Abstract
Tachyphylaxis of itch refers to a markedly reduced scratching response to consecutive exposures of a pruritogen, a process thought to protect against tissue damage by incessant scratching and to become disrupted in chronic itch. Here, we report that a strong stimulation of the Mas-related G-protein-coupled receptor C11 by its agonist, Ser-Leu-Ile-Gly-Arg-Leu-NH2 (SL-NH2) or bovine adrenal medulla 8-22 peptide, via subcutaneous injection in mice induces tachyphylaxis to the subsequent application of SL-NH2 to the same site. Notably, co-application of acid and SL-NH2 following the initial injection of the pruritogen alone counteracted itch tachyphylaxis by augmenting the scratching behaviors in wild-type but not in acid-sensing ion channel 3-null, animals. Using an activity-dependent silencing strategy, we identified that acid-sensing ion channel 3-mediated itch enhancement mainly occurred via the Mas-related G-protein-coupled receptor C11-responsive sensory neurons. Together, our results indicate that acid-sensing ion channel 3, activated by concomitant acid and certain pruritogens, constitute a novel signaling pathway that counteracts itch tachyphylaxis to successive pruritogenic stimulation, which likely contributes to chronic itch associated with tissue acidosis.
Keywords: Tachyphylaxis, chronic itch, acidosis, SL-NH2, acid-sensing ion channel 3, Mas-related G-protein-coupled receptor C11
Introduction
Chronic itch is a common and debilitating manifestation of a variety of dermatologic and systemic diseases1; however, its fundamental mechanisms remain unclear. The itch-scratch cycle has been proposed as an important factor underlying chronic itch.2 While under normal conditions, the acute itch response to the repeated exposure of a pruritogenic stimulus is often dampened by tachyphylaxis,3 as indicated by the markedly reduced scratching to the subsequent exposure of the pruritogen, under chronic itch conditions, this self-protective mechanism is severely disrupted by multiple pathological processes.
Itch is categorized as histaminergic itch and non-histaminergic itch. Although histamine is the best understood pruritogen,4 a sensory neuron-specific type of histamine-independent itch receptors belonging to the Mas-related G protein-coupled receptor (Mrgpr) family has also been well characterized during the past decade.5–8 These receptors can be activated by a large number of endogenous and exogenous pruritogens to evoke scratching behaviors. Among them, Mas-related G-protein-coupled receptor C11 (MrgprC11) accounts for the itch9 evoked by the endogenous peptide, bovine adrenal medulla 8-22 (BAM8-22), a proteolytic product of proenkephalin A,10 and a synthetic peptide, Ser-Leu-Ile-Gly-Arg-Leu-NH2 (SL-NH2),11 also known as an agonist of proteinase-activated receptor 2 (PAR2). Interestingly, Ser-Leu-Ile-Gly-Arg-Leu is the naturally occurring tethered ligand sequence that is unmasked by serine protease through cleavage at an N-terminal domain, leading to self-activation of the PAR2,12 which plays an important role in pain and inflammation.13 The synthetic SL-NH2 peptide is able to activate PAR2 in the absence of the protease.13
Both histaminergic and non-histaminergic itch have received a lot of attention and signaling mechanisms about their generation are being unveiled, especially with respect to chronic itch,14 which is caused by complex interplay among skin keratinocytes, pruritogenic molecules, immunomodulators, and cutaneous nerve fibers.15 However, the dynamic regulation of itch sensation under various physiological and pathological conditions, in particular the tachyphylaxis of itch and its counteraction, remains largely unexplored. An understanding of the mechanism by which the tachyphylaxis of itch is removed or overcome under pathological conditions will aid the development of therapeutic treatments for dermatologic and systemic diseases associated with chronic itch.
Recently, we showed that acid-sensing ion channel 3 (ASIC3) is involved in enhancing itch sensation16 under tissue acidosis to the pruritogenic peptide SL-NH2, or any one of the analogous neuropeptides that share the common C-terminal motif with SL-NH2. These included -RFamide, -RYamide, and -RYG, all of which can be released from mast cells.17 Interestingly, this effect of ASIC3 required coincident stimulation of the channel by protons and the pruritogenic peptide, which targeted the previously identified nonproton ligand sensing domain located in the extracellular region of ASIC3 channels,18 thus prolonging and potentiating channel activation in primary sensory neurons.16 The pathophysiological significance of the ASIC3-mediated potentiation of itch responses is highlighted by the finding that ablation of the ASIC3 gene reduced dry skin-induced scratching behaviors and pathological changes under conditions with concomitant inflammation16; however, the precise cellular mechanisms underlying the action of ASIC3 in chronic itch remain mysterious. As a polymodal receptor in the periphery neurons,19 ASIC3 operates as an ion channel gated by not only extracellular protons but also a number of nonproton ligands,16,18,20–22 including SL-NH2 and related peptides. However, it remains unclear how the itch component mediated by ASIC3 cooperates with that produced by the activation of the G-protein-coupled MrgprC11 receptors. Here, we extend our original investigation16 by characterizing how acidosis, through activation of ASIC3, counteracts itch tachyphylaxis produced by a prior stimulation with SL-NH2, pointing to an essential role of ASIC3 in signaling pathways that contribute to chronic itch under tissue acidosis.
Results
Association of acid and SL-NH2 counteracts itch tachyphylaxis via ASIC3
In a previous study,16 we demonstrated that although acidosis alone is not strongly pruritogenic, it significantly potentiates itch evoked by SL-NH2, but not BAM8-22, through an ASIC3-, rather than MrgprC11-, dependent mechanism. In order to dissect the contribution of ASIC3 to MrgprC11-dependent itch, particularly the cross-tachyphylaxis between acid and SL-NH2, which acts at both ASIC3 and MrgpC11, we examined the scratching behaviors of mice in response to two consecutive subcutaneous injections of the SL-NH2 solution into the nape of the neck in the absence or presence of acidosis.
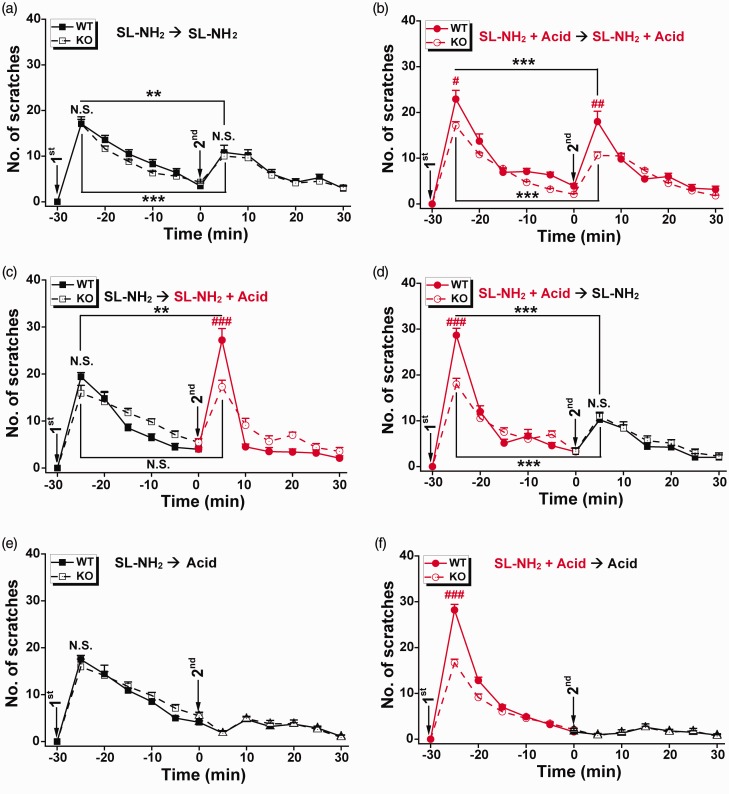
Application of SL-NH2 (7 mM in 50 µl) in wild-type (WT) mice elicited scratching behaviors, which gradually decreased over 30 min (Figure 1(a)). The subsequent application of SL-NH2 (14 mM, 25 µl) to the same site evoked another increase of the scratching behaviors, but the scratching frequency, as quantified by the number of scratching bouts in the first 5-min bin (Figure 1(a)), was reduced as compared to that in response to the initial SL-NH2 application. A two-way analysis of variance (ANOVA) conducted on the scratching bouts within each 5-min bin over the 30-min period indicated a significant difference between the scratching bouts following the first and second injections of SL-NH2 in the same animal (first vs. second SL-NH2, F(1, 132) = 29.51, P < 0.001, Figure 1(a)). When the animal was given the identical volume of SL-NH2 (both are 14 mM in 25 µl), the itch tachyphylaxis to the second injection also occurred (first vs. second SL-NH2, F(1, 96) = 51.168, P < 0.001, data not shown). Similarly, if the WT mice were given SL-NH2 plus acid (0.6% acetic acid, v/v) in the initial injection, then the subsequent injection of the same amount of SL-NH2 plus acid generated significantly less scratching response (Figure 1(b), first vs. second SL-NH2 plus acid: F(1, 132) = 13.414, P < 0.001, by two-way ANOVA for each 5-min bin over the 30-min period). Thus, tachyphylaxis indeed occurs to itch induced by SL-NH2 at either neutral or acidic pH when the same amount of stimulus was applied again within 30 min. This endogenous tachyphylaxis mechanism may help protect the animal from severe scratching.
Figure 1.
Effects of acidosis on itch tachyphylaxis to SL-NH2. Itch-related behaviors in mice were determined by the number of scratching bouts in 5-min bins during a 30-min period following injection of pruritogens (as indicated) into the rostral back/neck in WT (filled symbols) and ASIC3-KO mice (open symbols). (a) First injection: SL-NH2 (7 mM) in saline (50 µl); second injection: SL-NH2 (14 mM) in saline (25 µl). (b) First injection: SL-NH2 (7 mM) and acid (0.6% acetic acid, v/v) in saline (50 µl); second injection: SL-NH2 (14 mM) and acid (1.2% acetic acid, v/v) in saline (25 µl). (c) First injection: SL-NH2 (7 mM) in saline (50 µl); second injection: SL-NH2 (14 mM) and acid (1.2% acetic acid, v/v) in saline (25 µl). (d) First injection: SL-NH2 (7 mM) and acid (0.6% acetic acid, v/v) in saline (50 µl); second injection: SL-NH2 (14 mM) in saline (25 µl). (e) First injection: SL-NH2 (7 mM) in saline (50 µl); second injection: acid (1.2% acetic acid, v/v) in saline (25 µl). (f) First injection: SL-NH2 (7 mM) and acid (0.6% acetic acid, v/v) in saline (50 µl); second injection: acid (1.2% acetic acid, v/v) in saline (25 µl). Data are means ± SEM; n = 8–11. N.S.: not significant difference, **P < 0.01, ***P < 0.001, first vs. second, paired Student’s t test. N.S.: not significant difference, #P < 0.05, ##P < 0.01, ###P < 0.001, WT vs. ASIC3-KO, unpaired Student’s t test.
To assess the contribution of ASIC3 in the tachyphylaxis of itch induced by SL-NH2, we also examined the scratching behaviors of ASIC3 knockout (ASIC3-KO) mice to two consecutive injections of the SL-NH2 solutions. Application of SL-NH2 at neutral pH elicited comparable scratching in ASIC3-KO mice as their WT littermates following both first (WT vs. ASIC3-KO, F(1, 126) = 3.448, P = 0.066) and second (WT vs. ASIC3-KO, F(1, 126) = 0.631, P = 0.429) injections (Figure 1(a)). Similar to the tachyphylaxis observed in WT mice, there was also a significant difference between the first and second SL-NH2 applications to the ASIC3-KO mice (first vs. second SL-NH2, F(1, 120) = 66.347, P < 0.001, Figure 1(a)), suggestive of an insignificant role of ASIC3 in the itch tachyphylaxis to SL-NH2 at neutral pH. Consistent with the previous observation that acid potentiated SL-NH2-evoked itch through an ASIC3-dependent mechanism,16 both the first and second injections of the same amount of SL-NH2 in the acidic solution produced less numbers of scratching bouts in ASIC3-KO than WT mice (first SL-NH2 plus acid, WT vs. ASIC3-KO, F(1, 126) = 23.667, P < 0.001; second SL-NH2 plus acid, WT vs. ASIC3-KO, F(1, 126) =6.59, P = 0.012). However, the itch tachyphylaxis to SL-NH2 in the acidic solution was still quite evident in ASIC3-KO mice (first vs. second SL-NH2 plus acid, F(1, 120) =31.441, P < 0.001, Figure 1(b)), suggesting that although ASIC3 enhances the itch response to the pruritogen, it is not necessary for the generation of itch tachyphylaxis under acidic pH. These separable roles raise a possibility that ASIC3 activation by the combined acid and SL-NH2 may help overcome itch tachyphylaxis developed following the prior pruritogen exposure.
We therefore examined the cross-tachyphylaxis of itch behaviors to SL-NH2 alone versus SL-NH2 plus acid. Notably, in WT mice, the itch response to SL-NH2 plus acid following a prior treatment with SL-NH2 alone in the same site was enhanced (Figure 1(c)). With the prior SL-NH2 injection, the subsequent response to SL-NH2 plus acid (Figure 1(c), second injection) was not different from that to SL-NH2 plus acid alone (Figure 1(d), first injection, P > 0.05, by Student’s t test for the first 5 min following pruritogen injection). However, the facilitating effect to SL-NH2-induced itch by acid was seen only in WT, but not ASIC3-KO, mice (Figure 1(c), dashed line), indicating that ASIC3 is required for the counteracting effect of acid on itch tachyphylaxis. Thus, in addition to enhancing the itch response to SL-NH2 (Figure 1(b) and (c)),16 acid may also trigger an ASIC3-dependent signaling cascade that counteracts the itch tachyphylaxis to this pruritogen. Interestingly, pretreatment with SL-NH2 plus acid largely reduced the number of scratching bouts evoked by a subsequent injection of SL-NH2 in both WT and ASIC3-KO mice (Figure 1(d)), consistent with the development of tachyphylaxis through mechanism(s) independent of ASIC3.
To dissect mechanisms underlying the counteraction of SL-NH2-induced itch tachyphylaxis by association of acid and SL-NH2, we then examined whether acid alone was sufficient to induce strong scratching behaviors after SL-NH2 conditioning. As shown in the previous study,16 although acid alone is not a typical pruritogen like SL-NH2 or BAM8-22, it elicited significant scratching behaviors compared with the saline control (WT: acid vs. saline, F(1, 138) = 58.497, P < 0.001; ASIC3-KO: acid vs. saline, F(1, 138) = 68.175, P < 0.001), up to a similar extent in WT and ASIC3-KO mice (acid: WT vs. ASIC3-KO, F(1, 138) = 0.007, P = 0.933).16 This agreed well with a recent study23 that identified the involvement of transient receptor potential cation channel, subfamily V, member 1 (TRPV1) and T cell death-associated gene 8 receptor, but not ASIC3, in pruriception associated with citric acid. Moreover, SL-NH2 conditioning did not alter the acid-evoked scratching in both WT and ASIC3-KO mice (acid vs. acid following SL-NH2 conditioning, WT: F(1, 126) =1.529, P = 0.219; ASIC3-KO: F(1, 126) = 1.476, P = 0.227, Figure 1(e)).16 Due to the acid-mediated, ASIC3-independent itch component,16,23 it is not surprising that the itch response as the second injection to SL-NH2 alone differs from that to SL-NH2 plus acid in ASIC3-KO mice (P < 0.001, by Student’s t test for the first 5 min following pruritogen injection, Figure 1(a) and (c)), each following SL-NH2 conditioning. Nevertheless, in WT mice, the combined SL-NH2 and acid is capable of inducing more striking scratching than SL-NH2 alone (P < 0.001, by Student’s t test for the first 5 min following pruritogen injection, Figure 1(a) and (c)), each following SL-NH2 conditioning, which strengthens the notion that acid has an ASIC3-dependent synergistic effect with SL-NH2 to counteract itch tachyphylaxis induced by SL-NH2 alone. Interestingly, following SL-NH2 plus acid application, the acid-induced scratching behaviors were greatly reduced (acid following SL-NH2 vs. acid following SL-NH2 plus acid conditioning, WT: F(1, 120) =39.567, P < 0.001; ASIC3-KO: F(1, 108) = 19.1, P < 0.001, Figure 1(e) and (f)). This effect was ASIC3-independent, as the scratching induced by acid as the second injection was alike in WT and ASIC3-KO mice (acid following SL-NH2 plus acid conditioning: WT vs. ASIC3-KO, F(1, 114) = 0.021, P = 0.886, Figure 1(f)). These data were thus compatible with the different itch response to the second injection of SL-NH2 plus acid in ASIC3 KO mice with the prior treatment of SL-NH2 and that of SL-NH2 plus acid (P < 0.001, by Student’s t test for the first 5 min following pruritogen injection, Figure 1(b) and (c)), respectively. Overall, these data collectively point to a view that although acidosis itself contributes to itch behavior independent of ASIC3, the concurrent association of SL-NH2 and acid indeed ASIC3-dependently counteracts the SL-NH2-induced itch tachyphylaxis.
TRPV1 is not required for acid counteraction of itch tachyphylaxis to SL-NH2
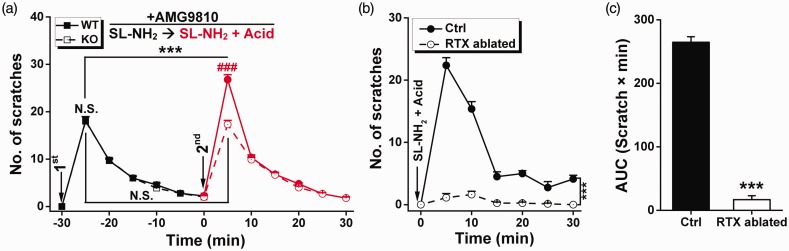
Like ASIC3 (see literature16,21), TRPV1 represents another acid-responsive channel type in sensory neurons, which has also been suggested to play significant roles in itch sensation.24–26 Therefore, we tested whether TRPV1 also plays a role in acid counteraction of itch tachyphylaxis to SL-NH2 with the use of AMG9810 (100 µM), a TRPV1-selective antagonist capable of blocking proton-induced TRPV1 channel activation.27 In the cross-tachyphylaxis tests of itch behaviors to SL-NH2 alone versus SL-NH2 plus acid, the inclusion of AMG9810 did not alter the effect of acidic pH on enhancing the itch response of WT mice to the second SL-NH2 injection to the same site that had been previously injected with SL-NH2 alone (Figure 2(a)). In ASIC3-KO mice, the acidic pH remained ineffective in counteracting the itch tachyphylaxis to SL-NH2 even in the presence of AMG9810 (Figure 2(a), dashed line), confirming that ASIC3, but not TRPV1, is involved in the acid-counteracting effect on tachyphylaxis.
Figure 2.
TRPV1-positive sensory neurons but not TRPV1 channels are involved in itch evoked by SL-NH2 plus acid. (a) Itch-related behaviors in mice were determined as in Figure 1(c) but with a TRPV1 antagonist, AMG9810 (100 µM), injected together with SL-NH2. First injection: SL-NH2 (7 mM) and AMG9810 (100 µM) in saline (50 µl); second injection: SL-NH2 (14 mM), AMG9810 (100 µM), and acid (1.2% acetic acid, v/v) in saline (25 µl). (b) Itch-related behaviors determined by scratching bouts in 5-min bins during the 30-min test period following injection of SL-NH2 plus acid into the rostral back/neck of control (Ctrl, filled) or RTX-pre-treated (RTX ablated; 100 µg/ml RTX in 50 µl 0.05% ascorbic acid and 7% Tween 80, open symbols) mice. Data are means ± SEM; n = 7–8. ***P < 0.001, Ctrl vs. RTX-ablated, by two-way repeated measures ANOVA. Group, F(1, 90) = 504.597, P < 0.001; time, F(5, 90) = 92.187, P < 0.001; interaction, F(5, 90) =71.342, P < 0.001. (c) Area under curve (AUC, scratching bout × min) calculated from the same set of data in (b). ***P < 0.001, Ctrl vs. RTX-ablated, by unpaired Student’s t test. N.S.: not significant difference, ***P < 0.001, first vs. second, paired Student’s t test. N.S.: not significant difference, ###P < 0.001, WT vs. ASIC3-KO, unpaired Student’s t test.
A subset of sensory neurons is involved in itch response to SL-NH2 plus acid
To understand the mechanisms underlying itch tachyphylaxis and the effect of ASIC3, we then examined the cellular basis for the ASIC3-dependent itch evoked by SL-NH2 plus acid. Since primary afferent neurons expressing TRPV1 and transient receptor potential cation channel, subfamily A, member 1 (TRPA1) (expressed in a subset of TRPV1-positive fibers) are required for transducing itch signaling in response to both histamine25 and non-histamine pruritogens,28,29 such as chloroquine and BAM8-22, we examined whether the TRPV1-expressing fibers are also needed for the acid potentiation of SL-NH2-evoked itch. To ablate the TRPV1- and TRPV1-/TRPA1-positive sensory neurons, we used the super potent capsaicin analogue resiniferatoxin (RTX), which has been shown to suppress acute pain and itch in animal models via axonopathy.25,30–32 We found that a treatment with RTX on the neck rendered the mice insensitive to the injection of acid plus SL-NH2 (F(1, 90) = 504.597, P < 0.001, Ctrl vs. RTX, two-way ANOVA, Figure 2(b) and (c)). These data demonstrate that at least a subset of the TRPV1-positive sensory neurons is responsible for the acid potentiation of SL-NH2-evoked itch, although the TRPV1 channels are not necessarily involved in producing such an effect, as shown in Figure 2(a).
Enhanced activation of pruriceptors accounts for acidosis-potentiated itch
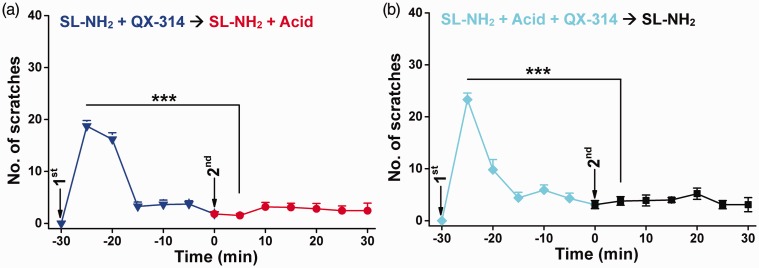
Our previous study16 identified that the combined acidosis and SL-NH2 promote the activation of ASIC3 (by slowing down desensitization of acid-induced ASIC3 channel opening) independent on MrgprC11, suggesting that the enhanced ASIC3 channel activity to co-stimulation by SL-NH2 and acid as compared with each stimulus alone would be responsible for the acid potentiation of SL-NH2-evoked itch behaviors and the counteraction of itch tachyphylaxis to SL-NH2. However, it remained unclear whether the itch response to SL-NH2 plus acid and its counteraction on itch tachyphylaxis to SL-NH2 require the same population of sensory neurons that express MrgprC11 to account for SL-NH2-evoked itch.11 For this purpose, we took an activity-dependent silencing strategy29 to dissect the itch-generating sensory neurons. It has been shown that SL-NH2 activates MrgprC11 (see literature11), which then opens the downstream TRPA1 channels to cause itch.28 Notably, the activation of TRPA1 channels permits the entry of, if present, the charged, membrane-impermeable sodium-channel blocker, QX-314, which can then silence the targeted sensory neurons by preventing sodium channel-mediated excitation.29 We reasoned that if acidosis sensitized pruriceptors to the pruritogen by recruiting SL-NH2-irresponsive (MrgprC11-negative) sensory neurons to potentiate itch, then the simultaneous delivery of QX-314 and SL-NH2 in the absence of acidosis should not affect the itch elicited by the subsequent injection of SL-NH2 plus acid. However, when co-injected with SL-NH2 in the absence of acidosis, QX-314 completely abolished the scratching behaviors evoked by the subsequent co-injection of SL-NH2 plus acid (Figure 3(a)). In reverse, QX-314 also abolished the SL-NH2-elicited scratching behaviors when it was included during the pre-exposure to SL-NH2 plus acid (Figure 3(b)). Given that QX-314 did not alter the initial itch responses to the co-injected SL-NH2, or SL-NH2 plus acid, an activity-dependent process that must be involved to allow its inhibitory action. Therefore, it is likely that SL-NH2 evokes itch through activation of the same population of pruritogen-responsive sensory neurons, regardless of the absence or presence of acidosis.
Figure 3.
Effects of administration of pruritogens together with QX-314 on subsequent pruritogen-evoked scratching. (a) First injection: SL-NH2 (7 mM) and QX-314 (1%, w/v) in saline (50 µl); second injection: SL-NH2 (14 mM) and acid (1.2% acetic acid, v/v) in saline (25 µl). (b) First injection: SL-NH2 (7 mM), acid (0.6% acetic acid, v/v), and QX-314 (1%, w/v) in saline (50 µl); second injection: SL-NH2 (14 mM) in saline (25 µl). Data are means ± SEM; n = 10–11 each group. ***P < 0.001, first vs. second, paired Student’s t test.
Acidosis counteracts cross-tachyphylaxis of itch by different MrgprC11 agonists
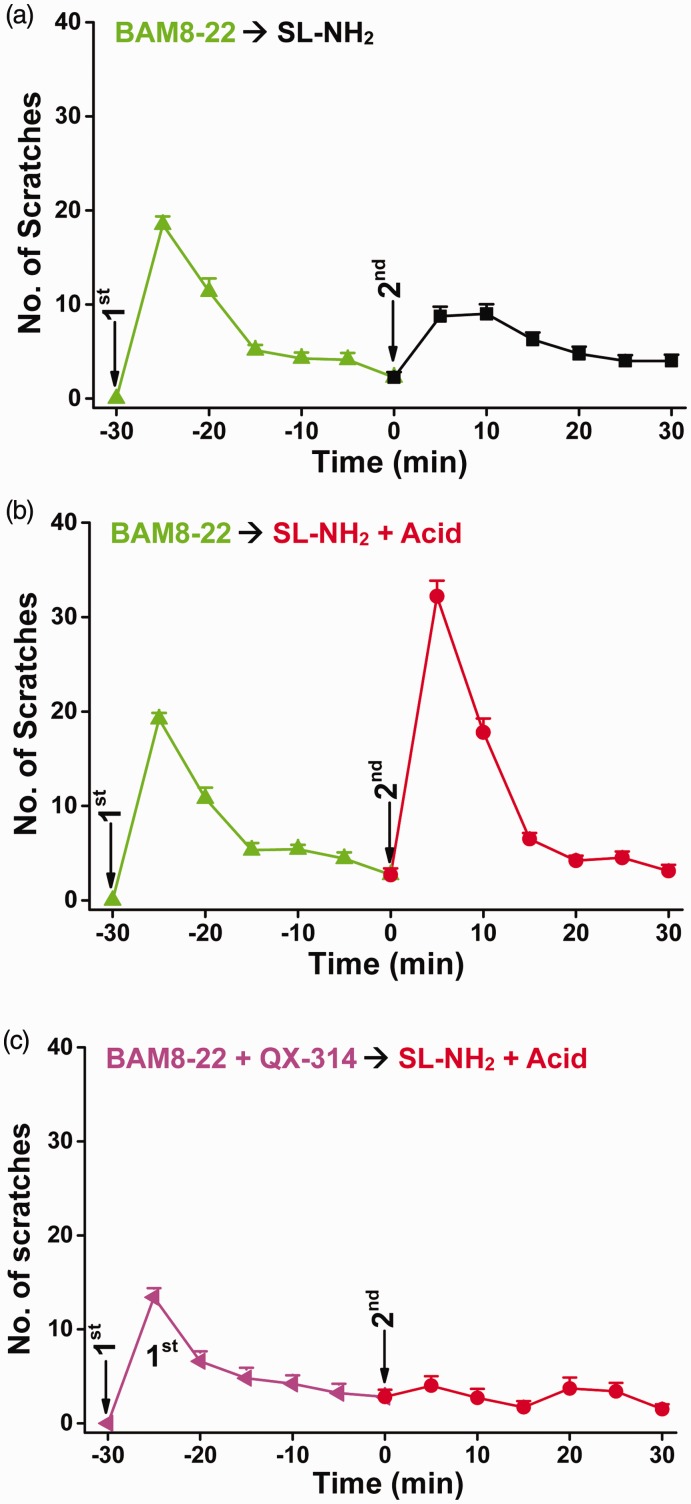
The above observation strongly supports the notion that acidosis potentiates SL-NH2-evoked itch behaviors through an ASIC3-dependent signaling cascade, which also counteracts itch tachyphylaxis to SL-NH2 alone. These actions appear to rely on activation of a solitary population of SL-NH2-responsive sensory neurons, which likely express MrgprC11. However, the fact that SL-NH2 can act at ASIC3 in the absence of MrgprC1116 entails a possibility that during the 30-min pre-exposure to QX-314 and SL-NH2, some of the SL-NH2−/ASIC+ sensory neurons could also take up QX-314. To rule this out, we used a different MrgprC11 agonist, BAM8-22, which does not act at ASIC3 channels,16 to ensure that the itch response to SL-NH2 and its tachyphylaxis counteraction by acidosis were all mediated by the MrgprC11-expressing neurons. Similar to SL-NH2, a prior application of BAM8-22 (3 mM in 50 µl) also suppressed the itch response to the subsequent injection of SL-NH2 (14 mM in 25 µl) to the same site (Figure 4(a)). In the BAM8-22 pre-treated animals, the scratching frequency in response to the subsequent SL-NH2 injection (Figure 6(a)) was significantly lower than in those that received SL-NH2 for the first time without the BAM8-22 pretreatment (Figure 1(a)) (first SL-NH2 vs. SL-NH2 with BAM8-22 pretreatment: F(1, 114) = 42.224, P < 0.001, by two-way ANOVA for each 5-min bin over the 30-min period; P < 0.001, by Student’s t test for the first 5 min following pruritogen injection); however, it was not different from the response to the second SL-NH2 injection in animals that were treated with SL-NH2 as the first injection (second SL-NH2 with SL-NH2 vs. BAM8-22 as pretreatment: F(1, 114) =0.649, P = 0.422, by two-way ANOVA for each 5-min bin over the 30-min period; P > 0.05, by Student’s t test for the first 5 min following pruritogen injection). Therefore, a prior stimulation of MrgprC11 with either SL-NH2 (Figure 1(a)) or BAM8-22 (Figure 4(a)) produced similar tachyphylaxis in the itch response to the subsequent application of SL-NH2.
Figure 4.
Cross-tachyphylaxis of itch elicited by administration of different MrgprC11 agonists. (a) First injection: BAM8-22 (3 mM) in saline (50 µl); second injection: SL-NH2 (14 mM) in saline (25 µl). (b) First injection: BAM8-22 (3 mM) in saline (50 µl); second injection: SL-NH2 (14 mM) and acid (1.2% acetic acid, v/v) in saline (25 µl). (c) First injection: BAM8-22 (3 mM) and QX-314 (1%, w/v) in saline (50 µl); second injection: SL-NH2 (14 mM) and acid (1.2% acetic acid, v/v) in saline (25 µl). Data are means ± SEM; n = 8–11 each group.
Remarkably, the delivery of SL-NH2 plus acid also completely counteracted the itch tachyphylaxis induced by the prior exposure to BAM8-22 (Figure 4(b)). In the BAM8-22-pre-treated animals, the scratching frequency in response to the injection of SL-NH2 plus acid (Figure 4(b)) was significantly higher than that to SL-NH2 alone (Figure 4(a)) (SL-NH2 vs. SL-NH2 plus acid: F(1, 108) = 87.909, P < 0.001, by two-way ANOVA for each 5-min bin over the 30-min period; P < 0.001, by Student’s t test for the first 5 min following pruritogen injection). Thus, the cross-tachyphylaxis between the two distinct MrgprC11 agonists, BAM8-22 and SL-NH2, occurred when the two different stimuli were applied sequentially within 30 min, but in the presence of acidosis, the BAM8-22-induced itch tachyphylaxis to subsequent application of SL-NH2 was completely overcome.
Using the activity-dependent silencing strategy29 as shown above, we found that co-application of QX-314 with BAM8-22 also abolished the scratching behaviors evoked by the subsequent application of SL-NH2 plus acid (Figure 4(c)). This supports the notion that the ASIC3-mediated itch elicited by SL-NH2 under acidosis, which also counteracts tachyphylaxis to MrgprC11-mediated itch by SL-NH2 alone, mainly occurred via the same population of MrgprC11-responsive sensory neurons. Taken together, our findings establish a pivotal role of ASIC3 (Figure 5), which when activated by concomitant acid and specific pruritogens that directly act at the channel,16 constitute a novel and essential component of signaling pathways that retain persistent itch behaviors. This pathway likely contributes to the chronic itch associated with tissue acidosis.
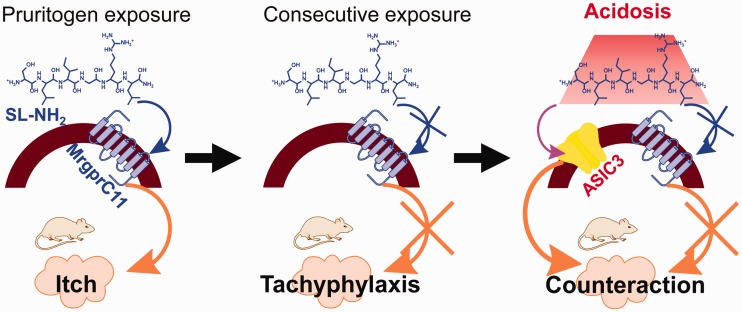
Figure 5.
A hypothetical scheme for acid-mediated, ASIC3-dependent, counteraction of tachyphylaxis to successive pruritogenic itch. (Left) SL-NH2 evokes itch via activation of MrgprC11 in sensory neurons; (middle) consecutive exposure of SL-NH2 causes itch tachyphylaxis, likely due to the desensitization of MrgprC11 signaling cascades; (right) SL-NH2 in the presence of acidosis activates ASIC3 in the same neuron, inducing itch via a different pathway, which counteracts tachyphylaxis and contributes to chronic itch.
Discussion
While the tachyphylaxis of itch may serve as a protective mechanism for the skin or body during repeated exposure to pruritogens, there must be counter-tachyphylaxis mechanism(s) under conditions of chronic itch. The existing itch pathway may be enhanced or additional itch mechanisms may be recruited to counteract or substitute for the desensitized signaling pathway in various dermatologic and systemic diseases in which chronic itch is one of the major irritating symptoms. Acidosis is a common condition associated with tissue injury and/or inflammation.33,34 In a previous study, we demonstrated that preventing the potential acidosis by increasing tissue pH or deletion of ASIC3 effectively decreased the spontaneous scratching behaviors in a dry skin pruritus model.16 Here, we further characterized the roles of acidosis and ASIC3 in the counteraction of itch tachyphylaxis to the pruritogen, SL-NH2, which can act at both the MrgprC11 and ASIC3, with the latter occurring only under acidosis. We found that acidosis, via ASIC3, could overcome itch tachyphylaxis to SL-NH2 produced by the prior stimulation of MrgprC11, regardless of the MrgprC11 agonist used, demonstrating a novel pathological mechanism that prolongs scratching behaviors under tissue acidosis.
Unlike the common pruriceptors, such as histamine receptors4 and a number of Mrgpr family members, such as MrgprA3 (see literature9) and MrgprC11 (see literature11), which mediate the itch evoked by chloroquine, BAM8-22, and SL-NH2, respectively. ASIC3 is involved in both itch16,21 and nociception.35–38 This calls into a question on the specific cell types that mediate ASIC3-dependent itch versus pain. Based on the activity-dependent silencing experiments, we favored the view that the same population of itch-generating sensory neurons responsive to SL-NH2 or BAM8-22 via the MrgprC11-TRPA1 pathway are responsible for ASIC3-mediated itch to SL-NH2 under acidosis, and hence able to counteract the tachyphylaxis of MrgprC11-TRPA1 signaling. Namely, the same population of sensory neurons transduces itch to the identical pruritogen via distinct molecular mechanisms, through activation of either ASIC3 or MrgprC11-TRPA1, in the presence or absence of acidosis (Figure 5), respectively.
We cannot completely rule out a possibility that aberrant activation of MrgprC11-negative sensory neurons by the combined acidosis and SL-NH2 may also have a role in shaping the itch response of the animal. As itch can be inhibited by painful chemical or mechanical stimuli,39–42 the ASIC3-dependent scratching behaviors measured here may reflect an underestimate of itch resulting from simultaneous activation of both pruritoceptive and nociceptive neurons. Specifically, ASIC3 may play a prominent role in mechanotransduction,43,44 a mechanism probably related to mechanical itch45 or mechanical modulation of pruritogenic itch. Surprisingly, when the MrgprC11-positive sensory neurons were silenced by the co-application of QX-314 and SL-NH2 or BAM8-22, the itch response to the co-injection of SL-NH2 and acid was completely eliminated even though the combined stimulation should still be able to activate a large number of MrgprC11-negative sensory neurons.16 Although other modalities of unpleasant sensation in these mice cannot be excluded, we speculate that either these neurons are not pruritoceptive or the activation of additional unidentified nociceptive and pruritoceptive neurons by acid plus SL-NH2 masked the itch-generating effects of ASIC3. These possibilities need to be carefully examined in the future.
In summary, we showed that MrgprC11-dependent itch tachyphylaxis was counteracted by the co-application of acid and SL-NH2. This occurred in WT, but not ASIC3-KO, animals, suggesting that ASIC3 is necessary for the acidosis-induced effect. Meanwhile, the MrgprC11-expressing sensory neurons contribute to the ASIC3-mediated itch. We propose that through coincident sensing of protons and certain pruritogens, ASIC3 constitutes an essential signaling component that causes chronic itch associated with tissue acidosis.
Methods
Animals
Animal care and the experimental protocols were approved by the Animal Ethics Committee of Shanghai Jiao Tong University School of Medicine, Shanghai, China. All efforts were made to minimize animal suffering and to reduce the number of animals used. Mice were housed under standard laboratory conditions (12/12 h light/dark, temperature 22℃–26℃, air humidity 55%–60%) with food and water ad libitum. Animal procedures were carried out in accordance with the guidelines for the Care and Use of Laboratory Animals of Shanghai Jiao Tong University School of Medicine and approved by the Institutional Animal Care and Use Committee (Department of Laboratory Animal Science, Shanghai Jiao Tong University School of Medicine) (Policy Number DLAS-MP-ANIM. 01–05).
Itch-related behavioral assays
All behavioral measurements were performed in awake and unrestrained C57BL/6 J, WT, and ASIC3-KO littermates.43 Animals were acclimatized for 30 min before all behavioral experiments. For itch responses, the pruritic compounds were subcutaneously injected into the nape of the neck after acclimatization, and scratching behaviors were observed for 30 min. For sequential injection experiments, the intradermal bullae of the conditioning (first) injection was outlined with a fine-tip permanent marker to denote the extent of intradermal drug distribution, thereby providing visible drug distribution boundaries for the second injection.29 Due to the potential consideration on the volume limit of the nape of the neck in a mouse, a different volume and concentration but the same amount of pruritogens from first to second injections were used. For the RTX treatment, RTX (100 µg/ml RTX, 50 µl in 0.05% ascorbic acid, and 7% Tween 80 vehicle) was injected subcutaneously into the neck three days before the behavioral assessment.
All the behavioral experiments were videotaped and scored, respectively, with a trained experimenter blinded to the genotype and the drug treatment. A bout of scratching was defined as continuous scratch movements with hind paws directed at the area around the injection site. Scratching behavior was quantified by recording the number of scratching bouts in 5-min intervals over the 30-min observation period.9 All experiments were performed under the policies and recommendations of the International Association for the Study of Pain and the Animal Care and Use Committee of Shanghai Jiao Tong University School of Medicine.
Reagents
All drugs were purchased from Sigma-Aldrich unless otherwise mentioned. SL-NH2 and BAM8-22 were synthesized by GL Biochem. Detailed preparation of injections to evoke scratching behaviors was mentioned in Figure legends.
Data analysis
Results are expressed as means ± SEM. Statistical comparisons were performed using unpaired or paired Student’s t tests or two-way analysis of variance. *P < 0.05, **P < 0. 01, and ***P < 0.001 represent statistically significant differences.
Acknowledgment
The authors thank Dr. James Celentano for proof-reading of this manuscript and also thank Ms. Fan Liu and Ms. Xin Qi for their technical assistance.
Author Contributions
TLX, WGL, and ZP designed the project. ZP, YMJ, and CH performed behavioral testing. CH and SLH performed genotyping. ZP, YMJ, and WGL did data analysis. WGL, MXZ, and TLX wrote the manuscript. All authors read and approved the final manuscript. YMJ and CH contributed equally to this work.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by grants from the National Basic Research Program of China (2014CB910300), the National Natural Science Foundation of China (31230028, 81400870, 81500941, and 91632304), and National Institutes of Health of USA (NS102452).
References
- 1.Ikoma A, Steinhoff M, Stander S, et al. The neurobiology of itch. Nat Rev Neurosci 2006; 7: 535–547. [DOI] [PubMed] [Google Scholar]
- 2.Mihara K, Kuratani K, Matsui T, et al. Vital role of the itch-scratch response in development of spontaneous dermatitis in NC/Nga mice. Br J Dermatol 2004; 151: 335–345. [DOI] [PubMed] [Google Scholar]
- 3.Akiyama T, Merrill AW, Zanotto K, et al. Scratching behavior and Fos expression in superficial dorsal horn elicited by protease-activated receptor agonists and other itch mediators in mice. J Pharmacol Exp Ther 2009; 329: 945–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thurmond RL, Kazerouni K, Chaplan SR, et al. Antihistamines and itch. Handb Exp Pharmacol 2015; 226: 257–290. [DOI] [PubMed] [Google Scholar]
- 5.LaMotte RH, Dong X, Ringkamp M. Sensory neurons and circuits mediating itch. Nat Rev Neurosci 2014; 15: 19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bautista DM, Wilson SR, Hoon MA. Why we scratch an itch: the molecules, cells and circuits of itch. Nat Neurosci 2014; 17: 175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han L, Dong X. Itch mechanisms and circuits. Annu Rev Biophys 2014; 43: 331–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McNeil B, Dong X. Peripheral mechanisms of itch. Neurosci Bull 2012; 28: 100–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Q, Tang Z, Surdenikova L, et al. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell 2009; 139: 1353–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lembo PM, Grazzini E, Groblewski T, et al. Proenkephalin A gene products activate a new family of sensory neuron–specific GPCRs. Nat Neurosci 2002; 5: 201–209. [DOI] [PubMed] [Google Scholar]
- 11.Liu Q, Weng HJ, Patel KN, et al. The distinct roles of two GPCRs, MrgprC11 and PAR2, in itch and hyperalgesia. Sci Signal 2011; 4: ra45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramachandran R, Hollenberg MD. Proteinases and signalling: pathophysiological and therapeutic implications via PARs and more. Br J Pharmacol 2008; 153: S263–S282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bunnett NW. Protease-activated receptors: how proteases signal to cells to cause inflammation and pain. Semin Thromb Hemost 2006; 32: 39–48. [DOI] [PubMed] [Google Scholar]
- 14.Zhou FM, Cheng RX, Wang S, et al. Antioxidants attenuate acute and chronic itch: peripheral and central mechanisms of oxidative stress in pruritus. Neurosci Bull 2017. doi:10.1007/s12264-016-0076-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mollanazar NK, Smith PK, Yosipovitch G. Mediators of chronic pruritus in atopic dermatitis: getting the itch out? Clin Rev Allergy Immunol 2016; 51: 263–292. [DOI] [PubMed] [Google Scholar]
- 16.Peng Z, Li WG, Huang C, et al. ASIC3 mediates itch sensation in response to coincident stimulation by acid and nonproton ligand. Cell Rep 2015; 13: 387–398. [DOI] [PubMed] [Google Scholar]
- 17.Lee MG, Dong X, Liu Q, et al. Agonists of the MAS-related gene (Mrgs) orphan receptors as novel mediators of mast cell-sensory nerve interactions. J Immunol 2008; 180: 2251–2255. [DOI] [PubMed] [Google Scholar]
- 18.Yu Y, Chen Z, Li WG, et al. A nonproton ligand sensor in the acid-sensing ion channel. Neuron 2010; 68: 61–72. [DOI] [PubMed] [Google Scholar]
- 19.Li WG, Xu TL. ASIC3 channels in multimodal sensory perception. ACS Chem Nurosci 2011; 2: 26–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marra S, Ferru-Clement R, Breuil V, et al. Non-acidic activation of pain-related acid-sensing ion channel 3 by lipids. EMBO J 2016; 35: 414–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lei Z, Sami Shaikh A, Zheng W, et al. Non-proton ligand-sensing domain of acid-sensing ion channel 3 is required for itch sensation. J Neurochem 2016; 139: 1093–1101. [DOI] [PubMed] [Google Scholar]
- 22.Li WG, Yu Y, Zhang ZD, et al. ASIC3 channels integrate agmatine and multiple inflammatory signals through the nonproton ligand sensing domain. Mol Pain 2010; 6: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin SH, Steinhoff M, Ikoma A, et al. Involvement of TRPV1 and TDAG8 in pruriception associated with noxious acidosis. J Invest Dermatol 2017; 137: 170–178. [DOI] [PubMed] [Google Scholar]
- 24.Shim WS, Tak MH, Lee MH, et al. TRPV1 mediates histamine-induced itching via the activation of phospholipase A2 and 12-lipoxygenase. J Neurosci 2007; 27: 2331–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Imamachi N, Park GH, Lee H, et al. TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proc Natl Acad Sci U S A 2009; 106: 11330–11335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim S, Barry DM, Liu XY, et al. Facilitation of TRPV4 by TRPV1 is required for itch transmission in some sensory neuron populations. Sci Signal 2016; 9: ra71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gavva NR, Tamir R, Qu Y, et al. AMG 9810 [(E)-3 -(4-t-butylphenyl)-N-(2,3-dihydrobenzo[b][1,4] dioxin-6-yl)acrylamide], a novel vanilloid receptor 1 (TRPV1) antagonist with antihyperalgesic properties. J Pharmacol Exp Ther 2005; 313: 474–484. [DOI] [PubMed] [Google Scholar]
- 28.Wilson SR, Gerhold KA, Bifolck-Fisher A, et al. TRPA1 is required for histamine-independent, Mas-related G protein-coupled receptor-mediated itch. Nat Neurosci 2011; 14: 595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roberson DP, Gudes S, Sprague JM, et al. Activity-dependent silencing reveals functionally distinct itch-generating sensory neurons. Nat Neurosci 2013; 16: 910–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karai L, Brown DC, Mannes AJ, et al. Deletion of vanilloid receptor 1-expressing primary afferent neurons for pain control. J Clin Invest 2004; 113: 1344–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mishra SK, Hoon MA. Ablation of TrpV1 neurons reveals their selective role in thermal pain sensation. Mol Cell Neurosci 2010; 43: 157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitchell K, Bates BD, Keller JM, et al. Ablation of rat TRPV1-expressing Adelta/C-fibers with resiniferatoxin: analysis of withdrawal behaviors, recovery of function and molecular correlates. Mol Pain 2010; 6: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reeh PW, Steen KH. Tissue acidosis in nociception and pain. Prog Brain Res 1996; 113: 143–151. [DOI] [PubMed] [Google Scholar]
- 34.Steen KH, Reeh PW. Sustained graded pain and hyperalgesia from harmless experimental tissue acidosis in human skin. Neurosci Lett 1993; 154: 113–116. [DOI] [PubMed] [Google Scholar]
- 35.Deval E, Noel J, Lay N, et al. ASIC3, a sensor of acidic and primary inflammatory pain. EMBO J 2008; 27: 3047–3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen CC, Zimmer A, Sun WH, et al. A role for ASIC3 in the modulation of high-intensity pain stimuli. Proc Natl Acad Sci U S A 2002; 99: 8992–8997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sluka KA, Winter OC, Wemmie JA. Acid-sensing ion channels: a new target for pain and CNS diseases. Curr Opin Drug Discov Devel 2009; 12: 693–704. [PMC free article] [PubMed] [Google Scholar]
- 38.Sluka KA, Price MP, Breese NM, et al. Chronic hyperalgesia induced by repeated acid injections in muscle is abolished by the loss of ASIC3, but not ASIC1. Pain 2003; 106: 229–239. [DOI] [PubMed] [Google Scholar]
- 39.Liu Y, Abdel Samad O, Zhang L, et al. VGLUT2-dependent glutamate release from nociceptors is required to sense pain and suppress itch. Neuron 2010; 68: 543–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lagerstrom MC, Rogoz K, Abrahamsen B, et al. VGLUT2-dependent sensory neurons in the TRPV1 population regulate pain and itch. Neuron 2010; 68: 529–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kardon AP, Polgar E, Hachisuka J, et al. Dynorphin acts as a neuromodulator to inhibit itch in the dorsal horn of the spinal cord. Neuron 2014; 82: 573–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao ZQ, Liu XY, Jeffry J, et al. Descending control of itch transmission by the serotonergic system via 5-HT1A-facilitated GRP-GRPR signaling. Neuron 2014; 84: 821–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Price MP, McIlwrath SL, Xie J, et al. The DRASIC cation channel contributes to the detection of cutaneous touch and acid stimuli in mice. Neuron 2001; 32: 1071–1083. [DOI] [PubMed] [Google Scholar]
- 44.Lin SH, Cheng YR, Banks RW, et al. Evidence for the involvement of ASIC3 in sensory mechanotransduction in proprioceptors. Nat Commun 2016; 7: 11460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bourane S, Duan B, Koch SC, et al. Gate control of mechanical itch by a subpopulation of spinal cord interneurons. Science 2015; 350: 550–554. [DOI] [PMC free article] [PubMed] [Google Scholar]