Kidney diseases are generally classified on the basis of the specific renal compartment that is initially and principally afflicted by the disease process. Sickle cell nephropathy (SCN) challenges this classification as all renal compartments—vascular, glomerular, tubular, and interstitial—may be adversely altered early in the life of patients with sickle cell disease (SCD).1,2 Hematuria and impaired urinary concentrating ability, largely reflecting medullary vasoocclusion, may appear as early as in infancy, a period when renal hypertrophy and hyperfiltration (resulting from, in part, increased renal blood flow and altered glomerular hemodynamics) may be also apparent. Proteinuria due to the loss of glomerular permselectivity and/or tubular injury may develop in childhood, can progress with age, and may be driven by an incipient glomerulopathy, usually focal segmental glomerulosclerosis.1,2 Hyperfiltration is eventually followed by a steady decline in GFR as the glomerulopathy progresses, the tubulointerstitium is increasingly enveloped by inflammation and fibrosis, and iron is deposited in renal tubules. At any age, the kidney in SCD is susceptible to episodes of subclinical or clinical acute kidney injury (AKI), the latter contributing to the progression of SCN.1,2
Attempts to retard the initiation and progression of SCN are challenged not only by the early involvement of multiple renal compartments, but also by the diverse pathobiologic processes present in SCD, all of which may be relevant to SCN. Vasoocclusion, the defining lesion in SCD, occurs when sickle hemoglobin (HbS) polymerizes, red blood cells (RBCs) stiffen, sickle, and adhere to the endothelium, and flow through the increasingly congested microvasculature is progressively impeded. Such occlusion, its eventual resolution, and its recurrence entrain cycles of ischemia, hypoxia, reperfusion-related phenomena, endothelial activation, nitric oxide (NO) deficiency, inflammation, hemolysis, oxidative stress, procoagulant processes, and microparticle formation.1,2 These processes are interconnected, and the triggering of one may provoke the appearance of another; yet, the relative contribution of each participant in the ensuing tissue injury is unresolved.1,2 Against this backdrop of SCN as a disease involving multiple renal compartments and, potentially, multiple pathobiologic processes, the present study of Kasztan et al. published in this issue of JASN, is fascinating: this study demonstrates that the antagonism of a single, specific receptor, the endothelin-A (ETA) receptor, can effectively retard the initiation and progression of SCN in a murine model of SCD.3
Prior studies support a pathogenetic role for endothelin-1 (ET-1) in human SCD. Exposure of endothelial cells to RBCs from SCD patients induces ET-1,4 and conditioned media from cells so exposed provoke ETA-dependent vasoconstriction.5 Plasma ET-1 levels are elevated in patients with SCD in steady state and more so during crisis; plasma ET-1 levels decrease in patients treated with hydroxyurea, a therapy that reduces the frequency of vasoocclusive disease.6 Both endothelial dysfunction and albuminuria in human SCD associate with plasma ET-1 levels.7 Studies in a murine SCD model attest to the pathogenetic role of ET-1 in SCD by demonstrating that bosentan, a dual ETA/ETB receptor antagonist, attenuates renal vasoocclusion, vasoconstriction, and inflammation induced in this model by hypoxia/reoxygenation; bosentan also reduces vasoocclusion and inflammation in the lungs and mortality following such hypoxic stress.8
Using a murine SCD model that develops progressive SCN (without the need for an imposed hypoxic or other insult), Kasztan et al. demonstrate that the administration of ambrisentan (an ETA receptor antagonist), begun at the time of weaning and continued for 10 weeks, prevented glomerular dysfunction, inflammation, and structural injury; and that such antagonism also prevented tubulointerstitial inflammation and fibrosis.3 When the administration of ambrisentan was delayed until SCN became established, and then continued for a much shorter period (2 weeks), ambrisentan still significantly reduced glomerular and tubular injury. In contrast to the striking nephroprotective effects of the ETA receptor antagonist, a nonselective ETA/ETB receptor antagonist, administered in companion studies in both the long-term and short-term protocols, exerted considerably less nephroprotection.3 To explain these findings, Kasztan et al. drew upon the recognized vasoconstrictive, pro-inflammatory, proliferative, and pro-oxidant effects of ETA receptor signaling, and the mitigating or opposing effects commonly countered by ETB receptor signaling. Thus, blocking, specifically, the ETA receptor in SCD preserves the renal hemodynamic profile, the integrity of podocytes, and glomerular permselectivity; lessens microvascular congestion; and reduces inflammatory processes. The generally countervailing (and salutary) effects of ETB receptor signaling, however, are left entirely unchecked by ETA receptor antagonists.3
An intriguing question is how does the interruption of signaling through a single receptor confer such protection in multiple renal compartments in which multiple pathobiologic processes are likely at work. It is notable that ETA receptor signaling instigates processes (vasoconstriction, inflammation, and oxidative stress) that not only promote sickling and tissue injury in SCD, but are ones that also stimulate cellular production of ET-1. Specifically, ischemia and hypoxia (resulting from vasoconstriction), and oxidant stress, certain cytokines, and NF-kB activation (likely components of an inflammatory milieu elicited by ETA receptor signaling) are all recognized inducers of ET-1.9 ETA receptor signaling in SCD thus instigates a positive feedback loop: such signaling engenders pathobiologic processes that not only promote the sickle milieu, but also stimulate the production of ET-1, the agonist for the ETA receptor itself. The observations of Kasztan et al. support this speculation as the markedly elevated plasma ET-1 levels that occur in this model are normalized by chronic administration of ambrisentan.3
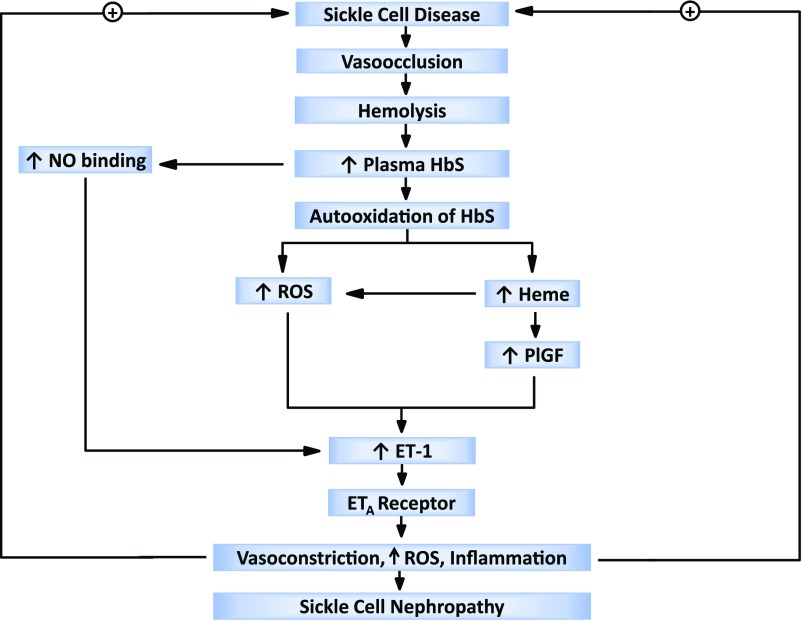
Akin to what is envisioned above for vasoconstriction, inflammation, and oxidant stress, other pathobiologic processes in SCD may also provide a positive feedback for ETA receptor signaling. We suggest that one such process is hemolysis because of the following considerations. Kasztan et al. demonstrate that iron deposits were prominent in renal tubules in the murine model, and that long-term administration of ambrisentan substantially reduced such deposition. Iron deposition in renal tubules in SCD generally reflects hemolysis in SCD: HbS, released from RBCs, undergoes glomerular filtration, tubular incorporation, and subsequent denaturation, with the resulting release of iron from the heme group1,2; as shown in prior studies, renal heme content is increased in murine SCD.10 Thus, the reduction in tubular iron deposits in the murine model following chronic administration of ambrisentan suggests that ambrisentan reduces hemolysis in this model. In support of this speculation are the findings that splenic enlargement occurs in this SCD model, and such enlargement is mitigated by ambrisentan. Evaluating hemolysis in this model and the effect of ETA receptor antagonism would thus be of interest. Hemolysis can provide a positive feedback loop because HbS, an unstable protein, is autooxidized when free in plasma; this leads to oxidant generation, the denaturing of HbS, and the release of heme; heme is a prooxidant, proinflammatory species. As ET-1 is an oxidant-inducible cytokine, heme may thus drive the synthesis of ET-1. Additionally, heme stimulates production of placenta growth factor (PlGF), an angiogenic growth factor that is increased in SCD, is an inducer of ET-1, and is implicated in the pathogenesis of tissue injury in SCD.11–14 Thus, either directly or via PlGF, heme can stimulate cellular production of ET-1 in SCD. Vascular ET-1 production may also increase as vascular NO is depleted due to binding of NO by plasma HbS, NO being a suppressor of ET-1 synthesis.1,2 Figure 1 depicts this hypothesized, hemolysis-dependent, positive feedback loop.
Figure 1:
Positive feedback loop involving endothelin-1 (ET-1) and hemolysis in sickle cell nephropathy. In sickle cell disease vasoocclusion predisposes to hemolysis and the release of sickle hemoglobin (HbS) into plasma. HbS binds nitric oxide (NO), and such loss of NO from the vasculature increases the synthesis of ET-1. HbS undergoes autooxidation leading to the generation of reactive oxygen species (ROS), HbS denaturation, and the liberation of heme. Heme, through the generation of ROS and induction of placenta growth factor (PlGF), induces cellular synthesis of ET-1. By engaging the endothelin-A (ETA) receptor, ET-1 causes vasoconstriction, generates ROS, and induces inflammation, all of which may provide a positive feedback loop that sustains this hemolysis-dependent cycle.
Dehydration of sickle RBCs elevates the concentration of HbS in RBCs, and thus promotes HbS polymerization and RBC sickling. ET-1 causes RBC dehydration by activating the Gardos channel present in the plasma membrane of RBCs.15 This effect of ET-1 is mediated through the ETB receptor.15 In attempting to reconcile this effect of ET-1 with the present findings of Kasztan et al., it is possible that the dehydrating effect of ET-1 on sickle RBCs may not be prominent in vivo in this particular murine model; or, if they are, it appears that the dehydrating effect on sickle RBCs resulting from unrestrained ETB receptor signaling, when a specific ETA receptor antagonist is administered, are entirely overshadowed by the beneficial effects resulting from specific blockade of the ETA receptor.
In addressing the pathobiologic significance of ETA receptor signaling, the present studies of Kasztan et al. also disclose a novel therapeutic strategy. Diseases such as SCD with myriad, interacting pathobiologic processes may be likened to inhomogeneous, “scale-free” networks. The vast majority of nodes in such networks have relatively few connections, whereas rare and exceptional nodes (“hubs”) have very high connectivity.16 Survival analyses of inhomogenous, scale-free networks reveal that these networks remain largely functional despite the loss of a substantial number of individual nodes; however, network integrity and functionality cannot endure the loss of hubs.16 In this regard, the present studies of Kasztan et al. identify ETA receptor signaling as a hub in the network of pathobiologic processes underlying the progression of murine SCN. Targeting this hub with ETA receptor antagonists thus offers a novel and feasible therapeutic strategy in SCD patients with, or at risk for, SCN. The efficacy and safety of such therapeutic approaches should thus be examined by appropriately designed, prospective clinical trials.
Disclosures
None.
Acknowledgments
K.A.N. and Z.S.K. gratefully acknowledge support from National Institutes of Health grants DK 70124 and DK 47060 and the Mayo Foundation.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related article, “Long-Term Endothelin-A Receptor Antagonism Provides Robust Renal Protection in Humanized Sickle Cell Disease Mice,” on pages 2443–2458.
References
- 1.Nath KA, Katusic ZS, Gladwin MT: The perfusion paradox and vascular instability in sickle cell disease. Microcirculation 11: 179–193, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Nath KA, Hebbel RP: Sickle cell disease: Renal manifestations and mechanisms. Nat Rev Nephrol 11: 161–171, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kasztan M, Fox BM, Speed JS, De Miguel C, Gohar EY, Townes TM, Kutlar A, Pollock JS, Pollock DM: Long-term endothelin-A receptor antagonism provides robust renal protection in humanized sickle cell disease mice. J Am Soc Nephrol 28: 2443–2458, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phelan M, Perrine SP, Brauer M, Faller DV: Sickle erythrocytes, after sickling, regulate the expression of the endothelin-1 gene and protein in human endothelial cells in culture. J Clin Invest 96: 1145–1151, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ergul S, Brunson CY, Hutchinson J, Tawfik A, Kutlar A, Webb RC, Ergul A: Vasoactive factors in sickle cell disease: In vitro evidence for endothelin-1-mediated vasoconstriction. Am J Hematol 76: 245–251, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Fox BM, Kasztan M: Endothelin receptor antagonists in sickle cell disease: A promising new therapeutic approach. Life Sci 159: 15–19, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ataga KI, Derebail VK, Caughey M, Elsherif L, Shen JH, Jones SK, Maitra P, Pollock DM, Cai J, Archer DR, Hinderliter AL: Albuminuria is associated with endothelial dysfunction and elevated plasma endothelin-1 in sickle cell anemia. PLoS One 11: e0162652, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sabaa N, de Franceschi L, Bonnin P, Castier Y, Malpeli G, Debbabi H, Galaup A, Maier-Redelsperger M, Vandermeersch S, Scarpa A, Janin A, Levy B, Girot R, Beuzard Y, Leboeuf C, Henri A, Germain S, Dussaule JC, Tharaux PL: Endothelin receptor antagonism prevents hypoxia-induced mortality and morbidity in a mouse model of sickle-cell disease. J Clin Invest 118: 1924–1933, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodríguez-Pascual F, Busnadiego O, Lagares D, Lamas S: Role of endothelin in the cardiovascular system. Pharmacol Res 63: 463–472, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Nath KA, Grande JP, Haggard JJ, Croatt AJ, Katusic ZS, Solovey A, Hebbel RP: Oxidative stress and induction of heme oxygenase-1 in the kidney in sickle cell disease. Am J Pathol 158: 893–903, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel N, Gonsalves CS, Malik P, Kalra VK: Placenta growth factor augments endothelin-1 and endothelin-B receptor expression via hypoxia-inducible factor-1 alpha. Blood 112: 856–865, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sundaram N, Tailor A, Mendelsohn L, Wansapura J, Wang X, Higashimoto T, Pauciulo MW, Gottliebson W, Kalra VK, Nichols WC, Kato GJ, Malik P: High levels of placenta growth factor in sickle cell disease promote pulmonary hypertension. Blood 116: 109–112, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brittain JE, Hulkower B, Jones SK, Strayhorn D, De Castro L, Telen MJ, Orringer EP, Hinderliter A, Ataga KI: Placenta growth factor in sickle cell disease: Association with hemolysis and inflammation. Blood 115: 2014–2020, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X, Mendelsohn L, Rogers H, Leitman S, Raghavachari N, Yang Y, Yau YY, Tallack M, Perkins A, Taylor JG 6th, Noguchi CT, Kato GJ: Heme-bound iron activates placenta growth factor in erythroid cells via erythroid Krüppel-like factor. Blood 124: 946–954, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rivera A: Reduced sickle erythrocyte dehydration in vivo by endothelin-1 receptor antagonists. Am J Physiol Cell Physiol 293: C960–C966, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Albert R, Jeong H, Barabasi AL: Error and attack tolerance of complex networks. Nature 406: 378–382, 2000 [DOI] [PubMed] [Google Scholar]