Cunha TM, Ferreira SH, Wei X, Leung BP, Fraser A, McInnes IB, Liew FY, Cunha FQ: IL-15 mediates antigen-induced neutrophil migration by triggering IL-18 production. Eur J Immunol 37: 3373–3380, 2007 [DOI] [PubMed] [Google Scholar]
- 200.Menke J, Bork T, Kutska B, Byrne KT, Blanfeld M, Relle M, Kelley VR, Schwarting A: Targeting transcription factor Stat4 uncovers a role for interleukin-18 in the pathogenesis of severe lupus nephritis in mice. Kidney Int 79: 452–463, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hewins P, Morgan MD, Holden N, Neil D, Williams JM, Savage CO, Harper L: IL-18 is upregulated in the kidney and primes neutrophil responsiveness in ANCA-associated vasculitis. Kidney Int 69: 605–615, 2006 [DOI] [PubMed] [Google Scholar]
- 202.Koenig KF, Groeschl I, Pesickova SS, Tesar V, Eisenberger U, Trendelenburg M: Serum cytokine profile in patients with active lupus nephritis. Cytokine 60: 410–416, 2012 [DOI] [PubMed] [Google Scholar]
- 203.Chen DY, Hsieh CW, Chen KS, Chen YM, Lin FJ, Lan JL: Association of interleukin-18 promoter polymorphisms with WHO pathological classes and serum IL-18 levels in Chinese patients with lupus nephritis. Lupus 18: 29–37, 2009 [DOI] [PubMed] [Google Scholar]
- 204.Leng L, Chen L, Fan J, Greven D, Arjona A, Du X, Austin D, Kashgarian M, Yin Z, Huang XR, Lan HY, Lolis E, Nikolic-Paterson D, Bucala R: A small-molecule macrophage migration inhibitory factor antagonist protects against glomerulonephritis in lupus-prone NZB/NZW F1 and MRL/lpr mice. J Immunol 186: 527–538, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Lan HY, Yang N, Nikolic-Paterson DJ, Yu XQ, Mu W, Isbel NM, Metz CN, Bucala R, Atkins RC: Expression of macrophage migration inhibitory factor in human glomerulonephritis. Kidney Int 57: 499–509, 2000 [DOI] [PubMed] [Google Scholar]
- 206.McColl SR, Paquin R, Ménard C, Beaulieu AD: Human neutrophils produce high levels of the interleukin 1 receptor antagonist in response to granulocyte/macrophage colony-stimulating factor and tumor necrosis factor alpha. J Exp Med 176: 593–598, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Tang WW, Feng L, Vannice JL, Wilson CB: Interleukin-1 receptor antagonist ameliorates experimental anti-glomerular basement membrane antibody-associated glomerulonephritis. J Clin Invest 93: 273–279, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Brugos B, Kiss E, Dul C, Gubisch W, Szegedi G, Sipka S, Zeher M: Measurement of interleukin-1 receptor antagonist in patients with systemic lupus erythematosus could predict renal manifestation of the disease. Hum Immunol 71: 874–877, 2010 [DOI] [PubMed] [Google Scholar]
- 209.Glowacka E, Banasik M, Lewkowicz P, Tchorzewski H: The effect of LPS on neutrophils from patients with high risk of type 1 diabetes mellitus in relation to IL-8, IL-10 and IL-12 production and apoptosis in vitro. Scand J Immunol 55: 210–217, 2002 [DOI] [PubMed] [Google Scholar]
- 210.De Santo C, Arscott R, Booth S, Karydis I, Jones M, Asher R, Salio M, Middleton M, Cerundolo V: Invariant NKT cells modulate the suppressive activity of IL-10-secreting neutrophils differentiated with serum amyloid A. Nat Immunol 11: 1039–1046, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Reglier H, Arce-Vicioso M, Fay M, Gougerot-Pocidalo MA, Chollet-Martin S: Lack of IL-10 and IL-13 production by human polymorphonuclear neutrophils. Cytokine 10: 192–198, 1998 [DOI] [PubMed] [Google Scholar]
- 212.Davey MS, Tamassia N, Rossato M, Bazzoni F, Calzetti F, Bruderek K, Sironi M, Zimmer L, Bottazzi B, Mantovani A, Brandau S, Moser B, Eberl M, Cassatella MA: Failure to detect production of IL-10 by activated human neutrophils. Nat Immunol 12: 1017–1018, author reply 1018–1020, 2011 [DOI] [PubMed] [Google Scholar]
- 213.Schotte H, Willeke P, Becker H, Poggemeyer J, Gaubitz M, Schmidt H, Schlüter B: Association of extended interleukin-10 promoter haplotypes with disease susceptibility and manifestations in German patients with systemic lupus erythematosus. Lupus 23: 378–385, 2014 [DOI] [PubMed] [Google Scholar]
- 214.Brandt E, Woerly G, Younes AB, Loiseau S, Capron M: IL-4 production by human polymorphonuclear neutrophils. J Leukoc Biol 68: 125–130, 2000 [PubMed] [Google Scholar]
- 215.Saleem S, Dai Z, Coelho SN, Konieczny BT, Assmann KJ, Baddoura FK, Lakkis FG: IL-4 is an endogenous inhibitor of neutrophil influx and subsequent pathology in acute antibody-mediated inflammation. J Immunol 160: 979–984, 1998 [PubMed] [Google Scholar]
- 216.Ifuku M, Miyake K, Watanebe M, Ito K, Abe Y, Sasatomi Y, Ogahara S, Hisano S, Sato H, Saito T, Nakashima H: Various roles of Th cytokine mRNA expression in different forms of glomerulonephritis. Am J Nephrol 38: 115–123, 2013 [DOI] [PubMed] [Google Scholar]
- 217.Grotendorst GR, Smale G, Pencev D: Production of transforming growth factor beta by human peripheral blood monocytes and neutrophils. J Cell Physiol 140: 396–402, 1989 [DOI] [PubMed] [Google Scholar]
- 218.Lianos EA, Orphanos V, Cattell V, Cook T, Anagnou N: Glomerular expression and cell origin of transforming growth factor-beta 1 in anti-glomerular basement membrane disease. Am J Med Sci 307: 1–5, 1994 [DOI] [PubMed] [Google Scholar]
- 219.Goumenos DS, Tsakas S, El Nahas AM, Alexandri S, Oldroyd S, Kalliakmani P, Vlachojannis JG: Transforming growth factor-beta(1) in the kidney and urine of patients with glomerular disease and proteinuria. Nephrol Dial Transplant 17: 2145–2152, 2002 [DOI] [PubMed] [Google Scholar]
- 220.Bliss SK, Marshall AJ, Zhang Y, Denkers EY: Human polymorphonuclear leukocytes produce IL-12, TNF-alpha, and the chemokines macrophage-inflammatory protein-1 alpha and -1 beta in response to Toxoplasma gondii antigens. J Immunol 162: 7369–7375, 1999 [PubMed] [Google Scholar]
- 221.Tsai CY, Wu TH, Yu CL, Tsai YY, Chou CT: Decreased IL-12 production by polymorphonuclear leukocytes in patients with active systemic lupus erythematosus. Immunol Invest 31: 177–189, 2002 [DOI] [PubMed] [Google Scholar]
- 222.Schwarting A, Tesch G, Kinoshita K, Maron R, Weiner HL, Kelley VR: IL-12 drives IFN-gamma-dependent autoimmune kidney disease in MRL-Fas(lpr) mice. J Immunol 163: 6884–6891, 1999 [PubMed] [Google Scholar]
- 223.Kikawada E, Lenda DM, Kelley VR: IL-12 deficiency in MRL-Fas(lpr) mice delays nephritis and intrarenal IFN-gamma expression, and diminishes systemic pathology. J Immunol 170: 3915–3925, 2003 [DOI] [PubMed] [Google Scholar]
- 224.Tucci M, Lombardi L, Richards HB, Dammacco F, Silvestris F: Overexpression of interleukin-12 and T helper 1 predominance in lupus nephritis. Clin Exp Immunol 154: 247–254, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Herber D, Brown TP, Liang S, Young DA, Collins M, Dunussi-Joannopoulos K: IL-21 has a pathogenic role in a lupus-prone mouse model and its blockade with IL-21R.Fc reduces disease progression. J Immunol 178: 3822–3830, 2007 [DOI] [PubMed] [Google Scholar]
- 226.Rinchai D, Khaenam P, Kewcharoenwong C, Buddhisa S, Pankla R, Chaussabel D, Bancroft GJ, Lertmemongkolchai G: Production of interleukin-27 by human neutrophils regulates their function during bacterial infection. Eur J Immunol 42: 3280–3290, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Summers SA, Phoon RK, Ooi JD, Holdsworth SR, Kitching AR: The IL-27 receptor has biphasic effects in crescentic glomerulonephritis mediated through Th1 responses. Am J Pathol 178: 580–590, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Shirafuji N, Matsuda S, Ogura H, Tani K, Kodo H, Ozawa K, Nagata S, Asano S, Takaku F: Granulocyte colony-stimulating factor stimulates human mature neutrophilic granulocytes to produce interferon-alpha. Blood 75: 17–19, 1990 [PubMed] [Google Scholar]
- 229.Denny MF, Yalavarthi S, Zhao W, Thacker SG, Anderson M, Sandy AR, McCune WJ, Kaplan MJ: A distinct subset of proinflammatory neutrophils isolated from patients with systemic lupus erythematosus induces vascular damage and synthesizes type I IFNs. J Immunol 184: 3284–3297, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Liu Z, Bethunaickan R, Huang W, Ramanujam M, Madaio MP, Davidson A: IFN-α confers resistance of systemic lupus erythematosus nephritis to therapy in NZB/W F1 mice. J Immunol 187: 1506–1513, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Liu Z, Bethunaickan R, Huang W, Lodhi U, Solano I, Madaio MP, Davidson A: Interferon-α accelerates murine systemic lupus erythematosus in a T cell-dependent manner. Arthritis Rheum 63: 219–229, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Markowitz GS, Nasr SH, Stokes MB, D’Agati VD: Treatment with IFN-alpha, -beta, or -gamma is associated with collapsing focal segmental glomerulosclerosis. Clin J Am Soc Nephrol 5: 607–615, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Obermoser G, Pascual V: The interferon-alpha signature of systemic lupus erythematosus. Lupus 19: 1012–1019, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Coit P, Yalavarthi S, Ognenovski M, Zhao W, Hasni S, Wren JD, Kaplan MJ, Sawalha AH: Epigenome profiling reveals significant DNA demethylation of interferon signature genes in lupus neutrophils. J Autoimmun 58: 59–66, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Ytterberg SR, Schnitzer TJ: Serum interferon levels in patients with systemic lupus erythematosus. Arthritis Rheum 25: 401–406, 1982 [DOI] [PubMed] [Google Scholar]
- 236.Bengtsson AA, Sturfelt G, Truedsson L, Blomberg J, Alm G, Vallin H, Rönnblom L: Activation of type I interferon system in systemic lupus erythematosus correlates with disease activity but not with antiretroviral antibodies. Lupus 9: 664–671, 2000 [DOI] [PubMed] [Google Scholar]
- 237.Evans R, Rudd P, Bass P, Harber M: Tip variant focal segmental glomerulosclerosis associated with interferon-beta treatment of multiple sclerosis. BMJ Case Reports, 2014, 2014. [DOI] [PMC free article] [PubMed]
- 238.Wallbach M, Gröne HJ, Kitze B, Müller GA, Koziolek MJ: Nephrotic syndrome in a multiple sclerosis patient receiving long-term interferon beta therapy. Am J Kidney Dis 61: 786–789, 2013 [DOI] [PubMed] [Google Scholar]
- 239.Ethuin F, Gérard B, Benna JE, Boutten A, Gougereot-Pocidalo MA, Jacob L, Chollet-Martin S: Human neutrophils produce interferon gamma upon stimulation by interleukin-12. Lab Invest 84: 1363–1371, 2004 [DOI] [PubMed] [Google Scholar]
- 240.Schwarting A, Wada T, Kinoshita K, Tesch G, Kelley VR: IFN-gamma receptor signaling is essential for the initiation, acceleration, and destruction of autoimmune kidney disease in MRL-Fas(lpr) mice. J Immunol 161: 494–503, 1998 [PubMed] [Google Scholar]
- 241.Haas C, Ryffel B, Le Hir M: IFN-gamma receptor deletion prevents autoantibody production and glomerulonephritis in lupus-prone (NZB x NZW)F1 mice. J Immunol 160: 3713–3718, 1998 [PubMed] [Google Scholar]
- 242.Jacob CO, van der Meide PH, McDevitt HO: In vivo treatment of (NZB X NZW)F1 lupus-like nephritis with monoclonal antibody to gamma interferon. J Exp Med 166: 798–803, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Kim K, Cho SK, Sestak A, Namjou B, Kang C, Bae SC: Interferon-gamma gene polymorphisms associated with susceptibility to systemic lupus erythematosus. Ann Rheum Dis 69: 1247–1250, 2010 [DOI] [PubMed] [Google Scholar]
- 244.Dubravec DB, Spriggs DR, Mannick JA, Rodrick ML: Circulating human peripheral blood granulocytes synthesize and secrete tumor necrosis factor alpha. Proc Natl Acad Sci USA 87: 6758–6761, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Bazzoni F, Cassatella MA, Laudanna C, Rossi F: Phagocytosis of opsonized yeast induces tumor necrosis factor-alpha mRNA accumulation and protein release by human polymorphonuclear leukocytes. J Leukoc Biol 50: 223–228, 1991 [DOI] [PubMed] [Google Scholar]
- 246.Parks CG, Pandey JP, Dooley MA, Treadwell EL, St Clair EW, Gilkeson GS, Feghali-Bostwick CA, Cooper GS: Genetic polymorphisms in tumor necrosis factor (TNF)-alpha and TNF-beta in a population-based study of systemic lupus erythematosus: Associations and interaction with the interleukin-1alpha-889 C/T polymorphism. Hum Immunol 65: 622–631, 2004 [DOI] [PubMed] [Google Scholar]
- 247.Puga I, Cols M, Barra CM, He B, Cassis L, Gentile M, Comerma L, Chorny A, Shan M, Xu W, Magri G, Knowles DM, Tam W, Chiu A, Bussel JB, Serrano S, Lorente JA, Bellosillo B, Lloreta J, Juanpere N, Alameda F, Baró T, de Heredia CD, Torán N, Català A, Torrebadell M, Fortuny C, Cusí V, Carreras C, Diaz GA, Blander JM, Farber CM, Silvestri G, Cunningham-Rundles C, Calvillo M, Dufour C, Notarangelo LD, Lougaris V, Plebani A, Casanova JL, Ganal SC, Diefenbach A, Aróstegui JI, Juan M, Yagüe J, Mahlaoui N, Donadieu J, Chen K, Cerutti A: B cell-helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nat Immunol 13: 170–180, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Huard B, McKee T, Bosshard C, Durual S, Matthes T, Myit S, Donze O, Frossard C, Chizzolini C, Favre C, Zubler R, Guyot JP, Schneider P, Roosnek E: APRIL secreted by neutrophils binds to heparan sulfate proteoglycans to create plasma cell niches in human mucosa. J Clin Invest 118: 2887–2895, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Vincent FB, Northcott M, Hoi A, Mackay F, Morand EF: Association of serum B cell activating factor from the tumour necrosis factor family (BAFF) and a proliferation-inducing ligand (APRIL) with central nervous system and renal disease in systemic lupus erythematosus. Lupus 22: 873–884, 2013 [DOI] [PubMed] [Google Scholar]
- 250.Scapini P, Nardelli B, Nadali G, Calzetti F, Pizzolo G, Montecucco C, Cassatella MA: G-CSF-stimulated neutrophils are a prominent source of functional BLyS. J Exp Med 197: 297–302, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Fletcher CA, Groom JR, Woehl B, Leung H, Mackay C, Mackay F: Development of autoimmune nephritis in genetically asplenic and splenectomized BAFF transgenic mice. J Autoimmun 36: 125–134, 2011 [DOI] [PubMed] [Google Scholar]
- 252.Ramanujam M, Bethunaickan R, Huang W, Tao H, Madaio MP, Davidson A: Selective blockade of BAFF for the prevention and treatment of systemic lupus erythematosus nephritis in NZM2410 mice. Arthritis Rheum 62: 1457–1468, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Zhao LD, Li Y, Smith MF Jr, Wang JS, Zhang W, Tang FL, Tian XP, Wang HY, Zhang FC, Ba DN, He W, Zhang X: Expressions of BAFF/BAFF receptors and their correlation with disease activity in Chinese SLE patients. Lupus 19: 1534–1549, 2010 [DOI] [PubMed] [Google Scholar]
- 254.Tarzi RM, Sharp PE, McDaid JP, Fossati-Jimack L, Herbert PE, Pusey CD, Cook HT, Warrens AN: Mice with defective Fas ligand are protected from crescentic glomerulonephritis. Kidney Int 81: 170–178, 2012 [DOI] [PubMed] [Google Scholar]
- 255.Cui JH, Qiao Q, Guo Y, Zhang YQ, Cheng H, He FR, Zhang J: Increased apoptosis and expression of FasL, Bax and caspase-3 in human lupus nephritis class II and IV. J Nephrol 25: 255–261, 2012 [DOI] [PubMed] [Google Scholar]
- 256.Nguyen V, Cudrici C, Zernetkina V, Niculescu F, Rus H, Drachenberg C, Rus V: TRAIL, DR4 and DR5 are upregulated in kidneys from patients with lupus nephritis and exert proliferative and proinflammatory effects. Clin Immunol 132: 32–42, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]