Abstract
Background
Epithelial ovarian cancer (EOC) is an aggressive and heterogeneous disease. Less than 10% of EOC demonstrate HER2/neu 3+ receptor over-expression. However, moderate to low (ie, 2+ and 1+) HER2/neu expression is reported in up to 50% of EOC. The objective of this study was to compare the anti-tumor activity of SYD985, a novel HER2-targeting antibody-drug conjugate (ADC), to trastuzumab emtansine (T-DM1) in EOC models with differential HER2/neu expression.
Methods
The cytotoxicity of SYD985 and T-DM1 was evaluated using ten primary EOC cell lines with 0/1+, 2+, and 3+ HER2/neu expression in antibody-dependent cellular cytotoxicity (ADCC), proliferation, viability and bystander killing experiments. Finally, the in vivo activity of SYD985 and T-DM1 was also studied in ovarian cancer xenografts
Results
SYD985 and T-DM1 induced similar ADCC in the presence of peripheral blood lymphocytes (PBL) against EOC cell lines with differential HER2/neu expression. In contrast, SYD985 was 3 to 42 fold more cytotoxic in the absence of PBL when compared to T-DM1 (p<0.0001). Unlike T-DM1, SYD985 induced efficient bystander killing of HER2/neu 0/1+ tumor cells when admixed with HER2/neu 3+ EOC cells. In vivo studies confirmed that SYD985 is significantly more active than T-DM1 against HER2/neu 3+ EOC xenografts.
Conclusions
SYD985 is a novel ADC with remarkable activity against EOC with strong (3+) as well as moderate to low (i.e., 2+ and 1+) HER2/neu expression. SYD985 is more potent than T-DM1 in comparative experiments and unlike T-DM1, it is active against EOC demonstrating moderate/low or heterogeneous HER2/neu expression.
Keywords: SYD985, T-DM1, antibody-drug conjugate, ovarian serous carcinoma, HER2, Ado-trastuzumab emtansine
Introduction
Epithelial ovarian carcinoma (EOC) is the most common cause of gynecological cancer death of women in the United States. According to the National Cancer Institute (Surveillance, Epidemiology, and End Results [SEER] Program), 22,280 EOC will be diagnosed in 2016 and 14,240 will succumb to the disease [1]. Due to inadequate screening tools and a lack of early clinical symptoms, approximately 70% of women with EOC are diagnosed with advanced stage of disease, which is associated with high morbidity and mortality [2]. Currently, standard primary therapy for patients with advanced EOC is either primary debulking surgery (PDS) to remove all visible tumor tissue or neoadjuvant chemotherapy (NACT) with paclitaxel and carboplatin [3, 4]. Despite treatment with these strategies, only 20–25% of cases are cured. Furthermore, 5-year survival rate of patients with advanced EOC has not seen a definite improvement in the last decade. The dismal prognosis of EOC patients emphasizes the unmet need to identify novel effective treatment options for patients with advanced or recurrent disease resistant to conventional chemotherapy.
The human HER2/neu (c-erbB2) gene product encodes a receptor protein that includes a cysteine-rich extracellular ligand binding domain, a hydrophobic transmembrane region, and an intracellular tyrosine kinase domain [5]. HER-2/neu functions as a preferred partner for heterodimerization with other members of the EGFR family (ErbB1, ErbB3 and ErbB4), and therefore plays a major role in coordinating the complex ErbB signaling network that is responsible for regulating cell growth and differentiation [5]. C-erbB2 gene amplifications and mutations have been identified in a variety of cancers, and it is predictive of response to adjuvant therapy and prognosis [6]. However, observed rates of HER2 overexpression/amplification in EOC show considerable variation ranging from 8% to 66% [7, 8] with significant intratumoral heterogeneity in receptor expression found in about 50% of the HER2 3+ and 2+ EOC samples [9]. The low incidence of c-erbB2 gene amplification and/or HER2 overexpression and the high HER2 intratumoral heterogeneity in EOC have so far significantly limited the impact of HER2-targeted therapies in the treatment of recurrent chemotherapy-resistant EOC.
Trastuzumab emtansine (T-DM1, Kadcyla, Genentech/Roche) is an antibody-drug conjugate (ADC) endowed with superior clinical activity when compared to trastuzumab naked antibody and accordingly, TDM-1 is currently FDA-approved for patients with HER2 positive breast cancer harboring trastuzumab-resistant disease [10, 11]. In T-DM1, the antibody and the cytotoxic agent are conjugated using a non-cleavable linker. This non-cleavable linker combines the HER2 targeting ability of trastuzumab with the cytotoxic effects of a maytansine derived anti-microtubule agent maytansine, DM1. Upon internalization of the ADC, DM1 binds to tubulin and inhibits microtubule assembly, leading to the cessation of mitosis and eventual apoptosis in dividing tumor cells [10, 12].
SYD985 (Synthon Biopharmaceuticals BV, Nijmegen, the Netherlands) is a novel HER2-targeting ADC which has a ‘cleavable’ linker-duocarmycin payload, valine-citrulline-seco Duocarmycin hydroxyBenzamide Azaindole (vc-seco-DUBA), conjugated to trastuzumab. Impressive preclinical results of SYD985 antitumor activity have been reported in breast cancer [13] and uterine serous cancer [10] followed by clinical activity of this ADC in Phase I clinical trial (NCT02277717). Importantly, the toxic payload in SYD985 (i.e., DUBA) binds to the minor groove of DNA and subsequently cause irreversible alkylation of DNA. This process disrupts the nucleic acid architecture, which leads to DNA damage, and ultimately cell death in both dividing and non-dividing cells [10, 13-15]. The linker cleavage in SYD985 and release of the membrane-permeable active toxin occur secondary to the activity of cysteine proteases such as cathepsin B present in endosomes and lysosomes which are released by human tumors. This property causes cell killing of not only HER2/neu positive cells but also of neighboring non-antigen-expressing tumor cells (i.e., bystander killing effect) [10].
The objective of this study was to evaluate the anti-tumor activity of SYD985 in comparison to T-DM1 in vitro and in vivo against primary EOC cell lines with different HER2/neu expression status. We report the first evidence that SYD985 is significantly more potent than T-DM1 in comparative in-vitro and in vivo experiments, especially against EOC with 2+ and 1+ HER2/neu expression. Importantly, our results also show that SYD985, unlike T-DM1, is able to induce a significant bystander effect against tumor cells with low/negligible HER2/neu expression when admixed with HER2/neu 3+ cells, suggesting potential clinical activity against patients harboring EOC with heterogeneous HER2/neu expression.
Materials and Methods
Establishment of EOC Cell Lines
Study approval was obtained from the Institutional Review Board (IRB), and all patients signed consent before tissue collection according to the institutional guidelines. Ten primary EOC cell lines (Table 1) were established from fresh tumor biopsy samples, as described previously [16]. Tumors were staged according to the International Federation of Gynecology and Obstetrics (FIGO) staging system. Patient characteristics are noted in Table 1. Primary EOC cell lines with limited passages (i.e. <50) were used in the experiments listed below and corresponding cell blocks were analyzed for HER2 surface expression by immunohistochemistry (IHC) and fluorescent in situ hybridization (FISH).
Table 1. Characteristics and demographic data of ovarian cancer cell lines.
| Cell line | Age | Race | FIGO* stage | Primary site | Histology | Flow cytometry (MFI)** | FISH*** | Flow cytometry/IHC score |
|---|---|---|---|---|---|---|---|---|
| KRCH31 | 69 | W | IV | Ovary | Serous | 119.68 | Amplified | 3+ |
| OVA11 | 79 | W | IC | Ovary | Clear cell | 290.76 | Amplified | 3+ |
| OVA10 | 51 | W | IIC | Ovary | Clear cell | 299.61 | Amplified | 3+ |
| OVA14 | 58 | W | IIIC | Ovary | Serous | 138.87 | Amplified | 3+ |
| OVA1 | 64 | W | IA | Ovary | Serous | 63.79 | Not amplified | 2+ |
| OVA5 | 60 | W | IIIC | Ovary | Serous | 85.31 | Not amplified | 2+ |
| OVA13 | 42 | W | IIIC | Ovary | Clear cell | 54.20 | Not amplified | 2+ |
| OVA12 | 32 | W | IC | Ovary | Clear cell | 27.10 | Not amplified | 1+/0 |
| OVA3 | 53 | W | IIIA | Ovary | Serous | 13.92 | Not amplified | 1+/0 |
| OVA4 | 64 | W | IIIC | Ovary | Serous | 20.65 | Not amplified | 1+/0 |
FIGO: International Federation of Gynecology and Obstetrics;
MFI: mean fluorescence intensity;
FISH: fluorescent in situ hybridization.
SYD985 and T-DM1
T-DM1 (batch N0001B02; Roche, Basel, Switzerland) was purchased by Synthon Biopharmaceuticals BV, Nijmegen, the Netherlands. SYD985 was prepared as previously described[13-15]. Briefly, vc-seco-DUBA was coupled to a cysteine residue of trastuzumab after partial reduction of the inter-chain disulfides. SYD985 was further purified to deliver a well-defined ADC predominantly consisting of species with a drug to antibody ratio (DAR) of 2 and 4, yielding a mean DAR of 2.8 [13, 14].
Immunostaining of Cell Blocks of Primary EOC
Cell blocks were obtained from all ten EOC cell lines and reviewed by a gynecologic surgical pathologist to confirm the presence of epithelial ovarian cancer cells. Briefly, HER2 immunohistochemical staining was performed on paraffin-embedded 5 μm sections of cell blocks after deparaffinisation and rehydration, using the c-erbB-2 antibody (Thermo Fisher Scientific, Fremont, CA) at 1:800 dilution. HER2 staining intensity was graded per the American Society of Clinical Oncology and the College of American Pathologists (ASCO/CAP) 2013 breast scoring criteria [17]. Appropriate positive and negative controls were used with each case.
Fluorescent In Situ Hybridization (FISH) of Cell Blocks From Primary EOC
Fluorescent in situ hybridization (FISH) analysis was performed using the PathVysion HER2 DNA FISH Kit (Abbott Molecular Inc., Abbott Park, IL, USA) according to the manufacturer's instructions. Cell block sections of 5 μm were deparaffinised and rehydrated, followed by acid pretreatment and proteinase K digestion. A probe mix containing an orange probe directed against the HER2 gene (Vysis, Inc., Downers Grove, IL, USA, LSI HER2) and a green probe directed against the pericentromeric region of chromosome 17 (Vysis CEP 17) were added and specimens were denatured for 5 minutes at 73°C. Slides were then incubated overnight in a humidity chamber at 37°C and washed the day after when a fluorescence mounting medium, containing 4, 6-diamidino-2-phenylindole (DAPI), was applied. Fluorescent signals in at least 30 non-overlapping interphase nuclei with intact morphology were scored using a Zeiss Axioplan 2 microscope (Carl Zeiss Meditec, Inc., Dublin, CA, USA) with a 100× planar objective, using a triple band-pass filter that permits simultaneous blue, green, and red colors. Tumor cells were scored for the number of orange (HER2) and green (chromosome 17) signals. A case was scored as amplified when the ratio of the number of fluorescent signals of HER2 to chromosome 17 was ≥2.
Tests for ADCC
A standard 4-hour chromium (51Cr) release assay was performed to measure the cytotoxic reactivity of Ficoll-Hypaque-separated PBLs from several healthy donors in combination with trastuzumab, T-DM1 and SYD985 against primary EOC target cell lines at an effector to target ratios (E:T) of 20:1 and 40:1. The release of 51Cr from target cells was measured as evidence of tumor cell lysis after exposure of the tumor cells to 2.0 μg/ml of trastuzumab or 2.0 μg/ml of T-DM1 and SYD985. Dose-response experiments were performed to determine the optimal antibody dosing for ADCC experiments. The negative control condition was the incubation of target cells alone. As a positive control condition, 0.1% sodium dodecyl sulfate (SDS) was used to achieve complete lysis of target cells. Chimeric anti-CD20 mAb rituximab 2.0 μg/ml was used as the negative control for trastuzumab, T-DM1 and SYD985 in all bioassays. The percentage cytotoxicity of trastuzumab, T-DM1 and SYD985 was calculated by the following formula: % cytotoxicity = 100 × (E-S)/(T-S), where E is the experimental release, S is the spontaneous release by target cells, T is the maximum release by target cells lysed with 0.1% SDS.
Cell Viability Assay
EOC cell lines were plated at log phase of growth in 6-well tissue culture plates at a density of 20,000-40,000 cells in RPMI 1640 media (Life Technologies, Carlsbad, CA) supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin (Mediatech, Manassas, VA), and 1% fungizone (Life Technologies, Carlsbad, CA). Cells were incubated at 37°C, 5% CO2. After 24 hours of incubation, cells were treated with SYD985, T-DM1, trastuzumab and isotype control ADC (i.e., rituximab conjugated to vc-seco-DUBA: SYD989). SYD985, T-DM1, and SYD989 were used at scalar concentrations of 0.005μg/ml, 0.05μg/ml, 0.5μg/ml, 2μg/ml and 8μg/ml. Trastuzumab was used at concentrations of 1μg/ml, 5μg/ml, 10μg/ml, 40μg/ml, and 100μg/ml. Three days after drug treatment, cells were harvested in their entirety, centrifuged and stained with propidium iodide (2μl of 500 μg/ml stock solution in PBS). The analysis was performed using a flow cytometry-based assay to quantify percent viable cells as a mean ± SEM relative to untreated cells as 100% viable controls. A minimum of three independent experiments per cell line was performed.
Bystander killing
Briefly, a 1:1 ratio of HER2/neu 3+ positive EOC cells (i.e., KRCH31) and HER2/neu negative USC cells (i.e., ARK-4) stably transfected with a Green Fluorescence Protein (GFP) plasmid (pCDH-CMV-MCSEF1-copGFP, a kind gift from Dr. Simona Colla, MDACC), were mixed (20,000 cells/well of each cell type) and plated in 6-well plates (3mL/well). After an overnight incubation, SYD985, T-DM1 or isotype control ADC at a concentration of 1μg/ml or vehicle were added. After a 72-hour incubation, cells were harvested in their entirety, centrifuged and stained with propidium iodide (2μl of 500 μg/ml stock solution in PBS) to facilitate the identification of dead cells. The analysis was performed using flow cytometry-based assay to recognize and quantify percent viable cells as a mean ± SEM relative to untreated cells as 100% viable controls. A minimum of three independent experiments was performed.
In vivo treatment
The in vivo antitumor activity of T-DM1 versus SYD985 was tested in xenograft models with 3+ HER2/neu expression. Briefly, six to eight week old CB-17/SCID mice were given a single subcutaneous injection of 7 × 106 OVA10 cells (HER2/neu 3+) in approximately 300 μL of a 1:1 solution of sterile PBS containing cells and Matrigel (BD Biosciences). Once the tumor volume was approximately 0.2 cm3, the mice were randomized into treatment groups (n=5); those treated with SYD985 (3mg/kg and 10mg/kg), T-DM1 (10 mg/kg), isotype control ADC (SYD989) (10mg/kg), and vehicle PBS. Drug dosages were chosen according to previous studies conducted on different xenograft models [10, 13, 14]. All treatment drugs were given as a single intravenous (IV) injection based on prior literature [10, 13, 14]. Mice were observed for overall survival as the primary outcome measure. Tumor measurements were recorded once weekly. Mice were sacrificed if tumor volume reached 1.5 cm3 using the formula (width)2 × height/2. Animal care and euthanasia were carried out according to the rules and regulations as set forth by the Institutional Animal Care and Use Committee (IACUC).
Statistical analysis
Statistical analysis was performed using GraphPad Prism version 6 (GraphPad Software, Inc. San Diego, CA). The differences in ADCC levels by 4-hr chromium release assays as well as the inhibition of proliferation in the EOC cell lines after exposure to SYD985 were evaluated by the two-tailed unpaired Student t-test. Overall survival data were analyzed and plotted using the Kaplan-Meier method. Survival curves were compared using the log-rank test. Differences in all comparisons were considered statistically significant at p-values < 0.05.
Results
HER2/neu expression in primary EOC cell lines
We evaluated erbB-2 gene amplification by FISH and HER2/neu protein expression by IHC and flow cytometry in ten primary EOC culture cell lines (Table 1). Gene amplification and high levels (3+ staining) of HER2 protein expression by IHC/flow cytometry were detected in 40% of the EOC cell lines (i.e. 4 out of 10). Three cell lines had moderate (2+) HER2 expression by IHC/flow cytometry, while the remaining three cell lines had 1+/0 expression (Table 1)(Figure 1). We utilized these ten primary EOC cell lines with similar growth rate and different HER2 expression for the additional in vitro and in vivo experiments described below.
Figure 1.
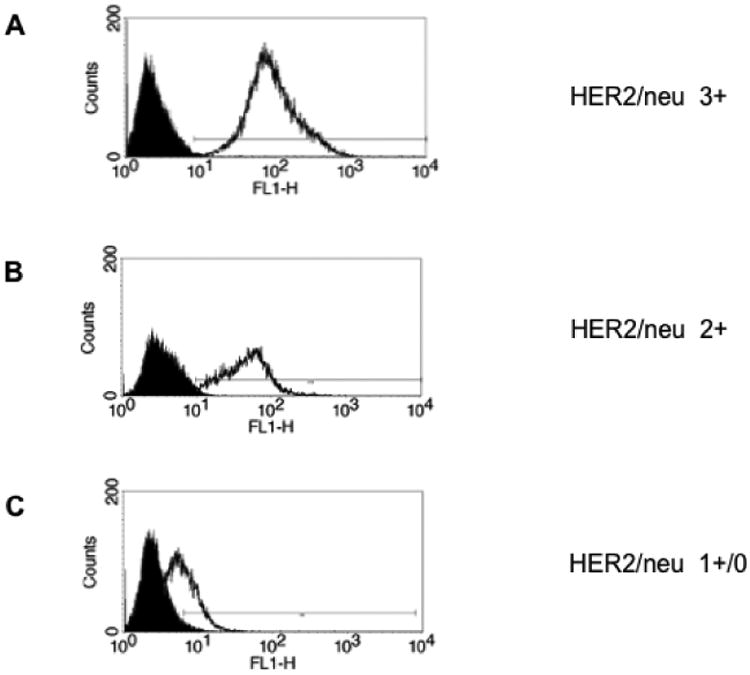
HER2/neu expression in representative EOC cell lines by flow cytometry: A) HER2 3+ cell line. B) HER2 2+ cell line. C) HER2 1+/0 cell line.
SYD985, T-DM1, and trastuzumab-mediated ADCC against HER2-positive primary EOC
Six representative primary EOC cell lines were tested for their sensitivity to PBL-mediated cytotoxicity when challenged with heterologous PBLs collected from several healthy donors in standard 4-h 51Cr release assays. EOC cell lines were consistently found to be resistant to PBL-mediated cytotoxicity when combined with PBLs and isotype control ADC (2.0 μg/mL) at E:T ratios of 20:1 and 40:1 (mean ± SEM cytotoxicity of 1.5± 0.1% with PBL alone and mean ± SEM cytotoxicity of 2.7 ± 0.1% in the presence of isotype control ADC + PBL, respectively)(Figure 2). We then investigated the sensitivity of EOC cell lines to heterologous PBLs in the presence of trastuzumab, SYD985, and T-DM1 at 2.0 μg/mL (Figure 2). SYD985, T-DM1, and trastuzumab (T) were similarly effective in inducing strong ADCC against primary EOC cell lines expressing HER2/neu at high levels [i.e., KRCH31, OVA10] with mean cytotoxicity ± SEM = 80.4 ± 1.53% for SYD985 vs. 75.6 ± 1.95% for T-DM1 vs. 84.45 ± 0.35% for Trastuzumab, p = 0.17. Similarly, no significant differences between SYD985, T-DM1, or trastuzumab in the induction of ADCC were detected against EOC cell lines with moderate (2+) or low/negligible (1+/0) HER2 expression (p=0.12 and p=0.52, respectively, Figure 2).
Figure 2.
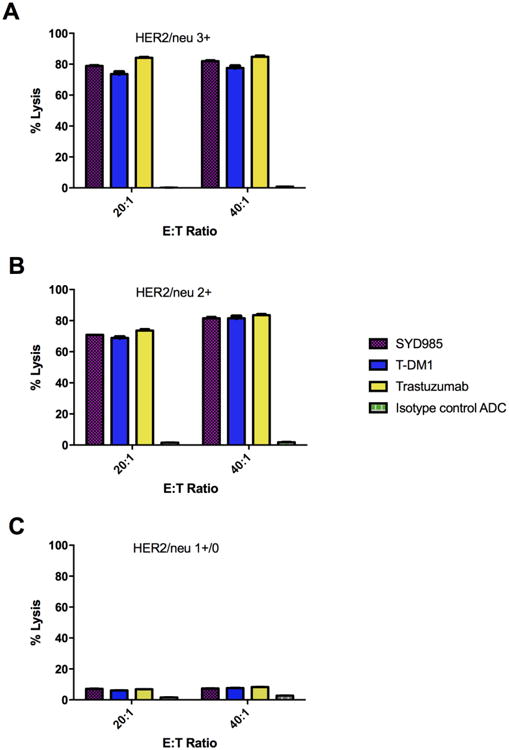
ADCC results (mean ± SD) of SYD985, T-DM1, Trastuzumab and ADC isotype control in a representative HER2 3+ expressing cell line (KRCH31, panel A), a representative 2+ HER2 expressing cell line [OVA5, panel B] and a representative 1+/0 HER2 expressing cell line (OVA3, panel C).
Cytotoxicity of SYD985 versus T-DM1 in vitro
Next, we exposed ten cell lines with different HER2/neu expression (Table 1) to scalar concentrations of ADC for a total of 3 days. As representatively demonstrated in Figure 3, SYD985 was more potent in inducing cell death than T-DM1 in all EOC cell lines tested regardless of the level of HER2/neu expression. In the HER2/neu 3+ cell lines, SYD985 exhibited a mean IC50 of 0.024 μg/mL while T-DM1 exhibited a mean IC50 = 0.088 μg/mL (p<0.0001). In the HER2/neu 2+ cell lines, SYD985 exhibited a mean IC50 of 0.054 μg/mL while T-DM1 exhibited a mean IC50 = 1.168 μg/mL (p<0.0001). Finally, in HER2/neu 1+/0 cell lines, SYD985 exhibited a mean IC50 = 0.072 μg/mL while T-DM1 a mean IC50 = 3.035 μg/mL (p<0.0001). Consistently, SYD985 was 3 to 42 fold more cytotoxic in the absence of PBL when compared to T-DM1 against primary EOC cell lines with 3+, 2+ and 1+/0 HER2/neu expression.
Figure 3.
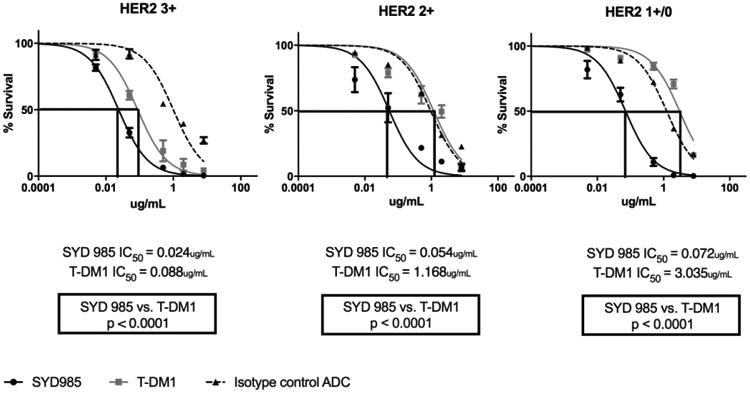
IC50 dose response curves of SYD985, T-DM1 and ADC isotype control in all EOC cell lines tested in vitro (i.e., HER2 3+ cell lines, p = <0.0001, HER2 2+ cell lines, p = <0.0001 and HER2 1+ cell lines, p = <0.0001) at 3 days.
Bystander killing in vitro
Next, we evaluated the ability of SYD985 and T-DM1 to induce bystander cytotoxicity of cells with low/negligible HER2 expression (i.e., ARK-4 cells) when admixed with KRCH31 (HER2/neu 3+) cells for 96 hours. As shown in the Figure 4a for SYD985, while no increase in the KRCH31 killing was noted when KRCH31 cells were co-cultured with ARK-4 (p=0.2929), KRCH31/ARK-4 co-cultures yielded a significant increase (ie, 25%, p=0.032) in the amount of bystander killing of HER2 low/negligible ARK-4 cells when compared to controls. In contrast, minimal bystander cytotoxicity was detected when KRCH31/ARK-4 co-cultures were challenged with T-DM1 (i.e., 1%, p = 0.55) or isotype control ADC for 96 hours (Figure 4b and 4c, respectively).
Figure 4.
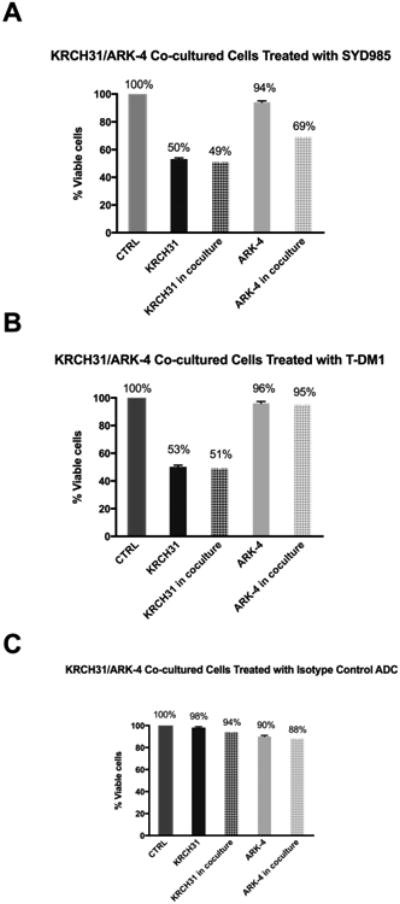
A) Cytotoxicity induced on HER2 3+ EOC cells (KRCH31), HER2 1+/0 USC cells (ARK-4), and KRCH31/ARK-4 co-cultures with 1μg/ml of SYD985. A significant increase of killing of HER2 1+/0 USC cells was detected when compared to control (p= 0.001) after treatment with SYD985. B) Cytotoxicity induced on HER2 3+ EOC cells (KRCH31), HER2 1+/0 USC cells (ARK-4), and KRCH31/ARK-4 co-cultures with 1μg/ml of SYD995 (T-DM1). No significant increase of killing of HER2 1+/0 USC cells was detected when compared to control (p= 0.29) after treatment with T-DM1. C) Cytotoxicity induced on HER2 3+ EOC cells (KRCH31), HER2 1+/0 USC cells (ARK-4), and KRCH31 /ARK-4 co-cultures with 1μg/ml of SYD989 (isotype control ADC). No significant increase of killing of HER2 1+/0 USC cells was detected when compared to control (p= 0.51) after treatment with isotype control ADC.
In vivo antitumor activity of SYD985 versus T-DM1
The in vivo effects of SYD985 and T-DM1 were determined by establishing xenografts of primary EOC cell lines with 3+ HER2/neu expression [i.e., OVA10 (HER2/neu 3+)]. After the tumors had reached the goal volume (i.e., 0.2 cm3), animals were randomized into treatment groups and treated as described previously [10]. Tumors were assessed once weekly, and mice were sacrificed if tumors became necrotic, reached a volume of 1.5cm3, or mice appeared to be in poor health. Treatment with a single injection of SYD985 showed remarkable inhibition of tumor growth in mice xenografts with 3+ HER2/neu expression (Figure 5). In OVA10 (HER2/neu 3+) xenografts, we detected a significant difference in growth inhibition between animals treated with SYD985 3 mg/kg (p=0.0002 at 22 days) and 10 mg/kg (p=0.0001 at 15 days) when compared to T-DM1 at 10 mg/kg (Figure 5).
Figure 5.
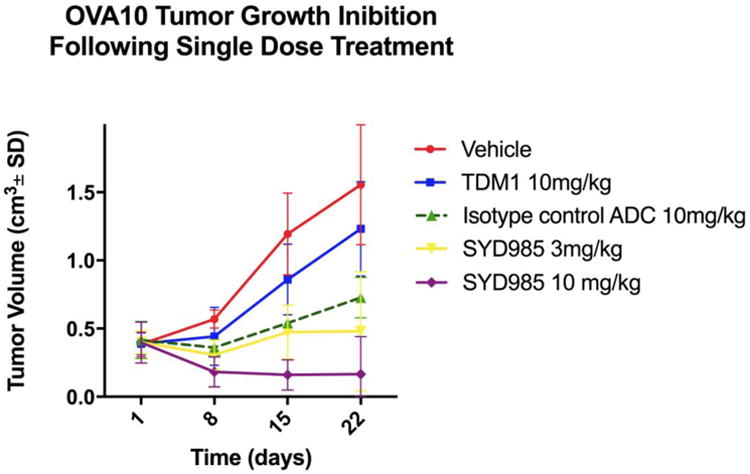
Antitumor activity of SYD985 compared to T-DM1 and ADC isotype control in EOC xenograft tumor models with OVA10 (HER2/neu 3+). Mice were treated with a single dose administered intravenously as described in Methods. A significant difference in tumor growth inhibition was detected in SYD985-treated groups at the dose of 3mg/kg and 10 mg/kg when compared to the other treatment groups.
A significant survival advantage was also seen in SYD985 when compared to TDM-1-treated xenografts (Figure 6). Specifically, when comparing SYD985 10mg/kg single injection to T-DM1 10mg/kg single injection, there was a significant difference in mean overall survival of 117 days versus 30 days, respectively, (p=0.028). Notably, 2 out of 5 (40%) of the mice were alive and disease-free at 180 days after a single injection of SYD985 at a dose of 10mg/kg. In contrast, there was no statistically significant difference in mean overall survival when comparing SYD985 3 mg/kg single injection to T-DM1 10 mg/kg single injection (mean overall survival of 32.5 days versus 30.75 days, respectively). In agreement with our in vitro results, isotype control ADC rituximab provided a superior survival benefit when compared to T-DM1 in the HER2/neu 3+ xenograft models (mean overall survival of 50 days versus 30.75 days, respectively, p=0.028). Mice treated with 10 mg/kg of isotype control ADC demonstrated a significantly shorter survival when compared to mice treated with SYD985 at 10 mg/kg (Figure 6).
Figure 6.
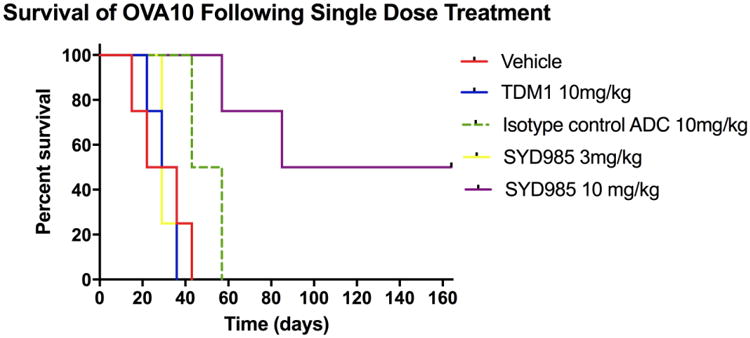
Overall survival in mice inoculated with EOC xenografts with OVA10 (HER2/neu 3+) after treatment with vehicle, single injection SYD985 (3mg/kg and 10mg/kg), and single injection T-DM1 (10mg/kg) and ADC isotype control. Significantly prolonged overall survival with SYD985 10 mg/kg treated group was detected when compared to the other treatment groups.
Discussion
Human epithelial growth factor receptor 2 (HER2/ErbB2) is a prominent member of the epidermal growth factor receptor (EGFR) family of trans-membrane receptors, as it plays a significant role in the development and prognosis of multiple human tumors [18-20]. Because of its overexpression in a variety of biologically aggressive malignancies, HER2 is a recognized target for antibody mediated treatment and tyrosine kinase inhibitor therapy [21]. In HER2 positive metastatic breast cancer, small molecules inhibiting both EGFR (erbB1) and HER2 receptors (i.e., lapatinib), along with humanized Mab targeting HER2 (i.e., trastuzumab and pertuzumab), have been shown to be clinically effective. Unfortunately, these HER2 antibodies and small molecule inhibitors demonstrated limited clinical benefits in patients with recurrent ovarian cancer, both alone or in combination with chemotherapy during phase I/II clinical trials [22-24].
Antibody-drug conjugates (ADCs) are sophisticated drug delivery systems that provide one of the most promising approaches to improving the therapeutic window of existing chemotherapy agents [25]. Moreover, the population of cancer patients who may benefit from HER2/neu-targeted therapy could be significantly increased by the use of more effective HER2/neu targeting ADCs, which can trigger killing of neighboring bystander cells with moderate, low and no HER2/neu expression. Accordingly, this is the first study to evaluate the pre-clinical activity of SYD985, a novel HER2-targeting ADC composed of trastuzumab linked to the highly potent DNA-alkylating agent, seco-DUBA, with a cleavable linker, against multiple EOC primary cell lines with different HER2/neu expression (i.e., 1+/0, 2+, and 3+). Our experiments consistently demonstrate that SYD985, when compared to T-DM1, is significantly more cytotoxic against primary EOC cell lines with HER2/neu expression of 1+/0, 2+ and 3+ in the absence of PBLs (i.e., effector cells). Specifically, in HER2/neu 3+ cell lines, we found SYD985 to be over ten folds more potent than T-DM1 (p = 0.0498) in inducing tumor cell death. In HER2/neu 2+ cell lines, SYD985 was 34 folds more potent than T-DM1 (p = 0.0468), while in HER2/neu 1+ cell lines, SYD985 was 75 folds more potent than T-DM1 (p= 0.0356). These results are most likely explained by the higher toxicity of the duocarmycin payload when compared to maytansine payload when internalized in tumor cell cytoplasm as well as its ability, unlike maytansine, to cause cell death in both dividing and non-dividing cells.
One of the biggest challenges in EOC treatment using highly targeted agents such as ADC is represented by the reported high HER2 intratumoral heterogeneity present in both primary and metastatic sites of the disease [7]. Importantly, in previous studies, we have shown that the cleavage of duocarmycin from its linker, unlike the cleavage of DM1, may take place in tumor cells not only within HER2/neu overexpressing tumor cells after antibody internalization, [13, 14] but also extracellularly within the tumor microenvironment, inducing a potent bystander effect. Consistent with this view, and similarly to our recently reported research work with SYD985 in biologically aggressive USC [10], we found this ADC to be significantly more potent than T-DM1 in its ability to induce bystander killing of low/negative HER2/neu expressing tumor cells admixed with HER2/neu positive tumor cells. These results strongly suggest that SYD985 may represent a significantly more effective therapeutic tool when compared to T-DM1 or naked trastuzumab in the treatment of recurrent EOC patients harboring tumors with heterogenous HER2/neu expression.
Importantly, in vivo experiments using as a model a 3+ HER2 positive EOC xenograft established in our laboratory, a single injection of SYD985 was sufficient to induce complete tumor regression in 40% of the mice harboring established tumors. These results are confirmatory of the in vitro results and demonstrate the higher efficacy of SYD985 vs. TDM-1 against EOC with 3+ HER2/neu expression.
Taken together, our results strongly suggest that SYD985 may represent a significantly more effective therapeutic tool when compared to T-DM1 or trastuzumab in HER2/neu expressing EOC. Moreover, SYD985 may be effective against EOC with low to moderate HER2/neu expression and may significantly increase the number of EOC patients potentially benefitting from this targeted therapeutic approach. Consistent with this view, encouraging clinical results have recently been reported in breast cancer patients with disease resistant to trastuzumab and TDM-1 with the administration of SYD985 [26]. A phase I trial evaluating the safety and efficacy of SYD985 in the treatment of patients with locally advanced or metastatic solid tumors is currently ongoing (NCT02277717).
In conclusion, we have demonstrated that SYD985 is a novel ADC with remarkable activity against EOC with high (3+) as well as moderate to low (i.e., 2+/1+) HER2/neu expression. SYD985 is significantly more potent than T-DM1 in comparative experiments and unlike T-DM1, it may be active against EOC demonstrating heterogeneous HER2/neu expression. Clinical studies with SYD985 in EOC patients harboring disease resistant to standard salvage chemotherapy are warranted.
Highlights.
SYD985 is a novel ADC with remarkable activity against HER2 amplified EOC.
SYD985 is more potent than T-DM1 against EOC with HER2/neu expression.
Unlike T-DM1, SYD985 may be active against EOC with heterogeneous HER2/neu expression.
Acknowledgments
We thank Synthon Biopharmaceuticals for the supply of SYD985 and its isotype control ADC.
Financial support and Grant Support: This work was supported in part by R01 CA154460-01 and U01 CA176067-01A1 grants from NIH, and grants from the Deborah Bunn Alley Foundation, the Tina Brozman Foundation, the Discovery to Cure Foundation and the Guido Berlucchi Foundation to A.D. Santin. This investigation was also supported by NIH Research Grant CA-16359 from the NCI to A.D. Santin.
Footnotes
Conflict Of Interest Statement: The authors declare no conflict of interest or previous publication. All of the authors fulfill the conditions required for authorship.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010;19:1893–907. doi: 10.1158/1055-9965.EPI-10-0437. [DOI] [PubMed] [Google Scholar]
- 3.Harter P, du Bois A. The role of surgery in ovarian cancer with special emphasis on cytoreductive surgery for recurrence. Curr Opin Oncol. 2005;17:505–14. doi: 10.1097/01.cco.0000174166.06734.c7. [DOI] [PubMed] [Google Scholar]
- 4.Vergote I, Trope CG, Amant F, Kristensen GB, Ehlen T, Johnson N, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363:943–53. doi: 10.1056/NEJMoa0908806. [DOI] [PubMed] [Google Scholar]
- 5.Salvesen HB, Carter SL, Mannelqvist M, Dutt A, Getz G, Stefansson IM, et al. Integrated genomic profiling of endometrial carcinoma associates aggressive tumors with indicators of PI3 kinase activation. Proc Natl Acad Sci U S A. 2009;106:4834–9. doi: 10.1073/pnas.0806514106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noske A, Schwabe M, Weichert W, Darb-Esfahani S, Buckendahl AC, Sehouli J, et al. An intracellular targeted antibody detects EGFR as an independent prognostic factor in ovarian carcinomas. BMC Cancer. 2011;11:294. doi: 10.1186/1471-2407-11-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tuefferd M, Couturier J, Penault-Llorca F, Vincent-Salomon A, Broet P, Guastalla JP, et al. HER2 status in ovarian carcinomas: a multicenter GINECO study of 320 patients. PLoS One. 2007;2:e1138. doi: 10.1371/journal.pone.0001138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wen W, Chen WS, Xiao N, Bender R, Ghazalpour A, Tan Z, et al. Mutations in the Kinase Domain of the HER2/ERBB2 Gene Identified in a Wide Variety of Human Cancers. The Journal of molecular diagnostics : JMD. 2015;17:487–95. doi: 10.1016/j.jmoldx.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mittermeyer G, Malinowsky K, Beese C, Hofler H, Schmalfeldt B, Becker KF, et al. Variation in cell signaling protein expression may introduce sampling bias in primary epithelial ovarian cancer. PLoS One. 2013;8:e77825. doi: 10.1371/journal.pone.0077825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Black J, Menderes G, Bellone S, Schwab CL, Bonazzoli E, Ferrari F, et al. SYD985, a Novel Duocarmycin-Based HER2-Targeting Antibody-Drug Conjugate, Shows Antitumor Activity in Uterine Serous Carcinoma with HER2/Neu Expression. Mol Cancer Ther. 2016 doi: 10.1158/1535-7163.MCT-16-0163. [DOI] [PubMed] [Google Scholar]
- 11.Mathew J, Perez EA. Trastuzumab emtansine in human epidermal growth factor receptor 2-positive breast cancer: a review. Curr Opin Oncol. 2011;23:594–600. doi: 10.1097/CCO.0b013e32834b895c. [DOI] [PubMed] [Google Scholar]
- 12.Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–91. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Lee MM, Groothuis PG, Ubink R, van der Vleuten MA, van Achterberg TA, Loosveld EM, et al. The Preclinical Profile of the Duocarmycin-Based HER2-Targeting ADC SYD985 Predicts for Clinical Benefit in Low HER2-Expressing Breast Cancers. Mol Cancer Ther. 2015;14:692–703. doi: 10.1158/1535-7163.MCT-14-0881-T. [DOI] [PubMed] [Google Scholar]
- 14.Dokter W, Ubink R, van der Lee M, van der Vleuten M, van Achterberg T, Jacobs D, et al. Preclinical profile of the HER2-targeting ADC SYD983/SYD985: introduction of a new duocarmycin-based linker-drug platform. Mol Cancer Ther. 2014;13:2618–29. doi: 10.1158/1535-7163.MCT-14-0040-T. [DOI] [PubMed] [Google Scholar]
- 15.Elgersma RC, Coumans RG, Huijbregts T, Menge WM, Joosten JA, Spijker HJ, et al. Design, Synthesis, and Evaluation of Linker-Duocarmycin Payloads: Toward Selection of HER2-Targeting Antibody-Drug Conjugate SYD985. Mol Pharm. 2015;12:1813–35. doi: 10.1021/mp500781a. [DOI] [PubMed] [Google Scholar]
- 16.Zhao S, Choi M, Overton JD, Bellone S, Roque DM, Cocco E, et al. Landscape of somatic single-nucleotide and copy-number mutations in uterine serous carcinoma. Proc Natl Acad Sci U S A. 2013;110:2916–21. doi: 10.1073/pnas.1222577110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skirnisdottir I, Sorbe B, Seidal T. The growth factor receptors HER-2/neu and EGFR, their relationship, and their effects on the prognosis in early stage (FIGO I-II) epithelial ovarian carcinoma. Int J Gynecol Cancer. 2001;11:119–29. doi: 10.1046/j.1525-1438.2001.011002119.x. [DOI] [PubMed] [Google Scholar]
- 18.Garrido-Castro AC, Felip E. HER2 driven non-small cell lung cancer (NSCLC): potential therapeutic approaches. Transl Lung Cancer Res. 2013;2:122–7. doi: 10.3978/j.issn.2218-6751.2013.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seo AN, Kwak Y, Kim DW, Kang SB, Choe G, Kim WH, et al. HER2 status in colorectal cancer: its clinical significance and the relationship between HER2 gene amplification and expression. PLoS One. 2014;9:e98528. doi: 10.1371/journal.pone.0098528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sheng Q, Liu J. The therapeutic potential of targeting the EGFR family in epithelial ovarian cancer. Br J Cancer. 2011;104:1241–5. doi: 10.1038/bjc.2011.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iqbal N, Iqbal N. Human Epidermal Growth Factor Receptor 2 (HER2) in Cancers: Overexpression and Therapeutic Implications. Molecular biology international. 2014;2014:852748. doi: 10.1155/2014/852748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bookman MA, Darcy KM, Clarke-Pearson D, Boothby RA, Horowitz IR. Evaluation of monoclonal humanized anti-HER2 antibody, trastuzumab, in patients with recurrent or refractory ovarian or primary peritoneal carcinoma with overexpression of HER2: a phase II trial of the Gynecologic Oncology Group. J Clin Oncol. 2003;21:283–90. doi: 10.1200/JCO.2003.10.104. [DOI] [PubMed] [Google Scholar]
- 23.Weroha SJ, Oberg AL, Ziegler KL, Dakhilm SR, Rowland KM, Hartmann LC, et al. Phase II trial of lapatinib and topotecan (LapTop) in patients with platinum-refractory/resistant ovarian and primary peritoneal carcinoma. Gynecol Oncol. 2011;122:116–20. doi: 10.1016/j.ygyno.2011.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sims AH, Zweemer AJ, Nagumo Y, Faratian D, Muir M, Dodds M, et al. Defining the molecular response to trastuzumab, pertuzumab and combination therapy in ovarian cancer. Br J Cancer. 2012;106:1779–89. doi: 10.1038/bjc.2012.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perez HL, Cardarelli PM, Deshpande S, Gangwar S, Schroeder GM, Vite GD, et al. Antibody-drug conjugates: current status and future directions. Drug discovery today. 2014;19:869–81. doi: 10.1016/j.drudis.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Aftimos P, van Herpen C, Mommers E, Koper N, Goedings P, Oesterholt M, et al. SYD985, a novel anti-HER2 ADC, shows promising activity in patients with HER2-positive and HER2-negative metastatic breast cancer. Poster presented at the San Antonio Breast Cancer Symposium; San Antonio, TX. December 6-10, 2016; [Last accessed on December 17, 2016]. Available at: http://www.abstracts2view.com/sabcs/view.php?nu=SABCS16L_323. [Google Scholar]


