Abstract
Intravenous transfer of LPS-treated bone marrow-derived dendritic cells blocks development of autoimmunity induced by CD4+ T cells in vivo. However, cellular mechanisms of dendritic cell-mediated immune tolerance have not yet been fully elucidated. Here, we report that there are two new subpopulations of CD4+CD25+FoxP3+GITR+ regulatory T cells (CD127+3G11+ and CD127+3G11− cells). LPS-treated dendritic cells facilitate development of CD4+CD127+3G11− regulatory T cells but inhibit that of CD4+CD127+3G11+ regulatory T cells. LPS-induced tolerogenic dendritic cells may cause immune tolerance through modulating balance of different subsets of CD4+ regulatory T cells mediated by CD127 and 3G11. Our results imply a new potential cellular mechanism of dendritic cell-mediated immune tolerance.
Keywords: Dendritic cell, EAE, Immune tolerance, CD4+T cell
Introduction
Dendritic cells (DCs) play a central role in regulating autoimmunity and tolerance. However, regulatory mechanisms of autoimmunity and immune tolerance mediated by DCs have not been fully elucidated [1, 2]. Our previous results indicated that intravenous (i.v.) transfer of LPS-treated DCs (1 µg/ml) can cause immune tolerance in C57 BL/6J mice with experimental autoimmune encephalomyelitis (EAE), an inflammatory disease in central nervous system, through blocking development of T helper 17 (Th17) cells [3]. Treatment of LPS leads to apoptosis of DCs [3]. Living DCs engulf apoptotic cells and cause generation of tolerogenic DCs [4]. LPS-induced tolerogenic DCs inhibit development of EAE in vivo through suppressing activity of CD4+ T cells such as Th17 cells [3]. Further mechanisms are still unknown. We propose that LPS-induced tolerogenic DCs not only inhibit development of Th17 cells but may also affect that of other typical CD4+ T cells such as regulatory T cells. Therefore, we investigated whether or not LPS-treated DCs also modulate development and differentiation of CD4+ regulatory T cells in this project. Our results will show that LPS-treated DCs induce immune tolerance through modulating differentiation of CD4+ regulatory T cell subpopulations.
CD4+ regulatory T cells (Tregs) are important immune cells in vivo [5, 6]. Tregs were found in 1990s. Immune function of Tregs has been deeply investigated in the past 20 years [7]. Phenotype of conventional Tregs is CD4+CD25+FoxP3+ cells [7–12]. CD25 is α-chain of interleukin (IL)-2 receptor, and forkhead/winged helix transcription factor (FoxP3) is a transcriptional factor [7]. Multiple Treg-associated molecules have been found since 1990s. For instance, glucocorticoid-induced tumor necrosis factor receptor (GITR) is also a molecular marker expressed on Tregs [13]. 3G11, a sialylated carbohydrate epitope of the disialoganglioside molecule, is associated with definition of specific Treg subsets [14, 15]; however, regulatory mechanisms of Treg development and differentiation are still obscure. Our research is focused on the role of dendritic cells on the development and differentiation of Tregs. Experimental data will show whether or not immature or mature bone marrow-derived DCs induced by LPS stimulation can affect development of Tregs through modulating expression of Treg-associated molecules such as CD25, CD127, FoxP3, GITR, and 3G11.
Recent research indicated that there are multiple subsets of Tregs in vivo [8–10]. For example, CD4+CD25+CD127low Tregs play a central role in peripheral tolerance [16, 17]. Ukena et al. reported that deficiency of CD4+CD25+CD127low Tregs leads to acute graft-versus-host disease (GVHD) [18]. Shenghui et al. found that increased frequencies of CD4+CD25+CD127low Tregs are associated with poor prognosis of acute myeloidleukemia (AML) [19]. At present, regulatory mechanisms of CD4+CD127low Treg development have not been fully elucidated. Our research is focused on whether or not immature and mature bone marrow-derived DCs can modulate development of CD4+CD127low Tregs. Experimental data demonstrate that mature DCs induced by LPS stimulation can regulate development and differentiation of CD4+CD127+ Treg subsets, compared with results of T cells co-cultured with immature DCs.
3G11 is a ganglioside which is a marker of murine CD4+ T cells. Immune function of 3G11 on CD4+ T cells is still unknown [20]. Zhang et al. reported that downregulation of 3G11 expression on CD4+ T cells is associated with T cell anergy and leads to immune tolerance in mouse with experimental autoimmune encephalomyelitis [15]. Zhao et al. found that 3G11 is probably a marker for definition of different CD4+ Treg subsets [14]. Our research is focused on how DCs regulate differentiation of Treg subpopulations mediated by 3G11. Experimental data show that there are two new subsets of CD4+CD25+FoxP3+GITR+ Tregs: CD127+3G11+ and CD127+3G11− Tregs. LPS-treated DCs facilitate development of CD127+3G11− Tregs, but inhibit that of CD127+3G11+ Tregs. LPS-induced tolerogenic DCs may lead to immune tolerance through modulating balance of different subsets of CD4+ Tregs mediated by 3G11 and CD127. Our data may reveal a new cellular mechanism of DC-mediated immune tolerance by regulating differentiation of CD4+CD127+3G11+/−Treg subsets.
Materials and methods
Mice
Wild-type C57 BL/6J female mice (8–12 weeks) were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). All mice were bred in Thomas Jefferson Animal Care facilities, and all experimental procedures were approved by the Institutional Animal Care and Use Committee of Thomas Jefferson University [4, 21].
Immunogen and peptide
Mouse MOG35–55 peptide (MEVGWYRSPFSRVVHLYRNGK), a part of myelin oligodendrocyte glycoprotein (MOG), was provided by Invitrogen (Invitrogen, Carlsbad, CA, USA) [3, 4, 20–23].
Bone marrow-derived DC culture
As described previously, femurs and tibiae were isolated from muscle tissue of mice. The intact bones were then sterilized with 70% ethanol for 5 min and washed with phosphate-buffered saline (PBS). Bone ends were cut, and the bone marrow was flushed with PBS. Cellular clusters within the bone marrow suspension were disintegrated and washed with PBS [3, 4, 21–23].
Leukocytes from bone marrow were fed in bacteriological 100-mm Petri dishes (Falcon, Becton Dickinson, Heidelberg, Germany) at 2 × 106 cells per dish. Cells were cultured in RPMI1640 complete medium (Gibco-BRL, Eggenstein, Germany) including penicillin (100 U/ml, Sigma, St. Louis, MO, USA), streptomycin (100 U/ml, Sigma), L-glutamine (2 mM, Sigma), 2-mercaptoethanol (2-ME, 50 µM, Sigma), 10% heat-inactivated and filtered (0.22 µm, Milipore, Inc., Bedford, MA, USA), fetal calf serum (FCS, Sigma), and granulocyte-macrophage colony-stimulating factor (GM-CSF, PeproTech, Rocky Hill, NJ, USA) at 20 ng/ml at day 0 (10 ml medium per dish).
Ten milliliters of fresh medium with GM-CSF (20 ng/ml) was added to each dish at day 3, and half of the medium (about 10 ml supernatant) was collected and centrifuged at 300g for 5 min at day 6. Subsequently, cells were re-suspended in 10 ml fresh medium with GM-CSF (20 ng/ml) and re-fed in original dish (day 6). Only unadherent cells (DCs) were harvested and seeded in a fresh dish, and 10-ml fresh medium including GM-CSF (20 ng/ml) was added at day 8.
Cells were also treated with lipopolysaccharide (LPS, Sigma) for 24 h at 1 µg/ml. LPS was isolated from Klebsiella pneumoniae. DCs or LPS-treated DCs were pulsed with MOG peptide for 30 min and then washed twice with PBS at 300g for 5 min before i.v. transfer to EAE mice. Fresh unadherent DCs were then collected and washed with PBS at 300g for 5 min and then conducted i.v. transfer to EAE mice. More than 90% of cells express DC marker CD11c.
Flow cytometry
MOG-primed T lymphocytes were isolated from EAE mice and incubated with anti-mouse Pacific blue-CD4, PE-Cy7-anti-mouse CD25, PerCp-Cy5.5-anti-mouse CD127, FITC-anti-mouse GITR, and allophycocyanin (APC)-anti-mouse 3G11 antibodies for 24 h at 4 °C. Cells were washed twice with 5% FCS in PBS at 300g for 5 min, fixed with 5% formalin in PBS at 4 °C for 24 h and then permeated for intracellular staining.
For intracellular staining, spleen cells were conducted surface staining shown as above. After cells were washed with permeabilization buffer (Biolegend) twice at 300g for 10 min, anti-mouse PE-FoxP3 antibody (Biolegend) was incubated with cells at 4 °C for 24 h. Cells were then washed with permeabilization buffer twice at 300g for 5 min, resuspended in 0.5 ml cell staining buffer (Biolegend), and tested in a FACSAria (BD Biosciences, San Jose, CA, USA). Data were analyzed using FlowJo software (Treestar, Ashland, OR, USA) [3, 4, 21–23].
Generation of effector T cells in vitro
C57 BL/6J mice were immunized with MOG35–55 peptide (Invitrogen) 200 µg, QuilA (Sigma) 20 µg, and keyhole limpet hemocyanin (KLH, Sigma) 20 µg per mouse at day 1. Spleen cells were then isolated at day 10 after immunization. T lymphocytes were purified with mouse CD4+ T cell subset column kit (R&D Systems). CD4+ T cells (1 × 106 cells/per well) were co-cultured with DCs at 5:1 (T cells: DCs) and pulsed with MOG35–55 peptide at 0.1 µM in complete medium with mouse IL-2 at 1 ng/ml for 3 days. Cells were harvested, and MOG-primed CD4+ T cells were gated and analyzed by flow cytometry [3, 4, 21–23].
EAE induction and treatment
C57BL/6J mice (female, 8–12 weeks) were immunized with MOG35–55 peptide/complete Freund’s adjuvant (CFA, Sigma) at 200 µg/200 µl/per mouse (subcutaneous injection (s.c.)). Pertussis toxin (PT, Sigma) was simultaneously injected at 200 ng/per mouse (intraperitoneal injection), and the second PT injection was conducted after 48 h. EAE was assessed following standard clinical scores: 0.5, paralysis of half the tail; 1, paralysis of whole tail; 2, paralysis of tail and one leg; 3, paralysis of tail and two legs; 4, moribund; and 5, death.
DCs were washed with PBS twice and were immediately injected via tail vein (3 × 105 cells/per mouse/per time) on days 11, 14, and 17 post-immunization (p.i.). Mice were divided into three groups: (1) injected with unpulsed DCs (DCs), (2) injected with DCs pulsed with MOG peptide (DCs-MOG), and (3) injected with LPS-treated DCs pulsed with MOG peptide (DCs-MOG+LPS).
At day 24 p.i., splenocytes were isolated and stimulated with MOG35–55 peptide (0.1 µM) and mouse IL-2 (1 ng/ml) for 3 days. Cells were then harvested for flow cytometry [3, 4, 21–23].
Statistical analysis
Experimental data were analyzed using Prism software (GraphPad, La Jolla, CA, USA). A two-way ANOVA test was performed for the analysis of clinical score of EAE; t tests were conducted for analysis of flow cytometry data. Data represent the mean and standard deviation (SD) or standard error of arithmetic mean (SEM). Results were regarded as showing a significant difference if the P value is less than 0.05 [3, 4, 21–23].
Results
LPS-treated DCs do not affect expression of Treg-associated molecules on CD4+ T cells in vitro
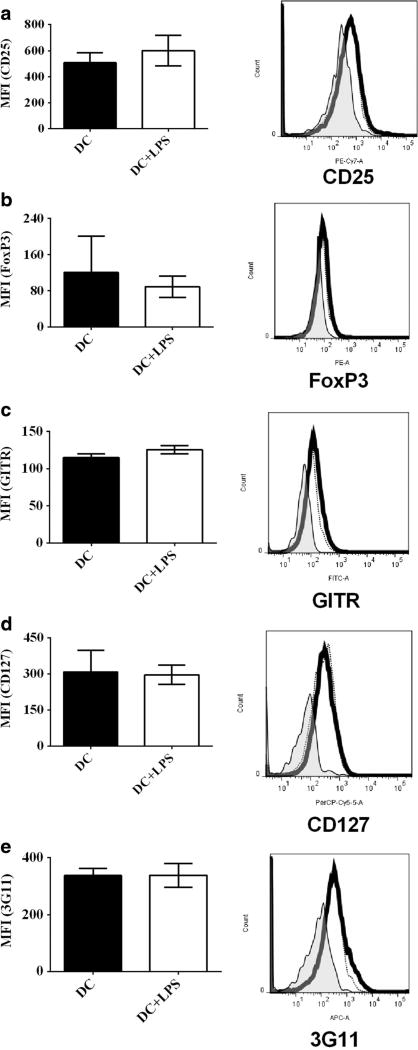
To test whether or not LPS-treated DCs can modulate protein expression of Treg-associated molecules on CD4+ T cells, DCs treated with LPS (DC+LPS) and LPS-untreated DCs (DC) were pulsed with MOG peptide (0.1 µM) and co-cultured with MOG-primed CD4+ T cells for 72 h at 37 °C. Protein expression of CD25 (Fig. 1a), FoxP3 (Fig. 1b), GITR (Fig. 1c), CD127 (Fig. 1d), and 3G11 (Fig. 1e) was detected using flow cytometry. Our results show that there is no difference in protein expression of CD25, FoxP3, GITR, CD127, and 3G11 on MOG-primed CD4+ T cells (Fig. 1a–e) after co-culture with DCs+LPS or DCs. It can be concluded that LPS treatment cannot affect protein expression of Treg-associated molecules on MOG-specific CD4+ T cells.
Fig. 1.
Protein expression of Treg-associated molecules on CD4+ T cells after co-culture with bone marrow-derived DCs treated with LPS or without LPS stimulation. Bone marrow-derived DCs were pulsed with MOG peptide (0.1 µM) and incubated with LPS (1 µg/ml) (thick line) or without LPS stimulation (thin line) as a control for 24 h at 37 °C. DCs incubated with isotype control antibody are negative control sample (shade). Cells were harvested and stained by anti-mouse CD4, CD25, CD127, GITR, FoxP3, and 3G11 antibodies. Expression of CD25 (a), FoxP3 (b), GITR (c), CD127 (d), and 3G11 (e) on CD4+ T cells was detected using flow cytometry. Error bars shown in this figure represent mean and SD of triplicate determinations of MFI of Treg-associated molecules on CD4+ T cells (*P < 0.05, n = 3, t test)
LPS-treated DCs modulate development and differentiation of CD4+CD127+3G11+ and CD4+CD127+3G11− regulatory T cell subpopulations in vitro
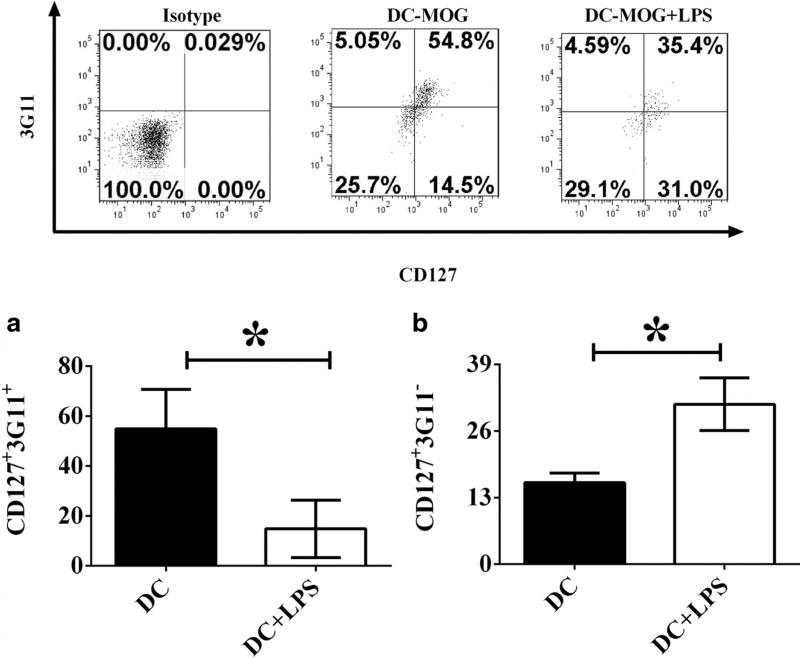
To investigate whether or not LPS-treated bone marrow-derived DCs can regulate development and differentiation of CD4+ regulatory T cell subpopulations, CD4+CD25+FoxP3+GITR+ (Tregs) cells shown in Fig. 1 as above were gated and frequencies of CD127+3G11+ and CD127+3G11− cells were shown (Fig. 2). Experimental data indicate that DCs-MOG+LPS upregulate frequency of CD127+3G11− regulatory T cells, but downregulate that of CD127+3G11+ regulatory T cells compared with that of regulatory T cells co-cultured with DCs-MOG (Fig. 2a, b). It implies that LPS-stimulated DCs may modulate development of different regulatory T cell subsets mediated by CD127 and 3G11.
Fig. 2.
LPS-treated DCs modulate development and differentiation of CD4+CD127+3G11+ and CD4+CD127+3G11− regulatory T cells. A flow cytometry assay was carried out. CD4+CD25+FoxP3+GITR+ Tregs were gated. Frequencies of CD127+3G11+ (a) and CD127+3G11− (b) regulatory T cells are indicated. Error bars demonstrated in this figure represent mean and SD of triplicate determinations of frequencies of CD127+3G11+ (a) and CD127+3G11− (b) regulatory T cells (*P < 0.05, n = 3, t test)
Intravenous transfer LPS-treated DCs blocks development of EAE in vivo
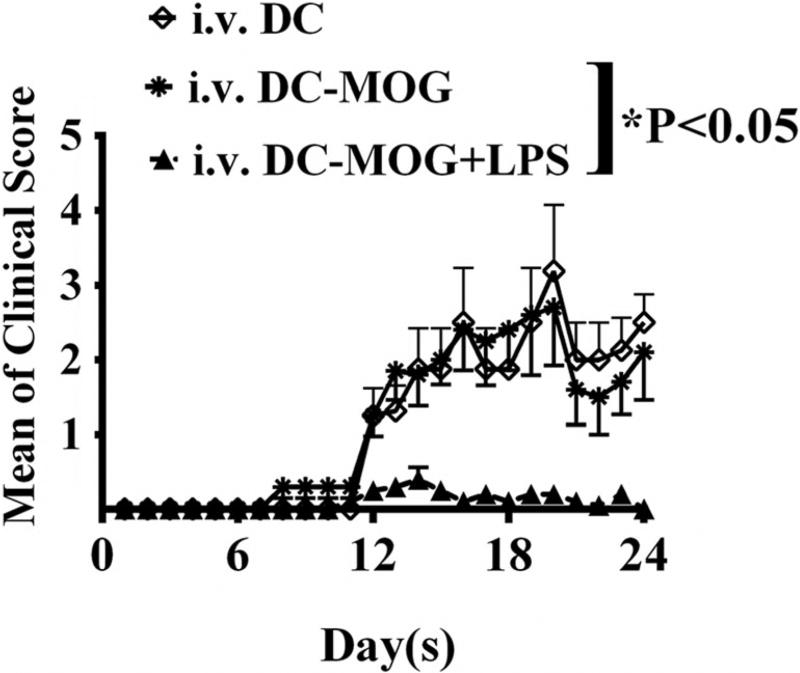
To investigate whether or not i.v. transfer of LPS-treated DCs can affect development of autoimmunity-mediated by CD4+ T cells in vivo, bone marrow-derived DCs treated with LPS or without LPS stimulation were pulsed with MOG peptide (0.1 µM) or without loading MOG peptide as a control. Cells were i.v. transferred into C57/BL6J mice immunized with MOG peptide/CFA (Fig. 3). Clinical scores of EAE development were recorded. Our results demonstrated that DCs-MOG+LPS significantly inhibit development of EAE in vivo compared with EAE mice by which were i.v. transferred DCs-MOG (Fig. 3). By contrast, i.v. transfer of DCs-MOG does not significantly affect development of EAE compared with mice with EAE in control group by which were i.v. transferred DCs (Fig. 3). It suggests that DCs-MOG+LPS inhibit CD4+ T cell-mediated immune responses in mice with EAE.
Fig. 3.
Intravenous transfer of DCs-MOG+LPS blocks development of EAE in vivo. LPS-treated DCs and LPS-untreated DCs were i.v. transferred into mice immunized with MOG peptide/CFA at days 11, 14, and 17 (3 × 105 cells/per mouse/per time). Clinical scores of EAE are shown. Error bars indicated in this figure represent mean and SEM of clinical scores of EAE (two-way-ANOVA test, n = 10, *P < 0.05)
LPS-stimulated DCs do not affect expression of Treg-associated molecules on CD4+ T cells ex vivo
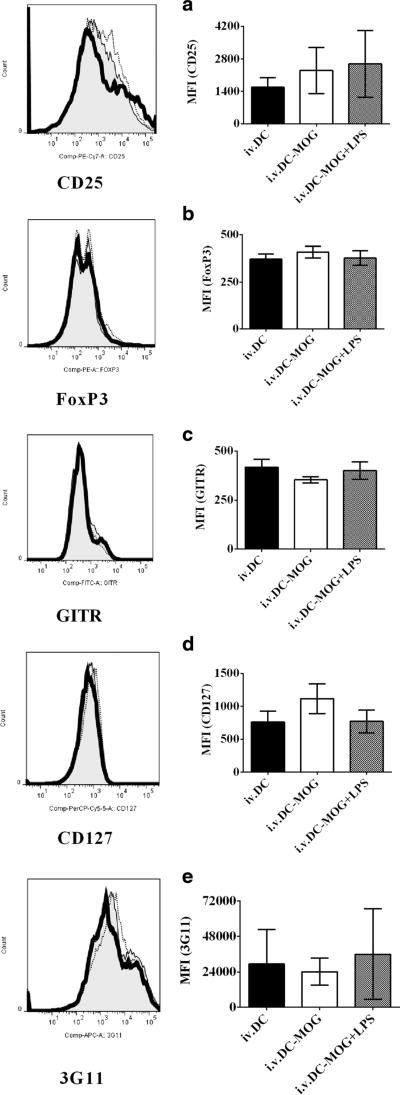
To test whether or not LPS-treated DCs can modulate expression of Treg-associated molecules on CD4+ T cells in vivo, cells isolated from mice in Fig. 3 were collected and stained by anti-mouse antibodies to target Treg-associated molecules. A flow cytometry assay was then conducted. Our results indicate that either i.v. transfer of DCs-MOG+LPS or DCs-MOG does not affect expression of Treg-associated molecules including CD25, FoxP3, GITR, CD127, and 3G11 on CD4+ T cells compared with them onCD4+ T cells isolated from mice by which were i.v. transferred DCs as a control ex vivo (Fig. 4a–e). It can be concluded that LPS-induced mature DCs cannot modulate Treg-associated molecules on CD4+ T cells ex vivo.
Fig. 4.
LPS-treated DCs do not affect expression of Treg-associated molecules on CD4+ T cells ex vivo. Splenocytes were isolated from mice treated with i.v. transfer of LPS-stimulated DCs loaded with MOG peptide (thick line) or DCs pulsed with MOG peptide without LPS stimulation (thin line) and DCs without incubation with MOG peptide and LPS as a control (shade) shown in Fig. 3. Cells were harvested and incubated with anti-mouse CD4, CD25, CD127, FoxP3, GITR, and 3G11 antibodies. CD4+ T cells were gated. Expression of CD25 (a), FoxP3 (b), GITR (c), CD127 (d), and 3G11 (e) on CD4+ T cells was detected using flow cytometry. Error bars demonstrated in this figure represent mean and SD of triplicate determinations of MFI of Treg-associated molecule expression in three independent experiments (*P < 0.05, n = 3, t test)
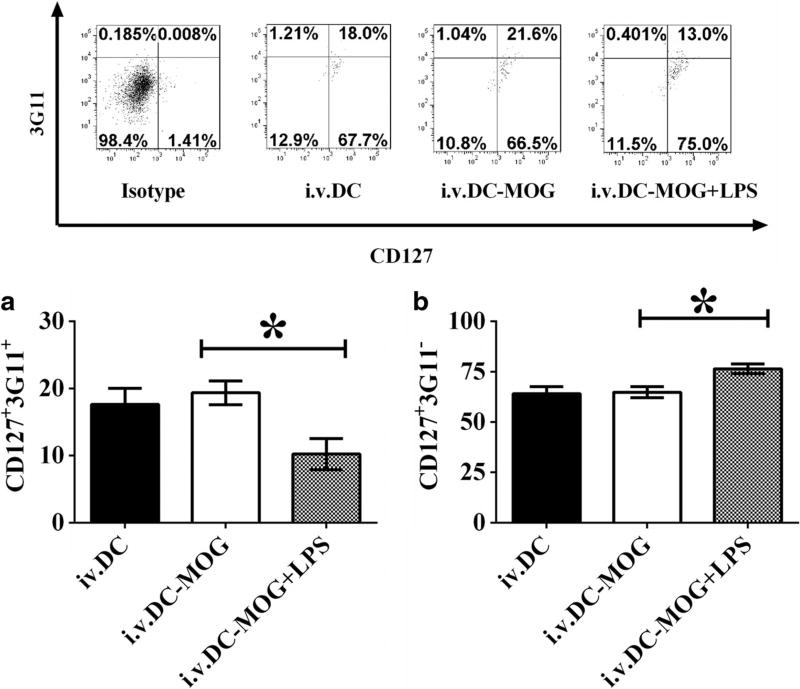
LPS-treated DCs modulate development of Treg subpopulations mediated by 3G11 and CD127 ex vivo
To investigate whether or not LPS-treated DCs can also regulate development and differentiation of Treg subpopulations in vivo, cells isolated from mice in Fig. 3 were stained anti-mouse CD4, CD25, CD127, 3G11, FoxP3, and GITR antibodies, and a flow cytometry assay was conducted. Experimental data demonstrate that i.v. transfer of DCs-MOG+LPS leads to upregulation of frequency of CD4+CD127+3G11− Tregs, but downregulation of percentage of CD4+CD127+3G11+ Tregs compared with that of Tregs isolated from mice treated with DCs-MOG. By contrast, DCs-MOG cannot modulate frequencies of Treg populations mediated by CD127 and 3G11 compared with that of Tregs isolated from mice treated with DCs as a control (Fig. 5). It can be concluded that LPS-stimulated bone marrow-derived DCs not only regulate development and differentiation of Treg subpopulations in vitro but also modulate those of Treg subpopulation mediated by CD127 and 3G11 ex vivo. It implies that LPS-treated DCs may inhibit autoimmunity through modulation development of Treg subsets mediated by CD127 and 3G11 in vivo.
Fig. 5.
LPS-treated DCs modulate development and differentiation of Treg subpopulations mediated by CD127 and 3G11. Splenocytes were isolated from mice which were i.v. transferred into LPS-treated DCs-MOG+LPS or DCs-MOG and DCs without incubation with LPS and MOG peptide shown in Fig. 3. Cells were re-stimulated with mouse IL-2 (1 ng/ml) and MOG peptide (0.1 µM) for 72 h at 37 °C. T lymphocytes were stained by anti-mouse CD4, CD25, CD127, GITR, FoxP3, and 3G11 antibodies. CD4+CD25+FoxP3+GITR+ Tregs were gated. Protein expression of 3G11 and CD127 on CD4+CD25+FoxP3+GITR+ Tregs was detected using flow cytometry. Error bars indicated in this figure represent mean and SD of triplicate determinations of frequencies of CD127+3G11+ (a) and CD127+3G11− (b) regulatory T cell subpopulations in three independent experiments (*P < 0.05, n = 3, t test)
Discussion
CD4+ regulatory T cells play an important role in induction of immune tolerance [24–27]. Multiple specific markers of Tregs have been found after Tregs were reported in 1990s [24–27]. It has been known that phenotype of conventional Tregs is CD4+CD25+FoxP3+ T cells [11, 28]. Moreover, GITR is also expressed on surface of typical CD4+ Tregs [13]. In addition, 3G11 is a marker for specific subsets of CD4+ Tregs [14, 15, 20, 29]. It is unclear whether or not DCs regulate development of Tregs through modulating expression of Treg-associated molecules. There is no difference in expression of CD25, CD127, FoxP3, GITR, and 3G11 after co-culture with immature or mature DCs stimulated by LPS. It suggests that DCs do not affect development of Tregs via regulating expression of Treg-associated molecules on CD4+ T cells. DCs may affect development of Tregs through other unknown pathways.
Multiple subpopulations of CD4+ Tregs were reported [30, 31]. For example, CD4+ CD25+CD127low Tregs are necessary for blocking autoimmunity in vivo [32, 33]. Experimental data of Bao et al. demonstrated that frequency of CD4+CD25+CD127low Tregs is decreased in patients with unexplained recurrent spontaneous miscarriage (URSM) [34]. CD127low Tregs may inhibit proliferation of effector T cells and block development of URSM induced by autoimmune T cells. Ukena etal. found that numbers of CD4+CD25+CD127low Tregs in healthy people are always more than those in patients with acute and chronic GVHD [18]. It suggests that deficiency of CD4+CD25+CD127low Tregs may be one of the reasons to induce GVHD in vivo. However, regulatory mechanisms of development and differentiation of Tregs are still obscure. Therefore, we focus on the role of immature and mature DCs on development of Treg subsets.
Our previous results indicated that LPS-treated bone marrow-derived DCs inhibit development of autoimmunity in vivo through blocking development of Th17 cells [3]. We propose that LPS-treated DCs may not only affect differentiation of Th17 cells but also regulate other CD4+ T cell subsets such as Tregs.
3G11 is a sialylated carbohydrate epitope of the disialoganglioside molecule and expressed on murine CD4+ T cells. Zhang et al. reported that deficiency of 3G11 induces T cell anergy. CD4+3G11− T cells indicate property of Tregs since they inhibit Th1 activation but CD4+3G11+ T cells still keep autoantigen-induced proliferation and IL-2 production [15]. Zhao et al. found that CD25+3G11− Tregs inhibit antigen-specific immune responses and EAE in vivo [14]. Intravenous transfer of LPS-treated DCs blocks development of EAE. Simultaneously, LPS-treated DCs upregulate frequency of CD127+3G11− but downregulate that of CD127+3G11+ Tregs. Since CD127+3G11− and CD127+3G11+ cells are two new subsets of conventional CD4+CD25+FoxP3+GITR+ Tregs, details of their immune functions are still unknown. Since data of Zhang et al. indicate that CD4+3G11− Tregs can lead to T cell anergy [15], CD4+CD127+3G11− Tregs may lead to Th17 cell anergy. LPS-treated DCs may block Th17 activity through facilitation of CD127+3G11− Tregs and induce Th17 anergy in vivo; however, this hypothesis has not yet been tested and we will investigate it in the future.
In summary, our data show that LPS-treated DCs can modulate development of CD4+CD127+3G11− and CD4+CD127+3G11+ Tregs in vitro and in vivo. Our results may reveal a new cellular mechanism of LPS-treated DC-mediated immune tolerance and suggest a potential possibility of immunotherapy using CD4+CD127+3G11− Tregs to target autoimmune diseases such as multiple sclerosis (MS).
Acknowledgments
We appreciate Mrs. Katherine Regan who carried out proofreading for this manuscript.
Abbreviations
- APC
Allophycocyanin
- CD
Cluster of differentiation
- CFA
Complete Freund’s adjuvant
- DC
Dendritic cell
- EAE
Experimental autoimmune encephalomyelitis
- FCS
Fetal calf serum
- FITC
Fluorescein isothiocyanate
- FoxP3
Forkhead/winged helix transcription factor
- GITR
Glucocorticoid-induced tumor necrosis factor receptor
- IL
Interleukin
- IFN-γ
Interferon gamma
- i.p.
Intraperitoneal
- i.v.
Intravenous
- KLH
Keyhole limpet hemocyanin
- LPS
Lipopolysaccharide
- MOG
Myelin oligodendrocyte glycoprotein
- 2-ME
2-Mercaptoethanol
- MS
Multiple sclerosis
- PBS
Phosphate-buffered saline
- pDCs
Plasmacytoid dendritic cells
- PE
Phycoerythrin
- PE-Cy7
Phycoerythrin-cyanine 7
- PerCP-Cy5.5
Peridinin-chlorophylla-protein–cyanine 5.5 complexes
- PT
Pertussis toxin
- s.c.
Subcutaneous
- SD
Standard deviation
- SEM
Standard error of arithmetic mean
- Th
T helper cells
- Treg
Regulatory T cell
Footnotes
Author contributions Dr. F. Zhou designed and conducted experiments. Dr. G.X. Zhang revised manuscript and Prof. A.M. Rostami supervised research.
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Baratin M, Foray C, Demaria O, Habbeddine M, Pollet E, Maurizio J, Verthuy C, Davanture S, Azukizawa H, Flores-Langarica A, Dalod M, Lawrence T. Homeostatic NF-kappaB signaling in steady-state migratory dendritic cells regulates immune homeostasis and tolerance. Immunity. 2015;42:627–39. doi: 10.1016/j.immuni.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Li W, Deng F, Wang Y, Ma C, Wang Y. Immune tolerance of mice allogenic tooth transplantation induced by immature dendritic cells. Int J Clin Exp Med. 2015;8:5254–62. [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou F, Ciric B, Zhang GX, Rostami A. Immunotherapy using lipopolysaccharide-stimulated bone marrow-derived dendritic cells to treat experimental autoimmune encephalomyelitis. Clin Exp Immunol. 2014;178:447–58. doi: 10.1111/cei.12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou F, Zhang GX, Rostami A. Apoptotic cell-treated dendritic cells induce immune tolerance by specifically inhibiting development of CD4(+) effector memory T cells. Immunol Res. 2016;64:73–81. doi: 10.1007/s12026-015-8676-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacobo P, Guazzone VA, Perez CV, Lustig L. CD4+ Foxp3+ regulatory T cells in autoimmune orchitis: phenotypic and functional characterization. Am J Reprod Immunol. 2015;73:109–25. doi: 10.1111/aji.12312. [DOI] [PubMed] [Google Scholar]
- 6.Laidlaw BJ, Cui W, Amezquita RA, Gray SM, Guan T, Lu Y, Kobayashi Y, Flavell RA, Kleinstein SH, Craft J, Kaech SM. Production of IL-10 by CD4 regulatory T cells during the resolution of infection promotes the maturation of memory CD8 T cells. Nat Immunol. 2015;16(8):871–9. doi: 10.1038/ni.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Germain RN. Special regulatory T-cell review: a rose by any other name: from suppressor T cells to Tregs, approbation to unbridled enthusiasm. Immunology. 2008;123:20–7. doi: 10.1111/j.1365-2567.2007.02779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chevalier MF, Didier C, Petitjean G, Karmochkine M, Girard PM, Barre-Sinoussi F, Scott-Algara D, Weiss L. Phenotype alterations in regulatory T-cell subsets in primary HIV infection and identification of Tr1-like cells as the main interleukin 10-producing CD4+ T cells. The Journal of infectious diseases. 2015;211:769–79. doi: 10.1093/infdis/jiu549. [DOI] [PubMed] [Google Scholar]
- 9.Collier FM, Tang ML, Martino D, Saffery R, Carlin J, Jachno K, Ranganathan S, Burgner D, Allen KJ, Vuillermin P, Ponsonby AL. The ontogeny of naive and regulatory CD4(+) T-cell subsets during the first postnatal year: a cohort study. Clinical & translational immunology. 2015:4–e34. doi: 10.1038/cti.2015.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun W, Li WJ, Fu QL, Wu CY, Lin JZ, Zhu XL, Hou WJ, Wei Y, Wen YH, Wang YJ, Wen WP. Functionally distinct subsets of CD4(+) regulatory T cells in patients with laryngeal squamous cell carcinoma are indicative of immune deregulation and disease progression. Oncol Rep. 2015;33:354–62. doi: 10.3892/or.2014.3553. [DOI] [PubMed] [Google Scholar]
- 11.Diaz A, Santucci N, Bongiovanni B, D’Attilio L, Massoni C, Lioi S, Radcliffe S, Didoli G, Bottasso O, Bay ML. Increased frequency of CD4+ CD25+ FoxP3+ T regulatory cells in pulmonary tuberculosis patients undergoing specific treatment and its relationship with their immune-endocrine profile. Journal of immunology research. 2015;2015:985302. doi: 10.1155/2015/985302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang J, Yu L, Jiang C, Fu X, Liu X, Wang M, Ou C, Cui X, Zhou C, Wang J. Cerebral ischemia increases bone marrow CD4+CD25+ FoxP3+ regulatory T cells in mice via signals from sympathetic nervous system. Brain Behav Immun. 2015;43:172–83. doi: 10.1016/j.bbi.2014.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petrillo MG, Ronchetti S, Ricci E, Alunno A, Gerli R, Nocentini G, Riccardi C. GITR+ regulatory T cells in the treatment of autoimmune diseases. Autoimmun Rev. 2015;14:117–26. doi: 10.1016/j.autrev.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 14.Zhao Z, Ciric B, Yu S, Li H, Yang J, Kamoun M, Zhang GX, Rostami A. Expression of 3G11 epitope defines subpopulations of regulatory T cells with different suppressive potency. J Neurol Sci. 2010;295:66–74. doi: 10.1016/j.jns.2010.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang GX, Yu S, Calida D, Zhao Z, Gran B, Kamoun M, Rostami A. Loss of the surface antigen 3G11 characterizes a distinct population of anergic/regulatory T cells in experimental autoimmune encephalomyelitis. J Immunol. 2006;176:3366–73. doi: 10.4049/jimmunol.176.6.3366. [DOI] [PubMed] [Google Scholar]
- 16.Venigalla RK, Tretter T, Krienke S, Max R, Eckstein V, Blank N, Fiehn C, Ho AD, Lorenz HM. Reduced CD4+, CD25− T cell sensitivity to the suppressive function of CD4+, CD25 high, CD127−/low regulatory T cells in patients with active systemic lupus erythematosus. Arthritis Rheum. 2008;58:2120–30. doi: 10.1002/art.23556. [DOI] [PubMed] [Google Scholar]
- 17.Thiruppathi M, Rowin J, Ganesh B, Sheng JR, Prabhakar BS, Meriggioli MN. Impaired regulatory function in circulating CD4(+)CD25(high)CD127(low/−) T cells in patients with myasthenia gravis. Clin Immunol. 2012;145:209–23. doi: 10.1016/j.clim.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ukena SN, Grosse J, Mischak-Weissinger E, Buchholz S, Stadler M, Ganser A, Franzke A. Acute but not chronic graft-versus-host disease is associated with a reduction of circulating CD4(+)CD25 (high)CD127 (low/−) regulatory T cells. Ann Hematol. 2011;90:213–8. doi: 10.1007/s00277-010-1068-0. [DOI] [PubMed] [Google Scholar]
- 19.Shenghui Z, Yixiang H, Jianbo W, Kang Y, Laixi B, Yan Z, Xi X. Elevated frequencies of CD4(+) CD25(+) CD127lo regulatory T cells is associated to poor prognosis in patients with acute myeloid leukemia. International journal of cancer Journal international du cancer. 2011;129:1373–81. doi: 10.1002/ijc.25791. [DOI] [PubMed] [Google Scholar]
- 20.Zhou F, Zhang GX, Rostami A. 3G11 expression in CD4+ T cell-mediated autoimmunity and immune tolerance. Int Immunopharmacol. 2011;11:593–6. doi: 10.1016/j.intimp.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou F, Ciric B, Li H, Yan Y, Li K, Cullimore M, Lauretti E, Gonnella P, Zhang GX, Rostami A. IL-10 deficiency blocks the ability of LPS to regulate expression of tolerance-related molecules on dendritic cells. Eur J Immunol. 2012;42:1449–58. doi: 10.1002/eji.201141733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou F, Lauretti E, di Meco A, Ciric B, Gonnella P, Zhang GX, Rostami A. Intravenous transfer of apoptotic cell-treated dendritic cells leads to immune tolerance by blocking Th17 cell activity. Immunobiology. 2013;218:1069–76. doi: 10.1016/j.imbio.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou F, Ciric B, Zhang GX, Rostami A. Immune tolerance induced by intravenous transfer of immature dendritic cells via upregulating numbers of suppressive IL-10(+) IFN-gamma(+)-producing CD4(+) T cells. Immunol Res. 2013;56:1–8. doi: 10.1007/s12026-012-8382-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campbell DJ, Koch MA. Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nat Rev Immunol. 2011;11:119–30. doi: 10.1038/nri2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Papatriantafyllou M. Regulatory T cells: MARCHing for tolerance. Nat Rev Immunol. 2013;13:472. doi: 10.1038/nri3486. [DOI] [PubMed] [Google Scholar]
- 26.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–32. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.von Herrath MG, Harrison LC. Antigen-induced regulatory T cells in autoimmunity. Nat Rev Immunol. 2003;3:223–32. doi: 10.1038/nri1029. [DOI] [PubMed] [Google Scholar]
- 28.Kwiatek M, Geca T, Krzyzanowski A, Malec A, Kwasniewska A. Peripheral dendritic cells and CD4+CD25+Foxp3+ regulatory T cells in the first trimester of normal pregnancy and in women with recurrent miscarriage. PLoS One. 2015;10:e0124747. doi: 10.1371/journal.pone.0124747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greer JM, Koerner TA, Hayakawa K, Hardy RR, Kemp JD. The 3G11+ antigen, a marker for murine CD4+ TH1 lymphocytes, is a ganglioside. Glycobiology. 1993;3:391–401. doi: 10.1093/glycob/3.4.391. [DOI] [PubMed] [Google Scholar]
- 30.Simonetta F, Bourgeois C. CD4+FOXP3+ regulatory T-cell subsets in human immunodeficiency virus infection. Front Immunol. 2013;4:215. doi: 10.3389/fimmu.2013.00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hall BM, Verma ND, Tran GT, Hodgkinson SJ. Distinct regulatory CD4+ T cell subsets; differences between naive and antigen specific T regulatory cells. Curr Opin Immunol. 2011;23:641–7. doi: 10.1016/j.coi.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 32.Guo Y, Wu CZ, Liao Y, Zhang QY. The expression and significance of CD4+CD25+CD127 low/− regulatory T cells and Foxp3 in patients with portal hypertension and hypersplenism. Hepato-Gastroenterology. 2013;60:581–4. doi: 10.5754/hge11381. [DOI] [PubMed] [Google Scholar]
- 33.Geraldes L, Morgado J, Almeida A, Todo-Bom A, Santos P, Paiva A, Cheira C, Pais ML. Expression patterns of HLA-DR+ or HLA-DR− on CD4+/CD25++/CD127 low regulatory T cells in patients with allergy. Journal of investigational allergology & clinical immunology. 2010;20:201–9. [PubMed] [Google Scholar]
- 34.Bao SH, Wang XP, De Lin Q, Wang WJ, Yin GJ, Qiu LH. Decidual CD4 + CD25 + CD127dim/− regulatory T cells in patients with unexplained recurrent spontaneous miscarriage. Eur J Obstet Gynecol Reprod Biol. 2011;155:94–8. doi: 10.1016/j.ejogrb.2010.11.007. [DOI] [PubMed] [Google Scholar]