Abstract
Purpose
To investigate the relationship between rest-activity rhythm (RAR) patterns and risk of falls/fractures in older men. We hypothesized that dysregulated RAR would be associated with incident fall/fractures.
Methods
We used wrist-worn actigraphy to measure RAR over 4.8±0.8 24 hour periods in men (≥67 years) enrolled in the multicenter Outcomes of Sleep Disorders in Men (MrOS Sleep) Study (n=3001). Men were contacted every 4 months to report occurrence of falls/fractures. RAR parameters included amplitude (difference between peak and nadir activity in counts/minute), mesor (activity counts/minute), acrophase (time of day of peak activity) and pseudo-F statistic (rhythm robustness) and were evaluated as continuous variables with associations reported per SD increase/decrease in models adjusted for confounders. Logistic regression was used to estimate the likelihood (odds ratio, OR) of recurrent falls in the year after the visit. Proportional hazards models were used to estimate the risk (hazard ratio, HR) of fractures.
Results
One year after the visit, 417 men (14%) had recurrent (≥2) falls). Later acrophase (OR 1.18, 95% CI: 1.06–1.32) was associated with a modestly greater likelihood of falls. In 8.6 years (SD 2.6 years) of >97% complete follow up, 256 men (8.53%) had a major osteoporotic fracture, 85 (2.8%) had a clinical spine fracture, and 110 (3.7%) had a hip fracture. No consistent, significant associations were observed between RAR patterns and fractures.
Conclusions
Later acrophase was associated with a modestly greater risk of falls; this association did not translate into a higher fracture risk in this cohort of elderly men.
Keywords: rest-activity rhythm, falls, fractures, aging, epidemiology
Introduction
Falls are common in the elderly [1], especially in patients with chronic conditions, functional disabilities and history of previous falls [2]. Over 50% of falls in the elderly lead to injury [3] and increased healthcare costs [4]. One of the most severe consequences of falls is fracture, which affects approximately 50% of women and 25% of men after age 50 [5,6]. Health care costs related to osteoporotic fracture are projected to reach $25.3 billion in the US by 2025 [7]. Associations between poor sleep quality and postmenopausal osteoporosis/osteopenia [8] and short sleep duration and risk of osteoporosis [9] suggest that sleep impacts bone health. Dysregulated sleep has been associated with increased risk of falls and fracture in the elderly [10–12], which may be mediated by factors such as impaired cognitive function, reduced muscle strength, medications, depression and other co-morbidities [10]. Balance problems, drowsiness upon waking and increased nighttime activity due to insomnia have also been implicated in the relationship between dysregulated sleep and falls [12].
Bone turnover exhibits circadian variation [13], and the relationships between bone, circadian rhythm and sleep are emerging areas of research [14]. Wrist actigraphy has been validated as a measurement tool for entrained sleep/wake rhythm, and it is particularly well-suited for assessing circadian activity rhythms [15]. Dysregulation in circadian activity patterns, which are also known as rest-activity rhythm (RAR) patterns, has been associated with increased risk of mortality [16,17], dementia [18] and cardiovascular disease [19] in older adults. The relationship between circadian activity rhythms and risk of falls has only been evaluated in one published study. In a cohort of very old (87–89 years) adults in the United Kingdom, wrist accelerometers were used to measure activity patterns over 5–7 days. Abnormal sleep-wake cycles (defined as minimal differences between the most and least active periods of the day) were significantly associated with number of falls (p=0.04). However, the associations were cross-sectional and were not adjusted for potential confounders [20]. To our knowledge, the association of RAR and incident fractures has not been reported.
Based on these observations, we hypothesized that RAR patterns would be associated with recurrent falls and fractures in older men. To test this hypothesis, we measured RAR in men aged 67 and older enrolled in the multicenter Outcomes of Sleep Disorders in Men (MrOS Sleep) Study and prospectively assessed for falls and fractures. The MrOS Sleep Study provides a unique opportunity to study this question in a large cohort of community-dwelling older men who are well-characterized for RAR parameters, fall and fracture incidence, as well as for potentially important confounding factors such as medication use, physical function, and comorbidities.
Materials and Methods
Study participants
The Osteoporotic Fractures in Men (MrOS) is a prospective cohort study following ambulatory men ≥65 years (n=5994) at six U.S. clinics (Birmingham, Alabama; Minneapolis, Minnesota; Palo Alto, California; Pittsburgh, Pennsylvania; Portland, Oregon; and San Diego, California). Men who were unable to walk without assistance of another person and men who had a history of bilateral hip replacement were excluded. Subjects were enrolled from March 2000 through April 2002 [21,22].
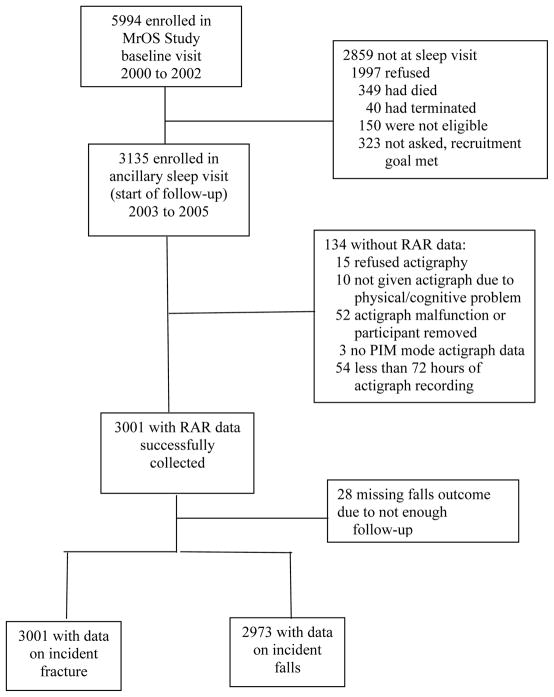
The Outcomes of Sleep Disorders in Older Men (MrOS Sleep) study is an ancillary MrOS study focused on sleep disorders in older men; men were enrolled in an ancillary “Sleep Visit” from December 2003 through March 2005. Of the 5994 men enrolled in MrOS, 3135 were enrolled in MrOS Sleep (105% of recruitment goal). Of the MrOS participants who did not enroll in MrOS Sleep, 1997 declined participation, 150 were not eligible, 349 died prior to the first visit, 40 terminated enrollment prior to the visit, and 323 were not contacted since recruitment goals were met. Technically adequate measures of RAR patterns (≥ 72 consecutive hours of data collection) were obtained for 3001 men. This restriction was used because it is the minimum current requirement for actigraphy monitoring for the Centers for Medicare Services (CMS) (Current Procedural Technology (CPT® code 95803) [23]. All 3001 men with technically adequate measures of RAR had data on the fracture outcomes, while 2973 men had enough follow-up data to ascertain incident falls (Figure). The 798 (26.6%) men who reported a prior fracture of the hip, wrist, shoulder, or spine and are included in this analysis.
Figure.
Recruitment and inclusion of participants.
Reasons for not including participants are given on the right.
RAR, rest-activity rhythm
The study was approved by the Institutional Review Board at each clinic site, and all participants provided written informed consent.
Assessment of falls and fractures
Participants were contacted every four months via postcard or telephone to report occurrence of falls and fractures (≥97% response rate). Falls reported in the first year after the Sleep Visit were included in this analysis. Men with at least two falls in the year after the Sleep Visit were classified as “recurrent fallers” in the dichotomous falls outcome (versus men with 0–1 fall). Fractures and their dates of occurrence were centrally adjudicated by physician review of radiology reports; mean follow up for fractures was 8.7 years (SD 2.6 years).
Rest-activity patterns
Actigraphy (SleepWatch-O; Ambulatory Monitoring, Inc., Ardsley, New York, USA) was used to collect activity count data from participants with over 4.8±0.8 consecutive 24-hour periods; the minimum current requirement for actigraphy monitoring is 3 days, per the Centers for Medicare Services (CMS) (Current Procedural Technology (CPT®) code for actigraphy monitoring (code 95803) [23]. Men wore the actigraph on the non-dominant wrist and were instructed to remove the actigraph only when performing activities that would submerse it in water. Movement was detected via a piezoelectric bimorph-ceramic cantilever beam that generates a voltage each time the actigraph was moved. These voltages were gathered continuously and summarized over one-minute intervals, with movement measured in arbitrary counts per minute [24]. Actigraphy data was measured in three modes but are reported here using proportional integration mode (PIM), which is the preferred mode of this device for measuring activity [25]. An extension of the traditional cosine curve was used to fit RAR to the activity data. Activity data often assumes a shape more similar to a squared wave than a cosine curve. This 5-parameter extended cosine model allows for this shape.[15,26]
Four RAR parameters were evaluated in this analysis: amplitude, mesor, acrophase and pseudo F-statistic. Amplitude (measured in activity counts/minute) is defined as the difference between peak and nadir in the fitted curve and indicates strength of the rhythm. Mesor (activity counts/minute) represents the mean level of the fitted curve. Acrophase (time of day) indicates the time of day of peak activity; earlier or later times indicate a tendency toward advanced or delayed activity rhythm. The pseudo F-statistic (no unit) indicates rhythm robustness and is a measure of how well the activity fits the cosine model; higher values indicate a better fit (more organization, increased circadian rhythmicity) [16,19].
Other measures
At the Sleep Visit, men completed a questionnaire about their medical history, which included self-report of diagnoses of diabetes mellitus, coronary heart disease, hypertension, and stroke, fall history, smoking status, caffeine and alcohol use. Depressive symptoms were assessed with the Geriatric Depression Scale (GDS) [27], with the standard cut point of ≥6 depressive symptoms used to define depression [28]. Instrumental Activities of Daily Living (IADL) were used to measure functional status and included walking two to three blocks on level ground, climbing up to ten steps, preparing meals, doing heavy housework, and shopping for groceries or clothing [29,30]. Current prescription and over-the-counter medications used in the past 30 days were categorized by a computerized medication coding dictionary [31]; prescription medication was stored electronically in a mediations inventory database (San Francisco Coordinating Center, San Francisco, CA). The Iowa Drug Information Service (IDIS) Drug Vocabulary (College of Pharmacy, University of Iowa, Iowa City, IA) was used to match each medication to its ingredient(s). Self-reported caffeine intake was calculated based on answers to questions regarding intake of caffeinated coffee, tea and soda [32]. Nocturia was defined as reporting difficulty sleeping due to nocturnal bathroom use at least 3 times per week. Gait speed (meters/second) was measured over a distance of six meters. Participants’ body weight was measured with a digital scale or with a standard regularly calibrated balance beam scale. A wall-mounted stadiometer was used to measure participants’ height without shoes, using a standard held-expiration technique. Body mass index (BMI; kg/m2) was calculated from height and weight measurements. Dual energy x-ray absorptiometry (DXA) (QDR 4500W; Hologic Inc, Bedford, MA) was used to measure bone mineral density (BMD, g/cm2), and T-scores were calculated using female white norms, per descriptive epidemiology recommendations from the Committee of Scientific Advisors of the International Osteoporosis Foundation [33,34].
Statistical analyses
There are no standard clinical cut-points for the RAR parameters. The RAR predictor variables amplitude, mesor and pseudo-F statistic were expressed as quintiles as was done in prior work in this cohort, with the highest quintile serving as the referent category; we hypothesized that those with highest values in these measures would have the lower risk of subsequent outcomes [16]. The acrophase variable, however, was analyzed differently. Low values for acrophase indicate early shifting of the time of activity (“early birds”) while higher values for acrophase indicate late shifting of the time of activity (“night owls”). Therefore, acrophase was examined in terms of the deviation from the population mean. We identified three categories based on having a peak time of more than one standard deviation (SD) above and below the population mean (2:18 PM±73 minutes) for the study population. Phase advanced participants (“early birds”) were defined as having an acrophase of <1:04 PM (<1 SD from the mean) and phase delayed participants (“night owls”) were defined as having an acrophase of >3:29 PM (>1 SD from the mean); those with acrophase between 1:04 pm and 3:29 pm comprised the referent group. Previous studies have reported U-shaped associations between acrophase and health conditions such as dementia [18]. Analyses were also performed with the effect estimates for the RAR parameters expressed as continuous variables per standard deviation increase (acrophase) or decrease (amplitude, mesor, pseudo-F statistic).
Participant characteristics were compared across category of the pseudo-F statistic and acrophase using ANOVA for continuous normally distributed variables, Kruskal-Wallis tests for skewed continuous variables, and chi-square tests for categorical variables. Similar analyses were performed for amplitude and mesor for use in covariate selection (data not shown). These results were used for covariate selection for multivariable models. Logistic regression was used to estimate the likelihood (odds ratio, OR) of recurrent falls. Proportional hazards models were used to estimate the risk (hazard ratio, HR) of fractures (major osteoporotic fracture [hip, wrist, shoulder, clinical spine], hip, clinical spine).
We aimed to understand if there were any associations between the RAR predictors and falls or fractures, and if so, whether these associations were explained by potentially confounding factors. Therefore, we first ran models adjusted only for age and clinical site. Then, we ran multivariate models. Multivariable models were further adjusted for potential confounders that were related to one or more RAR predictors at p<0.10, including race (white vs. non-white), BMI, any medical condition (from the list of diabetes mellitus, coronary heart disease, hypertension, stroke), smoking (none, past, current), alcohol use (<1 drink/week, 1–13 drinks/week, ≥14 drinks/week), caffeine use, depression (GDS ≥6), use of benzodiazepines, use of prescription sleep medications (excluding benzodiazepines), presence of an IADL impairment, and gait speed. Nocturia (disturbed sleep due to bathroom use ≥3 times per week) was included as a covariate based on previous reports [35,36]. Additionally, fracture models were adjusted for femoral neck BMD. In secondary analyses, models were further adjusted for activity level to examine if associations between RAR parameters and falls were independent of physical activity.
Unless otherwise noted, two-sided significance levels were set at p<0.05, and all analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
The analysis cohort was comprised of 3001 primarily Caucasian (89.9%) men with an average age of 76.4 ± 5.5 years at the RAR assessment and an average BMI of 27.2 ± 3.9 kg/m2.
Participant characteristics stratified by quintile of pseudo F-statistic and acrophase category are presented in Table 1a and Table 1b, respectively. Compared to men with lower values, men in higher pseudo F-statistic quintiles (more robust RAR) were generally younger, had lower BMI, and were less likely to report a history of diabetes mellitus, hypertension, stroke, coronary heart disease, or a history of falling. Men with lower values of the pseudo-F statistic (less robust RAR) were more likely to report depression, to use antidepressants or benzodiazepines, and to have a functional limitation compared to men in higher quintiles. In addition, men in higher quintiles of the pseudo-F statistic generally reported greater caffeine intake, greater alcohol intake, higher activity level, and had faster gait speed compared to men in lower quintiles. There were no differences across the quintiles of pseudo-F statistic for race, femoral neck BMD, femoral neck T-score, smoking status, use of prescription sleep medication, and nocturia. Compared to phase advanced or phase delayed men, men in the middle acrophase category were more likely to have a lower BMI, a faster gait speed, greater mean daytime activity count, and lower rates of CHD and stroke. Additionally, phase delayed men were more likely to smoke, to be non-white, to have higher rates of diabetes mellitus or depression, more likely to use anti-depressants or benzodiazepines, have a history of falling or to have functional limitations, and consumed less alcohol. There were no differences across category of acrophase for age, hypertension, caffeine use, use of prescription sleep medication, femoral neck BMD, femoral neck T-score or nocturia.
Table 1a.
Characteristics of older men in the MrOS cohort by quintiles of pseudo F-statistic (rest-activity rhythm robustness)
| Characteristic | Quintile 1 (<640.3; n=600) | Quintile 2 (640.3 – <853.5; n=600) | Quintile 3 (853.5– <1092.8; n=600) | Quintile 4 (1092.8 – <1415.1; n=600) | Quintile 5 (≥ 1415.1; n=601) | p-value |
|---|---|---|---|---|---|---|
| Age (years), mean ± SD | 77.7 ± 5.8 | 77.0 ± 5.7 | 76.1 ± 5.3 | 75.9 ± 5.3 | 75.0 ± 5.0 | <0.001 |
| Non-white race, n (%) | 77 (12.8) | 59 (9.8) | 58 (9.7) | 58 (9.7) | 51 (8.5) | 0.138 |
| BMI (kg/m2), mean ± SD | 28.3 ± 4.4 | 27.5 ± 4.0 | 27.1 ± 3.7 | 26.8 ± 3.4 | 26.4 ± 3.3 | <0.001 |
| Smoking status, n (%) | 0.188 | |||||
| Never | 217 (36.2) | 252 (42) | 236 (39.3) | 251 (41.8) | 223 (37.1) | |
| Past | 364 (60.8) | 340 (56.7) | 354 (59) | 339 (56.5) | 366 (60.9) | |
| Current | 18 (3.0) | 8 (1.3) | 10 (1.7) | 10 (1.7) | 12 (2) | |
| Alcohol intake (drinks/week), n (%) | <0.001 | |||||
| < 1 | 318 (53.4) | 318 (53.2) | 275 (46.1) | 261 (43.6) | 231 (38.7) | |
| 1–13 (moderate) | 251 (42.1) | 256 (42.8) | 286 (47.9) | 307 (51.3) | 314 (52.6) | |
| ≥ 14 (heavy) | 27 (4.5) | 24 (4.0) | 36 (6.0) | 31 (5.2) | 52 (8.7) | |
| History of diabetes mellitus, n (%) | 108 (18.0) | 82 (13.7) | 80 (13.3) | 60 (10) | 71 (11.8) | 0.001 |
| History or coronary heart disease,* n (%) | 272 (45.6) | 198 (33) | 190 (31.7) | 177 (29.6) | 157 (26.2) | <0.001 |
| History of hypertension, n (%) | 346 (57.8) | 309 (51.5) | 313 (52.2) | 288 (48) | 242 (40.3) | <0.001 |
| History of stroke, n (%) | 31 (5.2) | 29 (4.8) | 15 (2.5) | 19 (3.2) | 16 (2.7) | 0.031 |
| Caffeine intake (mg/d), mean ± SD | 220.4 ± 256.4 | 227.0 ± 254.3 | 225.6 ± 227.4 | 246.3 ± 242.5 | 259.6 ± 250.7 | 0.002 |
| Actigraphic mean daytime activity level (counts),** mean ± SD | 2815.8 ± 596.4 | 3308.6 ± 524.1 | 3545.9 ± 519.7 | 3915.9 ± 539.8 | 4337.1 ± 549.3 | <0.001 |
| Depression (GDS ≥ 6), n (%) | 70 (11.7) | 48 (8.0) | 30 (5.0) | 22 (3.7) | 26 (4.3) | <0.001 |
| Benzodiazepine use, n (%) | 40 (6.7) | 25 (4.2) | 24 (4) | 17 (2.8) | 28 (4.7) | 0.026 |
| Antidepressant use, n (%) | 69 (11.5) | 39 (6.5) | 55 (9.2) | 32 (5.3) | 40 (6.7) | <0.001 |
| Use of prescription sleep medication, n (%) | 18 (3) | 10 (1.7) | 9 (1.5) | 11 (1.8) | 12 (2) | 0.374 |
| Femoral neck BMD (g/cm2), mean ± SD | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.617 |
| Femoral neck BMD T-score, mean ± SD | −0.60 ± 1.11 | −0.66 ± 1.12 | −0.60 ± 1.13 | −0.66 ± 1.04 | −0.67 ± 1.01 | 0.617 |
| Femoral neck T-score Category, n (%) | 0.382 | |||||
| ≥−1 (Normal) | 365 (62) | 359 (60) | 375 (63) | 346 (58) | 356 (60) | |
| −1< T-score <−2.5 (Osteopenic) | 206 (35) | 219 (37) | 203 (34) | 239 (40) | 228 (38) | |
| ≤−2.5 (Osteoporotic) | 19 (3.2) | 16 (2.7) | 16 (2.7) | 10 (1.7) | 13 (2.2) | |
| ≥ 1 IADL impairments, n (%) | 226 (37.7) | 131 (21.8) | 107 (17.8) | 86 (14.3) | 81 (13.5) | <0.001 |
| Nocturia (≥ 3 times/week), n (%) | 454 (75.8) | 460 (76.7) | 450 (75) | 433 (72.2) | 428 (71.2) | 0.137 |
| Gait speed (m/s), mean ± SD | 1.1 ± 0.2 | 1.1 ± 0.3 | 1.2 ± 0.2 | 1.2 ± 0.2 | 1.2 ± 0.2 | <0.001 |
P-values for continuous variables are from ANOVA for normally distributed data; the Kruskal Wallis test was used for skewed data. P-values for categorical data from a chi-square test.
Coronary heart disease defined as a history of myocardial infarction, angina, congestive heart failure, bypass surgery, angioplasty or pacemaker placement.
From the out-of-bed interval; the time men were not in bed trying to sleep (includes naps)
Table 1b.
Characteristics of older men in the MrOS cohort by category of acrophase (time of day of peak activity)
| Characteristic | <1:04PM (n= 422) | 1:04–3:29PM (n= 2169) | >3:29PM (n= 410) | p-value |
|---|---|---|---|---|
| Age (years), mean ± SD | 76.5 ± 5.3 | 76.2 ± 5.5 | 76.8 ± 5.8 | 0.109 |
| Non-white race, n(%) | 28 (6.6) | 221 (10.2) | 54 (13.2) | 0.007 |
| Body mass index, kg/m2, mean ± SD | 27.8 ± 4.0 | 27.1 ± 3.7 | 27.4 ± 4.2 | 0.0004 |
| Smoking status, n(%) | ||||
| Never | 157 (37.2) | 860 (39.7) | 162 (39.5) | 0.007 |
| Past | 260 (61.6) | 1272 (58.7) | 231 (56.3) | |
| Current | 5 (1.2) | 36 (1.7) | 17 (4.2) | |
| Alcohol intake (drinks/week), n(%) | ||||
| <1 | 216 (51.7) | 981 (45.4) | 206 (50.5) | 0.017 |
| 1–13 (moderate) | 179 (42.8) | 1046 (48.4) | 189 (46.3) | |
| ≥14 (heavy) | 23 (5.5) | 134 (6.2) | 13 (3.2) | |
| History of diabetes mellitus, n(%) | 51 (12.1) | 278 (12.8) | 72 (17.6) | 0.025 |
| History of coronary heart disease,* n(%) | 152 (36.0) | 689 (31.8) | 153 (37.5) | 0.034 |
| History of hypertension, n(%) | 231 (54.7) | 1060 (48.9) | 207 (50.5) | 0.087 |
| History of stroke, n(%) | 18 (4.3) | 69 (3.2) | 23 (5.6) | 0.044 |
| Caffeine intake (mg/d), mean ± SD | 238.0 ± 248.3 | 238.3 ± 243.8 | 220.6 ± 260.3 | 0.408 |
| Actigraphic mean daytime activity level (counts), mean ± SD** | 3329.1 ± 775.0 | 3652.1 ± 728.1 | 3492.6 ± 793.1 | <0.001 |
| Depression (GDS≥6), n(%) | 23 (5.5) | 128 (5.9) | 45 (11.0) | <0.001 |
| Benzodiazepine use, n(%) | 14 (3.3) | 88 (4.1) | 32 (7.8) | 0.002 |
| Antidepressant use, n(%) | 33 (7.8) | 145 (6.7) | 57 (13.9) | <0.001 |
| Use of prescription sleep medication, n(%) | 8 (1.9) | 43 (2.0) | 9 (2.2) | 0.946 |
| Femoral neck BMD, g/cm2, mean ± SD | 0.78 ± 0.13 | 0.78 ± 0.13 | 0.78 ± 0.13 | 0.658 |
| Femoral neck BMD T-score, mean ± SD | −0.63 ± 1.10 | −0.63 ± 1.08 | −0.68 ± 1.10 | 0.658 |
| Femoral neck T-score Category, n (%) | 0.542 | |||
| ≥−1 (Normal) | 254 (60.8) | 1315 (61.3) | 232 (57.0) | |
| −1< T-score <−2.5 (Osteopenic) | 155 (37.1) | 775 (36.1) | 165 (40.5) | |
| ≤−2.5 (Osteoporotic) | 9 (2.2) | 55 (2.6) | 10 (2.5) | |
| ≥1 IADL impairments, n(%) | 96 (22.8) | 407 (18.8) | 128 (31.2) | <0.001 |
| Nocturia (≥3 times/week), n(%) | 318 (75.4) | 1610 (74.3) | 297 (72.4) | 0.619 |
| Gait speed (m/s), mean ± SD | 1.1 ± 0.2 | 1.2 ± 0.2 | 1.1 ± 0.3 | <0.001 |
P-values for continuous variables are from ANOVA for normally distributed data; a Kruskal Wallis test was used for skewed data. P-values for categorical data are from a chi-square test for homogeneity.
Coronary heart disease was defined as a history of myocardial infarction, angina, congestive heart failure, bypass surgery, angioplasty or pacemaker placement.
From the out-of-bed interval; the time men were not in bed trying to sleep (includes naps)
The year after the visit, 417 men (14.0%) had recurrent (≥2) falls. Associations of RAR variables and likelihood of recurrent falls (odds ratio as assessed by logistic regression) are presented in Table 2. In models adjusted for age and clinic site, lower amplitude (indicating weaker circadian rhythm strength), later acrophase (later time of daily peak activity) and lower pseudo F-statistic values (indicating greater disorganization of circadian activity) were associated with a modestly greater likelihood of falls. After adjustment for covariates, later acrophase remained associated with a modestly greater likelihood of falls, while significance of the pseudo-F statistic association was attenuated (p=0.06). The association between lower amplitude (weaker rhythm strength) and likelihood of falls no longer maintained significance after multivariable adjustment. After further adjustment for activity level, the association between later acrophase and incident falls remained unchanged, while the association between pseudo-F statistic and falls regained significance.
Table 2.
Associations of rest-activity parameters and likelihood of recurrent falls (≥2 in 1 year of follow-up)
| Predictor | Model | Odds Ratio (95 % Confidence Interval) | |||
|---|---|---|---|---|---|
| Events N (%) |
Age+Site Adjusted | Multivariable Adjusted* | Multivariable + Activity Adjusted** | ||
| Amplitude | Continuous per SD decrease (−1090 counts/min) Quintiles |
417 (14.03) | 1.21 (1.08, 1.36) | 1.07 (0.95, 1.20) | 1.11 (0.95, 1.31) |
| Q1:<2730.71 | 107 (18.20) | 1.60 (1.13, 2.27) | 1.09 (0.75, 1.58) | 1.08 (0.65, 1.80) | |
| Q2:2730.71 to <3315.83 | 92 (15.41) | 1.41 (0.99, 2.01) | 1.35 (0.93, 1.94) | 1.35 (0.88, 2.05) | |
| Q3:3315.83 to <3778.50 | 82 (13.71) | 1.25 (0.88, 1.79) | 1.17 (0.81, 1.70) | 1.17 (0.79, 1.73) | |
| Q4:3778.50 to <4361.92 | 76 (12.73) | 1.23 (0.86, 1.77) | 1.28 (0.88, 1.85) | 1.27 (0.87, 1.84) | |
| Q5:≥4361.92 | 60 (10.12) | reference | reference | reference | |
| P-trend | 0.0069 | 0.6684 | 0.6309 | ||
| Mesor | Continuous per SD decrease (−502 counts/min) Quintiles |
417 (14.03) | 1.11 (0.99, 1.24) | 1.01 (0.91, 1.14) | 1.02 (0.88, 1.19) |
| Q1:<1789.82 | 100 (16.92) | 1.28 (0.91, 1.79) | 0.94 (0.66, 1.34) | 0.76 (0.46, 1.23) | |
| Q2:1789.82 to <2030.22 | 78 (13.11) | 1.00 (0.71, 1.42) | 0.93 (0.64, 1.33) | 0.81 (0.54, 1.24) | |
| Q3:2030.22 to <2235.98 | 78 (13.02) | 1.04 (0.73, 1.47) | 0.99 (0.69, 1.41) | 0.92 (0.62, 1.34) | |
| Q4:2235.98 to <2500.61 | 91 (15.29) | 1.27 (0.90, 1.77) | 1.28 (0.90, 1.81) | 1.23 (0.87, 1.76) | |
| Q5:≥2500.61 | 70 (11.80) | reference | reference | reference | |
| P-trend | 0.503 | 0.2405 | 0.0783 | ||
| Acrophase | Continuous per SD increase (1 hr, 12 min) | 417 (14.03) | 1.23 (1.11, 1.36) | 1.18 (1.06, 1.32) | 1.18 (1.06, 1.32) |
| <1:04PM | 52 (12.56) | 0.90 (0.65, 1.24) | 0.86 (0.62, 1.20) | 0.86 (0.62, 1.21) | |
| 1:04–3:29PM | 282 (13.11) | reference | reference | reference | |
| >3:29PM | 83 (20.34) | 1.67 (1.27, 2.21) | 1.46 (1.08, 1.97) | 1.46 (1.08, 1.97) | |
| Pseudo-F statistic | Continuous per SD decrease (−512.9) Quintiles |
417 (14.03) | 1.24 (1.10, 1.40) | 1.12 (0.99, 1.27) | 1.23 (1.04, 1.46) |
| Q1:<640.319 | 111 (18.91) | 1.68 (1.19, 2.36) | 1.27 (0.88, 1.84) | 1.53 (0.95, 2.47) | |
| Q2:640.319 to <853.460 | 86 (14.41) | 1.25 (0.88, 1.78) | 1.11 (0.77, 1.61) | 1.27 (0.83, 1.94) | |
| Q3:853.460 to <1092.75 | 88 (14.72) | 1.35 (0.95, 1.91) | 1.33 (0.93, 1.91) | 1.48 (1.00, 2.20) | |
| Q4:1092.75 to <1415.08 | 68 (11.41) | 1.02 (0.70, 1.46) | 1.06 (0.73, 1.53) | 1.13 (0.77, 1.66) | |
| Q5:≥1415.08 | 64 (10.76) | reference | reference | reference | |
| P-trend | 0.0011 | 0.2109 | 0.1049 | ||
SD, standard deviation.
Adjusted for age, clinic site, race, body mass index, any medical condition (diabetes mellitus, coronary heart disease, hypertension, stroke), smoking, alcohol use, caffeine use, depression, use of benzodiazepines, use of prescription sleep medications, presence of an IADL, walking speed, nocturia.
In secondary analyses, models were further adjusted for activity level to examine if associations between RAR parameters and falls were independent of physical activity.
After 8.6 years (SD 2.6 years) of follow up from the Sleep visit, 256 men (8.53%) had a major osteoporotic fracture, 85 (2.8%) had a clinical spine fracture, and 110 (3.7%) had a hip fracture. In proportional hazards models adjusted for age and clinic site, the hazard ratio for amplitude (rhythm strength) suggested a modestly increased risk of clinical spine fracture (Table 3). Some statistically significant associations were observed between quintiles 2 and 3 of the RAR parameters and fractures, such a pseudo F-statistic quintile 3 and hip fracture (Table 3). However, these associations were inconsistent, and RAR parameters were not associated with fracture risk after multivariate adjustment (data not shown).
Table 3.
Associations of rest-activity parameters and incident fracture, adjusted by age and clinic site
| Predictor | Model | Clinical Spine Fracture | Hip Fracture | Major Osteoporotic Fracture* | |||
|---|---|---|---|---|---|---|---|
| Events N (%) |
HR (95% CI) | Events N (%) |
HR (95% CI) | Events N (%) |
HR (95% CI) | ||
| Amplitude | Continuous per SD decrease (−1090 counts/min) Quintiles |
85 (2.83) | 1.29 (1.01, 1.66) | 110 (3.67) | 0.98 (0.81, 1.18) | 256 (8.53) | 1.09 (0.95, 1.25) |
| Q1:<2730.71 | 17 (2.83) | 1.71 (0.77, 3.79) | 21 (3.50) | 0.92 (0.48, 1.77) | 45 (17.58) | 1.13 (0.72, 1.78) | |
| Q2:2730.71 to <3315.83 | 20 (3.33) | 1.8 (0.84, 3.87) | 30 (5.00) | 1.36 (0.75, 2.45) | 65 (25.39) | 1.60 (1.06, 2.41) | |
| Q3:3315.83 to <3778.50 | 25 (4.17) | 2.34 (1.12, 4.91) | 20 (3.33) | 0.90 (0.47, 1.71) | 57 (22.27) | 1.41 (0.93, 2.15) | |
| Q4:3778.50 to <4361.92 | 13 (2.17) | 1.25 (0.55, 2.85) | 21 (3.50) | 1.03 (0.55, 1.93) | 53 (20.70) | 1.37 (0.90, 2.10) | |
| Q5:≥4361.92 | 10 (1.66) | reference | 18 (3.00) | reference | 36 (14.06) | reference | |
| P-trend | 0.13 | 0.80 | 0.44 | ||||
| Mesor | Continuous per SD decrease (−502 counts/min) Quintiles |
85 (2.83) | 1.14 (0.89, 1.45) | 110 (3.67) | 1.06 (0.87, 1.30) | 256 (8.53) | 1.07 (0.93, 1.22) |
| Q1:<1789.82 | 17 (2.83) | 1.4 (0.65, 3.02) | 32 (5.33) | 1.65 (0.91, 3.02) | 55 (21.48) | 1.30 (0.86, 1.97) | |
| Q2:1789.82 to <2030.22 | 25 (4.17) | 2.11 (1.04, 4.31) | 22 (3.67) | 1.14 (0.60, 2.15) | 58 (22.66) | 1.37 (0.91, 2.06) | |
| Q3:2030.22 to <2235.98 | 15 (2.50) | 1.29 (0.59, 2.81) | 20 (3.33) | 1.09 (0.57, 2.08) | 55 (21.48) | 1.34 (0.89, 2.02) | |
| Q4:2235.98 to <2500.61 | 17 (2.83) | 1.5 (0.70, 3.22) | 19 (3.17) | 0.98 (0.51, 1.89) | 49 (19.14) | 1.17 (0.77, 1.78) | |
| Q5:≥2500.61 | 11 (1.83) | reference | 17 (2.83) | reference | 39 (15.23) | reference | |
| P-trend | 0.23 | 0.07 | 0.16 | ||||
| Acrophase | Continuous per SD increase (1 hr, 12 min) Category |
85 (2.83) | 1.12 (0.90, 1.39) | 110 (3.67) | 0.97 (0.80, 1.18) | 256 (8.53) | 0.99 (0.87, 1.13) |
| <1:04PM | 13 (3.08) | 1.21 (0.66, 2.21) | 18 (4.27) | 1.26 (0.75, 2.11) | 42 (9.95) | 1.24 (0.88, 1.73) | |
| 1:04–3:29PM | 58 (2.67) | reference | 74 (3.41) | reference | 178 (8.21) | reference | |
| >3:29PM | 14 (3.41) | 1.47 (0.81, 2.64) | 18 (4.39) | 1.19 (0.71, 2.01) | 36 (8.78) | 1.08 (0.75, 1.55) | |
| Pseudo-F statistic | Continuous per SD decrease (−512.9) Quintiles |
85 (2.83) | 1.13 (0.88, 1.44) | 110 (3.67) | 1.19 (0.96, 1.48) | 256 (8.53) | 1.13 (0.98, 1.29) |
| Q1:<640.319 | 17 (2.83) | 1.60 (0.72, 3.55) | 22 (3.67) | 1.64 (0.80, 3.36) | 51 (19.92) | 1.32 (0.86, 2.04) | |
| Q2:640.319 to <853.460 | 14 (2.33) | 1.30 (0.57, 2.94) | 23 (3.83) | 1.72 (0.85, 3.48) | 46 (17.97) | 1.19 (0.76, 1.85) | |
| Q3:853.460 to <1092.75 | 22 (3.67) | 1.94 (0.92, 4.12) | 28 (4.67) | 2.20 (1.12, 4.34) | 65 (25.39) | 1.69 (1.12, 2.55) | |
| Q4:1092.75 to <1415.08 | 22 (3.67) | 1.95 (0.92, 4.14) | 25 (4.17) | 1.91 (0.96, 3.81) | 58 (22.66) | 1.49 (0.98, 2.27) | |
| Q5:≥1415.08 | 10 (1.66) | reference | 12 (2.00) | reference | 36 (14.06) | reference | |
| P-trend | 0.73 | 0.40 | 0.63 | ||||
HR, hazard ratio; CI, confidence interval; SD standard deviation.
Major Osteoporotic Fracture includes hip, wrist, shoulder, and clinical spine fracture.
Discussion
Consistent with previous work that has suggested a deleterious relationship between later acrophase (later timing of peak circadian activity) and health outcomes [16,17,19,18,37], in this cohort of older men, we found a significant association between later acrophase and a modestly greater risk of falls, independent of traditional risk factors. Associations between low pseudo F-statistic (greater disorganization of activity) and fall risk approached significance. RAR parameters were not associated with fracture risk after adjusting for multiple confounders. These results suggest that some dysregulated circadian patterns may have influenced the risk of falls in healthy elderly men, but this association did not translate into a higher fracture risk in this cohort.
Associations between sleep parameters and fall risk and fracture risk have been reported in previous studies. Self-reported napping and sleeping more than 10 hours per day were associated with higher risk of falls and fractures in a cohort of elderly women [10], and self-reported night shift work, which can lead disrupted sleep and circadian rhythms, was associated with increased risk of hip and wrist fractures among women enrolled in the Nurse’s Health Study [38]. Differences in gender and method of sleep/24 hour rhythm activity assessment may partially explain the differing results from the present study compared to these previous studies [10,38]. Gender differences in the effects of sleep deprivation on bone mineral density (BMD) have been previously reported [39], and subjective methods such as self-report rely on subjects’ recall and are considered less reliable than objective methods such as actigraphy [15].
In the MrOS cohort, Stone and colleagues employed a variety of sleep measures including subjective (Epworth Sleepiness Scale, Pittsburgh Sleep Quality Index, self-reported total sleep time), actigraphic total sleep time and sleep efficiency, and sleep disordered breathing measured by polysomnography (PSG). Findings included an increased risk of falls for men with excessive daytime sleepiness, less than five hours of sleep per night, poor sleep efficiency or nocturnal hypoxemia [11]. Cauley and colleagues used exposure variables related to sleep disordered breathing/oxygenation and found associations with fall risk and non-spine fracture risk in MrOS participants [40]. Given these previous associations between disordered sleep and falls and fractures in the MrOS cohort [11,40], expanding this research to circadian activity rhythms was a logical next step [14]. This study is the first to report associations of RAR patterns and falls and incident fractures in elderly men.
However, the short-term nature of RAR data may have been insufficient to correspond to a fracture, which is a long-term measure of bone health. To more precisely examine the effects of circadian rhythm disturbance on bone metabolism, biomarkers of bone turnover could be utilized as an intermediate outcome measure in future studies. Bone turnover biomarkers are associated with changes in bone mass over time [41] and are also known to exhibit diurnal variation [42]. Although we found no independent associations between RAR and fracture risk, it is conceivable that RAR patterns may be related to bone turnover changes, and over time, bone turnover changes may result in bone loss and resultant fracture. Additionally, since weaker RAR patterns have been associated with poorer executive function [37], dementia and cognitive impairment [18] in women, the association with falls found among MrOS participants could also suggest an effect of RAR on neuromuscular function, leading to subsequent falls. Thus, RAR may have a completely different role in osteoporosis that is not along the fracture continuum. Additional research is needed.
This study has a number of strengths, including the large sample size, nearly complete follow-up on all surviving cohort participants, use of validated outcome measures, and study participants were enrolled from the community and were not selected on the basis of a sleep complaints or fracture risk. Results were adjusted for multiple potential confounding factors, so it is likely that associations were not explained by depression, comorbidities, medication use, or lifestyle factors. However, there were also a number of shortcomings in this study. First, we performed statistical adjustment for numerous covariates, but it is possible that unmeasured confounders may have affected the results. Also, study participants were predominately a population of relatively healthy white men, so further studies are needed to confirm these results in other populations, such as women and minorities. Additionally, RAR as measured by wrist actigraphy may be subject to masking, and RAR may not be as stable as melatonin or temperature rhythms as markers of suprachiasmatic nucleus (SCN) circadian output [15]. Lastly, the present analysis subset was limited to men with three or more continuous days of actigraphy recordings, and the duration of the actigraphy recordings was on average five 24-hr periods. The three day restriction was used because it is the minimum current requirement for actigraphy monitoring for the Centers for Medicare Services (CMS) (Current Procedural Technology (CPT® code 95803) [23].
Conclusions
In partial support of our hypothesis, later acrophase (later timing of peak circadian activity) was independently associated with risk of falls in older men. However, later acrophase and other disordered circadian activity patterns were not significantly associated with higher fracture risk after multivariate adjustment. Associations between circadian activity patterns and osteoporosis risk warrant further investigation.
Statement of Human Rights.
Ethical approval: All procedures performed in the MrOS study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the Institutional Review Board at each clinic site, and all participants provided written informed consent. For this type of retrospective analysis, additional formal consent was not required.
Acknowledgments
Funding:
The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health (NIH) funding. The following institutes provide support: the National Institute on Aging (NIA), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Center for Advancing Translational Sciences (NCATS), and NIH Roadmap for Medical Research under the following grant numbers: U01 AG027810, U01 AG042124, U01 AG042139, U01 AG042140, U01 AG042143, U01 AG042145, U01 AG042168, U01 AR066160, and UL1 TR000128.
The National Heart, Lung, and Blood Institute (NHLBI) provides funding for the MrOS Sleep ancillary study “Outcomes of Sleep Disorders in Older Men” under the following grant numbers: R01 HL071194, R01 HL070848, R01 HL070847, R01 HL070842, R01 HL070841, R01 HL070837, R01 HL070838, and R01 HL070839.
Additional funding was provided by NIH/NIAMS grant P50 AR063043.
The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Institute on Aging (NIA), the National Center for Research Resources (NCRR), and NIH Roadmap for Medical Research under the following grant numbers: U01 AR45580, U01 AR45614, U01 AR45632, AR45647, AR45654, AR45583, AG18197, AG027810, and TR000128. The National Heart, Lung, and Blood Institute (NHLBI) provides funding for the MrOS Sleep ancillary study “Outcomes of Sleep Disorders in Older Men” under the following grant numbers: HL071194, HL070848, HL070847, HL070842, HL070841, HL070837, HL070838, and R01 HL070839. Additional funding was provided by NIH/NIAMS grant P50 AR063043.
Footnotes
No supplemental data have been included in this submission.
Disclosures
Drs. Rogers, Lane, Tranah, Orwoll, Cauley, Stone, Cummings, Cawthon and Ms. Blackwell have no conflicts of interest to disclose.
Dr. Ancoli-Israel consults for Marck, Easai, Jansen, Pfizer.
Authors’ roles:
Study concept and design: SAI, ESO, JAC, KLS, SRC, PMC
Data collection: ESO, JAC, KLS, SRC
Data analysis and interpretation: TLB, PMC, TSR
Drafting manuscript: TSR, TLB, PMC, NEL, SAI
Critical review and final approval of manuscript content: TLB, NEL, GJT, ESO, JAC, SAI, KLS, SRC, PMC
Statistical Analysis: Ms. Terri Blackwell performed the statistical analyses and is independent of any commercial funder. She had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analyses.
References
- 1.O’Loughlin JL, Robitaille Y, Boivin JF, Suissa S. Incidence of and risk factors for falls and injurious falls among the community-dwelling elderly. Am J Epidemiol. 1993;137(3):342–354. doi: 10.1093/oxfordjournals.aje.a116681. [DOI] [PubMed] [Google Scholar]
- 2.Nevitt MC, Cummings SR, Kidd S, Black D. Risk factors for recurrent nonsyncopal falls. A prospective study. JAMA. 1989;261(18):2663–2668. [PubMed] [Google Scholar]
- 3.Nevitt MC, Cummings SR, Hudes ES. Risk factors for injurious falls: a prospective study. J Gerontol. 1991;46(5):M164–170. doi: 10.1093/geronj/46.5.m164. [DOI] [PubMed] [Google Scholar]
- 4.Rizzo JA, Friedkin R, Williams CS, Nabors J, Acampora D, Tinetti ME. Health care utilization and costs in a Medicare population by fall status. Med Care. 1998;36(8):1174–1188. doi: 10.1097/00005650-199808000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Peters BS, Martini LA. Nutritional aspects of the prevention and treatment of osteoporosis. Arq Bras Endocrinol Metabol. 2010;54(2):179–185. doi: 10.1590/s0004-27302010000200014. [DOI] [PubMed] [Google Scholar]
- 6.Moyer VA. Vitamin D and calcium supplementation to prevent fractures in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;158(9):691–696. doi: 10.7326/0003-4819-158-9-201305070-00603. [DOI] [PubMed] [Google Scholar]
- 7.Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, Lindsay R. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporos Int. 2014;25(10):2359–2381. doi: 10.1007/s00198-014-2794-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Albayrak I, Aydogmus M, Ozerbil OM, Levendoglu F. The association between bone mineral density, quality of life, quality of sleep and fatigue. Acta Clin Belg. 2016:1–7. doi: 10.1179/2295333715y.0000000061. [DOI] [PubMed] [Google Scholar]
- 9.Cunningham TD, Di Pace BS. Is Self-Reported Sleep Duration Associated with Osteoporosis? Data from a 4-Year Aggregated Analysis from the National Health and Nutrition Examination Survey. J Am Geriatr Soc. 2015;63(7):1401–1406. doi: 10.1111/jgs.13477. [DOI] [PubMed] [Google Scholar]
- 10.Stone KL, Ewing SK, Lui LY, Ensrud KE, Ancoli-Israel S, Bauer DC, Cauley JA, Hillier TA, Cummings SR. Self-reported sleep and nap habits and risk of falls and fractures in older women: the study of osteoporotic fractures. J Am Geriatr Soc. 2006;54(8):1177–1183. doi: 10.1111/j.1532-5415.2006.00818.x. [DOI] [PubMed] [Google Scholar]
- 11.Stone KL, Blackwell TL, Ancoli-Israel S, Cauley JA, Redline S, Marshall LM, Ensrud KE. Sleep disturbances and risk of falls in older community-dwelling men: the outcomes of Sleep Disorders in Older Men (MrOS Sleep) Study. J Am Geriatr Soc. 2014;62(2):299–305. doi: 10.1111/jgs.12649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brassington GS, King AC, Bliwise DL. Sleep problems as a risk factor for falls in a sample of community-dwelling adults aged 64–99 years. J Am Geriatr Soc. 2000;48(10):1234–1240. doi: 10.1111/j.1532-5415.2000.tb02596.x. [DOI] [PubMed] [Google Scholar]
- 13.Dudek M, Meng QJ. Running on time: the role of circadian clocks in the musculoskeletal system. Biochem J. 2014;463(1):1–8. doi: 10.1042/bj20140700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swanson CM, Shea SA, Stone KL, Cauley JA, Rosen CJ, Redline S, Karsenty G, Orwoll ES. Obstructive sleep apnea and metabolic bone disease: insights into the relationship between bone and sleep. J Bone Miner Res. 2015;30(2):199–211. doi: 10.1002/jbmr.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26(3):342–392. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 16.Paudel ML, Taylor BC, Ancoli-Israel S, Blackwell T, Stone KL, Tranah G, Redline S, Cummings SR, Ensrud KE. Rest/activity rhythms and mortality rates in older men: MrOS Sleep Study. Chronobiol Int. 2010;27(2):363–377. doi: 10.3109/07420520903419157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tranah GJ, Blackwell T, Ancoli-Israel S, Paudel ML, Ensrud KE, Cauley JA, Redline S, Hillier TA, Cummings SR, Stone KL. Circadian activity rhythms and mortality: the study of osteoporotic fractures. J Am Geriatr Soc. 2010;58(2):282–291. doi: 10.1111/j.1532-5415.2009.02674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tranah GJ, Blackwell T, Stone KL, Ancoli-Israel S, Paudel ML, Ensrud KE, Cauley JA, Redline S, Hillier TA, Cummings SR, Yaffe K. Circadian activity rhythms and risk of incident dementia and mild cognitive impairment in older women. Ann Neurol. 2011;70(5):722–732. doi: 10.1002/ana.22468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paudel ML, Taylor BC, Ancoli-Israel S, Stone KL, Tranah G, Redline S, Barrett-Connor E, Stefanick ML, Ensrud KE. Rest/activity rhythms and cardiovascular disease in older men. Chronobiol Int. 2011;28(3):258–266. doi: 10.3109/07420528.2011.553016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson KN, Catt M, Collerton J, Davies K, von Zglinicki T, Kirkwood TB, Jagger C. Assessment of sleep and circadian rhythm disorders in the very old: the Newcastle 85+ Cohort Study. Age Ageing. 2014;43(1):57–63. doi: 10.1093/ageing/aft153. [DOI] [PubMed] [Google Scholar]
- 21.Orwoll E, Blank JB, Barrett-Connor E, Cauley J, Cummings S, Ensrud K, Lewis C, Cawthon PM, Marcus R, Marshall LM, McGowan J, Phipps K, Sherman S, Stefanick ML, Stone K. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study--a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26(5):569–585. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 22.Blank JB, Cawthon PM, Carrion-Petersen ML, Harper L, Johnson JP, Mitson E, Delay RR. Overview of recruitment for the osteoporotic fractures in men study (MrOS) Contemp Clin Trials. 2005;26(5):557–568. doi: 10.1016/j.cct.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Coding FAQ. [Accessed 3 October 2016];American Academy of Sleep Medicine. 2014 http://www.aasmnet.org/codingfaq.Aspx.
- 24.Motionlogger User’s Guide: Act Millenium. Ambulatory Monitoring, Inc; Ardsley, NY: [Google Scholar]
- 25.Blackwell T, Ancoli-Israel S, Redline S, Stone KL. Factors that may influence the classification of sleep-wake by wrist actigraphy: the MrOS Sleep Study. J Clin Sleep Med. 2011;7(4):357–367. doi: 10.5664/jcsm.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marler MR, Gehrman P, Martin JL, Ancoli-Israel S. The sigmoidally transformed cosine curve: a mathematical model for circadian rhythms with symmetric non-sinusoidal shapes. Stat Med. 2006;25(22):3893–3904. doi: 10.1002/sim.2466. [DOI] [PubMed] [Google Scholar]
- 27.Yesavage JA, Sheikh JI. 9/Geriatric Depression Scale (GDS) Clin Gerontol. 1986;5(1–2):165–173. doi: 10.1300/J018v05n01_09. [DOI] [Google Scholar]
- 28.Almeida OP, Almeida SA. Short versions of the geriatric depression scale: a study of their validity for the diagnosis of a major depressive episode according to ICD-10 and DSM-IV. Int J Geriatr Psychiatry. 1999;14(10):858–865. doi: 10.1002/(sici)1099-1166(199910)14:10<858::aid-gps35>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 29.Fitti JE, Kovar MG. The Supplement on Aging to the 1984 National Health Interview Survey. Vital Health Stat. 1987;1(21):1–115. [PubMed] [Google Scholar]
- 30.Pincus T, Summey JA, Soraci SA, Jr, Wallston KA, Hummon NP. Assessment of patient satisfaction in activities of daily living using a modified Stanford Health Assessment Questionnaire. Arthritis Rheum. 1983;26(11):1346–1353. doi: 10.1002/art.1780261107. [DOI] [PubMed] [Google Scholar]
- 31.Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, Carbonin P. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol. 1994;10(4):405–411. doi: 10.1007/BF01719664. [DOI] [PubMed] [Google Scholar]
- 32.Barone JJ, Roberts HR. Caffeine consumption. Food Chem Toxicol. 1996;34(1):119–129. doi: 10.1016/0278-6915(95)00093-3. [DOI] [PubMed] [Google Scholar]
- 33.Looker AC, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP, Johnston CC, Jr, Lindsay R. Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int. 1998;8(5):468–489. doi: 10.1007/s001980050093. [DOI] [PubMed] [Google Scholar]
- 34.Kanis JA, Adachi JD, Cooper C, Clark P, Cummings SR, Diaz-Curiel M, Harvey N, Hiligsmann M, Papaioannou A, Pierroz DD, Silverman SL, Szulc P. Standardising the descriptive epidemiology of osteoporosis: recommendations from the Epidemiology and Quality of Life Working Group of IOF. Osteoporos Int. 2013;24(11):2763–2764. doi: 10.1007/s00198-013-2413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Asplund R. Nocturia in relation to sleep, health, and medical treatment in the elderly. BJU Int. 2005;96(Suppl 1):15–21. doi: 10.1111/j.1464-410X.2005.05653.x. [DOI] [PubMed] [Google Scholar]
- 36.Endeshaw Y. Correlates of self-reported nocturia among community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2009;64(1):142–148. doi: 10.1093/gerona/gln009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walsh CM, Blackwell T, Tranah GJ, Stone KL, Ancoli-Israel S, Redline S, Paudel M, Kramer JH, Yaffe K. Weaker circadian activity rhythms are associated with poorer executive function in older women. Sleep. 2014;37(12):2009–2016. doi: 10.5665/sleep.4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feskanich D, Hankinson SE, Schernhammer ES. Nightshift work and fracture risk: the Nurses’ Health Study. Osteoporos Int. 2009;20(4):537–542. doi: 10.1007/s00198-008-0729-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Specker BL, Binkley T, Vukovich M, Beare T. Volumetric bone mineral density and bone size in sleep-deprived individuals. Osteoporos Int. 2007;18(1):93–99. doi: 10.1007/s00198-006-0207-x. [DOI] [PubMed] [Google Scholar]
- 40.Cauley JA, Blackwell TL, Redline S, Ensrud KE, Ancoli-Israel S, Fink HA, Orwoll ES, Stone KL. Hypoxia during sleep and the risk of falls and fractures in older men: the Osteoporotic Fractures in Men Sleep Study. J Am Geriatr Soc. 2014;62(10):1853–1859. doi: 10.1111/jgs.13069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Civitelli R, Armamento-Villareal R, Napoli N. Bone turnover markers: understanding their value in clinical trials and clinical practice. Osteoporos Int. 2009;20(6):843–851. doi: 10.1007/s00198-009-0838-9. [DOI] [PubMed] [Google Scholar]
- 42.Qvist P, Christgau S, Pedersen BJ, Schlemmer A, Christiansen C. Circadian variation in the serum concentration of C-terminal telopeptide of type I collagen (serum CTx): effects of gender, age, menopausal status, posture, daylight, serum cortisol, and fasting. Bone. 2002;31(1):57–61. doi: 10.1016/s8756-3282(02)00791-3. [DOI] [PubMed] [Google Scholar]