Abstract
The Eighth Scientific Meeting of The TMJ Association, Ltd. was held in Bethesda, Maryland, September 11–13, 2016. As in the past, the meeting was cosponsored by components of the National Institutes of Health with speakers invited to review the state of temporomandibular disorder science and propose recommendations to further progress. The theme of precision medicine, which aims to tailor disease treatment and prevention to match the characteristics of an individual patient (genetic, epigenetic, environmental, lifestyle) underscored the current consensus that temporomandibular disorders are no longer viewed as local conditions of jaw pain and dysfunction. Rather, they represent a complex family of biopsychosocial disorders that can progress to chronic pain, most often accompanied by one or more other chronic pain conditions. Temporomandibular disorders and these comorbidities, called chronic overlapping pain conditions, predominantly or exclusively affect women in their childbearing years and reflect central nervous system sensitization. Presenters at the meeting included leaders in temporomandibular disorder and pain research, temporomandibular disorder patients and advocates, and experts in other fields or in the use of technologies that could facilitate the development of precision medicine approaches in temporomandibular disorders.
Keywords: Precision medicine, chronic overlapping pain conditions, temporomandibular disorders, recommendations, TMJ Association
The U.S. precision medicine initiative and applications to pain patients
As background, the new U.S. Precision Medicine Initiative was described. This multidecade federal research program charges the National Institutes of Health (NIH) with recruiting a million demographically diverse Americans whose genomes will be obtained, along with nongenetic data from electronic health records, lifestyle information, and physiological variables, to be assembled into databases for precision medicine analysis. Several speakers then addressed research to stratify pain patients to permit more personalized therapies. In the case of the long-running Orofacial Pain: Prospective Evaluation and Risk Assessment (OPPERA) study, investigators have been able to cluster newly diagnosed temporomandibular disorder (TMD) patients in the study into mild, moderate, and severe groups, the latter group often with overlapping pain conditions. Importantly, the analysis enabled the reduction of the many data elements collected initially into four factors that can be used to assign new patients to a cluster. OPPERA’s leading geneticist also reported the discovery of several new immune system-related genes in association with TMD pathology, based on genome-wide association studies and single nucleotide polymorphism (SNP)/expression quantitative trait loci (eQTL) analyses. In another study using brain imaging, investigators followed newly diagnosed low-back pain patients, noting extensive new connections between the amygdala and the cortex in patients who progressed to chronic low-back pain compared with those who recovered. Structural differences in the size of selected brain elements also distinguished the difference between the two groups.
More precise clinical trials
The idea of using a single patient as the focus of a clinical trial—an “N-of-1” trial—was highlighted as a mean of furthering precision medicine goals by tailoring therapies to the needs and outcomes specified by an individual patient. The patient would test successive treatments (including placebos) in a series of crossovers for fixed lengths of time with measured outcomes. For this to work the problem being treated needs to return to baseline conditions for each crossover and a washout period inserted in case a treatment might persist into the next crossover period. Also discussed were in silico approaches exploiting computer modeling of disease using patient registries as rich data resources, as well as current efforts to improve the design and conduct of traditional clinical trials. The emphasis was on patient-centric approaches, engaging patients and patient advocacy organizations in the design of protocols and recruitment of volunteers who would be better informed and valued for their contributions. The session included a TMD patient’s story of failed treatment and a description by the cofounder of the recently organized Chronic Pain Research Alliance, an initiative of The TMJ Association, of the role that advocacy organizations have played in documenting their members’ experience of overlapping pain conditions.
New therapeutic targets along signaling pathways
Cyclin-dependent kinase 5 (Cdk5) was identified as an enzyme not only involved in synaptic transmission but also implicated in several deleterious changes in the nervous system. Mice, whose Cdk5 gene was knocked out or inhibited by a drug, were better, faster learners, less depressed, and suffered less neuronal cell death from stroke or traumatic injury. Considerable discussion focused on the role of the immune system and glial cells in either exacerbating or mitigating the effects of chronic pain. In that regard, it was suggested that selected chemokines be therapeutic targets—in one case, to prevent the infiltration of immune cells to a site of nerve injury and continued inflammation, and in another, to promote healing by attracting mesenchymal bone marrow stem cells to the injured site to reduce neuroinflammation and pain. Microglia were identified as a cause of neuropathic pain but only in male mice. Several presenters cited a causative role for immune factors in conditions as varied as irritable bowel disease and neurodegenerative diseases such as Alzheimer’s and Parkinson’s, with proposals for countering these effects.
A positive contribution of immune cells figured in presentations on chemobrain, the combination of fatigue, neuropathy, and cognitive deficits associated with the use of potent anticancer drugs. CD3+, CD4+ and CD8+ T cells were found to be protective in a study of mice treated with the anticancer drug paclitaxel through production of interleukin 10. Nasal infusion of mesenchymal stem cells also proved restorative in another mouse model of chemobrain produced by cisplatin.
New technologies: Organs on a chip and induced pluripotent stem cells
Speakers pointed to the utility of new technologies in aiding precision medicine. Organs on a chip, for example, provide a microscopic model of a functioning organ of the body. To study disease in a particular patient, the chip can incorporate cells derived from the patient’s own stem cells. These induced pluripotent stem cells (iPSCs) are based on technology that allows adult human cells to be reprogrammed to a primitive stage and stimulated to become any cell type in the body. A chip modeling the blood brain barrier in a particular patient could be used to measure how effectively or not a pain drug is able to enter and exit the brain.
Sex differences
The question of sex differences in the perception and response to pain came up in a number of presentations and was specifically addressed by several speakers. Quantitative sensory tests of young adults as well as in a larger sample of adults across the life span consistently showed that females generally were more sensitive than males, reporting lower thresholds and more intense pain in response to graded applications of heat, cold, or pressure stimulation. And in an ingenious rat model of overlapping pain conditions simulating TMD and irritable bowel syndrome, female rats similarly proved to be more sensitive in tests which also showed that their sensitivity varied with their estrous cycle, being highest when estrogen peaked.
Epigenetic effects
Two examples of epigenetic influences on pain perception were presented. A mouse model of neuropathic pain, produced by a chronic constriction nerve injury, showed increased expression of a histone methyltransferase, effectively silencing gene expression of selected dorsal root ganglion potassium channels and opioid receptors important in ameliorating pain. A rat study comparing responses to induced inflammatory or nerve constriction injury found similarities in the animals’ sensory responses to pain but wide variation in their emotional responses. The differences corresponded to levels of DNA methyltransferase which were highest in the resilient rats and associated with silencing genes associated with anxiety behavior.
Anatomical findings
The meeting included several new nervous system discoveries. One investigator reported finding a small population of neurons in the ventrolateral medulla with potent analgesic properties. Another investigator, studying touch sensation in the orofacial area, found that Merkel’s discs, cells in contact with sensory nerve endings, exert a mechanical force on the nerve ending that results in the release of the neurotransmitter serotonin. A third presenter provided details on how to program an iPSC to form a nociceptor, suggesting that a patient’s own stem cells reprogrammed in this way would provide an efficient means of testing the patient’s response to new drugs.
Concluding remarks
As the meeting drew to an end, the organizers and a key NIH administrator and leading researcher informally summarized the “arc of research” over the past decade, concluding that the time was ripe for translating findings to the private sector for clinical application. There was a brief presentation by the research director of a small private company collecting specimens for a biobank, working on a TMD diagnostic questionnaire and a pain perception sensitivity score—all described as “works in progress.” The remainder of the morning was devoted to a group discussion to develop the research recommendations described below.
Research recommendations to further progress on how precision medicine can be applied to TMD and its comorbidities
The TMJ Association’s Eighth Scientific Meeting explored the role that “precision medicine” can play in improving the treatment of TMD and comorbid chronic pain conditions. Past research has affirmed that TMD represents a family of complex pain conditions in which environmental, behavioral, and genetic factors interact to generate psychological stress and pain hypersensitivity. The result can lead to a worsening of TMD symptoms and the development of chronic pain conditions elsewhere in the body. Current diagnostic methods and treatments fail to reflect the new state of science and fail to meet the needs of patients. The presentations and discussions at the meeting led to recommendations which could significantly advance the diagnosis, treatment, and prevention of TMD and associated overlapping pain conditions using precision medicine approaches.
Next-generation sequencing (NGS) using low-cost technology can now be used to sequence an individual’s genome. The genome can then be scanned with the goal of identifying those variants in sequence segments, which could provide relevant information about the state of the individual’s health, risk of disease, and appropriate treatment choices. At this time, most diagnostic tests follow a one-test–one-disease paradigm despite the ability of NGS to produce a wealth of data about a patient. While NGS may lead to the identification of disease-causing DNA variants or associated susceptibility genes, such results provide only a one-dimensional starting point toward understanding the multiple dimensions of the pathological process. Expanding studies to a more comprehensive precision medicine approach, as emphasized below, will enable more subtle delineations of the patient population into subgroups amenable to different types of treatment.
At this time, NGS and precision medicine approaches are only just beginning to be considered for TMD and its associated comorbidities—and for the chronic pain field in general. Progress has been slow, in part as a consequence of limited availability of:
Appropriate animal models of these complex conditions;
Molecular and cellular tools to identify and functionally annotate susceptibility or disease genes;
Physiological approaches to determine the signaling pathways which lead to the progressive development of TMD;
Analytical tools to analyze big data in efficient ways; and
Appropriately trained physicians/dentists and basic/translational scientists who can assemble and integrate NGS data with data related to the regulation of gene function and the expanding knowledge of the cell and molecular physiology of chronic pain.
Despite these challenges, there are now exciting scientific opportunities to identify new genes and effector pathways that contribute to the pathogenesis of TMD and associated chronic pain conditions. Application of technologies which can produce an abundance of data about a patient should yield a deeper understanding of the biological basis of TMD and help identify new drug targets to delay or reverse the onset or the progression of these disorders. Importantly, the research recommendations below have been driven by patient needs as reflected by their involvement in the planning and attendance at the meeting. The recommendations thus reflect a patient-centric approach that will help advance more meaningful and personalized diagnostic and therapeutic measures, which take into account individual variability in genes, family history, environment, and lifestyle.
Recommendation 1. Bring TMD and chronic overlapping pain conditions stakeholders together to develop and implement precision medicine approaches for diagnosis and treatment
There is a need to form integrated research groups to coordinate basic research, human data collection and sharing, and translation of basic and clinical science into therapeutic advances. These integrated groups should reflect patient needs, population diversity (race and sex), and recognition that the majority of patients are female. The key stakeholders should be patients and their advocates, academic units, government agencies, and the private sector. Strategies of “implementation science” must be employed so that patients, families, and other stakeholders can interact with scientists and physicians in the development of scientific protocols and studies.
Physicians, researchers, and patients should come together in academic medical centers to form Regional Centers of Excellence. To encourage this approach, NIH could provide competitive planning grants for centers that involve participation by medical, dental, and other professional schools in a region. External advisory committees could then guide and coordinate center activities, as is the case with NIH cooperative agreements. The centers should represent different areas of expertise and disease focus related to TMD and its comorbidities, the most prominent area being chronic pain. The centers should be sufficiently flexible to respond to local needs to focus on specific areas of research, enable coordination of local resources, develop pilot grant support for preliminary data, and be able to link to other centers to develop national data coordinating centers.
As the Regional Centers of Excellence are formed, their research and training initiatives should be supported by NIH research, research training grants, and cooperative agreements, as well as grants and contracts from other government agencies, including the United States Department of Veterans Affairs, the Department of Defense, and private sector foundations and corporations.
Examples of several regional Research Centers are emerging at Duke University, the University of Toronto, and the University of Alabama, each of which is currently developing focus areas of research on TMD and comorbid conditions.
A specific model of the collaborative and integrative research needed in this field is one currently being supported by the VA: The VA Spinal Cord Injury (SCI) Consortium represents a cooperative effort involving UC San Francisco, Palo Alto VAMC, UC Irvine, San Diego VA/UCSD, and UC Davis. The consortium brings together patients, clinical trials and rehabilitation experts, neurosurgeons, stem cell biologists, cell therapists, informatics experts, and experimental rodent biologists. Similar planning and integration of diverse scientific and clinical expertise, working in a patient-centric manner, should be developed and supported by NIH or interagency initiatives.
Recommendation 2. Advance understanding of the molecular, genetic and neural mechanisms that mediate persistent pain conditions in individuals with TMD
To implement a precision medicine approach toward this end, the following areas should be addressed:
Expand cohorts in prospective studies to obtain richer phenotypic and genomic databases. These data will enable more detailed elucidation of molecular pathways, molecular markers, and pathophysiological mechanisms to characterize risk factors that predispose to TMD and chronic pain.
Advance opportunities to apply NGS approaches in order to obtain more personalized diagnostic and therapeutic measures with the goal of accounting for individual variability in genes, family history, environment, and lifestyle.
Support studies to assess the epigenomic impact of both natural environmental exposures and those related to implant devices that can lead to great harm to genetically susceptible individuals.
Encourage the affordable use of sequencing and informatics applications to carry out whole genome methylation and histone analysis studies.
Support research on how the oral microbiome affects the development of TMD and related comorbidities such as chronic pain conditions.
Encourage the development and application of phenotyping tools for collection of environmental exposure data as a part of the medical history.
Recommendation 3. Integrate the brain imaging structure-function database together with studies related to TMD and chronic overlapping pain conditions
Recommendation 4. Study the role of the immune system in the development of chronic overlapping pain conditions
Support research on immunopharmacological approaches for diagnosis and treatment of chronic pain.
Support research on the role of the peripheral immune system in TMD and related chronic pain conditions.
Recommendation 5. Develop strategies/methodologies/protocols for disease modeling to advance precision medicine
Research is needed to develop guidelines for identifying subgroups of patients with distinct mechanisms of disease and particular responses to treatments. The research design of a precision medicine approach should incorporate the following:
Expansion of existing patient cohorts to enable collection of more detailed patient phenotypes with longitudinal follow-up and collection of associated biological specimens for precision medicine.
Emphasis on the importance of shared decision-making between the clinician and patient to assess the patient’s potential for benefit or harm from treatment proposed.
Establishment of additional cohorts to increase power, replicate findings, and extend the findings to more diverse patient populations. Replication is of critical importance for genetic studies, and support in this area will strengthen the efforts in other target areas such as biomarker identification and the development of more relevant disease models.
Increase efforts toward developing synergies with other NIH initiatives such as the Precision Medicine Initiative cohorts. Study designs for the above-mentioned cohorts should build and utilize the infrastructure of these other initiatives.
Develop animal models which better recapitulate the disease phenotypes.
Recommendation 6. Develop induced pluripotent stem cell technologies to provide a tool for basic and translational investigators studying chronic pain
Strategies should be developed to encourage the development, validation, and application of iPSC cell lines from chronic pain patients to discover new mechanisms of disease and new therapeutic targets.
Support investigators in the field to develop a collection of iPSC models and leverage genome editing technologies (e.g. CRISPR-cas9) and use these cell models from pain patients in conjunction with other emerging technologies such as three-dimensional culture systems (organs-on-chips) and xenografts.
Support careful characterization of disease iPSC lines (maintenance, authentication, and distribution) coupled with support for a dedicated repository.
Encourage research utilizing iPSCs for candidate drug testing, compound screening, drug repurposing, and toxicity testing (clinical trials in a dish) in order to assess effectiveness of therapeutic compounds on an individual’s own genetic background.
Emphasize the development of novel drug treatments. Building on the concepts of precision medicine, these efforts should incorporate the above outlined platforms; integration of genetic findings in patient cohorts, biomarker development based on samples collected from various patient cohorts as well as leveraging the Precision Medicine Initiative cohorts, when integrated with iPSC cell technology and animal models has a high likelihood to yield more targeted, disease relevant compounds.
Developing the infrastructure for this will require significant administrative oversight and guidance based on the achievement of milestones in each area.
Recommendation 7. Address the dire lack of appropriately trained physicians and dentists at medical, dental, and other professional schools in the U.S.
Increase the numbers of appropriately trained physicians/dentists and basic/translational scientists who understand the technologies required to carry out precision medicine.
Encourage the development of educational materials and guidelines for students, health-care providers, patients, and the public.
Encourage workshops that include medical and dental students/fellows, practicing physicians and allied health nurses and assistants, patients, and administrators.
Encourage the implementation of “patient-centric” educational processes. Medical treatments and diagnostics for TMD are currently designed for the average patient as a “one-size-fits-all” approach. A precision medicine approach will require an individual patient’s data regarding health, lifestyle, and environment.
Acknowledgments
The authors are grateful to John Kusiak, PhD, Acting Deputy Director, National Institute of Dental and Craniofacial Research, National Institutes of Health for his participation in the planning of the scientific meeting. They thank The TMJ Association, Ltd., Milwaukee, WI, staff and volunteers; Terrie Cowley, Deanne Clare, Steven Pusztai, Laurie Friedrich and Karen McQuestion for their editorial expertise and Greg McQuestion for videography.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The Eighth Scientific Meeting of The TMJ Association, Ltd., September 11–13, 2016, was supported by The TMJ Association and the National Institute of Dental and Craniofacial Research, grant number R13DE026363.
Meeting Abstracts
The U.S. precision medicine initiative
Eric D Green
National Human Genome Research Institute, National Institutes of Health, Bethesda, MD, USA
Starting with the launch of the Human Genome Project in 1990, the past quarter-century has brought spectacular achievements in genomics that dramatically empower the study of human biology and disease. Augmenting the advances in human genomics have been innovations in electronic health records, data science, and technologies for capturing environmental, physiological, and lifestyle information. Together, these provide opportunities of unprecedented scale and scope for investigating the underpinnings of health and disease.
To capitalize on these developments, the United States recently launched a major new research endeavor—the Precision Medicine Initiative. This bold effort is framed around several key aims, which include accelerating the use of genomically informed approaches to cancer care, making important policy and regulatory changes, and establishing a large research cohort of >1 million volunteers to facilitate precision medicine research. The latter will include making the partnership with all participants a centerpiece of the cohort’s design and development. The Precision Medicine Initiative represents a broad-based research program that will allow new approaches for individualized medical care to be tested in a rigorous fashion, so as to establish a new evidence base for advancing clinical practice and, eventually, human health.
Key issues for advancing precision medicine for TMD and chronic overlapping pain conditions: Case definitions and phenotypic measures
William Maixner
Department of Anesthesiology, Duke University School of Medicine, Durham, NC, USA
There is increasing recognition that many, if not most, common chronic pain conditions are heterogeneous with a high degree of overlap or coprevalence.
There is an increased recognition that we should begin to think of common chronic pain conditions as Chronic Overlapping Pain Conditions (COPCs). 1 There are at least two features of COPCs that will be presented by Dr. Maixner: (1) That the etiology/mechanisms of COPCs are multifactorial and (2) that the clinical manifestations of COPCs are diverse, common, and shared across COPCs.2–4
Dr. Maixner will discuss findings that support the view that there are shared biological mechanisms that contribute to the manifestation of COPCs. A unifying theme of his presentation is that multiple biological processes, when coupled with environmental exposures (e.g. injury, infections, physical, and psychological stress), contribute to the signs and symptoms that underlie COPCs. Concepts and methods that can be used to identify subpopulations of COPCs patients that contribute to advancing precision pain medicine will be presented. 5
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Equity shareholder and consultant—Proove Biosciences, Orthogen, and Algynomics Holdings.
References
- 1.Veasley C, Nguyen R, Clare D, et al. Impact of chronic overlapping pain conditions on public health and the urgent need for safe and effective treatment: 2015 analysis and policy recommendations, http://chronicpainresearch.org/public/CPRA_WhitePaper_2015-FINAL-Digital.pdf (2015, accessed 19 June 2017).
- 2.Diatchenko L, Nackley AG, Slade GD, et al. Idiopathic pain disorders – pathways of vulnerability. Pain 2006; 123: 226–230. [DOI] [PubMed] [Google Scholar]
- 3.Slade GD, Bair E, Greenspan JD, et al. Signs and symptoms of first-onset TMD and sociodemographic predictors of its development: the OPPERA prospective cohort study. J Pain 2013; 14. T20-32 e1–e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slade GD, Ohrbach R, Greenspan JD, et al. Painful temporomandibular disorder: Decade of Discovery from OPPERA studies. J Dent Res 2016; 95(10): 1084–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bair E, Gaynor S, Slade GD, et al. Identification of clusters of individuals relevant to temporomandibular disorders and other chronic pain conditions: the OPPERA study. Pain 2016; 157: 1266–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
Longitudinal gene-brain mapping to guide diagnosis and treatment of mechanistically distinct types of chronic pain
Apkar Vania Apkarian
Department of Physiology, Feinberg School of Medicine, Northwestern University, Chicago, IL, USA; Departments of Anesthesia and Surgery, Feinberg School of Medicine, Northwestern University, Chicago, IL, USA
The pursuit of longitudinal gene-brain mapping has immense potential to guide diagnosis and treatment of mechanistically distinct types of chronic pain. Our work has established that the interaction between the brain and chronic pain gives rise to distinct brain states, characterized by specific anatomical and physiological features, for different clinical pain conditions. Specifically, neocortical grey matter dynamically changes with chronic pain, and this reorganization is pain-type specific. In the only longitudinal study of the transition to chronic pain, we used brain imaging to identify functional and structure properties of the limbic brain that predict which chronic back pain patients would transition to chronicity one year later. Findings reveal that risk for chronicity is conferred by subcortical-limbic functional connections between key regions and parallel the transition to the chronic pain state, as well as stable subcortical anatomical circuits that likely reflect predisposition for developing chronic pain. These observations dovetail with longitudinal brain imaging results from the Multidisciplinary Approach to the Study of Chronic Pelvic Pain Research Network, wherein baseline resting state functional connectivity of frontoparietal regions predicts future reductions in urologic pelvic pain three months later. Collectively, these results emphasize the potential of multimodal brain imaging to parse brain properties that induce risk (predisposition), their interaction with injury (transition), and the resulting new brain state (maintenance) in clinically undifferentiated chronic pain populations. Our more recent rodent brain imaging and electrophysiological studies that closely parallel the human studies are beginning to provide complementary mechanistic insights regarding cellular, synaptic, and circuit reorganization with transition to and maintenance of chronic pain.
N-of-1 trials for personalized decision-making
Christopher Schmid
Center for Evidence Based Medicine, Brown University School of Public Health, Providence, RI, USA
N-of-1 trials are a promising tool to enhance decision-making and improve outcomes. These trials are single-participant multiple-crossover studies for determining the relative comparative effectiveness of two or more treatments for one individual. The individual selects treatments and outcomes of interest and, possibly working with a health-care provider, carries out the trial, before making a final treatment decision based on its results. This talk will discuss the advantages and challenges in conducting N-of-1 trials, along with some of the design and analytic considerations. A study to test the effectiveness of the N-of-1 trial as a clinical decision tool comparing patients randomized to N-of-1 versus usual care is ongoing. The challenges of implementing the decision strategy in such a context and the pros and cons of combining information from different patients in order to provide a better estimate of each individual’s effect than from his or her own data alone will be discussed.
In silico approach to developing TMD-related precision medicine applications: Narrowing the gap between currently available and urgently needed
Yelizaveta Torosyan
Division of Epidemiology of the Center for Devices and Radiological Health, Food and Drug Administration, Silver Spring, MD, USA
Temporomandibular disorders (TMDs) are known for clinical heterogeneity and multifactorial etiology, and possible adverse outcomes from treatments such as temporomandibular joint (TMJ) arthroplasty can impose an additional layer of complexity on the already complicated sum effect. In the meantime, a proper management of TMD patients requires distinguishing among Patient–Procedure–Device-related inputs which ultimately define treatment success or failure. Variations in nociceptive threshold and underlying inflammatory conditions, including osteoarthritis as one of the main TMD causes, can not only define the extent of initial—functional and clinical—presentation of TMD, but can also predetermine the treatment outcome after TMJ arthroplasty. Both of these patient-related characteristics—tolerance to pain and susceptibility to inflammation—are prone to interindividual variations due to environmental factors (e.g. health habits) and demographic/genetic factors (e.g. sex, race/ethnicity, single nucleotide polymorphisms (SNPs)). Genome-wide association studies (GWAS) suggested that a number of SNPs in the genes involved in pain perception and inflammation may help identifying patients who are more prone to development of severe TMDs or who have TMDs with certain phenotypic features. However, many of putative biomarkers for the initial TMD are also identified as potential drivers of the adverse treatment outcomes in TMD/TMJ arthroplasty patients. As a result, potential use of these biomarkers is complicated by uncertainty whether the positivity for such a biomarker would indicate the initial inflammatory condition, or whether it would demonstrate the post-implantation inflammatory response that may lead to an implant-elicited adverse reaction resulting in revision surgery. In addition, many of the currently identified putative TMD biomarkers are influenced by patient’s race/ethnicity, which further complicates their potential utility in TMD/TMJ arthroplasty subpopulations with different ethnic backgrounds.
This presentation will outline the current field of TMD/TMJ arthroplasty biomarkers and will discuss the existing challenges impeding development of actionable biomarkers. The presentation will particularly highlight the need for developing different types of biomarkers, ranging from diagnostic TMD biomarkers that would be specific for the underlying causal mechanisms (e.g. functional pain vs. degenerative process) to prognostic TMJ arthroplasty biomarkers that would be indicative of the implant-specific outcomes (e.g. wound healing vs. osteolytic loosening). As possible solutions for developing TMD/TMJ arthroplasty biomarkers that could be applicable in clinical and regulatory settings, the presentation will discuss possibilities for in silico research using GWAS and expression profiling data in association with the clinical and epidemiological evidence gathered from observational studies and patient queries.
Effective engagement with patient groups around clinical trials
Jamie Roberts
Clinical Trials Transformation Initiative, Duke Clinical Research Institute, Durham, NC, USA
Background
Patient groups are developing diverse skill sets and assets to provide valuable trial services, funding, and the ability to enhance collaboration, as well as providing the necessary information to ensure that precision medicine trials become a reality. Research sponsors across the clinical trials enterprise are recognizing the benefits of continuous and meaningful patient group engagement, but all stakeholders need further guidance on operationalizing this new model. CTTI’s best practices consolidate actionable recommendations for establishing strong, active patient group engagement during all phases of the research and development lifecycle. Our evidence gathering, along with further consultation with experts, has led to the identification of best practices and tools for patient group engagement in the clinical trial enterprise. Additional work is underway to develop a framework for assessing the value and impact of patient group engagement.
Importance
Patient-centered clinical research demands greater participant engagement as patients become more empowered in their health-care decision-making. To ensure a high level of patient participation and engagement in clinical trials, the medical therapeutic research and development community must elevate the patient voice in research planning. Organizations are beginning to incorporate processes and practices that allow patients to become more actively involved in precision medicine clinical trials, from design through execution and dissemination of results. However, best practices and a value proposition for engaging with patients and patient groups are necessary to further the cause of deeper, more meaningful levels of patient engagement in the clinical research enterprise. CTTI has developed widely accepted Best Practices and a proposed value model for measuring the impact of various engagement methods.
Objectives
An introduction to The Clinical Trials Transformation Initiative and the Best Practices for Patient Group Engagement in Clinical Trials Project;
An understanding of best practices, recommendations, and tools to maximize patient engagement in the development of precision medicine research and trials;
A brief introduction to how such engagement might be measured.
TMD treatment and precision medicine—The past, present, and future
Michele Kaseta1, Danica Marnica-Dabic2 and Christin Veasley3
1The TMJ Association, Ltd., Milwaukee, WI, USA
2Division of Epidemiology of the Center for Devices and Radiological Health, Food and Drug Administration, Silver Spring, MD, USA
3Chronic Pain Research Alliance, The TMJ Association, Ltd., Milwaukee, WI, USA
Currently, we know very little about the genetic, environmental, psychophysical, and other factors that affect an individual’s responsiveness to treatment. This has left health-care providers and patients with little to no information on which to base important treatment decisions, and many describe the process of selecting a safe and effective treatment for temporomandibular disorder (TMD) and overlapping conditions from the myriad that are available as a “roll of the dice.” This session will explore the promise that precision medicine holds in transforming this situation. Michele Kaseta will describe her experiences in being diagnosed with TMD and undergoing temporomandibular joint (TMJ) implant (and other) surgical procedures and how her treatment outcome may have been improved if advances in precision medicine were available a decade ago. Dr. Danica Marinac-Dabic, Director of the Division of Epidemiology at the Food and Drug Administration (FDA), will provide a summary of a new FDA initiative being undertaken with multiple stakeholder groups to advance individualized, patient-centered diagnosis and treatment of TMD. Specifically, the long-term goals of this initiative are to: (i) develop outcome assessment and reporting tools based on patient input; (ii) understand the complex interplay of patient biology, anatomy, genetics, and physiology with TMJ medical devices and clinical patient-centered outcomes in order to better target therapies toward the patients most likely to benefit from them; and (iii) develop evidence to incorporate patient-centered data into clinical care. Christin Veasley, Co-Founding Director of the Chronic Pain Research Alliance, will conclude the session with an exploration of the complexity of the overlap among TMD and other chronic pain disorders, and how precision medicine techniques will aid in more accurate and adequate diagnostic approaches that are based on underlying pathophysiology, as well as the selection of safe and effective treatment that is individualized, patient-centered, and based on scientific evidence.
Precision medicine strategies to selectively alter intracellular signaling mechanisms: A new generation of targets
James A Bibb
Departments of Psychiatry, and Neurology and Neurotherapeutics, Harold C. Simmons Comprehensive Cancer Center, The University of Texas Southwestern Medical Center, Dallas, TX, USA
Therapeutic development based on targeting cell surface receptors or the catalytic activity of enzymatic proteins has provided many effective drugs for disease treatments. More recently, protein–protein interactions have provided additional targeting strategies. However, lack of specificity, unwanted side effects, and pharmacokinetic limitations have hindered advances. Post-translational regulatory mechanisms present a vast and largely undeveloped pool of targets for which more precise or patient-specific strategies hold promise. In studying protein phosphorylation/dephosphorylation in brain function, we have found that the targeting of phosphorylation sites of the protein kinase Cdk5 can have wide ranging effects including neuroprotection from ischemic and traumatic brain injury, enhancement of cognition, and antidepression. New data have also revealed Cdk5-dependent pathways as targets for the development of anticancer therapies. Relevant to this meeting, Cdk5 dysregulation has been suggested to contribute to pain sensation and craniofacial pain. This presentation will provide an overview of the roles of Cdk5 in these diverse processes and show how these mechanisms may be specifically targeted as a possible way to treat disease. New data on novel systemic Cdk5 inhibitors now being tested will also be presented. From these studies, we hope to show novel ways that intracellular signaling mechanisms may be targeted for more precise and effective treatment strategies.
Targeting chemokine and protease signaling for the control of neuroinflammation and chronic pain
Ru-Rong Ji
Departments of Anesthesiology and Neurobiology, Duke University Medical Center, Durham, NC, USA
Current analgesics predominately modulate pain transduction and transmission in neurons and have limited success in controlling disease progression. Accumulating evidence suggests that neuroinflammation, which is characterized by infiltration of immune cells, activation of glial cells (e.g. microglia and astrocytes), and production of inflammatory mediators in the peripheral and central nervous systems, has an important role in the induction and maintenance of chronic pain including temporomandibular joint (TMJ)-associated pain. 1 My talk focuses on emerging therapeutic targets such as chemokines and proteases that promote spinal cord neuroinflammation and chronic pain via neuron–glia interactions. In particular, I will discuss how proteases (e.g. caspase-6) and chemokines (e.g. CXCL1) regulate microglial and astroglial signaling and synaptic plasticity in inflammatory and neuropathic pain.2,3 Targeting excessive neuroinflammation could offer new therapeutic opportunities for chronic pain including TMJ-associated pain and neuroinflammation-related neurological and psychiatric disorders. I will also show the data that bone marrow stem cells can effectively control neuroinflammation and chronic pain. 4
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study is partially supported NIH R01 grants DE17794 and DE22743.
References
- 1.Ji RR, Xu ZZ, Gao YJ. Emerging targets in neuroinflammation-driven chronic pain. Nat Rev Drug Discov 2014; 13: 533–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berta T, Park CK, Xie RG, et al. Extracellular caspase-6 drives murine inflammatory pain via microglial TNF-alpha secretion. J Clin Invest 2014; 124: 1173–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen G, Park CK, Xie RG, et al. Connexin-43 induces chemokine release from spinal cord astrocytes to maintain late-phase neuropathic pain in mice. Brain 2014; 137: 2193–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen G, Park CK, Xie RG, et al. Intrathecal bone marrow stromal cells inhibit neuropathic pain via TGF-beta secretion. J Clin Invest 2015; 125: 3226–3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
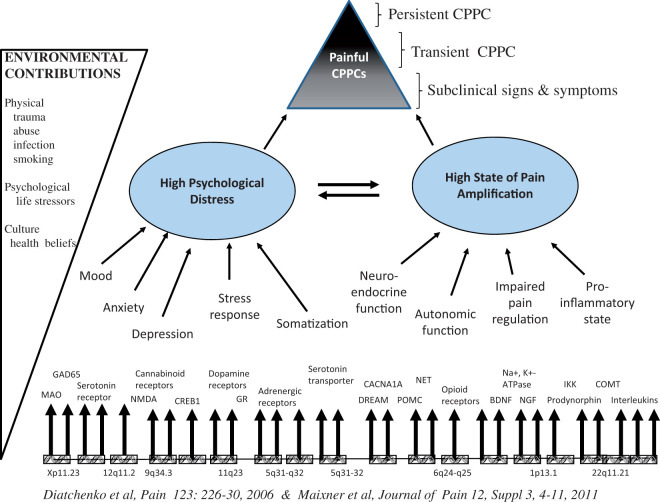
Human chronic pain conditions: Genome-wide analysis and pathways of vulnerabilities
Luda Diatchenko1, Marc Parisien1, Samar Khoury1, Anne-Julie Chabot-Dore1, Gary Slade2,3, Eric Bair2,4, Joel Greenspan5, William Maixner6, Shad Smith6, Roger Fillingim7, Richard Ohrbach8 and Inna Belfer1
1The Alan Edwards Centre for Research on Pain, Faculty of Dentistry, McGill University, Montreal, QC, Canada
2Center for Pain Research and Innovation, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA
3Department of Dental Ecology, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA
4Department of Biostatistics, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA
5Department of Neural and Pain Sciences, University of Maryland School of Dentistry, Baltimore, MD, USA; Brotman Facial Pain Center, University of Maryland School of Dentistry, Baltimore, MD, USA
6Center for Translational Pain Medicine, Duke University, Durham, NC, USA
7Department of Community Dentistry and Behavioral Science, Pain Research and Intervention Center of Excellence, University of Florida College of Dentistry, Gainesville, FL, USA
8Department of Oral Diagnostic Sciences, University at Buffalo, Buffalo, NY, USA
Background
Genome-wide association studies (GWAS) successfully identified genetic variants that affect risk of a wide range of human psychiatric and neurological conditions, but human chronic pain conditions have only started to be evaluated on the GWAS basis. Functionality of many identified single nucleotide polymorphism (SNP) variants can be assessed and confirmed through their regulation of RNA transcript expression levels, so called expression quantitative trait loci (eQTL). Manifestation of chronic pain conditions largely depends on the functioning of the nervous system; thus, the identification of eQTLs in the relevant transcription system is crucial. One of the critical organs in gating pain stimuli is dorsal root ganglions (DRG).
Materials and methods
We performed a GWAS of temporomandibular disorder (TMD) tested on 1082 chronic TMD cases and 2144 TMD-free controls enrolled in the Orofacial Pain: Prospective Evaluation and Risk Assessment (OPPERA) study. The Omni2.5 M platform was used with 1KG phase 3 imputation creating over 30 million genetic markers. The replication of the results has been done in four independent TMD cohorts. We then used genome-wide data from human DRG eQTL dataset obtained from 300 subjects to assess functionality of identified hits.
Results
Our analysis identified three new genome-wide loci that contribute to the risk of TMD. One locus has been replicated and showed association with multiple tras DRG eQTLs. Pathway analysis on eQTL hits identified new potentially causative biological pathways for TMD that involves the activation of T and B cells.
Conclusions
Our results suggest that at least a portion of chronic pain patients develop their conditions through immune, rather than neurological, processes. Elucidation of the biological mechanisms by which genetic markers contribute to the perception of pain in chronic pain patients will enlarge our understanding of pathophysiology of chronic pain conditions and enable the development of novel effective drugs and methodologies that permit better diagnoses and approaches to personalized medicine.
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr. Diatchenko is a Medical Advisory Board member and shareholder at Proove Biosciences, Inc.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institutes of Health and the National Institute of Dental and Craniofacial Research (grants U01-DE017018 and R0-1DE016155), Center For Inherited Disease Research (CIDR) (High Throughput Genotyping and Sequencing Resource Access 1X01-HG007586), and by the Canadian Excellence Research Chairs (CERC) Program (http://www.cerc.gc.ca/home-accueil-eng.aspx, grant CERC09) to Dr. Diatchenko.
Intranasal mesenchymal stem cell transplantation for the repair of neuronal damage in chemobrain
Cobi J Heijnen, Gabriel Chiu, Nabila Boukelmoune and Annemieke Kavelaars
Laboratory of Neuroimmunology, Division of Internal Medicine, Department of Symptom Research, The University of Texas MD Anderson Cancer Center, Houston, TX, USA
Background
Progress has been made in the treatment of cancer leading to a sharp increase in the number of survivors. However, cancer treatment poses severe side effects including the consequences of neurotoxicities like pain, fatigue, and cognitive deficits which can persist long into survivorship. Currently, there are no pharmacologic treatments that have proven value in the management of cancer treatment-induced neurotoxicities. Cisplatin is a platinum-based drug and is widely used for many cancer types. Over the past years, mesenchymal stem cells (MSCs) have become a potentially attractive therapeutic option for peripheral and cerebral neuronal damage.
Methods
C57/Bl6 mice were treated with two cycles of 2.3 mg/kg of cisplatin (five daily doses followed by five days rest per cycle). Cognition was determined by the novel object and place recognition task and the Puzzle box test. Neuronal arborization was measured in Golgi-stained brains, and MBP staining was used as a measure of white matter damage. Mitochondrial function was analyzed by Seahorse technology. Chemotherapy-induced peripheral neuropathy was measured as mechanical hyperalgesia using von Frey hairs.
Results
Cisplatin treatment induced persistent mechanical hyperalgesia and a decrease in cognitive function long after cessation of treatment. Cisplatin also induced a neuronal mitochondrial dysfunction such as a decrease in mitochondrial oxygen consumption rate in the peripheral as well as central nervous system. In the brain, cisplatin treatment was associated with a decrease in complexity of white matter and a decrease in neurogenesis as shown by the number of doublecortin-positive precursors aligning the subventricular zone. Two intranasal administrations of two million MSCs after completion of cisplatin treatment reversed the cognitive impairment and the associated structural and functional defects.
In search of a mechanism, we observed that intranasally administered MSCs can be traced in the brain 12–24 h after nasal administration. MSCs did not survive long-term in the brain and were not incorporated into the network. We propose that MSCs act by transferring intracellular information to damaged neurons leading to repair of adult neurons and neuronal stem cells.
Conclusion
Intranasal administration of MSC is an attractive noninvasive option for treatment of neurotoxic side effects of chemotherapy.
Probing the complexities of biology and medicine: Closing the hermeneutic circle with in vitro models to study nerve pain and neural responses to pain medication
John P Wikswo1,2,3,4, Jacquelyn A Brown1,3, M Diana Neely5,6,7, Aaron B Bowman5,6,7,8, Ethan S Lippmann1,9, Dmitry A Markov1,2, Lisa J McCawley1,2,10, Philip C Samson1,3, Ronald S Reiserer1,3, Clayton M Britt1,3, Orlando S Hoilett1, Mingjian Shi11, Donna J Webb1,11, Simona G Codreanu12,13, Stacy D Sherrod12,13,14 and John A McLean12,13,14
1Vanderbilt Institute for Integrative Biosystems Research and Education, Vanderbilt University, Nashville, TN, USA
2Department of Biomedical Engineering, Vanderbilt University, Nashville, TN, USA
3Department of Physics and Astronomy, Vanderbilt University, Nashville, TN, USA
4Department of Molecular Physiology and Biophysics, Vanderbilt University, Nashville, TN, USA
5Departments of Pediatrics and Neurology, Vanderbilt University Medical Center, Nashville, TN, USA
6Vanderbilt Kennedy Center, Vanderbilt University, Nashville, TN, USA
7Vanderbilt Brain Institute, Vanderbilt University Medical Center, Nashville, TN, USA
8Department of Biochemistry, Vanderbilt University, Nashville, TN, USA
9Department of Chemical and Biomolecular Engineering, Vanderbilt University, Nashville, TN, USA
10Department of Cancer Biology, Vanderbilt University Medical Center, Nashville, TN, USA
11Department of Biological Sciences, Vanderbilt University, Nashville, TN, USA
12Department of Chemistry, Vanderbilt University, Nashville, TN, USA
13Center for Innovative Technology, Vanderbilt University, Nashville, TN, USA
14Vanderbilt Institute of Chemical Biology, Vanderbilt University, Nashville, TN, USA
In vitro organ-on-chip (OoC) models can recapitulate human physiology more realistically than planar monocultures on plastic. Multiple groups are working to couple together two or more OoCs, populated with human cells, to create a homunculus—a miniature, in vitro representation of key organs in a human.1,2 Homunculi are of particular interest for studying how one organ metabolizes drugs and environmental toxins and creates metabolites that are toxic to other organs. Constructing OoCs with cells derived from human-induced pluripotent stem cells (hiPSCs) that in turn are derived from a specific person, including patients with genetic or acquired diseases, will lead to personalized homunculi that serve as patient-specific in vitro disease models. OoCs could thereby help realize several of the potential long-term benefits of National Institutes of Health’s Precision Medicine Initiative,3,4 including matching a treatment to the disease of a specific patient, elucidating the underlying mechanisms of a disease, and designing better treatments.
OoCs and other three-dimensional (3D) cultures and their associated technologies and hardware present opportunities to advance our understanding and treatment of nerve pain and chronic overlapping pain conditions (COPC). While it is unlikely that OoCs will recreate a temporomandibular joint or recapitulate its dysfunction, these tools are immediately applicable for studying the response of human neurons, the blood–brain barrier, and the neurovascular unit to drugs 5 and to study genetic differences and environmental factors that influence drug influx and efflux for pain treatment. Mass spectrometry for untargeted neuroimmune metabolomics 6 will enable exploration of the metabolic aspects of COPC. The ability to grow human central or peripheral nerves in perfused 3D microenvironments will be critical for understanding neuroimmune interactions. OoCs could recapitulate other operational components of nociception, including the neuromuscular junction, the dorsal root ganglion (DRG), and spinal sensory neurons. Critical to understanding nerve pain and COPC is the ready availability of human neurons. The differentiation of DRG neurons from human embryonic pluripotent stem cells (hePSCs) is an important first step.7,8 The shift from hePSCs to hiPSCs is critical for personalized nociception models, and a MicroFormulator being developed to formulate aliquots of customized cocktails of small molecules and growth factors that are delivered to and removed from each well of a 96-well plate should improve the yield and phenotypic purity of hiPSC-derived cells. Neuroelectric recording arrays will allow measurement of neural activity in a DRG-on-a-chip. Mass spectrometric metabolomics will provide information about the COPC metabolic and signaling pathways. These tools should help remediate COPC.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded in part by Assistance Agreement No. 83573601 awarded by the U.S. Environmental Protection Agency (EPA) to Vanderbilt University, by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UH3TR000491 and contract HHSN271201600009C (to CFDRC), AstraZeneca, NIH grants R01HL118392, R01 HL095813, and 5R01-AR056138, Department of Veterans Affairs, and Defense Threat Reduction Agency (DTRA) grants HDTRA1-09-0013 and CBMXCEL-XL1-2-001. Prior support was provided by DARPA and DTRA. The views expressed in this document are solely those of the authors and do not necessarily reflect those of any of the funding agencies. EPA does not endorse any products or commercial services mentioned in this publication.
References
- 1.Wikswo JP. The relevance and potential roles of microphysiological systems in biology and medicine. Exp Biol Med 2014; 239: 1061–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wikswo JP, Porter AP. Biology coming full circle: joining the whole and the parts. Exp Biol Med 2015; 240: 3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.U.S. National Library of Medicine. What are some potential benefits of precision medicine and the Precision Medicine Initiative?, https://ghr.nlm.nih.gov/primer/precisionmedicine/potentialbenefits (2016, accessed 28 July 2016).
- 4.Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med 2015; 372: 793–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown JA, Pensabene V, Markov DA, et al. Recreating blood-brain barrier physiology and structure on chip: a novel neurovascular microfluidic bioreactor. Biomicrofluidics 2015; 9: Article 054124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown JA, Sherrod SD, Goodwin CR, et al. Metabolic consequences of interleukin-6 challenge in developing neurons and astroglia. J Neuroinflamm 2014; 11: Article 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Young GT, Gutteridge A, De Fox H, et al. Characterizing human stem cell-derived sensory neurons at the single-cell level reveals their ion channel expression and utility in pain research. Mol Ther 2014; 22: 1530–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meyer K, Kaspar BK. Making sense of pain: are pluripotent stem cell-derived sensory neurons a new tool for studying pain mechanisms? Mol Ther 2014; 22: 1403–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Induced pluripotent stem cells for disease modeling
Ulrich Broeckel
Department of Pediatrics, Medicine and Physiology, Medical College of Wisconsin, Milwaukee, WI, USA
The development of novel therapeutics is often hampered by the availability of relevant disease models. In particular, the identification of novel drugs through high throughput screening relies on the availability of cells or tissues resembling a disease phenotype. Recent technological advances in stem cell technologies, the availability of induced pluripotent stem cells (iPSCs), and the development of protocols to differentiate these cells into a broad spectrum of tissue types provide now an unprecedented resource for disease modeling. Human-induced pluripotent stem cells and derived cell types have been shown to not only resemble various disease phenotypes but also reflect the genetic risk and disease susceptibility.
In this presentation, we will describe the conceptual framework, biological mechanisms, and potential applications for using iPSC-derived cells in precision medicine. Incorporating genetic and phenotypic diversity will require the development of a large number of iPSCs. We will present results from our recent study, which generated 250 iPSC lines from participants in a large biracial epidemiological cohort. Building on extensive phenotypic and genetic data available, we discuss approaches for disease modeling, disease gene identification, and methodology for complex tissue interaction analysis between different cell types or materials. Finally, we will demonstrate how this approach can be used to identify underlying genetic factors in vivo in order to guide drug and biomarker development as well as toxicology testing.
Role of the immune system in resolution of pain
Annemieke Kavelaars1, Geoffroy Laumet1, Karen Krukowski1, Niels Eijkelkamp2, Robert Dantzer1, Cobi J Heijnen1
1Laboratory of Neuroimmunology, Department of Symptom Research, University of Texas MD Anderson Cancer Center, Houston, TX, USA
2Laboratory of Neuroimmunology and Developmental Origins of Disease, University Medical Center, Utrecht, The Netherlands
Chronic pain and its comorbidities, including depression and anxiety, are among the most disabling and costly disorders. Transient pain commonly develops in response to chemotherapeutic treatment, tissue damage, and inflammation. The associated behavioral responses such as reduced activity, guarding of damaged tissue, and social withdrawal serve an adaptive purpose, and pain should resolve after tissues heal and inflammation resolves. We hypothesize that the resolution of pain depends on an active regulatory process involving endogenous resolution pathways. Dysregulation of these resolution processes results in transition into maladaptive chronic pain.
We investigated the contribution of T cells and endogenous interleukin (IL)-10 signaling to chemotherapy-induced peripheral neuropathy and inflammatory pain. The results show that chemotherapy-induced mechanical allodynia was prolonged in T cell-deficient (Rag1−/− or Rag2−/−) male and female mice compared with wild-type (WT) mice. Similarly, inflammatory pain was prolonged in T cell-deficient mice. There were no differences between WT and T cell-deficient mice in onset or severity of mechanical allodynia. Adoptive transfer of either CD3+ or CD8+, but not CD4+, T cells to Rag1−/− mice normalized resolution of pain. In the model of paclitaxel-induced neuropathic pain, the number of T cells in lumbar dorsal root ganglia (DRG) increased, and CD8+ T cells were the major subset. Inhibition of endogenous IL-10 signaling by intrathecal injection of anti-IL-10 to WT mice or Rag1−/− mice reconstituted with CD8+ T cells delayed recovery of paclitaxel-induced mechanical allodynia. Intrathecal anti-IL-10 treatment also prolonged inflammatory pain. In addition, recovery was also delayed in IL-10 knock-out mice. Conversely, administration of exogenous IL-10 attenuated paclitaxel-induced allodynia and promoted resolution of inflammatory pain. In vitro, IL-10 suppressed abnormal paclitaxel-induced spontaneous discharges in DRG neurons. Paclitaxel increased IL-10 receptor expression in the DRG but only in the presence of CD8+ T cells. In conclusion, we identified a novel mechanism for resolution of pain that requires CD8+ T cells and endogenous IL-10. We propose that CD8+ T cells increase DRG IL-10 receptor expression and that IL-10 suppresses the abnormal paclitaxel-induced spontaneous discharges by DRG neurons to promote resolution. Clinically, peripheral blood CD8+ T cell function and/or the capacity of individuals to produce IL-10 may represent biomarkers of risk for developing persistent peripheral neuropathy after completion of cancer treatment.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants NS073939 and NS074999 from the National Institutes of Health.
Bridge between neuroimmunity and traumatic brain injury; immunopharmacology approaches for diagnosis/treatment of neurodegenerative diseases
Howard E Gendelman
Department of Pharmacology and Experimental Neuroscience, University of Nebraska Medical Center, Omaha, NE, USA
Aberrant innate and adaptive immune responses are effectors of neurodegenerative, neuroinfectious, and temporomandibular joint (TMJ) disorders. Disease is heralded by a generalized, but subtle immune activation orchestrated by the release of extracellular prion-like aggregated and oxidized or otherwise modified proteins. These are responsible for an inflammatory neurotoxic cascade. The perpetrators of such events include effector T cells and activated microglia. What ensues are, for example, Alzheimer’s and Parkinson’s disease, amyotrophic lateral sclerosis, and stroke with changed frequencies of effector T cell and reduced numbers or function of regulatory lymphocytes. The control of such immune responses could lead to new therapeutic strategies and the means to effectively combat a composite of diseases that currently have quite limited therapeutic options.
Specification and maturation of nociceptive neurons from human pluripotent stem cells
Erin Boisvert1,2, Sandra J Engle3, Shawn E Hallowell3, Ping Liu2, Zhao-Wen Wang2 and Xue-Jun Li4,5
1Department of Genetics and Developmental Biology, University of Connecticut Health Center, Farmington, CT, USA
2Department of Neuroscience, University of Connecticut Health Center, Farmington, CT, USA
3Pharmacokinetics, Dynamics, Metabolism-New Chemical Entities, Pfizer Worldwide Research and Development, Pfizer Inc., Groton, CT, USA
4Department of Biomedical Sciences, University of Illinois College of Medicine at Rockford, Rockford, IL, USA
5Department of Bioengineering, University of Illinois at Chicago, Chicago, IL, USA
Background
Chronic pain is a debilitating condition, which directly affects about one-fifth of the global population. Nociceptive neurons play an essential role in pain sensation by transmitting painful stimuli to the central nervous system. However, investigations of nociceptive neuron biology have been hampered by the lack of accessibility of human nociceptive neurons.
Materials and methods
Based on our successful generation of neural lineage cells and spinal progenitors from human pluripotent stem cells (hPSCs), we investigated the specification and maturation of nociceptive neurons from hPSCs by establishing a chemically defined system. The mRNA and protein expressions of various neural markers at different time points after differentiation were examined by reverse transcription polymerase chain reaction and immunostaining, respectively. The function of sensory neurons was evaluated using calcium imaging.
Results
We established a system for efficiently guiding hPSCs into nociceptive neurons by first inducing these cells to the neural lineage. Subsequent addition of retinoic acid and BMP4 at specific time points and concentrations yielded a high population of neural crest progenitor cells (AP2α+, P75+), which further differentiated into nociceptive neurons (TRKA+, Nav1.7+, P2X3+). The overexpression of Neurogenin 1 (Neurog1) promoted the neurons to express genes related to sensory neurons (Peripherin, TrkA) and to further mature into TRPV1+ nociceptive neurons. Importantly, the overexpression of Neurog1 increased the response of these neurons to capsaicin stimulation, a hallmark of mature functional nociceptive neurons.
Conclusions
Taken together, this study reveals the important role that Neurog1 plays in generating functional human nociceptive neurons and provides a potential tool for high throughput screening of therapeutic agents for pain.
Pain begets pain: Toward a mechanistic understanding of chronic overlapping pain
Richard J Traub1,2, Yaping Ji1,2, David Seminowicz1,2, Susan Dorsey2,3, Feng Wei1,2 and Dean Dessem1,2
1Department of Neural and Pain Sciences, University of Maryland School of Dentistry, Baltimore, MD, USA
2University of Maryland Center to Advance Chronic Pain Research, Baltimore, MD, USA
3Department of Pain and Translational Symptom Science, University of Maryland School of Nursing, Baltimore, MD, USA
Background
Pain and stress have a reciprocal interaction and are potentially modifiable risk factors for poor health outcomes including chronic pain. However, the interaction between pain and stress to generate de novo pain is not fully understood. Many chronic pain conditions have minimal identifiable origins in organic disease, tend to overlap in presentation, and are referred to as Chronic Overlapping Pain Conditions (COPCs). Generally these conditions are more prevalent or exclusive to women, and symptoms can be exacerbated or triggered by stress. Current thinking focuses on modifications to shared neural, immune, and endocrine mechanisms underlying altered central nervous system function contributing to pain hypersensitivity. Our studies look at the effects of orofacial pain and stress to induce de novo visceral hypersensitivity.
Materials and methods
Intact male and female rats and ovariectomized rats with E2 replacement were stressed using a three-day forced swim paradigm. Direct (visceromotor response to colorectal distention (VMR)) and indirect (referred pain) visceral sensitivity and orofacial mechanosensitivity were measured. Orofacial pain was produced by either intramuscular CFA or infraorbital nerve injury.
Results
Stress induced visceral hypersensitivity with longer duration in female rats. The hypersensitivity was estrogen dependent, independent of sex and tempered by testosterone. The presence of orofacial pain followed by stress further increased the duration of visceral hypersensitivity (comorbid pain). Multiple mechanisms contribute to the stress- and comorbid-induced pain. Peripherally restricted corticotropin-releasing factor receptor antagonists and mast cell stabilizers prevented, but did not reverse, the stress- and comorbid-induced visceral hypersensitivity. Colonic lidocaine attenuated the VMR but referred pain persisted, indicating that central sensitization contributes to the visceral hypersensitivity. Functional magnetic resonance imaging revealed changes in supraspinal processing of noxious visceral stimuli during stress-induced visceral hypersensitivity. This likely reflects changes in descending 5-HT function following orofacial pain and stress. Finally, RNA deep sequencing revealed significant changes in gene expression in the spinal cord during stress-induced and comorbid pain-induced visceral hypersensitivity.
Conclusions
Stress induces visceral hypersensitivity that is more robust in females, likely due to action by estradiol. However, the duration of stress-induced visceral hypersensitivity is relatively short—days to weeks. When the nervous system is primed by a prior painful event, the same stressor induces visceral hypersensitivity that persists for months, a transition from acute to chronic pain. This animal model of overlapping pain (mimicking TMD and IBS) can be useful to investigate neural, immune, and hormonal mechanisms that contribute to the generation and maintenance of chronic overlapping pain conditions in afflicted patients.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by NIH grants R01 NR015472, R01 NS37424, and R21 DE022235.
Comparing experimental pain sensitivity and endogenous pain modulatory processes in men and women
Hailey W Bulls1, Burel R Goodin1,2, Myriah McNew3, Ethan W Gossett1,4, Laurence A Bradley1,4 and Roger B Fillingim5
1Department of Psychology, University of Alabama at Birmingham, Birmingham, AL, USA
2Department of Anesthesiology, University of Alabama at Birmingham, Birmingham, AL, USA
3Department of Psychology, Florida International University, Miami, FL, USA
4Division of Clinical Immunology and Rheumatology, Department of Medicine, University of Alabama at Birmingham, Birmingham, AL, USA
5College of Dentistry, Department of Community Dentistry and Behavioral Science, University of Florida, Gainesville, FL, USA
Background
Previous research indicates that women experience a variety of clinical pain syndromes at a rate and severity higher than their male counterparts, including orofacial pain (among others). One explanation may be that increased pain sensitivity and disruption of endogenous pain modulatory processes contribute to these sex differences. However, previous studies addressing this hypothesis have resulted in mixed findings. Additionally, possible sex differences in pain sensitivity across the adult lifespan have not been fully assessed. Thus, two studies are presented in an effort to evaluate sex differences in pain sensitivity and endogenous pain modulation using quantitative sensory testing (QST).
Materials and methods
In both studies, healthy men and women each completed a QST battery. In Study 1, 48 young, healthy participants (ages 19–45) completed an ischemic pain task that used a submaximal effort tourniquet procedure as well as a conditioned pain modulation (CPM) procedure for the assessment of endogenous pain inhibition. 1 Study 2 involved a separate sample of 241 healthy participants from across the adult lifespan (ages 19–76). Participants in the second study underwent QST procedures that assessed pain sensitivity including endogenous pain facilitation via temporal summation (TS) of heat and mechanical pain stimuli.
Results
In both studies, analyses revealed significant sex differences such that women demonstrated lower thresholds and tolerances to multiple modalities of pain stimuli, including ischemia (Study 1), heat (Study 2), and pressure (Study 2) when compared to men. Additionally, women demonstrated significantly decreased CPM (Study 1) and increased TS (Study 2) in comparison to their male counterparts (all p’s < 0.05).
Conclusions
This study provides evidence suggesting that women may be more pain sensitive and demonstrate less efficient pain inhibition as well as greater pain facilitation than their male counterparts. This is important, as women are often at increased risk for the development of chronic pain. It has been suggested that responses to QST may be better related to the clinical pain experiences of women compared with men. As such, it may be that less efficient endogenous pain modulation plays a contributory role to increased prevalence of clinical pain conditions—including orofacial pain—in women. Further research is necessary to confirm or refute such a hypothesis. Additionally, further investigations into interventions that may decrease pain sensitivity and enhance pain inhibition in women are warranted, particularly when considering possible impacts on clinical pain outcomes.
Reference
- 1.Bulls HW, Freeman EL, Anderson AJB, et al. Sex differences in experimental measures of pain sensitivity and endogenous pain inhibition. J Pain Res 2015; 8: 311–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
Epigenetic regulation of neuropathic pain
Lingli Liang1, Jianyuan Zhao2, Xiyao Gu1, Brianna M Lutz1, Shaogen Wu1, Xuerong Miao1, Alex Bekker1 and Yuan-Xiang Tao1,3
1Department of Anesthesiology, New Jersey Medical School, Rutgers, The State University of New Jersey, Newark, NJ, USA
2State Key Laboratory of Genetic Engineering, Collaborative Innovation Center for Genetics and Development, School of Life Sciences, Fudan University, Shanghai, China
3Departments of Cell Biology & Molecular Medicine and Physiology, Pharmacology & Neuroscience, New Jersey Medical School, Rutgers, The State University of New Jersey, Newark, NJ, USA
Neuropathic pain, which is related to peripheral or central nervous system injury, is difficult to treat since it does not respond well to typical analgesics. Previous studies have shown that peripheral nerve injury can reduce the expression of pain-related genes, such as potassium channels and opioid receptors, in primary sensory neurons.1,2 Epigenetic mechanisms, including DNA methylation and histone modification, have been linked to the alteration of gene expression.3,4 Here, we studied histone methylation in neuropathic pain genesis. Using quantitative real-time reverse transcription polymerase chain reaction analysis, Western blot analysis, or immunohistochemistry, we found increased mRNA and protein expression of G9a, a histone methyltransferase, in injured mouse dorsal root ganglion (DRG) after L4 spinal nerve ligation (SNL). A G9a inhibitor or genetic knockdown of G9a relieved SNL-induced mechanical allodynia, thermal hyperalgesia, and cold allodynia. We further found that G9a is required for nerve injury-induced epigenetic silencing of potassium channels (Kv1.2 and Kv1.4) and opioid receptors (mu, kappa, and delta), which are key players in pain transmission. Functionally, DRG G9a overexpression increased mu opioid receptor-gated primary afferent neurotransmitter release, reduced voltage-gated potassium channel current, increased excitability in the DRG, and led to pain hypersensitivities. Conversely, DRG G9a inhibition/knockdown restored the decrease of morphine analgesia, prevented the development of morphine-induced tolerance, and mitigated neuropathic pain. Finally, we concluded that G9a may be a potential target for future neuropathic pain treatment.
Acknowledgments
We thank Eric J Neslter (Icahn School of Medicine at Mount Sinai) for G9a flox mice, HSV-G9a, and HSV-GFP virus; Fan Wang (Duke University Medical Center) for Advillin Cre/+ mice; and Han-Rong Weng (University of Georgia College of Pharmacy) for electrophysiological data analysis.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by NIH grants (NS072206, DA033390, and HL117684).
References
- 1.Zhao X, Tang Z, Zhang H, et al. A long noncoding RNA contributes to neuropathic pain by silencing Kcna2 in primary afferent neurons. Nat Neurosci 2013; 16: 1024–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee CY, Perez FM, Wang W, et al. Dynamic temporal and spatial regulation of mu opioid receptor expression in primary afferent neurons following spinal nerve injury. Eur J Pain 2011; 15: 669–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liang L, Lutz BM, Bekker A, et al. Epigenetic regulation of chronic pain. Epigenomics 2015; 7: 235–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kouzarides T. Chromatin modifications and their function. Cell 2007; 128: 693–705. [DOI] [PubMed] [Google Scholar]
Exploring the epigenetic mechanisms for individual pain vulnerability
You-Qing Cai, Wei Wang and Zhizhong Z Pan
Department of Anesthesiology and Pain Medicine, The University of Texas MD Anderson Cancer Center, Houston, TX, USA
Background
Clinical data clearly show that there is considerable individual variance in response to pain stimuli and to pain drugs, and consequently in vulnerability to developing chronic pain. Many factors, including genetic background, prior pain experience, and previous stressful life events, may contribute to the pain vulnerability. These psychological and environmental events could influence pain vulnerability by markedly changing an individual’s epigenetic landscape that regulates gene expression in adaptive responses to those stressful events. However, individual variance in pain responses and pain vulnerability has been rarely addressed in current preclinical studies on animals. In this study, we examined individual variations in behavioral responses of both sensory and affective pain in rats and explored a pain-induced epigenetic modification that may contribute to the pain variation and vulnerability for chronic pain development.
Materials and methods
Chronic condition of neuropathic pain was induced in rats by partial nerve ligation. Sensory pain responses were measured by the thermal paw withdrawal test and mechanical von Frey test. Affective emotion responses to pain were measured by the open filed test and elevated plus maze test. An AAV-CaMKII-ChR2-EGFP viral vector was injected into the parabrachial (PB) nucleus for optogenetic stimulation of the glutamatergic projections from PB neurons to central nucleus of the amygdala (CeA).
Results
We found that, while individual rats with chronic pain were not much variable in their behavioral response to sensory pain, the animals displayed considerable variance in their affective emotion behaviors, ranging from significant anxiety behavior to absence of the behavior at 30 days after the pain induction. In CeA, the protein level of DNA methyltransferases that catalyze de novo DNA methylation for gene repression was significantly altered by the pain condition. Photostimulation of the excitatory PB-CeA glutamatergic projections in CeA induced significant anxiety-like behavior in naïve rats.
Conclusions
The results indicate that individual variance in pain responses is mostly manifested in the affective component of pain in rats; and maladaptation in DNA methylation and its potential target of glutamate receptors in CeA may play an important role in the individual variance of affective pain.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by NIH grant R01DE025943.
Mechanisms underlying the sense of touch in orofacial regions of rodents—Implications for treating and preventing disease states that affect touch sensation such as TMD
Jianguo G Gu
Department of Anesthesiology and Perioperative Medicine, School of Medicine, University of Alabama at Birmingham, Birmingham, AL, USA
Background
Pathological pain conditions in orofacial regions such as temporomandibular disorder (TMD) are often manifested by mechanical allodynia, a severe pain state that can be triggered by a gentle touch. Orofacial mechanical allodynia is a main clinical pain problem and is poorly managed by the currently available pain medicines. One main reason for the poor management of orofacial mechanical allodynia is the lack of scientific knowledge about how a gentle touch is sensed by our sensory system in orofacial regions.
Materials and methods
Rodent whisker hair follicles, important touch-sensing structures in orofacial regions, were used in our studies to explore cellular and molecular mechanisms underlying the sense of gentle touch in orofacial regions. Multiple approaches including electrophysiology, molecular biology, immunohistochemistry, pharmacology, and mouse genetics were used in the studies.
Results
Our studies show that epidermal Merkel cells in whisker hair follicles are equipped with the recently cloned mammalian mechanical transducers Piezo2 ion channels and that Merkel cells are primary sites of sensory impulse initiation in response to a gentle touch to a whisker hair. We also demonstrate that tactile signals are synaptically transmitted from Merkel cells to their associated Aβ-afferent fibers to lead to the sense of touch. 1
Conclusions
Our studies provide novel insights into the transduction and transmission of gentle touch within whisker hair follicles and shall have implications in orofacial mechanical allodynia observed in TMD and other pathological pain conditions in orofacial regions.
Acknowledgments
Many researchers in Dr. Jianguo G Gu’s laboratory are involved in the studies described above, but Drs. Ryo Ikeda and Weipang Chang made major contributions to the experimental work.
Reference
- 1.Ikeda R, Cha M, Ling J, et al. Merkel cells transduce and encode tactile stimuli to drive Aβ-afferent impulses. Cell 2014; 157: 664–675. [DOI] [PMC free article] [PubMed]
Exploiting the immune response to illuminate host-microbiota interactions
Noah W Palm
Human and Translational Immunology Program, Yale University School of Medicine, New Haven, CT, USA
The composition of the gut microbiota is thought to have dramatic effects on the development and progression of a variety of diseases, including inflammatory bowel disease (IBD), autoimmunity, and metabolic syndrome. However, identifying the specific bacteria that preferentially affect disease susceptibility and severity in humans remains a major challenge. In response to this problem, we developed a novel technology that uses the host’s own immune response to the microbiota as a guide to identify specific members of the gut microbiota that preferentially modulate disease development. This approach specifically identified known disease-causing intestinal bacteria in a mouse model of microbiota-driven colitis. Furthermore, we were able to use this approach to identify specific bacterial strains from IBD patients that selectively conferred susceptibility to severe colitis when transplanted into germ-free mice. These studies thus: (i) establish a new strategy for the identification of disease- and immune-modulating members of the microbiota in humans; (ii) identify potentially disease-driving members of the intestinal microbiota in humans with IBD; and (iii) begin to establish causal, rather than correlative, connections between specific changes in the microbiota and human disease. Future studies using similar approaches will allow us to elucidate the full spectrum of reciprocal interactions between the microbiota and the host immune system. These studies will lead to a more complete understanding of the role of microbiota composition in human health and disease and will eventually enable the development of novel and specific microbiota-targeted therapeutics.
The impact of genetic testing for pain perception in the clinical management of chronic noncancer pain
Ashley Brenton, Svetlana Kantorovich and Brian Meshkin
Proove Biosciences, Irvine, CA, USA
Genetic markers of pain sensitivity, such as single nucleotide polymorphisms in the catechol-O-methyltransferase gene, have been shown to be associated with pain perception and have been used to provide objective information about a patient’s pain.
A prospective, longitudinal study was conducted with 134 chronic noncancer pain patients genotyped for pain perception-related catechol-O-methyltransferase haplotypes. Physicians were provided with patients’ results and asked to document (1) their assessment of benefit of the genetic test; (2) treatment changes made based on the genetic test; and (3) patient clinical responses to changes implemented.
Based on genetic testing results, physicians adjusted treatment plans for 40% of patients. When medication changes were made based on genetic testing results, 72% of patients showed improvement in clinical status. When nonpharmacological actions were performed, 69% of physicians felt their patients’ clinical status improved. Moreover, physicians believed the genetic test results were consistent with patient pain levels in 85% of cases.
These results demonstrate that providing personalized medicine with genetic information related to pain perception affected physician clinical decision-making for a substantial proportion of patients in this study and that the availability and utilization of this information was a contributing factor in clinical improvement.
Our aim is to combine phenotypic and proven genetic factors that influence pain to produce a test that will: (1) identify individuals at risk of TMJ/TMD and (2) for those individuals at risk or suffering from TMJ/TMD, determine specific, actionable treatment recommendations based on their individual genetics.
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: AB and SK are employees of Proove Biosciences; BM is the CEO and founder of Proove Biosciences.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by Proove Biosciences.
A prediction error model of placebo analgesia and its extinction
Ben Colagiuri1, Evan Livesey1 and Luana Colloca2
1School of Psychology, University of Sydney, Sydney, Australia
2Department of Pain Translation Symptom Science, University of Maryland School of Nursing, Baltimore, MD, USA
Background
Placebo analgesia can ameliorate the experience of pain.1,2 There has yet to be a model created that can predict how an individual will respond to a placebo analgesic procedure and how long lasting these effects will be. 3 Therefore, the aims for this study were to use a prediction error model to: (1) predict placebo analgesia on an iterative trial-by-trial basis, (2) identify factors that lead to extinction or nonextinction of placebo analgesia, and (3) identify predictors of whether individuals experience placebo analgesia or not.
Materials and methods
A prediction error model of placebo analgesia was developed and applied to data from two separate placebo analgesia experiments in humans using painful-like electrical stimulations and a placebo conditioning procedure. Both of these experiments measured expectancy and pain rating before and directly after the introduction of a placebo manipulation. We developed a model to provide predictions of placebo analgesic responses based on the specific treatment administered on each trial (placebo vs. control) and level of pain stimulation received by individuals during the conditioning. The model computed a perceived pain index as a simple weighted average of expectancy and actual pain stimulation.
Results
The implemented prediction error model provided a strong quantitative fit to the placebo analgesia data obtained from the two experiments. When fit separately, the ideal parameters across the two experiments were generally highly concordant, indicating that the model is generalizable across experiments that varied in nonessential aspects of procedure, indicating good external validity. Initial expectancy was one of the most important parameters in terms of the likelihood of an individual exhibiting placebo analgesia, whereas parameters such as learning rate did not appear to predict whether an individual experienced placebo analgesia or not. Initial expectancy also predicted the extent to which any placebo analgesia extinguished, with higher initial expectancies leading to slower extinction. However, learning rate was a significant predictor of extinction, with individuals with a slower learning rate demonstrating increased resistance to extinction, i.e. longer lasting placebo analgesia during test.
Conclusions
In this project, we tested the extent to which a prediction error model can account for placebo analgesia and its rate of extinction in two distinct experiments. The developed model was effective in predicting placebo analgesia and its extinction. These results point to a potential advantage in understanding the mechanisms of placebo analgesia. Researchers and clinicians may be able to develop clinical interventions that capitalize on the pain modulation to improve patient pain outcomes. 4
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The research was supported by Australian Research Council grants DP150104026 (BC) and DE160100864 (BC) and University of Maryland Baltimore and NIDCR 1R01DE025946-01 (LC).
References
- 1.Colloca L. Placebo, nocebo, and learning mechanisms. Handb Exp Pharmacol 2014; 225: 17–35. [DOI] [PubMed] [Google Scholar]
- 2.Colloca L, Benedetti F. Placebos and painkillers: is mind as real as matter? Nat Rev Neurosci 2005; 6: 545–552. [DOI] [PubMed] [Google Scholar]
- 3.Buchel C, Geuter S, Sprenger C, et al. Placebo analgesia: a predictive coding perspective. Neuron 2014; 81: 1223–1239. [DOI] [PubMed] [Google Scholar]
- 4.Colloca L, Enck P, DeGrazia D. Relieving pain using dose-extending placebos: a scoping review. Pain 2016; 157: 1590–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
Clinical characteristics of TMD at onset and predictors of persistence: Preliminary results
Carolina Meloto1, Gary Slade2,3, Ryan Lichtenwalter1, Eric Bair2,4, Nuvan Rathnayaka4, Ronald Dubner5, Joel Greenspan5, William Maixner6, Roger Fillingim7, Luda Diatchenko1 and Richard Ohrbach8
1The Alan Edwards Centre for Research on Pain, Faculty of Dentistry, McGill University, Montreal, QC, Canada
2Center for Pain Research and Innovation, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA
3Department of Dental Ecology, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA
4Department of Biostatistics, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA
5Department of Neural and Pain Sciences, University of Maryland School of Dentistry, Baltimore, MD, USA; Brotman Facial Pain Center, University of Maryland School of Dentistry, Baltimore, MD, USA
6Center for Translational Pain Medicine, Duke University, Durham, NC, USA
7Department of Community Dentistry and Behavioral Science, Pain Research and Intervention Center of Excellence, University of Florida College of Dentistry, Gainesville, FL, USA
8Department of Oral Diagnostic Sciences, University at Buffalo, Buffalo, NY, USA
The prospective study of first-onset temporomandibular disorder (TMD) that is part of Orofacial Pain: Prospective Evaluation and Risk Assessment (OPPERA) has assessed initially TMD-free individuals and identified clinical characteristics that are associated with higher risk of developing TMD. 1 In the context of clinical care, such characteristics are more likely to be assessed when individuals are symptomatic and first present for care. However, it is not known whether any incidence risk factors assessed at initial consultation are useful for predicting TMD persistence. Thus, we evaluated clinical characteristics of the individual assessed at the time of TMD onset in order to develop a prediction model using variables that can be easily and reliably assessed by clinicians using the DC/TMD protocol 2 in order to identify first-onset TMD patients who are likely to have their symptoms persist versus those who are likely to recover within the subsequent six months. In addition, this study will also describe the clinical characteristics of TMD at onset and at the six-month follow-up visit. The data presented here are from a nested case-control study of first-onset TMD that was part of the OPPERA project, which enrolled 260 incident cases of first-onset TMD and followed them over an approximately six-month period, when TMD case classification was re-assessed. TMD case classification was determined by a calibrated examiner using the RDC/TMD protocol. Additional variables that fulfill the DC/TMD protocol were also collected. At follow-up, 147 (56.6%) incident cases were re-examined, of which 72 (49%) presented “persistent” TMD and 75 (51%) no longer had examiner-verified TMD and were labeled “transient” TMD cases. Preliminary results show that a multivariable regression model including the number of masticatory muscles with pain on maximum unassisted opening, number of masticatory muscles tender to palpation, and count of comorbid conditions, performs best at distinguishing persistent from transient TMD. Specifically, the model provides an area under the receiver operating characteristic (ROC) curve (AUC) of 0.77, as opposed to an AUC of 0.61 obtained with demographic variables only, suggesting that these clinical variables may be useful as predictive markers for the progression of the disorder. Clinically, this means that the time of onset represents a pragmatic point at which clinicians would be able to identify those at higher risk of persistence, allowing informed decision-making about who should receive treatment—and presumably which type of treatment—in order to avoid TMD persistence and eventual chronification of the disorder.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institutes of Health and the National Institute of Dental and Craniofacial Research (grants U01-DE017018, R0-1DE016155, R03-DE022595, R03DE023592), by the Canadian Excellence Research Chairs (CERC) Program (http://www.cerc.gc.ca/home-accueil-eng.aspx, grant CERC09) to Dr. Diatchenko, and by the Catherine Bushnell Fellowship in Pain Research to Dr. Meloto.
References
- 1.Ohrbach R, Bair E, Fillingim RB, et al. Clinical orofacial characteristics associated with risk of first-onset TMD: the OPPERA prospective cohort study. J Pain 2013; 14(12 Suppl): T33–T50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schiffman E, Ohrbach R, Truelove E, et al. Diagnostic criteria for temporomandibular disorders (DC/TMD) for clinical and research applications: recommendations of the International RDC/TMD Consortium Network and Orofacial Pain Special Interest Group. J Oral Fac Pain Headache 2014; 28: 6–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
The behavioral and neural effects of multipotent stromal cells in rodent models of persistent pain
Wei Guo, Yu-Xia Chu, Satoshi Imai, Jia-Le Yang, Shiping Zou, Zaid Mohammad, Feng Wei, Ronald Dubner and Ke Ren
Department of Neural and Pain Sciences, School of Dentistry & Program in Neuroscience, University of Maryland, Baltimore, MD, USA
Multipotent stromal cells (MSCs) have shown potential to treat chronic pain. However, their efficacy and mechanisms of action under different pain conditions are still elusive. To extend our previous observation by utilizing bone marrow stromal cells (BMSCs), a major type of MSCs, we provide further evidence on the pain-attenuating effect of BMSCs in three rodent pain models. Women exhibit higher prevalence of orofacial pain than men, but it is unclear whether BMSCs produce pain relief in females as that in males. In a model of orofacial pain involving injury of a tendon of the masseter muscle, rat BMSCs (1.5 M cells, i.v.) reduced mechanical hyperalgesia and conditioned place avoidance behavior in female rats. The pain-attenuating effect of BMSCs in females lasted for 28–56 days, apparently shorter than that seen in males. To coincide preclinical findings with clinical conditions, we used human BMSCs in rats after L5 spinal nerve ligation (SNL) injury. Human BMSC (1.5 M cells, i.v.) also attenuated mechanical and thermal pain hypersensitivity and suppressed SNL-induced aversive behavior, and the effect persisted through the eight-week observation period. In a trigeminal slice preparation derived from mice with chronic constriction injury of the infraorbital nerve, BMSC-treated animals showed reduced amplitude and frequency of spontaneous miniature excitatory postsynaptic currents, compared to naïve and culture medium-treated mice. Electrical stimulation-evoked and N-methyl-D-aspartate receptor (NMDA) receptor-dependent synaptic current was also reduced in BMSC-treated animals. These results suggest inhibition of trigeminal neuronal hyperexcitability and nociceptive primary afferent input. Further, GluN2A tyrosine phosphorylation and PKCΓ immunoreactivity in the rostral ventromedial medulla (RVM), a key site for descending pain modulation, was suppressed at eight-week after BMSC in tendon-injured rats. As PKCΓ activity related to NMDA receptor activation is critical in opioid tolerance, these results may explain long-term pain-relieving effect of BMSCs, which requires opioid receptors in RVM and apparently lacks the development of tolerance. These results provide convergent evidence that supports the use of BMSC in pain control.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Maryland Stem Cell Foundation grant 2014-MSCRFI-0584 (KR); National Institutes of Health grants: DE025137 (KR), NS019296 (FW), DE021804 (RD).
Structural and functional abnormalities in chronic orofacial pain disorders: A meta-analytic study
Shariq A Khan1, Lizbeth Ayoub2, David A Seminowicz1 and Massieh Moayedi2
1Department of Neural and Pain Sciences, University of Maryland School of Dentistry, Baltimore, MD, USA
2Faculty of Dentistry, University of Toronto, Toronto, ON, Canada
Chronic orofacial pain (COFP) disorders are prevalent and debilitating pain conditions affecting the head, face, and neck areas. COFPs comprise several etiologically different disorders, either arising from the periphery, such as musculoskeletal abnormalities in the orofacial region and neuropathic and neuralgic conditions, or from the central nervous system (CNS), such as functional pain disorders. A notable clinical challenge in the treatment of these disorders is the interindividual variability in the manifestation and clinical measures of the chronic pain. The diversity of chronic orofacial pains and interindividual factors that contribute to the clinical presentation of these disorders make them particularly difficult to diagnose and to treat. COFPs are associated with complex patterns of central neural mechanisms reflected in the structure and function of the brain. Finding convergent structural and functional brain abnormalities across these phenotypically different COFPs could allow us to understand the CNS mechanisms common to all these conditions. Here, we review the COFP neuroimaging literature and perform three coordinate-based meta-analyses to examine (1) structural and (2) functional brain abnormalities of patients with COFP compared to healthy, pain-free subjects. The structural magnetic resonance imaging meta-analysis identified 12 peer-reviewed articles met the study criteria and revealed widespread gray matter abnormalities in COFP: gray matter increases in nociceptive processing regions, including the orofacial region of the primary somatosensory and motor cortices (S1, M1), thalamus, the basal ganglia, medial and dorsolateral prefrontal cortices (mPFC, dlPFC), the anterior cingulate cortex (ACC), and the insula (INS). COFP patients had lesser gray matter in the hand region of S1/M1, the hippocampus, the bilateral superior temporal gyrus, and the posterior cingulate cortex. The functional brain imaging meta-analysis identified 22 peer-reviewed studies comparing COFP patients and pain-free subjects that met study criteria and showed that patients had greater activation in several regions, including the STG, DLPFC, S1, M1, the secondary somatosensory cortex (S2), the basal ganglia, and the hippocampus. Patients, however, had less activation in bilateral thalamus and INS. These results suggest that the structural and functional abnormalities may be related and that there is increased nociceptive drive in COFP and decreased pain modulation. Overall, these findings of structural and functional abnormalities in the brain of COFP patients could be used to develop novel diagnostic and prognostic tools.
Lack of evidence for ectopic sprouting of genetically labeled Aβ touch afferents in inflammatory and neuropathic trigeminal pain
Yong Chen1, Yi Zhang2, Wolfgang B Liedtke1 and Fan Wang2
1Department of Neurology, Duke University, Durham, NC, USA
2Department of Neurobiology, Duke University, Durham, NC, USA
Background
Mechanical and in particular tactile allodynia is a hallmark of chronic pain in which innocuous touch becomes painful. Previous cholera toxin B-based neural tracing experiments and electrophysiology studies had suggested that aberrant axon sprouting from touch sensory afferents into pain-processing laminae after injury is a possible anatomical substrate underlying mechanical allodynia. This hypothesis was later challenged by experiments using intra-axonal labeling of A-fiber neurons, as well as single-neuron labeling of electrophysiologically identified sensory neurons. However, no studies have used genetically labeled neurons to examine this issue, and most studies were performed on spinal but not trigeminal sensory neurons which are the relevant neurons for orofacial pain, where allodynia oftentimes plays a dominant clinical role.
Materials and methods
We recently discovered that parvalbumin::Cre (Pv::Cre) labels two types of Aβ touch neurons in trigeminal ganglion. Using a Pv::CreER driver and a Cre-dependent reporter mouse, we specifically labeled these Aβ trigeminal touch afferents by timed taxomifen injection prior to inflammation or infraorbital nerve injury (ION transection).
Results
We examined the peripheral and central projections of labeled axons into the brainstem caudalis nucleus after injuries vs. controls. We found no evidence for ectopic sprouting of Pv::CreER labeled trigeminal Aβ axons into the superficial trigeminal nocireceptive laminae after inflammation or nerve injury. Furthermore, there was also no evidence for peripheral sprouting.
Conclusions
CreER-based labeling prior to injury precluded the issue of phenotypic changes of neurons after injury. Our results suggest that touch allodynia in chronic orofacial pain is unlikely caused by ectopic sprouting of Aβ trigeminal afferents.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by NIDCR 1K12DE022793 (UNC-CPRI, YC), R01DE018549 (WL), R01DE19440 (FW), R01DE19440S1 (FW and WL), F33DE024668 (YC and WL); the Duke Institute for Brain Science (DIBS Incubator Award to FW and WL); the Duke Nicholas School for the Environment (Leon Goldberg Fellowship to YC).
Mechanical and inflammatory-mediated degeneration of mandibular cartilage is associated with altered NG2-type VI collagen colocalization and cytosolic NG2 residues in articular chondrocytes
Andrew E Bertagna1, Diyora Amanova1, Nageeb Hasan2, Scott Bicknell2, Shreya Thakkar2, Polina Gubareva2 and David A Reed3
1College of Dentistry, The University of Illinois at Chicago, Chicago, IL, USA
2Honors College, The University of Illinois at Chicago, Chicago, IL, USA
3Department of Oral, College of Dentistry, The University of Illinois at Chicago, Chicago, IL, USA
Background
Temporomandibular joint (TMJ) degenerative joint disease (DJD) is of an unknown etiology and has few available interventional options. One key aspect of DJD is pervasive chondrocyte cell death or chondroptosis that limits homeostatic repair of the condylar cartilage following mechanical or inflammatory damage. This is an unappreciated target for both clinical interventional and translational research. Cell matrix-derived signaling is an established regulator of both cell viability and tissue homeostasis. The major component of the pericellular matrix surrounding chondrocytes of the mandibular condylar cartilage (MCC) is type VI collagen. A known receptor for type VI collagen is the transmembrane proteoglycan, nerve/glial antigen 2 (NG2). Colocalized NG2-type VI collagen has been illustrated in various cell types and is known to be a key regulator of cell proliferation, migration, differentiation, and viability. NG2 has a hypothesized role as a transcriptional regulator through the cleavage of its ectodomain and subsequent nuclear translocation of the intracellular domain. Despite its regulatory roles in various cell types, the significance of the NG2-ype VI collagen relationship has yet to be elucidated in the proliferative chondrocytes of the MCC.
Materials and methods
Using a surgical instability mouse model, DJD was induced in the TMJ of male c57 mice. Tissue was collected at 0, 4, 8, 12, and 16 weeks post-operatively. NG2-type VI collagen colocalization was calculated from immunofluorescence confocal microscopy using WCIF_ImageJ plugin (Toronto Western Research Institute, Canada) and with a proximity ligation assay confirming the spatial correlation between residues of NG2 and its ligand, type VI collagen.
Results
In nonsurgical controls, NG2-type VI collagen colocalization coefficients were high in articular, prechondroblastic, and chondroblastic chondrocytes but not hypertrophic chondrocytes. On the medial aspect of four, eight, and 12 week post-operative joints, colocalization coefficients slightly decreased. At eight weeks post-operative, cytosolic localization of the NG2 intracellular domain was observed in articular cells. These changes were also associated with remarkable spatiotemporal changes in NG2 expression relative to structural matrix proteins such as type VI collagen. Type VI collagen was found to encompass proliferative chondrocytes in a tight band at zero and four weeks post-operative, become progressively more diffuse and spatially disorganized as degeneration progressed (n = 3). This degenerative change was concentrated on the medial aspect of the joint.
Conclusions
Together, these data support the hypothesis that the transmembrane proteoglycan NG2 colocalizes with type VI collagen in the pericellular matrix, that NG2-type VI collagen interactions mediate cell matrix-derived signaling, and that disruption of this signaling could promote cartilage degeneration.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Schour Scholars Fund and the UIC College of Dentistry; ACC 14-106.
Lateral thalamic control of nociceptive response after whisker pad injection of varicella zoster virus
Crystal Stinson1, Phillip Kramer1, Mikhail Umorin1, Mohong Deng2, Mahesh Rao1, Larry Bellinger1, Michael B Yee3 and Paul R Kinchington3
1Texas A&M University Baylor College of Dentistry, Dallas, TX, USA
2Department of Oral and Maxillofacial Surgery, The State Key Laboratory Breeding Base of Basic Science of Stomatology & Key Laboratory of Oral Biomedicine Ministry of Education, School & Hospital of Stomatology, Wuhan University, Wuhan, China
3Department of Ophthalmology and of Microbiology and Molecular Genetics, University of Pittsburgh, Pittsburgh, PA, USA
Background
Herpes Zoster (HZ) leads to post-herpetic neuralgia in 20% of patients causing chronic pain that can be debilitating. Currently, using a novel orofacial model for HZ-associated pain, we are able to investigate mechanisms controlling the pain responses that are not yet understood. HZ infection which results from reactivation of a latent varicella zoster virus (VZV) will produce orofacial pain in about a quarter of HZ patients. Studies suggest central pathways involving the thalamus could control pain related to HZ, and preliminary studies in our lab suggest vesicular GABA transporter (VGAT) in the lateral thalamus influences orofacial pain.
Methods
VZV was injected into the whisker pad of Sprague Dawley male and female rats. Affective and motivational aspects of pain were measured using the Place Escape/Avoidance Paradigm. Thalamic neuronal activity was modulated after injecting an adeno-associated virus (AAV) expressing an engineered acetylcholine Gi-protein-coupled receptor. This receptor inhibits neuronal burst firing when bound by clozapine-n-oxide (CNO). VGAT expression was attenuated in the thalamus by injecting an AAV construct that expressed a VGAT silencing shRNA.
Results
VZV-induced nociception was significantly decreased after administering CNO in both male and female rats. Nociception significantly increased concomitant with increased thalamic c-fos expression after attenuating thalamic VGAT expression.
Conclusions
This data establish that the lateral thalamus (posterior, ventral posteromedial, ventral posterolateral, and reticular thalamic nucleus) controls VZV-induced nociception in the orofacial region and that GABA in this region appears to reduce the response to VZV-induced nociception possibly by gating facial pain input.