Abstract
Hyperinsulinemic hypoglycemia (HI) and congenital polycystic kidney disease (PKD) are rare, genetically heterogeneous disorders. The co-occurrence of these disorders (HIPKD) in 17 children from 11 unrelated families suggested an unrecognized genetic disorder. Whole-genome linkage analysis in five informative families identified a single significant locus on chromosome 16p13.2 (logarithm of odds score 6.5). Sequencing of the coding regions of all linked genes failed to identify biallelic mutations. Instead, we found in all patients a promoter mutation (c.-167G>T) in the phosphomannomutase 2 gene (PMM2), either homozygous or in trans with PMM2 coding mutations. PMM2 encodes a key enzyme in N-glycosylation. Abnormal glycosylation has been associated with PKD, and we found that deglycosylation in cultured pancreatic β cells altered insulin secretion. Recessive coding mutations in PMM2 cause congenital disorder of glycosylation type 1a (CDG1A), a devastating multisystem disorder with prominent neurologic involvement. Yet our patients did not exhibit the typical clinical or diagnostic features of CDG1A. In vitro, the PMM2 promoter mutation associated with decreased transcriptional activity in patient kidney cells and impaired binding of the transcription factor ZNF143. In silico analysis suggested an important role of ZNF143 for the formation of a chromatin loop including PMM2. We propose that the PMM2 promoter mutation alters tissue-specific chromatin loop formation, with consequent organ-specific deficiency of PMM2 leading to the restricted phenotype of HIPKD. Our findings extend the spectrum of genetic causes for both HI and PKD and provide insights into gene regulation and PMM2 pleiotropy.
Keywords: polycystic kidney disease, hyperinsulinemic hypoglycemia, glycosylation, promoter, PMM2, ZNF143
Autosomal recessive polycystic kidney disease (ARPKD), a rare disorder with an estimated incidence of 1:20,000 live births, is characterized by the combination of polycystic kidneys and hepatic fibrosis.1 It is caused by mutations in PKHD1, although mutations in other ciliary disease genes may phenocopy the disorder.2,3 Causative mutations are identified in approximately 85% of cases.4 Here, we investigated patients with an ARPKD-like clinical presentation that were genetically unsolved and prominently characterized by a concurrent clinical diagnosis of hyperinsulinemic hypoglycemia (HI). HI in itself is also a rare disorder, with an estimated incidence of 1:50,000 and most commonly associated with mutations in the ABCC8 and KCNJ11 genes involved in the regulation of insulin release from pancreatic β cells.5,6 However, no causative mutations had been identified in these genes in any of the patients described here.
The co-occurrence of these two rare disorders in multiple patients and without identified mutations in known disease genes strongly suggested the existence of a yet undescribed Mendelian disorder with recessive inheritance.
Results
Patients
We noted the co-occurrence of the clinical diagnoses of HI and polycystic kidney disease (HIPKD) in 17 patients from 11 families of European descent (Supplemental Figure 1, Table 1), including a consanguineous family with four affected individuals. Multiple siblings were affected in three further pedigrees, consistent with autosomal recessive inheritance (Figure 1, Supplemental Figure 1).
Table 1.
Clinical details
| Variable | Patient ID | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.1 | 1.2 | 1.3 | 1.4 | 2.1 | 2.2 | 3.1 | 4.1 | 5.1 | 6.1 | 7.1 | 8.1 | 9.1 | 9.2 | 10.1 | 10.2 | 11.1 | 12.1 | |
| Sex | Male | Female | Female | Female | Female | Male | Male | Male | Female | Male | Female | Female | Male | Male | Female | Male | Female | Male |
| Mutation | ||||||||||||||||||
| Nucleotide | c.-167G>T/c.-167G>T | c.-167G>T/c.-167G>T | c.-167G>T/c.-167G>T | c.-167G>T/c.-167G>T | c.-167G>T/c.422G>A | c.-167G>T/c.422G>A | c.-167G>T/c.470T>C | c.-167G>T/c.422G>A | c.-167G>T/c.422G>A | c.-167G>T/c.422G>A | c.-167G>T/c.422G>A | c.-167G>T/c.255+1G>A | c.-167G>T/c.620T>C | c.-167G>T/c.620T>C | c.-167G>T/c.470T>C | c.-167G>T/c.470T>C | c.-167G>T/c.470T>C | c.-167G>T/c.24del |
| Predicted protein | p.?/p.? | p.?/p.? | p.?/p.? | p.?/p.? | p.?/p.Arg141His | p.?/p.Arg141His | p.?/Phe157Ser | p.?/p.Arg141His | p.?/p.Arg141His | p.?/p.Arg141His | p.?/p.Arg141His | p.?/p.? | p.?/p.Phe207Ser | p.?/p.Phe207Ser | p.?/p.Phe157Ser | p.?/p.Phe157Ser | p.?/p.Phe157Ser | p.?/p.Leu8fsX |
| Hyperinsulinism | ||||||||||||||||||
| Birth weight, g | 2880 | 3400 | 3000 | 3750 | 3850 | 3400 | 3325 | 2960 | N/A | 4200 | 4252 | 4500 | 3440 | 3450 | N/A | 3720 | N/A | 3160 |
| Age at diagnosis, mo | 6 | 10 | 48 | 10 | 0 | 8 | 21 | 0 | 4 | 0 | N/A | 12 | 14 | 0 | 18 | 11 | 14 | 24 |
| Treatment | None | None | None | None | Diazoxide | Diazoxide | Diazoxide | Diazoxide | N/A | Diazoxide | Diazoxide/cornstarch | N/A | Diazoxide | Diazoxide | N/A | N/A | Diazoxide | Diazoxide |
| Renal disease | ||||||||||||||||||
| Antenatal abnormalities | Cysts/polyhydramnios | No | No | No | No | No | No | No | N/A | Cysts | N/A | N/A | Cysts | Cysts | N/A | N/A | No | No |
| Age at renal diagnosis, mo | 0 | 6 | 48 | 11 | 48 | 0 | 21 | 0 | 36 | 0 | 36 | 15 | 0 | 0 | N/A | N/A | 20 | 0 |
| eGFR at diagnosis, ml/min per 1.73 m2 | 70 | 100 | 85 | 115 | 95 | 90 | 90 | 50 | 95 | 20 | 120 | 75 | 30 | 40 | N/A | N/A | 105 | N/A |
| Kidney size, percentile | >95th | >95th | >95th | >95th | >95th | >95th | >95th | >95th | >95th | >95th | >95th | >95th | N/A | N/A | N/A | N/A | >95th | >95th |
| Morphology | Cysts | Hyperechogenicity/cysts | Hyperechogenicity/cysts | Hyperechogenicity/cysts | Cysts | Cysts | Cysts | Cysts | Cysts | Cysts | Cysts | Cysts | Cysts | Cysts | Cysts | Cysts | Cysts | Cysts |
| Age at last f/u, yr | 10 | 15 | 20 | 14 | 11 | 4 | 1 | 2 | 14 | 6 | 11 | 7 | 4 | 0.3 | N/A | N/A | 11 | 19 |
| eGFR at last f/u, ml/min per 1.73m2 | 70 | 90 | <10 (18) | 75 | 80 | 40 | 90 | 50 | 85 | <10 (2) | 141 | 40 | 65 | 70 | N/A | N/A | 94 | <10 (7) |
| Liver disease | ||||||||||||||||||
| Morphology (ultrasound) | Normal | Normal | Normal | Normal | Cysts | Cysts | Normal | Normal | Coarse echotexture | Cysts | Cysts | Normala | Normal | Normal | Cysts | N/A | Cysts | Cysts |
| Liver function tests | Normal | Normal | Normal | Normal | Normal | Normal | Normal | Normal | N/A | Normal | Normal | Normal | Normal | Normal | Normal | Normal | N/A | Normal |
Shown are pertinent clinical data for the 17 patients. Note the presence of the PMM2 promoter mutation c.-167G>T in all patients. ID, identifier; N/A, not available; f/u, follow-up.
Ultrasound imaging of the liver was normal, yet a biopsy revealed ductal plate malformation (see Figure 3).
Figure 1.
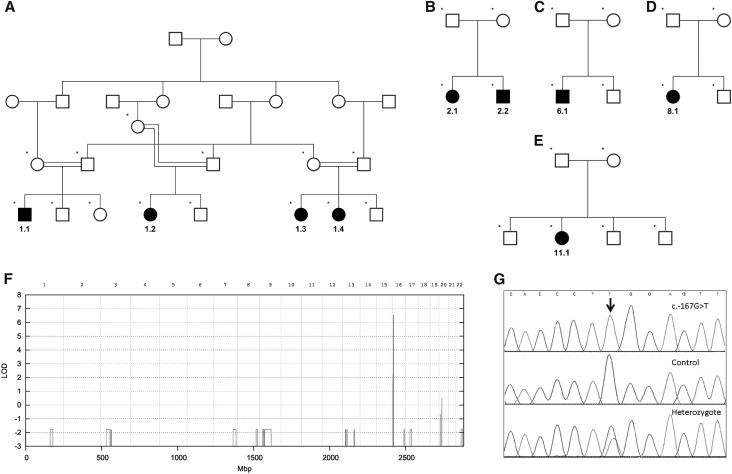
Genetic studies identify a shared mutation. (A–E) Pedigrees of informative families used for linkage analysis. (F) The combined parametric multipoint linkage analysis reveals a single significant peak with a maximum LOD score of 6.5 on chromosome 16. (G) Sequencing of the linked region reveals the presence of the mutation c.-167G>T in the promoter of PMM2 in all patients. Shown are electropherogram traces of the region c.-173_–c.161 from a patient homozygous for the mutation, a control (wild-type) subject, and a heterozygote parent.
Eight patients from seven families had been referred for genetic testing for HI through an international study of 1250 families. All patients had been tested for ABCC8 and KCNJ11 mutations, which are the most common cause of HI, yet none were found. Mutations in PKHD1, the causative gene for ARPKD, had been excluded by linkage and/or sequencing analysis in 14 patients.
Kidney Disease
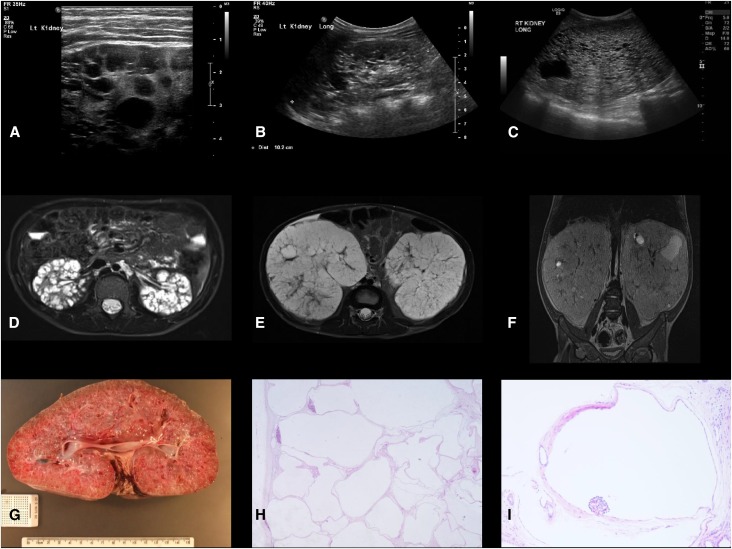
All patients were found to have enlarged kidneys with multiple cysts on imaging, prompting an initial clinical diagnosis of ARPKD (Figure 2). Severity of kidney disease was variable: three patients presented antenatally; in others kidney cysts were discovered incidentally during childhood. Blood tests were consistent with CKD stage 1 or 2 in nine patients, whereas two patients (1.1 and 6.1, see Table 1) progressed to ESRD at ages 20 and 2 years, respectively, with need for dialysis or transplantation. Native kidneys were removed at the time of transplant in patient 6.1 due to their massive size (20 cm longitudinal diameter, normal <7.7), and histology was consistent with a predominantly glomerulocystic disorder (Figure 2).
Figure 2.
Renal imaging and histology in children with HIPKD. (A) Ultrasound images of the kidney from patient 2.1, left kidney, age 11 years; (B) patient 2.2, right kidney, age 4 years; and (C) patient 6.1, right kidney, age 2 years. Note the presence of cysts of various sizes. Kidney length was >95th percentile for age in all patients. (D) Axial MRI image of the kidney from patient 2.1, age 11 years; (E and F) axial and coronal MRI images (without contrast) from patient 6.1, age 2 years. Note the massively enlarged kidneys with cysts of various sizes. (G) Macroscopic appearance of the nephrectomy specimen from patient 6.1, age 2 years, removed at the time of transplant. Note the large kidney size (20 cm longitudinal, normal <7.7 cm) and numerous macroscopic cysts. (H and I) Histology of the same kidney demonstrating multiple cysts lined by simple attenuated epithelium, with glomeruli noted in some (glomerulocystic disease; hematoxylin and eosin; original magnifications, ×20 and ×100, respectively). Dist, distance; Lt, left; Rt, right.
Liver Disease
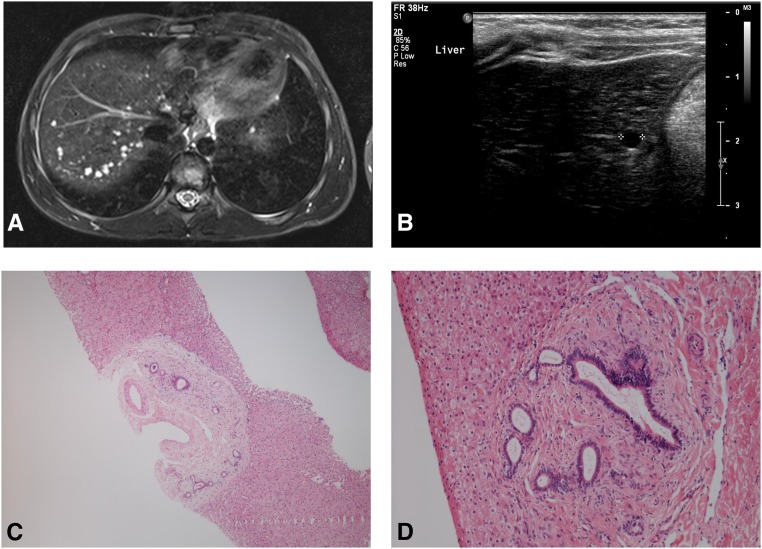
In addition, liver cysts were seen in eight patients. Other patients had no abnormalities on liver imaging (Table 1), including patient 8.1, whose liver biopsy (age 1 year) showed ductal plate malformation (Figure 3). Liver function tests were normal or only borderline elevated.
Figure 3.
Liver involvement in HIPKD. (A) Shown is an axial T2-weighted (STIR sequence) MRI image of patient 2.1 showing numerous small liver cysts. (B) Ultrasound image of the liver of patient 2.2 showing a cyst. (C and D) Photomicrographs of the liver biopsy specimen from patient 8.1, demonstrating abnormal and expanded portal tracts consistent with ductal plate malformation (hematoxylin and eosin; original magnifications, ×40 and ×100, respectively).
HI
HI was diagnosed at a median age of 10 months of life, often presenting with hypoglycemic seizures. The spectrum of severity varied and in one patient (1.3) diagnosis of HI was made only at 4 years of age. Most were treated with diazoxide and responded to this therapy, although some patients did not receive any treatment.
Other Organs
No manifestations in other organs or dysmorphologies were noted. Specifically, there was no evidence for neurologic impairment. Patients 2.1, 2.2, and 6.1 underwent detailed audiologic and ophthalmologic testing including electroretinogram, which revealed no abnormalities.
Serum transferrin glycosylation was assessed by isoelectric focusing in 11 patients and was normal in all, excluding a substantial global defect in glycosylation.
Genetic Analysis
Autozygosity analysis of SNP-chip genotype data in the consanguineous family revealed a homozygous 2.5 Mb region on chromosome 16p.13.2. Whole-genome multipoint parametric linkage analysis in this and four other informative families confirmed and refined this to a single significant locus of 2.3 Mb, including 14 annotated genes on chromosome 16p.13.2 with a combined LOD score of 6.5 (Figure 1).
Using next-generation sequencing, we identified a noncoding variant in the promoter region for PMM2 (encoding Phosphomannomutase 2, a key enzyme in N-glycosylation), c.-167G>T, not previously documented in databases (Figure 1). Sanger sequencing confirmed the presence of this mutation in all patients. It was found in homozygous state in the four patients from the consanguineous family and in compound heterozygote state with previously described deleterious coding mutations in PMM2 in all others (Table 1). Sequencing of the parents from families 2–11 confirmed that these mutations were inherited in trans.
Functional Studies of the Promoter Variant
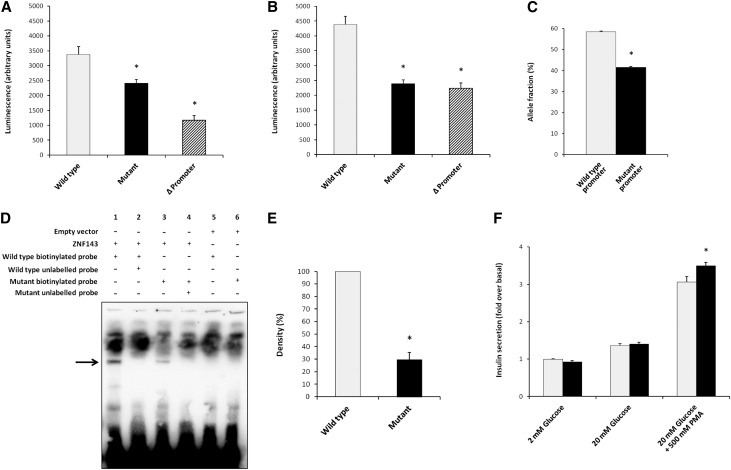
The identified c.-167G>T mutation lies in a region previously described as a promoter of PMM2.7 We assessed the effect of the promoter variant on PMM2 transcription in vitro, using human kidney and pancreatic β cell lines. Cells were transfected with luciferase under the control of either wild-type or mutant promoter. In both cell models, the promoter variant caused a significant reduction in luciferase activity (Figure 4, A and B). We also assessed PMM2 transcription in kidney cells derived from the nephrectomy from patient 6.1, compound heterozygous for the promoter mutation c.-167G>T and the missense mutation c.422G>A; p.R141H. Digital quantitative PCR revealed reduced expression of the allele containing the promoter variant compared with the other allele (Figure 4C). The promoter mutation lies in a recognized binding site for the transcription factor ZNF143.7 We therefore assessed binding of ZNF143 to the promoter using an Electrophoretic Mobility Shift Assay (EMSA), which demonstrated significantly reduced binding to the mutant promoter compared with wild type (Figure 4, D and E).
Figure 4.
The c.-167G>T mutation impairs promoter function. Luciferase activity is significantly reduced with mutant compared with wild-type promoter in (A) human pancreatic β cell line 1.1B4 and (B) human kidney cell line RPTEC/TERT1. Light gray bars show luciferase activity with wild-type promoter, black bars with the c.-167G>T mutation, and the crosshatched bars show activity with deletion of the ZNF143 binding site c.-163_-180del. Asterisk indicates significance (wild type versus respective mutation; P<0.01). (C) In kidney cells derived from patient 6.1, expression of the allele with the mutant promoter (c.-167G>T) is significantly reduced compared with the other allele. Asterisk indicates significance (P<0.01). (D) EMSA demonstrating reduced affinity of mutant promoter to ZNF143. Note the impaired binding of ZNF143 (indicated by arrow) to the mutant (lane 3) compared with wild-type (lane 1) PMM2 promoter. Binding is specific, because addition of excess unlabeled wild-type (lane 2) or mutant promoter probe (lane 4) abolishes binding to the labeled probes. Moreover, no specific binding is seen in the negative control (no ZNF143) to either wild-type (lane 5) or mutant (lane 6) promoter probe. (E) Densitometry confirms significantly reduced affinity of ZNF143 to mutant promoter (P<0.01). Bands from lane 1 and 3 indicated by arrow in (D) were measured by densitometry and results pooled from five independent experiments. (F) Deglycosylation alters insulin secretion in a murine pancreatic β cell line (MIN6). There is significant increase in insulin secretion after stimulation with high glucose (P<0.01), as expected. Note the significant (P<0.01) increase in insulin secretion with deglycosylation after further stimulation with phorbol-12 myristate-13 acetate (PMA).
To assess the consequences of decreased PMM2 activity on insulin secretion, we investigated the effect of deglycosylation in a murine pancreatic β cell line.8 We noted a significant increase in insulin secretion after stimulation with the protein kinase C activator, phorbol-12 myristate-13 acetate (Figure 4F), establishing a direct link between glycosylation and insulin secretion.
Discussion
HIPKD: A Disorder with Pancreatic, Kidney, and Liver Manifestations
We describe a previously unreported disorder presenting with ARPKD-like kidney disease and HI in childhood.
Most patients presented with hypoglycemia in the first year of life, consistent with congenital HI. Hypoglycemia was the first recognized manifestation of HIPKD in most patients, typically presenting with seizures. However, severity varied with delayed diagnosis in some.
All patients had enlarged cystic kidneys, which typically led to an initial diagnosis of ARPKD. Yet, kidney manifestations of HIPKD differ from ARPKD. Histology of the kidneys from patient 6.1 shows predominantly, if not exclusively, glomerular cysts, whereas in ARPKD cysts arise from fusiform dilatation of the collecting duct.3 Several of the patients had renal cysts seen on antenatal ultrasound, yet none had severe manifestations with oligohydramnios and consequent complications, such as pulmonary hypoplasia. Instead, two patients (1.1 and 6.1), including the one with the earliest progression to ESRD (age 2 years), had antenatal documentation of polyhydramnios. In contrast, about 30%–40% of patients with ARPKD die in the neonatal period from complications of oligohydramnios.4
Liver involvement is an obligate aspect of ARPKD and the presence of liver manifestations initially seemed to support a diagnosis of ARPKD in our patients. Liver abnormalities on imaging were noted in eight patients, mostly showing scattered cysts and/or a heterogeneous echotexture. Liver enzyme levels in plasma were normal or only borderline abnormal. Interestingly, liver imaging was normal in patient 8.1, who underwent a liver biopsy to establish a diagnosis, which showed ductal plate malformation (Figure 3). This is the only patient in our cohort who had a liver biopsy and it suggests that the absence of abnormalities on imaging or blood tests does not exclude the possibility of microscopic structural changes in the liver of patients with HIPKD. None of the patients reported here had evidence of portal hypertension, a complication seen in about a quarter of patients with ARPKD.9
HIPKD and the Promoter Mutation
The identification of a promoter mutation in PMM2 was unexpected, because there was no evidence for a systemic disorder of glycosylation in our patients. Autosomal recessive mutations in PMM2 are the cause of a devastating multisystemic disease called congenital disorder of glycosylation 1a (CDG1A). Neurologic problems usually predominate the phenotype of CDG1A, with affected patients presenting with severe developmental delay, strabism, and muscular hypotonia, none of which was noted in our patients. Moreover, typical dysmorphic features of CDG1A, such as protruding ears, inverted nipples, and abnormal subcutaneous fat pads, were not seen in any of our patients with HIPKD. Infantile demise is common.10 Later on, in surviving patients, other complications, such as thrombotic events, skeletal deformities, epilepsy, retinitis pigmentosa, hypogonadism, and peripheral neuropathy become apparent.11 None of our patients presented any evidence for such involvement, clearly distinguishing HIPKD from CDG1A.
A key diagnostic feature of CDG1A is the presence of specific abnormalities seen with isoelectric focusing of transferrin due to abnormal glycosylation. Importantly, this test was normal in all patients with HIPKD tested. Although normal, or only slightly abnormal, transferrin isoelectric patterns have been reported in a few patients with a mild form of CDG1A with isolated mild neurologic and no visceral involvement, this is clearly clinically distinct from patients with HIPKD.12,13
The phenotype of HIPKD appears to be restricted to kidneys and pancreatic β cells with additional liver involvement, which is distinct from CDG1A, where visceral involvement is only noted in the severe early-onset form with neurologic and dysmorphic manifestations. A potential explanation for this organ-specific involvement would be a unique sensitivity of kidney, pancreas, and liver to impaired PMM2 function. Under this hypothesis, the promoter mutation would affect PMM2 function to just such a degree that only kidney, pancreatic β cells, and liver are affected, whereas PMM2 activity was still sufficient to avoid manifestations in other organs. However, this is not consistent with observations in patients with CDG1A, as those with mild forms show isolated neurologic involvement only.12,13 This strongly suggests that the brain is most sensitive to loss of PMM2 function and mild impairment thus primarily causes neurologic problems. The severity of manifestations seen in patients with HIPKD in the three key organ systems involved, yet with no evidence of systemic involvement, clearly argues for a tissue-specific effect of the promoter mutation. Although specific neurologic investigations, such as an MRI scan of the brain, have not been performed due to a lack of clinical indication, previous reports of patients with CDG1A with visceral manifestations as seen in HIPKD also had associated debilitating neurologic and dysmorphic features, which are absent in HIPKD.
Mutations affecting PMM2 function or transcription can therefore cause either CDG1A or HIPKD and such pleiotropy is well recognized: mutations in TRPV4, for instance, are associated with at least eight different disorders, comprising such divergent clinical phenotypes as skeletal dysplasias or motor and sensory neuropathies.14 Mutations in OCRL cause either the systemic disorder Lowe syndrome or the kidney-specific Dent disease.15 Yet, although the pleiotropic effects of most of these genes are poorly understood, our data here clearly point to tissue-specific dysregulation of PMM2 by the promoter mutation as the key disease mechanism in HIPKD. Indeed, it has been suggested that altered chromatin looping allows for tissue-specific interactions between enhancers and promoters, thus constituting a key mechanism for genetic pleiotropy.16 Our promoter mutation appears to confirm this concept.
Promoter Mutation and Chromatin Loops
Gene expression can be modulated by regulatory regions many megabases away from the coding region and it is the three-dimensional architecture of chromatin that is critical to allow this interaction.17 This involves the formation of structural chromatin “loops,” typically between 40 kb and 3 Mb in size and flanked by binding sites for CCCTC–binding factor (CTCF) in convergent orientation.17 These loops, also called “topologically associated domains” (TAD), serve as fundamental regulatory units of transcription.18 Inspection of publicly available data for transcription-factor binding and chromatin interactions shows that the region around PMM2 contains a number of CTCF sites and functional promoters (Supplemental Figure 2). Interaction between pairs of CTCF sites has been observed in a variety of combinations, potentially dynamic and tissue-specific in nature, to form localized chromatin loops. Moreover, these data suggest an interaction between ZNF143 and specific CTCF sites, consistent with previous observations of functional interactions between these two DNA binding proteins, which affect the three-dimensional structure of such chromatin loops by linking CTCF with distal regulatory elements, facilitating specific gene regulation.19–21 Here, ZNF143 binds to the PMM2 promoter, as confirmed by our EMSA data (Figure 4). By simultaneously binding CTCF and the PMM2 promoter, ZNF143 could establish a functional chromatin loop enabling interaction between the promoter and a number of regulatory binding sites. This would allow specific spatiotemporal regulation of PMM2, depending on the expression profile of respective regulatory factors. Although the expression of all genes contained in the chromatin loop could be affected by the promoter mutation, the fact that it is found in trans with coding mutations in PMM2 clearly implicates specific impairment of PMM2 regulation as the key disease mechanism in HIPKD. Although other genes in the loop and especially TMEM167, which is regulated by the same promoter as PMM2, may also be affected, this effect is expected to be the same as in the asymptomatic heterozygous carriers of the promoter mutation without a PMM2 coding mutation in trans. The key role of PMM2 is further supported by the decreased PMM2 expression with the promoter mutation (Figure 4). Although PMM2 is expressed ubiquitously, there is evidence for additional tissue-specific regulation.22 A potential candidate involved in such tissue-specific regulation is the transcription factor HNF4A, which is expressed predominantly in the three organ systems involved in HIPKD: liver, pancreas, and kidney.23 Indeed, we previously linked mutations in HNF4A with HI and kidney disease.24 Clusters of HNF4A binding site are present in the loop (Supplemental Figures 2 and 3). HNF4A could affect PMM2 transcription either positively in the setting of the wild-type promoter, because it would require binding of ZNF143 to establish the functional loop, or negatively, by destabilizing a weakened interaction between mutant PMM2 promoter and ZNF143/CTCF. Under both scenarios, there would be decreased PMM2 transcription from the mutant promoter in HNF4A-expressing cells, compatible with tissue-specific disease as seen in HIPKD.
HIPKD and Glycosylation
Defective glycosylation has been previously linked to the development of HI or cystic kidney disease and both phenotypes have been described as part of the spectrum of clinical manifestations in the multivisceral form of CDG1A.25–29 Yet, given the predominance of neurologic problems, only few data on the renal involvement in CDG1A are available, mainly that cysts can be seen on imaging, which on antenatal ultrasound may manifest as increased echogenicity (“antenatal bright kidneys”), and in cases with postmortem examination tubular microcysts were noted.25,29,30 Interestingly, the latter is different to the only available histology in our HIPKD cohort, which shows predominantly, if not exclusively, glomerular cysts (Figure 2).
The consequences of impaired glycosylation for cystogenesis are evident from the recent identification of mutations in GANAB as a cause of kidney and liver cysts.31 Several animal studies provide further links: mice deleted for Aqp11 develop cystic kidney disease due to altered glycosylation of polycystin1,32 and defective glycosylation of polycystin2 leads to cyst formation.33 Thus, impaired glycosylation of key proteins involved in cystic kidney disease may be responsible for the development of renal cysts in HIPKD. There is also evidence for the importance of glycosylation for targeted membrane trafficking of the sulfonyl urea receptor (SUR), a key protein for regulated insulin secretion.34 This complements our own data, showing altered insulin secretion after deglycosylation (Figure 4E). Similarly, polycystic liver disease is associated with mutations in genes that cause abnormal glycosylation or processing of glycoproteins.35
In conclusion, we report a previously undescribed disease with the key manifestations of HI and polycystic kidney disease. All patients share a noncoding mutation, which disrupts binding of the transcription factor ZNF143 and impairs specific regulation of PMM2 expression. Our findings are consistent with a critical role of protein glycosylation for normal insulin secretion and kidney morphology. Selective organ involvement, as seen in the patients presented here, has to our knowledge not previously been associated with a promoter mutation and thus provides important insight into gene regulation. Identification of a disease-causing promoter mutation emphasizes the importance of assessing noncoding variants in the genetic analysis of disease.
Concise Methods
Full details of all methods can be found in the Supplemental Material.
Genetic Studies
All subjects and/or their parents gave informed consent for genetic testing and all studies were approved by the respective institutional research ethics review boards. Linkage analysis was performed as detailed previously.36
Next-generation sequencing was performed through Perkin Elmer (www.perkinelmer.com) and Illumina (www.illumina.com). Sequencing data were analyzed with an in-house pipeline, as well as Ingenuity variant analysis software (www.ingenuity.com). Identified mutations in PMM2 were confirmed with Sanger sequencing.
EMSA
EMSA was performed as described.37 ZNF143 was expressed in vitro and bound to biotinylated probes specific for either wild-type or mutant promoter. Specificity was assessed by competition with excess of unlabeled probes.
Insulin Secretion
Insulin-secreting MIN6 cells were maintained and insulin secretion measured as described previously.38,39 To assess the effect of deglycosylation, the cells were incubated in the absence or presence of Peptide N-Glycosidase F and Endoglycosidase H.
Luciferase Assay
Promoter activity was assessed by placing three different promoter constructs 5′ to the renilla luciferase gene: (1) wild type, (2) c.-167G>T, and (3) c.-180_-163del, the latter deleting the entire predicted ZNF143 binding site.7
Digital PCR
Analysis of allele-specific PMM2 expression was performed in primary renal cells from patient 6.1 obtained after nephrectomy. Cells were prepared and cultured as described previously.40 RNA was isolated and cDNA generated as described and used as template for digital PCR with primers specific for the alleles containing either the promoter or missense mutation.41
Bioinformatics
The genomic region around the PMM2 promoter was assessed for chromosomal segmentation, transcription factor, and CTCF binding sites using the University of California in Santa Cruz Human Genome browser with publicly available tracks. Chromatin loop formation was assessed using available Hi-C data.42
Statistical Analyses
Appropriate statistical assays were used as indicated in the Supplemental Material for the respective experimental data. A P<0.05 was considered significant.
Disclosures
None.
Supplementary Material
Acknowledgments
This work was supported by the Medical Research Council (grant number 98144); the Great Ormond Street Hospital Children’s Charity; Kids Kidney Research; St Peter’s Trust for Kidney, Bladder and Prostate Research; The David and Elaine Potter Foundation; and the European Union, FP7 (grant agreement 2012-305608 “European Consortium for High-Throughput Research in Rare Kidney Diseases [EURenOmics]”). S.E.F. has a Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society (grant number: 105636/Z/14/Z). S.E. is a Wellcome Trust Senior Investigator.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2016121312/-/DCSupplemental.
References
- 1.Zerres K, Mücher G, Becker J, Steinkamm C, Rudnik-Schöneborn S, Heikkilä P, Rapola J, Salonen R, Germino GG, Onuchic L, Somlo S, Avner ED, Harman LA, Stockwin JM, Guay-Woodford LM: Prenatal diagnosis of autosomal recessive polycystic kidney disease (ARPKD): Molecular genetics, clinical experience, and fetal morphology. Am J Med Genet 76: 137–144, 1998 [PubMed] [Google Scholar]
- 2.Gunay-Aygun M, Parisi MA, Doherty D, Tuchman M, Tsilou E, Kleiner DE, Huizing M, Turkbey B, Choyke P, Guay-Woodford L, Heller T, Szymanska K, Johnson CA, Glass I, Gahl WA: MKS3-related ciliopathy with features of autosomal recessive polycystic kidney disease, nephronophthisis, and Joubert Syndrome. J Pediatr 155: 386–392.e1, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hartung EA, Guay-Woodford LM: Autosomal recessive polycystic kidney disease: A hepatorenal fibrocystic disorder with pleiotropic effects. Pediatrics 134: e833–e845, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guay-Woodford LM, Bissler JJ, Braun MC, Bockenhauer D, Cadnapaphornchai MA, Dell KM, Kerecuk L, Liebau MC, Alonso-Peclet MH, Shneider B, Emre S, Heller T, Kamath BM, Murray KF, Moise K, Eichenwald EE, Evans J, Keller RL, Wilkins-Haug L, Bergmann C, Gunay-Aygun M, Hooper SR, Hardy KK, Hartung EA, Streisand R, Perrone R, Moxey-Mims M: Consensus expert recommendations for the diagnosis and management of autosomal recessive polycystic kidney disease: Report of an international conference. J Pediatr 165: 611–617, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flanagan SE, Kapoor RR, Hussain K: Genetics of congenital hyperinsulinemic hypoglycemia. Semin Pediatr Surg 20: 13–17, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Mohamed Z, Arya VB, Hussain K: Hyperinsulinaemic hypoglycaemia: Genetic mechanisms, diagnosis and management. J Clin Res Pediatr Endocrinol 4: 169–181, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anno YN, Myslinski E, Ngondo-Mbongo RP, Krol A, Poch O, Lecompte O, Carbon P: Genome-wide evidence for an essential role of the human Staf/ZNF143 transcription factor in bidirectional transcription. Nucleic Acids Res 39: 3116–3127, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishihara H, Asano T, Tsukuda K, Katagiri H, Inukai K, Anai M, Kikuchi M, Yazaki Y, Miyazaki JI, Oka Y: Pancreatic beta cell line MIN6 exhibits characteristics of glucose metabolism and glucose-stimulated insulin secretion similar to those of normal islets. Diabetologia 36: 1139–1145, 1993 [DOI] [PubMed] [Google Scholar]
- 9.Shneider BL, Magid MS: Liver disease in autosomal recessive polycystic kidney disease. Pediatr Transplant 9: 634–639, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Grünewald S: The clinical spectrum of phosphomannomutase 2 deficiency (CDG-Ia). Biochim Biophys Acta 1792: 827–834, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Monin ML, Mignot C, De Lonlay P, Héron B, Masurel A, Mathieu-Dramard M, Lenaerts C, Thauvin C, Gérard M, Roze E, Jacquette A, Charles P, de Baracé C, Drouin-Garraud V, Khau Van Kien P, Cormier-Daire V, Mayer M, Ogier H, Brice A, Seta N, Héron D: 29 French adult patients with PMM2-congenital disorder of glycosylation: Outcome of the classical pediatric phenotype and depiction of a late-onset phenotype. Orphanet J Rare Dis 9: 207, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casado M, O’Callaghan MM, Montero R, Pérez-Cerda C, Pérez B, Briones P, Quintana E, Muchart J, Aracil A, Pineda M, Artuch R: Mild clinical and biochemical phenotype in two patients with PMM2-CDG (congenital disorder of glycosylation Ia). Cerebellum 11: 557–563, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Vermeer S, Kremer HP, Leijten QH, Scheffer H, Matthijs G, Wevers RA, Knoers NA, Morava E, Lefeber DJ: Cerebellar ataxia and congenital disorder of glycosylation Ia (CDG-Ia) with normal routine CDG screening. J Neurol 254: 1356–1358, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Nilius B, Voets T: The puzzle of TRPV4 channelopathies. EMBO Rep 14: 152–163, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoopes RR Jr, Shrimpton AE, Knohl SJ, Hueber P, Hoppe B, Matyus J, Simckes A, Tasic V, Toenshoff B, Suchy SF, Nussbaum RL, Scheinman SJ: Dent disease with mutations in OCRL1. Am J Hum Genet 76: 260–267, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lonfat N, Montavon T, Darbellay F, Gitto S, Duboule D: Convergent evolution of complex regulatory landscapes and pleiotropy at Hox loci. Science 346: 1004–1006, 2014 [DOI] [PubMed] [Google Scholar]
- 17.Dekker J, Mirny L: The 3D genome as moderator of chromosomal communication. Cell 164: 1110–1121, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dixon JR, Selvaraj S, Yue F, Kim A, Li Y, Shen Y, Hu M, Liu JS, Ren B: Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature 485: 376–380, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bailey SD, Zhang X, Desai K, Aid M, Corradin O, Cowper-Sal Lari R, Akhtar-Zaidi B, Scacheri PC, Haibe-Kains B, Lupien M: ZNF143 provides sequence specificity to secure chromatin interactions at gene promoters. Nat Commun 2: 6186, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heidari N, Phanstiel DH, He C, Grubert F, Jahanbani F, Kasowski M, Zhang MQ, Snyder MP: Genome-wide map of regulatory interactions in the human genome. Genome Res 24: 1905–1917, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phanstiel DH, Boyle AP, Heidari N, Snyder MP: Mango: A bias-correcting ChIA-PET analysis pipeline. Bioinformatics 31: 3092–3098, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grünewald S, Schollen E, Van Schaftingen E, Jaeken J, Matthijs G: High residual activity of PMM2 in patients’ fibroblasts: Possible pitfall in the diagnosis of CDG-Ia (phosphomannomutase deficiency). Am J Hum Genet 68: 347–354, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drewes T, Senkel S, Holewa B, Ryffel GU: Human hepatocyte nuclear factor 4 isoforms are encoded by distinct and differentially expressed genes. Mol Cell Biol 16: 925–931, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamilton AJ, Bingham C, McDonald TJ, Cook PR, Caswell RC, Weedon MN, Oram RA, Shields BM, Shepherd M, Inward CD, Hamilton-Shield JP, Kohlhase J, Ellard S, Hattersley AT: The HNF4A R76W mutation causes atypical dominant Fanconi syndrome in addition to a β cell phenotype. J Med Genet 51: 165–169, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strøm EH, Strømme P, Westvik J, Pedersen SJ: Renal cysts in the carbohydrate-deficient glycoprotein syndrome. Pediatr Nephrol 7: 253–255, 1993 [DOI] [PubMed] [Google Scholar]
- 26.Shanti B, Silink M, Bhattacharya K, Howard NJ, Carpenter K, Fietz M, Clayton P, Christodoulou J: Congenital disorder of glycosylation type Ia: Heterogeneity in the clinical presentation from multivisceral failure to hyperinsulinaemic hypoglycaemia as leading symptoms in three infants with phosphomannomutase deficiency. J Inherit Metab Dis 32[Suppl 1]: S241–S251, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Chang Y, Twiss JL, Horoupian DS, Caldwell SA, Johnston KM: Inherited syndrome of infantile olivopontocerebellar atrophy, micronodular cirrhosis, and renal tubular microcysts: Review of the literature and a report of an additional case. Acta Neuropathol 86: 399–404, 1993 [DOI] [PubMed] [Google Scholar]
- 28.de Lonlay P, Seta N, Barrot S, Chabrol B, Drouin V, Gabriel BM, Journel H, Kretz M, Laurent J, Le Merrer M, Leroy A, Pedespan D, Sarda P, Villeneuve N, Schmitz J, van Schaftingen E, Matthijs G, Jaeken J, Korner C, Munnich A, Saudubray JM, Cormier-Daire V: A broad spectrum of clinical presentations in congenital disorders of glycosylation I: A series of 26 cases. J Med Genet 38: 14–19, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hertz-Pannier L, Déchaux M, Sinico M, Emond S, Cormier-Daire V, Saudubray JM, Brunelle F, Niaudet P, Seta N, de Lonlay P: Congenital disorders of glycosylation type I: A rare but new cause of hyperechoic kidneys in infants and children due to early microcystic changes. Pediatr Radiol 36: 108–114, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Pérez-Dueñas B, García-Cazorla A, Pineda M, Poo P, Campistol J, Cusí V, Schollen E, Matthijs G, Grunewald S, Briones P, Pérez-Cerdá C, Artuch R, Vilaseca MA: Long-term evolution of eight Spanish patients with CDG type Ia: Typical and atypical manifestations. Eur J Paediatr Neurol 13: 444–451, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Porath B, Gainullin VG, Cornec-Le Gall E, Dillinger EK, Heyer CM, Hopp K, Edwards ME, Madsen CD, Mauritz SR, Banks CJ, Baheti S, Reddy B, Herrero JI, Banales JM, Hogan MC, Tasic V, Watnick TJ, Chapman AB, Vigneau C, Lavainne F, Audrezet MP, Ferec C, Le Meur Y, Torres VE; Genkyst Study Group, HPoPKDG, Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease ; Harris PC: Mutations in GANAB, encoding the glucosidase IIalpha subunit, cause autosomal-dominant polycystic kidney and liver disease. Am J Hum Genet 98: 1193–1207, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Inoue Y, Sohara E, Kobayashi K, Chiga M, Rai T, Ishibashi K, Horie S, Su X, Zhou J, Sasaki S, Uchida S: Aberrant glycosylation and localization of polycystin-1 cause polycystic kidney in an AQP11 knockout model. J Am Soc Nephrol 25: 2789–2799, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hofherr A, Wagner C, Fedeles S, Somlo S, Köttgen M: N-glycosylation determines the abundance of the transient receptor potential channel TRPP2. J Biol Chem 289: 14854–14867, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Conti LR, Radeke CM, Vandenberg CA: Membrane targeting of ATP-sensitive potassium channel. Effects of glycosylation on surface expression. J Biol Chem 277: 25416–25422, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Janssen MJ, Waanders E, Woudenberg J, Lefeber DJ, Drenth JP: Congenital disorders of glycosylation in hepatology: The example of polycystic liver disease. J Hepatol 52: 432–440, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Bockenhauer D, Feather S, Stanescu HC, Bandulik S, Zdebik AA, Reichold M, Tobin J, Lieberer E, Sterner C, Landoure G, Arora R, Sirimanna T, Thompson D, Cross JH, van’t Hoff W, Al Masri O, Tullus K, Yeung S, Anikster Y, Klootwijk E, Hubank M, Dillon MJ, Heitzmann D, Arcos-Burgos M, Knepper MA, Dobbie A, Gahl WA, Warth R, Sheridan E, Kleta R: Epilepsy, ataxia, sensorineural deafness, tubulopathy, and KCNJ10 mutations. N Engl J Med 360: 1960–1970, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garner MM, Revzin A: A gel electrophoresis method for quantifying the binding of proteins to specific DNA regions: Application to components of the Escherichia coli lactose operon regulatory system. Nucleic Acids Res 9: 3047–3060, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jones PM, Salmon DM, Howell SL: Protein phosphorylation in electrically permeabilized islets of Langerhans. Effects of Ca2+, cyclic AMP, a phorbol ester and noradrenaline. Biochem J 254: 397–403, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu B, Barbosa-Sampaio H, Jones PM, Persaud SJ, Muller DS: The CaMK4/CREB/IRS-2 cascade stimulates proliferation and inhibits apoptosis of β-cells. PLoS One 7: e45711, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuo NT, Norman JT, Wilson PD: Acidic FGF regulation of hyperproliferation of fibroblasts in human autosomal dominant polycystic kidney disease. Biochem Mol Med 61: 178–191, 1997 [DOI] [PubMed] [Google Scholar]
- 41.Klootwijk ED, Reichold M, Helip-Wooley A, Tolaymat A, Broeker C, Robinette SL, Reinders J, Peindl D, Renner K, Eberhart K, Assmann N, Oefner PJ, Dettmer K, Sterner C, Schroeder J, Zorger N, Witzgall R, Reinhold SW, Stanescu HC, Bockenhauer D, Jaureguiberry G, Courtneidge H, Hall AM, Wijeyesekera AD, Holmes E, Nicholson JK, O’Brien K, Bernardini I, Krasnewich DM, Arcos-Burgos M, Izumi Y, Nonoguchi H, Jia Y, Reddy JK, Ilyas M, Unwin RJ, Gahl WA, Warth R, Kleta R: Mistargeting of peroxisomal EHHADH and inherited renal Fanconi’s syndrome. N Engl J Med 370: 129–138, 2014 [DOI] [PubMed] [Google Scholar]
- 42.Rao SS, Huntley MH, Durand NC, Stamenova EK, Bochkov ID, Robinson JT, Sanborn AL, Machol I, Omer AD, Lander ES, Aiden EL: A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell 159: 1665–1680, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.